Abstract
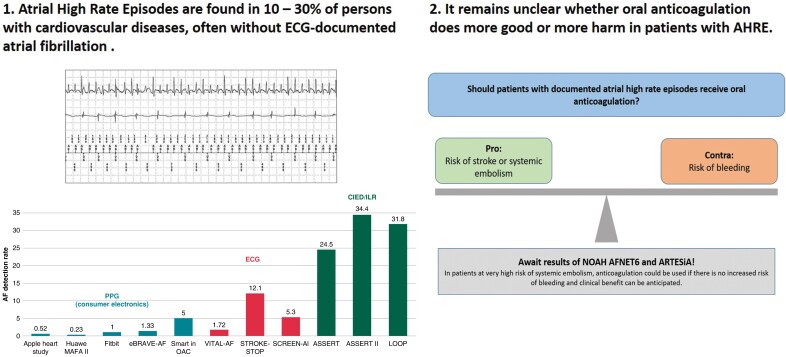
Atrial high-rate episodes (AHRE) are atrial tachyarrhythmias detected by continuous rhythm monitoring by pacemakers, defibrillators, or implantable cardiac monitors. Atrial high-rate episodes occur in 10–30% of elderly patients without atrial fibrillation. However, it remains unclear whether the presence of these arrhythmias has therapeutic consequences. The presence of AHRE increases the risk of stroke compared with patients without AHRE. Oral anticoagulation would have the potential to reduce the risk of stroke in patients with AHRE but is also associated with a rate of major bleeding of ∼2%/year. The stroke rate in patients with AHRE appears to be lower than the stroke rate in patients with atrial fibrillation. Wearables like smart-watches will increase the absolute number of patients in whom atrial arrhythmias are detected. It remains unclear whether anticoagulation is effective and, equally important, safe in patients with AHRE. Two randomized clinical trials, NOAH-AFNET6 and ARTESiA, are expected to report soon. They will provide much-needed information on the efficacy and safety of oral anticoagulation in patients with AHRE.
Keywords: Atrial fibrillation, Atrial high-rate episodes, Sub-clinical atrial fibrillation, Pacemaker, Implantable cardiac monitor, Continuous monitoring, Stroke, Thrombo-embolic risk, Anticoagulation
Graphical Abstract
Graphical abstract.
Introduction
Atrial fibrillation (AF) often remains undiagnosed until a first stroke occurs, especially in elderly populations with comorbidities.1–4 Early detection of AF and subsequent initiation of oral anticoagulation has the potential to reduce these arrhythmia-related strokes.5 On the other hand, the effectiveness of oral anticoagulation has been shown in patients with electrocardiogram (ECG)-documented AF,5,6 leaving the effectiveness and safety of oral anticoagulation in patients with atrial arrhythmias detected by systematic rhythm monitoring an evidence gap.5 This evidence gap will require addressing due to the growing availability of consumer electronics and wearables capable of recording and analysing the cardiac rhythm.7,8 To assess the effectiveness of modern oral anticoagulation in the context of systematic screening, several clinical trials pairing systematic ECG screening for AF with the initiation of oral anticoagulation in high-risk patient groups, e.g. stroke survivors, and in elderly populations have recently been completed, with some signals for effectiveness and some neutral outcomes.2,9,10 One of the reasons for these more ambiguous outcomes could be that a reduced arrhythmia burden carries a lower risk of stroke.8,11
An almost ideal population to evaluate the role of infrequent atrial arrhythmias for outcomes in elderly populations are patients with implanted pacemakers, defibrillators, or loop recorders. These devices can reliably capture and quantity atrial high-rate episodes (‘AHRE’), the biological equivalents of short and rare bouts of AF. Atrial high-rate episodes are associated with an increased thrombo-embolic risk, even though it is lower than that of clinical AF.12 The thrombo-embolic risk appears to be influenced by the duration and frequency of AHRE episodes and by the number and severity of comorbidities.13 Other research has provided a more detailed picture of the proportion of patients with AHRE in elderly cohorts of patients with cardiovascular conditions. This review summarizes the current knowledge of stroke risk.
Definition and prevalence of atrial high-rate episodes/sub-clinical atrial fibrillation and atrial fibrillation and effects of associated conditions on prevalence
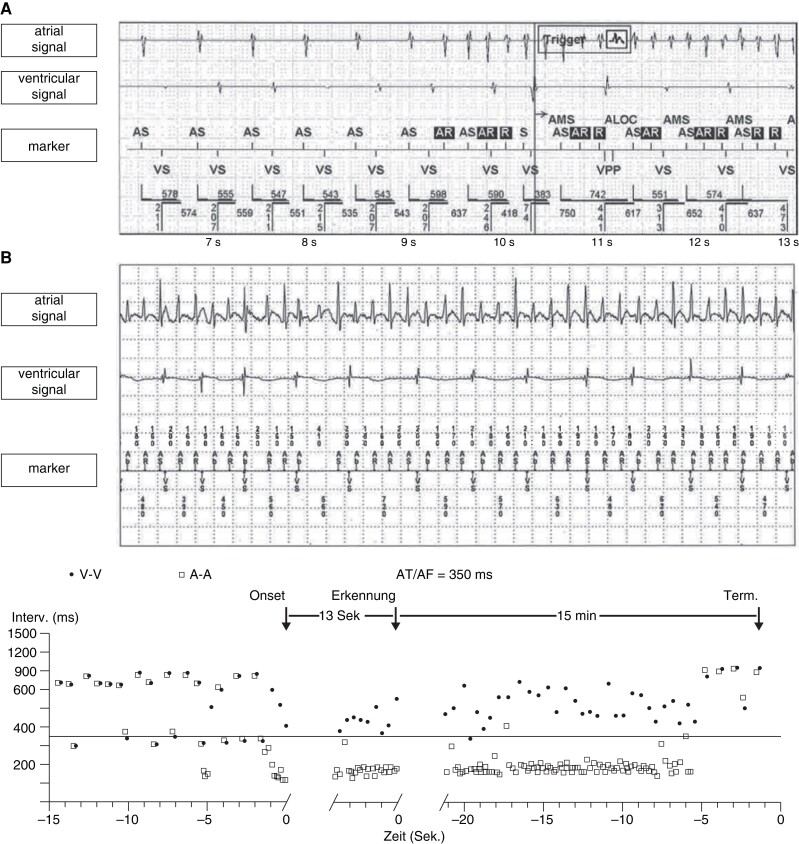
While the terms AHRE and sub-clinical atrial fibrillation (SCAF) have been used with slightly different meanings, they refer essentially to the same observations, relatively rare and short bouts of atrial arrhythmias that resemble short episodes of AF recorded on intracardiac or subcutaneous electrograms in patients without clinically diagnosed AF. These arrhythmias can reliably be detected with long-term continuous cardiac monitoring by implanted devices, provided that each episode lasts a few minutes (Figure 1). Intermittent monitoring will only record a small fraction of these episodes, resulting in populations enriched for patients with frequent and long AHRE. For this review, we consider ‘patients with AHRE’ as patients with AHRE, but without ECG-diagnosed AF, thereby focusing on the population in whom the best treatment for stroke prevention is not yet established.5
Figure 1.
