Abstract
Background
Recent studies suggest that metformin use may be associated with improved infectious disease–related outcomes, whereas other papers suggest potentially worse outcomes in serious bacterial infections. Our purpose was to examine the association of prior outpatient prescription of metformin on 30- and 90-day mortality for older veterans with pre-existing diabetes hospitalized with pneumonia.
Methods
We conducted a retrospective cohort study using national Department of Veterans Affairs data of patients ≥65 years with a prior history of diabetes who were hospitalized with pneumonia over a 10-year period (fiscal years 2002–2012.) For our primary analysis, we created a propensity score and matched metformin users to nonusers 1:1.
Results
We identified 34 759 patients who met the inclusion criteria, 20.3% of whom were prescribed metformin. Unadjusted 30-day mortality was 9.6% for those who received metformin versus 13.9% in nonusers (P < .003), and 90-day mortality was 15.8% for those who received metformin versus 23.0% for nonusers (P < .0001). For the propensity score model, we matched 6899 metformin users to 6899 nonusers. After propensity matching, both 30-day (relative risk [RR]: .86; 95% confidence interval [CI]: .78–.95) and 90-day (RR: .85; 95% CI: .79–.92) mortality was significantly lower for metformin users.
Conclusions
Prior receipt of metformin was associated with significantly lower mortality after adjusting for potential confounders. Additional research is needed to examine the safety and potential benefits of metformin use in patients with respiratory infections.
Keywords: metformin, pneumonia, diabetes mellitus, AMP-activated protein kinase, mortality
Patients with diabetes are at higher risk for serious infections and worse outcomes. We found that, for veterans with diabetes who were hospitalized with pneumonia, prior metformin use was associated with improved short-term survival.
(See the Editorial Commentary by Goto and Perencevich on pages 1245–6.)
Community-acquired pneumonia (CAP) continues to be one of the most common causes of hospitalization in the United States [1]. It is estimated that, by 2040, the rate of pneumonia-related hospitalizations will increase by 96% and related healthcare costs will increase by $2.5 billion annually [2]. Diabetes mellitus (DM) has been observed to be a risk factor driving CAP-related hospitalizations [3], and prior studies have shown that a prior history of DM is associated with worse CAP-related outcomes including prolonged length of stay and higher mortality, potentially up to 1 year [4–7].
Metformin, a biguanide, is the most frequently prescribed antidiabetic agent and is known to have pleiotropic properties, allowing it to be used in a wide variety of domains including cardiovascular disease protection [8], anticancer [9], geroprotection (anti-aging) [10], and weight management [11]. Recent studies have highlighted the usefulness of metformin in infections as a supportive agent due to its ability to potentiate innate immune responses against pathogens [12]. It is postulated that metformin activates the AMP-activated protein kinase (AMPK) pathway, a catabolic cascade that leads to downstream production of mitochondrial reactive oxygen species (mROS), resulting in the inhibition of mammalian target of rapamycin (mTOR), facilitating phagosome–lysosome fusion and bacterial death [13, 14]. Although metformin has a short serum half-life (<4–8 hours), the intracellular half-life of metformin is much longer (>24 hours) than serum/plasma, and a prolonged effect of up 2 weeks is conceivable [15]. Among patients with Mycobacterium tuberculosis infections on metformin, decreased severity in illness and improved clinical outcomes have been documented [13, 16]. Similar anti-pathogenic activity has been observed in pneumonia caused by Pseudomonas aeruginosa [12] and Legionella pneumophila [14].
Given the slow progress in developing new antibiotic treatments and the rapid rise in antibiotic resistance, it is especially important to identify potential adjunctive therapies for those with pneumonia. The pleiotropic therapeutic potential of metformin could expand its clinical application and help improve health outcomes among its users.
In this study, we examined the association of prior outpatient prescription of metformin on 30- and 90-day mortality for older veterans with pre-existing diabetes hospitalized with pneumonia. Our a priori hypothesis was that outcomes among patients prescribed metformin for DM prior to hospitalization with CAP would be significantly improved compared with nonusers, after adjusting for potential confounders.
METHODS
We conducted a retrospective cohort study using the clinical and administrative databases of the Department of Veterans Affairs (VA) Health Care System. These databases are repositories of clinical data from the nationwide VA Health Care System. The VA North Texas Institutional Review Board approved this study.
Inclusion Criteria
We included patients who met the following criteria:
Hospitalization between 1 October 2001 and 30 September 2012
Sixty-five years or older on the date of admission
Discharged with a diagnosis of pneumonia defined as either a primary diagnosis of pneumonia (International Classification of Diseases, Ninth Revision [ICD-9], codes 480.0–483.99 or 485.0–487.0) or a secondary diagnosis of pneumonia with a primary diagnosis of respiratory failure (ICD-9 code 518.81) or sepsis (ICD-9 code 0.38xx)
Diagnosis of diabetes (ICD-9 codes 250.0–250.9) in the year prior to the admission for pneumonia
Had at least 1 dose of antimicrobial therapy within the first 48 hours of admission.
Had at least 3 or more VA outpatient clinic visits in the year preceding admission.
