Abstract
Early-phase HIV remission (“cure”) trials aim to test interventions developed to eradicate HIV or to sustainably control HIV without antiretroviral treatment (ART). Many remission trials include analytic treatment interruption (ATI) to evaluate interventions, which increases the risk to participants and their sexual partners. We conducted an online questionnaire of international HIV remission trial investigators and other study team members to assess their expectations regarding the time to achieve long-term control of HIV replication without treatment (functional cure) or complete eradication of replication-competent HIV virus (sterilizing cure); attitudes toward HIV remission research and the feasibility, acceptability, and efficacy of six HIV transmission risk mitigation strategies during trials with ATI of fixed duration.
Nearly half of respondents (47%) reported expecting a functional cure for HIV to be achieved in 5–10 years, and one-third (35%) reported 10–20 years for a sterilizing cure to be achieved. On a scale of −3 to 3, mean scores indicated greater respondent concern about the risk of HIV transmission to partners during ATI (Time to rebound Mean: 0.4 and Fixed duration Mean: 11), compared to participant health risks from ATI (Time to Rebound Mean: -.9 and Fixed duration Mean: 0.0). With regard to feasibility, acceptability, and efficacy respectively, mitigation efforts rated positively included: requiring counseling for potential participants (Means: 2.3; 2.1; and 1.1), providing partner referrals for PrEP (Means: 1.3; 1.3; 1.5), providing pre-exposure proxylaxis directly to partners (Means: 1.0; 1.5; 1.6), and monitoring participants for new sexually transmitted disease acquisition (Means: 1.9; 1.4; 1.0). Respondents were less positive about requiring that participants’ sexual partner(s) participate in risk counseling or limiting participation to those who commit to abstaining from sex during the entire ATI period.
Our study demonstrates that HIV remission trial investigators and study team members are concerned about the risk of transmission to sexual partners during ATI. Separating the assessment of risk mitigation strategies for transmission risk into feasibility, acceptability, and efficacy allows the discovery of strategies that may best achieve all three outcomes. Additional research is needed to compare these more fine-grained assessments with views held by other investigators, people living with HIV, and trial participants.
Keywords: HIV cure, HIV remission Trials, Analytic treatment interruption, Transmission to sexual partners, Time to rebound, Fixed duration
1. Introduction
Given the costs and challenges that lifelong antiretroviral therapy (ART) presents, the search for a cure for HIV is increasingly seen as an important and potentially feasible approach. HIV remission (“cure”) trials aim to develop and test interventions to either completely eradicate HIV from the body, or to sustainably suppress HIV sufficiently enough to avoid immunologic damage without ART)1 Most such studies are in early stages of investigation. HIV remission trials often require some form of analytic treatment interruption (ATI), which is the halting of ART for a fixed or variable time to assess whether suppression in its absence has been achieved.2,3 However, controversy exists over the use of ATI, because such interruptions involve health risks for people with HIV (PWH) participating in such trials as well as their partners.2,4, 5, 6, 7 Concerns have been generated particularly over trials with longer durations of pausing before re-starting ART, as the longer the duration, the greater the risk. Mitigation of partner risk is a particularly critical challenge.8,9 Partners are not involved in the consenting process and may or may not be involved in knowing about the risk, which complicates strategies for mitigating that risk.
To address such challenges ethically and balance them with advancing science, it is important to understand the attitudes and views of the many stakeholders involved in such trials. These individuals can inform us of actual challenges and best strategies and therefore, should be part of the solution. The extant literature on stakeholder views includes studies of how PWH might respond to trials with ATI. These studies included both larger surveys of the general HIV population that demonstrated interest and hypothetical willingness to consider participation and smaller studies of individuals who considered participation in actual ATI trials and declined or joined. In those studies of actual trial recruits, the risk of ATI was a concern among some decliners.4,5 One survey of over 400 members of an HIV research cohort in Thailand compared attitudes toward hypothetical ATI trials of shorter versus longer durations and found greater willingness to consider participation for trials with shorter ATI duration than longer10; another investigated attitudes before and after HIV treatment interruption of 11 remission trial participants in the HIV-STAR study in Belgium, finding 10/11 had high satisfaction with ATI, but most had underestimated the emotional impact, which was associated with “feelings of uncertainty and loss of control”. Risk of HIV transmission due to viral rebound was also judged as burdensome.11 A compelling personal account by Freshwater (2019) described his decision to participate in a “cure” trial with ATI.12 Each of the aforementioned revealed the relative willingness of PWH to participate in remission trials and to accept the risks involved in order to advance science.
