Summary
Background
Domesticated animal ownership is an understudied aspect of the human environment that influences mosquito biting behaviour and malaria transmission, and is a key part of national economies and livelihoods in malaria-endemic regions. In this study, we aimed to understand differences in Plasmodium falciparum prevalence by ownership status of common domesticated animals in DR Congo, where 12% of the world's malaria cases occur and anthropophilic Anopheles gambiae vectors predominate.
Methods
In this cross-sectional study, we used survey data from individuals aged 15–59 years in the most recent (2013–14) DR Congo Demographic and Health Survey and previously performed Plasmodium quantitative real-time PCR (qPCR) to estimate P falciparum prevalence differences by household ownership of cattle; chickens; donkeys, horses, or mules; ducks; goats; sheep; and pigs. We used directed acyclic graphs to consider confounding by age, gender, wealth, modern housing, treated bednet use, agricultural land ownership, province, and rural location.
Findings
Of 17 701 participants who had qPCR results and covariate data, 8917 (50·4%) of whom owned a domesticated animal, we observed large differences in malaria prevalence across types of animals owned in both crude and adjusted models. Household chicken ownership was associated with 3·9 (95% CI 0·6 to 7·1) more P falciparum infections per 100 people, whereas cattle ownership was associated with 9·6 (–15·8 to –3·5) fewer P falciparum infections per 100 people, even after accounting for bednet use, wealth, and housing structure.
Interpretation
Our finding of a protective association conferred by cattle ownership suggests that zooprophylaxis interventions might have a role in DR Congo, possibly by drawing An gambiae feeding away from humans. Studies of animal husbandry practices and associated mosquito behaviours could reveal opportunities for new malaria interventions.
Funding
The National Institutes of Health and the Bill & Melinda Gates Foundation.
Translations
For the French and Lingala translations of the abstract see Supplementary Materials section.
Introduction
Despite substantial research investment and attention, malaria cases and deaths are increasing in high-burden countries, with an estimated 247 million cases and 619 000 deaths worldwide in 2021.1 96% of these deaths occurred in the WHO African region, where the most virulent species of the parasite that causes malaria, Plasmodium falciparum, predominates.1 Malaria infections involve a complex interplay of environment, pathogen, vector and human host biology, and human behaviour. Substantial research and public health programming have been allocated to examining parasite–vector–host biology, but many questions remain about how local environmental factors influence P falciparum transmission.
DR Congo accounts for the second highest percentage of malaria cases worldwide (12%) and 54% of the cases in central Africa.2 Anthropophilic Anopheles gambiae predominate, with An gambiae sensu stricto most commonly found, followed by Anopheles coluzzii.3 As the second largest country in Africa by area and one of the most biodiverse regions of the world, DR Congo has high ecological and population diversity across a large geographical area.4 Although previous research identified demographic and environmental factors associated with low P falciparum prevalence, such as wealth, modern housing, and low agricultural cover,5, 6 the relationship between livestock and farm animal ownership—a key source of income and the country's overall economy7—and P falciparum infection remains undefined.
Human–animal interactions influence local disease ecology, and the effect of livestock on malaria is an important question given that the introduction of livestock as zooprohylatic decoys (ie, approaches to draw mosquito bites away from humans and towards animals) or as a means of using veterinary endectocides has been proposed as an intervention (ie, a One Health strategy) to reduce malaria infections.8, 9 A 2015 systematic review found that zooprophylaxis shows promise in reducing malaria infections, particularly in areas where zoophilic mosquito vectors predominate, livestock are kept away from areas where humans sleep, and bednets are used.10 Other studies have found that proximity of cattle to human dwellings is protective, such as in a region of Tanzania where Anopheles arabiensis dominates,11 whereas other studies have found that cattle have no effect on malaria transmission, such as in The Gambia where An gambiae sensu stricto, An melas, and An arabiensis predominate.12 In other areas, livestock have been found to increase malaria prevalence (ie, zoopotentiation), potentially by creating an environment near the household that is hospitable to mosquito breeding.13 Thus, the effect of livestock ownership on malaria risk is unclear but appears to be context specific.
Research in context.
Evidence before this study
We searched PubMed using search terms (“domesticated animal” OR “household animal ownership” OR “animal”) AND (“malaria” OR “plasmodium”) for any articles in any language published from database inception to May 24, 2022. None of the studies identified investigated this question in DR Congo. Several studies have evaluated aspects of zooprophylaxis and zoopotentiation for malaria control in Ethiopia, The Gambia, Ghana, Mozambique, Senegal, Tanzania, and Zambia, or included specific animals owned in malaria risk factor analyses (eg, in Burkina Faso, Ethiopia, Guinea-Bissau). The evidence is mixed across these settings: in Macha, Zambia and southern Tanzania, where the moderately zoophilic Anopheles arabiensis predominates, cattle ownership was protective against malaria infection, but had no effect in anthropophilic Anopheles gambiae sensu stricto regions of The Gambia or zoophilic An arabiensis regions of Ethiopia. Chicken ownership appears protective in Ethiopia, but chicken DNA is not frequently found in bloodmeals across contexts. In a feeding analysis of 1886 An gambiae sensu lato in Senegal, 37·1% of An gambiae sensu lato feedings involved single-animal blood meals and a similar proportion were observed to be single human origin. A 2015 systematic review of zooprophylaxis for malaria control concluded that zooprophylaxis has potential as an effective strategy—particularly in contexts of zoophilic vectors, large distances between where animals are kept at night and where humans sleep, and frequent bednet use—but more evidence is needed for specific environmental conditions. Further, increasing use of insecticide-treated bednets is predicted to increase feeding on cattle by anthropophilic An gambiae sensu stricto vectors.
