Abstract
Understanding neural circuits that support mood is a central goal of affective neuroscience, and improved understanding of the anatomy could inform more targeted interventions in mood disorders. Lesion studies provide a method of inferring the anatomical sites causally related to specific functions, including mood. Here, we performed a large-scale study evaluating the location of acquired, focal brain lesions in relation to symptoms of depression. Five hundred and twenty-six individuals participated in the study across two sites (356 male, average age 52.4 ± 14.5 years). Each subject had a focal brain lesion identified on structural imaging and an assessment of depression using the Beck Depression Inventory-II, both obtained in the chronic period post-lesion (>3 months). Multivariate lesion–symptom mapping was performed to identify lesion sites associated with higher or lower depression symptom burden, which we refer to as ‘risk’ versus ‘resilience’ regions. The brain networks and white matter tracts associated with peak regional findings were identified using functional and structural lesion network mapping, respectively. Lesion–symptom mapping identified brain regions significantly associated with both higher and lower depression severity (r = 0.11; P = 0.01). Peak ‘risk’ regions include the bilateral anterior insula, bilateral dorsolateral prefrontal cortex and left dorsomedial prefrontal cortex. Functional lesion network mapping demonstrated that these ‘risk’ regions localized to nodes of the salience network. Peak ‘resilience’ regions include the right orbitofrontal cortex, right medial prefrontal cortex and right inferolateral temporal cortex, nodes of the default mode network. Structural lesion network mapping implicated dorsal prefrontal white matter tracts as ‘risk’ tracts and ventral prefrontal white matter tracts as ‘resilience’ tracts, although the structural lesion network mapping findings did not survive correction for multiple comparisons. Taken together, these results demonstrate that lesions to specific nodes of the salience network and default mode network are associated with greater risk versus resiliency for depression symptoms in the setting of focal brain lesions.
Keywords: depression, lesion–symptom mapping, lesion–network mapping, affective neuroscience, mood
Trapp et al. present data from a large-scale lesion–symptom mapping and lesion–network mapping study of depression. Using a multivariate approach, they identify distinct brain regions and networks which, when lesioned, are associated with either higher or lower levels of depression.
See Klingbeil and Saur (https://doi.org/10.1093/brain/awad067) for a scientific commentary on this article.
See Klingbeil and Saur (https://doi.org/10.1093/brain/awad067) for a scientific commentary on this article.
Introduction
Depression is the leading cause of disability worldwide and the leading cause of disease burden in the US.1 A central goal of translational research on depression is to understand the anatomy of neural networks that contribute to symptoms of depression. With recent advances in therapeutic neuromodulation there is a very direct path for translating advances in understanding the neuroanatomical substrates of depression to improved treatments. Yet, our current understanding of which anatomical regions and networks to target to alleviate specific symptoms of depression is incomplete. One of the challenges with this line of research is that work in humans relies heavily on correlational data from functional imaging,2–7 which limits causal inferences. Thus, there is a critical need to revisit these questions on the functional neuroanatomy of mood and depression using methods that facilitate causal inferences and have the potential to improve upon existing models.
Human lesion studies provide a strong method for drawing causal inferences relating brain anatomy to function. Lesion studies have provided important insights upon which current models of human brain function have been built, with seminal contributions in domains of memory, language, motor, and attention. This approach, termed lesion–symptom mapping, has also been successfully utilized for symptoms relevant to psychiatric disorders, including executive dysfunction,8 hallucinations9,10 and emotion regulation.11,12 Yet, there is a relative paucity of large-scale systematic efforts to leverage modern methods for mapping the anatomy of depressed mood following focal brain lesions. Prior studies have examined whether lesion location relates to one’s risk for developing depression13–18 or resistance to depression.14 Some studies implicate lesions of the left frontal lobe closer to the frontal pole as increasing risk for post-stroke depression, whereas lesions of the ventromedial prefrontal cortex have been associated with lower depression ratings. However, these findings have not been reliably replicated, matching the overall trend in the literature.17,19–36 Potential reasons for this include the complexity of depressive symptoms themselves, combined with heterogeneity of behavioural rating scales across studies, small sample sizes or crude lesion location information. Another limitation relates to the challenge of ‘localizing’ a constellation of symptoms that may map onto spatially distributed networks as opposed to a single anatomical location; this can result in a lack of lesion localization due to insufficient power.37
Symptoms associated with focal brain lesions can also be considered in relation to their role in disrupting anatomically distributed brain networks.38 Newer imaging tools have been utilized to investigate the network effects of focal lesions in relation to depressed mood. This includes studies that have performed functional imaging of individuals with lesions39 as well as studies that have used connectivity information from healthy cohorts to infer the network effects of focal lesions, termed lesion–network mapping (LNM).16,18 One recent study of depressed mood, which had a null result for lesion–symptom mapping of depression ratings, used LNM to demonstrate that lesions functionally connected to the left prefrontal cortex (PFC) were associated with higher levels of depression than lesions not connected to this site.16 This brain-wide network spanned multiple regions beyond the left PFC, and while this is an important advance, it has the same limitations of functional MRI in that it is unclear which regions within the brain-wide functional network are the most critical for depression.
Our study was designed such that it may overcome some of the challenges of prior lesion studies of depression. We utilize a larger sample size than prior studies and employ a widely used and validated depression rating scale across all participants, the Beck Depression Inventory-II (BDI-II).40 This study also uses multivariate lesion–symptom mapping for depression, a statistical approach for linking lesion location and outcomes that has advantages over a mass univariate approach when multiple nodes of a network may be critical for the expression of symptoms, as is likely the case for depression.41 Leveraging a combination of large sample size, uniform assessment method and multivariate statistical approach, we investigate whether there are brain regions that, when damaged, are significantly associated with higher levels of depression—here referred to as depression ‘risk’—and whether there are brain regions that, when damaged, are associated with a relative lack of depression, or ‘resilience’. In addition, we perform LNM of brain regions having the strongest association with depression ratings from the lesion–symptom mapping analysis.42 Structural and functional networks are derived from these regions using normative data to identify the networks most associated with depressed mood following focal acquired brain lesions.
Materials and methods
Subjects
Participants included 526 individuals who met study criteria, selected from the Patient Registry of the Division of Behavioral Neurology and Cognitive Neuroscience at the University of Iowa Department of Neurology (Iowa cohort; n = 330) and the Vietnam Head Injury Study (VHIS cohort; n = 196).43 For the University of Iowa Registry, inclusion criteria were presence of a stable, acquired focal brain lesion and depression assessment using the BDI-II, performed as part of a neuropsychological testing battery.44 Each participant was enrolled approximately 3 months or greater after the lesion onset. Exclusion criteria for the Patient Registry included a history of significant alcohol or substance abuse, psychiatric disorder prior to the brain lesion, medically intractable epilepsy, or other neurologic disorder unrelated to the lesion. For the VHIS cohort, all subjects were drawn from the W.F. Caveness Vietnam Head Injury Study registry, which consists of military veterans who suffered penetrating head trauma while in combat during the Vietnam War era (1967–1970). Subjects were included if they had neuroimaging of their lesion and had completed a BDI-II in the chronic phase post-injury, defined as >3 months later. All VHIS combat veterans had been declared fit for duty at the time of their enlistment in the military prior to their head injury. In accordance with federal and institutional guidelines, all procedures including informed consent were approved by the Institutional Review Boards of the participating institutions and are in accordance with the Declaration of Helsinki.
Mood assessment
The BDI-II is a 21-item self-reported questionnaire that evaluates characteristic attitudes and symptoms of depression experienced over the preceding 2 weeks. The items correspond to affective, cognitive, somatic and vegetative symptoms of depression that align with the criteria used to diagnose major depression in the fourth edition of the Diagnostic and Statistical Manual (DSM-IV). Test–retest reliability is high at 0.93, which suggests a robustness against daily mood variations; the validity is further supported by high correlations with Hamilton Depression Rating Scale (r = 0.71) and Minnesota Multiphasic Personality Inventory—Depression Subscale (r = 0.77).40 For the current study, if participants had repeat assessments, the highest score was selected.
Lesion segmentation
Each participant included in the analysis had a focal brain lesion with visible boundaries evident from research-quality structural imaging from T1 and T2 sequences on MRI. CT scans were used in the VHIS and in rare cases in the Iowa Registry when MRI was contraindicated (n = 35 of 330 Iowa subjects). All imaging was performed in the chronic epoch (>3 months since onset) to ensure relative stability of the lesion. Lesions were manually segmented in three dimensions by a rater blind to mood ratings, and anatomical accuracy of each tracing was reviewed by a neurologist (A.D.B.) in both native space and upon transformation to Montreal Neurologic Institute Structural MRI Template (MNI152) 1-mm template brain using a combination of linear and non-linear registration techniques. Additional details of lesion segmentation are provided in Supplementary material, Appendix 1.