We excluded any patients with a history of end-stage liver disease or end-stage renal disease as these conditions are contraindications to metformin therapy.
Data Sources and Definitions
We controlled for race and ethnicity categories (White, Black, and Hispanic), tobacco use (ICD-9 codes 305.1 and V15.82, smoking cessation clinic use, and/or use of medications for the treatment of nicotine dependence such as bupropion (Zyban, GlaxoSmithKline), nicotine replacement, or varenicline), alcohol abuse (ICD-9 codes 291, 303, 305.0), and illicit drug use (ICD-9 codes 292, 304, 305, excluding 305.0-.1). We used the Charlson–Deyo comorbidity system to identify pre-existing comorbid conditions [17] and the VA priority status for socioeconomic status [18]. Mechanical ventilation was defined as being admitted to the intensive care unit (ICU) during the admission with ICD-9 codes 93.9x or 96.7x.
We defined a patient as a metformin user if they had received at least 1 prescription for metformin within 90 days prior to the admission for pneumonia. Non–metformin users did not receive any metformin prescriptions during this period of time or during the hospitalization for pneumonia.
Potential confounding medications were controlled for by using a count of unique drugs in each of the following classes for outpatient prescriptions filled within 90 days prior to presentation: insulin, antiplatelet agents, antipsychotics, statins, angiotensin II receptor blockers (ARBs), angiotensin-converting enzyme (ACE) inhibitors, cardiac antiarrhythmics, B-blockers, calcium channel blockers, diuretics, nitrate antianginal medications, other cardiac medications, other lipid-lowering medications, B-agonist bronchodilators, other bronchodilators, and prior outpatient antibiotics.
Outcomes
The primary outcomes of this study were all-cause mortality within 30 and 90 days of admission. Death was identified using the VA Vital Status file, which has a 98% accuracy in identifying mortality [19]. Secondary outcomes were surrogates of severity, such as ICU admission, use of mechanical ventilation, use of vasopressors, and length of hospital stay.
Statistical Analysis
For the primary analyses, we used propensity score matching to balance measured confounders between groups (metformin users vs nonusers). Logistic regression was used to create the propensity score and then nearest-number matching with a caliper of 0.01 with no replacement was performed [20]. We selected candidate variables that we believed would be potentially associated with severity of illness (eg, ICU admission, use of mechanical ventilation), outcomes (eg, age, nursing home residence), or comorbid illness that may impact both severity or outcome (eg, cardiovascular disease, dementia.) Variables included in the propensity score are displayed in Table 1. We created a graph that demonstrates the frequency of the values of propensity score by metformin versus nonuse (Supplementary Figure 1). Relative risks (RRs) were calculated to determine the association between metformin use and the outcomes using a generalized linear model (GLM).
Table 1.
Comparison of Metformin Users and Nonusers Hospitalized With Pneumonia
| Variables | Metformin (n = 7039) | No Metformin (n = 27 720) | P |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD), years | 74.9 (6.6) | 77.3 (7.1) | <.0001 |
| Male | 6945 (98.7%) | 27 325 (98.6%) | .6 |
| Race | |||
| White | 5839 (83.0%) | 21 993 (79.3%) | <.0001 |
| Black | 691 (9.8%) | 3698 (13.3%) | <.0001 |
| Hispanic | 537 (7.6%) | 2236 (8.1%) | .2 |
| Nursing home residence | 105 (1.5%) | 562 (2.0%) | .003 |
| Socioeconomic proxies | |||
| VA priority group 1 | 1600 (22.7%) | 6651 (24.0%) | <.0001 |
| VA priority groups 2–6 | 4693 (66.7%) | 18 549 (67.0%) | |
| VA priority groups 7–8 | 746 (10.5%) | 2520 (9.1%) | |
| No. of outpatient visits the year prior to admission, mean (SD) | 18.0 (14.1) | 18.9 (14.6) | <.0001 |
| Characteristics of hospitalization | |||
| ICU admission | 1217 (17.3%) | 5260 (19.0%) | .001 |
| Invasive mechanical ventilation | 473 (5.3%) | 1632 (5.5%) | .009 |
| Noninvasive mechanical ventilation | 370 (5.3%) | 1512 (5.5%) | .5 |
| Vasopressors | 349 (5.0%) | 1577 (5.7%) | .002 |
| Guideline-concordant antibiotics | 5842 (83.0%) | 22 193 (80.1%) | .001 |
| Comorbid conditions | |||
| Tobacco use | 3039 (43.2%) | 10 498 (37.9%) | <.0001 |
| Alcohol abuse | 194 (2.8%) | 940 (3.4%) | .007 |