Added value of this study
Our study investigates whether zooprophylaxis could offer protection against malaria in DR Congo, where Plasmodium falciparum remains highly prevalent despite ongoing control efforts and where the anthropophilic An gambiae sensu stricto vector is widespread and the anthropophilic Anopheles coluzzi is also common. In the largest analysis of malaria zooprophylaxis in Africa conducted to date, we found a protective effect of cattle ownership against P falciparum infection, even after accounting for household wealth, treated bednet use, agricultural land, and modern housing. This finding raises the possibility that cattle kept near household sleeping quarters could draw mosquitos away from humans and reduce household P falciparum transmission risk. We also found increased P falciparum infection prevalence with chicken ownership. Although rare in this population, ownership of horses, donkeys, or mules was also associated with an increased P falciparum prevalence; however, this result was not statistically significant in adjusted models. Goat, sheep, pig, and duck ownership did not result in a difference in infection prevalence.
Implications of all the available evidence
These findings suggest that cattle ownership in the household compound offers protection against P falciparum infection and that chicken ownership confers risk of P falciparum infection in DR Congo. There might be a role for zooprophylaxis in DR Congo and other settings where P falciparum transmission via An gambiae vectors is high. Future studies of animal ownership and husbandry practices could reveal opportunities for novel malaria control interventions, such as optimising distance between humans and household animals combined with the use of veterinary endectocides.
Feeding preferences of the mosquito species are an important determinant of an animal's zooprophylaxis or zoopotentiation effect on human malaria infection. These effects are evaluated through analysis of mosquito blood meal content to establish the proportion from humans (ie, human blood indices). In Tanzania, human blood meals have been found to be less frequent in An arabiensis and Anopheles funestus sensu lato vectors where livestock are present in the household compared with households without livestock, whereas human blood meals in An gambiae sensu stricto appeared unaffected by the presence of household livestock.14 Although this finding is consistent with previous research establishing anthropophilic preferences of An gambiae sensu stricto and zoophilic tendencies of An arabiensis and An funestus,15 human blood indices are highly variable. The human blood indices of An arabiensis ranges from 0% to 80% across surveys, suggesting adaptability of the zoophilic vector to feed across hosts, including an increased proportion from humans.16 Vector biting preferences are further predicted to change with malaria control measures. Anthropophilic An gambiae sensu stricto might be driven towards a preference for cattle feeding with increasing use of insecticide-treated bednets.17
This adaptability of mosquito vectors underscores the need to move beyond human-centred malaria prevention, and consider the broader environment, including non-human hosts. Improved understanding of the relationship between household animals and malaria prevalence in DR Congo is needed to identify One Health strategies that might reduce DR Congo's high malaria burden. In this study, we aimed to investigate the association between P falciparum prevalence and ownership status of common domesticated animals in DR Congo.
Methods
Study design and population
In this population-based, nationally representative, cross-sectional study with a two-stage cluster design, we investigated the association between animal ownership and P falciparum infection using the most recent (2013–14) DR Congo Demographic and Health Survey (DHS) and previously collected dried blood spots.18
In the first stage, 540 sampling clusters across 26 DR Congo provinces were sampled, and in the second stage, households were randomly sampled within each cluster. Men (aged 15–59 years) and women (aged 15–49 years) from the selected households were offered enrolment, including collection of a dried blood spot specimen. Consent for participation, specimen collection, and specimen analysis for other biomarkers was more than 95%.
Additional ethics approval was not required for this analysis of publicly available, de-identified data. The parent studies from which data used in this analysis were generated were approved by Kinshasa School of Public Health (ESP/CE/015/14) and the University of North Carolina at Chapel Hill Institutional Review Board (number 14-0077).