Multivariate lesion–symptom mapping
Lesion–symptom mapping analyses were performed on the BDI-II mood rating results using sparse canonical correlation analysis (SCCAN) as implemented in LESYMAP,45 a package available in R (https://github.com/dorianps/LESYMAP). The SCCAN method involves an optimization procedure that finds voxel weights that maximize the multivariate correlation between voxel values and BDI-II depression scores. The predictive value and sparseness of the model is derived empirically using a 4-fold, within-sample correlation between model-predicted and actual BDI-II scores. LESYMAP deems a map ‘valid’ if it is associated with a statistically significant predictive correlation. Briefly, SCCAN builds a model using 75% of the sample, applies it to the remaining 25% of the sample in order to predict the BDI-II scores from lesion location and correlates these predictions with actual BDI-II scores. Thus, this approach tests the statistical significance of the entire map at once and avoids the pitfalls associated with voxel-wise (i.e. mass univariate) methods, such as inflated rates of false-positive errors. This previously validated method has been demonstrated as more accurate than mass univariate methods and is better able to identify when multiple brain regions are associated with a behavioural variable.45
Functional lesion network mapping
Functional lesion network mapping was performed similarly to prior work,42,46 in which networks were derived from regional peak brain–behaviour associations identified from the lesion–symptom map. This differs from the approach of using each lesion mask to ‘seed’ the network analysis38 to avoid some of the problems associated with signal averaging from large lesions.47,48 To identify peak regions of interest with the strongest association with BDI-II ratings from the lesion–symptom map, a cluster tool in FSL was used. Peaks were identified for ‘risk’ and ‘resilience’ maps and assigned to grey or white matter using brain masks for each tissue class.
Four-millimetre spherical regions of interest were placed at each of the peak grey matter coordinates and used to seed separate functional connectivity analyses. Resting state functional connectivity MRI (rs-fcMRI) data from a normative database (n = 98) were used, as in previous work.38,49–51 The rs-fcMRI data were processed in accordance with previously described methods and are described in detail in Supplementary material, Appendix 1.52–55 Global signal regression was included in the primary analysis. For all datasets the time course of the average blood oygen level-dependent (BOLD) signal within each spherical region of interest was compared with the BOLD signal time course of other brain voxels to identify regions with positive and negative correlations. Pearson correlation coefficients were converted to normally distributed Z-scores using the Fisher transformation.
A group mean t-test was performed separately for the positive and negative maps of Z-scores using FSL flameo using ordinary least-squares, and then clustered for significance at an alpha level of 0.05, t > 3.1. These maps of significant positive and negative rs-fcMRI correlations were combined to generate a single positive and a single negative rs-fcMRI map for each individual in the normative dataset derived from the spherical regions of interest. Next, the individual positive and negative rs-fcMRI maps were entered into separate weighted principal components analyses (PCA) using MATLAB (2012b, Natick, MA, USA)42 in order to identify the principal component networks that explain the most variance in network maps derived from ‘risk’ and ‘resilience’ regions. These network maps were compared to the Yeo et al. canonical resting state functional MRI 7-network parcellation using spatial correlation analysis to relate our findings to previously described rs-fcMRI networks.56 Additional details of LNM methods are provided in Supplementary material, Appendix 1. To ensure the results were not unique to the specific methodological approach to lesion network mapping, two secondary analyses were conducted: one using ‘each lesion mask as a seed’ and another using the first principal component of the rs-fcMRI signal computed from within the lesioned area as the seed.57 The resulting relationship between the rs-fcMRI and BDI scores was evaluated with a voxel-wise permutation analysis of linear models (FSL PALM), as performed previously58,59 and described in more detail in Supplementary material, Appendix 1.
Structural lesion network mapping
To evaluate structural networks associated with lesion location, each lesion mask was used to seed an individual deterministic tractography analysis using Lead-DBS software60 as performed previously.42,61,62 This method employs a normative dataset of neurologically healthy individuals with high-quality diffusion tensor imaging data included in the Human Connectome Project’s MGH 32-fold group connectome (https://ida.loni.usc.edu/login.jsp).63 For structural lesion–network mapping the challenges of signal averaging within a large lesion mask that occur with BOLD do not apply, and thus individual lesion masks were used to seed networks rather than regional peaks derived from the lesion–symptom maps. The direction of streamlines was not constrained to any other region of interest beyond the starting ‘seed’ region of interest. The 526 unthresholded individual lesion-derived tractography maps were evaluated in FSL using a voxel-wise permutation analysis of linear models (PALM, https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Randomise/UserGuide). The BDI rating was the behavioural variable in the general linear model, with lesion volume entered as a covariate and statistical significance evaluated with threshold free cluster enhancement, 2-tailed significance and 2000 permutations. Regional findings for the ‘risk’ and ‘resilience’ peaks were compared to the HCP-842 and JHU white matter tractography atlases using spatial correlation analysis to relate our findings to common white matter tracts.64–68 A visual outline of the lesion–symptom mapping and lesion–network mapping methods is provided in Fig. 1.
Figure 1.
Overview of methods demonstrating the procedures used for lesion symptom mapping, functional lesion network mapping (top row) and structural lesion network mapping (bottom row) in the primary analysis. Details are further described in the ‘Materials and methods’ section and Supplementary material, Appendix 1.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request. The data are not publicly available, and some data cannot be made available due to containing information that could compromise the privacy of research participants.
Results
Demographics
Across the two datasets, 526 lesion subjects were included in the primary analysis (67.7% male, average age 52.4 years at time of assessment). The Iowa cohort (n = 330) was 48.5% male, average age 48.9 years. In contrast, the VHIS cohort was entirely male (100%), average age 58.3 years. Both populations were predominantly right-handed (88.2 and 83.7%, respectively) and predominantly White (97.6 and 90.8%, respectively). The Iowa cohort consisted of various lesion aetiologies, with ischaemic stroke representing the largest proportion (43.0%); the VHIS cohort consisted entirely of lesions due to penetrating head injury. Additional demographic details can be found in Table 1.
Table 1.
Demographics
| Iowa cohort (n = 330) |
VHIS cohort (n = 196) |
Combined cohort (n = 526) |
|
|---|---|---|---|
| Demographic | |||
| ȃPopulation | Civilian | Military | – |
| ȃAge at assessment, years (SD) | 48.9 (17.2) | 58.3 (3.1) | 52.4 (14.5) |
| ȃGender | 160 M (48.5%) | 196 M (100%) | 356 M (67.7%) |
| ȃTime from lesion to scan, years (SD) | 4.0 (8.1)a | >30 | – |
| ȃTime from lesion to BDI-II assessment, years (SD) | 4.9 (8.8) | >30 | – |
| ȃBDI-II total score, average (SD) | 12.8 (9.3) | 9.3 (9.1) | 11.5 (9.4) |
| ȃHandedness | 291 R (88.2%) | 164 R (83.7%) | 455 R (86.5%) |
| 32 L | 27 L | 59 L (11.2%) | |
| 7 ambidextrous | 5 ambidextrous | 12 ambidextrous | |
| ȃRace | 322 White (97.6%) | 178 White (90.8%) | 500 White (95.0%) |
| 3 Black | 14 Black | 17 Black (3.2%) | |
| 3 American Indian | 2 Asian American | 9 Other (1.7%) | |
| 1 other | 1 American Indian | ||
| 1 unidentified | 1 other | ||
| ȃEthnicity | 329 Non-Hispanic (99.7%) | 187 Non-Hispanic (95.4%) | 516 Non-Hispanic (98.1%) |
| ȃYears of education (SD) | 13.8 (2.4) | 14.8 (2.5)b | 14.2 (2.5) |
| Lesion aetiology (% of sample) | |||
| ȃStroke, ischaemic | 43.0% | 0% | 27.0% |
| ȃStroke, haemorrhagic | 19.7% | 0% | 12.4% |
| ȃTumour resection (primarily benign meningiomas) | 18.8% | 0% | 11.8% |
| ȃSubarachnoid haemorrhage | 6.1% | 0% | 3.8% |
| ȃHead trauma | 3.9% | 100% | 39.7% |
| ȃOther (AVM, cavernoma resection, encephalitis, cyst ± resection, abscess resection, developmental) | 8.5% | 0% | 5.3% |
Demographics for the two cohorts included in the primary analysis, reported separately and combined. Bold font in the lesion aetiology section indicates the most highly represented aetiology for each cohort.
