Abstract
BACKGROUND:
Nociceptin (N/OFQ), which binds to the nociceptive opioid peptide receptor (NOP), regulates stress and reward in addiction. In a previous [11C]NOP-1A PET study, we found no differences in NOP in non-treatment-seeking AUD relative to healthy controls (HC). Here, we evaluated NOP in treatment-seeking AUD to document its relationship with relapse to alcohol.
METHODS:
[11C]NOP-1A distribution volume (VT) was measured in recently abstinent AUD and HC(n=27/group) using an arterial-input function based kinetic analysis in brain regions that regulate reward and stress behaviors. Recent heavy drinking before PET was quantified using hair ethyl glucuronide (ETG+ ≥30pg/mg). To document relapse, twenty-two AUD subjects were followed with urine ETG tests (x3/week) for 12-weeks after PET, where they were incentivized with money to abstain.
RESULTS:
There were no differences in [11C]NOP-1A VT between AUD and HC. AUD who drank heavily before the study had significantly lower VT than those with no recent heavy drinking history. Significant negative correlations between VT and the number of drinking days and the number of drinks consumed per drinking day in the past thirty days before enrollment were also present. AUD who relapsed (and dropped-out) had significantly lower VT than those who abstained for 12-weeks.
CONCLUSIONS:
Lower NOP VT in heavy drinking AUD predicted relapse to alcohol during a 12-week follow-up. The results of this PET study support the need to investigate medications that act at NOP to prevent relapse in AUD.
Keywords: [11C]NOP-1A, positron emission tomography, nociceptin (N/OFQ) nociceptive opioid peptide receptors (NOP), alcohol use disorders (AUD), relapse, heavy drinking
INTRODUCTION
N/OFQ, which binds to NOP blocks the rewarding properties of alcohol in the conditioned place preference behavioral paradigm and reduces alcohol consumption (1, 2). It prevents the somatic and affective signs of alcohol withdrawal in alcohol-dependent rodents (3). N/OFQ also prevents both cue- and stress-induced reinstatement in animals self-administering alcohol (4, 5). N/OFQ blocks ethanol- and corticotrophin-releasing factor (CRF)- induced increases GABA in the amygdala to prevent reinstatement in alcohol dependent animals (6-8). Consistent with these results, numerous studies have documented the therapeutic potential of NOP agonists in rodent models of AUD (9). Counterintuitively, NOP antagonists have also been shown decrease alcohol reward, intake, binge drinking, and self-administration in rodents (9). N/OFQ stimulates ventral tegmental area NOP to decrease nucleus accumbens dopamine release and constraint motivation for natural rewards (10-13). N/OFQ-induced decreases in dopamine can promote a state of hyperkatifeia (a greater intensity of negative emotion/motivation) and contribute to negative reinforcement and relapse (9, 14). N/OFQ also enhances the rewarding and anxiolytic effects of alcohol when co-administered with alcohol in the amygdala (15). These mechanisms support using a NOP antagonist to block N/OFQ transmission to prevent relapse in AUD. Despite promising rodent data, no NOP agonists have been investigated as a therapeutic in humans with AUD. LY2940094, the only NOP antagonist investigated in AUD, was found to be no better than placebo in reducing the number of alcoholic drinks consumed per day (primary endpoint) (16-19). However, in the same 8-week clinical trial, LY2940094 was superior to placebo in decreasing the number of heavy drinking days and increasing the number of abstinent days (secondary endpoints). Exploratory subgroup analysis also favored using LY2940094 over placebo in reducing the number of drinks consumed daily in light to moderate drinkers and females. In a broad sense, it is unclear whether a successful strategy to prevent relapse in AUD should involve a NOP agonist or antagonist. It is also unclear whether AUD with specific characteristics (light/moderate vs. heavy drinkers, males vs. females, etc.) may benefit from a NOP agonist vs. antagonist treatment.
Our previous [11C]NOP-1A PET study in non-treatment-seeking AUD and matched controls, found no significant between-group differences in the in vivo binding to NOP receptors (VT) in brain regions involved in stress and reward pathways (20). They were partially consistent with a human postmortem study in AUD that reported lower NOP in the amygdala but not in the hippocampus and prefrontal cortex (2). However, they were inconsistent with higher NOP observed in another addictive disorder, i.e., cocaine use disorder patients, compared to controls (21). Here, we were interested in using [11C]NOP-1A PET to contrast NOP binding in a clinically representative treatment-seeking group of AUD with that in HC. We purposefully enrolled more females with AUD as they were underrepresented (33%) in our prior study (20). Our secondary objective was to investigate the relationship between NOP VT and relapse to alcohol in a 12-week follow-up during which AUD were incentivized to abstain from alcohol. Consistent with an observation in non-treatment-seeking AUD in whom lower OFC [11C]NOP-1A VT was associated with higher cravings for alcohol (20), we hypothesized that lower NOP would predict relapse. Lastly, we were also interested in exploring the relationships between VT and the clinical features of AUD.
METHODS
Human subjects
The University of Pittsburgh Human Research Protection Office and Radioactive Drug Research Committee approved the study. All subjects provided written informed consent. Subjects were recruited via advertisements and a University of Pittsburgh research registry (Pitt+Me). Study criteria for AUD included: (1) males and females between 18-55 years old; (2) fulfill DSM-5 criteria for AUD (3) no current DSM-5 major depressive, bipolar, psychotic or substance use disorders (tobacco use was not exclusionary); (4) no medical or neurological illness; (5) no medications that may bind to NOP (e.g., buprenorphine, or morphine); (6) not pregnant; (7) no recent research or occupational radioactivity exposure; and (8) no contraindications for magnetic resonance imaging (MRI). Study criteria for age- and sex- matched healthy controls included: (1) absence of DSM-5 psychiatric and substance use disorders; (2) no history of current heavy or binge drinking as defined by NIAAA criteria; and criteria 4 to 8 above. Inclusion/exclusion was determined using the Structured Clinical Interview for DSM-5, a physical exam, routine blood work, urine drug screen and pregnancy tests. Clinical assessments performed included the: Barratt Simplified Measure of Social Status, Michigan Alcohol Screening Test (MAST), Alcohol Dependence Scale (ADS), Penn Alcohol Craving Scale (PACS), and Fagerstrom Test for Nicotine Dependence, Perceived Stress Scale (PSS), and the Hamilton Anxiety Rating Scale (HAM-A). AUD were monitored three times per week using urine ETG (alcohol metabolite) tests for a minimum of ten days for abstinence before the PET scans. The absence of any recent drug use (including cannabis) was also confirmed using a urine drug screen on scan day in both AUD and controls. Hair sampling for ETG (or finger nails if hair was unavailable) in AUD was also performed on PET scan day to identify individuals with heavy alcohol use in the past 8- to 12-weeks before PET (22). Hair ETG analyses identified AUD with ETG ≥ 30 pg/mg (ETG+), which corresponds to the use of greater than 60g of pure ethanol/day, i.e., an equivalent of 30+ drinks per week as per the 2019 consensus for the use of alcohol markers in hair (23, 24).