Exposure, outcome, and covariate classification
We defined the exposure as a binary variable of each type of livestock, herd, or farm animal owned versus not owned by the household, including cattle; chickens; ducks; goats; horses, donkeys, or mules; pigs; and sheep. We defined the outcome as a binary variable reflecting quantitative real-time PCR (qPCR)-confirmed P falciparum infection identified using participant dried blood spot samples. As described elsewhere,5 DNA was extracted using Chelex 100 (Bio-Rad, Hercules, CA, USA) and evaluated using a qPCR assay targeting P falciparum lactate dehydrogenase with a limit of detection of 5–10 parasites per μL.19, 20 Gender was determined by self-report with binary male or female options. Bednet use was collapsed into a single variable comparing if the participant had slept under a long-lasting insecticidal treated bednet the previous night versus using an untreated bednet or no bednet. Wealth was operationalised as quintiles calculated by the DHS programme; briefly, these quintiles originate from a principal components analysis of household attributes reflecting the standard of living.21 Rural location was a binary categorisation determined by country administrators. Modern housing was dichotomised as a composite binary variable reflecting houses with floor, wall, and roof material that is more insulated and less penetrable by mosquitoes for inhabitants compared with traditional housing materials, as described elsewhere.5
Statistical analysis
We used a directed acyclic graph (appendix 3 pp 2–5) to identify confounders and modifiers of the relationship between animal ownership and malaria. We then sought to reduce the model by comparing the change in estimate and precision across multiple iterations (appendix 3 pp 2–5). Confounders included in the adjusted models were gender, treated bednet use, wealth, rural location, and modern housing.
The survey did not include information on where animals were kept overnight, but we used past qualitative research to inform a subanalysis with cattle ownership—ie, households with fewer cattle might be more likely to keep animals within the household compound, as they are less likely to be stolen when kept near the household.22 As a small but unknown number of cattle can be feasibly kept on the compound, we tested the relationship between different thresholds of herd size and P falciparum prevalence. We also conducted a post-hoc analysis of different flock sizes for chickens.
We used linear binomial regression models with generalised estimating equations to account for the clustered data structure to estimate crude and adjusted prevalence differences in P falciparum infections between participants from households that owned each animal compared with participants from households that did not own each respective animal. We applied survey sample weights to provide estimates of the target population based on the sampling design. For subanalyses, we used the log and logit links to calculate prevalence ratios and odds ratios to enhance model convergence given small sample sizes. We calculated profile likelihood 95% CIs.
We conducted all analyses in R (version 4.0.3).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
A total of 17 703 participants were eligible for inclusion and 17 701 (99·9%) had non-missing values for all covariates (appendix 3 p 6), representing 18 091 individuals when weighted to the total DR Congo population. When weighted, 31·1% (95% CI 29·0–33·0) of participants included in the analysis had PCR-confirmed P falciparum malaria (table 1).
Table 1.
Characteristics of the study population, accounting for survey sample weights
| Plasmodium falciparum infection* | No P falciparum infection* | Overall | ||||
|---|---|---|---|---|---|---|
| Total | 5625 (31·1%) | 12 466 (68·9%) | 18 091 | |||
| Household members | 6·70 (0·09) | 6·77 (0·09) | 6·75 (0·08) | |||
| Children aged <5 years in household | 1·46 (0·03) | 1·47 (0·03) | 1·47 (0·02) | |||
| Age, years | 28·2 (0·20) | 30·4 (0·17) | 29·7 (0·12) | |||
| Gender | ||||||
| Male | 2901 (51·6%) | 5685 (45·6%) | 8586 (47·5%) | |||
| Female | 2724 (48·4%) | 6781 (54·4%) | 9505 (52·5%) | |||
| Slept under treated bed net previous night | ||||||
| Yes | 2798 (49·7%) | 6860 (55·0%) | 9658 (53·4%) | |||
| No | 2827 (50·3%) | 5606 (45·0%) | 8433 (46·6%) | |||
| Modern housing | ||||||
| Yes | 618 (11·0%) | 2600 (20·9%) | 3218 (17·8%) | |||
| No | 5007 (89·0%) | 9866 (79·1%) | 14 873 (82·2%) | |||
| Wealth quintile | ||||||
| Lowest | 1222 (21·7%) | 2111 (16·9%) | 3333 (18·4%) | |||
| Lower-middle | 1198 (21·3%) | 2285 (18·3%) | 3483 (19·3%) | |||
| Middle | 1322 (23·5%) | 2352 (18·9%) | 3674 (20·3%) | |||
| Upper-middle | 1157 (20·6%) | 2439 (19·6%) | 3596 (19·9%) | |||
| Highest | 726 (12·9%) | 3279 (26·3%) | 4005 (22·1%) | |||
| Rurality | ||||||
| Rural location | 3917 (69·6%) | 7605 (61·0%) | 11 522 (63·7%) | |||
| Urban location | 1708 (30·4%) | 4861 (39·0%) | 6569 (36·3%) | |||
| Agricultural land ownership | ||||||
| Yes | 3744 (66·6%) | 7169 (57·5%) | 10 914 (60·3%) | |||
| No | 1879 (33·4%) | 5297 (42·5%) | 7176 (39·7%) | |||
| Livestock, herd, or farm animal ownership | ||||||
| Owns any animal | 2993 (53·2%) | 5923 (47·5%) | 8917 (49·3%) | |||
| Cattle | 62 (1·1%) | 300 (2·4%) | 362 (2·0%) | |||
| Median owned | 4 (2–7) | 3 (2–5) | 3 (2–6) | |||
| Chickens | 2596 (46·2%) | 4936 (39·6%) | 7532 (41·6%) | |||
| Median owned | 5 (2–9) | 4 (2–8) | 5 (2–8) | |||
| Horses† | 10 (0·2%) | 20 (0·2%) | 31 (0·2%) | |||
| Median owned | 10 (3–10) | 10 (3–10) | 10 (3–10) | |||
| Goats | 1127 (20·0%) | 2445 (19·6%) | 3572 (19·8%) | |||
| Median owned | 2 (1–4) | 2 (1–4) | 2 (1–4) | |||
| Sheep | 209 (3·7%) | 482 (3·9%) | 690 (3·8%) | |||
| Median owned | 2 (1–4) | 2 (1–3) | 2 (1–3) | |||
| Pigs | 481 (8·6%) | 1134 (9·1%) | 1617 (8·9%) | |||
| Median owned | 2 (1–3) | 2 (1–4) | 2 (1–4) | |||
| Ducks | 597 (10·6%) | 1129 (9·1%) | 1726 (9·5%) | |||
| Median owned | 3 (2–5) | 3 (2–5) | 3 (2–5) | |||
Data are mean (SD), median (IQR), or n (%).