Sixty-two cases were missing data, average time from lesion to scan was calculated for remaining n = 268.
Three cases were missing data, average years of education was calculated for remaining n = 193.
Lesion coverage
Lesion overlap maps show greatest lesion coverage in the medial PFC (mPFC), with slightly greater coverage on the right hemisphere in both cohorts of patients (maximum overlap 60; Fig. 2). Areas with the least coverage include the brainstem, cerebellum and thalamus.
Figure 2.
Lesion groups and overlap maps. Lesion cohorts demonstrate lesion coverage over most of the brain, with highest representation of medial prefrontal cortex lesions. Posterior medial structures and deep midline structures such as the thalamus, brainstem and cerebellum show the least representation.
Beck Depression Inventory
Figure 3 shows the distribution of BDI-II scores across the two samples. Individual BDI-II scores ranged from 0 to 48 (scale maximum is 63) across the two samples, with fair representation across all depression severities. Three hundred and forty-seven patients qualified as having ‘minimal depression’ by BDI-II standards (score <14), 84 patients had ‘mild depression’ (BDI-II score 14–19) and 95 patients met criteria for ‘moderate to severe depression’ (BDI-II score >19).40
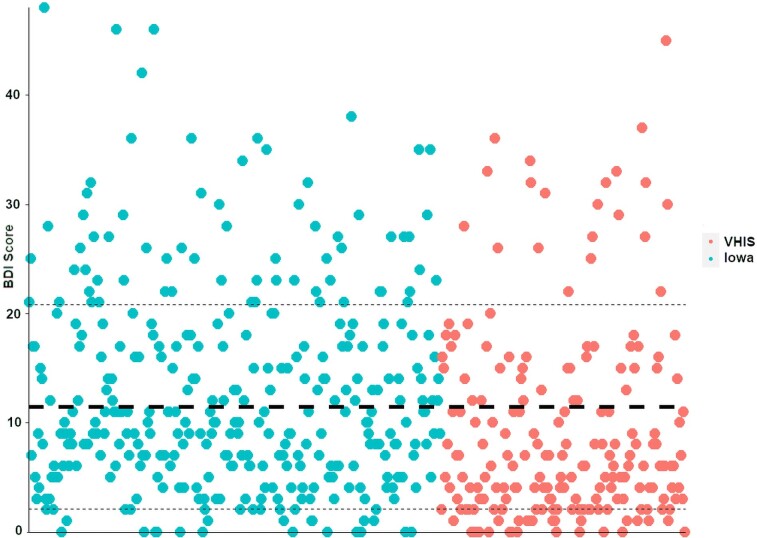
Figure 3.
Distribution of BDI-II scores. The Iowa cohort (teal, left) and VHIS cohort (red, right) are represented here as a scatter plot. The thick dashed line represents the mean BDI-II score and the thin dashed lines represent the range based on a single standard deviation above or below. Individual BDI-II scores ranged from 0 to 48 (scale maximum is 63) across the two samples. Three hundred and forty-seven patients qualified as having ‘minimal depression’ (BDI-II score <14), 84 patients had ‘mild depression’ (score 14–19) and 95 patients met criteria for ‘moderate to severe depression’ (score >19).
Lesion–symptom mapping results
Multivariate lesion–symptom mapping results demonstrated several brain regions that were significantly associated with higher and lower levels of depression severity (r = 0.11, P = 0.013; Fig. 4A). The most robust ‘risk’ regional findings were in the bilateral mid- to anterior insula and the left prefrontal deep white matter. Many regions of the left and right dorsolateral PFC and underlying white matter were included, as well as the left dorsomedial PFC. The most robust ‘resilience’ peaks were in the R > L orbitofrontal cortex (OFC) and medial PFC (mPFC) and the right inferolateral temporal cortex. In total, the clustering analysis identified 24 ‘risk’ peak regions of interest (15 grey matter peaks, 9 white matter peaks) and 20 ‘resilience’ peak regions of interest (13 grey matter peaks, 7 white matter peaks), shown in Fig. 4B and Supplementary Fig. 1. The cross-validated correlation value, a measure of the strength of the correlation, for this dataset is modest (r = 0.11), and running each dataset individually fails to reach statistical significance (Iowa r = 0.09, P = 0.12; VHIS r = 0.13, P = 0.06).
Figure 4.
Lesion-symptom mapping results for combined Iowa and VHIS cohorts. (A) Brain surface image showing all cortical regions where a lesion was significantly associated with a higher (red–yellow) or lower (blue–green) BDI-II depression scale score (n = 526, P = 0.013). We refer to the red regions as ‘risk’ regions and the blue regions as ‘resilience’ regions based on these associations. (B) Axial brain slices highlighting regions of interest with peak LESYMAP correlations for both ‘risk’ (red circles, left images) and ‘resilience’ (blue circles, right images) lesions. See Supplementary Table 1 for the MNI coordinates associated with peak clusters.
A post hoc analysis of the lesion–symptom mapping findings was undertaken to evaluate whether ‘resilience’ regions in fact reflected distinctly ‘sub-normal’ depression levels, as opposed to representing an artefact associated with ‘lack of risk’. We assessed the percentage of patients with clinically significant depression (defined as a BDI-II score >13)40 based on the proportion of the ‘risk’ or ‘resilience’ map that was lesioned. As shown in Fig. 5, patients with lesions that involve an increasing proportion of the ‘risk’ map had a higher rate of clinically significant depression; the opposite is true for ‘resilience’ regions, where patients with lesions overlapping with a greater proportion of the ‘resilience’ map had a lower rate of clinically significant depression. The average BDI-II scores from patients that overlap with >10% of the ‘risk’ or ‘resilience’ maps were significantly higher (BDI-II 17.7 ± 10.3, n = 66, P = 0.0000082) and lower (BDI-II 5.7 ± 5.9, n = 49, P = 0.000020) than a comparison group of patients whose lesions did not overlap either the ‘risk’ or ‘resilience’ regions (n = 135, BDI-II 10.8 ± 9.0). The disparity between the BDI-II scores in the ‘risk’ and ‘resilience’ groups and a lesion comparison group suggest that the lesions associated with ‘risk’ and ‘resilience’ are each uniquely associated with higher and lower levels of depression than average, respectively. This supports the notion that lesions involving ‘resilience’ regions are associated with significantly lower overall depression symptoms, rather than simply being regions not associated with higher depression severity. Further support comes by way of a large normative community-dwelling sample69 having average BDI-II scores of 8.6 ± 7.7 (n = 356), which is significantly greater than 5.7 ± 5.9 (P = 0.011).
Figure 5.
The prevalence of clinically significant depression relates to the extent a lesion overlaps with the ‘risk’ or ‘resilience’ lesion-symptom maps. Lesions affecting increasingly larger portions of the ‘risk’ map lead to higher rate of depressive symptoms (red bars, beginning with n = 320), whereas lesions affecting increasing portions of the ‘resilience’ map lead to lower rates of depressive symptoms (blue bars, beginning with n = 290). The yellow bar (n = 135) depicts the rate of depression in a sample of patients with brain lesions that do not overlap with either the ‘risk’ or ‘resilience’ maps. The numbers at the bottom of each bar indicate the number of subjects included in the analysis. For example, the rate of clinically significant depression was more than 60% when >20% of the ‘risk’ regions were lesioned (n = 23, average BDI-II = 19.6). This is in stark contrast to subjects with >20% of the ‘resilience’ regions lesioned, where 0% had significant depressive symptoms (n = 5, average BDI-II 3.6).
