Abstract
Background
Randomized controlled trials evaluated monoclonal antibodies for the treatment (Study 2067) and prevention (Study 2069) of coronavirus disease 2019 (COVID-19). Household contacts of the infected index case in Study 2067 were enrolled in Study 2069 and prospectively followed; these cohorts provided a unique opportunity to evaluate correlates of transmission, specifically viral load.
Methods
This post hoc analysis was designed to identify and evaluate correlates of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission, adjusting for potential confounding factors related to source SARS-CoV-2 viral load and risk of SARS-CoV-2 acquisition in this population. Correlates of transmission were evaluated in potential transmission pairs (any infected household member plus susceptible household contact).
Results
In total, 943 participants were included. In multivariable regression, 2 potential correlates were determined to have a statistically significant (P < .05) association with transmission risk. A 10-fold increase in viral load was associated with a 40% increase in odds of transmission; sharing a bedroom with the index participant was associated with a 199% increase in odds of transmission.
Conclusions
In this prospective, post hoc analysis that controlled for confounders, the 2 key correlates for transmission of SARS-CoV-2 within a household are sharing a bedroom and increased viral load, consistent with increased exposure to the infected individual.
Keywords: acquisition, COVID-19, household, SARS-CoV-2, transmission
In this post hoc, prospective analysis of placebo-treated participants from 2 related COVID-19 treatment and prevention studies, sharing a bedroom with an infected individual and increased viral load were the key correlates for transmission of SARS-CoV-2 within a household.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), is primarily spread through exposure to respiratory droplets from an infected individual [1]. Secondary attack rates within households have been estimated to be as high as 51.2% [2, 3], driving onward transmission at the community level. Previous cross-sectional studies have demonstrated a number of potential contributors to household transmission of SARS-CoV-2 [4]. However, the breadth of the current data is based on retrospective, observational studies from healthcare databases or contact tracing [5, 6]. Neither study type can ensure proper attribution of direction or source of transmission. Prospective evaluation of SARS-CoV-2 household transmission with repeated testing is needed to more accurately evaluate timing and order of infections to identify transmission events.
Clinical trials that enroll household members with frequent monitoring offer a unique opportunity to evaluate correlates of SARS-CoV-2 transmission. Casirivimab and imdevimab (CAS + IMD) are coadministered anti–SARS-CoV-2 monoclonal antibodies that were previously used for treatment and prevention of COVID-19 [7–11]. CAS + IMD is no longer authorized for use as the combination is not active against current variants [12]. The efficacy and safety of CAS + IMD for treatment of outpatients with COVID-19 was evaluated in a randomized, double-blind, placebo-controlled trial, referred to herein as Study 2067 [13]. Additionally, the efficacy and safety of CAS + IMD for prevention of COVID-19 in household contacts of an infected individual was evaluated in a randomized, double-blind, placebo-controlled trial, referred to herein as Study 2069 [14, 15]. Household contacts of the infected index case in Study 2067 were able to be enrolled in Study 2069, presenting a unique opportunity to prospectively evaluate correlates of SARS-CoV-2 acquisition and transmission, specifically to investigate a dose response for SARS-CoV-2 viral load and transmission, within a household in participants from these trials.
METHODS
Analysis Overview
Two related studies, described below, are included in this post hoc analysis: a COVID-19 outpatient treatment study (Study 2067) and a COVID-19 prevention study (Study 2069).
Study 2069 Design
The design of this 2-part, randomized, double-blind, placebo-controlled, phase 3 trial (also referred to as COVID-19 Prevention Network 3502) assessing the efficacy and safety of subcutaneously administered CAS + IMD in preventing SARS-CoV-2 infections among uninfected household contacts of infected individuals (part A) and in recently infected individuals (part B) was previously published (ClinicalTrials.gov identifier NCT04452318) [14, 15]. Asymptomatic household contacts of an index case were treated with a single dose of CAS + IMD or placebo and followed through day 29 with weekly nasopharyngeal swabs for SARS-CoV-2 reverse-transcription quantitative polymerase chain reaction (RT-qPCR) testing and weekly interviews to assess for COVID-19 symptoms. Part A participants were included in this analysis if they were seronegative at baseline and randomized to placebo. Part B participants were included, regardless of serostatus, if they had a household member enrolled in Study 2067 and had another household member in part A of Study 2069 randomized to placebo. Study 2069 data utilized in this analysis were collected from 13 July 2020 to 12 February 2021, prior to the emergence of the Delta and Omicron variants.