Atrial high-rate episode detected by a dual-chamber pacemaker and the corresponding intracavitary electrogram. (A) Electrogram of a dual-chamber pacemaker (Abbott). AF, atrial fibrillation; AMS, atrial mode switch episode; AR, atrial signal in the refractory period; AS, atrial sensing; AT, atrial tachycardias; VS, ventricular sensing; VPP, ventricular backup pacing. (B) Electrogram and plot of a dual-chamber pacemaker (Medtronic).
The detection of atrial high-rate episodes depends on the duration of monitoring and on age and comorbidities
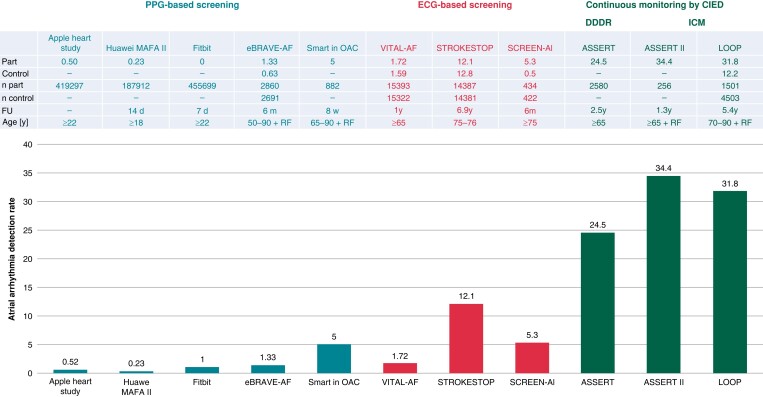
The prevalence of AHRE highly differs among published trials and is influenced by age, comorbidities, and the detection threshold programmed by the device14 and, in case of wearables, the detection algorithm. Overall, continuous monitoring identifies AHRE in 25–30% of elderly patient populations.15 The ASSERT trial defined AHRE (termed ‘SCAF’ by the authors) as an atrial rate >190 b.p.m. exceeding 6 min and found these in 251 out of 2580 (10.1%) pacemaker patients without a history of AF during a screening period of 3 months. The presence of AHRE was associated with an increased future risk of clinical AF [hazard ratio (HR) 5.56, 95% confidence interval (CI) 3.78–8.17; P < 0.001] and ischaemic stroke or systemic embolism (SE) (HR 2.49, 95% CI 1.28–4.85; P = 0.007) during a mean follow-up of 2.5 years.16
The ASSERT II trial included patients without an implanted pacemaker or defibrillator aged ≥65 years without a history of AF and a CHA2DS2-VASc score ≥2.17 For the detection of SCAF (defined by a duration of ≥5 min), an implantable cardiac monitor (ICM) was used. Sub-clinical atrial fibrillation was found in 34.4% (90 out of 256) patients during a follow-up of 16.3 ± 3.8 months. This rate increased to 51.9% per year in patients with a left atrial volume above the median value of 73.5 mL.
In the Implantable Loop Recorder to prevent Stroke in High-risk Individuals (LOOP) study, 6004 patients aged 70–90 years (mean age 74.7 ± 4.1 years) with at least one additional stroke risk factor (arterial hypertension, diabetes, stroke, and heart failure) were 1:3 randomized to ICM monitoring or standard of care.10 During more than 5 years of follow-up, detection rates were remarkably high both in the intervention (32%) and in the control group (12%).
A recent systematic review and meta-analysis found a point estimate of 17.56 AHRE cases per 100 person-years (95% CI 8.61–35.79; n = 4322).18 If studies including a subgroup of patients (<50%) with a history of AF were taken into account as well, the pooled prevalence of asymptomatic mode switch episodes increased to even 28.1% (95% CI 24.3–32.1%; n = 72 784 patients).19
The detection rates of AF in short-term or intermittent monitoring are as expected much lower than in implanted devices. It detects atrial arrhythmias in 1–10% of populations. Healthcare professional-led approaches to screening for AF yielded relevant numbers of arrhythmia detection, depending on the amount of screening, devices, and study population: 2, 5, and 12% in the VITAL-AF,20 SCREEN-AF,4 and STROKESTOP,9 respectively.
In the STROKESTOP study, 28 768 eligible subjects between the age of 75 and 76 years being the only inclusion criteria were randomly invited for an AF screening program using a handheld ECG device twice daily for 14 days. Minimum and median follow-up periods were 5.6 and 6.9 years, respectively. A total of 13 979 were invited to the screening of whom 7165 participants followed the invitation whereas 6814 did not. In both groups, the AF-detection rate was 12%. The primary endpoint was a combination of all-cause death, stroke, or bleeding and just reached statistical significance in the intention-to-treat analysis: 4456 events (31.9%) in the intervention group and 4616 events (33.0%) in the control group (HR 0.96, 95% CI 0.92–1.00; P = 0.045). The secondary outcome parameter—ischaemic stroke—was significantly reduced in participants vs. controls (HR 0.76, 95% CI 0.68–0.87; P < 0.001). Using a lifetime perspective per 1000 invited to screening, 11 strokes can be avoided and 65 QALYs can be gained making this a cost-effective intervention already after 3 years.21,22
In the VITAL-AF trial, 30 715 participants aged ≥65 years without a history of AF were randomized to screening (n = 15 393; 91% active participation) using a handheld single-lead ECG (AliveCor KardiaMobile) at primary care visits or usual care (n = 15 322). This approach did not affect new AF diagnosis within 1 year (1.72 vs. 1.59%; risk difference 0.13%; 95% CI −0.16 to 0.42; P = 0.38). In the subgroup of individuals aged ≥85 years, new AF was diagnosed in 5.56% in the screening arm vs. 3.76% in the control arm (risk difference 1.80%; 95% CI 0.18–3.30).20
In contrast to healthcare professional-led approaches to screen for AF, the rate of atrial arrhythmias detected by consumer electronics is remarkably low: 0.04, 0.12, and 0.08% in the Apple Heart Study,23 Huawei Heart Study,24 and Fitbit Heart Study,25 respectively. Importantly, when an irregular heart rhythm detection occurred, participants were sent a patch ECG to confirm the diagnosis. In the Apple Heart Study, the patch was returned by 21% of participants with atrial arrhythmias, of whom 34% were confirmed as AF.