In evaluating potential confounding relationships that may impact our results, we evaluated the roles of lesion size, education level (as an available correlate for socioeconomic status) and post-lesion IQ (as a correlate for overall cognitive function). We first evaluated the potential role of lesion size. Lesion volume was not correlated with BDI-II scores (r = 0.02, P = 0.64). Controlling for lesion size in the analysis had minimal bearing on the statistical map (spatial correlation with the primary map = 0.98). We next evaluated the effect of education level and found this to have a weak but statistically significant correlation with BDI-II scores in the patients for whom education level was recorded (n = 522, r = 0.14, P = 0.001). When SCCAN was performed while controlling for lesion volume and education level, the cross-validation correlation value improved from 0.11 in the primary finding to 0.13 (n = 522, P = 0.002), and the map looked largely similar to the primary lesion–symptom map (spatial correlation = 0.92). We also evaluated the relationship of our findings to cognitive function as estimated with full scale IQ post-lesion. We again observed a weak but significant correlation between IQ and BDI-II scores in a subset of subjects with available IQ scores (n = 248, r=-0.18, P = 0.005). However, a lesion symptom mapping analysis of full-scale IQ using SCCAN was not statistically significant and localized to different brain regions than BDI-II (spatial correlation <0.01), suggesting that lesion localization of depressed mood was unlikely to be associated with lesion-associated differences in cognition. Unfortunately, additional measures of post-lesion cognitive status and functional status were not systematically available or readily accessible for the majority of subjects at parallel time points to allow for more extensive evaluation of the relationship between post-lesion functional status and depressive symptom burden.
Lesion–network mapping results
Functional LNM
The first principal components derived from the 15 ‘risk’ grey matter peaks and the 13 ‘resilience’ peaks are shown in Fig. 6B. They accounted for 43 and 39% of the variance of individual maps, respectively. The ‘risk’ PCA network was most similar to the salience/ventral attention network (r = 0.805) and the ‘resilience’ PCA network was most similar to the default mode network (DMN, r = 0.914; Fig. 6B and C). The results of the other components and their relation to canonical networks are shown in Supplementary Figs 2–4. The topography of ‘risk’ and ‘resilience’ networks was similar when employing alternate approaches, such as using each lesion mask to ‘seed’ the rs-fcMRI network (secondary analysis 1, r = 0.742 and −0.762 for ‘risk’ and ‘resilience’, respectively, Supplementary Fig. 5) or using the first principal component of the rs-fcMRI signal within the lesion as the seed (secondary analysis 2, r = 0.747 and −0.752 for ‘risk’ and ‘resilience’, respectively, Supplementary Fig. 5). These alternate methods generated almost identical maps to one another (r = 0.996) and also highlight the salience/ventral attention network and default mode networks as the most strongly correlated and anticorrelated networks within the Yeo 7-network parcellation (r = 0.749 and 0.765 for salience/ventral attention, −0.832 and −0.836 for default mode, respectively). To assess whether our findings were being driven by one cohort of subjects over another (Iowa cohort versus VHIS cohort), the above analyses were re-run for each individual cohort. The uncorrected lesion network mapping results were highly correlated between the Iowa and VHIS cohorts (Supplementary Fig. 6) using both the results of secondary analysis 1 (r = 0.368) and secondary analysis 2 (r = 0.423), and each demonstrated similar regions of significance which were relevant to the peak findings in our primary lesion symptom mapping result. No findings in either individual cohort survived multiple comparisons correction.
Figure 6.
Identifying ‘risk’ and ‘resilience’ networks—functional lesion network mapping results. (A) Lesion symptom mapping (LESYMAP) results demonstrate brain regions with positive (red–yellow, peak in right insula) or negative (blue–green, peaks in right orbitofrontal and lateral anterior temporal regions) associations with BDI-II depression scores post-lesion, reproduced from Fig. 4. This is used to identify peak regions with the most robust relationships to depressive symptoms (24 positive ‘risk’ regions of interest and 20 negative ‘resilience’regions of interest). (B) Principal component analysis of individual functional connectivity maps was used to identify an aggregate ‘risk’ and ‘resilience’ network (explaining 43.2 and 39.1% of the variance, respectively). In each image, hot colours (e.g. red–yellow) denote regions with positive functional connectivity to the region of interest group whereas cool colours (e.g. blue–green) denote regions with negative connectivity to the region of interest group. (C) The ‘risk’ and ‘resilience’ networks depicted in (B) appear to overlap closely with the salience/ventral attention (‘risk’, spatial correlation 0.805) and default mode (‘resilience’, spatial correlation 0.914) networks described by Yeo et al.56
Structural LNM
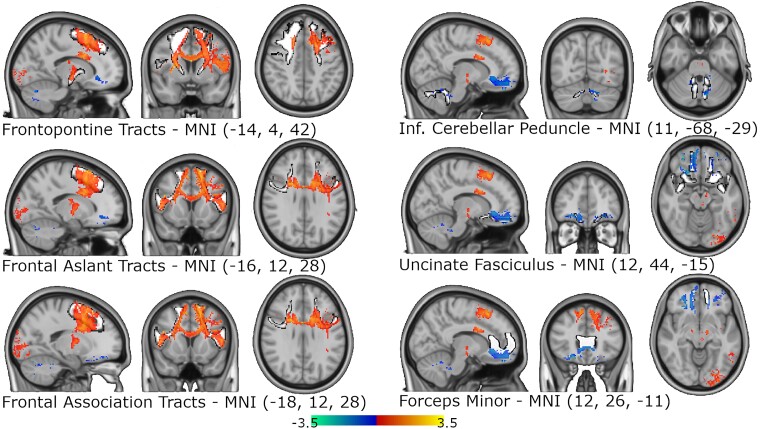
White matter tracts maximally associated with ‘risk’ for depression included dorsal frontal white matter pathways on the left more so than the right, including frontopontine fibre tracts, frontal aslant tracts and association fibre tracts (r = 0.354, 0.335, 0.334, respectively). ‘Resilience’ tracts included cerebellar outflow tracts as well as ventral frontal white matter tracts, primarily forceps minor and the uncinate fasciculus, with all findings stronger in the right hemisphere (r = 0.139, 0.128, and 0.111, respectively; Fig. 7). These findings were each significant at P values <0.000001, but no findings survived a whole-brain voxel-wise correction for multiple comparisons using family-wise error correction. Additional details and images are provided in Supplementary Fig. 7.
Figure 7.
Structural lesion network mapping results. t-Stat map highlighting the significant white matter findings from the voxel-wise permutational analysis of linear models for both ‘risk’ (red–yellow) and ‘resilience’ (blue–green) and how they align with common white matter tracts (white outlined in black). The three white matter tracts with the highest spatial correlation for both the ‘risk’ and ‘resilience’ stat maps are shown with representative brain slices. ‘Risk’ findings tended to align with dorsal prefrontal pathways and ‘resilience’ findings tended to overlap with ventral prefrontal tracts and cerebellar outflow tracts.
Discussion
Our results demonstrate that specific brain regions, when lesioned, have an association with higher depressive symptoms. The most prominent of these ‘risk’ regions are the bilateral anterior insula and dorsolateral PFC, including grey and white matter. We also identified regions that, when lesioned, were associated with lower depressive symptoms. These ‘resilience’ regions are the right OFC, right mPFC and right inferolateral temporal lobe. Moreover, our functional LNM results suggest that these regions are not randomly distributed, but fall primarily within two functional networks, with lesions of the salience network associated with increased depressive symptoms (‘risk’ nodes) and lesions of the DMN associated with reduced depressive symptoms (‘resilience’ nodes). Activity patterns in these networks are negatively correlated with each other at rest (Fig. 6B and Supplementary Fig. 4) and our results support an inverse relationship with mood when lesioned. This may help to inform the robust literature that exists on the role of these two networks in depression,70–75 as discussed below.
Our results also highlight why lesion studies of depression have been challenging. The strength of association in the brain–behaviour relationship is weak compared to other neurological functions, with an r value of 0.11. By comparison, we obtained an r-value of 0.62 for naming, using the same lesion–symptom mapping approach in a similarly large sample.42 Prior work with conflicting or null findings could potentially be explained by insufficient power, which is underscored by the fact that dividing our two samples into individual cohorts fails to detect a significant relationship, despite similar correlation values to the combined sample (0.09 and 0.13). Moreover, the regional findings were distributed across several brain areas spanning the cerebral cortex, white matter and subcortical sites. As such, the multivariate approach used here was likely important for identifying the distributed regional patterns. For example, another large-scale lesion symptom mapping study employing a multivariate analysis with a similar sample size identified some similar regions of interest (e.g. right basal ganglia and right mesial temporal lobe structures; see Supplementary Fig. 1B for reference) as associated with greater post-lesion depression symptoms.76 However, this study was limited to include only subjects with ischaemic stroke and lacked lesion coverage of medial prefrontal regions, likely limiting its ability to detect some of the ‘resilience’ regions identified here. In the next paragraphs we discuss our strongest individual findings and relate them to other literature on depression.