Infection confirmed by P falciparum lactate dehydrogenase quantitative PCR.
Donkeys, horses, or mules.
This subset was similar to the overall DHS cohort that has been described elsewhere,5 but, in brief, participants in this analysis with P falciparum infection tended to be slightly younger than those who were P falciparum negative, and more participants with P falciparum infection were men than women (table 1). About half of participants had slept under a treated bednet the previous night, with a lower percentage of participants with P falciparum infection having slept under a treated bednet than participants without P falciparum infection (table 1). Only about a tenth of those with P falciparum infection had modern housing, compared with two-fifths of those without P falciparum infection (table 1). Of P falciparum-positive participants, the smallest proportion were in the highest wealth quintile (12·9%), whereas of P falciparum-negative participants, the largest proportion were in the highest wealth quintile (26·3%; table 1). Living in a rural area was more common among P falciparum-positive participants than P falciparum-negative participants, as was agricultural land ownership (table 1).
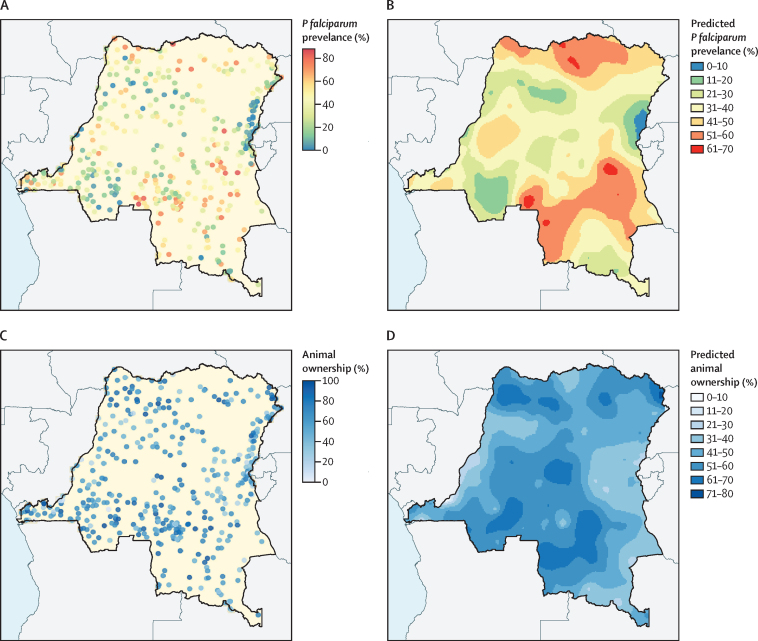
About half of all participants lived in households that owned any domesticated animal (table 1). Chickens were the most common animal owned (41·6% of participants, 95% CI 38·9–44·0), followed by goats (19·8%, 17·9–22·0). Only 2·0% (1·4–3·0) of all participants lived in households that owned cattle, and 0·2% (0·1–0·2) lived in households that owned horses, donkeys, or mules. Except for horses, donkeys, or mules, ownership of each animal type followed a right-skewed distribution, with most individuals living in households with five or fewer of a given animal (appendix 3 p 7). The overlap of animals owned is depicted in appendix 3 (p 8). P falciparum prevalence and animal ownership varied spatially, with higher proportions of animal ownership seen in the rural south-central and northern regions than in other regions of the country (figure 1; appendix 3 pp 9–10).
Figure 1.
Geographical prevalence of animal ownership and Plasmodium falciparum infection in DR Congo
Geographical prevalence of P falciparum and animal ownership by Demographic and Health Survey cluster (A, C). Predicted regional distribution of P falciparum and animal ownership (B, D). Respondents with missing data on animal ownership or geographical location were excluded.
