Abstract
Influenza-associated pulmonary aspergillosis (IAPA) is a feared complication in patients with influenza tracheobronchitis, especially those receiving corticosteroids. Herein, we established a novel IAPA mouse model with low-inoculum Aspergillus infection and compared outcomes in mice with and without cortisone acetate (CA) immunosuppression. CA was an independent predictor of increased morbidity/mortality in mice with IAPA. Early antifungal treatment with liposomal amphotericin B was pivotal to improve IAPA outcomes in CA-immunosuppressed mice, even after prior antiviral therapy with oseltamivir. In summary, our model recapitulates key clinical features of IAPA and provides a robust preclinical platform to study the pathogenesis and treatment of IAPA.
Keywords: pneumonia, amphotericin B, aspergillosis, corticosteroids, influenza, mouse model, oseltamivir
We established a novel corticosteroid-immunosuppressed murine model of influenza-associated pulmonary aspergillosis (IAPA) that underscores the detrimental effect of corticosteroid therapy on the outcomes of IAPA and provides a preclinical platform to study pathogenesis and therapy of this clinically important entity.
In addition to bacterial superinfections (eg, due to Staphylococcus aureus), secondary Aspergillus pneumonia is a feared complication in patients with influenza pneumonia [1,2]. Impaired epithelial barrier integrity, local hyperinflammation in the respiratory tract, and systemic immune paralysis due to influenza infection can predispose even otherwise immunocompetent hosts to influenza-associated pulmonary aspergillosis (IAPA) [1, 2]. Although the routine use of corticosteroids is discouraged and is associated with increased mortality in patients with severe influenza pneumonia, corticosteroids are frequently used in patients who develop acute respiratory distress syndrome and have been identified as a major risk factor for both the development and poor outcome of IAPA [1–5].
While there is increasing interest in the development of mammalian models of IAPA [6, 7], these models typically use a supraphysiological Aspergillus inoculum and lack the background of corticosteroid immunosuppression, hampering efforts to characterize the immunopathology and study treatments of this clinically important entity. Therefore, we established a novel IAPA mouse model that utilizes low-inoculum Aspergillus infection, incorporates cortisone acetate (CA) immunosuppression, and recapitulates the clinical timelines of infection and treatment events.
METHODS
IAPA Infection Model and Monitoring of Infection Severity
Eight- to ten-week-old female BALB/c mice were infected with 1.5% or 7.5% of the 90% lethal dose (LD90) of a mouse-adapted influenza A/Hong Kong/1968 (H3N2) strain [8] by delivering approximately 5000 or 25 000 plaque-forming units of influenza A virus (IAV), respectively, to cohorts of up 30 mice in a nebulization chamber [8]. Aerosolized saline was used as a control. Mice then received 2 intraperitoneal injections of 400 mg/kg CA (Sigma-Aldrich) or mock injections on days 5 and 8 after IAV infection. On day 9, mice were anesthetized with isoflurane (Aspen Veterinary Resources, Ltd) and intranasally challenged with 50 000 Aspergillus fumigatus AF-293 conidia or mock-infected with saline. Severity of IAV infection and IAPA were scored using the viral pneumonia score (VPS, range 0–12) [9] and the murine sepsis score (MSS, 0 = healthy to 3 = moribund, 4 = death prior to the time of assessment), with previously described modifications [10].
Therapeutic Interventions
Mice received daily tail vein injections of 5 mg/kg liposomal amphotericin B (LamB; Gilead Sciences) or empty liposomes (Gilead Sciences) dissolved in 5% glucose, or mock treatment with 5% glucose on days 9 through 13 (0–4 days after A. fumigatus infection, early therapy) or days 10 through 13 (late therapy). We chose LamB over triazoles due to the known beneficial immunomodulatory properties of the liposomal formulation in corticosteroid-immunosuppressed mice with invasive aspergillosis [11]. In some experiments, some mice additionally received oseltamivir (OST, 10 mg/kg; Sigma) or 5% glucose (solvent, mock treatment) twice daily by oral gavage on days 3 through 6 after IAV infection.
Downstream Assessment of Lung Tissue
Fungal burden was determined in lung tissue homogenates on day 16 (7 days after A. fumigatus infection) or upon earlier natural death using an 18S quantitative polymerase chain reaction assay [12]. Histopathological staining is described in Supplementary Methods.