All Study 2069 participants completed a questionnaire on household member status (1 per household) including information on general age, the presence/absence of COVID-19 symptoms, COVID-19 testing status, relationship to index case, index case participation in Study 2067, and monoclonal antibody treatment (Supplementary Table 1) and a questionnaire on their interaction with the index case including sharing of household spaces, masking in the home, the presence/absence of COVID-19 symptoms, and COVID-19 treatment(s) (Supplementary Table 2).
Study 2067 Design
The phase 3 portion of this randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of intravenous CAS + IMD in outpatients with COVID-19 was previously published (ClinicalTrials.gov identifier NCT04425629) [16]. In brief, SARS-CoV-2–positive patients (from a diagnostic sample collected ≤72 hours prior to randomization) were treated with a single dose of CAS + IMD or placebo and followed through day 29. Patients were enrolled in the phase 3 portion of Study 2067 between 24 September 2020 and 17 January 2021, prior to the emergence of the Omicron variant. Only Study 2067 participants with a household member enrolled in Study 2069 part A and randomized to placebo were evaluated in this analysis.
Patient Consent Statement
The original studies were conducted in accordance with the Declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice guidelines, and applicable regulatory requirements. The local institutional review board or ethics committee at each study center oversaw trial conduct and documentation. All patients provided written informed consent before participating in the trial.
Analysis Data Sets
Participants enrolled in the United States (to reduce country-specific sources of heterogeneity that cannot be adjusted for) from Studies 2067 and 2069 were eligible for inclusion in this analysis. Three analysis data sets were defined to assess the main outcome of SARS-CoV-2 household transmission and 2 supporting analyses of SARS-CoV-2 viral load in the potential source and SARS-CoV-2 acquisition in household contacts.
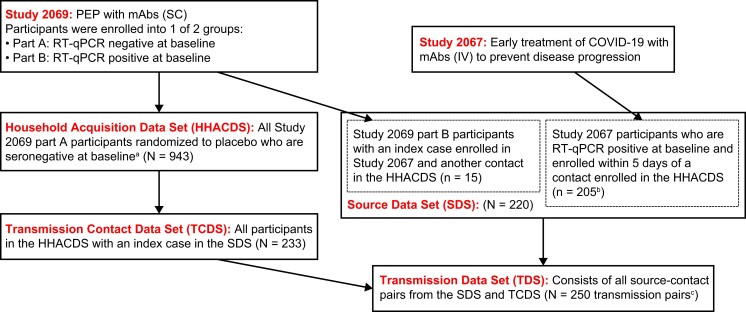
Following the order of Figure 1, which describes the data set derivations, the household acquisition data set (HHACDS) included Study 2069 part A participants who were randomized to the placebo arm in part A of Study 2069, were seronegative, and had a RT-qPCR result in the first 10 days of follow-up and was used to assess the endpoint of SARS-CoV-2 infection (acquisition) in household contacts. The transmission contact data set (TCDS) includes the subset of participants in HHACDS who had a household member enrolled in Study 2067 within 5 days of the contact's enrollment. To be included in this data set, the contact had to have at least 1 household member who is a potential source (enrolled in Study 2067 or Study 2069 part B with detectable SARS-CoV-2 virus at enrollment). The source data set (SDS) includes all Study 2067 and Study 2069 part B participants who shared a household with a participant in the TCDS; correlates of SARS-CoV-2 viral load were analyzed in this data set. The transmission data set (TDS) consists of all possible transmission pairs (infected household member plus susceptible household contact) from the TCDS and SDS to assess the transmission endpoint.
Figure 1.
Derivation of analysis data sets. Derivation of study data sets from index cases enrolled in Study 2067 and household contacts enrolled in Study 2069. aMust have been enrolled in the United States and had a reverse-transcription quantitative polymerase chain reaction result in the first 10 days of follow-up. bTwo households in Study 2069 had the same index case from Study 2067. cTransmission pairs were derived from 220 sources in the source data set (205 index cases from Study 2067 and 15 infected household contacts from Study 2069 part B) and 233 uninfected contacts from the transmission contact data set. Abbreviations: COVID-19, coronavirus disease 2019; HHACDS, household acquisition data set; IV, intravenous; mAbs, monoclonal antibodies (casirivimab and imdevimab); PEP, postexposure prophylaxis; RT-qPCR, reverse-transcription quantitative polymerase chain reaction; SC, subcutaneous; SDS, source data set; TCDS, transmission contact data set; TDS, transmission data set.