| Illicit drug abuse | 78 (1.1%) | 361 (1.3%) | .2 |
| Myocardial infarction | 515 (7.3%) | 2732 (9.9%) | <.0001 |
| Heart failure | 1668 (23.7%) | 10 274 (37.1%) | <.0001 |
| Peripheral vascular disease | 1242 (17.6%) | 6162 (22.2%) | <.0001 |
| Stroke | 1241 (17.6%) | 6460 (23.3%) | <.0001 |
| Chronic obstructive pulmonary disease | 3474 (49.4%) | 143 473 (48.6%) | .3 |
| Rheumatologic disease | 146 (2.1%) | 755 (2.7%) | .002 |
| Mild liver disease | 36 (0.5%) | 252 (0.9%) | .001 |
| Peptic ulcer disease | 164 (2.3%) | 769 (2.8%) | .04 |
| Dementia | 222 (3.2%) | 1433 (5.2%) | <.0001 |
| Diabetes | 6964 (99.0%) | 27 183 (98.1%) | <.0001 |
| Diabetes with complications | 1950 (27.9%) | 8840 (31.9%) | <.0001 |
| Hemiplegia | 103 (1.5%) | 467 (1.7%) | .2 |
| Renal disease | 430 (6.1%) | 8891 (32.1%) | <.0001 |
| Any prior malignancy | 265 (3.8%) | 1084 (3.9%) | .6 |
| Metastatic solid tumor | 1616 (23.0%) | 6436 (23.2%) | .6 |
| Hematologic malignancy | 167 (2.4%) | 673 (2.5%) | .8 |
| HIV | 4 (0.1%) | 23 (0.1%) | .5 |
| Other medications | |||
| Antipsychotic agents | 462 (6.6%) | 1655 (6.0%) | .023 |
| Antiplatelet agents | 766 (10.9%) | 3238 (11.7%) | .05 |
| Statins | 4140 (58.8%) | 12 171 (43.9%) | <.0001 |
| ARBs | 810 (11.5%) | 2671 (9.6%) | <.0001 |
| ACE inhibitors | 4008 (57.0%) | 11 183 (40.3%) | <.0001 |
| Antiarrhythmics | 228 (3.2%) | 987 (3.6%) | .2 |
| B-Blockers | 3282 (46.6%) | 11 262 (40.6%) | <.0001 |
| Calcium channel blockers | 2308 (32.8%) | 8289 (29.9%) | <.0001 |
| Diuretics | 4317 (61.3%) | 16 005 (57.7%) | <.0001 |
| Oral antidiabetic medications | 5832 (50.9%) | 8988 (32.4%) | <.0001 |
| Nitrate antianginal medications | 1339 (19.0%) | 5996 (21.6%) | <.0001 |
| Other antihypertensive medications | 1767 (25.1%) | 7067 (25.4%) | <.0001 |
| Insulin 90 days prior | 1684 (24.0%) | 8612 (31.1%) | <.0001 |
| B-Agonist bronchodilators | 2538 (36.1%) | 8616 (31.1%) | <.0001 |
| Theophylline | 214 (3.0%) | 650 (2.3%) | .003 |
| Other cardiac medications | 5908 (83.9%) | 21 295 (76.8%) | <.0001 |
| Other inhaled medications | 1813 (25.8%) | 5784 (20.9%) | <.0001 |
| Other injectable medications | 395 (5.6%) | 1535 (5.5%) | .9 |
| Oral corticosteroids | 1351 (19.2%) | 5117 (18.5%) | .4 |
| Other pulmonary medications | 2461 (35.0%) | 8184 (29.5%) | <.0001 |
| Other bronchodilators | 2708 (38.4%) | 9340 (33.7%) | <.0001 |
| Antibiotics within 90 days prior to hospitalization | 1984 (28.2%) | 7509 (27.1%) | .07 |
Data are presented as no. (%) unless otherwise indicated. Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; HIV, human immunodeficiency virus; ICU, intensive care unit; SD, standard deviation; VA, Veterans Affairs.
To analyze time-to-event for mortality outcomes by receipt of metformin, we used Kaplan–Meier plots to display the survivor functions and assessed statistical significance using the log-rank test.
For secondary analyses, we used Cox proportional hazard models to examine the association of metformin use prior to admission with 90-day mortality in the following predefined analyses: all patients with prior history of diabetes (entire cohort), those with diabetic complications (eg, retinopathy, neuropathy, and/or nephropathy), patients with a history of chronic obstructive pulmonary disease (COPD), and those who were prescribed insulin. These models were adjusted for all of the covariates in the propensity score, except for ICU stay, receipt of mechanical ventilation, vasopressor use, and receipt of noninvasive mechanical ventilation as these variables violated the proportional hazards assumption. For each model, the proportional hazards assumption was verified by checking Schoenfeld residuals over time.
Statistical significance was defined as a 2-tailed P-value of .05 or less. All analyses were performed using STATA 17MP (StataCorp, College Station, TX).
RESULTS
Of the 34 759 patients who were eligible for inclusion, 7039 (20.3%) were prescribed metformin and 27 720 (79.8%) were not. Table 1 demonstrates the breakdown by receipt of metformin. For metformin users, the mean age of patients was 74.9 (standard deviation [SD]: 6.6) years, and 98.7% were male. For non–metformin users, the mean age of patients was 77.3 (SD: 7.1) years, and 98.6% were male. Unadjusted 30-day mortality was 9.6% for those who received metformin versus 13.9% in nonusers (P < .0001), and 90-day mortality was 15.8% for those who received metformin versus 23.0% for nonusers (P < .0001).
