Abstract
Objectives
To develop alginate nanoparticles functionalized with polysorbate 80 (P80) as miltefosine carriers for brain targeting in the oral treatment of cryptococcal meningitis.
Methods
Miltefosine-loaded alginate nanoparticles functionalized or not with P80 were produced by an emulsification/external gelation method and the physicochemical characteristics were determined. The haemolytic activity and cytotoxic and antifungal effects of nanoparticles were assessed in an in vitro model of the blood–brain barrier (BBB). A murine model of disseminated cryptococcosis was used for testing the efficacy of oral treatment with the nanoparticles. In addition, serum biomarkers were measured for toxicity evaluation and the nanoparticle biodistribution was analysed.
Results
P80-functionalized nanoparticles had a mean size of ∼300 nm, a polydispersity index of ∼0.4 and zeta potential around −50 mV, and they promoted a sustained drug release. Both nanoparticles were effective in decreasing the infection process across the BBB model and reduced drug cytotoxicity and haemolysis. In in vivo cryptococcosis, the oral treatment with two doses of P80 nanoparticles reduced the fungal burden in the brain and lungs, while the non-functionalized nanoparticles reduced fungal amount only in the lungs, and the free miltefosine was not effective. In addition, the P80-functionalization improved the nanoparticle distribution in several organs, especially in the brain. Finally, treatment with nanoparticles did not cause any toxicity in animals.
Conclusions
These results support the potential use of P80-functionalized alginate nanoparticles as miltefosine carriers for non-toxic and effective alternative oral treatment, enabling BBB translocation and reduction of fungal infection in the brain.
Introduction
Cryptococcal meningitis (CM) is an opportunistic fungal infection mainly caused by Cryptococcus neoformans that occurs commonly in immunocompromised patients, such as those with advanced HIV infection.1,2 Inadequate or no treatment is associated with 81% mortality; however, the mortality rate of patients who have received appropriate antifungal treatment reduces to 20%–30%.1 The infection begins in the lung through inhalation of yeasts or spores present in the environment. Following the establishment of pulmonary infection, cryptococci disseminate primarily to the CNS upon crossing the blood–brain barrier (BBB).3–5
The recommended therapy for CM is the combination of amphotericin B with flucytosine in the induction phase followed by fluconazole in the maintenance phase.6,7 Besides antifungal therapy being limited to a few drugs, there are problems of significant toxicity and increased resistance.8 Furthermore, treatment of CNS infections is often difficult because the BBB limits the diffusion of molecules to the CNS and efflux pumps can reduce drug concentrations in the tissue, resulting in therapeutic failure.9 Thus, the need for new treatment options for CM is evident.
Previous in vitro and in vivo studies showed that miltefosine, an oral FDA-approved treatment of leishmaniosis (2014),10 also has broad-spectrum activity and fungicidal effects against dimorphic, filamentous and yeast fungi.11–21 In female mice with disseminated cryptococcosis, oral miltefosine increased animal survival and reduced the fungal burden;21 however, a limited effect was observed in male mice models of CM and disseminated cryptococcosis.22 Indeed, data on miltefosine antifungal activity in in vivo models are scarce and still inconclusive.
Recently, miltefosine was granted ‘Orphan Drug’ designation by the FDA for the treatments of invasive candidiasis (2021) and primary amoebic meningoencephalitis (2016).23 However, this drug has some disadvantages, such as limited penetration across the BBB in humans (only 2%–4% of plasma concentration) and high affinity for serum proteins, thus limiting tissue distribution.24,25 In addition, miltefosine causes gastrointestinal adverse effects when orally administered and presents renal and hepatic toxicities, a teratogenic effect and high haemolytic activity.24
The use of drug delivery systems has increased in the last few decades as alternative treatments for many diseases, mainly to overcome drug toxicity and pharmacokinetic limitations. Nanocarriers have been increasingly investigated to deliver drugs and macromolecules to the brain as a non-invasive approach to promote transport through the BBB.26 In this regard, nanocarriers can undergo surface modifications to improve drug delivery, and the use of surfactants, such as polysorbate 80 (P80), can increase the ability of nanocarriers to cross the BBB.27 Recently, our research group demonstrated standardized alginate-based nanoparticles as miltefosine carriers as an alternative treatment of cryptococcosis, candidiasis and aspergillosis in a larval model of infection and a murine model of vaginal candidiasis.12,28,29 Furthermore, the alginate nanoparticles released miltefosine in a sustained manner, decreased drug toxicity in the larval model, and no haemolytic effect was observed compared with free miltefosine.29 Building upon this work, we functionalized miltefosine-loaded alginate nanoparticles with P80 for brain targeting, and assessed their toxicity, biodistribution and antifungal activity in a human in vitro BBB model and in a murine model of systemic cryptococcosis.
Materials and methods
Alginate nanoparticle production
Alginate nanoparticles were produced by emulsification using the external gelation method. The unloaded alginate nanoparticles (AN) and miltefosine-loaded alginate nanoparticles (MFS-AN) were produced according to the protocol previously described.29 The P80-functionalized alginate nanoparticles (P80-AN and P80-MFS-AN) were standardized using the same protocol, except that P80 was included. Briefly, an emulsion with 1.35 g of 1% alginate (MP Biomedicals, USA) and 2.04 g of sunflower oil containing 3% SPAN 80 (Sigma–Aldrich, USA) was prepared, homogenized for 1 min and probe sonicated for 10 min (50 s on—10 s off). Under stirring, 0.2 M calcium chloride with 0.5% P80 (Synth, Brazil) was added and sonicated for 5 min (50 s on—10 s off). After 30 min of stirring, the emulsion was centrifuged for 10 min at 3000 g and the supernatant was removed. Then, 10% trehalose was added to the samples for freeze-drying for 24 h. To obtain P80-MFS-AN, 3 mg of miltefosine (Cayman Chemical, USA) was added to the alginate dispersion.
P80-MFS-AN and MFS-AN nanoparticles were stored at −22°C for 1 to 90 days to evaluate the stability by determination of average diameter (Dz), polydispersity index (Pdi) and zeta potential. Nanoparticles were diluted in distilled water (1:1000, v/v) to determine Dz and Pdi by dynamic light scattering (DLS), while the zeta potential was measured by electrophoresis using a Nano ZS (Malvern Instruments, UK).
Encapsulation efficiency and in vitro miltefosine release assay
The encapsulation efficiency of miltefosine in the P80-functionalized alginate nanoparticles was obtained by quantification of miltefosine remaining in the supernatant. To assess drug release, P80-MFS-AN nanoparticles were dispersed in 1 mL of sterile distilled water and incubated at 37°C under constant agitation (200 rpm). At 6, 12 and 24 h, the samples were centrifuged for 5 min at 3000 g and the supernatants were collected for miltefosine quantification. Miltefosine was quantified colorimetrically at 460 nm (Epoch 2, BioTek, USA) based on a miltefosine calibration curve (15.62 to 2000 mg/L).30 The encapsulation percentage was calculated using the formula: 100 − (miltefosine in supernatant × 100/amount of initial miltefosine).29
Antifungal susceptibility test
The MIC values of miltefosine, in its free form and loaded in nanoparticles (P80-MFS-AN) were determined by the broth microdilution technique31 against C. neoformans H99. MIC was defined as the lowest concentration that inhibited 90% of fungal growth by visual inspection.