The arrhythmia detection rates are substantially lower than those from studies of healthcare professional-led screening for AF. The main reason is that the populations screened were relatively young and healthy, but also the photoplethysmography (PPG)-based irregular heart rhythm detection algorithm may play a role, as it is only active at rest. However, there are reports showing that PPG technology still needs improvement and only a minority of PPG samples lasting for 30 s are analysable.26 It is also important to note that the effectiveness of consumer-led AF screening on AF outcomes remains unknown.27
There are two more recent studies with PPG-based screening for AF. The recently published eBRAVE-AF study28 combined PPG-based screening using a smartphone app with a remote recruitment approach from the pool of policyholders of a large German health insurance. There was no in-person contact with study participants (aged 50–90 years without known AF and without previous oral anticoagulation therapy and a CHA2DS2-VASc score of ≥1 in males and ≥2 in women) who were randomized to repeated 1-min PPG screening during 6 months or usual care, followed by a second cross-over phase if the primary endpoint (newly diagnosed AF leading to initiation of oral anticoagulation by an independent physician not involved in the study) had not been reached. Digital screening more than doubled the detection rate of treatment-relevant AF in both phases of the trial, with odds ratio (OR) of 2.12 (95% CI 1.19–3.76; P = 0.01) and 2.75 (95% CI 1.42–5.34; P = 0.003) in the first and second phases, respectively. A similar trial, the Smart in OAC-AFNET9 study enrolled 882 participants ≥65 years without known AF, not receiving oral anticoagulation.29 Consenting participants received a wristband with a PPG sensor to be coupled to their smartphone. The primary outcome was the detection of atrial arrhythmias lasting 6 min or longer in the first 4 weeks of monitoring. Atrial arrhythmias were detected in 44 participants (5%) within 28 days, and in 53 (6%) within 8 weeks (Figure 2).
Figure 2.
Atrial arrhythmia detection rates in studies using PPG-based screening, ECG-based screening and continuous monitoring by a cardiac electronic implanted device (CIED). ECG, electrocardiogram; PPG, photoplethysmography
Association with outcomes of atrial high-rate episodes/sub-clinical atrial fibrillation
Stroke and systemic embolism
There is a growing body of evidence on the stroke and systemic embolism risk in patients with AHRE. In the ancillary study of the MOST trial, there were 10 strokes, of which 8 occurred in patients with AHRE.30 More details are reported in the TRENDS study.31 In this study, the median value for daily atrial tachycardias (AT)/AF burden among 30-day windows was 5.5 h. Accordingly, the authors used the observed median value of AT/AF burden as the cut-off between low and high AT/AF burden. The annualized rate of stroke and SE was 0.5% (95% CI 0.3–0.9%) for patients without AT/AF, 1.1% (95% CI 0.4–2.8%) for patients with an AT/AF burden <5.5 h, and 1.8% (95% CI 0.9–3.8%) for patients with an AT/AF burden ≥5.5 h. The risk remained elevated even after adjustment for other risk factors.31 Similarly, in the ASSERT study, the annual thrombo-embolic event rate was 1.7% in patients with AHRE within 3 months after inclusion, compared with 0.7% in patients who did not show AHRE. These numbers are comparable to those obtained in a recent systematic review where patients with AHRE had an annual stroke rate of 1.9%, compared with 0.9% in patients without AHRE.32 This is much lower than the stroke risk that can be expected in patients with a similar stroke risk profile and ECG-documented AF. Interestingly, strokes occur equally during periods with and without AHRE in patients with AHRE suffering a stroke.33
Cardiovascular death and mortality
The risk due to AHRE may extend beyond the increased risk of stroke. In the ancillary study of the MOST trial, the primary trial endpoint of death or non-fatal stroke occurred in 20.6% of patients with AHRE and 10.5% of those without AHRE. A total of 17.5% of patients in the group with AHRE and 10.5% of patients in the no-AHRE group died. Multivariable analyses demonstrated that the presence of AHRE was an independent predictor of total mortality (HR AHRE vs. no AHRE 2.48, 95% CI 1.25–4.91; P = 0.009).30 A study looking at 224 patients with no history of AF who underwent dual-chamber pacemaker implant, discovered that AHRE were associated with a significant increase in cardiovascular mortality (HR 2.80, 95% CI 1.24–6.31; P = 0.013) and stroke mortality (HR 1.79, 95% CI 0.98–3.26; P = 0.059).34 A recent study by Pastori et al.35 demonstrated that patients with AHRE show a significant risk for major adverse cardiovascular events including acute heart failure, myocardial infarction, cardiovascular hospitalization, and ventricular tachycardia/fibrillation, which depends on the burden of AHRE.
On the contrary, in a more recent small study, the death rate from all or cardiovascular causes was not different between patients with or without AHRE. Cardiovascular death occurred in 3.9 per 100-person-years in the AHRE group, compared with 3.4 per 100-person-years in the control group (HR 1.1, 95% CI 0.3–3.8; P = 0.90). All-cause death occurred in 8.5 per 100-person-years in the AHRE group, compared with 9.5 per 100-person-years in the control group (HR 0.8, 95% CI 0.4–2.0; P = 0.70).36
Does arrhythmia duration interact with the risk of stroke and cardiovascular death?
In patients with AHRE, the risk of thrombo-embolic complications is probably a quantitative function of arrhythmia burden, i.e. the time not in sinus rhythm per observation period. In addition, thrombo-embolic risk increases with age and with the number and severity of comorbidities. The intersection between these clinical conditions and AHRE burden that identifies patients at high thrombo-embolic risk to justify oral anticoagulation is still undefined.37
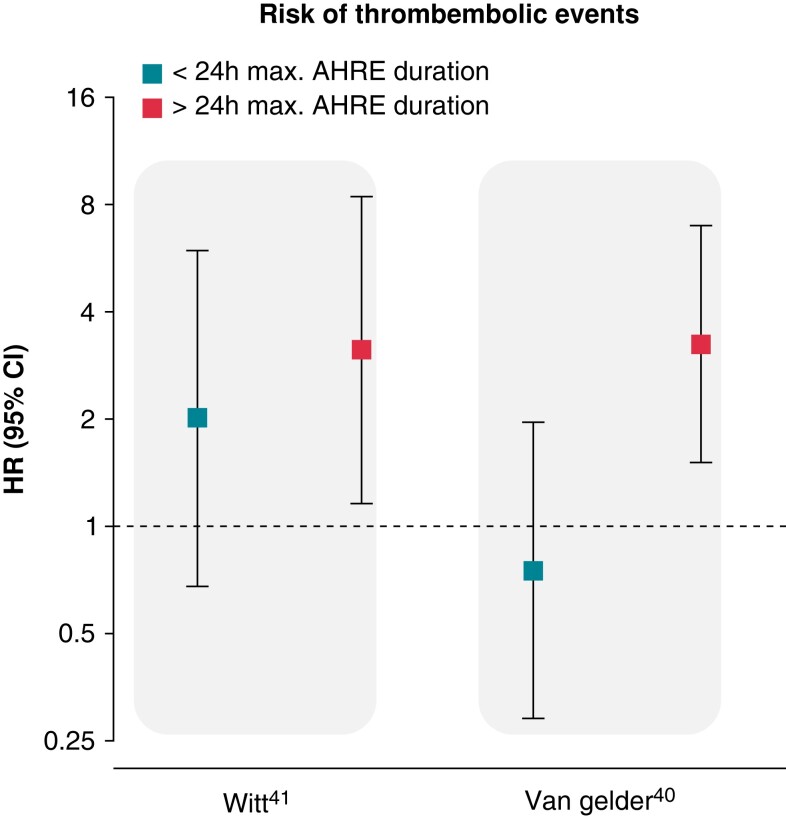