Statistical Analysis
SARS-CoV-2 Viral Load Analysis
This analysis was performed on the SDS. Linear regression was used to model the first observed viral load in the potential source as an outcome with predictors including region, ethnicity, age, sex, body mass index, and serostatus univariably and in a multivariable model including covariates selected by adaptive lasso with 10-fold cross-validation to select the tuning parameter. Notably, the first observed viral load was also the observed peak viral load in approximately 90% of evaluated participants.
SARS-CoV-2 Acquisition Analysis
Correlates of and risk factors for acquisition were modeled in the HHACDS using univariable and multivariable generalized estimating equations to account for household correlation assuming working independence with a log link to estimate the relative risk of acquisition. Covariates for the multivariable model were selected using adaptive lasso [17]. Acquisition, the endpoint, was defined as SARS-CoV-2 infection detected by day 10. This analysis was repeated on the TCDS to identify potential confounders in the final TDS.
Correlates of SARS-CoV-2 Transmission Analysis
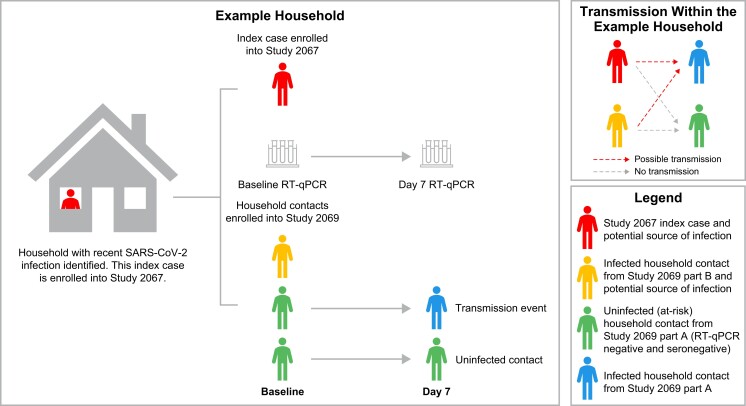
To assess correlates of transmission, a competing risk type model was employed that allocated each negative household member a risk of transmission from each infected household member while allowing that the negative household member can only be infected by 1 household member. Each potential transmission pair (infected household member [potential source] with negative household member [contact]) constituted the unit of analysis (TDS). Figure 2 provides an example of potential transmission within a household. The prospective design of these studies, with household contacts enrolled early in the index case's infection, allows us to determine the sequence of infection. The model determines the likelihood of transmission to susceptible household contacts from each potential source. We included viral load from each potential source in a transmission pair as a predictor of transmission. Other covariates were included if they were selected via adaptive lasso in the acquisition or viral load analyses on the subset of participants in the TDS (analyses performed on the TCDS and SDS, respectively). All correlates selected for the multivariable acquisition model (in the HHACDS) were included in univariable models. Two sensitivity analyses were conducted. The first explored potential bias from including households where some members were excluded by refitting the multivariable model to only those households for whom all participants were enrolled. The second assessed further potential confounding factors by building a second multivariable model including all covariates that were significant at P < .05 in univariable analyses. A cubic B-spline was included to determine if there was a nonlinear relationship between SARS-CoV-2 viral load and the log odds of transmission increases. The secondary attack rate within pairs was estimated using an intercept-only form of the model. Additional details on the statistical model for SARS-CoV-2 transmission are provided in the Supplementary material.
Figure 2.
Possible transmission within an example household. In this example, there are 4 household members—2 potential sources (an index case [Study 2067] and a household contact that was infected at baseline [Study 2069 part B]) and 2 at-risk household contacts enrolled in Study 2069 part A—yielding 4 transmission pairs. This graphic also illustrates the prospective nature of the studies. Abbreviations: RT-qPCR, reverse-transcription quantitative polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
RESULTS
Correlates of SARS-CoV-2 Viral Load in the Potential Sources
Baseline characteristics of the 220 potential sources (205 enrolled in Study 2067 and 15 enrolled in Study 2069 part B) are described in Supplementary Table 3 with the derivation of the potential infectors (SDS) described in Figure 1.
We examined correlates of viral load in the potential infectors to determine potential confounders for the transmission analysis. Seropositivity and being of Hispanic or Latino ethnicity are correlates of decreased viral load (Supplementary Table 4). These characteristics may confound the relationship between viral load and risk of transmission.