The anterior to mid-insula is the ‘risk’ region with the strongest findings in both hemispheres. Interestingly, few lesion–symptom mapping studies implicate the insula as a cortical region associated with depression when lesioned, despite its known role in emotion processing. This brain region is associated with prediction,77 emotion regulation after stroke,78 autonomic functioning, interoception and integration of emotional valence with our physiological state. Furthermore, the anterior insula demonstrates altered grey matter volume and abnormal functional connectivity in studies of depression79,80 and has been implicated in other forms of psychopathology,73,81,82 as well as in the generation of ecstatic or blissful experiences with stimulation.83
Interestingly, the anterior insula also serves as a hub for the salience/ventral attention network of the brain,84–87 which has nodes in other ‘risk’ regions identified in this study including the dorsolateral PFC, dorsomedial PFC and dorsal anterior cingulate cortex.88 Additionally, one of the key white matter tracts connecting cortical regions of the salience network is the frontal aslant tract, also a primary tract associated with white matter ‘risk’ regions in the structural LNM results.89 This relationship between ‘risk’ regions and the salience network is especially informative from a treatment perspective—studies suggest salience network connectivity abnormalities correlate with depressive symptoms90 and are predictive of antidepressant response in patients undergoing transcranial magnetic stimulation (TMS).91 The dorsolateral PFC was included as a ‘risk’ region, as implicated by others in lesion studies.13,14,16 The dorsolateral PFC includes a node of the salience network92 with connectivity to the anterior insula; this salience node of the PFC is in the region of the TMS target for treating depression.93–97 Finally, the dorsomedial PFC and dorsal anterior cingulate, both ‘risk’ regions, have also been implicated as potential targets for neuromodulation of mood, with stimulation inducing antidepressant responses, laughter, and the will to persevere.98–100 The dorsolateral PFC, dorsomedial PFC and dorsal cingulate cortex regions have all been associated with increases in activity in depression remitters following antidepressant medication administration.101
The ‘resilience’ regions—those locations where a lesion was associated with less depressive symptom burden—were the OFC, mPFC (right > left) and the right inferolateral anterior temporal lobe. These brain regions are involved in impulse control, decision making, reward and emotion processing (OFC, mPFC)102–106 and higher-order visual processing (inferolateral anterior temporal lobe).107 They are also notably hubs of the DMN, a network involved in self-referential thinking.108 In depressed patients, research suggests hyperconnectivity within the DMN is associated with negative rumination and depressive symptoms,71,109–112 and excessive connectivity between the DMN and attentional, externally focused brain networks also correlates with depression, possibly related to patients struggling to ‘disengage’ from their internally focused state. Some studies suggest that stimulating brain networks anticorrelated with the DMN can have an antidepressant effect,52,97,113–117 and lesioning or ‘virtually lesioning’ DMN structures such as the mPFC and nearby white matter tracts has been hypothesized to have antidepressant effects in prior studies.14 Indeed, the ‘resilience’ findings from the structural LNM results here implicate two of the three mPFC white matter tracts targeted with deep brain stimulation for treatment-refractory depression in some studies, a technique thought to induce a virtual lesion or disruption of pathological information processing.118
The purported functional contributions of the salience and DMN to mood and depression offer a potentially parsimonious explanation for our findings. The salience network, here found to be the primary ‘risk’ network for post-lesion depression, is considered important for task-switching to reallocate neural processing resources towards meaningful stimuli. In fact, the strongest regional ‘risk’ findings of anywhere in the brain correspond closely to the right dorsal anterior insula, which has a hypothesized role of acting as a network ‘switch’.119 In doing so, the salience network may have a role in coordinating transitions in attention between internally mediated thought processes supported by the DMN and externally focused processes supported by other ‘task positive’ brain networks.75 Damage to the integrity of the salience network (such as via an insula lesion) theoretically would result in impairments disengaging from internally focused processing in favour of orienting one’s attention to the environment, as is observed in association with depressive symptoms. Indeed, one study identified emotional processing deficits as the most commonly reported symptom category after salience network disturbance.120 In contrast, the DMN supports self-referential thinking, including negative rumination and depressive symptoms.71,109–112 Prior work from our lab demonstrated that lesions of the DMN reduce mind wandering,121 and one could postulate that a lesion to this network may similarly reduce negative rumination in a way that is protective against depressed mood.
This study is not without limitations. First, although the sample size was large, we did not have sufficient coverage of some brain areas, particularly subcortical sites like the brainstem, thalamus and cerebellum; as sample size can influence the results of LNM,122 we cannot draw conclusions about the relationship of depression to lesions in poorly sampled regions.123
Second, our sample also incorporated some subjects that were included in other lesion analyses, and thus we must be cautious in interpreting our findings in light of other published studies using overlapping datasets.14,16 For example, Koenigs et al.14 conducted seminal work identifying ventral mPFC lesions as associated with lower depression scores, and this dataset included n = 18 subjects that are in our Iowa (n = 7) and VHIS (n = 11) patient samples. To ensure our findings were not being unduly influenced by this cohort, we also conducted our lesion symptom mapping analysis excluding these 18 subjects (n = 508 remaining). Our findings in this unique cohort showed statistically significant findings (r = 0.09, P = 0.037) largely similar to our primary analysis results, with a spatial correlation of 0.93.
Next, although the Iowa cohort incorporated patients with no known psychiatric disorders, the pre-lesion psychiatric history of patients in the VHIS cohort is unknown; however, all VHIS subjects were deemed fit for combat in Vietnam. Thus, interindividual differences in mood, personality/temperament, psychosocial circumstance (combat exposure, other trauma history, family history) or structural and functional connectivity that preceded the onset of the lesion may reduce the strength of the brain–behaviour relationship124–126; this could be addressed in future studies by assessing these pre-lesion factors at the time of the injury or studying a population with pre-lesion clinical and neuroimaging data available.
The studies had a variable degree of time from the lesion occurrence to the depression assessment and imaging. Assessments were obtained in the chronic phase (>3 months post-injury), which attempts to minimize the confounds of acute functional or psychosocial effects of the lesion on depressive symptom reporting, but simultaneously also limits any inferences that can be made about direct causality of the lesion on depressive symptoms. The timing of assessments was not standardized beyond this and thus cannot control for chronic effects of brain network reorganization or other post-lesion psychosocial or biological changes affecting depression ratings. For example, damage to the OFC, mPFC or DMN could influence one’s emotional reasoning,14,127 moral judgement105 and associated self-referential introspection. This could alter one’s appraisal and self-report on his or her internal state, although family reports tend to confirm self-reports in some studies.128 Similarly, clinical depressive disorders are often episodic in nature. Although some studies suggest that depressive symptoms persisting 6 months post-lesion frequently develop a chronic course,29,129 the natural history of post-lesion depression can be variable.130 Thus, the severity of symptoms captured by a depression inventory provides only a snapshot of active symptoms that cannot characterize the longitudinal nature of symptoms or the effect of ongoing treatments. Although we were unable to thoroughly evaluate the relationship between post-lesion depression and post-lesion functional impairment in our sample, our evaluation of confounding cognitive status was reassuring. In general, the relationship between post-lesion cognitive or functional impairment and post-lesion depressive symptoms is mixed in prior literature,17,21,131,132 and our lack of depression ‘risk’ findings in regions classically associated with the most obvious functional impairments (e.g. Broca’s area, primary motor cortices) provides further reassurance that the findings in this study are not confounded by functional impairment. Furthermore, despite the identification of mood-relevant brain regions and networks in our analyses, the findings and conclusions are restricted to depression manifested following focal brain lesions; we cannot generalize or extrapolate to make conclusions about primary mood disorders such as major depressive disorder, which likely have unique pathophysiology.
Due to the variety of lesion aetiologies included in this study, there is a chance that the underlying pathology leading to the lesion may have an influence on the development of depression (i.e. subjects with cardiovascular disease may be at higher risk for both ischaemic stroke with a specific regional distribution and depression, which could present a confound).
Finally, this study was conducted on a cohort of subjects that was predominantly White and predominantly male. Further study of gender, racial and ethnic differences is prudent, as these findings require validation in other demographic groups before they can be generalized.
In conclusion, we present findings from one of the largest lesion–symptom mapping studies of depressive symptoms to date. Our results suggest that lesions to certain brain regions are associated with a higher post-lesion depression symptom burden, including the anterior insula, dorsolateral PFC and left dorsomedial PFC—regions that are nodes of the salience network. Equally intriguing, certain brain lesion locations are associated with lower than expected post-lesion depressive symptoms, including lesions to the OFC, mPFC and inferolateral anterior temporal lobe; these best correspond with regions of the DMN. Future studies will focus on lesion–symptom mapping of specific depression subtypes or symptom categories as well as longitudinal evaluation of the relationship between salience network or DMN connectivity and development of depressive symptoms after lesion onset.