Haemolytic activity
A 4% suspension of RBCs (v/v, in 5% glucose-PBS) was subjected to treatment with various concentrations of miltefosine and P80-MFS-AN nanoparticles for 2 h in a bath at 37°C. The negative (no treatment) and positive (0.1% Triton X-100) controls were included in the test for haemolytic activity (HA) determination,29 and 50% HA (HA50) was obtained by linear prediction.
In vitro BBB model
Antifungal effect
Endothelial cells (hCMEC/D3 passages 25–30) were seeded at 50% confluent density on collagen-coated permeable transwell inserts (Corning, USA; 8 µm pore diameter) in endothelial basal media (Lonza, USA) supplemented with human fibroblast growth factor (Gibco, USA; 1 ng/mL), 2.5% FBS and antibiotics. After reaching confluence at 4 days, growth factors were reduced first to 50% for 24 h, then to 25% 24 h prior to treatment. Transwells were treated with miltefosine (2 mg/L) or miltefosine-loaded alginate nanoparticles (MFS-AN or P80-MFS-AN; 100 mg/L miltefosine ) added to the top of inserts, along with 3.3 × 104C. neoformans H99. After 12 h incubation at 37°C and 5% CO2, cfu count beneath the transwell inserts were determined by plating on Sabouraud dextrose agar.
Cytotoxicity
Endothelial cells were grown in 96-well, opaque-wall, clear-bottom plates under the conditions described above. Miltefosine (1.56 to 25 mg/L), MFS-AN or P80-MFS-AN (50 to 800 mg/L miltefosine) were diluted in serum-free media and introduced to triplicate wells. After 24 h incubation at 37°C, 5% CO2, cytotoxicity was evaluated with an MTT assay kit (Abcam, USA), and calculated as %cytotoxicity = 100×[(absorbance of control)−(absorbance of treatments)]/(control absorbance).
In vivo experiments
Male BALB/c mice aged 6–8 weeks with an average weight of 25 g were kept in pathogen-free conditions with water and food ad libitum in the Animal Experimentation Vivarium of the Departments of Parasitology and Microbiology (ICB/USP). All experimental protocols were previously approved by the Ethics Committee for Animal Use of the Institute of Biomedical Sciences (CEUA-ICB/USP, Reg 68/2014).
Antifungal activity and toxicity of alginate nanoparticles in a murine model of systemic cryptococcosis
C. neoformans H99 yeast was cultivated twice in Sabouraud dextrose broth for 72 h at 35°C. A yeast suspension was adjusted to 1 × 107 cfu/mL of PBS for inoculation of 100 μL into the tail vein of the mice. After 1 h of infection, the animals (five per group) were orally treated by gavage with 100 µL of three treatments: (i) 10 mg/kg miltefosine, in water, once a day for 5 days (miltefosine group); (ii) 20 mg/kg miltefosine-loaded alginate nanoparticles, in PBS, after 1 and 72 h of infection (MFS-AN group); and (iii) 20 mg/kg miltefosine-loaded P80-functionalized alginate nanoparticles, in PBS, after 1 and 72 h of infection (P80-MFS-AN group).
On the sixth day after infection, the animals were euthanized with a lethal dose of anaesthetics (xylazine 30 mg/kg and ketamine 900 mg/kg). The organs (lung and brain) were excised, weighed, macerated in PBS, and plated on Sabouraud dextrose agar with 50 mg/L chloramphenicol and incubated at 35°C for 72 h for cfu count. During organ removal, a fraction of each tissue was separated, fixed in 10% formalin and processed for histopathological analysis using Gomori–Grocott staining.28
Additionally, after euthanasia the blood was collected and the serum obtained was used for measurement of glucose, triglycerides, cholesterol, creatinine, urea, alkaline phosphatase (ALP), AST and ALT. In addition, a non-infected and non-treated group (NINT), i.e. mice that did not suffer any procedure (n = 5), was included in this assay. The analyses were carried out by the wet biochemistry method (Equipment Urit 8210, URIT Medical, China).
Biodistribution of alginate nanoparticles
Production of fluorescent nanoparticles
Alginate nanoparticles were produced according to the protocols described above, except that 5 mg of rhodamine B (Rod) (Sigma–Aldrich, USA) was added to the alginate dispersion to obtain the fluorescent nanoparticles, modified or not with polysorbate 80 (P80-AN-Rod and AN-Rod, respectively).
Biodistribution assay
One hundred microlitres of P80-AN-Rod or AN-Rod, dispersed in PBS, were orally administered by gavage. Animals that received PBS-Rod solution (Rod group) or only PBS (PBS group) were included as control groups. After 12 and 24 h, the animals were euthanized with a lethal dose of anaesthetics. The organs were removed for evaluation of fluorescence in a bioimaging system (IVIS Spectrum System, Perkin-Elmer Life Sciences, USA) using an exposure time of 1 s and excitation/emission wavelengths of 535/580 nm. After that, the organs were macerated with 1 mL of PBS and 100 µL added to the wells of the 96-well polystyrene flat-bottom plate for fluorescence quantification in the plate reader (Synergy/H1, BioTek, USA) at excitation/emission wavelengths of 535/580 nm, resulting in fluorescence measured in relative fluorescence units (RFU).
Statistical analyses
The statistical analyses were performed using the GraphPad Prism version 8.0 (GraphPad Software, USA) and a P value less than 0.05 was considered significant.
Results
P80-functionalization does not alter the average size and polydispersity of alginate nanoparticles and increases stability
Both nanoparticles P80-MFS-AN and MFS-AN had similar Dz (∼300 nm) and Pdi (∼0.4), and the P80 functionalization increased the absolute value of zeta potential. In addition, the freeze-drying step did not interfere with these parameters (Table S1, available as Supplementary data at JAC Online).
The size and Pdi values of P80-MFS-AN nanoparticles did not change during 60 days of storage, but after 75 and 90 days, the size increased 1.2-fold (P < 0.05) although no changes to the dispersion were noticed (Figure S1). Compared with P80-functionalized nanoparticles, the MFS-AN displayed more pronounced increases in size and Pdi at shorter periods of time. More specifically, 1.8- and 1.92-fold increases in size and Pdi, respectively, at 60 days, and 1.92- and 2.28-fold in size and Pdi, at 90 days, respectively, were observed (P < 0.001) (Figure S1).