Some information on AHRE duration and burden has been generated. Mizayawa noted in a sub-analysis of the IMPACT study38 that older age and hypertension are risk factors for the occurrence of AHRE with longer duration.39 A sub-analysis from ASSERT analysing stroke risk by duration of the longest AHRE episode found that only AHRE >24 h were associated with an increased risk of stroke compared with the absence of AHRE.40 In the TRENDS study, in patients with a daily cut-off ≥5.5 h, the HR for stroke and systemic embolism was 2.20 (95% CI 0.96–5.05; P = 0.06) compared with the zero AT/AF burden subset, while in patients with a lower AT/AF burden, the hazard ratio was 0.98 (95% CI 0.34–2.82; P = 0.97) compared with the zero AT/AF burden subset.31 Data from TRENDS and ASSERT are further supported by smaller prospective trials which assessed the relationship between AHRE and thrombo-embolic events in patients with implantable devices.41 Capucci et al.42 looked at 725 patients with dual-chamber pacemaker and discovered that AHRE lasting <24 h did not significantly increase embolic risk, while episodes >24 h did. Botto et al.43 in a separate study looked at 562 patients with dual-chamber pacemaker and followed them for 1 year post-implantation. These patients were stratified using a combination of AHRE burden and CHADS2 score. This stratification allowed to identify two separate populations with different stroke risk: patients with AHRE >5 min and CHADS2 score of ≥ 2 and cumulative AHRE >24 h with CHADS2 score >1 had an annualized thrombo-embolic event rate of as high as 5%.43 In the paper by Uittenbogaart et al., two different meta-analysis showed different results regarding the minimum AHRE duration associated with a significant risk of stroke and systemic embolism. In the first meta-analysis, patients with an AHRE burden over 6 min had an increased risk of a thrombo-embolic event when compared with patients without AHRE but this risk did not increase for an AHRE burden over 6 h (HR 1.82 vs. 1.78). In a second meta-analysis, only patients with an AHRE burden over 24 h presented an increased risk for stroke (HR 3.2, 95% CI 1.75–5.86), while patients with an AHRE burden <24 h did not44 (Figure 3). More recently, in another sub-analysis of ASSERT among 2470 patients without previous known AF, an AF burden >6 min in a single simulated 14-day ECG monitor was associated with a stroke risk of over 2% per year, in comparison to an event rate of 0.70%/year in patients with total AF burden <6 min.45
Figure 3.
Hazard ratio for risk of thrombo-embolic event in patients with AHRE compared with patients without AHRE according to the longest duration of AHRE adapted from the observational studies by Witt et al. and van Gelder et al. (please see references). AHRE, atrial high-rate episode.
Anticoagulation in patients with AHRE: knowns and known unknowns
The risk and benefit of oral anticoagulation in atrial fibrillation
Vitamin K antagonists compared with placebo reduce stroke risk in patients with AF by ∼64%.46 A meta-analysis of four large randomized trials47–50 (42 411 patients receiving non-vitamin K antagonist and 29 272 receiving warfarin) showed a significantly higher efficacy of new oral anticoagulants (NOACs) vs. warfarin in the prevention of stroke/systemic embolism (HR 0.81, 95%CI 0.73–0.91; P < 0.0001), and a strong trend toward fewer major bleedings (HR 0.86, 95% CI 0.73–1.00; P = 0.06). Mortality was also lower with NOAC than with warfarin (HR 0.90, 95% CI 0.85–0.95).51 The data on safety and efficacy and the relative benefit of NOACs compared with vitamin K antagonists have been reinforced by large, non-randomized registries.52–56
Efficacy and safety of oral anticoagulation in patients with atrial high-rate episodes
Detection of AHRE in patients with a pacemaker was associated with an increased risk of clinically detected AF and stroke, but in those patients experiencing a stroke, there was no clear temporal relation between the timing of AHRE detection and the occurrence of stroke.16 In a large case-cross-over study linking a national electronic health record to the database of remote monitoring of pacemakers and implantable cardioverter-defibrillators, there was, conversely, an excess of stroke risk within 5 days of an episode of AF lasting longer than 5.5 h.57 Particularly, episodes of more than 24-h duration were associated with stroke risk, whereas shorter episodes carried a similar stroke risk as that in patients without AHRE.40 In older patients (n = 256, mean age 74 ± 6 years, mean CHA2DS2-VASc score of 4.1 ± 1.4) without a history of AF, implantable loop recorder detected SCAF in 34.4%/year of patients.17 However, AHRE lasting longer than 24 h were detected only in 2.7%/year, and ischaemic stroke occurred in four patients, none of whom experienced SCAF. One patient who was anticoagulated following the detection of AHRE experienced a haemorrhagic stroke.17
In the above-mentioned LOOP study,10 the use of anticoagulants to prevent stroke in individuals at high risk was also examined. Oral anticoagulation was initiated in 1036 patients (445 in the ICM group and 591 in the control group) but this did not result in a reduction of first stroke or systemic embolism, which occurred in 67 patients in the ICM group (4.5%) and in 251 patients in the control group (5.6%) (HR 0.80, 95% CI 0.61–1.05; P = 0.11). Major bleeding occurred in 4.3% of patients in the ICM group compared with 3.5% in the control group (HR 1.26, 95%CI 0.95–1.69; P = 0.11). Hence, although the detection rate of (treatment requiring) AHRE was three times higher in the ICM group than in the control group, neither this detection nor the instituted anticoagulation resulted in a lower risk of efficacy or safety endpoints.10
The already mentioned eBRAVE study employed the use of intermittent photoplethysmography recording compared with standard of care in 5551 patients older than 65 years.28 The primary endpoint was newly diagnosed AF within 6 months, treated with oral anticoagulation. After 6 months, patients were invited to cross over to photoplethysmography recording. Digital screening resulted in the detection of more treatment-relevant AF in both phases of the trial and more prescriptions of oral anticoagulants. Note that there was no difference in the occurrence of stroke [11 (0.47%) vs. 7 (0.29%) in usual care vs. digital screening, respectively, OR 0.64, 95% CI 0.25–1.65; P = 0.35]; thrombo-embolic events [9 (0.38%) vs. 11 (0.46%), OR 1.22, 95%CI 0.50–2.95; P = 0.66]; or major bleeding [6 (0.25%) vs. 12 (0.50%) in usual care vs. digital, HR 2.00, 95% CI 0.75–5.34; P = 0.17]. The rate of death and occurrence of MACCE were not different either.28
Efficacy and safety of prescribing oral anticoagulants post-embolic stroke of unknown source
Given the high prevalence of newly detected AF after stroke,58,59 two large-scale randomized trials tested prescribing a NOAC in all post-stroke patients: the New Approach Rivaroxaban Inhibition of Factor Xa in a Global Trial vs. Aspirin to Prevent Embolism in Embolic Stroke of Undetermined Source (NAVIGATE ESUS) and the Randomized, Double-Blind, Evaluation in Secondary Stroke Prevention Comparing the Efficacy and Safety of the Oral Thrombin Inhibitor Dabigatran Etexilate vs. Acetylsalicylic Acid in Patients with Embolic Stroke of Undetermined Source (RESPECT ESUS).