PET acquisition and analysis
[11C]NOP-1A PET scans, which lasted 70 minutes, were conducted using the Siemens Biograph64 mCT with an arterial line for a metabolite-corrected arterial input function as previously described (21, 25, 26). [11C]NOP-1A plasma free fraction (fp) was not pursued in this study because it has poor reproducibility (27), which is likely due to its high adherence to the ultracentrifugation filter as demonstrated in our earlier studies using a saline buffer (20, 28, 29). PMOD was used to correct head motion between frames, co-register the MR to PET, and generate time-activity curves. Regions of interest (ROI), including the amygdala, hippocampus, midbrain, cerebellum, striatum (ventral striatum, caudate, and putamen), and prefrontal (dorsolateral, orbital, medial, and anterior cingulate) cortical subregions, were generated based on the AAL-VOIs atlas using PMOD’s PNEURO Tool (30-32). ROIs were restricted to the same regions examined in our previous [11C]NOP-1A PET studies in addictive and stress disorders (20, 21, 25, 26). All ROIs generated by the PNEURO tool were visually inspected and adjusted as deemed necessary by an image analyst trained in manual region drawing. Derivation of [11C]NOP-1A volume of distribution were performed using a two-tissue compartment kinetic analysis using the arterial input function implemented in MATLAB (27, 33, 34). VT, which includes both the receptor-bound specific and non-specific binding, was used as the outcome measure because no region in the brain can be used to estimate [11C]NOP-1A non-specific binding (35).
Relapse monitoring protocol for AUD
In order to document relapse, AUD were enrolled in a 12-week follow-up protocol after the [11C]NOP-1A PET scan. This follow-up protocol used contingency management to encourage abstinence, as in cocaine use disorder (CUD) PET studies (21, 36). AUD were monitored with urine ETG tests three times a week, for which they earned voucher points on an escalating schedule for negative results. Subjects earned bonus points for every three-consecutive ETG-free urine sample (one week of abstinence). Missed appointments reset the voucher points to a value that was lower by 10 points. Subjects had the potential to earn a maximum of $1197.00 for providing ETG-free urine samples for all (36 visits) scheduled monitoring visits. The money earned was disbursed to them on a weekly basis via a debit card. Subjects were terminated from the research protocol and referred to a treatment program (as they required more intensive treatment than offered in the research to remain abstinent) for testing positive for ETG three times (i.e., were allowed only three distinct relapses) or missing three consecutive scheduled appointments (i.e., were lost to follow-up for a week). Subjects were also monitored for psychiatric symptoms and de-briefed on the progress of their abstinence once a week. No psychotherapy was provided in addition to contingency management in the study.
Statistical Analysis
All statistical analyses were conducted using IBM SPSS v.27. Group demographic and baseline scan parameter (such as injected dose, mass, plasma clearance) comparisons were performed with unpaired t-tests. Between-group differences in [11C]NOP-1A VT were examined with a linear mixed model analysis (LMM) using ROIs as a repeated measure and diagnostic group (AUD vs. HC) as the fixed factor. The effect of tobacco use status, comorbid disorders, and psychotropic medications on VT and diagnostic group were subsequently examined in a second level LMM analyses by including them as fixed factors in the model. LMM analyses using ROIs as a repeated measure were also used to examine the effect of factors, such as a history of heavy drinking prior to the scan (ETG+ vs. ETG−) and follow-up outcome (abstained vs. relapsed vs. dropped out) on VT in AUD. Post-hoc unpaired t-tests in the individual ROIs were also conducted. Pearson product-moment correlations were used to explore the relationships between VT and clinical variables of interest such as stress (PSS), anxiety (HAM-A), craving (PACS), alcohol use severity (MAST, ADS scores, number of drinking days and the mean number of drinks consumed per drinking day in the past thirty days prior to enrollment, amount of voucher money earned (level of abstinence accomplished) and duration of abstinence before PET. A two-tailed probability value of p ≤ 0.05 was selected as the significance level for the LMM analyses that included all regions of interest. A Bonferroni corrected p-value of < 0.00454 (n=0.05/11) was used as the significance level to correct for multiple comparisons for the analyses that evaluated VT in the individual regions of interest. No further multiple comparison corrections were implemented for the number of clinical correlations examined.
RESULTS
Twenty-seven AUD (6 male, 21 female) and 27 HC subjects matched for age, sex, and tobacco use were scanned with [11C]NOP-1A. Subjects had no overlap with a published [11C]NOP-1A PET study in AUD (20). Seven AUD had a comorbid psychiatric and/or chronic pain disorder in the past twelve months (three with generalized anxiety disorder, one with post-traumatic stress disorder, and four with chronic pain; Note: one AUD had both PTSD and chronic pain). Eight AUD were on psychotropic medications (5 serotonin reuptake inhibitors, 1 serotonin-norepinephrine reuptake inhibitor, 1 tricyclic antidepressant, and 1 was on both bupropion and topiramate).
Twenty-two (out of 27) AUD were enrolled in the 12-week follow up to document relapse and relate it to VT. COVID19 pandemic-related modifications implemented by the University to reduce the number of visits to the lab during the study did not allow for the inclusion of 5 AUD in the follow-up protocol. These modifications also led to three AUD completing part of their follow-up in an honor system via phone. Baseline demographic and clinical characteristics of the 27 AUD and 27 HC matched on age, sex, and tobacco use are shown in Table 1.