Correlates of SARS-COV-2 Acquisition in the Household Contacts
This post hoc analysis evaluated correlates of SARS-CoV-2 acquisition in the first week of observation in placebo-treated participants from Study 2069 who were uninfected (RT-qPCR negative) and seronegative at baseline (HHACDS). This includes 943 participants and is defined as the uninfected household contacts (Figure 1). Demographics and baseline characteristics for participants in uninfected household contacts and the subset of those participants in all uninfected household contacts living with an index case (TCDS) are presented in Supplementary Table 5.
Supplementary Table 6 includes estimates of relative risk of SARS-CoV-2 acquisition univariably for all characteristics considered for inclusion in a multivariable model as well as estimates from the final multivariable model. In the final multivariable model, male sex, Hispanic or Latino ethnicity, and sharing a bedroom with an index case are statistically significant correlates of SARS-CoV-2 infection, with male sex and Hispanic or Latino ethnicity associated with decreased risk and bedroom sharing associated with increased risk. Other variables selected for the model may still play an important role in SARS-CoV-2 acquisition. Most notably, having another person in the household, besides the index case, who is symptomatic and infected was estimated to increase the risk of infection >2-fold, although this was not statistically significant in the multivariable model. Age was significant univariably but was not selected for the multivariable model, suggesting confounding with other covariates.
Correlates of SARS-CoV-2 Transmission in Households
The aim of this analysis was to evaluate the correlates of SARS-CoV-2 transmission from any potential household source—the index case or an infected household member—to susceptible household contacts. The analysis is conducted on the potential transmission pairs (TDS) and includes 250 transmission pairs (Figure 1) with a secondary attack rate, defined as the probability that transmission occurs within an infected/susceptible pair, estimated to be 12.9% (95% confidence interval [CI], 9.3–17.7).
In this analysis, only viral load and sharing a bedroom with the index case were associated with an increased risk of transmission (Table 1), with a 10-fold increase in SARS-CoV-2 viral load being associated with a 40% (OR, 1.40 [95% CI, 1.07–1.85]) increase in the odds of transmission and sharing a bedroom being associated with a 199% (ie, 2.99-fold) (95% CI, 1.35–6.71) increase in the odds of transmission. Notably, transmission was not mitigated by randomization of the potential source to an active product (CAS + IMD). On average, households in this analysis had an average of 3.36 members with an average of 2.71 enrolled into the 2 studies. Results were similar when subset to households for which all participants were enrolled (average household size = 2.99).
Table 1.
Correlates of Severe Acute Respiratory Syndrome Coronavirus 2 Transmission in Households
| Correlate | Univariable | Multivariable | Multivariable Whole Household Enrolled |
|||
|---|---|---|---|---|---|---|
| OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | |
| VL (in the source at enrollment), log10 copies/mL | 1.37 | (1.06–1.76) | 1.40 | (1.07–1.85) | 1.54 | (1.08–2.19) |
| Contact shares bedroom with index case | 2.88 | (1.32–6.32) | 2.99 | (1.35–6.71) | 3.80 | (1.34–10.68) |
| Contact age (10-y increase) | 1.19 | (.97–1.49) | … | … | … | … |
| Contact is male | 1.16 | (.55–2.45) | … | … | … | … |
| Contact identifies as Hispanic/Latino ethnicity | 1.30 | (.62–2.73) | … | … | … | … |
| Source identifies as Hispanic/Latino ethnicity | 1.25 | (.58–2.68) | 1.85 | (.81–4.19) | 1.33 | (.46–3.81) |
| Does not wear a face mask indoors (excluding within the household) | 1.68 | (.58–4.85) | … | … | … | … |
| No. of household members positive (excluding index case) 0 1 ≥2 | … 1.51 1.35 | … (.57–4.01) (.36–5.02) | … … … | … … … | … … … | … … … |
| ≥2 symptomatic COVID-19 cases in the household (excluding index case) | 1.11 | (.40–3.06) | … | … | … | … |
| BMI ≥30 kg/m2 (contact) | 1.25 | (.59–2.70) | … | … | … | … |
| BMI ≥30 kg/m2 (source) | 1.86 | (.85–4.07) | … | … | … | … |
| South-central region of United States | 1.89 | (.87–4.15) | … | … | … | … |
| Source is seropositive at baseline | 0.55 | (.21–1.41) | 0.96 | (.35–2.66) | 1.15 | (.35–3.80) |
| Source randomized to placebo arm | 0.95 | (.42–2.17) | … | … | … | … |
Significant correlates (95% CI excludes 1.00) are shown in bold.