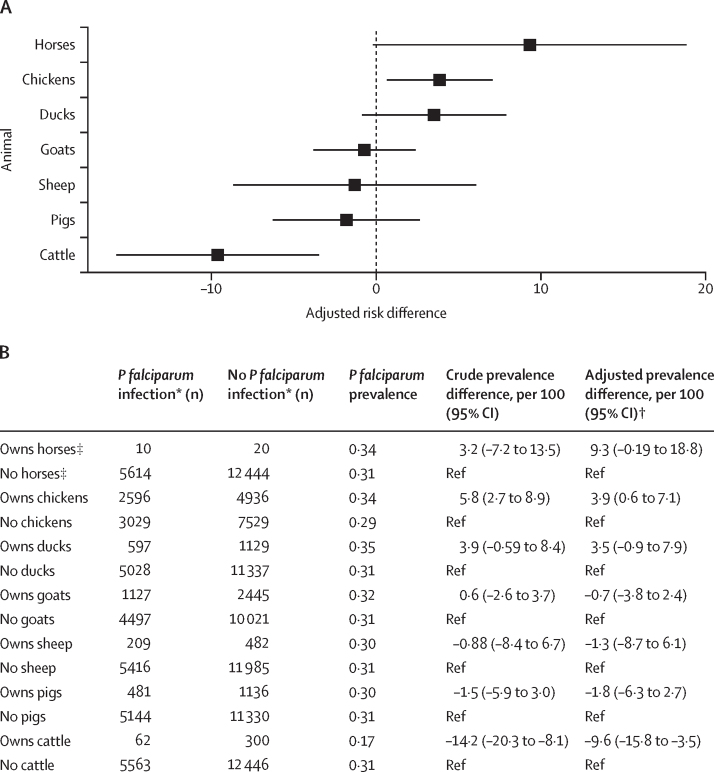
Participants from households that owned chickens had 3·9 (95% CI 0·6 to 7·1) more P falciparum infections per 100 people compared with participants from households without chickens, after adjusting for gender, treated bednet use, modern housing, wealth, and rurality (figure 2). Participants from households that owned cattle had 9·6 (95% CI –15·8 to –3·5) fewer P falciparum infections per 100 people compared with participants from households without cattle, after adjusting for the same variable set. The remaining animal types (ie, ducks, goats, sheep, pigs, and horses, donkeys, or mules) did not show a significant protective or harmful association with P falciparum infection (figure 2).
Figure 2.
Crude and adjusted associations between animal ownership and Plasmodium falciparum infection
(A) Adjusted P falciparum prevalence difference (95% CI) by each animal owned versus not owned. (B) Weighted counts and crude and adjusted associations for each animal and P falciparum infection. Prevalence difference associations were estimated with generalised estimating equation models to account for clustering within households. Survey weights are incorporated. *Infection confirmed by P falciparum lactate dehydrogenase quantitative PCR. †Adjustment set includes gender, treated bednet, modern housing, wealth, and rurality. ‡Donkeys, horses, or mules.
When both chickens and cattle were owned by a household, the protective association of cattle ownership remained (appendix 3 p 11). P falciparum prevalence among individuals living in households with chickens and cattle was 20% (appendix 3 p 11), similar to the prevalence among all cattle owners (17%), but lower than the prevalence among all chicken owners (34%; figure 2).
In crude models, participants in households with between one and four cattle had 17·8 fewer (95% CI –24·8 to –10·8) P falciparum infections per 100 people compared with households that owned no cattle. We observed no signficant association between ownership of five or more cattle and P falciparum infection (table 2). When the threshold of ten cattle was used, a similar prevalence difference was observed for owning one to nine cattle and ten or more cattle (data not shown). These associations were similar in adjusted models, suggesting a beneficial effect of owning fewer than five cattle on the risk of P falciparum infection and no evidence of an effect when five or more cattle are owned (data not shown). All chicken flock sizes (ie, 1–4, 5–9, 10–14, and ≥15 chickens) were associated with increased P falciparum prevalence compared with households that owned no chickens in unadjusted models, with an increasing prevalence ratio with more chickens owned (table 2). All except the smaller two flock sizes (<10 chickens) were significantly associated with increased P falciparum prevalence in adjusted models (table 2).
Table 2.
Crude and adjusted associations between number of cattle owned and Plasmodium falciparum infection, and between number of chickens owned and P falciparum infection
| n | Crude prevalence difference, per 100 (95% CI) | Crude prevalence ratio (95% CI) | Adjusted prevalence ratio (95% CI)* | |
|---|---|---|---|---|
| Cattle | ||||
| ≥5 cows or bulls | 124 | −7·3 (−16·0 to 1·4) | 0·77 (0·54 to 1·10) | 0·98 (0·66 to 1·46) |
| 1–4 cows or bulls | 238 | −17·8 (−24·8 to −10·8) | 0·43 (0·26 to 0·72) | 0·44 (0·26 to 0·75) |
| No cows or bulls | 17 726 | Ref | Ref | Ref |
| Chickens | ||||
| ≥15 chickens | 753 | 11·5 (5·1 to 18·0) | 1·40 (1·18 to 1·66) | 1·40 (1·18 to 1·66) |
| 10–14 chickens | 792 | 8·8 (4·0 to 13·6) | 1·31 (1·14 to 1·50) | 1·27 (1·10 to 1·46) |
| 5–9 chickens | 2258 | 5·0 (0·9 to 9·1) | 1·17 (1·03 to 1·34) | 1·11 (0·98 to 1·26) |
| 1–4 chickens | 3730 | 4·4 (0·8 to 8·1) | 1·15 (1·03 to 1·30) | 1·06 (0·94 to 1·19) |
| No chickens | 10 557 | Ref | Ref | Ref |
Prevalence associations were estimated with generalised estimating equation models to account for clustering within households, with survey weights applied.