Table 1.
Demographic and clinical characteristics of alcohol use disorder and healthy control subjects
| Alcohol use disorder n = 27 |
Healthy controls n = 27 |
|
|---|---|---|
| Age | 36 ± 10 | 34 ± 9 |
| Sex | ||
| Females | 21 | 21 |
| Males | 6 | 6 |
| Ethnicity | ||
| African American | 4 | 3 |
| Caucasian | 21 | 22 |
| Asian | 2 | 2 |
| Barratt Simplified Measure of Social Status | ||
| Education | 16 ± 3 | 16 ± 4 |
| Occupation | 26 ± 10 | 26 ± 10 |
| Total | 42 ± 12 | 42 ± 13 |
| Tobacco use | 12 | 15 |
| Fagerstrom Test for Nicotine Dependence moderate/high dependence (score ≤ 4) | 5 | 4 |
| Hamilton Anxiety Rating Scale (range 0 to 56) | 6 ± 6 | 2 ± 3 * |
| Perceived Stress Scale (range 0 to 40) | 11 ± 8 | 7 ± 6 * |
| DSM-5 Alcohol use disorders (mild / moderate / severe) | 2/3/22 | |
| Michigan Alcohol Screening Test (range 0 to 22) | 10 ± 5 | - |
| Alcohol Dependence Scale (range 0 to 47) | 19 ± 7 | - |
| Penn Alcohol Craving Scale (range 0 to 30) | 15 ± 7 | - |
| Age of first use of alcohol (years) | 16 ± 3 | - |
| Frequency of alcohol use in past 30 days prior to enrollment (days/month) | 16 ± 9 | - |
| Amount of alcohol use in past 30 days prior to enrollment (drinks/drinking day) | 6.7 ± 4.5 | - |
| Duration abstinent from alcohol before PET (days) | 27 ± 20 | - |
| 12-week follow-up protocol to monitor for relapse following PET scan (n = 22†) | ||
| Final Outcome | ||
| Abstinent | 6 | |
| Relapse confirmed with ETG+ urine sample | 8 | |
| Drop-out | 8 | |
| Time to first relapse (days) | 8 ± 7 | |
| Time to drop-out (days) | 11 ± 18 | |
| Amount of money earned in vouchers (dollars) | 421 ± 514 |
p < 0.05, unpaired t-tests for numerical variables and Chi-square test for categorical variables.
Includes three subjects (one in abstinent and two in relapse group) who completed part of their follow-up in an honor system via phone due to COVID19 pandemic. Note: Both subjects in the relapse group had a urine ETG+ test confirmed relapse before the follow-up protocol was converted to an honor system.
[11C]NOP-1A scan parameters
No significant differences in [11C]NOP-1A injected dose (AUD 12.0 ± 1.1mCi; HC 12.6 ± 1.0mCi), mass (AUD 2.4 ± 0.9μg; HC 2.3 ± 0.6μg), or plasma clearance (AUD 151 ± 47L/h; HC 147 ± 33L/h) were present between the AUD and HC groups. There were also no significant between-group differences in the MR-based ROI volumes (Table S1).
[11C]NOP-1A distribution volume (VT)
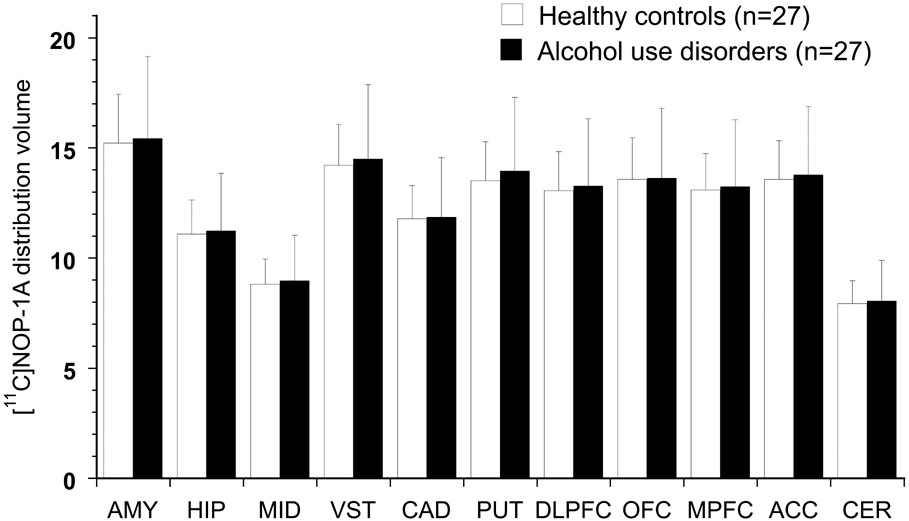
[11C]NOP-1A VT was not significantly different between the AUD and HC groups (LMM, effect of diagnosis, F (1, 52) = 0.09, p = 0.77; effect of region, F (10, 520) = 601.95, p < 0.001; region X diagnosis, F (10, 520) = 0.33, p = 0.97, see Figure 1). VT was significantly higher in males than females, and a trend towards a higher VT in tobacco users relative to non-users, especially in AUD, was observed (supplement analyses and Table S2). Nevertheless, the inclusion of sex or tobacco use as factors did not alter the significance of diagnosis in the LMM. Unpaired t-tests conducted to examine group differences in the individual regions of interest were also not significant (data not shown). Comorbid psychiatric/ chronic pain disorders and psychotropic medications had no significant effect on VT in the AUD group (supplement analyses).
Figure 1.
show no significant differences in [11C]NOP-1A VT (mean and standard deviation) between AUD and HC. Regions included are AMY: amygdala, HIP: hippocampus, MID: midbrain, VST: ventral striatum, CAD: caudate, PUT: putamen, DLPFC: dorsolateral prefrontal cortex, OFC: orbital frontal cortex, MPFC: medial prefrontal cortex, ACC: anterior cingulate cortex, CER: cerebellum.
