Abstract
Background
Persistent infection with high-risk human papillomavirus (HPV) is associated with development of invasive cervical cancer.
Methods
Longitudinal data was collected from 174 Senegalese women. We employed marginal Cox proportional hazards models to examine the effect of human immunodeficiency virus (HIV) status (HIV positive vs HIV negative) and HIV type (HIV-1 vs HIV-2 vs dual HIV-1/HIV-2) on clearance of type-specific HPV infection. Analyses were stratified by incident versus prevalent HPV infection.
Results
Incident HPV infections in HIV-positive women were less likely to clear than those in HIV-negative women (adjusted hazard ratio [HR] = 0.60; 95% confidence interval [CI], .38–.94). Among HIV-positive women, HIV-2–infected women and HIV-1/2 dually infected women were more likely to clear HPV incident infections than HIV-1–infected women (HR = 1.66; 95% CI, .95–2.92 and HR = 2.17; 95% CI, 1.12–4.22, respectively). Incident HPV infections in HIV-positive women with CD4 cell count ≤500 cells/μL were less likely to clear than those in HIV-positive women with CD4 cell count >500 cells/μL (HR = 0.65; 95% CI, .42–1.01). No significant associations were observed for prevalent HPV infections.
Conclusions
HIV infection reduced the likelihood of clearance of incident HPV infection. Furthermore, among HIV-positive women, low CD4 cell count and dual HIV infection were each associated with reduced likelihood of clearance.
Keywords: human immunodeficiency virus, HIV, HIV-2, HPV, clearance, human papillomavirus, women
In Senegalese women, incident HPV infections in HIV-positive women were less likely to clear than those in HIV-negative women and HIV-2–infected or dually infected women were more likely to clear incident HPV infections than HIV-1–infected women.
Infection with high-risk human papillomavirus (HPV) is a universally recognized causative agent of invasive cervical cancer (ICC) and its precursor lesions, cervical intraepithelial neoplasia (CIN) [1, 2]. At least 40 genotypes of HPV can infect the genital area [3] and are classified on the basis of oncogenic potential with high-risk types 16 and 18 accounting for over 70% of cervical cancers [4]. Most HPV infections are self-limited and asymptomatic [3], but persistent detection of high-risk HPV is associated with an increased risk of CIN and ICC [5, 6]. There is evidence that infection with human immunodeficiency virus (HIV) is associated with persistence of HPV infection [6–8]. HIV-induced immunosuppression can limit the ability of the immune system to effectively control HPV infection, leading to increased risk of persistent infection [9]. Despite numerous studies describing the increased risk of HPV detection and cervical disease in HIV-positive women [10, 11], few have examined the natural history of HPV infection longitudinally or provided a direct comparison to HIV-negative women.
More complicated questions arise in West Africa regarding the impact of HIV infection on HPV infection and subsequent development of cervical cancer. HIV-1 is the most common type of HIV and extends worldwide [12]. Distinct from HIV-1, HIV-2 is endemic in West Africa and is often neglected in the global campaign to end the AIDS epidemic [12]. Despite many similarities, there are important differences between HIV-1 and HIV-2, including lower transmissibility, lower viral loads, and slower CD4 cell loss and progression to AIDS in the natural history of HIV-2 infection compared to HIV-1 infection [13]. There is limited information on the influence of HIV type on HPV infection [8, 14]. HIV-2 infection may have less of an effect on clearance of HPV infection due to less severe immunosuppression. Several articles suggested that the adjustment for CD4 cell count attenuated the association between HIV type and HPV infection [8, 14]. Furthermore, the situation has become more elusive in the era of antiretroviral therapy (ART) [15] because the effect of ART on HPV infection and cervical lesions is still debatable [16]. In spite of advances in cervical cancer screening and HPV vaccine development, implementing screening and vaccination programs in sub-Saharan Africa has proven to be challenging due to financial, logistical, and sociocultural factors [17]. Therefore, understanding the relationship between HIV infection and HPV infection is important for effective ICC prevention strategies.
A previous study assessed the impact of HIV status and types on HPV clearance from cohorts of Senegalese women, but it used prevalent cases of HPV infection to assess HPV clearance, where left-censoring may produce bias [8]. In the present longitudinal analyses, we assessed the effect of HIV status and HIV type on the clearance of type-specific HPV infection among women in Senegal, West Africa. We also assessed the difference in the effect between incident and prevalent HPV infections. Furthermore, we examined the role of CD4 cell count in clearing HPV infection among HIV-infected women.