Statistical Analyses
Morbidity/mortality scores and fungal burden were compared using the Mann-Whitney U test for 2-group comparisons and Kruskal-Wallis test with Dunn posttest for multigroup comparisons. Survival curves were compared using the Mantel-Cox log-rank test. Correlation was assessed using Spearman rank correlation coefficients (2 continuous variables) or rank-biserial correlation coefficients (continuous vs dichotomous variables). Nonparametric general linear regression was used to evaluate the independent impact of each challenge/intervention on day 16 MSS.
RESULTS
Establishment of a Reliable IAPA Infection Model in Corticosteroid-Immunosuppressed Mice
For model optimization (Figure 1A ), we compared infection of mice with 2 different IAV inocula. Influenza infection alone caused modest and temporary inoculum-dependent distress, with median VPS peaks of 2.0 (1.5% LD90) and 3.0 (7.5% LD90) by day 7 postinfection (Supplementary Figure 1A). IAV-associated morbidity fully resolved in nonimmunosuppressed mice by day 10. As expected, CA immunosuppression exacerbated the severity of IAV infection, with median VPS plateaus at 5.0 (1.5% LD90) and 6.0 (7.5% LD90) by day 10–11 postinfection, respectively (Supplementary Figure 1A). Accordingly, lungs from CA-immunosuppressed mice showed more pronounced IAV-induced diffuse alveolar damage than those from non-CA–treated mice (Supplementary Figure 2A).
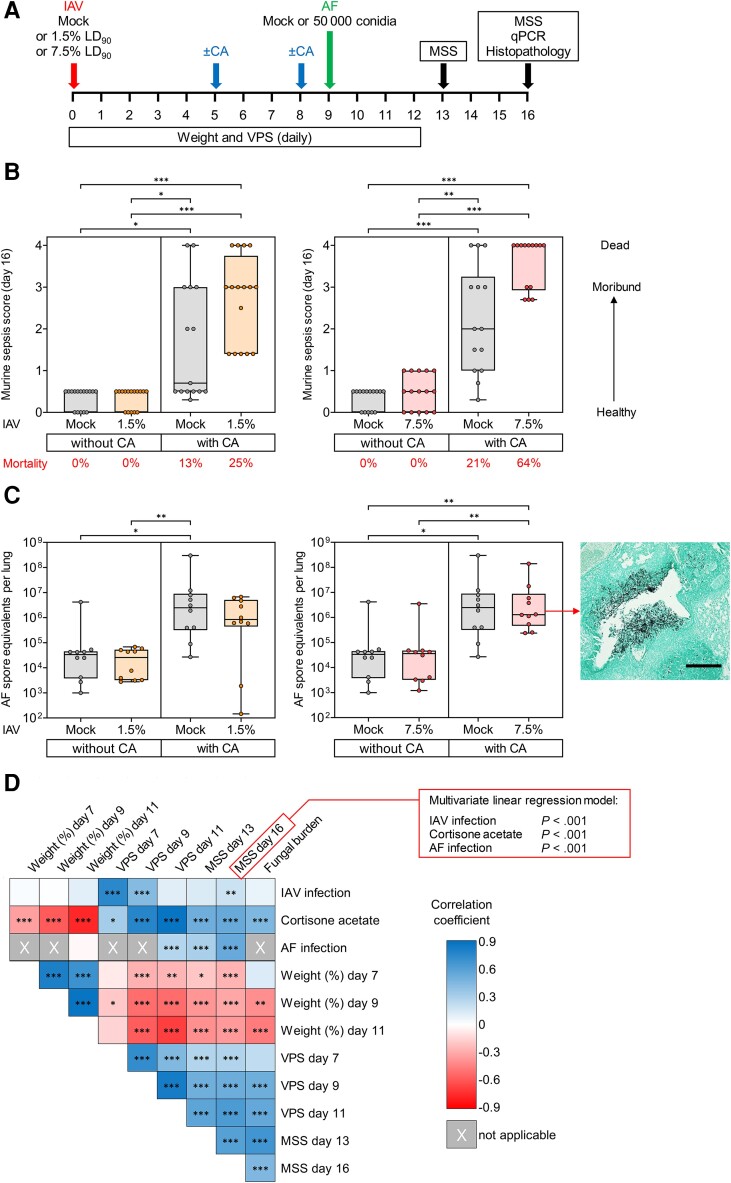
Figure 1.