Abbreviations: BMI, body mass index; CI, confidence interval; COVID-19, coronavirus disease 2019; OR, odds ratio; VL, viral load.
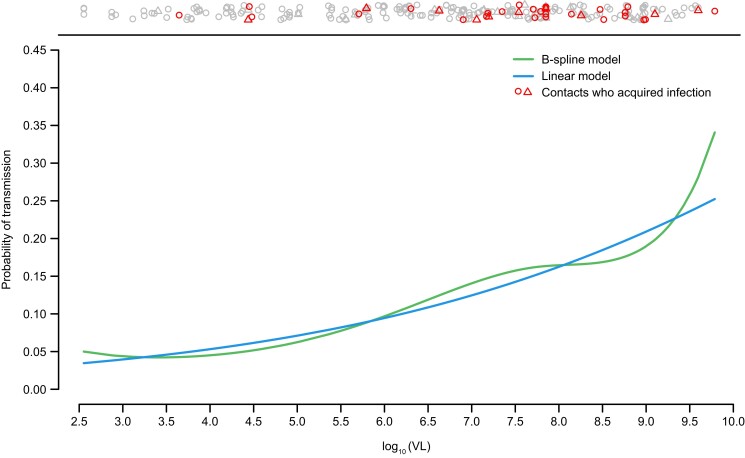
Figure 3 plots the relationship between risk of transmission and viral load based on 2 univariable models: 1 with a linear relationship between log odds of transmission and viral load and the other replacing the linear model with a spline model. The markers at the top of the plot show that transmission occurs across all levels of viral load, with increasing risk as viral load levels increase. There was no evidence of a nonlinear relationship between viral load and transmission; thus, we could not identify a clear inflection/change point at which risk of transmission starts to increase more rapidly with increased vial load levels. The sensitivity analysis did not indicate any evidence of unaccounted-for confounding factors (data not shown).
Figure 3.
Relationship between viral load (VL) in the index case and risk of transmission to susceptible contact under linear and B-spline models. Markers at the top of the plot indicate transmission outcomes in the contacts at their source's VL. Contacts may be represented multiple times depending on the number of sources in the household. Those contacts who acquired infection are indicated in red with the number of sources indicated by the shape (circle = 1; triangle = 2; + = 3).
DISCUSSION
This is the first prospective study of case-contact pairs to assess drivers of SARS-CoV-2 household transmission. While other studies have examined the propensity of an infected person to transmit SARS-CoV-2 [4] or the risk factors of a susceptible person to acquire SARS-CoV-2 infection [18, 19], none have followed the potential transmission pairs prospectively for transmission accounting for characteristics of both the source and susceptible contact. In this post hoc analysis, we prospectively evaluated correlates of SARS-CoV-2 acquisition, viral load in the potential source, and transmission in uninfected, placebo-treated participants in a COVID-19 prevention trial (Study 2069) who were in the same household as participants in a COVID-19 treatment trial (Study 2067) and known to be uninfected at the time that potential sources in the household were infectious.
Overall, we identified 2 key correlates associated with increased risk for SARS-CoV-2 transmission in our multivariable analysis: SARS-CoV-2 viral load and sharing a bedroom with the index participant. Every log10 increase in SARS-CoV-2 viral load (copies/mL) was associated with a 40% increase in risk of transmission. Thus, COVID-19 treatment and vaccination strategies that decrease viral load have the potential to decrease onward transmission. Sharing a bedroom with the index case was associated with approximately 3-fold increased odds of SARS-CoV-2 infection after adjustment for viral load in the source, consistent with previous reports [20], quantifying the potential benefit of isolation to reduce transmission. It is notable that while isolation was recommended in the study (consistent with public health guidance at the time of study conduct), it might not have been possible, indicating the feasibility of isolation and the potential role of pharmacological periexposure prophylaxis to limit transmission. Questions about practices on mask-wearing in the home were assessed the week prior to the start of follow-up (the time before the potential exposure, not reflective of postexposure practices); thus, we were not able to appropriately assess a decrease in transmission with mask-wearing. Male sex and Hispanic or Latino ethnicity were associated with an increased risk of SARS-CoV-2 transmission (not statistically significant), consistent with reports in Hispanic/Latino patients showing increased risk of COVID-19 illness and severity of COVID-19 [21] and men having an increased risk of severe outcomes with COVID-19 [22].