In NAVIGATE ESUS, 7213 patients with a recent stroke of embolic origin were randomized between rivaroxaban 15 mg od and aspirin 100 mg od. The trial was prematurely terminated after a median of 11 months when the primary outcome of ischaemic or haemorrhagic stroke or systemic embolism was not different between the treatment arms (annualized risk 5.1 and 4.8% in rivaroxaban and aspirin-treated patients, respectively, (HR 1.07, 95% CI 0.87–1.33). There was, however, an access of major bleeding events with 1.8%/year in the rivaroxaban group and 0.7%/year in the aspirin group (HR 2.72, 95% CI 1.68–4.39). Also, life-threatening or fatal bleeding, intracranial haemorrhagic stroke, and clinically relevant non-major bleeding occurred consistently and significantly more in the rivaroxaban group.60
RESPECT ESUS randomized 5390 patients with an embolic stroke of undetermined source to receiving dabigatran at a dose of 150 or 110 mg bid or aspirin 100 mg od. Recurrent strokes occurred in 4.1%/year in the dabigatran-treated patients vs. 4.8% in the aspirin group (HR 0.85, 95% CI 0.69–1.03; P = 0.10). Major bleeding occurred in 1.7%/year in the dabigatran group vs. 1.4%/year in the aspirin-treated patients (HR 1.19, 95% CI 0.85–1.66), with significantly more clinically relevant non-major bleedings (HR 1.73, 95% CI 1.17–2.54) or the composite of major or clinically relevant non-major bleedings (HR 1.44, 95% CI 1.12–1.85) in the dabigatran group. In subgroup analysis, patients treated with dabigatran 110 mg and the subgroup of patients older than 75 years had significantly fewer recurrent strokes with dabigatran compared with aspirin (HR 0.57, 95% CI 0.39–0.83 and HR 0.63, 95% CI 0.43–0.94, respectively).61
Thus, in patients with embolic stroke of undetermined source but without AF, both trials demonstrated futility of the use of rivaroxaban and dabigatran compared with aspirin. In patients treated with rivaroxaban, even more major and life-threatening or fatal bleedings occurred.
What do the guidelines say?
The ESC guidelines for the diagnosis and management of AF acknowledge that the risk of stroke may be lower in patients with AHRE detected by an implantable device and suggest that the temporal dissociation between stroke and the detection of AHRE represents a risk marker for stroke, rather than an actual cause.5 Available evidence that oral anticoagulation is justified in patients with AHRE is not available yet, but it may be considered to prescribe oral anticoagulants in patients with AHRE lasting longer than 24 h, especially when patients are at high stroke risk.5,12,40 Similarly, the Canadian Cardiovascular Society/Canadian Heart Rhythm Society for the management of AF emphasize the moderately increased stroke risk in patients with AHRE and states that it is reasonable to prescribe oral anticoagulants for patients older than 65 years, or with a CHADS2 score >1 who have AHRE lasting longer than 24 h.62 The American Heart Association/American College of Cardiology/Heart Rhythm Society guidelines of 2019 state that the detection of AHRE should prompt further evaluation to document clinically relevant AF, but that the indication for oral anticoagulation should be based on clinical risk assessment.63
Hence, the stroke risk of patients with AHRE or SCAF is increased compared with patients without device-detected atrial arrhythmias, but not to the same extent as in patients with clinically overt AF. Therefore, whether oral anticoagulation is indicated with the same risk factors remains a matter of debate and several studies indicate that despite the detection of AHRE the benefit of oral anticoagulation does not outweigh the risk.10,17,28 Consensus is that there is, in the absence of randomized data, clinical equipoise as to anticoagulate or not in patients with device-detected AHRE or SCAF, particularly if these episodes are shortlasting.5
Ongoing trials
The increase in stroke risk with AHRE appears to be substantially lower than that associated with clinical AF. At the same time, the stroke risk is increased compared with patients without AHRE. Therefore, whether anticoagulation is beneficial in patients with sub-clinical, short asymptomatic AF episodes remains disputable. Randomized trials are required to clarify if this therapeutic approach is beneficial in this setting. In fact, there were very few SCAF patients included in any of the landmark anticoagulation trials, although AHRE are a common finding with prolonged monitoring with a cardiac electronic device. Two studies, NOAH-AFNET664 and ARTESiA,65 will address this question and provide guidance on how to treat patients with AHRE. Both are likely to report soon.
These prospective multicentre randomized trials enrolled thousands of patients with AHRE ≥6 min in duration but with no history of clinical AF and no requirement for anticoagulation at the time of enrolment (AHRE episodes >24 h at any time before enrolment are also an exclusion criterion in ARTESiA). Patients with pacemakers, implantable cardioverter-defibrillators, cardiac resynchronization therapy devices, or implantable monitors with a feature of detection of AHRE were eligible. In both trials, patients are censored when they develop clinical AF and offered open-label anticoagulation. In ARTESiA, patients were randomized to receive apixaban or aspirin, while in NOAH-AFNET6 patients received either edoxaban or aspirin/placebo. A contraindication for anticoagulation in general or for any of the trial drugs in specific, or indication for anticoagulation for any other unrelated reason (e.g. recent deep vein thrombosis or pulmonary embolism) constitute exclusion criteria in both ARTESiA and NOAH-AFNET6. Patients in these trials qualified for oral anticoagulation if they had been diagnosed with clinical AF in routine clinical practice, as determined by their CHA2DS2-VASc scores. Patients in ARTESiA will be ≥55 years old and have additional risk factors for stroke (i.e. minimum CHA2DS2-VASc score of 2 in those ≥75 years or with a history of stroke or transient ischaemic attack, or a score ≥3 in those younger than 75 and without a history of cerebrovascular event). Similarly, in NOAH-AFNET6 patients will be >65 years and have at least one additional risk factor. Slightly different primary endpoints have been established for these two studies, with ARTESiA considering the composite of stroke, transient ischaemic attack with magnetic resonance imaging evidence of cerebral infarction, and systemic embolism, while the primary outcome parameter in NOAH-AFNET6 will be a composite of stroke, systemic embolism, or cardiovascular death. Both trials will report major bleeding events.
On 2 September 2022, the sponsor of NOAH-AFNET6 decided to terminate the trial, following a recommendation by the DSMB. The reasons for early termination given by the DSMB are an observed trend towards futility for efficacy combined with safety concerns.
Until the results of these two trials are available, decisions on anticoagulation prescription in patients with SCAF will have to be individualized, and oral anticoagulants will not be routinely recommended until further ECG monitoring documents AF.