Corticosteroid therapy is an independent predictor of poor morbidity and mortality outcomes in a murine model of influenza-associated pulmonary aspergillosis. A, Experimental procedures and timelines. Mice were infected with aerosolized influenza A virus (IAV) at 1.5% or 7.5% of the 90% lethal dose (LD90) or mock-infected with aerosolized saline. Mice then received 2 intraperitoneal injections of 400 mg/kg cortisone acetate (CA) or mock injections on days 5 and 8 after IAV infection. On day 9, mice were intranasally challenged with 50 000 Aspergillus fumigatus AF-293 (AF) conidia or mock-infected with saline. Survival, weight, and infection severity were monitored daily until day 16. Infection severity was scored using the viral pneumonia score (VPS) [9] and the modified murine sepsis score (MSS) [10]. Fungal burden was determined by quantitative polymerase chain reaction (qPCR) on day 16 or upon earlier natural death. In addition, lung tissue was stained with hematoxylin and eosin (Supplementary Figure 2) and Grocott's methenamine silver (GMS) stain (Supplementary Figure 3) for histopathological examination. B, Comparison of day 16 MSS (n = 14–16 mice per group from 3 independent experiments) and (C) fungal burden in lung tissue (n = 10 mice per group from 2 independent experiments) of Aspergillus-infected mice depending on preceding IAV infection and CA therapy. Boxes and whiskers denote interquartile range (with median bars) and minimum-to-maximum range, respectively. Kruskal-Wallis test with Dunn posttest. In addition, a representative GMS-stained lung tissue section from an IAV- and A. fumigatus-infected, CA-immunosuppressed mouse is shown, documenting peribronchiolar A. fumigatus invasion extending into the surrounding alveolar tissue. Scale, 100 µm. D, Univariate rank correlation analysis comparing individual challenges (IAV, CA, A. fumigatus) and surrogates of infection severity (weight, morbidity/mortality scores, fungal burden). Color scale represents rank correlation coefficients. X denotes nonapplicable comparisons, ie, readouts either determined prior to A. fumigatus infection or not performed in non-A. fumigatus–infected mice (fungal burden). Individual comparisons are based on n = 58–218 mice, depending on the applicability and availability of the respective readouts. Additionally, the box in the upper right corner summarizes the results of a multivariate nonparametric logistic regression model testing the independent impact of individual challenges (IAV, CA, A. fumigatus) on day 16 MSS values (n = 216 valid data sets). *P < .05, **P < .01, ***P < .001.
CA plus A. fumigatus infection caused low mortality (13%–21%) and modest morbidity in non-IAV–infected mice (Figure 1B and Supplementary Figure 1A and 1B), although considerable pulmonary fungal burden was seen (1.5 × 106 median conidial equivalents per lung; Figure 1C ). Mice with IAPA but no CA immunosuppression mostly recovered by day 16 (7 days after A. fumigatus infection) and showed minimal signs of distress (median MSS, 0.5) and universal survival (Figure 1B and Supplementary Figure 1B and 1C). Consistently, pulmonary histology revealed few and mostly degenerated fungal cells in the airways and minimal alveolar lesions (Supplementary Figure 2B and 2C, Supplementary Figure 3A).
When all 3 challenges (7.5% LD90 IAV, CA, and A. fumigatus) were combined, considerable morbidity and 64% 16-day mortality was seen (Figure 1B and Supplementary Figure 1B and 1C). All surviving triple-challenged mice displayed severe distress or were in moribund condition (median MSS, 4.0; median among survivors, 2.7; P < .01 vs all other conditions; Figure 1B and Supplementary Figure 1B and 1C). Congruently, this cohort had high fungal burden (0.83 × 106 median conidial equivalents per lung; Figure 1C ) and showed extensive peribronchiolar A. fumigatus invasion as well as multiple fungal lesions in alveolar tissue that were surrounded by massive granulocyte infiltrates and areas of tissue necrosis (Supplementary Figure 2B and 2C, Supplementary Figure 3B–3G).
While IAPA also caused considerable morbidity (median MSS 3.0, median among survivors 2.8) and 25% mortality in CA-immunosuppressed mice infected with the lower 1.5% LD90 IAV inoculum, morbidity and fungal burden were less consistent than in mice challenged with the higher IAV inoculum (Figure 1B and 1C ). Therefore, we used the 7.5% LD90 IAV inoculum for all subsequent experiments.