Propensity Matched Cohort
Prior to matching, there were some statistically significant differences between groups. Some examples include metformin users being younger, more likely to be White, have more tobacco use, less renal disease and heart failure, and more likely to be on ACE inhibitors, statins, and other oral antidiabetic medications.
We matched 6899 patients who were prescribed metformin containing antidiabetic therapy to 6899 patients who received other antidiabetic treatment regimes. Table 2 shows the balance between key variables after propensity matching. There were no statistically significant differences between groups for any of the key characteristics after matching.
Table 2.
Comparison of Propensity Matched Metformin Users and Nonusers Hospitalized With Pneumonia
| Variables | Metformin (n = 6899) | No Metformin (n = 6899) | P | Standardized Difference |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean (SD) | 75.1 (6.6) | 75.1 (6.6) | .97 | 0.0005 |
| Men | 6806 (98.7%) | 6806 (98.7%) | 1.0 | 0.0000 |
| Race | ||||
| White | 5710 (82.3%) | 5729 (83.0%) | .20 | 0.007 |
| Black | 691 (10.0%) | 686 (9.9%) | .90 | −0.0024 |
| Hispanic | 526 (7.6%) | 525 (7.6%) | .97 | −0.0006 |
| Nursing home residence | 103 (1.5%) | 104 (1.5%) | .94 | 0.001 |
| Socioeconomic proxies | .91 | 0.005 | ||
| VA priority group 1 | 1553 (22.5%) | 1533 (22.2%) | ||
| VA priority groups 2–6 | 4619 (67.0%) | 4634 (67.1%) | ||
| VA priority groups 7–8 | 727 (10.5%) | 732 (10.6%) | ||
| No. of outpatient visits the year prior to admission, mean (SD) | 17.9 (13.3) | 18.0 (14.2) | .76 | −0.005 |
| Characteristics of hospitalization | ||||
| ICU admission | 1201 (17.4%) | 1156 (16.8%) | .31 | −0.02 |
| Invasive mechanical ventilation | 468 (6.9%) | 465 (6.7%) | .91 | −0.002 |
| Noninvasive mechanical ventilation | 367 (5.3%) | 353 (5.1%) | .60 | −0.009 |
| Vasopressors | 342 (5.0%) | 336 (4.9%) | .81 | −0.004 |
| Guideline-concordant antibiotics | 5727 (83.0%) | 5724 (83.0) | .95 | −0.001 |
| Comorbid conditions | ||||
| Tobacco use | 2952 (42.8%) | 2964 (43.0%) | .84 | 0.003 |
| Alcohol abuse | 193 (2.8%) | 190 (2.8%) | .88 | −0.003 |
| Illicit drug abuse | 77 (1.1%) | 76 (1.1%) | .94 | 0.001 |
| Myocardial infarction | 511 (7.4%) | 509 (7.4%) | .95 | −0.001 |
| Heart failure | 1661 (24.1%) | 1714 (24.8%) | .30 | 0.02 |
| Peripheral vascular disease | 1228 (18.0%) | 1271 (18.4%) | .34 | 0.02 |
| Stroke | 1232 (17.9%) | 1283 (18.6%) | .26 | 0.02 |
| Chronic obstructive pulmonary disease | 3398 (49.3%) | 3475 (50.4%) | .19 | 0.02 |
| Rheumatologic disease | 144 (2.1%) | 165 (2.4%) | .23 | 0.02 |
| Mild liver disease | 36 (0.5%) | 22 (0.3%) | .07 | −0.03 |
| Peptic ulcer disease | 163 (2.4%) | 171 (2.5%) | .66 | 0.008 |
| Dementia | 222 (3.2%) | 232 (3.4%) | .63 | 0.0078 |
| Diabetes | 6824 (98.9%) | 6834 (99.1%) | .40 | 0.01 |
| Diabetes with complications | 1904 (27.6%) | 1866 (27.1%) | .47 | −0.01 |
| Hemiplegia | 103 (1.5%) | 88 (1.3%) | .31 | −0.02 |
| Renal disease | 430 (6.2%) | 431 (6.3%) | .97 | 0.0006 |
| Any prior malignancy | 1583 (22.9%) | 1569 (22.7%) | .78 | −0.005 |
| Metastatic solid tumor | 261 (3.8%) | 72 (3.9%) | .63 | 0.008 |
| Hematologic malignancy | 163 (2.4%) | 180 (2.6%) | .35 | 0.02 |
| HIV | 4 (0.1%) | 5 (0.1%) | .73 | 0.005 |
| Outpatient medications | ||||
| Antipsychotic agents | 451 (6.5%) | 465 (6.7%) | .20 | 0.01 |
| Antiplatelet agents | 753 (10.9%) | 779 (11.2%) | .44 | 0.007 |