What of the researchers who conduct remission trials with ATI? To our knowledge, there is only one prior study exploring researcher or care provider attitudes about remission trials. Lau and colleagues (2020) describe their online international surveys of PWH and care providers, finding that both were concerned about risk of HIV transmission to trial participants’ partners during ATI.13 These surveys also included a question about when respondents thought a cure for HIV would be achievable, though without differentiating functional from sterilizing cure. PWH were more optimistic than care providers about prospects for cure.13 In this article, we contribute to this limited literature, reporting on a survey of international HIV remission trial investigators and other study team members with these specific aims:
-
1.
Assess expectations about timeline to achieve an HIV functional or sterilizing cure.
-
2.
Compare trials with time to rebound ATI (restart ART after confirmed viremia of 1000 copies/mL) and those with fixed duration ATI (restart ART after confirmed viremia at 1000 copies/mL that lasts for 4 weeks); assess degree of investigators’ concerns regarding participants’ developing virologic resistance, HIV symptoms or opportunistic infections, reluctance to restart ART after ATI, and risk of transmission to sexual partners during ATI.
-
3.
Measure perceived feasibility, acceptability, and efficacy of transmission risk mitigation approaches.
-
4.
Examine attitudes about whether monitoring participants’ sexual activity during a trial with ATI would strain the researcher/participant relationship.
2. Methods
Potential respondents for our online survey were identified and selected in two batches. Current and recently completed clinical trials and observational studies that included analytical treatment interruptions (ATI) were initially identified if they were listed as a study on the Treatment Action Group (TAG) Research Toward a Cure Trials study list.14 For each likely relevant ATI study identified, clinicaltrials.gov15 was used to identify individuals serving in the role of Principal Investigator (PI), Study Chair, Recruiter, or contact person(s). These initial efforts yielded a total of 110 researchers (Batch 1) anticipated to meet respondent eligibility criteria, who were contacted and invited to participate via email addresses obtained through publicly available online resources. A second batch of researchers anticipated to meet eligibility criteria was identified through researchers involved in active AIDS Clinical Trials Group (ACTG) HIV “cure” studies, by consulting colleagues and study tracking resources from the Southeast Asia Research Collaboration in HIV (SEARCH), and through referrals from TAG's Basic Science, Vaccines, and Cure Project Director, Richard Jeffries. Sixty additional researchers from this batch were identified and recruited via email. In sum, 170 professionals working in HIV remission research were recruited to take the anonymous survey, including individuals from North America, Europe, Asia, and Africa. The study was reviewed by UNC's Office of Human Research Ethics (OHRE) and determined to be exempt.
2.1. Measures
A questionnaire was administered in English using Qualtrics and took 20 min or less to complete. It remained open to participants from March 17-June 3, 2021, with up to 3 follow-up emails sent as needed. The questions included in this analysis are available as Supplement 1. Items assessed the following domains.
2.1.1. Socio-demographic characteristics
Respondents were asked about their gender identity, racial identity/ethnicity, age, level of educational attainment, current geographical location, and clinical trial/professional role (e.g., principal investigator, nurse-investigator, and clinician investigator; study coordinator; or other trial personnel; and other).
2.1.2. HIV trials experience
Respondents were asked about the number of completed and ongoing HIV remission trials that they had been part of in the past five years, what year they were first and most recently involved in a trial as part of a research team, and the number of people they had personally recruited and/or consented to participate in trials over the past five years. They were then asked to select all applicable ATI approaches used by the HIV remission trials they were involved in. These questions included response options developed for this questionnaire based on expert advice from our research team.
Respondents were also asked about the number of upcoming HIV remission trials that they had assisted in planning, for which recruitment was expected to start within the next 12 months, and the year the trials were expected to start. They were then asked the same questions about trial interventions and ATI approaches they planned to use.