Univariate rank correlation analysis revealed that each individual challenge contributed significantly to increased day 16 MSS (Figure 1D ), with rank correlation coefficients of 0.19 for IAV (P = .005), 0.54 for CA (P < .001), and 0.54 for A. fumigatus (P < .001). Furthermore, all 3 challenges were independent predictors of day 16 morbidity/mortality in a multivariate general linear regression model (P < .001; Figure 1D and Supplementary Table 1). CA was also strongly associated with other adverse outcome of IAPA, that is weight loss and high fungal burden (P < .001; Figure 1D ).
Of note, the severity of influenza pneumonia at the time of A. fumigatus infection (day 9 VPS), which was significantly influenced by both the IAV inoculum and CA immunosuppression, strongly correlated with subsequent surrogates of IAPA severity (MSS, fungal burden, P < .001; Figure 1D ). Altogether, these results underscore the detrimental impact of corticosteroids on the course and outcomes of IAPA.
Impact of Anti-infective Treatment on the Course of IAPA in Corticosteroid-Immunosuppressed Mice
To further validate our model, we tested the impact of antifungal and antiviral therapy on the outcomes of IAPA (Figure 2A ). Compared to mock treatment with 5% glucose, a 5-day course of LamB monotherapy, starting on the day of A. fumigatus infection, significantly reduced mortality (13% vs 50%; P = .020), morbidity (median day 16 MSS, 0.8 vs >3.0; P < .001), and fungal burden (4.14 × 104 vs 3.37 × 107 median conidial equivalents per lung; P = .002) in CA-immunosuppressed mice with IAPA (Figure 2B ). Although both morbidity/mortality scores (P = .118) and fungal clearance (P = .073) were improved by empty liposomes versus mock treatment (glucose), the therapeutic benefit of liposomes without the amphotericin B compound was less consistent and did not reach significance (Figure 2B ).
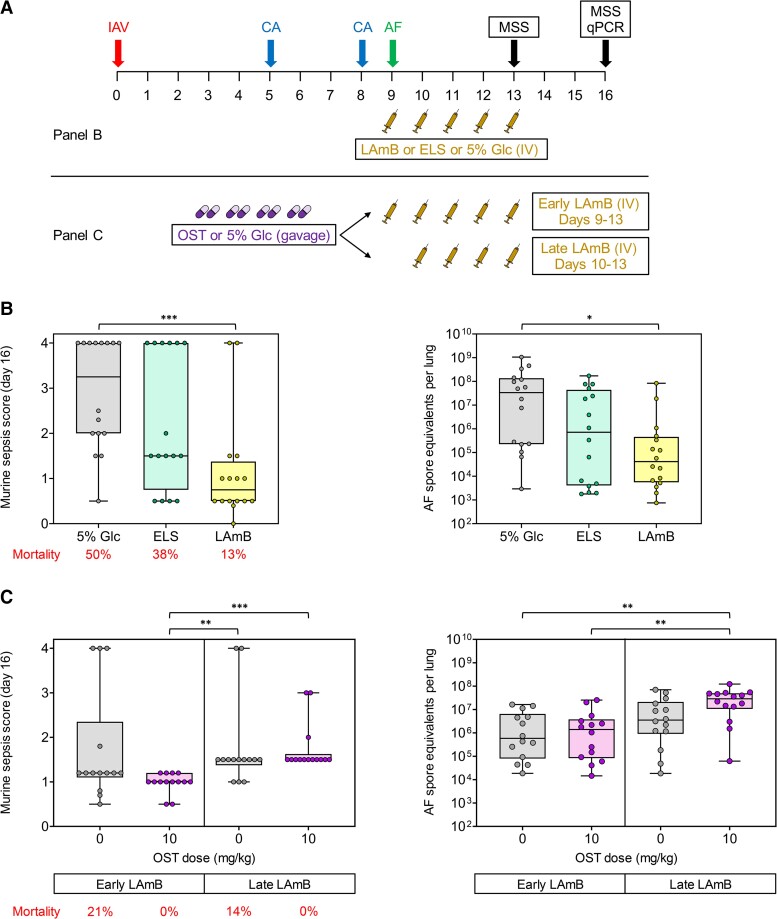
Figure 2.