| Statins | 4004 (58.0%) | 3963 (57.4%) | .14 | −0.007 |
| ARBs | 780 (11.3%) | 768 (12.5%) | .47 | −0.004 |
| ACE inhibitors | 3888 (56.4%) | 3920 (56.8%) | .31 | 0.01 |
| Antiarrhythmics | 223 (3.2%) | 253 (3.7%) | .24 | 0.02 |
| B-Blockers | 3184 (46.2%) | 3202 (46.4%) | .22 | 0.009 |
| Calcium channel blockers | 2230 (32.3%) | 2253 (32.7%) | .45 | 0.01 |
| Diuretics | 4199 (60.9%) | 4324 (62.7%) | .15 | 0.03 |
| Nitrate antianginal medications | 1317 (19.0%) | 1357 (19.7%) | .69 | 0.01 |
| Other antihypertensive medications | 1708 (24.9%) | 1624 (23.5%) | .07 | −0.02 |
| Insulin | 1675 (24.3%) | 1679 (24.3%) | .94 | 0.001 |
| B-Agonist bronchodilators | 2468 (35.8%) | 2558 (37.0%) | .59 | 0.02 |
| Theophylline | 208 (3.0%) | 213 (3.1%) | .81 | 0.004 |
| Other cardiac medications | 5716 (82.9%) | 5658 (82.0%) | .13 | 0.02 |
| Other inhaled medications | 1822 (26.4%) | 1753 (25.4%) | .66 | 0.03 |
| Other injectable medications | 390 (5.7%) | 422 (6.1%) | .43 | 0.02 |
| Oral corticosteroids | 1324 (19.2%) | 1358 (19.7%) | .23 | 0.01 |
| Other pulmonary medications | 2634 (38.2%) | 2732 (39.6%) | .28 | 0.02 |
| Other bronchodilators | 2390 (34.6%) | 2449 (35.5%) | .27 | 0.01 |
| Antibiotics within 90 days prior to hospitalization | 1930 (28.0%) | 1964 (28.4%) | .52 | 0.01 |
Data are presented as no. (%) unless otherwise indicated. Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; HIV, human immunodeficiency virus; ICU, intensive care unit; SD, standard deviation; VA, Veterans Affairs.
Primary Outcomes
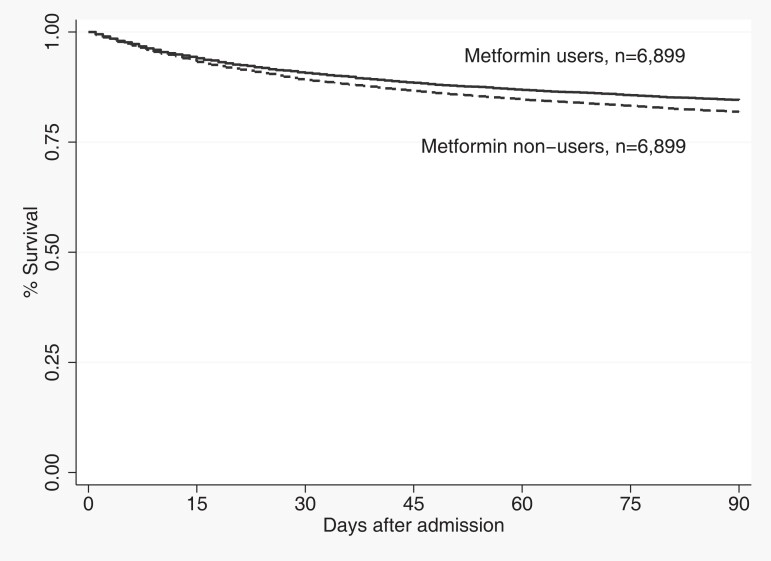
Table 3 show the primary and secondary outcome results. After propensity matching, 30-day mortality was 9.6% (n = 667) for those who received metformin versus 13.9% (n = 775) in nonusers (P < .003), and 90-day mortality was 15.8% (n = 1098) for those who received metformin versus 23.0% (n = 1285) for nonusers (P < .0001). Both 30-day (RR: .86; 95% confidence interval [CI]: .78–.95) and 90-day (RR: .85; 95% CI: .79–.92) mortality were significantly lower for metformin users. The number needed to treat (NNT) at 30 days was 23.3 and at 90 days was 13.9. Figure 1 shows mortality over the first 90 days after admission and demonstrates that those who were previously prescribed metformin had significantly lower mortality (P < .001).
Table 3.
Primary and Secondary Outcomes
| Relative Risk or Hazard Ratio | 95% Confidence Interval | |
|---|---|---|
| Propensity matched modelsa | ||
| 30-Day mortality | .86 | (.78–.95) |
| 90-Day mortality | .85 | (.79–.92) |
| Secondary analyses, 90-day mortalityb | ||
| Entire cohort | .85 | (.80–.91) |
| Diabetes with complications | .76 | (.66–.87) |
| Prior history of COPD | .85 | (.77–.93) |
| Prescribed insulin | .76 | (.66–.87) |
Abbreviation: COPD, chronic obstructive pulmonary disease.
Relative risks.
Hazard ratios.
Figure 1.
Mortality over the first 90 days after admission by metformin use versus non-use (P < .001).
Secondary Analyses
For the entire cohort (N = 34 759), in the Cox proportional hazards models, prescription of metformin was associated with lower 90-day (hazard ratio [HR]: .85; 95% CI: .80–.91) mortality. In addition, 90-day mortality was significantly lower for patients prescribed metformin in those who had diabetes with complications (HR: .76; 95% CI: .66–.87), prior history of COPD (HR: .85; 95% CI: .77–.93), or prescribed insulin (hazards ratio: .76; 95% CI: .66–.87).