METHODS
Data Collection
Data for the present analysis were obtained from a cohort study conducted in Dakar, Senegal, West Africa between 2006 and 2010, which recruited women older than 18 years presenting to either an outpatient primary care clinic (Pikine) with low HIV prevalence (<1%) or an outpatient infectious disease clinic (Le service des Maladies Infectieuses et Tropicales [SMIT], Centre Hospitalier NationalUniversitaire [CHNU]-Fann, Dakar) with high HIV prevalence (>10%) [18]. Subjects were excluded from participation if they were pregnant or did not have an intact cervix. All participants provided written informed consent upon enrollment. The protocol was approved by the Institutional Review Boards of both the University of Washington and the Université Cheikh Anta Diop de Dakar, Sénégal.
Upon enrollment, a physical examination and a structured interview soliciting demographic and health information, including reproductive and sexual history, were given. Blood samples were collected for HIV-1 and HIV-2 testing, and for lymphocyte subset analysis (CD4 cell count). At baseline and at each 4-month follow-up visit, cervical swab samples were collected for HPV detection and typing.
HIV Serology and Lymphocyte Testing
Serologic assays for HIV-1 and HIV-2 were performed on all participants’ blood samples collected at baseline using a 2-test sequence, as described elsewhere [19, 20]. Initial testing was performed for the presence of either HIV-1 or HIV-2 antibodies. Positive samples were confirmed with a peptide-based membrane immunoassay that distinguishes HIV-1 and HIV-2 antibodies. Whole blood collected in EDTA tubes from HIV-infected women was analyzed using a fluorescence activated cell sorter (FACS) count analyzer to determine the number of CD4+ cells per microliter of blood.
HPV DNA Detection and Typing
Cervical swab samples were tested for HPV DNA with a polymerase chain reaction (PCR) assay using MY09 and MY11 L1 consensus primers, HPV type-specific oligonucleotide probes, and a generic probe, with amplification of the cellular β-globin gene as a control, as previously described [14, 20, 21]. The presence of any HPV DNA was determined by PCR amplification followed by dot blot hybridization using a generic probe. Positive samples were subsequently reamplified to assess presence of 38 HPV types (6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 57, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, IS39 [a subtype of HPV-82], and CP6108) mainly using a liquid bead microarray assay [22, 23].
Statistical Analysis
Analyses were restricted to women with HPV detected at baseline or during follow-up and with at least 2 subsequent visits with HPV test results. Demographic and behavioral characteristics of subjects at enrollment were summarized with frequency distributions of categorical variables separately for HIV-negative and HIV-positive subjects.
Given that an individual can be infected by multiple HPV genotypes simultaneously or sequentially, type-specific HPV infection, rather than the individual, was treated as the unit of analysis and robust variance estimates were considered to account for within-subject correlations. Untyped infections were excluded from the analyses. Cumulative probability of clearance of type-specific HPV infection by HIV status and HIV type was estimated by Kaplan-Meier methods. To reduce the likelihood of misclassification induced by false-negative results, HPV clearance was defined as 2 consecutive HPV-negative test results after an HPV-positive test. The time at risk was calculated from initial detection of HPV to the time of the second nondetection or the last sample in the study.
Marginal Cox proportional hazards models were fitted to evaluate the effect of HIV status (HIV positive vs HIV negative) on clearance of HPV infection among all subjects and the effect of HIV type (HIV-1 vs HIV-2 vs dual HIV-1/HIV-2) on clearance of HPV infection among HIV-positive subjects. Analyses were stratified by HPV infection status (prevalent vs incident detection) by including the interaction term of exposure and HPV infection status in the models. Prevalent infection was defined as detection of type-specific HPV infection at baseline and incident infection was defined as initial detection of type-specific HPV infection during follow-up. Furthermore, we evaluated the effect of CD4 cell count on HPV clearance among HIV-positive subjects. A priori, HIV-positive subjects were classified into 2 categories based on CD4 cell count at enrollment: ≤ 500 and >500 cells/μL. Results were reported as hazard ratios (HRs) for HPV clearance with their 95% confidence intervals (CIs). Potential confounding factors were chosen a priori, including age at enrollment (<35, 35–49, or ≥50 years), lifetime number of sex partners at enrollment (1, 2, or ≥3) and concurrent infection with ≥1 additional HPV type at time of initial detection of type-specific HPV infection (yes/no).
Separate models were fitted to assess the effect of HIV status on clearance of high-risk HPV (HR-HPV) infection and clearance of low-risk HPV (LR-HPV) infection, with the following 18 types classified as HR-HPV types based on their oncogenic potential: 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82, and IS39 [24]. Analyses were performed utilizing R version 4.0.2. All statistical tests were 2-tailed with significance level α = .05.