Adjustment set includes sex, treated bednet, modern housing, wealth, and rurality.
The protective association between cattle ownership and P falciparum infection remained significant after assessing agricultural land ownership as a modifier (appendix 3 p 12). A protective association was observed among participants with land, while a non-significant association was found among participants without land usable for agriculture (appendix 3 p 12).
Discussion
To our knowledge, this study is the largest investigation of household animal ownership and P falciparum prevalence done in Africa to date. We found that cattle ownership confers a protective association with P falciparum infection in DR Congo, resulting in 9·6 fewer P falciparum infections per 100 people. This protective association held after accounting for wealth, housing structure, bednet use, and modification by agricultural land. We found ownership of chickens to be associated with an increased prevalence of P falciparum infection. We did not observe a significant relationship between P falciparum prevalence and horse, donkey, or mule; goat; sheep; duck; and pig ownership. In a setting where cattle ownership and chicken ownership are common across the country, our findings suggest new opportunities for integrated vector management in DR Congo.
The protective association of cattle ownership was strongest when the herd size was small. Although the DHS did not collect information about where animals were kept overnight, we used herd size as a proxy for where cattle might be kept overnight, based on past research in DR Congo indicating that households with a small number of cattle were more likely to keep animals within the household compound.22 Keeping cattle closer to the household's sleeping area could yield an increased zooprophylactic effect by drawing mosquito feeding away from humans. This effect requires further investigation given the modification observed by agricultural land ownership. Previous research in An arabiensis or Anopheles pharoensis regions of Ethiopia found that large cattle herds (>20 cows per human) did not provide a zooprophylactic effect;23 however, other research has shown that increased cattle population density appeared to reduce vectors entering indoor areas of households, thus being potentially protective against malaria.24 In other settings, household cattle ownership appeared protective, and was associated with reduced odds of malaria infection in regions of Zambia with An Arabiensis.25 In Ghana, cattle being within 20 meters of humans was associated with a 66% reduction in the number of An gambiae sensu stricto landings on humans.26 Together, these findings highlight the context-dependent interplay among mosquito vectors, environment, and animal husbandry, and provide a biological basis for the protective association of cattle observed in this study.
We further observed that household chicken ownership was associated with increased P falciparum prevalence, and that prevalence increased with flock size. An gambiae sensu lato bloodmeals from chickens are found least frequently,27 suggesting that chickens have less zoophilic potential than do other household animals. Our finding contrasts with other studies that found a protective effect of chicken ownership in the context of An arabiensis vectors in Ethiopia.28 Although chickens were not a desired source of bloodmeals for female An arabiensis, volatile compounds secreted by chickens acted as medium-to-long range deterrents for female An arabiensis.28 Our contrasting findings have a few potential biological explanations. One is that the olfactory and other volatile compounds secreted by chickens might have little effect on An gambiae sensu stricto species, a common vector in DR Congo. Second, living conditions in DR Congo for chickens might provide additional mosquito breeding habitats, amplifying vector abundance. This amplification could be particularly plausible given the increasing prevalence ratios with larger flock sizes.
Although we did not observed a significant increase in P falciparum prevalence in households with horses, donkeys, or mules compared with households without these animals, ownership of these animals was rare and this estimate was imprecise. In contexts with An gambiae, donkeys have been associated with reduced malaria in univariate analyses,29 but because these animals are not frequently owned in DR Congo, interventions focused on them might not be widely effective. We estimated no noticeable association between P falciparum infection with each of the remaining animals (goats, sheep, ducks, and pigs). In contexts with anthropophilic An gambiae sensu stricto and An funestus, pig ownership has been found to be associated with increased odds of positivity on malaria rapid diagnostic test among children (aged 1–15 years).30 In An arabiensis regions of Ethiopia, sheep and goats have not been associated with an increased risk of P falciparum infection.31
This study has a few notable limitations. First, the cross-sectional design limits investigation of temporal trends between the exposure and outcome, malaria seasonality, and migratory patterns of some animals, particularly cattle. Second, although multiple iterations of the models resulted in estimates of similar magnitude and direction as the models presented, unmeasured confounding or measurement error of the self-reported exposure could nonetheless induce bias. Third, refusal to participate or provide a blood sample could result in selection bias; however, refusal rates were very low (<5%) across this nationally representative study and it is unlikely to substantially bias results. Finally, absence of information about household-specific animal husbandry restricts our ability to identify the behaviours or practices most amenable to intervention.