P80-alginate nanoparticles encapsulate miltefosine and promote its sustained in vitro release
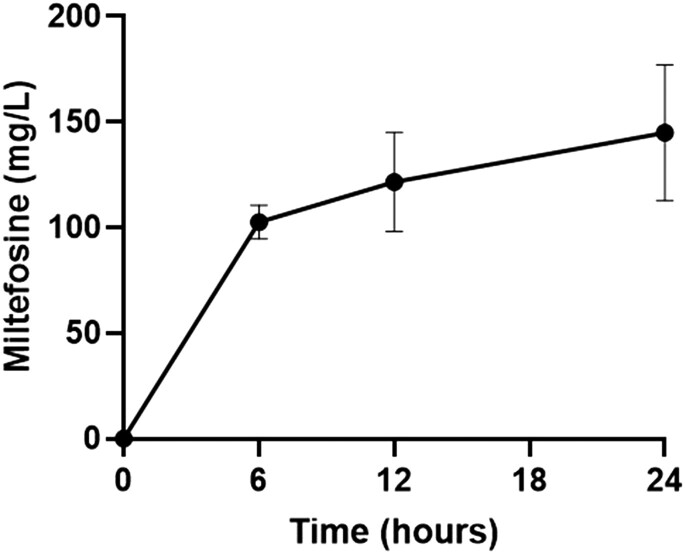
The encapsulation efficiency of miltefosine in P80-functionalized alginate nanoparticles was 73.20% ± 9.89%. P80-MFS-AN nanoparticles promoted a sustained release of miltefosine with an increase in concentration over the first 6 h, obtaining a peak release of approximately 102.65 mg/L in 6 h. After this period, the drug release was slow and prolonged with a peak value (∼144.87 mg/L) at 24 h (Figure 1).
Figure 1.
In vitro miltefosine release from polysorbate 80-functionalized alginate nanoparticles. The miltefosine quantification, in the supernatant, was performed by the colorimetric method with ammonium ferrothiocyanate.30 The experiment was performed three times in triplicate.
Alginate nanoparticles reduce miltefosine haemolytic activity and cytotoxicity on endothelial cells
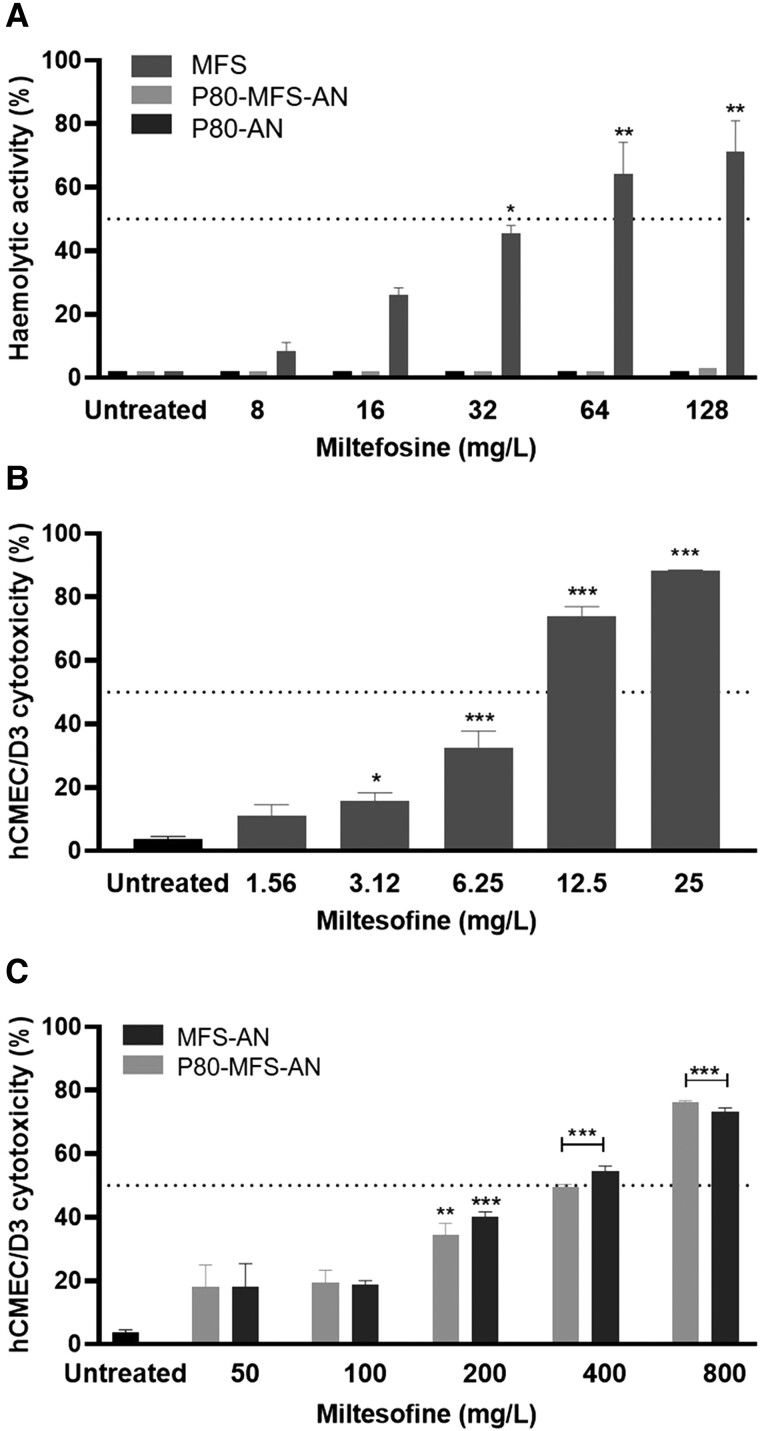
The haemolytic effect of miltefosine was concentration dependent (Figure 2a) and its HA50 value was ∼43.6 mg/L. Unloaded and miltefosine-loaded nanoparticles (P80-MFS-AN) did not cause any haemolytic effect, even at the highest tested miltefosine concentration (128 mg/L), which is consistent with previously obtained results for MFS-AN.29
Figure 2.
Cytotoxicity of miltefosine (MFS) in its free form or loaded on alginate nanoparticles. (a) Haemolytic activity of MFS and MFS-loaded alginate nanoparticles, functionalized or not with polysorbate 80 (P80-MFS-AN and MFS-AN, respectively). (b) Cytotoxicity of MFS and (c) P80-MFS-AN and MFS-AN on hCMEC/D3 endothelial cells. *P < 0.05, **P < 0.01 and ***P < 0.001 versus untreated group (one-way ANOVA with Dunnett’s post-test). The assays were performed three times in triplicate.
Increases in miltefosine concentration also increased its cytotoxic effects in an in vitro BBB model with hCMEC/D3 endothelial cells (Figure 2b and c). Compared with miltefosine, which presented a CC50 of 8.67 mg/L, drug encapsulation in nanoparticles drastically reduced its cytotoxic effect and increased the CC50 by 38.4- and 45.12-fold for MFS-AN and P80-MFS-AN, respectively. In addition, concentrations less than or equal to 3.12 mg/L miltefosine and 100 mg/L miltefosine-loaded nanoparticles (P80-MFS-AN and MFS-AN) resulted in low and insignificant cell damage (P > 0.05, Figure 2b and c).
In vitro antifungal activity using a BBB model
The MIC values of miltefosine, in its free form and loaded in nanoparticles (P80-MFS-AN) were 2 and 100 mg/L for C. neoformans. Unloaded P80-AN nanoparticles were evaluated as a control and no inhibitory effect was observed.