RESULTS
Characteristics of Participants and Infections
Among 174 women, we identified 664 HPV infections first detected at baseline or during follow-up with at least 2 subsequent HPV results after initial detection. The median age of the 174 women was 43 years, ranging from 20 to 70 years. The majority of the participants did not use contraceptives (86.2%) and did not smoke (98.3%). Compared to HIV-negative women (n = 75), women with HIV-1 and/or HIV-2 infection (n = 99) were more likely to be recruited at the SMIT, CHNU-Fann clinic, be younger, be single, have fewer children, have a greater lifetime number of sex partners, and were more likely to use condoms at baseline (Table 1). Among the 664 HPV infections, 183 (27.6%) were in HIV-negative women, 395 (59.5%) were in HIV-1–infected women, 54 (8.1%) were in HIV-2–infected women, and 32 (4.8%) were in HIV-1/2 dually infected women. Of these HPV infections, 275 (41.4%) were incident and 389 (58.6%) were prevalent, 397 (59.8%) were HR-HPV types, and 267 (40.2%) were LR-HPV types.
Table 1.
Baseline Demographic, Behavioral, and Health Characteristics of 174 Senegalese Women with Human Papillomavirus Infections, in 2006–2010, by Human Immunodeficiency Virus (HIV) Status, n (%)
| Characteristics | HIV Negative (n = 75) | HIV Positive (n = 99) | Overall (n = 174) |
|---|---|---|---|
| Clinic | |||
| ȃSMIT, CHNU-Fann | 14 (18.7) | 97 (98.0) | 111 (63.8) |
| ȃPikine | 61 (81.3) | 2 (2.0) | 63 (36.2) |
| Age, y | |||
| ȃ<35 | 14 (18.7) | 19 (19.2) | 33 (19.0) |
| ȃ35–49 | 33 (44.0) | 66 (66.7) | 99 (56.9) |
| ȃ≥50 | 28 (37.3) | 14 (14.1) | 42 (24.1) |
| Marital status | |||
| ȃNot married currently | 10 (13.5) | 44 (45.4) | 54 (31.6) |
| ȃMono marrieda | 20 (27.0) | 30 (30.9) | 50 (29.2) |
| ȃPoly marriedb | 44 (59.5) | 23 (23.7) | 67 (39.2) |
| ȃMissing | 1 (1.3) | 2 (2.0) | 3 (1.7) |
| Parity, No. | |||
| ȃ0 | 7 (9.4) | 12 (12.4) | 19 (11.1) |
| ȃ1–4 | 17 (23.0) | 61 (62.9) | 78 (45.6) |
| ȃ≥5 | 50 (67.6) | 24 (24.7) | 74 (43.3) |
| ȃMissing | 1 (1.3) | 2 (2.0) | 3 (1.7) |
| Lifetime sex partners, No. | |||
| ȃ1 | 45 (60.0) | 42 (43.8) | 87 (50.9) |
| ȃ2 | 20 (26.7) | 36 (37.5) | 56 (32.7) |
| ȃ≥3 | 10 (13.3) | 18 (18.7) | 28 (16.4) |
| ȃMissing | 0 (0) | 3 (3.0) | 3 (1.7) |
| Current birth control method | |||
| ȃCondom | 1 (1.3) | 8 (8.1) | 9 (5.2) |
| ȃOther | 12 (16.0) | 3 (3.0) | 15 (8.6) |
| ȃNone | 62 (82.7) | 88 (88.9) | 150 (86.2) |
| Current smoking | |||
| ȃYes | 1 (1.3) | 2 (2.0) | 3 (1.7) |
| ȃNo | 74 (98.7) | 96 (98.0) | 170 (98.3) |
| ȃMissing | 0 (0) | 1 (1.0) | 1 (0.6) |
| HIV type | |||
| ȃHIV-1 | … | 79 (79.8) | … |
| ȃHIV-2 | … | 15 (15.1) | … |
| ȃHIV-1/2 | … | 5 (5.1) | … |
| CD4 cell count, cells/μL | |||
| ȃ<200 | … | 21 (22.3) | … |
| ȃ200–500 | … | 42 (44.7) | … |
| ȃ>500 | … | 31 (33.0) | … |
| ȃMissing | … | 5 (5.1) | … |
Data are No. (%).
Mono married is defined as in a marital relationship involving one spouse at a time.
Poly married is defined as in a marital relationship involving multiple spouses at a time.