DISCUSSION
Our study demonstrates that recent prior prescription of metformin among patients with a history of DM and admitted with pneumonia was associated with significantly lower 30- and 90-day mortality. Additionally, similar results were observed in those with complicated DM, history of COPD, and concurrent insulin use associated with metformin prescription.
Community-acquired pneumonia has consistently ranked as the top cause of infection-related hospitalization in developed countries and is directly responsible for increased healthcare utilization and mortality among the elderly population [20, 21]. Several studies have shown an increasing prevalence of DM in patients admitted with CAP and significantly worse outcomes [22–24]. Therefore, ensuring that patients with DM, without contraindications to metformin, are prescribed this medication may improve outcomes for this vulnerable population.
Based on the evidence from preclinical studies, we hypothesize that this significant association between metformin and better CAP outcomes is related to the modulation of the AMPK pathway. The role of AMPK in terms of regulating the carbohydrate and lipid metabolism is well established [25], and extensive in vitro studies undertaken to understand the pathobiology behind metformin's pleiotropic effects have shed insights into the numerous beneficial effects of activation of AMPK [25–29]. Animal studies have shown that metformin prolongs the life of mice and their health span by promoting healthy aging [30] and that metformin is also protective against the damage induced by cigarette smoking in the lungs and other tissues through the AMPK pathway [27–30]. In humans, a study from the COPDGene cohort demonstrated a slower progression of emphysema among participants taking metformin [26].
In CAP, bacterial toxins may cause acute lung inflammation from an influx of neutrophils and heightened pulmonary proinflammatory cytokine synthesis due to alveolar macrophage activation [31]. This proinflammatory state induces inactivation of AMPK through glycogen synthase kinase-3β (GSK-3β), further promoting lung tissue injury [32]. In preclinical models of acute lung injury, metformin counteracted this process, resulting in reduced damage to the lung parenchyma and mortality [31, 32].
In a prospective cohort study from Italy evaluating the association of hospitalization for CAP with type 2 DM (T2DM), metformin therapy was associated with lower 30-day mortality, consistent with our findings [5]. However, there were no differences in mortality at 1 year, possibly because the patients who had diabetes and CAP had significantly higher rates of concurrent comorbidities, including heart failure, COPD, and chronic kidney disease. Gorricho et al [33] observed no difference in the risk of CAP among patients with T2DM among patients on metformin when compared with patients not receiving any antidiabetic treatment.
Potential benefits of metformin have also been demonstrated for other pulmonary conditions. For instance, metformin use was associated with reduced respiratory complications including pneumonia and improved quality of life from reduced symptom exacerbation among patients with asthma–COPD overlap [34]. However, there are conflicting results for CAP outcomes among patients with COPD and T2DM on metformin. Some studies reported that metformin use reduced adverse outcomes among patients with COPD [34–36]. Sexton et al [37] reported decreased symptom burden and overall improved health status among patients with a similar risk profile. Yen et al [38] reported a higher incidence of bacterial pneumonia and increased risk of hospitalizations and invasive mechanical ventilation use. A limitation of this study was that patients were not medically optimized, which may contribute to the higher mortality. During the coronavirus disease 2019 (COVID-19) pandemic, several studies also documented better outcomes in patients with COVID-19 pneumonia who were on metformin [38–40].
We acknowledge that our results may be due to other factors that are only indirectly related to metformin use, such as healthy user behaviors (eg, frequent healthcare visits, adherence to medications). While we controlled for measured factors that are potentially associated with healthy behaviors (eg, sex, smoking status), there were many other factors that we were unable to appropriately measure and control for. Future prospective studies may be able to explore these possible relationships more fully.
Our study has several limitations. We did not have microbiological data, vaccination information, or information on other healthy user behaviors. The vast majority of VA patients in general, and in our cohort, are White males, which is not representative of the general US population. We lacked the necessary data to calculate severity-of-illness scores such as the Acute Physiology and Chronic Health Evaluation (APACHE) or the Pneumonia Severity Index because when this dataset was created laboratory and physiologic variables (eg, blood pressure) were unreliable in the data source. We had to use ICD-9 codes to define mechanical ventilation, which potentially may miss some patients who received this therapy. In addition, our dataset collected information until the end of 2012, so there is the potential that it may be less applicable to patients today. However, we believe that, as metformin continues to be one of the most used medications for T2DM, this is unlikely. We were also unable to reliably determine if patients had type 1 DM or T2DM. However, we do not believe that this would have significantly impacted our results as type 1 DM is usually an excluding factor for military service. Finally, we were unable to examine the impact of metformin continuation during hospitalization because during this period the recommendation was to stop metformin during hospitalization due to concerns related to lactic acidosis [41].
Our study also has several strengths. It has a large sample size with good matching of measured confounders using propensity scores techniques. The electronic medical record of the VA healthcare system allows complete capture of all medical information for the entire healthcare system. Additionally, our large cohort allowed us to assess subgroups with different comorbid conditions, such as complicated DM and COPD, and the effect of metformin with concurrent insulin use.