Lower [11C]NOP-1A VT in AUD subjects predicts relapse to alcohol (and early drop-out) in a 12-week follow up
During the 12-week follow up of twenty-two AUD subjects in which abstinence from alcohol was promoted using contingency management, n=6 abstained, n=8 relapsed (defined as having tested positive on at least one urine ETG test during follow-up), and n=8 dropped-out. Hair ETG+ heavy drinkers (total, n=13) were more likely to relapse (n=7) and drop out (n=4) than abstain (n=2) during follow-up. Hair ETG− AUD subjects (total, n=8 in whom follow-up data were available) were more likely to abstain (n=4) and drop out (n=4) than relapse (n=0). These differences in follow-up outcome, ETG+ vs ETG− AUD subjects were significant (Chi-Square, p=0.03, Table S3). Trend level differences in the duration of abstinence before PET (i.e., time between last drink and PET) were also observed between AUD groups that abstained vs. relapsed during follow-up (unpaired t-test, p=0.08, Table S3).
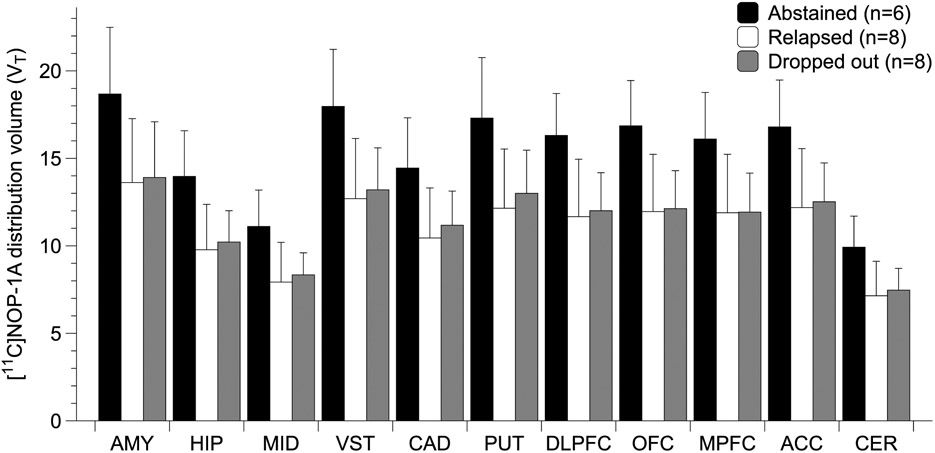
Significant follow-up outcome (abstained, relapsed, dropped-out) based differences in VT were present in AUD (LMM, effect of final outcome status, F (2, 19)= 5.6, p = 0.012; effect of region, F (10, 190)=199.40, p < 0.001; region * final outcome interaction, F (20, 190)= 2.67, p <0.001, see Figure 2). The relatively small number of subjects in the individual groups precluded us from conducting meaningful statistics to correct for the differences in the clinical characteristics (Table S3), including heavy drinking and duration of abstinence before PET.
Figure 2.
shows significantly lower VT (~ 25%, p = 0.012) in AUD subjects who relapsed (or dropped out) compared to AUD who abstained during the 12-week follow-up period (data shown in the graph are mean ± standard deviation).
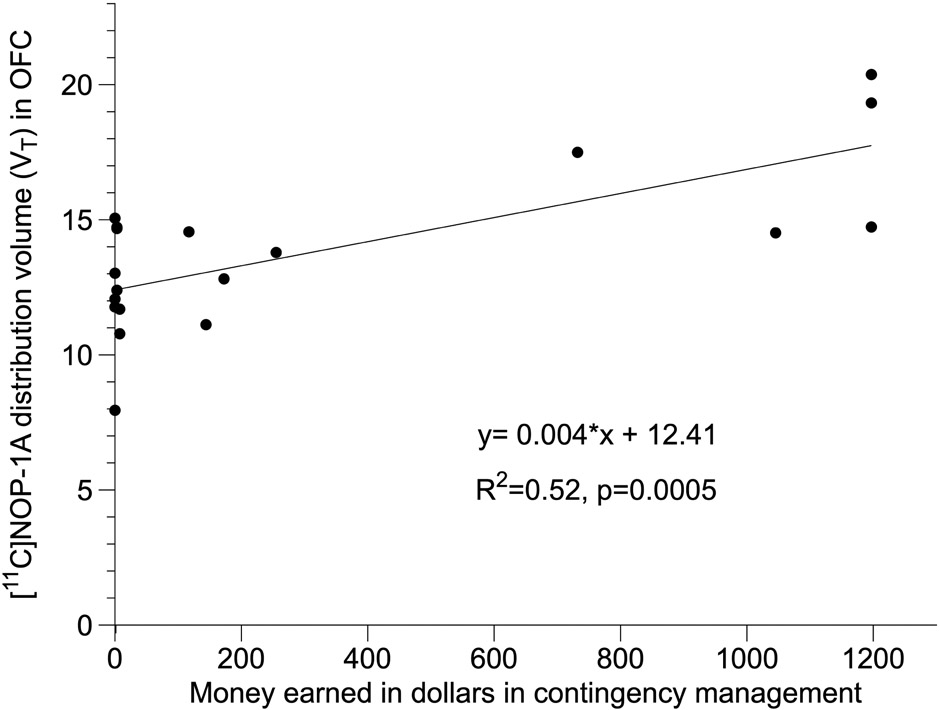
Significant positive correlations between ROI VT and total money earned in the contingency management protocol (Figure 3 shows this correlation with OFC VT), which corresponds to the level of abstinence accomplished during follow-up, were also present. These correlations remained significant following a Bonferroni correction for multiple comparisons in all ten ROIs except the amygdala (Table S4). Controlling for heavy drinking status (ETG+ vs ETG−) and duration of abstinence before PET using partial correlations did not alter the Bonferroni-corrected significance of these relationships in the OFC (Tables S5 and S6).
Figure 3.
shows the positive relationship between OFC VT and total money earned during follow up (data shown is from n=19 subjects because it excludes 3 AUD who completed the follow-up in an honor system via phone due to COVID19 pandemic).
Lower [11C]NOP-1A VT in AUD was related to recent heavy alcohol use.