2.2. Aim 1: Expectations about timeline to achieve an HIV cure
Respondents were asked how long they thought it would be until research had identified an intervention that achieved a functional cure (meaning treatment-free remission without complete viral eradication) and a sterilizing cure (meaning complete viral eradication) for HIV, using 2 questions modified from one used by Lau et al., 202013 to add a differentiation of functional vs. sterilizing cure. Response options included, “<5 years”, “5–10 years”, “10–20 years”, “>20 years” and “never”.
2.3. Aim 2: Attitudes about risks associated with HIV remission trials and use of ATI
In this section, two trial situations were described: “1) A trial that uses ATI to measure ‘time to rebound’. In this trial, participants would restart ART after confirmed viremia of 1000 copies/mL. 2) A trial that uses ATI of a ‘fixed duration’. In this trial, participants restart ART after confirmed viremia at 1000 copies/ml that lasts for 4 weeks.” Then, for each of the two trial situations, we assessed researcher concerns about risk to trial participants of developing: 1) antiretroviral resistance, 2) symptoms of HIV or opportunistic infections, 3) reluctance to restart ART after a trial, and 4) the risk of transmitting HIV to sexual partners during a trial. Respondents’ concerns were assessed using 7-point scales (−3; 0; +3) between bipolar, contrasting descriptors (i.e., not at all to extremely), and a neutral zero point. The scale's intervals were assumed to be of equal distance.
2.4. Aim 3: Attitudes about transmission risk mitigation approaches during ATI trials of fixed duration
Using implementation science concepts from Proctor et al.16 respondents were asked to rate mitigation approaches based on how feasible each action was, how acceptable it was to them as a researcher, and how efficacious they anticipated each action to be at reducing transmission risk during trials with ATI, of fixed duration, respectively. Six actions were listed: 1) require counseling for potential participants that is focused on reducing transmission risk during ATI of fixed duration; 2) require that participants’ sexual partner(s) participate in risk counseling targeted to reducing transmission risk during ATI of fixed duration; 3) limit participation to those who commit to abstaining from sex during the entire ATI period; 4) provide referrals so that all HIV negative partners of participants can obtain pre-exposure proxylaxis (PrEP); 5) provide PrEP directly to all HIV negative partners of participants without cost; and 6) monitor participants for new sexual transmitted disease (STD) acquisition as an effort to assess sexual activity. For each, a 7-point scale (−3; 0; +3) was used.
2.5. Aim 4: Attitudes about efforts to limit and monitor participants’ sexual activity during ATI trials
Respondents were asked to rate their agreement with the following statement: “Efforts to limit and monitor participants’ sexual activity during a trial with ATI would strain the trusting relationship between researcher and participant.” The same 7-point scale (−3; 0; +3) was used, with contrasting anchoring descriptors of strongly disagree to strongly agree.
3. Analysis
Of the 170 respondents who were invited to take the survey, 157 of the invitation emails were successfully delivered. Sixty-six respondents started the survey, of which 10 did not answer any questions and were therefore excluded. Of the remaining 56 respondents, four were ineligible. After excluding those four, 52 eligible respondents who started the survey were included in the analysis data set, including 11 who had partially completed the survey. The response rate was (52/157) 33%. Quantitative analysis was conducted using IBM SPSS Statistics 27. All variables were analyzed descriptively. Frequencies and percentages were presented for categorical variables and means, or medians were presented for continuous and scale variables unless already categorized.
4. Results
4.1. Socio-demographic characteristics
Sixty-five percent of the survey respondents were white, 50% female, 33% had a medical degree only, and 25% had both PhD and medical degrees (Table 1). Fifty-six percent of the respondents resided in North America, and 75% were investigators for their clinical trials, a category that included principal investigator, nurse-investigator, and clinician investigator. The minimum and maximum age of participants was 35 and 65 years old, respectively, with a median age of 54 years and a range of 30 years.
Table 1.