The ATTICUS trial66 is a further trial studying the effect of anticoagulation in patients with AHRE in an ICM. It is a randomized trial in patients with an embolic stroke of undetermined source (ESUS) in ∼500 patients, which are 1:1 randomized to apixaban or acetylsalicylic acid. The primary outcome is the occurrence of at least one new ischaemic lesion identified by axial T2-weighted FLAIR magnetic resonance imaging and/or axial diffusion-weighted magnetic resonance imaging at 12 months. In this trial, AHRE are only one risk factor for cardiac embolism qualifying for inclusion, others are left atrium size >45 mm (parasternal axis), spontaneous echo contrast in left atrial appendage (LAA), LAA flow velocity <0.2 m/s, a persistent foramen ovale or a CHA2DS2-VASc score ≥4.
Future perspective
Improvements in rhythm monitoring through implantable devices or continuous non-invasive monitoring systems have helped to identify AHRE episodes and other short atrial arrhythmias that resemble atrial fibrillation in many individuals. However, the management of such rare and typically asymptomatic episodes remains unclear, mainly in relation to anticoagulation as described above. This review highlights the uncertainty in this area, including in the therapeutic consequences. The underlying question that arises is whether AHRE should be considered as asymptomatic or ‘sub-clinical’ AF. The immediate challenges apply to the decision to anticoagulation. In view of the effectiveness and safety of early rhythm control therapy,6,67 classifying AHRE episodes as similar to AF may even suggest speculation about the possibility of treating AHRE when progression to atrial fibrillation is considered very likely, in the hope of reducing disease progression and the risk of cardiovascular events. Should AHRE episodes not respond to oral anticoagulation therapy as clinical atrial fibrillation does, one could alternatively argue that the arrhythmia burden in patients with AHRE is already very low, with little room for improvement using rhythm control therapy. We hope that the data from NOAH-AFNET6 and ARTESIA will shed some more light on the subject and that further studies will follow.
Contributor Information
Tobias Toennis, Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistr. 52, Hamburg 20246, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Luebeck, Martinistr. 52, 20246 Hamburg, Germany.
Emanuele Bertaglia, Department of Cardiac, Vascular, Thoracic and Public Health Sciences, Azienda Ospedaliera, 35128 Padua, Italy.
Axel Brandes, Department of Clinical Research, University of Southern Denmark, 5230 Odense, Denmark; Department of Cardiology, Odense University Hospital, 5230 Odense, Denmark.
Wolfgang Dichtl, University Hospital of Internal Medicine III, Medical University Innsbruck, 6020 Innsbruck, Austria.
Nina Fluschnik, Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistr. 52, Hamburg 20246, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Luebeck, Martinistr. 52, 20246 Hamburg, Germany.
Joris R de Groot, Department of Cardiology, Heart Center, Amsterdam University Medical Center, University of Amsterdam, 1081 HV Amsterdam, The Netherlands.
Eloi Marijon, Cardiac Electrophysiology Section, European Georges Pompidou Hospital, 75015 Paris, France.
Lluis Mont, Cardiovascular Clinical Institute, Hospital Clinic, Universitat de Barcelona, 08036 Barcelona, Catalonia, Spain.
Carina Blomström Lundqvist, Faculty of Medicine and Health, Department of Cardiology, School of Medical Sciences, Örebro University, 701 85 Örebro, Sweden; Department of Medical Science, Uppsala University, 751 85 Uppsala, Sweden.
Nuno Cabanelas, Arrhythmias Unit of Cardiology Department, Hospital Prof. Dr. Fernando Fonseca, 2720-276 Amadora-Sintra, Portugal.
G Andrei Dan, Department 5, Colentina University Hospital, Medicine University ‘Carol Davila’, Bucharest 020021, Romania.
Andrzej Lubinski, Department of Cardiology and Internal Diseases, Medical University of Gdańsk, 80-210 Gdańsk, Poland.
Béla Merkely, Heart and Vascular Center, Semmelweis University Budapest, 1122 Budapest, Hungary.
Kim Rajappan, Cardiac Department, John Radcliffe Hospital, Oxford OX3 9DU, UK.
Andrea Sarkozy, Ventricular Arrhythmia and Sudden Death Management Unit, Heart Rhythm Management Center, University Hospital of Brussels, 1090 Brussels, Belgium.
Vasil Velchev, Cardiology Clinic, St. Anna University Hospital, Medical University Sofia, 1750, Sofia, Bulgaria.
Dan Wichterle, Department of Cardiology, Institute for Clinical and Experimental Medicine, 140 21 Prague 4, Czech Republic.
Paulus Kirchhof, Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistr. 52, Hamburg 20246, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Luebeck, Martinistr. 52, 20246 Hamburg, Germany; Institute of Cardiovascular Sciences, University of Birmingham, UHB and Sandwell & West Birmingham Hospitals NHS Trusts, IBR 126a, Wolfson Drive, Birmingham B15 2TT, UK; Atrial Fibrillation NETwork (AFNET), 48149 Muenster, Germany.