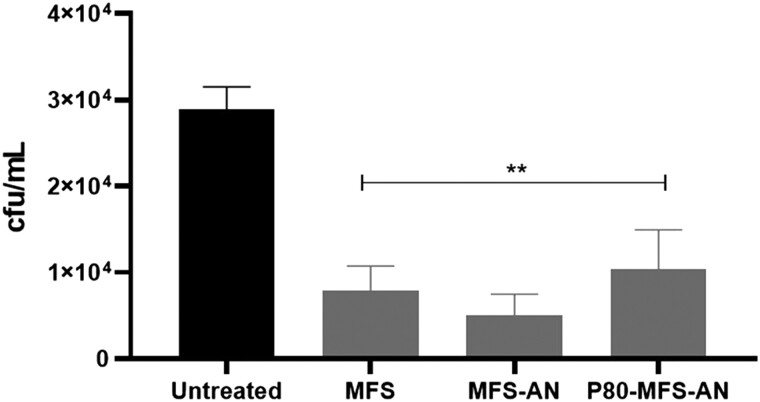
After cytotoxicity assays and MIC determination, the antifungal activity of 2 mg/L miltefosine and 100 mg/L miltefosine-loaded nanoparticles (MFS-AN and P80-MFS-AN) was evaluated in the in vitro BBB model infected with C. neoformans. After 12 h, all treatments were significantly effective in reducing fungal viability compared with the untreated group (P < 0.001, Figure 3).
Figure 3.
Antifungal activity of miltefosine (MFS), in its free form or loaded on alginate nanoparticles, during C. neoformans infection across the BBB transwell model (hCMEC/D3 cells). Fungal burden (cfu/mL) was recovered from the basal compartment of the transwell after 12 h of infection and treatment with free MFS (2 mg/L) and MFS-loaded alginate nanoparticles, functionalized or not with polysorbate 80 (P80-MFS-AN and MFS-AN, respectively) (100 mg/L MFS). **P < 0.001 versus untreated group (one-way ANOVA with Dunnett’s post-test). The experiment was performed three times in triplicate.
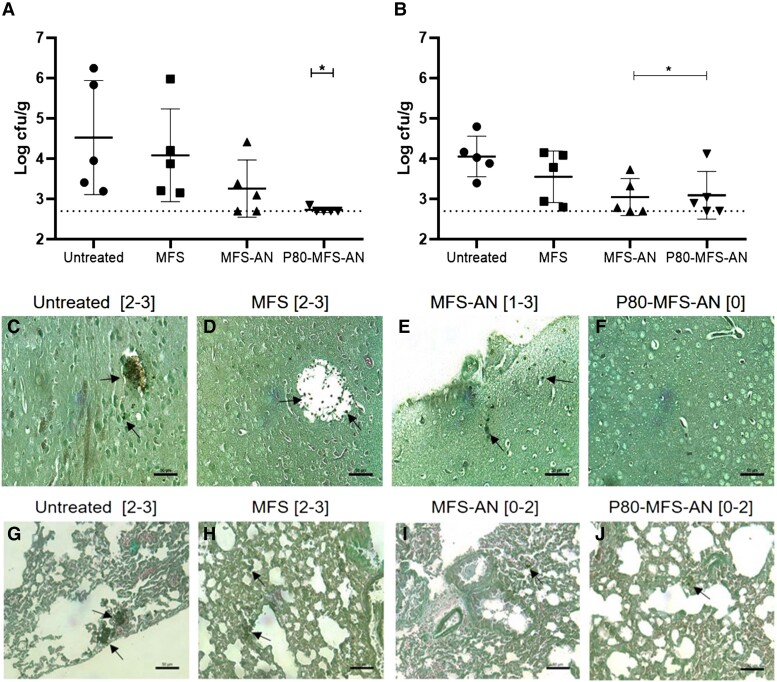
P80-functionalized alginate nanoparticles decrease fungal burden in the brain and lung in a murine model of systemic cryptococcosis
The oral treatment with P80-MFS-AN nanoparticles significantly decreased the fungal burden in the brain (∼1.8 log, P < 0.05) and lung (∼1 log, P < 0.05) when compared with the untreated group (Figure 4a and b). MFS-AN nanoparticles significantly decreased the fungal burden in the lungs (∼1 log, P < 0.05), but failed to significantly reduce it in the brain when compared with the untreated group (Figure 4a and b). On the other hand, the oral miltefosine treatment was the least effective, reducing only ∼0.5 log of fungal burden in the lung and brain of animals (Figure 4a and b). These cfu results corroborated the semi-quantitative data obtained from histopathological analysis, which demonstrated that P80-MFS-AN treatment led to a lower amount of yeast in the brain and lung of the animals, highlighting it as the best antifungal therapy in the murine model of systemic cryptococcosis (Figure 4c–j). In addition, no significant difference was observed in hepatic (AST, ALT and ALP) and renal (creatinine and urea) toxicity biomarkers as well as glucose, triglycerides and cholesterol levels when compared with the NINT group (Table 1).
Figure 4.
Antifungal effect of miltefosine (MFS), in its free form or loaded on alginate nanoparticles, in the murine model of systemic cryptococcosis. Fungal burden in the brain (a) and lungs (b) of male mice infected with C. neoformans H99 untreated and treated with MFS or MFS-loaded alginate nanoparticles (MFS-AN and P80-MFS-AN). *P < 0.05 versus untreated group (one-way ANOVA with Dunnett’s post-test). Gomori–Grocott-stained histopathological sections of brain (c–f) and lungs (g–j) of the animals untreated and treated with MFS, MFS-AN or P80-MFS-AN. Results of semi-quantitative fungal load analysis from brain and lung tissue sections are indicated in brackets according to the scale: 0, no fungal load; 1, up to 5 fungal elements per section; 2, ≥6 fungal elements per section; 3, from 6 to 50 fungal elements per field; and 4, more than 50 fungal elements per field.28 Bars = 50 µm. Arrows indicate the fungal cells. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Table 1.
Serum biomarkers of mice infected by C. neoformans and treated with miltefosine (MFS) or MFS-loaded alginate nanoparticles functionalized or not with polysorbate 80 (P80-MFS-AN and MFS-AN, respectively)
| Biomarkers | NINT | Untreated | MFS | MFS-AN | P80-MFS-AN |
|---|---|---|---|---|---|
| Urea (mg/dL) | 56.76 ± 7.77 | 49.84 ± 2.70 | 64.32 ± 18.56 | 49.76 ± 9.35 | 52.70 ± 9.66 |
| Creatinine (mg/dL) | 0.69 ± 0.08 | 0.68 ± 0.15 | 0.80 ± 0.06 | 0.78 ± 0.12 | 0.67 ± 0.09 |
| AST (U/L) | 268 ± 102.9 | 263.2 ± 249.7 | 265.8 ± 222.4 | 329.2 ± 283.8 | 197.2 ± 128.5 |
| ALT (U/L) | 49.50 ± 6.6 | 44.40 ± 18.62 | 52 ± 25.14 | 62.20 ± 23.44 | 43.20 ± 17.92 |
| ALP (U/L) | 518.4 ± 55.61 | 390.8 ± 83.79 | 361.6 ± 48.63 | 424.8 ± 114 | 375.2 ± 73.02 |
| Glucose (mg/dL) | 367.2 ± 94.28 | 258.8 ± 79.26 | 209.8 ± 52.96 | 271 ± 37.87 | 293.8 ± 32.15 |
| Triglycerides (mg/dL) | 211.4 ± 52.86 | 176 ± 19.52 | 208.3 ± 60.42 | 186.7 ± 44.83 | 194.7 ± 87.50 |
| Cholesterol (mg/dL) | 109.7 ± 8.57 | 106.6 ± 11.56 | 138.7 ± 47.13 | 121.6 ± 13.59 | 119.3 ± 6.43 |
All values are given as mean ± SD.