Respondent's socio-demographic characteristics.
| Socio-demographics | Count (N = 52) | Percent |
|---|---|---|
| Gender | ||
| Female | 26 | 50 |
| Male | 25 | 48 |
| Non-binary | 1 | 2 |
| Race | ||
| Asian or Asian American | 10 | 19 |
| Black or African American | 2 | 4 |
| Hispanic or Latinx | 4 | 8 |
| Hispanic or Latinx, White | 2 | 4 |
| White | 34 | 65 |
| Age group (years) (n = 50) | ||
| 30–39 | 3 | 6 |
| 40–49 | 16 | 32 |
| 50–59 | 19 | 38 |
| >60 | 12 | 24 |
| Education | ||
| Bachelors’ Degree | 5 | 10 |
| High School | 1 | 2 |
| Masters’ Degree | 8 | 15 |
| Medical Degree Only | 17 | 33 |
| PhD | 6 | 12 |
| PhD & Medical Degree | 13 | 25 |
| Other: | 2 | 4 |
| Respondent continent of residence (n = 50) | ||
| Unknown | 2 | 4 |
| Africa | 2 | 4 |
| Asia | 8 | 15 |
| Europe | 9 | 17 |
| North America | 29 | 56 |
| South America | 2 | 4 |
| Role (within Clinical Trials) | ||
| Investigator | 39 | 75 |
| Study Coordinator | 10 | 19 |
| Othera | 3 | 6 |
“Other” included Clinical Director, Research Program Director, and CRS Coordinator.
4.2. HIV trial experience
Forty-four percent (44%) of the respondents reported that they had been part of 2–4 HIV remission trials in the past five years and 27% reported planning at least 2–3 upcoming trials. Thirty-seven percent of the respondents reported not planning any upcoming trials, while 33% reported planning at least 1 trial.
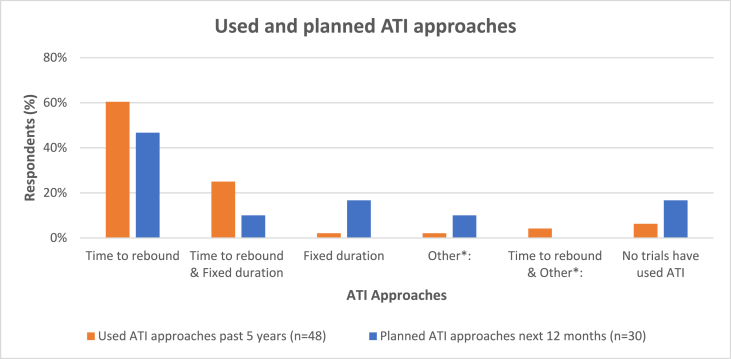
Fig. 1 shows that, in the past five years, 60% of the respondents reported using “time to rebound”, 25% reported using both “time to rebound” and “fixed duration”. Forty-seven percent (47%) of the respondents reported that “time to rebound” ATI approach will be used in the next planned trials and 17% reported that “fixed duration” ATI approach will be used.
Fig. 1.
Analytic treatment interruption (ATI) approaches used and planned**
**“Other” ATI approach reported as used in the last 5 years and in the planned trials was defined as “open duration to find set point and durability”. Both “time to rebound” and “Other” ATI approaches reported as used in the last 5 years was “ART resumption based on multiple or composite measures”. “Other” in the planned trials was “still under discussion”.
Thirty-one percent of 48 respondents reported personally recruiting and/or consenting more than 30 participants in the past five years. In contrast, 23% reported not personally recruiting and/or consenting participants—most of these (8/11) were investigators. Forty-five percent (45%) of 45 respondents reported that they were first involved in HIV remission trials between 2010 and 2014, and 33% reported getting involved between 2015 and 2019. Sixty-nine percent (69%) of the respondents reported being involved in an HIV remission trial as recently as 2021. At least 16 of respondents whose most recent trial involvement was 2021 also reported planned HIV remission trials expected to start in the same year, i.e., 2021. Overall, of the 29 respondents who reported planning upcoming HIV remission trials, the majority (79%) were expected to start in 2021.
4.3. Aim 1: Assess expectations about timeline to achieve an HIV cure
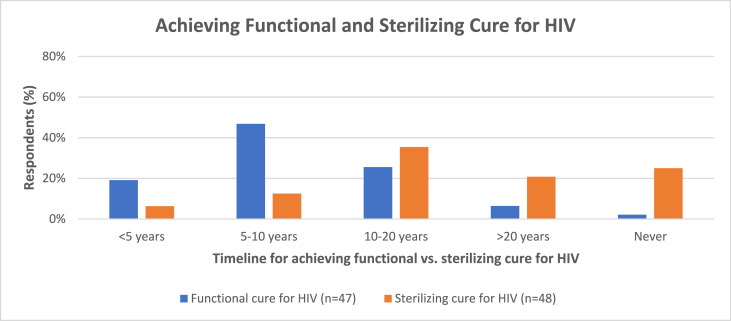
Forty-seven percent of respondents reported that a functional cure will be achieved within between 5 and 10 years, and 35% reported that a sterilizing cure will be achieved within between 10 and 20 years (Fig. 2). Twenty-six percent of the respondents reported that a functional cure will be achieved between 10 and 20 years and that a sterilizing cure will never be achieved (Fig. 2).