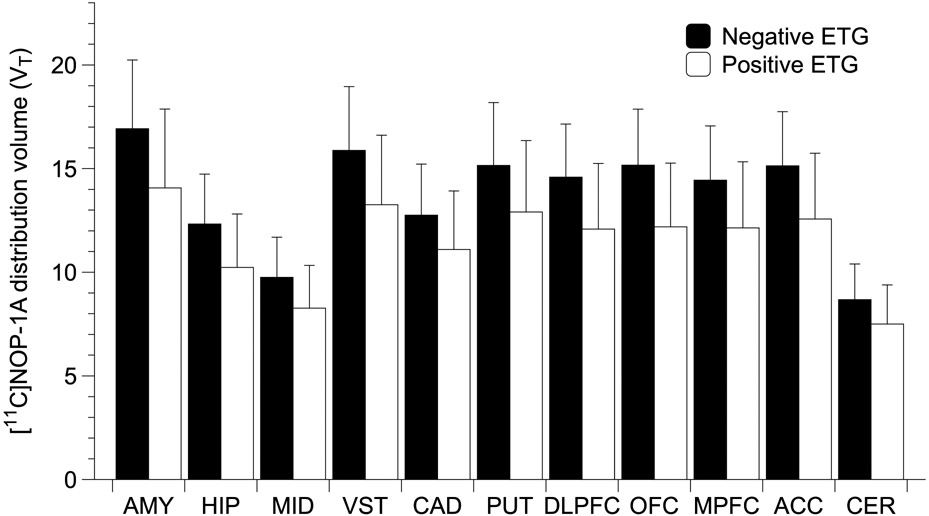
VT was significantly lower in hair ETG+ AUD vs. ETG− AUD (n=13/group, linear mixed model, LMM effect of ETG+, F (1,24)= 4.3, p =0.049; effect of region, F (10, 240)=246.4, p < 0.001; region x ETG interaction, F (10, 240)= 3.8, p < 0.001, see Figure 4). Post-hoc unpaired t-tests in the HIP, VST, DLPFC, OFC and ACC were nominally significant at the p<0.05 level. However, none survived a Bonferroni correction for multiple hypothesis testing in the ROIs. Further, consistent with the effect of heavy drinking, significant negative correlations (that survived multiple comparisons in several regions, see Table 2) between VT and the number of drinking days and mean drinks consumed per drinking day in the past 30 days prior to enrollment were observed. Several of these relationships remained significant when controlling for tobacco and sex using partial correlations (Table S7). Significant positive correlations that did not survive multiple comparisons (Table 2) between VT and the number of abstinent days before PET in AUD were also present.
Figure 4.
shows significantly lower VT (~ 15%, p = 0.049) in Hair ETG+ AUD with recent heavy alcohol use compared to ETG− AUD subjects (n=13/group). Error bars are standard deviation. Note: Hair ETG was not available for n=1 subject.
Table 2.
shows the Pearson’s correlation coefficients (r) for the relationships between VT and self-reported alcohol use in 27 AUD.
| Prior to PET scans | 30 days prior to enrollment | ||
|---|---|---|---|
| Region VT | Number of days abstinent | Drinking days | Drinks per drinking day |
| Amygdala | 0.282 | − 0.548** | − 0.562** |
| Hippocampus | 0.459* | − 0.544** | − 0.586** |
| Midbrain | 0.413* | − 0.472* | − 0.533** |
| Ventral Striatum | 0.415* | − 0.568** | − 0.560** |
| Caudate | 0.403* | − 0.474* | − 0.520* |
| Putamen | 0.403* | − 0.526* | − 0.542** |
| Dorsolateral prefrontal cortex | 0.366 | − 0.530** | − 0.548** |
| Orbitofrontal cortex | 0.374 | − 0.598** | − 0.593** |
| Medial prefrontal cortex | 0.361 | − 0.529* | − 0.542** |
| Anterior Cingulate cortex | 0.401* | − 0.547** | − 0.556** |
| Cerebellum | 0.374 | − 0.445* | − 0.530** |
Significant positive associations between VT and abstinent days before PET were present in six out of eleven ROIs (Figure S1). Significant negative associations between VT and number of drinking days (Figure S2), and mean drinks/drinking day (Figure S3) in the past thirty days prior to enrollment were present in all ROIs.
p ≤ 0.05
Bonferroni corrected p ≤ 0.00454.
Relationships between [11C]NOP-1A VT and clinical measures
No significant relationships were observed between regional VT and any stress (PSS, HAM-A) or AUD clinical measures (MAST, ADS), including cravings for alcohol (PACS).
DISCUSSION
In this PET study, we found no significant differences in the in vivo binding of [11C]NOP-1A in treatment-seeking AUD compared to HC. This result is consistent with our prior [11C]NOP-1A PET study that mostly included non-treatment-seeking males with no comorbid psychiatric and medical disorders (20). Our attempts to maximize the ability to detect differences in NOP binding in this study by investigating a clinically representative treatment-seeking sample of AUD with comorbid anxiety, trauma, and pain disorders (on psychotropic medications) and including more females (78% of the sample) was still unsuccessful. Congruent with these [11C]NOP-1A PET studies are the results of human postmortem studies that have also failed to find significant differences in NOP in brain regions (except for lower NOP in the amygdala in AUD in one out of two studies) in AUD relative to controls (2, 37). Contrary to these convergent human data in AUD, results of the basic studies measuring NOP (and N/OFQ) in AUD are mixed and inconclusive (Table S8). In these basic AUD studies, consideration for factors such as alcohol administration paradigm, duration of withdrawal, and regions examined do not allow for a discernible pattern to emerge. Notably, the only [11C]NOP-1A PET study investigating another substance use disorder found an ~ 10% higher VT in CUD relative to controls(21). A comparable observation that fell short of significance in the current PET study was a higher VT in tobacco users compared to non-users. This trend, which was driven by subjects with AUD (Table S2), was not observed in prior [11C]NOP-1A investigations(20, 21, 26). Future studies with larger samples should confirm whether NOP is higher in AUD who co-use tobacco and clarify its role in a relapse to alcohol. NOP binding is likely altered (or not) in humans depending on the substance (alcohol, cocaine, tobacco, etc.) or combination of substances they chronically use. Because N/OFQ broadly inhibits GABA, glutamate, and monoamines in the brain (38), the extent to which chronic alcohol vs. cocaine use (and comorbid tobacco use) alters these neurotransmitters probably influences NOP binding in these disorders.