Supplementary Material
Acknowledgements
We would like to acknowledge all the patients who so generously agreed to participate in this research study, and especially the military veterans who suffered injuries in the line of duty. We would also like to acknowledge Shuwen Li, Fatimah Albazron and Benjamin Pace for assistance with data organization.
Abbreviations
- BDI-II
Beck Depression Inventory-II
- DMN
default mode network
- LNM
lesion–network mapping
- mPFC
medial prefrontal cortex
- OFC
orbitofrontal cortex
- PFC
prefrontal cortex
- rs-fcMRI
resting state functional connectivity MRI
- VHIS
Vietnam Head Injury Study
Contributor Information
Nicholas T Trapp, Department of Psychiatry, University of Iowa, Iowa City, IA, USA; Iowa Neuroscience Institute, University of Iowa, Iowa City, IA, USA.
Joel E Bruss, Department of Neurology, University of Iowa, Iowa City, IA, USA.
Kenneth Manzel, Department of Neurology, University of Iowa, Iowa City, IA, USA; Department of Psychological and Brain Sciences, University of Iowa, Iowa City, IA, USA.
Jordan Grafman, Shirley Ryan AbilityLab, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Daniel Tranel, Iowa Neuroscience Institute, University of Iowa, Iowa City, IA, USA; Department of Neurology, University of Iowa, Iowa City, IA, USA; Department of Psychological and Brain Sciences, University of Iowa, Iowa City, IA, USA.
Aaron D Boes, Department of Psychiatry, University of Iowa, Iowa City, IA, USA; Iowa Neuroscience Institute, University of Iowa, Iowa City, IA, USA; Department of Neurology, University of Iowa, Iowa City, IA, USA; Department of Pediatrics, University of Iowa, Iowa City, IA, USA.
Funding
This research was partially supported by grants from the National Institutes of Mental Health (1K23MH125145 and 5T32-MH019113 to N.T.T.), the National Institutes of Health (P50 MH094258 to D.T.; R01NS114405 to A.D.B.; and R21MH120441 to A.D.B.) and the Kiwanis Spastic Paralysis and Allied Diseases of the Central Nervous System Research Foundation (to D.T.). The authors have no financial conflicts of interest regarding this manuscript.
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Friedrich MJ. Depression is the leading cause of disability around the world. JAMA. 2017;317:1517. [DOI] [PubMed] [Google Scholar]
- 2. Zhuo C, Li G, Lin X, et al. The rise and fall of MRI studies in major depressive disorder. Transl Psychiatry. 2019;9:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Castanheira L, Silva C, Cheniaux E, Telles-Correia D. Neuroimaging correlates of depression—Implications to clinical practice. Front Psychiatry. 2019;10:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mayberg HS. Targeted electrode-based modulation of neural circuits for depression. J Clin Invest. 2009;119:717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Terroni L, Amaro E, Iosifescu DV, et al. Stroke lesion in cortical neural circuits and post-stroke incidence of major depressive episode: A 4-month prospective study. World J Biol Psychiatry. 2011;12:539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hama S, Murakami T, Yamashita H, et al. Neuroanatomic pathways associated with monoaminergic dysregulation after stroke. Int J Geriatr Psychiatry. 2017;32:633–642. [DOI] [PubMed] [Google Scholar]
- 7. Kim NY, Lee SC, Shin JC, Park JE, Kim YW. Voxel-based lesion symptom mapping analysis of depressive mood in patients with isolated cerebellar stroke: A pilot study. Neuroimage Clin. 2017;13:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Robinson H, Calamia M, Glascher J, Bruss J, Tranel D. Neuroanatomical correlates of executive functions: A neuropsychological approach using The Examiner battery. J Int Neuropsychol Soc. 2014;20:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Braun CM, Dumont M, Duval J, Hamel-Hebert I, Godbout L. Brain modules of hallucination: An analysis of multiple patients with brain lesions. J Psychiatry Neurosci. 2003;28:432–449. [PMC free article] [PubMed] [Google Scholar]
- 10. Kim NY, Hsu J, Talmasov D, et al. Lesions causing hallucinations localize to one common brain network. Mol Psychiatry. 2019;26:1299–1309. [DOI] [PubMed] [Google Scholar]
- 11. Operskalski JT, Paul EJ, Colom R, Barbey AK, Grafman J. Lesion mapping the four-factor structure of emotional intelligence. Front Hum Neurosci. 2015;9:649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salas CE, Castro O, Yuen KSL, et al. ‘Just can't hide it’: A behavioral and lesion study on emotional response modulation after right prefrontal damage. Soc Cogn Affect Neurosci. 2016;11:1528–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koenigs M, Grafman J. The functional neuroanatomy of depression: Distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res. 2009;201:239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koenigs M, Huey ED, Calamia M, et al. Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. J Neurosci. 2008;28:12341–12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pardini M, Grafman J, Raymont V, et al. Left rostrolateral prefrontal cortex lesions reduce suicidal ideation in penetrating traumatic brain injury. CNS Spectr. 2020;25:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Padmanabhan JL, Cooke D, Joutsa J, et al. A human depression circuit derived from focal brain lesions. Biol Psychiatry. 2019;86:749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nickel A, Thomalla G. Post-stroke depression: Impact of lesion location and methodological limitations—A topical review. Front Neurol. 2017;8:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Siddiqi SH, Schaper FLWVJ, Horn A, et al. Brain stimulation and brain lesions converge on common causal circuits in neuropsychiatric disease. Nat Hum Behav. 2021;5:1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Folstein MF, Maiberger R, McHugh PR. Mood disorder as a specific complication of stroke. J Neurol Neurosurg Psychiatry. 1977;40:1018–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Robinson RG, Kubos KL, Starr LB, Rao K, Price TR. Mood disorders in stroke patients. Importance of location of lesion. Brain. 1984;107:81–93. [DOI] [PubMed] [Google Scholar]
- 21. Robinson RG, Price TR. Post-stroke depressive disorders: A follow-up study of 103 patients. Stroke. 1982;13:635–641. [DOI] [PubMed] [Google Scholar]
- 22. Robinson RG, Starkstein SE, Price TR. Post-stroke depression and lesion location. Stroke. 1988;19:125–126. [DOI] [PubMed] [Google Scholar]
- 23. Robinson RG, Szetela B. Mood change following left hemispheric brain injury. Ann Neurol. 1981;9:447–453. [DOI] [PubMed] [Google Scholar]
- 24. Starkstein SE, Robinson RG, Price TR. Comparison of cortical and subcortical lesions in the production of poststroke mood disorders. Brain. 1987;110:1045–1059. [DOI] [PubMed] [Google Scholar]
- 25. Yu L, Liu C-K, Chen JW, et al. Relationship between post-stroke depression and lesion location: A meta-analysis. Kaohsiung J Med Sci. 2004;20:372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carson AJ, MacHale S, Allen K, et al. Depression after stroke and lesion location: A systematic review. Lancet. 2000;356:122–126. [DOI] [PubMed] [Google Scholar]
- 27. MacHale SM, O'Rourke SJ, Wardlaw JM, Dennis MS. Depression and its relation to lesion location after stroke. J Neurol Neurosurg Psychiatry. 1998;64:371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Metoki N, Sugawara N, Hagii J, et al. Relationship between the lesion location of acute ischemic stroke and early depressive symptoms in Japanese patients. Ann Gen Psychiatry. 2016;15:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Astrom M, Adolfsson R, Asplund K. Major depression in stroke patients. A 3-year longitudinal study. Stroke. 1993;24:976–982. [DOI] [PubMed] [Google Scholar]
- 30. Berg A, Palomaki H, Lehtihalmes M, Lonnqvist J, Kaste M. Poststroke depression in acute phase after stroke. Cerebrovasc Dis. 2001;12:14–20. [DOI] [PubMed] [Google Scholar]
- 31. House A, Dennis M, Warlow C, Hawton K, Molyneux A. Mood disorders after stroke and their relation to lesion location. A CT scan study. Brain. 1990;113:1113–1129. [DOI] [PubMed] [Google Scholar]
- 32. Morris PL, Robinson RG, Carvalho ML, et al. Lesion characteristics and depressed mood in the stroke data bank study. J Neuropsychiatry Clin Neurosci. 1996;8:153–159. [DOI] [PubMed] [Google Scholar]
- 33. Nys GM, van Zandvoort MJE, van der Worp HB, et al. Early depressive symptoms after stroke: Neuropsychological correlates and lesion characteristics. J Neurol Sci. 2005;228:27–33. [DOI] [PubMed] [Google Scholar]
- 34. Snaphaan L, van der Werf S, Kanselaar K, de Leeuw FE. Post-stroke depressive symptoms are associated with post-stroke characteristics. Cerebrovasc Dis. 2009;28:551–557. [DOI] [PubMed] [Google Scholar]
- 35. Guiraud V, Gallarda T, Calvet D, et al. Depression predictors within six months of ischemic stroke: The DEPRESS study. Int J Stroke. 2016;11:519–525. [DOI] [PubMed] [Google Scholar]