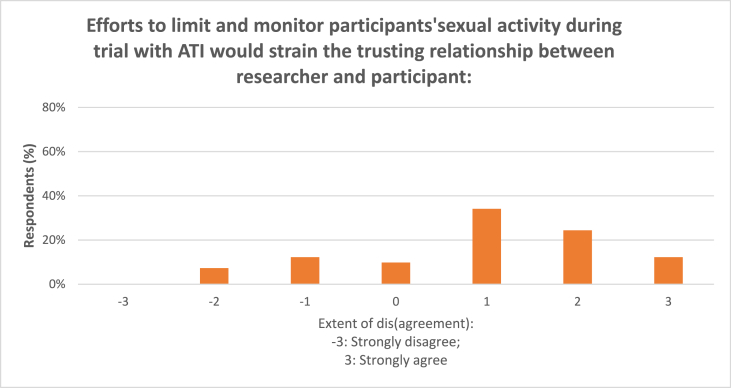
Fig. 3.
Monitoring participants’ sexual activity during ATI trials.
Fig. 2.
Timeline for functional and sterilizing HIV cure.
4.4. Aim 2: Attitudes about risks associated with HIV remission trials and use of ATI
Table 2 summarizes concern about risks associated with ATI. Overall, respondents reported low concern about ATI risk to participants (−1.3 time to rebound and −0.4 fixed duration for developing resistance; −0.9 time to rebound and 0 fixed duration for developing HIV symptoms). They also reported perceiving that the risk for reluctance to restart ART after an ATI was low (−0.8 time to rebound; −0.5 fixed duration). In contrast, they reported some concern about risk of HIV transmission during ATI (0.4 time to rebound and 1.1 fixed duration).
Table 2.
Respondents concerns regard use of an anlytical treatmetn interruption (ATI) in HIV remission trials.
| ATI Concerns about: (-3: Not at all; 3: Extremely) | Time to Rebound ATI |
Fixed Duration ATI |
|---|---|---|
| Mean; Range; (n) | Mean; Range; (n) | |
| Concern about risk to trial participants of developing antiretroviral resistance in a trial with ATI resumption defined by: | 1.3; 6; (42) | −.4; 6; (39) |
| Concern about participants' risk of developing symptoms of HIV or opportunistic infections in a trial with ATI resumption defined by: | −.9; 6; (37) | .0; 6; (38) |
| Concern about the potential for participants to be reluctant to restart ART after a trial with ATI resumption defined by: | −.8; 6; (41) | −.5; 6; (39) |
| Concern about the risk of trial participants transmitting HIV to sexual partners during a trial with ATI resumption defined by: | .4; 6; (45) | 1.1; 6; (44) |
4.5. Attitudes about transmission risk mitigation approaches during ATI trials of fixed duration
Table 3 presents respondents’ evaluations of strategies to mitigate transmission risk during ATI of fixed duration. Mitigation approaches that had positive mean ratings for feasibility and acceptability, respectively (above the midline of zero) were participant counseling (2.3 and 2.1), partner referrals for PrEP (1.3 and 1.3), direct PrEP provision (1.0 and 1.5), and STD monitoring (1.9 and 1.4). Mitigation approaches that had positive mean ratings for efficacy were participant counseling (1.1), partner counseling (0.2), referrals for PrEP (1.5), direct PrEP provision (1.6), and STD monitoring (1.0).
Table 3.