The lack of a difference in NOP binding in AUD vs. HC should not diminish its pursuit as a therapeutic in AUD because findings linking it with clinically relevant features were present. Higher baseline VT in subjects with AUD successfully predicted their ability to abstain from alcohol during 12-weeks of contingency management. AUD subjects with lower baseline VT values were more likely to relapse and drop-out during follow-up. Reaffirming this finding were significant positive relationships between VT in the ROIs and the total money earned during contingency management (which reflected both the level of abstinence and the number of visits for which the subject was present during follow-up). These findings with relapse were predicted by an inverse relationship between lower OFC NOP-1A VT and higher craving for alcohol in non-treatment-seeking alcoholics(20). AUD subjects who reported chronic heavy drinking (confirmed using hair ETG) in the months prior to the study had significantly lower VT in the ROIs than AUD who did not report such a history. An effect of recent alcohol use on VT was also supported by the positive relationships, which fell short of significance, between regional VT and the number of abstinent days before PET in subjects with AUD. In summary, the results of this study point towards lower NOP availability in AUD who drink heavily interferes with their ability to abstain from alcohol during treatment. They also suggest a possible recovery in NOP VT in AUD with prolonged abstinence.
Lower NOP binding was observed in heavy drinking AUD who routinely consumed more than 30 drinks/week (roughly 2- and 4-fold higher than the NIAAA heavy drinking definition for males and females) than AUD who drank less prior to enrollment. Lower NOP binding in AUD also predicted relapse to alcohol and drop-out in a three-month follow-up protocol that used contingency management to incentivize abstinence. PET data acquired in this study do not inform whether lower NOP VT in AUD who drink heavily and relapse results from higher (occupancy model) or lower (receptor/neurotransmitter expression model) N/OFQ levels. Higher N/OFQ in AUD who drink heavily (interpreted using an occupancy model) support using a NOP antagonist to block excessive N/OFQ transmission to promote abstinence. Proof for this approach was present in the failed LY2940094 AUD trial in which treatment with the NOP antagonist significantly decreased heavy drinking days and increased abstinent days relative to placebo in patients with AUD (16). Future NOP antagonist trials could enroll heavy drinking subjects with AUD as they are likely to have high N/OFQ levels (assumed from lower VT) and focus on heavy drinking and abstinence as the endpoints. Heavy drinking and abstinence as endpoints because they are linked with lower VT (i.e., high N/OFQ) and are secondary measures that the NOP antagonist, LY2940094, met in the AUD trial. Incorporating [11C]NOP-1A PET in NOP antagonist trials to screen and enroll AUD with low VT and, by extension, high N/OFQ levels could also be considered to maximize success. On the other hand, lower N/OFQ levels in AUD who drink heavily (interpreted using a low receptor /low neurotransmitter expression model) would support using a NOP agonist to promote abstinence. This approach is supported by most AUD rodent studies (17 out of 20) that have evaluated the efficacy of N/OFQ or NOP agonists(9). Basic and PET studies have also shown NOP receptors to upregulate, presumably to enhance N/OFQ transmission in response to increases in stress, cortisol, and CRF (25, 39, 40), all drivers of negative reinforcement in relapse. One unanswered question in the literature is whether NOP upregulation to enhance N/OFQ is an adaptive or maladaptive response to increases in stress and stress-promoting hormones. Albeit from a small sample, binding to NOP (VT) was higher in the six AUD who abstained from alcohol than in healthy controls in this study. Higher NOP in AUD who abstained supports the notion of it being an adaptive response to counter stress and CRF, which promote relapse. Unfortunately, the PET results do not inform the ongoing debate on whether to use a NOP agonist or antagonist in AUD. However, they underscore the need to clarify the role of N/OFQ in patients with AUD to inform NOP agonist and antagonist medication development efforts. Given the absence of clinical trials with NOP agonists and antagonists in humans, there may be value in repurposing drugs, such as buprenorphine, that show NOP agonism at high doses to treat AUD (41, 42).
In summary, consistent with our prior study in non-treatment seeking AUD, we found no between-group differences in [11C]NOP1A VT in a clinical representative treatment-seeking cohort of AUD relative to HC. Secondary analyses of the [11C]NOP-1A data found lower VT in heavy drinking AUD to predict relapse (and drop-out) during a contingency management protocol that promoted abstinence. Limitations of these secondary findings, which were generated using a relatively small number of subjects per group (e.g., relapsed vs. abstinent), include the inability to account for the differences in clinical characteristics (such as tobacco use, heavy drinking, and duration of abstinence from alcohol before PET) that existed between these groups. For example, we cannot exclude the possibility of a higher number of heavy drinkers or a lower duration of abstinence in the relapsed group driving the relationship between relapse and NOP VT. Studies in which heavy drinkers and duration of abstinence are more evenly distributed across follow-up outcomes (relapsed and abstinent) are necessary to clarify whether lower [11C]NOP-1A VT is an independent risk factor for relapse. Enrolling and maintaining AUD subjects in the follow-up protocol to document relapse during the COVID19 pandemic was another challenge that impacted the sample size. Other methodological limitations include using VT to quantify NOP because it cannot exclude the relative contribution of non-specific binding and plasma-free fraction, if any, to the findings. For example, we cannot exclude the contribution of between-group differences in fp to the finding of lower VT in ETG+ vs. ETG− AUD. Lastly, hair/nail ETG might have been vulnerable to various medical disorders, race, sex, genetic variation in enzymes metabolizing alcohol, use of medications, and recreational drugs(43). Despite these limitations, the results of this PET study support the investigation of medications that act at NOP to prevent relapse in AUD. These results also highlight the need to understand the clinical relevance of variability in PET studies that fail to find group differences in receptor binding measures.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | ||||
| Bacterial or Viral Strain | ||||
| Biological Sample | ||||
| Cell Line | ||||
| Chemical Compound or Drug | [C-11]NOP-1A precursor and standard | ABX advanced biochemical compounds | Custom synthesis ordered | |
| Chemical Compound or Drug | [C-11]NOP-1A synthesis | NIMH/SNIDD Database | https://kidbdev.med.unc.edu/databases/snidd/IND/nop1a.htmlRemoved | |
| Commercial Assay Or Kit | ||||
| Deposited Data; Public Database | ||||
| Genetic Reagent | ||||
| Organism/Strain | ||||
| Peptide, Recombinant Protein | ||||
| Recombinant DNA | ||||
| Sequence-Based Reagent | ||||
| Software; Algorithm | MATLABR-2021b | Mathworks | https://www.mathworks.com/products/matlab.html | |
| Software; Algorithm | PMOD v4.2 | PMOD Technologies | https://www.pmod.com/web/ | |
| Software; Algorithm | IBM SPSS Statistics v 27 | IBM SPSS | https://www.ibm.com/analytics/spss-statistics-software | |
| Transfected Construct | ||||
| Other |
ACKNOWLEDGEMENTS.