Funding
This work was largely written by voluntary contributions of time from the authors. There is no funding related to this article.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Grond M, Jauss M, Hamann G, Stark E, Veltkamp R, Nabavi Det al. Improved detection of silent atrial fibrillation using 72-hour Holter ECG in patients with ischemic stroke: a prospective multicenter cohort study. Stroke 2013;44:3357–64. [DOI] [PubMed] [Google Scholar]
- 2. Haeusler KG, Kirchhof P, Kunze C, Tutuncu S, Fiessler C, Malsch Cet al. Systematic monitoring for detection of atrial fibrillation in patients with acute ischaemic stroke (MonDAFIS): a randomised, open-label, multicentre study. Lancet Neurol 2021;20:426–36. [DOI] [PubMed] [Google Scholar]
- 3. Noubiap JJ, Thomas G, Middeldorp ME, Fitzgerald JL, Harper C, Sanders P. Atrial fibrillation detection using insertable cardiac monitor after stroke: a real-world cohort study. J Cardiovasc Electrophysiol 2023;34:142–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gladstone DJ, Wachter R, Schmalstieg-Bahr K, Quinn FR, Hummers E, Ivers Net al. Screening for atrial fibrillation in the older population: a randomized clinical trial. JAMA Cardiol 2021;6:558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist Cet al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 6. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan Aet al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–16. [DOI] [PubMed] [Google Scholar]
- 7. Jensen MT, Treskes RW, Caiani EG, Casado-Arroyo R, Cowie MR, Dilaveris Pet al. ESC Working group on e-cardiology position paper: use of commercially available wearable technology for heart rate and activity tracking in primary and secondary cardiovascular prevention-in collaboration with the European Heart Rhythm Association, European Association of Preventive Cardiology, Association of Cardiovascular Nursing and Allied Professionals, Patient Forum, and the Digital Health Committee. Eur Heart J Digit Health 2021;2:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. US Preventive Services Task Force; Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey ABet al. Screening for atrial fibrillation: US Preventive Services Task Force recommendation statement. JAMA 2022;327:360–7. [DOI] [PubMed] [Google Scholar]
- 9. Svennberg E, Friberg L, Frykman V, Al-Khalili F, Engdahl J, Rosenqvist M. Clinical outcomes in systematic screening for atrial fibrillation (STROKESTOP): a multicentre, parallel group, unmasked, randomised controlled trial. Lancet 2021;398:1498–506. [DOI] [PubMed] [Google Scholar]
- 10. Svendsen JH, Diederichsen SZ, Hojberg S, Krieger DW, Graff C, Kronborg Cet al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (the LOOP study): a randomised controlled trial. Lancet 2021;398:1507–16. [DOI] [PubMed] [Google Scholar]
- 11. Eckardt L, Sehner S, Suling A, Borof K, Breithardt G, Crijns Het al. Attaining sinus rhythm mediates improved outcome with early rhythm control therapy of atrial fibrillation: the EAST-AFNET 4 trial. Eur Heart J 2022;43:4127–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Camm AJ, Simantirakis E, Goette A, Lip GY, Vardas P, Calvert Met al. Atrial high-rate episodes and stroke prevention. Europace 2017;19:169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fabritz L, Crijns H, Guasch E, Goette A, Hausler KG, Kotecha Det al. Dynamic risk assessment to improve quality of care in patients with atrial fibrillation: the 7th AFNET/EHRA Consensus Conference. Europace 2021;23:329–44. [DOI] [PubMed] [Google Scholar]
- 14. Freedman B, Camm J, Calkins H, Healey JS, Rosenqvist M, Wang Jet al. Screening for atrial fibrillation: a report of the AF-SCREEN International Collaboration. Circulation 2017;135:1851–67. [DOI] [PubMed] [Google Scholar]
- 15. Bertaglia E, Blank B, Blomstrom-Lundqvist C, Brandes A, Cabanelas N, Dan GAet al. Atrial high-rate episodes: prevalence, stroke risk, implications for management, and clinical gaps in evidence. Europace 2019;21:1459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci Aet al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120–9. [DOI] [PubMed] [Google Scholar]
- 17. Healey JS, Alings M, Ha A, Leong-Sit P, Birnie DH, de Graaf JJet al. Subclinical atrial fibrillation in older patients. Circulation 2017;136:1276–83. [DOI] [PubMed] [Google Scholar]
- 18. Doundoulakis I, Gavriilaki M, Tsiachris D, Arsenos P, Antoniou CK, Dimou Set al. Atrial high-rate episodes in patients with devices without a history of atrial fibrillation: a systematic review and meta-analysis. Cardiovasc Drugs Ther 2022;36:951–8. [DOI] [PubMed] [Google Scholar]
- 19. Proietti M, Romiti GF, Vitolo M, Borgi M, Rocco AD, Farcomeni Aet al. Epidemiology of subclinical atrial fibrillation in patients with cardiac implantable electronic devices: a systematic review and meta-regression. Eur J Intern Med 2022;103:84–94. [DOI] [PubMed] [Google Scholar]
- 20. Lubitz SA, Atlas SJ, Ashburner JM, Lipsanopoulos ATT, Borowsky LH, Guan Wet al. Screening for atrial fibrillation in older adults at primary care visits: VITAL-AF randomized controlled trial. Circulation 2022;145:946–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aronsson M, Svennberg E, Rosenqvist M, Engdahl J, Al-Khalili F, Friberg Let al. Cost-effectiveness of mass screening for untreated atrial fibrillation using intermittent ECG recording. Europace 2015;17:1023–9. [DOI] [PubMed] [Google Scholar]
- 22. Lyth J, Lind L, Persson HL, Wirehn AB. Can a telemonitoring system lead to decreased hospitalization in elderly patients? J Telemed Telecare 2021;27:46–53. [DOI] [PubMed] [Google Scholar]
- 23. Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris Tet al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guo Y, Wang H, Zhang H, Liu T, Liang Z, Xia Yet al. Mobile photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol 2019;74:2365–75. [DOI] [PubMed] [Google Scholar]
- 25. Lubitz SA, Faranesh AZ, Selvaggi C, Atlas SJ, McManus DD, Singer DEet al. Detection of atrial fibrillation in a large population using wearable devices: the Fitbit heart study. Circulation 2022;146:1415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bashar SK, Han D, Hajeb-Mohammadalipour S, Ding E, Whitcomb C, McManus DDet al. Atrial fibrillation detection from wrist photoplethysmography signals using smartwatches. Sci Rep 2019;9:15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schnabel RB, Marinelli EA, Arbelo E, Boriani G, Boveda S, Buckley CMet al. Early diagnosis and better rhythm management to improve outcomes in patients with atrial fibrillation: the 8th AFNET/EHRA consensus conference. Europace 2023;25:6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rizas KD, Freyer L, Sappler N, von Stulpnagel L, Spielbichler P, Krasniqi Aet al. Smartphone-based screening for atrial fibrillation: a pragmatic randomized clinical trial. Nat Med 2022;28:1823–30. [DOI] [PubMed] [Google Scholar]
- 29. Fabritz LC, Czarnecki E, Dudek E, Guasch E, Haase D, Huebner Tet al. Smartphone and wearable detected atrial arrhythmias in Older Adults: results of a fully digital European Case finding study. Eur Heart J Digital Health 2022;3:610–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Glotzer TV, Hellkamp AS, Zimmerman J, Sweeney MO, Yee R, Marinchak Ret al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation 2003;107:1614–9. [DOI] [PubMed] [Google Scholar]
- 31. Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, Hilker Cet al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol 2009;2:474–80. [DOI] [PubMed] [Google Scholar]
- 32. Mahajan R, Perera T, Elliott AD, Twomey DJ, Kumar S, Munwar DAet al. Subclinical device-detected atrial fibrillation and stroke risk: a systematic review and meta-analysis. Eur Heart J 2018;39:1407–15. [DOI] [PubMed] [Google Scholar]