The current report prospectively and systematically tested all participants for infection at multiple time points, whereas the published reports are primarily cross-sectional in nature and thus cannot determine order of infection. They also do not account for potential biases/confounders, including structural barriers due to disparities in COVID-19 testing among Hispanic/Latino populations [23].
We also explored correlates of SARS-CoV-2 viral load in potential sources to identify potential confounders for the transmission analysis. We found that seropositivity was a correlate of decreased viral load, as previously described [24], indicating protection conferred by immunity. We also found that Hispanic or Latino ethnicity was a correlate of decreased viral load. One possibility for this finding is this population was enrolled slightly later in the course of infection, consistent with the higher seropositivity reported in this population [25, 26] and the decreased risk of acquisition identified in the Hispanic/Latino population in this report.
The analysis of acquisition risk in this population illustrates the potential pitfalls in performing analyses of risk in a nongeneralizable subset: in this case, placebo arm participants with a known household exposure. The results conflict with published literature as well as results within potential transmission pairs, indicating lower risk for male sex and Hispanic or Latino ethnicity, and illustrate the importance of properly characterizing exposures and outcomes in transmission research. The transmission analysis is based on prospective and systematic testing for all participants for infection at multiple time points, excluding participants for whom there was no enrolled household member with a quantifiable viral load during the period of time in which risk of infection was assessed.
Due to the complexity of both household and SARS-CoV-2 transmission studies, limited prospective data informing drivers of transmission within households currently exist. Our study designs and statistical analysis methods have several advantages over prior assessments, permitting us to evaluate household transmission of SARS-CoV-2. First, the design of Studies 2067 and 2069 allowed for prospective follow-up of household contacts, starting with the first known infection in the household. Second, we were able to include the index case as well as other infected individuals within the household in the competing risk type model at the time of transmission risk to account for multiple potential sources of transmission without having to attribute the infection to a single source. Third, these studies were conducted at the time in the pandemic when households were quarantining upon infection, making community acquisition unlikely (not a competing source of acquisition). Therefore, we likely captured all potential transmitters and their susceptible contacts. Fourth, an in-depth questionnaire about household and individual risk factors was completed by the household contacts at the time of greatest risk of household transmission (when the majority of index cases were at their peak viral load). Fifth, Study 2069 included repeat virologic assessment so we could determine order of transmission (in contrast to contact tracing). Sixth, household contacts underwent virologic testing irrespective of symptoms, allowing us to capture both symptomatic and asymptomatic infections. Seventh, we utilized specific analytic methods that differentiate our analysis from previous analyses and were able to quantify potential effect sizes, strengthening current data [4, 27].
In this analysis, we evaluated incident SARS-CoV-2 acquisition approximately 1 week after enrollment (where participants had to be randomized within 96 hours of collection of the index cases’ positive SARS-COV-2 diagnostic test sample). This timepoint was prospectively chosen based on (1) our current understanding of SARS-CoV-2 incubation following exposure [28]; and (2) the assumption that most index cases were recently infected, and therefore, at or around peak viral load during the initial exposure period to the household contact. To be included in the treatment study (Study 2067), patients had to be SARS-CoV-2 positive from a diagnostic sample collected ≤72 hours prior to randomization, and symptom onset needed to be ≤7 days before randomization. In the phase 3 analysis of Study 2067, the median time of symptom onset to randomization was 3 days [16], indicating that most patients were recently infected.
This analysis also shows the power of enrolling and following households prospectively. Although contacts were identified from Study 2067 index cases for enrollment into Study 2069 to assess the postexposure prophylactic efficacy of CAS + IMD, there are clear advantages to designing complementary studies of index cases and their household contacts. This study is proof of concept of the feasibility of such an endeavor, as well as the potential of such a study to answer important questions about transmission, including the potential benefit of a treatment intervention to impact transmission.
This post hoc analysis has several limitations. First, we cannot assess causation, only correlation of cofactors that may contribute to acquisition or transmission of SARS-CoV-2. Second, this study was not powered to evaluate a virologic threshold for transmission risk, which may partly explain why we were not able to identify a clear nonlinear relationship using this data set. Third, we may not have enrolled the entire household into the 2 studies. Fourth, we only collected information about the relationship of each person in the household to the index case but not to each other. Fifth, this analysis was conducted during the 28-day efficacy assessment period, prior to effective vaccine receipt, and transmission dynamics may differ in vaccinated individuals. Future studies are needed to address these outstanding questions.