Timing of antifungal therapy determines the morbidity of corticosteroid-immunosuppressed mice with influenza-associated pulmonary aspergillosis (IAPA). A, Experimental procedures and timelines. Mice were infected with 7.5% of the 90% lethal dose influenza A virus (IAV), immunosuppressed with 2 × 400 mg/kg cortisone acetate (CA), and subsequently infected with 50 000 Aspergillus fumigatus (AF) conidia, as described in “Methods.” Mice received daily intravenous injections of liposomal amphotericin B (LAmB, 5 mg/kg/injection), starting either on the day of A. fumigatus infection (day 9, early) or on the day following A. fumigatus infection (day 10, late). Empty liposomes (ELS) and 5% glucose (Glc, vehicle) were used as controls. For the experiment shown in (C), mice additionally received oseltamivir (OST, 10 mg/kg) or mock treatment with 5% glucose twice daily by oral gavage on days 3 through 6 after IAV infection. Severity of influenza pneumonia and IAPA was scored using the modified murine sepsis score (MSS). Fungal burden was determined by quantitative polymerase chain reaction (qPCR) on day 16 or upon earlier natural death. B, Day 16 MSS and pulmonary fungal burden depending on the treatment arm; n = 16 mice per treatment from 2 independent experiments. Kruskal-Wallis test with Dunn posttest. C, Day 16 MSS and pulmonary fungal burden depending on OST treatment and timing of LAmB therapy; n = 14 mice per treatment from 2 independent experiments. B and C, Boxes and whiskers denote interquartile range (with median bars) and minimum-to-maximum range, respectively. *P < .05, **P < .01, ***P < .001.
Lastly, we tested the combined effect of antiviral treatment of IAV infection with OST and early (starting on the day of A. fumigatus infection) or late (starting on the day after A. fumigatus infection) LamB treatment of IAPA on infection severity. OST significantly attenuated the severity of influenza pneumonia in CA-immunosuppressed mice (median day 9 VPS, 4.0 vs 6.0; P < .001; Supplementary Figure 4A) and, subsequently, the severity of IAPA (median day 13 MSS in mice with early LamB therapy, 0.5 vs 1.2, P = .006, Supplementary Figure 4B; median day 16 MSS, 1.0 vs 1.2, P = .118, Figure 2C ). Furthermore, treatment of influenza pneumonia with OST reduced the mortality of subsequent IAPA in mice receiving early LamB therapy from 21% to 0% (P = .150) but did not reduce the fungal burden (Figure 2C ). Notably, even when influenza pneumonia was treated with OST, delayed initiation of LamB therapy was associated with worsened morbidity (median day 13 MSS, 1.2 vs 0.5, P < .001, Supplementary Figure 2B; median day 16 MSS, 1.5 vs 1.0, P < .001, Figure 2C ) and higher fungal burden (2.88 × 107 vs 1.40 × 106 median conidial equivalents per lung, P = .005; Figure 2C ) in CA-immunosuppressed mice with IAPA.
DISCUSSION
Herein, we developed a new preclinical IAPA mouse model, focusing on the comparison of infection outcomes with and without underlying corticosteroid immunosuppression. We found that CA immunosuppression was strongly associated with morbidity, mortality, weight loss, and extensive pulmonary fungal infiltrates. This observation aligns with clinical evidence that identified corticosteroids as a major independent cause of poor outcomes of both influenza pneumonia and IAPA [3–5] and underscores a need to consider the effects of corticosteroids as a key pathogenetic factor in preclinical models of IAPA. Furthermore, we captured features of both airway disease and alveolar lesions in CA-immunosuppressed mice with IAPA, aligning with the proposed role of corticosteroids as a driver of peribronchiolar invasion and fungal tissue dissemination in human IAPA [2].
The use of high fungal inocula (millions of conidia) presents a common source of artificiality in preclinical mold infection models. Unlike the published IAPA models in immunocompetent mice [6, 7], we focused on low-inoculum Aspergillus infection. Despite the low fungal inoculum, the reproducibility of infection severity (morbidity and fungal burden) in CA-immunosuppressed mice was comparable to the published immunocompetent IAPA models that used high A. fumigatus inocula.
Furthermore, our model recapitulates real-life clinical scenarios, that is corticosteroid treatment was initiated when mice started to show signs of distress from influenza infection and aspergillosis was introduced shortly after corticosteroid therapy. These timelines are consistent with the clinical observations that IAPA usually is an early event, with an IAPA symptom onset at a median of 3 days after intensive care unit admission [3].