The project described above was funded by Award Numbers R01AA025247 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Recruitment of subjects were in part supported by a research registry (Pitt+Me), which is funded by the National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program, Award Number UL1 TR001857.
The content is solely the responsibility of the authors and does not necessarily represent the official views of NIAAA or the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURE
The authors report no biomedical financial interests or potential conflicts of interest.
References:
- 1.Ciccocioppo R, Panocka I, Polidori C, Regoli D, Massi M (1999): Effect of nociceptin on alcohol intake in alcohol-preferring rats. Psychopharmacology (Berl). 141:220–224. [DOI] [PubMed] [Google Scholar]
- 2.Kuzmin A, Bazov I, Sheedy D, Garrick T, Harper C, Bakalkin G (2009): Expression of pronociceptin and its receptor is downregulated in the brain of human alcoholics. Brain Res. 1305 Suppl:S80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Economidou D, Cippitelli A, Stopponi S, Braconi S, Clementi S, Ubaldi M, et al. (2011): Activation of brain NOP receptors attenuates acute and protracted alcohol withdrawal symptoms in the rat. Alcohol Clin Exp Res. 35:747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciccocioppo R, Economidou D, Fedeli A, Angeletti S, Weiss F, Heilig M, et al. (2004): Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behaviour by the antiopioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacology (Berl). 172:170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin-Fardon R, Ciccocioppo R, Massi M, Weiss F (2000): Nociceptin prevents stress-induced ethanol- but not cocaine-seeking behavior in rats. Neuroreport. 11:1939–1943. [DOI] [PubMed] [Google Scholar]
- 6.Cruz MT, Herman MA, Kallupi M, Roberto M (2012): Nociceptin/orphanin FQ blockade of corticotropin-releasing factor-induced gamma-aminobutyric acid release in central amygdala is enhanced after chronic ethanol exposure. Biol Psychiatry. 71:666–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberto M, Siggins GR (2006): Nociceptin/orphanin FQ presynaptically decreases GABAergic transmission and blocks the ethanol-induced increase of GABA release in central amygdala. Proc Natl Acad Sci U S A. 103:9715–9720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, et al. (2010): Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol Psychiatry. 67:831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciccocioppo R, Borruto AM, Domi A, Teshima K, Cannella N, Weiss F (2019): NOP-Related Mechanisms in Substance Use Disorders. Handb Exp Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker KE, Pedersen CE, Gomez AM, Spangler SM, Walicki MC, Feng SY, et al. (2019): A Paranigral VTA Nociceptin Circuit that Constrains Motivation for Reward. Cell. 178:653–671 e619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy NP, Ly HT, Maidment NT (1996): Intracerebroventricular orphanin FQ/nociceptin suppresses dopamine release in the nucleus accumbens of anaesthetized rats. Neuroscience. 75:1–4. [DOI] [PubMed] [Google Scholar]
- 12.Murphy NP, Maidment NT (1999): Orphanin FQ/nociceptin modulation of mesolimbic dopamine transmission determined by microdialysis. J Neurochem. 73:179–186. [DOI] [PubMed] [Google Scholar]
- 13.Norton CS, Neal CR, Kumar S, Akil H, Watson SJ (2002): Nociceptin/orphanin FQ and opioid receptor-like receptor mRNA expression in dopamine systems. J Comp Neurol. 444:358–368. [DOI] [PubMed] [Google Scholar]
- 14.Koob GF (2021): Drug Addiction: Hyperkatifeia/Negative Reinforcement as a Framework for Medications Development. Pharmacol Rev. 73:163–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knight CP, Hauser SR, Waeiss RA, Molosh AI, Johnson PL, Truitt WA, et al. (2020): The Rewarding and Anxiolytic Properties of Ethanol within the Central Nucleus of the Amygdala: Mediated by Genetic Background and Nociceptin. J Pharmacol Exp Ther. 374:366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Post A, Smart TS, Jackson K, Mann J, Mohs R, Rorick-Kehn L, et al. (2016): Proof-of-Concept Study to Assess the Nociceptin Receptor Antagonist LY2940094 as a New Treatment for Alcohol Dependence. Alcohol Clin Exp Res. 40:1935–1944. [DOI] [PubMed] [Google Scholar]
- 17.Statnick MA, Chen Y, Ansonoff M, Witkin JM, Rorick-Kehn L, Suter TM, et al. (2016): A Novel Nociceptin Receptor Antagonist LY2940094 Inhibits Excessive Feeding Behavior in Rodents: A Possible Mechanism for the Treatment of Binge Eating Disorder. J Pharmacol Exp Ther. 356:493–502. [DOI] [PubMed] [Google Scholar]
- 18.Toledo MA, Pedregal C, Lafuente C, Diaz N, Martinez-Grau MA, Jimenez A, et al. (2014): Discovery of a novel series of orally active nociceptin/orphanin FQ (NOP) receptor antagonists based on a dihydrospiro(piperidine-4,7'-thieno[2,3-c]pyran) scaffold. J Med Chem. 57:3418–3429. [DOI] [PubMed] [Google Scholar]
- 19.Raddad E, Chappell A, Meyer J, Wilson A, Ruegg CE, Tauscher J, et al. (2016): Occupancy of Nociceptin/Orphanin FQ Peptide Receptors by the Antagonist LY2940094 in Rats and Healthy Human Subjects. Drug Metab Dispos. 44:1536–1542. [DOI] [PubMed] [Google Scholar]