These findings suggest that interventions focused on household animals, particularly cattle and chickens kept in designated pens on the household compound, could have a role in DR Congo as a complement to current malaria control interventions. Future studies are needed to evaluate where animals are kept in relation to human sleeping quarters, as well as environmental and behavioural conditions in DR Congo that might affect the interplay between the vector, livestock, and human host. Promotion of husbandry practices found to be protective against malaria could complement ongoing malaria control activities in high-burden countries with dominant An gambiae sensu stricto vectors such as DR Congo.
Data sharing
Data are available in a public, open access repository and upon request from the DHS programme (https://dhsprogram.com/methodology/survey/survey-display-421.cfm), including malaria PCR results. R code used for the analysis is available at https://github.com/IDEELResearch/animalaria.
Declaration of interests
JBP reports research support from Gilead Sciences, non-financial support from Abbott Laboratories, consulting for Zymeron Corporation, and honoraria from Virology Education, all outside the scope of this work. JBP also reports malaria research support from WHO, unrelated to this work. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This study received partial support from the National Institutes of Health (F30AI169752 to CEM, R01AI139520 to JBP, R01AI129812 to MMK and AT, and K24AI134990 to JJJ). This work was supported, in whole or in part, by the Bill & Melinda Gates Foundation (OPP1161913 to ME and MMK). We would like to thank the participants who offered their time and information to the 2013–14 DHS in the Democratic Republic of Congo, and the field teams who conducted the study. We would also like to thank Kyaw Lay Thwai for performing P falciparum lactate dehydrogenase PCR laboratory assays. We would especially like to acknowledge the contribution of Dr Steve Meshnick posthumously for his mentorship throughout our malaria DHS analyses.
Editorial note: The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Contributors
CEM and JBP conceptualised the analysis. JM, ES, MMK, AT, JJJ, and JBP oversaw sample processing and biospecimen analysis. CEM, HMT, KB, and CM analysed the data. CEM wrote the first draft, with contributions from HMT, CM, TB, JBP, and ME. All authors edited and revised the final manuscript and approved the final version. All authors had access to the de-identified data used in the study and had final responsibility for the decision to submit for publication. CEM, HMT, KB, and CM accessed and verified all data used in the study.
Supplementary Materials
References
- 1.WHO Malaria. March 29, 2023. https://www.who.int/news-room/fact-sheets/detail/malaria
- 2.WHO World malaria report 2020: 20 years of global progress and challenges. Nov 30, 2020. https://www.who.int/publications-detail-redirect/9789240015791
- 3.Bobanga T, Umesumbu SE, Mandoko AS, et al. Presence of species within the Anopheles gambiae complex in the Democratic Republic of Congo. Trans R Soc Trop Med Hyg. 2016;110:373–375. doi: 10.1093/trstmh/trw035. [DOI] [PubMed] [Google Scholar]
- 4.Seyler JR, Thomas D, Mwanza N, Mpoyi A. Democratic Republic of Congo: biodiversity and tropical forestry assessment (118/119): final report. January, 2010. https://pdf.usaid.gov/pdf_docs/Pnads946.pdf
- 5.Deutsch-Feldman M, Brazeau NF, Parr JB, et al. Spatial and epidemiological drivers of Plasmodium falciparum malaria among adults in the Democratic Republic of the Congo. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-002316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janko MM, Irish SR, Reich BJ, et al. The links between agriculture, Anopheles mosquitoes, and malaria risk in children younger than 5 years in the Democratic Republic of the Congo: a population-based, cross-sectional, spatial study. Lancet Planet Health. 2018;2:e74–e82. doi: 10.1016/S2542-5196(18)30009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.United States Agency for International Development Agriculture and Food Security. Nov 10, 2016. https://www.usaid.gov/democratic-republic-congo/agriculture-and-food-security
- 8.Franco AO, Gomes MGM, Rowland M, Coleman PG, Davies CR. Controlling malaria using livestock-based interventions: a one health approach. PLoS One. 2014;9 doi: 10.1371/journal.pone.0101699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaccour C. Veterinary endectocides for malaria control and elimination: prospects and challenges. Philos Trans R Soc Lond B Biol Sci. 2021;376 doi: 10.1098/rstb.2019.0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donnelly B, Berrang-Ford L, Ross NA, Michel P. A systematic, realist review of zooprophylaxis for malaria control. Malar J. 2015;14:313. doi: 10.1186/s12936-015-0822-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahande A, Mosha F, Mahande J, Kweka E. Feeding and resting behaviour of malaria vector, Anopheles arabiensis with reference to zooprophylaxis. Malar J. 2007;6:100. doi: 10.1186/1475-2875-6-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bøgh C, Clarke SE, Walraven GEL, Lindsay SW. Zooprophylaxis, artefact or reality? A paired-cohort study of the effect of passive zooprophylaxis on malaria in The Gambia. Trans R Soc Trop Med Hyg. 2002;96:593–596. doi: 10.1016/s0035-9203(02)90320-2. [DOI] [PubMed] [Google Scholar]