- 36. Gozzi SA, Wood AG, Chen J, Vaddadi K, Phan TG. Imaging predictors of poststroke depression: Methodological factors in voxel-based analysis. BMJ Open. 2014;4:e004948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gajardo-Vidal A, Lorca-Puls DL, Crinion JT., et al. How distributed processing produces false negatives in voxel-based lesion-deficit analyses. Neuropsychologia. 2018;115:124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boes AD, Prasad S, Liu H, et al. Network localization of neurological symptoms from focal brain lesions. Brain. 2015;138:3061–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Balaev V, Orlov I, Petrushevsky A, Martynova O. Functional connectivity between salience, default mode and frontoparietal networks in post-stroke depression. J Affect Disord. 2018;227:554–562. [DOI] [PubMed] [Google Scholar]
- 40. Beck AT, Steer RA, Brown GK. Manual for the beck depression inventory. 2nd ed.Psychological Cooperation; 1996. [Google Scholar]
- 41. Mah YH, Husain M, Rees G, Nachev P. Human brain lesion–deficit inference remapped. Brain. 2014;137:2522–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bowren M, Bruss J, Manzel K, et al. Post-stroke outcomes predicted from lesion location and lesion network mapping. Brain 2022;145:1338–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Raymont V, Salazar AM, Krueger F, Grafman J. “Studying injured minds”—The Vietnam head injury study and 40 years of brain injury research. Front Neurol. 2011;2:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tranel D. The Iowa-Benton School of neuropsychological assessment. In: Grant I, Adams KM, eds. Neuropsychological assessment of neuropsychiatric and neuromedical disorders. Oxford University Press; 2009:66–83. [Google Scholar]
- 45. Pustina D, Avants B, Faseyitan OK, Medaglia JD, Coslett HB. Improved accuracy of lesion to symptom mapping with multivariate sparse canonical correlations. Neuropsychologia. 2018;115:154–166. [DOI] [PubMed] [Google Scholar]
- 46. Albazron FM, Bruss J, Jones RM, et al. Pediatric postoperative cerebellar cognitive affective syndrome follows outflow pathway lesions. Neurology. 2019;93:e1561–e1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Boes AD. Lesion network mapping: Where do we go from here? Brain. 2021;144:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Salvalaggio A, Pini L, De Grazia MF, et al. Reply: Lesion network mapping: Where do we go from here? Brain. 2021;144:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Boes AD, Fischer D, Geerling JC, et al. Connectivity of sleep- and wake-promoting regions of the human hypothalamus observed during resting wakefulness. Sleep. 2018;41:zsy108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Uitermarkt BD, Bruss J, Hwang K, Boes AD. Rapid eye movement sleep patterns of brain activation and deactivation occur within unique functional networks. Hum Brain Mapp. 2020;41:3984–3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Holmes AJ, Hollinshead MO, O’Keefe TM, et al. Brain genomics superstruct project initial data release with structural, functional, and behavioral measures. Sci Data. 2015;2:150031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fox MD, Halko MA, Eldaief MC, Pascual-Leone A. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS). Neuroimage. 2012;62:2232–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Van Dijk KR, Hedden T, Venkataraman A, et al. Intrinsic functional connectivity as a tool for human connectomics: Theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pini L, Salvalaggio A, De Grazia MF, et al. A novel stroke lesion network mapping approach: Improved accuracy yet still low deficit prediction. Brain Commun. 2021;3:fcab259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cotovio G, Talmasov D, Barahona-Corrêa JB, et al. Mapping mania symptoms based on focal brain damage. J Clin Invest. 2020;130:5209–5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ferguson MA, Schaper FLWVJ, Cohen A, et al. A neural circuit for spirituality and religiosity derived from patients with brain lesions. Biol Psychiatry. 2022;91:380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Horn A, Kuhn AA. Lead-DBS: A toolbox for deep brain stimulation electrode localizations and visualizations. Neuroimage. 2015;107:127–135. [DOI] [PubMed] [Google Scholar]
- 61. Bowren M Jr, Adolphs R, Bruss J, et al. Multivariate lesion–behavior mapping of general cognitive ability and its psychometric constituents. J Neurosci. 2020;40:8924–8937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Horn A, Neumann WJ, Degen K, Schneider GH, Kuhn AA. Toward an electrophysiological “sweet spot” for deep brain stimulation in the subthalamic nucleus. Hum Brain Mapp. 2017;38:3377–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Horn A, Reich M, Vorwerk J, et al. Connectivity predicts deep brain stimulation outcome in Parkinson disease. Ann Neurol. 2017;82:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hua K, Zhang J, Wakana S, et al. Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39:336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mori S, Wakana S, van Zijl PCM, Nagae-Poetscher LM. MRI Atlas of human white matter. 1st ed. Elsevier Science; 2005. [Google Scholar]
- 66. Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yeh FC, Panesar S, Fernandes D, et al. Population-averaged atlas of the macroscale human structural connectome and its network topology. Neuroimage. 2018;178:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Glasser MF, Sotiropoulos SN, Wilson JA, et al. The minimal preprocessing pipelines for the human connectome project. Neuroimage. 2013;80:105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Segal DL, Coolidge FL, Cahill BS, O'Riley AA. Psychometric properties of the Beck Depression Inventory II (BDI-II) among community-dwelling older adults. Behav Modif. 2008;32:3–20. [DOI] [PubMed] [Google Scholar]
- 70. Marchetti I, Koster EH, Sonuga-Barke EJ, De Raedt R. The default mode network and recurrent depression: A neurobiological model of cognitive risk factors. Neuropsychol Rev. 2012;22:229–251. [DOI] [PubMed] [Google Scholar]
- 71. Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol Psychiatry. 2015;78:224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Coutinho JF, Fernandesl SV, Soares JM, et al. Default mode network dissociation in depressive and anxiety states. Brain Imaging Behav. 2016;10:147–157. [DOI] [PubMed] [Google Scholar]
- 73. Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sha Z, Wager TD, Mechelli A, He Y. Common dysfunction of large-scale neurocognitive networks across psychiatric disorders. Biol Psychiatry. 2019;85:379–388. [DOI] [PubMed] [Google Scholar]
- 75. Fischer AS, Keller CJ, Etkin A. The clinical applicability of functional connectivity in depression: Pathways toward more targeted intervention. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:262–270. [DOI] [PubMed] [Google Scholar]
- 76. Weaver NA, Lim J-S, Schilderinck J, et al. Strategic infarct locations for poststroke depressive symptoms: A lesion– and disconnection–symptom mapping study. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;21:254–258. [DOI] [PubMed] [Google Scholar]
- 77. Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nat Rev Neurosci. 2015;16:419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Shi Y, Liu W, Liu R, et al. Investigation of the emotional network in depression after stroke: A study of multivariate Granger causality analysis of fMRI data. J Affect Disord. 2019;249:35–44. [DOI] [PubMed] [Google Scholar]
- 79. Kandilarova S, Stoyanov D, Kostianev S, Specht K. Altered resting state effective connectivity of anterior insula in depression. Front Psychiatry. 2018;9:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wise T, Marwood L, Perkins AM, et al. A morphometric signature of depressive symptoms in unmedicated patients with mood disorders. Acta Psychiatr Scand. 2018;138:73–82. [DOI] [PubMed] [Google Scholar]
- 81. Menon B. Towards a new model of understanding—The triple network, psychopathology and the structure of the mind. Med Hypotheses. 2019;133:109385. [DOI] [PubMed] [Google Scholar]
- 82. Menon V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn Sci. 2011;15:483–506. [DOI] [PubMed] [Google Scholar]
- 83. Nencha U, Spinelli L, Vulliemoz S, Seeck M, Picard F. Insular stimulation produces mental clarity and bliss. Ann Neurol. 2022;91:289–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Seeley WW. The salience network: A neural system for perceiving and responding to homeostatic demands. J Neurosci. 2019;39:9878–9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Menon V. Salience network. In: Toga AW, ed. Brain mapping: An encyclopedic reference. Academic Press; 2015:597–611. [Google Scholar]