Feasibility, acceptability, and efficacy of each action to reduce transmission during ATI trials.
| Anticipate feasibility, acceptability, and efficacy of the following actions to reduce transmission risk during trials with ATI of fixed duration. (-3: Not at all; 3: Extremely) | Feasible |
Acceptable |
Efficacious |
|---|---|---|---|
| Mean; Range; (n) | Mean; Range; (n) | Mean; Range; (n) | |
| Require counseling for potential participants that is focused on reducing transmission risk during ATI of fixed duration | 2.3; 3; (42) | 2.1; 5; (42) | 1.1; 6; (42) |
| Require that participants’ sexual partner(s) participate in risk counseling targeted to reducing transmission risk during ATI of fixed duration. | −.2; 6; (40) | −.0; 6; (39) | .2; 6; (40) |
| Limit participation to those who commit to abstaining from sex during the entire ATI period. | −.8; 6; (36) | −.7; 6; (37) | −.2; 6 (38) |
| Provide referrals so that all HIV negative partners of participants can obtain PrEP | 1.3; 5; (42) | 1.3; 5; (42) | 1.5; 5; (42) |
| Provide PrEP directly to all HIV negative partners of participants without cost. | 1.0; 5; (41) | 1.5; 5; (42) | 1.6; 6; (42) |
| Monitor participants for new STD acquisition as an effort to assess sexual activity. | 1.9; 3; (41) | 1.4; 6; (40) | 1.0; 6; (41) |
Seventy percent (70%) of the respondents agreed that efforts to limit and monitor participants’ sexual activity during the trial would strain the trusting relationship between researcher and participant (Fig. 3); the mean score was 0.9 (n = 41).
5. Discussion
In this study, we offer a unique set of self-reported data on international HIV remission trial researchers’ concerns about studies with ATI—a topic that has generated considerable controversy in the scientific and bioethics literature. Our respondents represented diverse professional backgrounds, geographies, and socio-demographic characteristics, with considerable historical and current experience conducting remission trials, and with ATI. Our findings inform ways to improve ATI trials and generate important areas for future research.
Our findings reveal which risks are most worrisome for researchers conducting HIV remission trials with ATI, and among those risks, what strategies are considered most promising. Our respondents, overall, expressed little concern regarding trial participants developing antiretroviral resistance or HIV symptoms or opportunistic infections, or of exhibiting a reluctance to restart ART following an ATI trial—whether defined by time to rebound or fixed duration. In contrast, the risk of transmitting to sexual partners elicited more concern, especially when ATI was of longer duration. This finding is in accordance with several other studies that indicate a high level of concern.8,9,17
Regarding the strategies for risk mitigation, four approaches were rated as acceptable, feasible and potentially efficacious by respondents: 1) counseling, 2) referral for PrEP, 3) provision of PrEP, and 4) STD monitoring. Partner counseling and requiring abstinence were not. While monitoring participants’ new STD acquisition to assess sexual activity was judged as potentially feasible, acceptable, and efficacious, most respondents also agreed with the statement, “Efforts to limit and monitor participants' sexual activity during a trial with ATI would strain the trusting relationship between researcher and participant.” Investigators may find monitoring STD acquisition to assess sexual activity acceptable from their viewpoint, while still be aware of the possible negative impact on their relationship with participants. This is an area for future research.
The feasibility, acceptability, and efficacy of risk mitigation strategies is further complicated by practicability for the clinical trial team and the social and economic contexts of potential trial participants. For example, in many parts of the world, it may be common for PWH not to disclose their status to partners, even regular or monogamous partners.18, 19, 20, 21 Thus, despite well-intentioned efforts, some risk mitigation strategies that depend upon talking with partners about ATI will be difficult to adopt. One choice investigators may make is to limit recruitment to individuals who have disclosed to partners, although this assumes single partnerships and honesty about the disclosure. In a recent article, Rennie and colleagues22 detailed the importance of trust in navigating recruitment, enrollment, and mitigating risk during and after ATI trials. They show how strengthening trust relationships and tackling sources of distrust can reduce, though not eliminate the risk of transmission. Rennie and colleagues’ recommendations include concrete examples of what the research teams and sexual partners trust that participants will do, and likewise what participants trust the research team will do.21
We make several contributions to the study of remission trial investigators' views. First, by assessing differences in their overall assessments of feasibility, acceptability, and efficacy of different mitigation strategies, we demonstrate the importance of more nuanced measures than have been used in previous studies—e.g., studies that assess only acceptability will be missing critical data on whether a risk mitigation approach is feasible and potentially efficacious. Second, we extend the findings of Lau and colleagues regarding the anticipated time it will take for scientists to discover a cure by demonstrating there is greater optimism regarding the promise of a functional cure, compared to a sterilizing cure. Future research would benefit from differentiating expectations regarding types of “cure”.