Our experimental approach also provided suitable therapeutic windows to study antifungal and antiviral treatment interventions. As expected, prompt LAmB therapy strongly reduced morbidity and mortality of IAPA in CA-immunosuppressed mice, aligning with the clinical observation that nonsurvivors of IAPA had significantly delayed onset of antifungal therapy than survivors [5]. Although statistical significance was not reached, treatment with empty liposomes yielded favorable trends of improved morbidity, mortality, and fungal clearance, suggesting that immunomodulatory properties of the liposomal packaging might contribute to the protective activity of LAmB against IAPA in a background of corticosteroid immunosuppression [11]. These findings encourage further detailed exploration of immunomodulatory effects of Aspergillus-active antifungals, both conventional ones (eg, triazoles or cell wall inhibitors) and investigational drugs [13], as well as a variety of other immunomodulatory agents in animal models of postviral mold infections.
The impact of antiviral therapy on the incidence and therapeutic outcomes of IAPA is incompletely understood. Interestingly, OST was shown to impair neutrophil-targeted cytokine signaling and neutrophil recruitment, thereby increasing the susceptibility of non-IAV–infected mice to “single-hit” pulmonary aspergillosis [14]. Although not significant, we also found a trend toward higher fungal burden in mice with IAPA after OST therapy (Figure 2C ). Nonetheless, our morbidity/mortality data that indicated a modest protective effect of OST align with the results of a recent study in an otherwise immunocompetent murine IAPA model, where early-onset OST therapy improved the outcomes of IAPA, even without subsequent antifungal therapy [7]. Taken together, these findings suggest that the beneficial impact of OST on the severity of influenza pneumonia outweighs potential adverse immunomodulatory effects of OST that could lead to impaired fungal clearance.
Given the known strain-specific differences in pathogenesis and virulence [15], a major limitation of this study is the use of a single influenza and A. fumigatus strain. Previous reports demonstrated the feasibility of IAPA mouse models with both H1N1 [6] and H3N2 [7] IAV strains; however, direct comparisons of infection severity are precluded by different timelines, inocula, modes of infection, and combinations with different A. fumigatus isolates. Additionally, we used inbred mice, which are cost-efficient and reduce experimental variability but cannot account for the heterogeneity of patients at particular risk for IAPA and for the impact of key comorbidities (eg, obesity and diabetes mellitus) [3].
Despite these limitations, we established a novel IAPA mouse model with low-inoculum A. fumigatus infection that complements existing preclinical IAPA models and facilitates comparative studies of the pathogenesis and therapy of IAPA with and without underlying CA immunosuppression. Furthermore, our study underscores the detrimental impact of corticosteroids on the course of IAPA and highlights the importance of early antifungal therapy, even after prior OST therapy. Our model may serve as a potent platform for preclinical studies of immunopharmacological effects of antivirals, antifungals, and adjunct investigational immunotherapies to prevent and treat IAPA, as well as for in-depth exploration of specific virulence attributes of Aspergillus (eg, gliotoxin) in the context of IAPA.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Sebastian Wurster, Department of Infectious Diseases, Infection Control and Employee Health, The University of Texas M.D. Anderson Cancer Center, Houston, Texas, USA.
Jezreel Pantaleón García, Department of Pulmonary Medicine, The University of Texas M.D. Anderson Cancer Center, Houston, Texas, USA.
Nathaniel D Albert, Department of Infectious Diseases, Infection Control and Employee Health, The University of Texas M.D. Anderson Cancer Center, Houston, Texas, USA.
Ying Jiang, Department of Infectious Diseases, Infection Control and Employee Health, The University of Texas M.D. Anderson Cancer Center, Houston, Texas, USA.
Keerthi Bhoda, Department of Pulmonary Medicine, The University of Texas M.D. Anderson Cancer Center, Houston, Texas, USA.
Vikram V Kulkarni, Department of Pulmonary Medicine, The University of Texas M.D. Anderson Cancer Center, Houston, Texas, USA.
Yongxing Wang, Department of Pulmonary Medicine, The University of Texas M.D. Anderson Cancer Center, Houston, Texas, USA.
Thomas J Walsh, Center for Innovative Therapeutics and Diagnostics, Richmond, Virginia, USA.