In summary, metformin was associated with improved short-term mortality in veterans with DM hospitalized with CAP. We believe that, given the increasing rates of antimicrobial resistance to commonly used antibiotics, novel methods of improving outcomes for patients with pneumonia are needed. Prospective, well-designed studies are needed to further examine the association of metformin, and other potentially protective medications, in populations that are at high risk of respiratory infections.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. T. M.: project administration, validation, resources, and writing—original draft and editing. M. B.: data curation, resources, and writing—original draft preparation. A. P.: data curation, resources. M. P.: supervision, reviewing, and editing. C. A. A.: software, validation, and formal analysis. E. M. M.: conceptualization, formal analysis, supervision, funding acquisition, review, and editing.
Financial support. The work was supported by the National Institute of Nursing Research (grant number R01NR010828). C. A. A. reports support from the National Institutes of Health/National Center for Advancing Translational Sciences (NIH/NCATS; grant number UL1TR003163) and the University of Texas Southwestern Center for Translational Medicine (paid to their institution). E. M. M. reports support from National Institute of Nursing Research (grant funds to their institution) and the Department of Veterans Affairs (study material).
Supplementary Material
Contributor Information
Turab Mohammed, Department of Medicine, University of Connecticut School of Medicine, Farmington, Connecticut, USA.
Michael Bowe, Department of Medicine, University of Connecticut School of Medicine, Farmington, Connecticut, USA.
Alexandria Plant, Department of Medicine, University of Connecticut School of Medicine, Farmington, Connecticut, USA.
Mario Perez, Department of Medicine, University of Connecticut School of Medicine, Farmington, Connecticut, USA.
Carlos A Alvarez, Department of Medicine, VA North Texas Health Care System, Dallas, Texas, USA; Department of Medicine, Texas Tech University Health Sciences Centre, Jerry H. Hodge School of Pharmacy, Dallas, Texas, USA.
Eric M Mortensen, Department of Medicine, University of Connecticut School of Medicine, Farmington, Connecticut, USA; Department of Medicine, VA North Texas Health Care System, Dallas, Texas, USA.
References
- 1.Agency for Healthcare Research and Quality. Most common diagnoses in hospital inpatient stays—HCUP Fast Stats [Internet]. Available at: https://www.hcup-us.ahrq.gov/faststats/NationalDiagnosesServlet. Accessed 6 October 2022.
- 2. Wroe PC, Finkelstein JA, Ray GT, et al. Aging population and future burden of pneumococcal pneumonia in the United States. J Infect Dis 2012; 205:1589–92. [DOI] [PubMed] [Google Scholar]
- 3. Brunetti VC, Ayele HT, Yu OHY, Ernst P, Filion KB. Type 2 diabetes mellitus and risk of community-acquired pneumonia: a systematic review and meta-analysis of observational studies. CMAJ Open 2021; 9:E62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martins M, Froes F, Boavida JM, et al. Prevalence and impact of diabetes mellitus (DM) among hospitalized community-acquired pneumonia (CAP) patients. Eur Respir J 2015; 46:PA1104. [Google Scholar]
- 5. Falcone M, Tiseo G, Russo A, et al. Hospitalization for pneumonia is associated with decreased 1-year survival in patients with type 2 diabetes: results from a prospective cohort study. Medicine 2016; 95:e2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bader MS, Yi Y, Abouchehade K, Haroon B, Bishop LD, Hawboldt J. Community-acquired pneumonia in patients with diabetes mellitus: predictors of complications and length of hospital stay. Am J Med Sci 2016; 352:30–5. [DOI] [PubMed] [Google Scholar]
- 7. Jensen AV, Faurholt-Jepsen D, Egelund GB, et al. Undiagnosed diabetes mellitus in community-acquired pneumonia—a prospective cohort study. Eur Respir J 2017; 50:PA4104. [DOI] [PubMed] [Google Scholar]
- 8. Han Y, Xie H, Liu Y, Gao P, Yang X, Shen Z. Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: a systematic review and an updated meta-analysis. Cardiovasc Diabetol 2019; 18:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aljofan M, Riethmacher D. Anticancer activity of metformin: a systematic review of the literature. Future Sci OA 2019; 5:FSO410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Piskovatska V, Stefanyshyn N, Storey KB, Vaiserman AM, Lushchak O. Metformin as a geroprotector: experimental and clinical evidence. Biogerontology 2019; 20:33–48. [DOI] [PubMed] [Google Scholar]
- 11. Seifarth C, Schehler B, Schneider HJ. Effectiveness of metformin on weight loss in non-diabetic individuals with obesity. Exp Clin Endocrinol Diabetes 2013; 121:27–31. [DOI] [PubMed] [Google Scholar]
- 12. Xiao Y, Liu F, Li S, et al. Metformin promotes innate immunity through a conserved PMK-1/p38 MAPK pathway. Virulence 2020; 11:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marupuru S, Senapati P, Pathadka S, Miraj SS, Unnikrishnan MK, Manu MK. Protective effect of metformin against tuberculosis infections in diabetic patients: an observational study of south Indian tertiary healthcare facility. Braz J Infect Dis 2017; 21:312–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kajiwara C, Kusaka Y, Kimura S, et al. Metformin mediates protection against Legionella pneumonia through activation of AMPK and mitochondrial reactive oxygen Species. J Immunol 2018; 200:623–31. [DOI] [PubMed] [Google Scholar]
- 15. Kajbaf F, Bennis Y, Hurtel-Lemaire A-S, Andréjak M, Lalau J-D. Unexpectedly long half-life of metformin elimination in cases of metformin accumulation. Diabet Med 2016; 33:105–10. [DOI] [PubMed] [Google Scholar]
- 16. Singhal A, Jie L, Kumar P, et al. Metformin as adjunct antituberculosis therapy. Sci Transl Med 2014; 6:263ra159. [DOI] [PubMed] [Google Scholar]
- 17. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45:613–9. [DOI] [PubMed] [Google Scholar]
- 18. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med 1998; 158:626–32. [DOI] [PubMed] [Google Scholar]
- 19. Sohn M-W, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr 2006; 4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leuven E, Sianesi B. PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing [Internet]. 2018. Available at: https://econpapers.repec.org/software/bocbocode/s432001.htm. Accessed 16 December 2021.