P80-functionalization increases the biodistribution of alginate nanoparticles and enhances their presence in the brain, suggesting improved translocation across the BBB
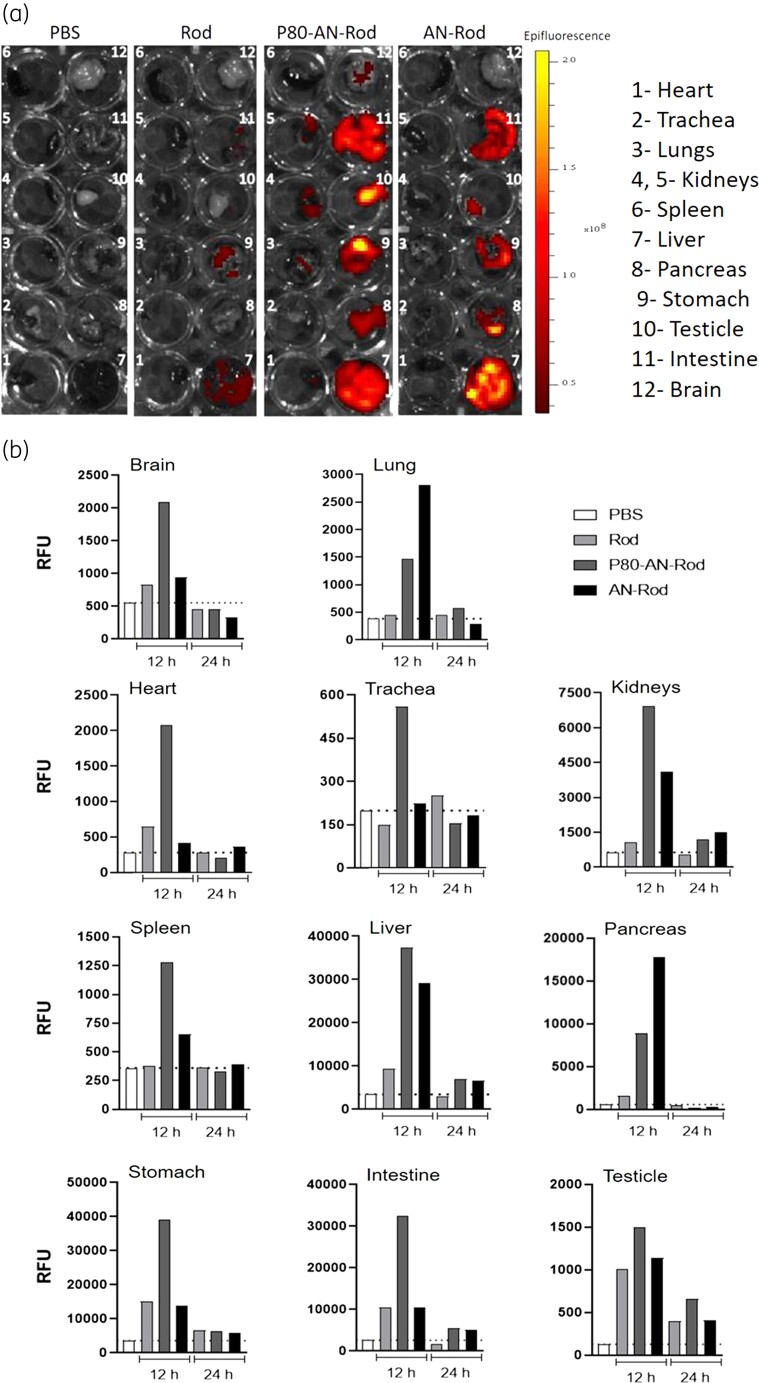
To evaluate the biodistribution of alginate nanoparticles, P80 functionalized or not, we encapsulated a fluorescent marker rhodamine. As expected, little or no fluorescence was detected in the organs of animals that received only PBS. While rhodamine, per se, was absorbed and distributed to some organs, the fluorescence intensity was much lower than for animals that received alginate nanoparticles (Figure 5).
Figure 5.
Biodistribution of alginate nanoparticles, functionalized or not with polysorbate 80. (a) Fluorescent images obtained in a bioimaging system of the organs of mice treated with rhodamine-loaded alginate nanoparticles without (AN-Rod) and with polysorbate 80 (P80-AN-Rod) at 12 h. (b) RFU of organs from mice treated with AN-Rod or P80-AN-Rod obtained at excitation/emission wavelengths of 535/580 nm at 12 and 24 h. Rhodamine B (Rod) and PBS were used as controls. These data are representative of an experiment carried out twice. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
The qualitative analysis of the organs’ fluorescence was performed in a bioimaging system at 12 h after oral administration of nanoparticles (Figure 5a). P80 functionalization of alginate nanoparticles (P80-AN-Rod) had a larger biodistribution, observed in almost all analysed organs (heart, lungs, kidneys, liver, pancreas, stomach, testicle, intestine and brain) (Figure 5a). On the other hand, alginate nanoparticles (AN-Rod) were detected in the liver, pancreas, stomach, testicle and intestine (Figure 5a). At 24 h, the organs’ fluorescence reduced to control PBS and rhodamine levels.
Importantly, the fluorescence results were confirmed in a plate reader as the method is more sensitive for quantification of fluorescence (Figure 5b). Indeed, P80-functionalized alginate nanoparticles were detected in all organs, with higher amounts in the brain, while non-functionalized alginate nanoparticles were found in the kidneys, spleen and liver; in the lungs and pancreas, they were found in greater amounts than P80 functionalized nanoparticles (Figure 5b).
Discussion
The use of nanoparticle-mediated drug delivery systems functionalized with ligands targeting the CNS for the treatment of brain diseases has been increasing.26 For fungal brain diseases, few antifungals have suitable CNS penetration with adequate concentrations.9 Here, we chose P80, due to its low cost, commercial availability and ease of handling.32 We produced alginate nanoparticles that were P80 functionalized as miltefosine carriers (P80-MFS-AN) that improved antifungal activity in CM treatment when compared with unfunctionalized alginate nanoparticles (MFS-AN).
The physicochemical characteristics of the nanoparticles are important parameters that have an impact on the systemic activities of the nanocarriers,33 and the P80-MFS-AN nanoparticles had a Dz and Pdi similar to those previously observed for MFS-AN.29 The P80-MFS-AN showed the absolute value of zeta potential increased to approximately −50 mV. This is an important feature since zeta potential higher than 30 mV (in module) has been associated with greater electrical stability and lower particle aggregation.34,35 The higher zeta potential of P80-MFS-AN compared with MFS-AN may have contributed to its enhanced stability.