- 13.Hasyim H, Dhimal M, Bauer J, et al. Does livestock protect from malaria or facilitate malaria prevalence? A cross-sectional study in endemic rural areas of Indonesia. Malar J. 2018;17:302. doi: 10.1186/s12936-018-2447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayagaya VS, Nkwengulila G, Lyimo IN, et al. The impact of livestock on the abundance, resting behaviour and sporozoite rate of malaria vectors in southern Tanzania. Malar J. 2015;14:17. doi: 10.1186/s12936-014-0536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takken W, Verhulst NO. Host preferences of blood-feeding mosquitoes. Annu Rev Entomol. 2013;58:433–453. doi: 10.1146/annurev-ento-120811-153618. [DOI] [PubMed] [Google Scholar]
- 16.Killeen GF, Kiware SS, Okumu FO, et al. Going beyond personal protection against mosquito bites to eliminate malaria transmission: population suppression of malaria vectors that exploit both human and animal blood. BMJ Glob Health. 2017;2 doi: 10.1136/bmjgh-2016-000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone C, Gross K. Evolution of host preference in anthropophilic mosquitoes. Malar J. 2018;17:257. doi: 10.1186/s12936-018-2407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The DHS Program Congo Democratic Republic: standard DHS, 2013–14. 2014. https://dhsprogram.com/methodology/survey/survey-display-421.cfm
- 19.Plowe CV, Djimde A, Bouare M, Doumbo O, Wellems TE. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: polymerase chain reaction methods for surveillance in Africa. Am J Trop Med Hyg. 1995;52:565–568. doi: 10.4269/ajtmh.1995.52.565. [DOI] [PubMed] [Google Scholar]
- 20.Mwandagalirwa MK, Levitz L, Thwai KL, et al. Individual and household characteristics of persons with Plasmodium falciparum malaria in sites with varying endemicities in Kinshasa Province, Democratic Republic of the Congo. Malar J. 2017;16:456. doi: 10.1186/s12936-017-2110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Croft TN, Marshall AMJ, Allen CK, et al. Guide to DHS Statistics DHS-7. 2018. https://dhsprogram.com/data/Guide-to-DHS-Statistics/index.htm#t=Wealth_Quintiles.htm
- 22.Kuhl J, Bisimwa L, Thomas ED, et al. Formative research for the development of baby water, sanitation, and hygiene interventions for young children in the Democratic Republic of the Congo (REDUCE program) BMC Public Health. 2021;21:427. doi: 10.1186/s12889-021-10246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tirados I, Gibson G, Young S, Torr SJ. Are herders protected by their herds? An experimental analysis of zooprophylaxis against the malaria vector Anopheles arabiensis. Malar J. 2011;10:68. doi: 10.1186/1475-2875-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan K, Cano J, Massebo F, Messenger LA. Cattle-related risk factors for malaria in southwest Ethiopia: a cross-sectional study. Malar J. 2022;21:179. doi: 10.1186/s12936-022-04202-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bulterys PL, Mharakurwa S, Thuma PE. Cattle, other domestic animal ownership, and distance between dwelling structures are associated with reduced risk of recurrent Plasmodium falciparum infection in southern Zambia. Trop Med Int Health. 2009;14:522–528. doi: 10.1111/j.1365-3156.2009.02270.x. [DOI] [PubMed] [Google Scholar]
- 26.Maia MF, Abonuusum A, Lorenz LM, et al. The effect of deltamethrin-treated net fencing around cattle enclosures on outdoor-biting mosquitoes in Kumasi, Ghana. PLoS One. 2012;7 doi: 10.1371/journal.pone.0045794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ngom HM, Ndione J-A, Ba Y, et al. Spatio-temporal analysis of host preferences and feeding patterns of malaria vectors in the sylvo-pastoral area of Senegal: impact of landscape classes. Parasit Vectors. 2013;6:332. doi: 10.1186/1756-3305-6-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaleta KT, Hill SR, Birgersson G, Tekie H, Ignell R. Chicken volatiles repel host-seeking malaria mosquitoes. Malar J. 2016;15:354. doi: 10.1186/s12936-016-1386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto SS, Louis VR, Sié A, Sauerborn R. The effects of zooprophylaxis and other mosquito control measures against malaria in Nouna, Burkina Faso. Malar J. 2009;8:283. doi: 10.1186/1475-2875-8-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Temu EA, Coleman M, Abilio AP, Kleinschmidt I. High prevalence of malaria in Zambezia, Mozambique: the protective effect of IRS versus increased risks due to pig-keeping and house construction. PLoS One. 2012;7 doi: 10.1371/journal.pone.0031409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghebreyesus TA, Haile M, Witten KH, et al. Household risk factors for malaria among children in the Ethiopian highlands. Trans R Soc Trop Med Hyg. 2000;94:17–21. doi: 10.1016/s0035-9203(00)90424-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in a public, open access repository and upon request from the DHS programme (https://dhsprogram.com/methodology/survey/survey-display-421.cfm), including malaria PCR results. R code used for the analysis is available at https://github.com/IDEELResearch/animalaria.