- 33. Brambatti M, Connolly SJ, Gold MR, Morillo CA, Capucci A, Muto Cet al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation 2014;129:2094–9. [DOI] [PubMed] [Google Scholar]
- 34. Gonzalez M, Keating RJ, Markowitz SM, Liu CF, Thomas G, Ip JEet al. Newly detected atrial high rate episodes predict long-term mortality outcomes in patients with permanent pacemakers. Heart Rhythm 2014;11:2214–21. [DOI] [PubMed] [Google Scholar]
- 35. Pastori D, Miyazawa K, Li Y, Szekely O, Shahid F, Farcomeni Aet al. Atrial high-rate episodes and risk of major adverse cardiovascular events in patients with cardiac implantable electronic devices. Clin Res Cardiol 2020;109:96–102. [DOI] [PubMed] [Google Scholar]
- 36. Marinheiro R, Parreira L, Amador P, Lopes C, Fernandes A, Mesquita Det al. Clinical impact of oral anticoagulation in patients with atrial high-rate episodes. J Stroke Cerebrovasc Dis 2019;28:971–9. [DOI] [PubMed] [Google Scholar]
- 37. Khan AA, Boriani G, Lip GYH. Are atrial high rate episodes (AHREs) a precursor to atrial fibrillation? Clin Res Cardiol 2020;109:409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martin DT, Bersohn MM, Waldo AL, Wathen MS, Choucair WK, Lip GYet al. Randomized trial of atrial arrhythmia monitoring to guide anticoagulation in patients with implanted defibrillator and cardiac resynchronization devices. Eur Heart J 2015;36:1660–8. [DOI] [PubMed] [Google Scholar]
- 39. Miyazawa K, Pastori D, Martin DT, Choucair WK, Halperin JL, Lip GYHet al. Characteristics of patients with atrial high rate episodes detected by implanted defibrillator and resynchronization devices. Europace 2022;24:375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Van Gelder IC, Healey JS, Crijns H, Wang J, Hohnloser SH, Gold MRet al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J 2017;38:1339–44. [DOI] [PubMed] [Google Scholar]
- 41. Witt CT, Kronborg MB, Nohr EA, Mortensen PT, Gerdes C, Nielsen JC. Early detection of atrial high rate episodes predicts atrial fibrillation and thromboembolic events in patients with cardiac resynchronization therapy. Heart Rhythm 2015;12:2368–75. [DOI] [PubMed] [Google Scholar]
- 42. Capucci A, Santini M, Padeletti L, Gulizia M, Botto G, Boriani Get al. Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with antitachycardia pacemakers. J Am Coll Cardiol 2005;46:1913–20. [DOI] [PubMed] [Google Scholar]
- 43. Botto GL, Padeletti L, Santini M, Capucci A, Gulizia M, Zolezzi Fet al. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol 2009;20:241–8. [DOI] [PubMed] [Google Scholar]
- 44. Uittenbogaart SB, Lucassen WAM, van Etten-Jamaludin FS, de Groot JR, van Weert H. Burden of atrial high-rate episodes and risk of stroke: a systematic review. Europace 2018;20:1420–7. [DOI] [PubMed] [Google Scholar]
- 45. McIntyre WF, Wang J, Benz AP, Johnson L, Connolly SJ, Van Gelder ICet al. Estimated incidence of previously undetected atrial fibrillation on a 14-day continuous electrocardiographic monitor and associated risk of stroke. Europace 2022;24:1058–64. [DOI] [PubMed] [Google Scholar]
- 46. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857–67. [DOI] [PubMed] [Google Scholar]
- 47. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh Aet al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- 48. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke Wet al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- 49. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna Met al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92. [DOI] [PubMed] [Google Scholar]
- 50. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JLet al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–104. [DOI] [PubMed] [Google Scholar]
- 51. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MDet al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–62. [DOI] [PubMed] [Google Scholar]
- 52. Camm AJ, Fox KAA, Virdone S, Bassand JP, Fitzmaurice DA, Berchuck SIet al. Comparative effectiveness of oral anticoagulants in everyday practice. Heart 2021;107:962–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Huisman MV, Rothman KJ, Paquette M, Teutsch C, Diener HC, Dubner SJet al. The changing landscape for stroke prevention in AF: findings from the GLORIA-AF Registry Phase 2. J Am Coll Cardiol 2017;69:777–85. [DOI] [PubMed] [Google Scholar]
- 54. Steinberg BA, Shrader P, Thomas L, Ansell J, Fonarow GC, Gersh BJet al. Factors associated with non-vitamin K antagonist oral anticoagulants for stroke prevention in patients with new-onset atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II (ORBIT-AF II). Am Heart J 2017;189:40–7. [DOI] [PubMed] [Google Scholar]
- 55. de Groot JR, Weiss TW, Kelly P, Monteiro P, Deharo JC, de Asmundis Cet al. Edoxaban for stroke prevention in atrial fibrillation in routine clinical care: 1-year follow-up of the prospective observational ETNA-AF-Europe study. Eur Heart J Cardiovasc Pharmacother 2021;7:f30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hanon O, Vidal JS, Le Heuzey JY, Kirchhof P, De Caterina R, Schmitt Jet al. Oral anticoagulant use in octogenarian European patients with atrial fibrillation: a subanalysis of PREFER in AF. Int J Cardiol 2017;232:98–104. [DOI] [PubMed] [Google Scholar]
- 57. Singer DE, Ziegler PD, Koehler JL, Sarkar S, Passman RS. Temporal association between episodes of atrial fibrillation and risk of ischemic stroke. JAMA Cardiol 2021;6:1364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall Jet al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014;370:2467–77. [DOI] [PubMed] [Google Scholar]
- 59. Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CAet al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478–86. [DOI] [PubMed] [Google Scholar]
- 60. Hart RG, Sharma M, Mundl H, Kasner SE, Bangdiwala SI, Berkowitz SDet al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med 2018;378:2191–201. [DOI] [PubMed] [Google Scholar]
- 61. Diener HC, Sacco RL, Easton JD, Granger CB, Bernstein RA, Uchiyama Set al. Dabigatran for prevention of stroke after embolic stroke of undetermined source. N Engl J Med 2019;380:1906–17. [DOI] [PubMed] [Google Scholar]
- 62. Andrade JG, Aguilar M, Atzema C, Bell A, Cairns JA, Cheung CCet al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society Comprehensive Guidelines for the Management of Atrial Fibrillation. Can J Cardiol 2020;36:1847–948. [DOI] [PubMed] [Google Scholar]
- 63. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jret al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration with the Society of Thoracic Surgeons. Circulation 2019;140:e125–51. [DOI] [PubMed] [Google Scholar]
- 64. Kirchhof P, Blank BF, Calvert M, Camm AJ, Chlouverakis G, Diener HCet al. Probing oral anticoagulation in patients with atrial high rate episodes: rationale and design of the non-vitamin K antagonist oral anticoagulants in patients with atrial high rate episodes (NOAH-AFNET 6) trial. Am Heart J 2017;190:12–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lopes RD, Alings M, Connolly SJ, Beresh H, Granger CB, Mazuecos JBet al. Rationale and design of the apixaban for the reduction of thrombo-embolism in patients with device-detected sub-clinical atrial fibrillation (ARTESiA) trial. Am Heart J 2017;189:137–45. [DOI] [PubMed] [Google Scholar]
- 66. Geisler T, Poli S, Meisner C, Schreieck J, Zuern CS, Nagele Tet al. Apixaban for treatment of embolic stroke of undetermined source (ATTICUS randomized trial): rationale and study design. Int J Stroke 2017;12:985–90. [DOI] [PubMed] [Google Scholar]
- 67. Eckardt L, Sehner S, Suling A, Borof K, Breithardt G, Crijns Het al. Attaining sinus rhythm mediates improved outcome with early rhythm control therapy of atrial fibrillation: the EAST—AFNET 4 trial. Eur Heart J 2022;43:4127–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.