The toxicity of miltefosine has been described in previous reports;24 the haemolytic effect and cytotoxicity on hCMEC/D3 endothelial cells at low concentrations corroborate previous observations. In contrast, the use of alginate nanoparticles as miltefosine carriers reduced its cytotoxicity considerably, abolishing the haemolytic effect and increasing the CC50 values in hCMEC/D3 cells 38–45 times. Drug delivery systems usually reduce the toxic effect of drugs, as observed in this work and by other authors who incorporated miltefosine in delivery systems.36–38
The in vitro antifungal activity of P80-MFS-AN was similar to that observed for MFS-AN with high values compared with miltefosine.29 This is expected in in vitro assays and can be explained by the slow and sustained release of the drug, also observed in other studies that tested antifungal nanocarriers.39,40 Additionally, in an in vitro BBB model, the miltefosine and miltefosine-loaded nanoparticles were effective in decreasing C. neoformans’ viability during infection. The hCMEC/D3 immortalized brain endothelial cell line is an alternative to in vivo studies of the BBB, which has already been used for research of C. neoformans invasion in the CNS.41 In this model, it was possible to observe that miltefosine and miltefosine-loaded alginate nanoparticles decreased the passage of fungus through the BBB, a very important step in the disease and that, for reasons not yet well known, this fungus has a special tropism for the brain and can invade the BBB by different and concomitant mechanisms.5
The use of nanoparticles for drug delivery to the brain is a promising alternative due to the possibility of surface multifunctionalization that can promote the targeting or/and crossing enhancement to the BBB.42 Among functionalization options is the use of surfactants, with polysorbates being the most efficient for targeting to the brain when compared with poloxamers, poloxamine 908, Cremophor® EZ, Cremophor® RH 40, polyoxyethylene-(23)-lauryl ether (Brij® 35).43 Consistent with previous reports, MFS-AN nanoparticles, which were prepared with poloxamer 407,29 did not reach the brain in adequate amounts to control the cerebral infection. In contrast, P80-MFS-AN nanoparticles, which were functionalized with P80, showed greater biodistribution and crossed the BBB, reaching the brain in higher amounts and resulting in a significant reduction in fungal burden to undetectable levels.
Our findings corroborated other studies that used P80 for functionalization of amphotericin B-loaded polymeric nanoparticles, enabling the nanoparticles to cross the BBB and increase drug concentrations in brains of mice,44 whereas free amphotericin B was not detected. As a result, treatment of cryptococcosis with these nanoparticles decreased the fungal burden and increased the survival rate of animals to 80%.45 In addition, a nanoemulsion with 3% surfactant blend containing Tween® 80 and Soluplus® and incorporating flubendazole (an anthelmintic reported to have antifungal activity) was effective when orally administered in murine systemic cryptococcosis, decreasing the fungal burden in the brain by 30%.46
Several mechanisms have been proposed to explain the efficiency of nanoparticles to pass through the BBB.47,48 For surfactant-functionalized nanoparticles, e.g. polysorbate 80, the previously proposed mechanism is based on the solubilization of lipids in the endothelial cell membrane that would lead to fluidization and destabilization of the membrane and greater permeability of the nanoparticles through the BBB.47,48 Additionally, this general effect of surfactants might contribute to the wider biodistribution of P80-functionalized alginate nanoparticles in mice compared with the non-functionalized nanoparticles. This finding might open up new possibilities for therapeutic applications of P80-MFS-AN nanoparticles, such as for the treatment of leishmaniasis and other systemic fungal infections. On the other hand, because wider distribution might lead to more side effects, we also addressed toxicological concerns by assessing markers of hepatic and renal damage. Biochemical analysis of serum after treatment with miltefosine and miltefosine-loaded alginate nanoparticles indicated that hepatic and renal markers, as well as markers of carbohydrate and lipid metabolism were not altered after treatments. These data support the idea that nanoparticles, in the therapeutic scheme used here, are safe to treat CM.
It is important to highlight that oral administration of P80-MFS-AN nanoparticles (two doses, 1 and 72 h after infection) were the best therapy to reduce fungal burden in the brain and lungs while the oral free miltefosine (five doses, 24/24 h) was ineffective to control the infection in systemic cryptococcosis as well as observed previously in CM and disseminated cryptococcosis models treated orally with miltefosine.22 Our data differ from results reported by Widmer and co-workers,21 although the murine model used here was similar, except the mice gender (they used female mice). It was reported that steroid hormones affect C. neoformans virulence,49 and oestradiol, a female hormone, is associated with a protective factor against Cryptococcus infection.50
Notably, the alginate nanoparticles, especially P80-functionalized ones, improved the in vivo antifungal effect of miltefosine when compared with miltefosine in solution. Moreover, the increase in dose interval used here resulted from the nanoparticle ability to modify and slow down the release of miltefosine. This is consistent with the drug release profile observed from non-functionalized nanoparticles (MFS-AN)29 and other alginate-based carriers.51,52 This feature visibly was relevant at decreasing Candida albicans infection, and a single dose of MFS-AN was sufficient to treat vaginal candidiasis in a murine model.28
In conclusion, P80-MFS-AN represents a safe oral delivery system to improve the brain delivery of miltefosine in CM treatment. In addition, the nanoparticles promoted sustained release of miltefosine, were more widely biodistributed, including in the brain, and decreased brain fungal burden without producing detectable damage to other organs. This study demonstrates that it is possible to obtain new management strategies for treatment of CM. Finally, our results open many other questions regarding the efficacy of combined treatment of P80-MFS-AN with standard antifungals (amphotericin B, flucytosine and/or fluconazole) and pharmacokinetic/pharmacodynamic data. Further studies must be conducted to bring promising results to improve CNS treatments and support the clinical trials.
Supplementary Material
Acknowledgements
We would like to thank Marcela Gonçalves for her assistance in preparatioin of mice samples for histopathological analysis. IVIS analysis was conducted at the CEFAP core facility (Institute of Biomedical Sciences, University of São Paulo).
Contributor Information
Cristina C Spadari, Institute of Biomedical Sciences, University of São Paulo, São Paulo, SP, Brazil.
Dylan M Lanser, Department of Pharmacology, School of Medicine, University of California, Davis, CA, USA.
Marcelo V Araújo, Institute of Biomedical Sciences, University of São Paulo, São Paulo, SP, Brazil.
Daniel F F De Jesus, Institute of Biomedical Sciences, University of São Paulo, São Paulo, SP, Brazil.
Luciana B Lopes, Institute of Biomedical Sciences, University of São Paulo, São Paulo, SP, Brazil.
Angie Gelli, Department of Pharmacology, School of Medicine, University of California, Davis, CA, USA.
Kelly Ishida, Institute of Biomedical Sciences, University of São Paulo, São Paulo, SP, Brazil.