In conclusion, using prospective data; detailed characteristics on the household, its cases, and susceptible members; and statistical approaches specifically tailored to household transmission with 1 or more potential sources, we present quantitative estimates for the most important correlates for acquisition and transmission of SARS-CoV-2 within a household, which are consistent with increased exposure to the infected individual by sharing a bedroom and increased viral load.
Supplementary Material
Contributor Information
Elizabeth R Brown, Vaccine and Infectious Disease and Public Health Services Divisions, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Department of Biostatistics, University of Washington, Seattle, Washington, USA.
Meagan P O’Brien, Global Development, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Brian Snow, Global Development, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Flonza Isa, Global Development, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Eduardo Forleo-Neto, Global Development, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Kuo-Chen Chan, Global Development, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Peijie Hou, Global Development, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Myron S Cohen, Institute for Global Health and Infectious Diseases, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Gary Herman, Global Development, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Ruanne V Barnabas, Division of Infectious Diseases, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. E. R. B., M. P. O., F. I., E. F.-N., M. S. C., G. H., and R. V. B. contributed to study concept and design. M. P. O., B. S., F. I., and E. F.-N. were involved in data collection. B. S. provided administrative, technical, or material support. E. R. B., K.-C. C., and P. H. provided statistical analysis. E. R. B., M. P. O., F. I., E. F.-N., M. S. C., G. H., and R. V. B. provided analysis and/or interpretation of the data. All authors provided critical revision of the manuscript for intellectual content and provided approval to submit the manuscript for publication.
Acknowledgment. We thank Caryn Trbovic, PhD, from Regeneron Pharmaceuticals, Inc. for assistance with development of the manuscript, and Prime, Knutsford, UK, for formatting and copy-editing suggestions.
Data availability. Qualified researchers may request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form, and statistical analysis plan) that support the methods and findings reported in this manuscript. Individual anonymized participant data will be considered for sharing once the product and indication have been approved by major health authorities (eg, Food and Drug Administration, European Medicines Agency, Pharmaceuticals and Medical Devices Agency), if there is legal authority to share the data and there is not a reasonable likelihood of participant reidentification. Requests should be submitted to https://vivli.org/.
Disclaimer. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. Study 2069 was supported by Regeneron Pharmaceuticals, Inc., and F. Hoffmann-La Roche Ltd. Study 2069 was conducted jointly with the COVID-19 Prevention Network (CoVPN) funded by the National Institute of Allergy and Infectious Diseases, NIH (award numbers UM1AI068619 and UM1AI148684). Study 2067 was supported by Regeneron Pharmaceuticals, Inc. Certain aspects of study 2067 were funded in whole or in part with federal funds from the Biomedical Advanced Research and Development Authority (BARDA), Office of the Administration for Strategic Preparedness and Response, Department of Health and Human Services (other transaction number HHSO100201700020C).
References
- 1. Centers for Disease Control and Prevention . Scientific brief: SARS-CoV-2 transmission. 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/sars-cov-2-transmission.html#anchor_1619805150492. Accessed 10 November 2022.
- 2. Madewell ZJ, Yang Y, Longini IM Jr, Halloran ME, Dean NE. Household secondary attack rates of SARS-CoV-2 by variant and vaccination status: an updated systematic review and meta-analysis. JAMA Netw Open 2022; 5:e229317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thompson HA, Mousa A, Dighe A, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) setting-specific transmission rates: a systematic review and meta-analysis. Clin Infect Dis 2021; 73:e754–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Madewell ZJ, Yang Y, Longini IM Jr, Halloran ME, Dean NE. Household transmission of SARS-CoV-2: a systematic review and meta-analysis. JAMA Netw Open 2020; 3:e2031756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arnedo-Pena A, Sabater-Vida S, Meseguer-Ferrer N, et al. COVID-19 secondary attack rate and risk factors in household contacts in Castellon (Spain): preliminary report. Enfermedades Emergentes 2020; 19:64–70. [Google Scholar]
- 6. Remón-Berrade M, Guillen-Aguinaga S, Sarrate-Adot I, et al. Risk of secondary household transmission of COVID-19 from health care workers in a hospital in Spain. Epidemiologia (Basel) 2022; 3:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. US Food and Drug Administration . Fact sheet for health care providers: emergency use authorization (EUA) of REGEN-COV (casirivimab with imdevimab). 2021. Available at: https://www.fda.gov/media/145611/download. Accessed 6 July 2022.