Our study has several limitations. One is regarding the limitation inherent in surveys that provide long and detailed definitions for concepts. For example, although we provided definitions of ‘fixed duration’ and ‘time to rebound’ designs, based on expert advice, given lack of specific detail regarding variability within categories, there may be differences in respondents’ interpretation of their meaning that we are not able to discern. Another limitation is the number of respondents in our survey. Although our sample is quite diverse, with considerable experience in HIV remission trials, it is nevertheless small; our response rate was 33%. Because the survey was anonymous, we have no way to assess for bias in those who responded to the survey compared to those who did not. A larger sample would have enabled us to model statistical relationships rather than the descriptive analyses presented in this report. Future studies should attempt to obtain a larger sample of HIV remission trial investigators and incorporate comparisons of investigator and participants' views where possible.
6. Conclusion
Investigators and clinical trial team members involved in HIV remission research expressed caution about how long it will take to achieve an HIV cure, particularly a sterilizing one. Reassuringly, they report, on average, low concern about the risk to participants in remission research with ATI. They report a higher concern about transmission risk to sexual partners during ATI but endorse several risk mitigation approaches in terms of feasibility, acceptability, and efficacy.
This study data generates important areas for future research. As more trials are conducted, investigators’ attitudes about the acceptability, feasibility, and efficacy of risk mitigation efforts may change. Continued research is needed to assess the most concerning risks and efforts to reduce those risks as our knowledge base grows. Those future studies should differentiate important implementation domains (such as acceptability, feasibility, and efficacy) to best inform future protocols.
It is also critically important to understand the attitudes and experiences of PWH. While direct comparisons between professionals and PWH can be informative, studies should be tailored to the experiences and expertise of each study population. Studies that compare attitudes about the acceptability of approaches, for example, may be important to inform ways to maintain and bolster trusting relationships in the clinical trial context. Additional insights will be gained by implementing social/behavioral research with trial participants, to understand their experiences during the trial and to carefully explore the actual impact of participation on participants and their partners, including responses to risk mitigation approaches that are employed.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the investigators who kindly participated in our survey, and two anonymous reviewers.
University of North Carolina Center for AIDS Research Social and Behavioral Research Core [P30 AI50410], Drs. Denise Hsu, Raj Gandhi, and Cindy Gay; and Joanne Hunt and Richard Jeffreys.
This work was supported by the National Institutes of Health NIMH [1R34MH123328] (G Henderson, H Peay Co-PIs) “Decision Support for Early Phase HIV Treatment Trials”, and a University of North Carolina School of Medicine BOOST funding award.
Data availability
Data will be made available on request.
References
- 1.Dubé K., Kanazawa J., Taylor J., et al. Ethics of HIV cure research: an unfinished agenda. BMC Med Ethics. 2021 Jun 30;22(1):83. doi: 10.1186/s12910-021-00651-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International AIDS Society Scientific Working Group on HIV Cure. Deeks S.G., Autran B., Berkhout B., Benkirane M., Cairns S., et al. Towards an HIV cure: a global scientific strategy. Nat Rev Immunol. 2012 Jul 20;12(8):607–614. doi: 10.1038/nri3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubé K., Kanazawa J., Roebuck C., et al. “We are looking at the future right now”: community acceptability of a home-based viral load test device in the context of HIV cure-related research with analytical treatment interruptions in the United States. HIV Res Clin Pract. 2022 Dec;23(1):120–135. [PMC free article] [PubMed] [Google Scholar]