Funding
This study was supported by Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP, Brazil, 2021/01279-5 and 2018/13877-1), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) and National Institute of Health (NINDS, USA, NS110800). C.C.S. was FAPESP fellow (2018/12149-2) and D.F.F.J. was CAPES fellow (finance code 001). K.I. and L.B.L. are research fellows of the CNPQ (303373/2019-9 and 306866/2020-0, respectively).
Transparency declarations
None to declare.
Author contributions
C.C.S. performed the experiments, analysed the results, and wrote the manuscript. M.V.A. and D.F.F.J. contributed with murine model assays. D.M.L. did the blood–brain barrier experiments. K.I., A.G. and L.B.L. designed the experiments, performed data critical review, and wrote and edited the manuscript. All authors have read and approved the manuscript before publication.
Data availability
All data will be made available from the corresponding author upon request.
Supplementary data
Figure S1 and Table S1 are available as Supplementary data at JAC Online.
References
- 1. Rajasingham R, Smith RM, Park BJet al. . Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17: 873–81. 10.1016/S1473-3099(17)30243-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stott KE, Loyse A, Jarvis JNet al. . Cryptococcal meningoencephalitis: time for action. Lancet Infect Dis 2021; 21: e259–71. 10.1016/S1473-3099(20)30771-4 [DOI] [PubMed] [Google Scholar]
- 3. Lin X, Heitman J. The biology of the Cryptococcus neoformans species complex. Annu Rev Microbiol 2006; 60: 69–105. 10.1146/annurev.micro.60.080805.142102 [DOI] [PubMed] [Google Scholar]
- 4. O’Meara TR, Alspaugh JA. The Cryptococcus neoformans capsule: a sword and a shield. Clin Microbiol Rev 2012; 25: 387–408. 10.1128/CMR.00001-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zaragoza O. Basic principles of the virulence of Cryptococcus. Virulence 2019; 10: 490–501. 10.1080/21505594.2019.1614383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. May RC, Stone NRH, Wiesner DLet al. . Cryptococcus: from environmental saprophyte to global pathogen. Nat Rev Microbiol 2016; 14: 106–17. 10.1038/nrmicro.2015.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perfect JR, Dismukes WE, Dromer Fet al. . Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2010; 50: 291–322. 10.1086/649858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Campoy S, Adrio JL. Antifungals. Biochem Pharmacol 2017; 133: 86–96. 10.1016/j.bcp.2016.11.019 [DOI] [PubMed] [Google Scholar]
- 9. Wirth F, Ishida K. Antifungal drugs: an updated review of central nervous system pharmacokinetics. Mycoses 2020; 63: 1047–59. 10.1111/myc.13157 [DOI] [PubMed] [Google Scholar]
- 10. Sunyoto T, Potet J, Boelaert M. Why miltefosine—a life-saving drug for leishmaniasis—is unavailable to people who need it the most. BMJ Glob Heal 2018; 3: e000709. 10.1136/bmjgh-2018-000709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barreto TL, Rossato L, de Freitas ALDet al. . Miltefosine as an alternative strategy in the treatment of the emerging fungus Candida auris. Int J Antimicrob Agents 2020; 56: 106049. 10.1016/j.ijantimicag.2020.106049 [DOI] [PubMed] [Google Scholar]
- 12. Barreto TL, Lopes LB, Melo ASAet al. . In vivo synergism of free miltefosine or in alginate-based nanocarrier combined with voriconazole on aspergillosis. Future Microbiol 2021; 16: 1153–60. 10.2217/fmb-2021-0056 [DOI] [PubMed] [Google Scholar]
- 13. Borba-Santos LP, Gagini T, Ishida Ket al. . Miltefosine is active against Sporothrix brasiliensis isolates with in vitro low susceptibility to amphotericin B or itraconazole. J Med Microbiol 2015; 64: 415–22. 10.1099/jmm.0.000041 [DOI] [PubMed] [Google Scholar]
- 14. Brilhante RSN, Malaquias ADM, Caetano ÉPet al. . In vitro inhibitory effect of miltefosine against strains of Histoplasma capsulatum var. capsulatum and Sporothrix spp. Med Mycol 2014; 52: 320–5. 10.1093/mmy/myt027 [DOI] [PubMed] [Google Scholar]
- 15. Compain F, Botterel F, Sitterlé Eet al. . In vitro activity of miltefosine in combination with voriconazole or amphotericin B against clinical isolates of Scedosporium spp. J Med Microbiol 2015; 64: 309–11. 10.1099/jmm.0.000019 [DOI] [PubMed] [Google Scholar]
- 16. Imbert S, Palous M, Meyer Iet al. . In vitro combination of voriconazole and miltefosine against clinically relevant molds. Antimicrob Agents Chemother 2014; 58: 6996–8. 10.1128/AAC.03212-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Loreto ES, Tondolo JSM, Oliveira DCet al. . In vitro activities of miltefosine and antibacterial agents from the macrolide, oxazolidinone, and pleuromutilin classes against Pythium insidiosum and Pythium aphanidermatum. Antimicrob Agents Chemother 2018; 62: e01678-17. 10.1128/AAC.01678-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rossi DCP, Spadari CC, Nosanchuk JDet al. . Miltefosine is fungicidal to Paracoccidioides spp. yeast cells but subinhibitory concentrations induce melanisation. Int J Antimicrob Agents 2017; 49: 465–71. 10.1016/j.ijantimicag.2016.12.020 [DOI] [PubMed] [Google Scholar]
- 19. Spadari CC, Vila T, Rozental Set al. . Miltefosine has a postantifungal effect and induces apoptosis in Cryptococcus yeasts. Antimicrob Agents Chemother 2018; 62: e00312-18. 10.1128/AAC.00312-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tong Z, Widmer F, Sorrell TCet al. . In vitro activities of miltefosine and two novel antifungal biscationic salts against a panel of 77 dermatophytes. Antimicrob Agents Chemother 2007; 51: 2219–22. 10.1128/AAC.01382-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Widmer F, Wright LC, Obando Det al. . Hexadecylphosphocholine (miltefosine) has broad-spectrum fungicidal activity and is efficacious in a mouse model of cryptococcosis. Antimicrob Agents Chemother 2006; 50: 414–21. 10.1128/AAC.50.2.414-421.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wiederhold NP, Najvar LK, Bocanegra Ret al. . Limited activity of miltefosine in murine models of cryptococcal meningoencephalitis and disseminated cryptococcosis. Antimicrob Agents Chemother 2013; 57: 745–50. 10.1128/AAC.01624-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Profounda, Inc . Profounda blog.https://www.profounda.com/blog.