- 86. Dosenbach NU, Visscher KM, Palmer ED, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: From environment to theory of mind. Neuron. 2008;58:306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Menon V, Uddin LQ. Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct. 2010;214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Briggs RG, Young IM, Dadario NB, et al. Parcellation-based tractographic modeling of the salience network through meta-analysis. Brain Behav. 2022;12:e2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Manoliu A, Meng C, Brandl F, et al. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front Hum Neurosci. 2013;7:930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fan J, Tso IF, Maixner DF, et al. Segregation of salience network predicts treatment response of depression to repetitive transcranial magnetic stimulation. Neuroimage Clin. 2019;22:101719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gordon EM, Laumann TO, Gilmore AW, et al. Precision functional mapping of individual human brains. Neuron. 2017;95:791–807 e797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Trapp NT, Bruss J, King Johnson M, et al. Reliability of targeting methods in TMS for depression: Beam F3 vs. 5.5 cm. Brain Stimul 2020;13, 578–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. McClintock SM, Reti IM, Carpenter LL, et al. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry. 2018;79:16cs10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Spitz NA, Ten Eyck P, Nizar K, Boes AD, Trapp NT. Similar outcomes in treating major depressive disorder with 10 Hz repetitive transcranial magnetic stimulation (rTMS) versus intermittent theta burst stimulation (iTBS): A naturalistic observational study. J Psychiatr Pract. 2022;28:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Perera T, George MS, Grammer G, et al. The clinical TMS society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016;9:336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Siddiqi SH, Trapp NT, Hacker CD, et al. Repetitive transcranial magnetic stimulation with resting-state network targeting for treatment-resistant depression in traumatic brain injury: A randomized, controlled, double-blinded pilot study. J Neurotrauma. 2019;36:1361–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Caruana F, Gerbella M, Avanzini P, et al. Motor and emotional behaviours elicited by electrical stimulation of the human cingulate cortex. Brain. 2018;141:3035–3051. [DOI] [PubMed] [Google Scholar]
- 99. Parvizi J, Rangarajan V, Shirer WR, Desai N, Greicius MD. The will to persevere induced by electrical stimulation of the human cingulate gyrus. Neuron. 2013;80:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bakker N, Shahab S, Giacobbe P, et al. rTMS of the dorsomedial prefrontal cortex for major depression: Safety, tolerability, effectiveness, and outcome predictors for 10 Hz versus intermittent theta-burst stimulation. Brain Stimul 2015;8:208–215. [DOI] [PubMed] [Google Scholar]
- 101. Cheng Y, Xu J, Arnone D, et al. Resting-state brain alteration after a single dose of SSRI administration predicts 8-week remission of patients with major depressive disorder. Psychol Med. 2017;47:438–450. [DOI] [PubMed] [Google Scholar]
- 102. Bechara A. The role of emotion in decision-making: Evidence from neurological patients with orbitofrontal damage. Brain Cogn. 2004;55:30–40. [DOI] [PubMed] [Google Scholar]
- 103. Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. [DOI] [PubMed] [Google Scholar]
- 104. Boes AD, Bechara A, Tranel D, et al. Right ventromedial prefrontal cortex: A neuroanatomical correlate of impulse control in boys. Soc Cogn Affect Neurosci 2009;4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Young L, Bechara A, Tranel D, et al. Damage to ventromedial prefrontal cortex impairs judgment of harmful intent. Neuron 2010;65:845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Pujara MS, Philippi CL, Motzkin JC, Baskaya MK, Koenigs M. Ventromedial prefrontal cortex damage is associated with decreased ventral striatum volume and response to reward. J Neurosci. 2016;36:5047–5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bonner MF, Price AR. Where is the anterior temporal lobe and what does it do? J Neurosci. 2013;33:4213–4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Perkins AM, Arnone D, Smallwood J, Mobbs D. Thinking too much: Self-generated thought as the engine of neuroticism. Trends Cogn Sci. 2015;19:492–498. [DOI] [PubMed] [Google Scholar]
- 110. Wise T, Marwood L, Perkins AM, et al. Instability of default mode network connectivity in major depression: A two-sample confirmation study. Transl Psychiatry. 2017;7:e1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Liston C, Chen AC, Zebley BD, et al. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry. 2014;76:517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Siddiqi SH, Weigand A, Pascual-Leone A, Fox MD. Identification of personalized transcranial magnetic stimulation targets based on subgenual cingulate connectivity: An independent replication. Biol Psychiatry. 2021;90:e55–e56. [DOI] [PubMed] [Google Scholar]
- 114. Weigand A, Horn A, Caballero R, et al. Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. Biol Psychiatry 2018;84:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ge R, Downar J, Blumberger DM, Daskalakis ZJ, Vila-Rodriguez F. Functional connectivity of the anterior cingulate cortex predicts treatment outcome for rTMS in treatment-resistant depression at 3-month follow-up. Brain Stimul. 2020;13:206–214. [DOI] [PubMed] [Google Scholar]
- 116. Cash RFH, Zalesky A, Thomson RH, Tian Y, Cocchi L, Fitzgerald PB. Subgenual functional connectivity predicts antidepressant treatment response to transcranial magnetic stimulation: Independent validation and evaluation of personalization. Biol Psychiatry. 2019;86:e5–e7. [DOI] [PubMed] [Google Scholar]
- 117. Cole EJ, Phillips AL, Bentzley BS, et al. Stanford Neuromodulation Therapy (SNT): A double-blind randomized controlled trial. Am J Psychiatry. 2021;179:132–141. [DOI] [PubMed] [Google Scholar]
- 118. Riva-Posse P, Choi KS, Holtzheimer PE, et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2014;76:963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Dadario NB, Sughrue ME. Should neurosurgeons try to preserve non-traditional brain networks? A systematic review of the neuroscientific evidence. J Pers Med. 2022;12:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Philippi CL, Bruss J, Boes AD, et al. Lesion network mapping demonstrates that mind-wandering is associated with the default mode network. J Neurosci Res. 2021;99:361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Sperber C, Dadashi A. The influence of sample size and arbitrary statistical thresholds in lesion–network mapping. Brain. 2020;143:e40. [DOI] [PubMed] [Google Scholar]
- 123. Poldrack RA, Huckins G, Varoquaux G. Establishment of best practices for evidence for prediction: A review. JAMA Psychiatry. 2020;77:534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Umarova R, Thomalla G. Indirect connectome-based prediction of post-stroke deficits: Prospects and limitations. Brain. 2020;143:1966–1970. [DOI] [PubMed] [Google Scholar]
- 125. Salvalaggio A, De Grazia MF, Zorzi M, de Schotten MT, Corbetta M. Post-stroke deficit prediction from lesion and indirect structural and functional disconnection. Brain. 2020;143:2173–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Salvalaggio A, De Grazia MF, Pini L, et al. Reply: Lesion network mapping predicts post-stroke behavioural deficits and improves localization. Brain. 2021;144:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Goel V, Lam E, Smith KW, et al. Lesions to polar/orbital prefrontal cortex selectively impair reasoning about emotional material. Neuropsychologia. 2017;99:236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. King ML, Manzel K, Bruss J, Tranel D. Neural correlates of improvements in personality and behavior following a neurological event. Neuropsychologia. 2020;145:106579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Dong L, Williams LS, Brown DL, et al. Prevalence and course of depression during the first year after mild to moderate stroke. J Am Heart Assoc. 2021;10:e020494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Parikh RM, Lipsey JR, Robinson RG, Price TR. Two-year longitudinal study of post-stroke mood disorders: Dynamic changes in correlates of depression at one and two years. Stroke. 1987;18:579–584. [DOI] [PubMed] [Google Scholar]
- 131. Hadidi N, Treat-Jacobson DJ, Lindquist R. Poststroke depression and functional outcome: A critical review of literature. Heart Lung. 2009;38:151–162. [DOI] [PubMed] [Google Scholar]
- 132. Srivastava A, Taly AB, Gupta A, Murali T. Post-stroke depression: Prevalence and relationship with disability in chronic stroke survivors. Ann Indian Acad Neurol. 2010;13:123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request. The data are not publicly available, and some data cannot be made available due to containing information that could compromise the privacy of research participants.