- 8. European Medicines Agency . Ronapreve: annex I summary of product characteristics. 2021. Available at: https://www.ema.europa.eu/en/documents/product-information/ronapreve-epar-product-information_en.pdf. Accessed 12 November 2021.
- 9. Roche Products Pty Ltd . Ronapreve (casirivimab and imdevimab) Australian product information. 2021. Available at: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2021-PI-02271-1&d=20220107172310101&d=20230609172310101. Accessed 6 June 2022.
- 10. Pharmaceuticals and Medical Devices Agency . Report on the deliberation results for Ronapreve for intravenous infusion. 2021. Available at: https://www.pmda.go.jp/files/000244786.pdf. Accessed 21 March 2022.
- 11. Medicines and Healthcare Products Regulatory Agency . Summary of product characteristics for Ronapreve. 2021. Available at: https://www.gov.uk/government/publications/regulatory-approval-of-ronapreve/summary-of-product-characteristics-for-ronapreve. Accessed 17 December 2021.
- 12. US Food and Drug Administration . Emergency use authorization 091. 2022. Available at: https://www.fda.gov/media/145610/download. Accessed 10 February 2022.
- 13. Norton T, Ali S, Sivapalasingam S, et al. REGEN-COV antibody combination in outpatients with COVID-19 – phase 1/2 results. medRxiv [Preprint]. 2022. doi: 10.1101/2021.06.09.21257915 [DOI]
- 14. O’Brien MP, Forleo-Neto E, Musser BJ, et al. Subcutaneous REGEN-COV antibody combination to prevent Covid-19. N Engl J Med 2021; 385:1184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O’Brien MP, Forleo-Neto E, Sarkar N, et al. Effect of subcutaneous casirivimab and imdevimab antibody combination vs placebo on development of symptomatic COVID-19 in early asymptomatic SARS-CoV-2 infection: a randomized clinical trial. JAMA 2022; 327:432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN-COV antibody combination and outcomes in outpatients with Covid-19. N Engl J Med 2021; 385:e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zou H. The adaptive lasso and its oracle properties. J Am Stat Assoc 2006; 101:1418–29. [Google Scholar]
- 18. Al Awaidy ST, Khamis F, Mahomed O, et al. Epidemiological risk factors for acquiring severe COVID-19: prospective cohort study. Oman Med J 2021; 36:e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pijls BG, Jolani S, Atherley A, et al. Demographic risk factors for COVID-19 infection, severity, ICU admission and death: a meta-analysis of 59 studies. BMJ Open 2021; 11:e044640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ng OT, Marimuthu K, Koh V, et al. SARS-CoV-2 seroprevalence and transmission risk factors among high-risk close contacts: a retrospective cohort study. Lancet Infect Dis 2021; 21:333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Magesh S, John D, Li WT, et al. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic-review and meta-analysis. JAMA Netw Open 2021; 4:e2134147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peckham H, de Gruijter NM, Raine C, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun 2020; 11:6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pond EN, Rutkow L, Blauer B, Aliseda Alonso A, Bertran de Lis S, Nuzzo JB. Disparities in SARS-CoV-2 testing for Hispanic/Latino populations: an analysis of state-published demographic data. J Public Health Manag Pract 2022; 28:330–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anantharaj A, Gujjar S, Verma N, et al. Resolution of viral load in mild COVID-19 patients is associated with both innate and adaptive immune responses. J Clin Virol 2022; 146:105060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yamaki J, Peled H, Mathews S, et al. Seroprevalence of novel coronavirus SARS-CoV-2 at a community hospital emergency department and outpatient laboratory in northern Orange County, California. J Racial Ethn Health Disparities 2021; 8:1551–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jones JM, Stone M, Sulaeman H, et al. Estimated US infection- and vaccine-induced SARS-CoV-2 seroprevalence based on blood donations, July 2020–May 2021. JAMA 2021; 326:1400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jajou R, Mutsaers-van Oudheusden A, Verweij JJ, Rietveld A, Murk JL. SARS-CoV-2 transmitters have more than three times higher viral loads than non-transmitters—practical use of viral load for disease control. J Clin Virol 2022; 150–151:105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu Y, Kang L, Guo Z, Liu J, Liu M, Liang W. Incubation period of COVID-19 caused by unique SARS-CoV-2 strains: a systematic review and meta-analysis. JAMA Netw Open 2022; 5:e2228008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.