- 24. Dorlo TPC, Balasegaram M, Beijnen JHet al. . Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J Antimicrob Chemother 2012; 67: 2576–97. 10.1093/jac/dks275 [DOI] [PubMed] [Google Scholar]
- 25. Roy SL, Atkins JT, Gennuso Ret al. . Assessment of blood–brain barrier penetration of miltefosine used to treat a fatal case of granulomatous amebic encephalitis possibly caused by an unusual Balamuthia mandrillaris strain. Parasitol Res 2015; 114: 4431–9. 10.1007/s00436-015-4684-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ahlawat J, Guillama Barroso G, Masoudi Asil Set al. . Nanocarriers as potential drug delivery candidates for overcoming the blood–brain barrier: challenges and possibilities. ACS Omega 2020; 5: 12583–95. 10.1021/acsomega.0c01592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sun W, Xie C, Wang Het al. . Specific role of polysorbate 80 coating on the targeting of nanoparticles to the brain. Biomaterials 2004; 25: 3065–71. 10.1016/j.biomaterials.2003.09.087 [DOI] [PubMed] [Google Scholar]
- 28. de Bastiani FWMDS, Spadari CC, de Matos JKRet al. . Nanocarriers provide sustained antifungal activity for amphotericin B and miltefosine in the topical treatment of murine vaginal candidiasis. Front Microbiol 2020; 10: 2976. 10.3389/fmicb.2019.02976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Spadari CC, de Bastiani FWMDS, Lopes LBet al. . Alginate nanoparticles as non-toxic delivery system for miltefosine in the treatment of candidiasis and cryptococcosis. Int J Nanomedicine 2019; 14: 5187–99. 10.2147/IJN.S205350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dorlo TPC, Eggelte TA, de Vries PJet al. . Characterization and identification of suspected counterfeit miltefosine capsules. Analyst 2012; 137: 1265. 10.1039/c2an15641e [DOI] [PubMed] [Google Scholar]
- 31. CLSI . Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts—Fourth Edition: M27. 2017. [Google Scholar]
- 32. Ravichandran V, Lee M, Nguyen Cao TGet al. . Polysorbate-based drug formulations for brain-targeted drug delivery and anticancer therapy. Appl Sci 2021; 11: 9336. 10.3390/app11199336 [DOI] [Google Scholar]
- 33. Raval N, Maheshwari R, Kalyane Det al. . Importance of physicochemical characterization of nanoparticles in pharmaceutical product development. In: Basic Fundamentals of Drug Delivery. Elsevier, 2019; 369–400. [Google Scholar]
- 34. Sundar S, Kundu J, Kundu SC. Biopolymeric nanoparticles. Sci Technol Adv Mater 2010; 11: 014104. 10.1088/1468-6996/11/1/014104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Honary S, Zahir F. Effect of zeta potential on the properties of nano-drug delivery systems—a review (Part 1). Trop J Pharm Res 2013; 12: 265–73. 10.4314/tjpr.v12i2.19 [DOI] [Google Scholar]
- 36. da Gama Bitencourt JJ, Pazin WM, Ito ASet al. . Miltefosine-loaded lipid nanoparticles: improving miltefosine stability and reducing its hemolytic potential toward erythtocytes and its cytotoxic effect on macrophages. Biophys Chem 2016; 217: 20–31. 10.1016/j.bpc.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 37. Valenzuela-Oses JK, García MC, Feitosa VAet al. . Development and characterization of miltefosine-loaded polymeric micelles for cancer treatment. Mater Sci Eng C 2017; 81: 327–33. 10.1016/j.msec.2017.07.040 [DOI] [PubMed] [Google Scholar]
- 38. Eissa MM, El-Moslemany RM, Ramadan AAet al. . Miltefosine lipid nanocapsules for single dose oral treatment of Schistosomiasis mansoni: a preclinical study. PLoS One 2015; 10: e0141788. 10.1371/journal.pone.0141788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tan TRM, Hoi KM, Zhang Pet al. . Characterization of a polyethylene glycol-amphotericin B conjugate loaded with free AMB for improved antifungal efficacy. PLoS One 2016; 11: e0152112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saldanha CA, Garcia MP, Iocca DCet al. . Antifungal activity of amphotericin B conjugated to nanosized magnetite in the treatment of paracoccidioidomycosis. PLoS Negl Trop Dis 2016; 10: e0004754. 10.1371/journal.pntd.0004754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vu K, Weksler B, Romero Iet al. . Immortalized human brain endothelial cell line HCMEC/D3 as a model of the blood-brain barrier facilitates in vitro studies of central nervous system infection by Cryptococcus neoformans. Eukaryot Cell 2009; 8: 1803–7. 10.1128/EC.00240-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nair KGS, Ramaiyan V, Sukumaran SK. Enhancement of drug permeability across blood brain barrier using nanoparticles in meningitis. Inflammopharmacology 2018; 26: 675–84. 10.1007/s10787-018-0468-y [DOI] [PubMed] [Google Scholar]
- 43. Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev 2012; 64: 213–22. 10.1016/j.addr.2012.09.015 [DOI] [PubMed] [Google Scholar]
- 44. Ren T, Xu N, Cao Cet al. . Preparation and therapeutic efficacy of polysorbate-80-coated amphotericin B/PLA-b-PEG nanoparticles. J Biomater Sci Polym Ed 2009; 20: 1369–80. 10.1163/092050609X12457418779185 [DOI] [PubMed] [Google Scholar]
- 45. Xu N, Gu J, Zhu Yet al. . Efficacy of intravenous amphotericin B-polybutylcyanoacrylate nanoparticles against cryptococcal meningitis in mice. Int J Nanomedicine 2011; 6: 905–13. 10.2147/IJN.S17503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yukuyama MN, Ishida K, de Araujo GLBet al. . Rational design of oral flubendazole-loaded nanoemulsion for brain delivery in cryptococcosis. Colloids Surfaces A Physicochem Eng Asp 2021; 630: 127631. 10.1016/j.colsurfa.2021.127631 [DOI] [Google Scholar]
- 47. Chacko BJ, Palanisamy S, Gowrishankar NLet al. . Effect of surfactant coating on brain targeting polymeric nanoparticles; a review. Indian J Pharm Sci 2018; 80: 215–22. 10.4172/pharmaceutical-sciences.1000348 [DOI] [Google Scholar]
- 48. Spadari CC, Wirth F, Lopes LBet al. . New approaches for cryptococcosis treatment. Microorganisms 2020; 8: 613. 10.3390/microorganisms8040613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McClelland EE, Hobbs LM, Rivera Jet al. . The role of host gender in the pathogenesis of Cryptococcus neoformans infections. PLoS One 2013; 8: e63632. 10.1371/journal.pone.0063632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Costa MC, Barros Fernandes H, Gonçalves GKNet al. . 17-β-Estradiol increases macrophage activity through activation of the G-protein-coupled estrogen receptor and improves the response of female mice to Cryptococcus gattii. Cell Microbiol 2020; 22: e13179. 10.1111/cmi.13179 [DOI] [PubMed] [Google Scholar]
- 51. Ahmad Z, Sharma S, Khuller GK. Chemotherapeutic evaluation of alginate nanoparticle-encapsulated azole antifungal and antitubercular drugs against murine tuberculosis. Biol Med 2007; 3: 239–43. 10.1016/j.nano.2007.05.001 [DOI] [PubMed] [Google Scholar]
- 52. Martín-Villena MJ, Fernández-Campos F, Calpena-Campmany ACet al. . Novel microparticulate systems for the vaginal delivery of nystatin: development and characterization. Carbohydr Polym 2013; 94: 1–11. 10.1016/j.carbpol.2013.01.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data will be made available from the corresponding author upon request.