Scott Evans, Department of Pulmonary Medicine, The University of Texas M.D. Anderson Cancer Center, Houston, Texas, USA.
Dimitrios P Kontoyiannis, Department of Infectious Diseases, Infection Control and Employee Health, The University of Texas M.D. Anderson Cancer Center, Houston, Texas, USA.
Notes
Disclaimer. The sponsor had no influence on the research design.
Financial support. This study was supported by Gilead Global Pharma (grant number IN-US-131-5756 investigator-initiated grant to D. P. K. and S. W.); the Robert C. Hickey Chair for Clinical Care Endowment (to D. P. K.); and the National Institutes of Health (grant number R35 HL144805 to S. E. E.).
References
- 1. Lamoth F, Lewis RE, Walsh TJ, Kontoyiannis DP. Navigating the uncertainties of COVID-19 associated aspergillosis (CAPA): a comparison with influenza associated aspergillosis (IAPA). J Infect Dis 2021; 224:1631–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van de Veerdonk FL, Brüggemann RJM, Vos S, et al. COVID-19-associated Aspergillus tracheobronchitis: the interplay between viral tropism, host defence, and fungal invasion. Lancet Respir Med 2021; 9:795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van de Veerdonk FL, Kolwijck E, Lestrade PP, et al. Influenza-associated aspergillosis in critically ill patients. Am J Respir Crit Care Med 2017; 196:524–7. [DOI] [PubMed] [Google Scholar]
- 4. Zhou Y, Fu X, Liu X, et al. Use of corticosteroids in influenza-associated acute respiratory distress syndrome and severe pneumonia: a systemic review and meta-analysis. Sci Rep 2020; 10:3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vanderbeke L, Spriet I, Breynaert C, Rijnders BJA, Verweij PE, Wauters J. Invasive pulmonary aspergillosis complicating severe influenza: epidemiology, diagnosis and treatment. Curr Opin Infect Dis 2018; 31:471–80. [DOI] [PubMed] [Google Scholar]
- 6. Tobin JM, Nickolich KL, Ramanan K, et al. Influenza suppresses neutrophil recruitment to the lung and exacerbates secondary invasive pulmonary aspergillosis. J Immunol 2020; 205:480–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seldeslachts L, Vanderbeke L, Fremau A, et al. Early oseltamivir reduces risk for influenza-associated aspergillosis in a double-hit murine model. Virulence 2021; 12:2493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kirkpatrick CT, Wang Y, Leiva Juarez MM, et al. Inducible lung epithelial resistance requires multisource reactive oxygen species generation to protect against viral infections. mBio 2018; 9:e00696-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rouxel RN, Mérour E, Biacchesi S, Brémont M. Complete protection against influenza virus H1N1 strain A/PR/8/34 challenge in mice immunized with non-adjuvanted novirhabdovirus vaccines. PLoS One 2016; 11:e0164245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wurster S, Albert ND, Bharadwaj U, et al. Blockade of the PD-1/PD-L1 immune checkpoint pathway improves infection outcomes and enhances fungicidal host defense in a murine model of invasive pulmonary mucormycosis. Front Immunol 2022; 13:838344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lewis RE, Chamilos G, Prince RA, Kontoyiannis DP. Pretreatment with empty liposomes attenuates the immunopathology of invasive pulmonary aspergillosis in corticosteroid-immunosuppressed mice. Antimicrob Agents Chemother 2007; 51:1078–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bowman JC, Abruzzo GK, Anderson JW, et al. Quantitative PCR assay to measure Aspergillus fumigatus burden in a murine model of disseminated aspergillosis: demonstration of efficacy of caspofungin acetate. Antimicrob Agents Chemother 2001; 45:3474–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lamoth F, Lewis RE, Kontoyiannis DP. Investigational antifungal agents for invasive mycoses: a clinical perspective. Clin Infect Dis 2022; 75:534–44. [DOI] [PubMed] [Google Scholar]
- 14. Dewi IMW, Cunha C, Jaeger M, et al. Neuraminidase and SIGLEC15 modulate the host defense against pulmonary aspergillosis. Cell Rep Med 2021; 2:100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen X, Liu S, Goraya MU, Maarouf M, Huang S, Chen JL. Host immune response to influenza A virus infection. Front Immunol 2018; 9:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.