- 20.Narendran R, Ciccocioppo R, Lopresti B, Paris J, Himes ML, Mason NS (2018): Nociceptin Receptors in Alcohol Use Disorders: A Positron Emission Tomography Study Using [(11)C]NOP-1A. Biol Psychiatry. 84:708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narendran R, Tollefson S, Himes ML, Paris J, Lopresti B, Ciccocioppo R, et al. (2019): Nociceptin Receptors Upregulated in Cocaine Use Disorder: A Positron Emission Tomography Imaging Study Using [(11)C]NOP-1A. Am J Psychiatry. 176:468–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berger L, Fendrich M, Jones J, Fuhrmann D, Plate C, Lewis D (2014): Ethyl glucuronide in hair and fingernails as a long-term alcohol biomarker. Addiction. 109:425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boscolo-Berto R, Favretto D, Cecchetto G, Vincenti M, Kronstrand R, Ferrara SD, et al. (2014): Sensitivity and specificity of EtG in hair as a marker of chronic excessive drinking: pooled analysis of raw data and meta-analysis of diagnostic accuracy studies. Ther Drug Monit. 36:560–575. [DOI] [PubMed] [Google Scholar]
- 24.Andraus M, Apppenzeller B, Baumgartner M, BInz T, Crunelle C, Favretto D, et al. (2019): Consensus for the use of alcohol markers in hair for supporting the assessment of abstinence and chronic alcohol consumption. Society of Hair Testing. Lille, France. [Google Scholar]
- 25.Flanigan M, Tollefson S, Himes ML, Jordan R, Roach K, Stoughton C, et al. (2020): Acute Elevations in Cortisol Increase the In Vivo Binding of [(11)C]NOP-1A to Nociceptin Receptors: A Novel Imaging Paradigm to Study the Interaction Between Stress- and Antistress-Regulating Neuropeptides. Biol Psychiatry. 87:570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narendran R, Tollefson S, Fasenmyer K, Paris J, Himes ML, Lopresti B, et al. (2019): Decreased Nociceptin Receptors Are Related to Resilience and Recovery in College Women Who Have Experienced Sexual Violence: Therapeutic Implications for Posttraumatic Stress Disorder. Biol Psychiatry. 85:1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lohith TG, Zoghbi SS, Morse CL, Araneta MD, Barth VN, Goebl NA, et al. (2014): Retest imaging of [11C]NOP-1A binding to nociceptin/orphanin FQ peptide (NOP) receptors in the brain of healthy humans. Neuroimage. 87:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narendran R, Tollefson S, Fasenmyer K, Paris J, Himes ML, Lopresti B, et al. (2019): Decreased Nociceptin Receptors Are Related to Resilience and Recovery in College Women Who Have Experienced Sexual Violence: Therapeutic Implications for Posttraumatic Stress Disorder. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narendran R, Tollefson S, Himes ML, Paris J, Lopresti B, Ciccocioppo R, et al. (2019): Nociceptin Receptors Upregulated in Cocaine Use Disorder: A Positron Emission Tomography Imaging Study Using [(11)C]NOP-1A. Am J Psychiatry.appiajp201918081007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Douaud G, Gaura V, Ribeiro MJ, Lethimonnier F, Maroy R, Verny C, et al. (2006): Distribution of grey matter atrophy in Huntington's disease patients: a combined ROI-based and voxel-based morphometric study. Neuroimage. 32:1562–1575. [DOI] [PubMed] [Google Scholar]
- 31.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 15:273–289. [DOI] [PubMed] [Google Scholar]
- 32.Collins DL, Zijdenbos AP, Kollokian V, Sled JG, Kabani NJ, Holmes CJ, et al. (1998): Design and construction of a realistic digital brain phantom. IEEE Trans Med Imaging. 17:463–468. [DOI] [PubMed] [Google Scholar]
- 33.Lohith TG, Zoghbi SS, Morse CL, Araneta MF, Barth VN, Goebl NA, et al. (2012): Brain and whole-body imaging of nociceptin/orphanin FQ peptide receptor in humans using the PET ligand 11C-NOP-1A. J Nucl Med. 53:385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. (2007): Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 27:1533–1539. [DOI] [PubMed] [Google Scholar]
- 35.Kimura Y, Fujita M, Hong J, Lohith TG, Gladding RL, Zoghbi SS, et al. (2011): Brain and whole-body imaging in rhesus monkeys of 11C-NOP-1A, a promising PET radioligand for nociceptin/orphanin FQ peptide receptors. J Nucl Med. 52:1638–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, et al. (2011): Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. The American journal of psychiatry. 168:634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lutz PE, Zhou Y, Labbe A, Mechawar N, Turecki G (2015): Decreased expression of nociceptin/orphanin FQ in the dorsal anterior cingulate cortex of suicides. Eur Neuropsychopharmacol. 25:2008–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schlicker E, Morari M (2000): Nociceptin/orphanin FQ and neurotransmitter release in the central nervous system. Peptides. 21:1023–1029. [DOI] [PubMed] [Google Scholar]
- 39.Rodi D, Zucchini S, Simonato M, Cifani C, Massi M, Polidori C (2008): Functional antagonism between nociceptin/orphanin FQ (N/OFQ) and corticotropin-releasing factor (CRF) in the rat brain: evidence for involvement of the bed nucleus of the stria terminalis. Psychopharmacology (Berl). 196:523–531. [DOI] [PubMed] [Google Scholar]
- 40.Ciccocioppo R, de Guglielmo G, Hansson AC, Ubaldi M, Kallupi M, Cruz MT, et al. (2014): Restraint stress alters nociceptin/orphanin FQ and CRF systems in the rat central amygdala: significance for anxiety-like behaviors. J Neurosci. 34:363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ciccocioppo R, Economidou D, Rimondini R, Sommer W, Massi M, Heilig M (2007): Buprenorphine reduces alcohol drinking through activation of the nociceptin/orphanin FQ-NOP receptor system. Biol Psychiatry. 61:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dematteis M, Pennel L, Toquero CS, Maillebuau M, Martin-Gatignol V (2018): High-dose buprenorphine: a last resort drug for treatment-resistant alcohol use disorder. Preliminary results of a compassionate observational pilot study. French Journal of Psychiatry.Voume 1, Supplement, S126–S127. [Google Scholar]
- 43.SAMHSA (2012): The Role of Biomarkers in the Treatment of Alcohol Use Disorders, 2012 Revision. Advisory, pp Volume 11, Issue 12. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.