- 21. Polsky D, Bonafede M, Suaya JA. Comorbidities as a driver of the excess costs of community-acquired pneumonia in U.S. commercially-insured working age adults. BMC Health Serv Res 2012; 12:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Millett ERC, Stavola BLD, Quint JK, Smeeth L, Thomas SL. Risk factors for hospital admission in the 28 days following a community-acquired pneumonia diagnosis in older adults, and their contribution to increasing hospitalisation rates over time: a cohort study. BMJ Open 2015; 5:e008737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Di Yacovo S, Garcia-Vidal C, Viasus D, et al. Clinical features, etiology, and outcomes of community-acquired pneumonia in patients with diabetes mellitus. Medicine (Baltimore) 2013; 92:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martins M, Boavida JM, Raposo JF, et al. Diabetes hinders community-acquired pneumonia outcomes in hospitalized patients. BMJ Open Diabetes Res Care 2016; 4:e000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Srivastava RAK, Pinkosky SL, Filippov S, Hanselman JC, Cramer CT, Newton RS. AMP-activated protein kinase: an emerging drug target to regulate imbalances in lipid and carbohydrate metabolism to treat cardio-metabolic diseases. J Lipid Res 2012; 53:2490–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Polverino F, Wu TD, Rojas-Quintero J, et al. Metformin: experimental and clinical evidence for a potential role in emphysema treatment. Am J Respir Crit Care Med 2021; 204:651–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sag D, Carling D, Stout RD, Suttles J. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol 2008; 181:8633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nath N, Khan M, Paintlia MK, Hoda MN, Giri S. Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. J Immunol 2009; 182:8005–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao X, Zmijewski JW, Lorne E, et al. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol 2008; 295:L497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martin-Montalvo A, Mercken EM, Mitchell SJ, et al. Metformin improves healthspan and lifespan in mice. Nat Commun 2013; 4:2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zmijewski JW, Lorne E, Zhao X, et al. Antiinflammatory effects of hydrogen peroxide in neutrophil activation and acute lung injury. Am J Respir Crit Care Med 2009; 179:694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Park DW, Jiang S, Liu Y, et al. GSK3β-dependent inhibition of AMPK potentiates activation of neutrophils and macrophages and enhances severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol 2014; 307:L735–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gorricho J, Garjón J, Alonso A, et al. Use of oral antidiabetic agents and risk of community-acquired pneumonia: a nested case–control study. Br J Clin Pharmacol 2017; 83:2034–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu TD, Fawzy A, Kinney GL, et al. Metformin use and respiratory outcomes in asthma-COPD overlap. Respir Res 2021; 22:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mendy A, Gopal R, Alcorn JF, Forno E. Reduced mortality from lower respiratory tract disease in adult diabetic patients treated with metformin. Respirology 2019; 24:646–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ho T-W, Huang C-T, Tsai Y-J, Lien AS-Y, Lai F, Yu C-J. Metformin use mitigates the adverse prognostic effect of diabetes mellitus in chronic obstructive pulmonary disease. Respir Res 2019; 20:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sexton P, Metcalf P, Kolbe J. Respiratory effects of insulin sensitisation with metformin: a prospective observational study. COPD 2014; 11:133–42. [DOI] [PubMed] [Google Scholar]
- 38. Yen F-S, Wei JC-C, Yang Y-C, Hsu C-C, Hwu C-M. Respiratory outcomes of metformin use in patients with type 2 diabetes and chronic obstructive pulmonary disease. Sci Rep 2020; 10:10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scheen AJ. Metformin and COVID-19: from cellular mechanisms to reduced mortality. Diabetes Metab 2020; 46:423–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kow CS, Hasan SS. Mortality risk with preadmission metformin use in patients with COVID-19 and diabetes: a meta-analysis. J Med Virol 2021; 93:695–7. [DOI] [PubMed] [Google Scholar]
- 41. Kodner C, Anderson L, Pohlgeers K. Glucose management in hospitalized patients. Am Fam Physician 2017; 96:648–54. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.