- 4.Henderson G.E., Peay H.L., Kroon E., et al. Ethics of treatment interruption trials in HIV cure research: addressing the conundrum of risk/benefit assessment. J Med Ethics. 2018 Apr;44(4):270–276. doi: 10.1136/medethics-2017-104433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henderson G.E., Waltz M., Meagher K., et al. Going off antiretroviral treatment in a closely monitored HIV “cure” trial: longitudinal assessments of acutely diagnosed trial participants and decliners. J Int AIDS Soc. 2019 Mar;22(3) doi: 10.1002/jia2.25260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Julg B., Dee L., Ananworanich J., et al. Recommendations for analytical antiretroviral treatment interruptions in HIV research trials—report of a consensus meeting. The Lancet HIV. 2019 Apr;6(4):e259–e268. doi: 10.1016/S2352-3018(19)30052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eyal N., Deeks S.G. Risk to nonparticipants in HIV remission studies with treatment interruption: a symposium. J Infect Dis. 2019 Jul 2;220(220 Suppl 1):S1–S4. doi: 10.1093/infdis/jiz173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eyal N., Holtzman L. Symposium on risks to bystanders in clinical research: an introduction. Bioethics. 2020 Nov;34(9):879–882. doi: 10.1111/bioe.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peluso M.J., Dee L., Campbell D., et al. A collaborative, multidisciplinary approach to HIV transmission risk mitigation during analytic treatment interruption. J Virus Erad [Internet. 2020 Feb 20;6(1):34–37. doi: 10.1016/S2055-6640(20)30009-1. https://pubmed.ncbi.nlm.nih.gov/32175090 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peay H.L., Rennie S., Cadigan R.J., et al. Attitudes about analytic treatment interruption (ATI) in HIV remission trials with different antiretroviral therapy (ART) resumption criteria. AIDS Behav. 2022 May;26(5):1504–1516. doi: 10.1007/s10461-021-03504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Scheerder M.A., van Bilsen W.P.H., Dullaers M., Martinez-Picado J., Davidovich U., Vandekerckhove L. Motivations, barriers and experiences of participants in an HIV reservoir trial. J Virus Erad [Internet. 2021;7(1) doi: 10.1016/j.jve.2021.100029. https://www.sciencedirect.com/science/article/pii/S2055664021000029 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freshwater W. From early AIDS vaccine to HIV cure research with analytical treatment interruption trials: a study participant testimonial. J Virus Erad. 2019 Nov 4;5(4):231–233. doi: 10.1016/S2055-6640(20)30028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau J.S.Y., Smith M.Z., Allan B., et al. Acceptability, motivation and the prospect of cure for people living with HIV and their healthcare providers in HIV cure-focused treatment interruption studies. AIDS Res Ther. 2020 Nov 10;17(1):65. doi: 10.1186/s12981-020-00321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Treatment Action Group. Group. T.A Treatment action Group (TAG) research toward a cure trials study list. https://www.treatmentactiongroup.org/cure/trials/ [Internet]. [cited 2023 Mar 26]. Available from:
- 15.Clinical trials. https://clinicaltrials.gov/ [Internet]. [cited 2023 Mar 26]. Available from:
- 16.Proctor E., Silmere H., Raghavan R., et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011 Mar;38(2):65–76. doi: 10.1007/s10488-010-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Julg B., Dee L., Ananworanich J., et al. The Lancet HIV. 2019. Recommendations for analytical antiretroviral treatment interruptions in HIV research trials—report of a consensus meeting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalichman S., Mathews C., Banas E., Kalichman M. HIV status disclosure and sexual transmission risks among people who are living with HIV and receiving treatment for non-HIV sexually transmitted infections, cape town, South Africa. J Acquir Immune Defic Syndr. 2020 Mar 1;83(3):223–229. doi: 10.1097/QAI.0000000000002256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Damian D.J., Ngahatilwa D., Fadhili H., et al. Factors associated with HIV status disclosure to partners and its outcomes among HIV-positive women attending Care and Treatment Clinics at Kilimanjaro region, Tanzania. PLoS One. 2019;14(3) doi: 10.1371/journal.pone.0211921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Obermeyer C.M., Baijal P., Pegurri E. Facilitating HIV disclosure across diverse settings: a review. Am J Publ Health. 2011 Jun;101(6):1011–1023. doi: 10.2105/AJPH.2010.300102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mekonnen F.A., Lakew A.M., Muchie K.F., Teshome D.F. Sero-positive HIV result disclosure to sexual partner in Ethiopia: a systematic review and meta-analysis. BMC Publ Health. 2019 Dec 27;19(1):1743. doi: 10.1186/s12889-019-8097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rennie S., Henderson G.E., Phanuphak N., et al. The essential need for trust when transmission risk cannot be eliminated in HIV remission trials (in press) Ethics Hum Res. 2023 doi: 10.1002/eahr.500172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.