Association of HIV Status and HPV Clearance
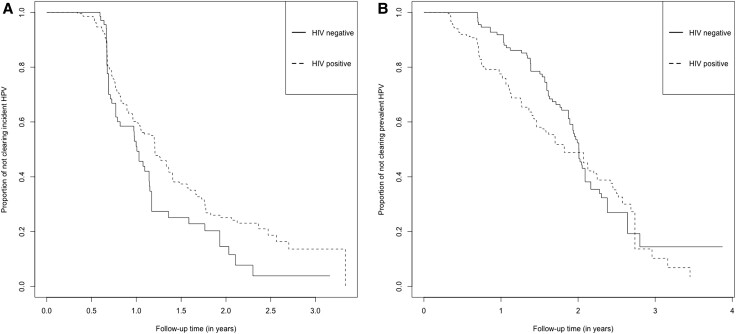
Among incident HPV infections, the 2-year cumulative probability of HPV clearance was 85.5% (95% CI, 73.6%–93.9%) in HIV-negative women and 74.9% (95% CI, 67.6%–81.7%) in HIV-positive women. Among prevalent HPV infections, the 2-year cumulative probability of HPV clearance was 47.6% (95% CI, 38.3%–57.9%) in HIV-negative women and 51.2% (95% CI, 44.0%–58.8%) in HIV-positive women (Figure 1). After adjustment for age at enrollment, lifetime number of sex partners at enrollment, and concurrent infection with ≥1 additional HPV type at time of initial HPV detection, prevalent HPV infections showed a significant reduction in likelihood of clearance compared with incident HPV infections (adjusted HR = 0.51; 95% CI, .38–.68). HR-HPV infections had a similar likelihood of clearance to LR-HPV infections (adjusted HR = 1.03; 95% CI, .83–1.27).
Figure 1.
Cumulative probability of clearing human papillomavirus (HPV) infection by human immunodeficiency virus (HIV) status. Data are for 174 Senegalese women with HPV infections, in 2006–2010. Cumulative probability of clearing HPV was estimated by Kaplan-Meier methods. A, In total, 275 type-specific incident HPV infections were analyzed, including 68 from HIV-negative women and 207 from HIV-positive women. A total of 320 person-years were at risk of clearance. We observed 192 HPV infections were cleared, including 52 from HIV-negative women and 140 from HIV-positive women. The result of log-rank test indicated that the distribution of time until clearance of incident HPV infection is different for women with and without HIV infection at the .05 significance level (P = .02). B, In total, 389 type-specific prevalent HPV infections were analyzed, including 115 from HIV-negative women and 274 from HIV-positive women. A total of 547 person-years were at risk of clearance. We observed 196 HPV infections were cleared, including 70 from HIV-negative women and 126 from HIV-positive women. The result of log-rank test indicated that the distribution of time until clearance of prevalent HPV infection is the same for women with and without HIV infection at the .05 significance level (P = .3).
In the multivariate model stratified by HPV infection status (Table 2), we observed that the association between HIV status and clearance of HPV infection differed significantly between incident and prevalent HPV infections. Among incident HPV infections, those in HIV-infected women were less likely to clear than those in HIV-uninfected women (adjusted HR = 0.60; 95% CI, .38–.94). Among prevalent HPV infections, no significant association between HIV status and HPV clearance was observed (adjusted HR = 1.16; 95% CI, .81–1.67).
Table 2.
Hazard Ratios for the Association of HIV Status and Clearance of HPV Infection Among 174 Senegalese Women With HPV Infections, in 2006–2010
| HPV and HIV Status | Incident HPV Infectionsa | Prevalent HPV Infectionsa | P Valued | ||||
|---|---|---|---|---|---|---|---|
| Eventsb | Person-Years | HR (95% CI)c | Eventsb | Person-Years | HR (95% CI)c | ||
| Any HPV | |||||||
| ȃHIV negative | 52 | 71 | Ref. | 70 | 203 | Ref. | … |
| ȃHIV positive | 140 | 249 | 0.60 (.38–.94) | 126 | 344 | 1.16 (.81–1.67) | .02 |
| Low-risk HPVe | |||||||
| ȃHIV negative | 26 | 38 | Ref. | 20 | 64 | Ref. | … |
| ȃHIV positive | 59 | 121 | 0.55 (.33–.93) | 49 | 127 | 1.45 (.82–2.58) | .02 |
| High-risk HPVf | |||||||
| ȃHIV negative | 26 | 33 | Ref. | 50 | 138 | Ref. | … |
| ȃHIV positive | 81 | 128 | 0.64 (.36–1.13) | 77 | 217 | 1.01 (.67–1.53) | .20 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; HPV, human papillomavirus; HR, hazard ratio; Ref., reference category.
Incident HPV infections are type-specific HPV infections initially detected during follow-up; prevalent HPV infections are type-specific HPV infections present at baseline.
Events are number of clearances of type-specific HPV infection.
Adjusted for the following covariates: age at enrollment, lifetime number of sex partners at enrollment, and concurrent infection with ≥1 additional HPV type at time of initial detection of type-specific HPV infection.
P value for difference in HRs between incident and prevalent HPV infection.
Low-risk HPV types include 6, 11, 26, 40, 42, 54, 55, 57, 61, 62, 64, 67, 69, 70, 71, 72, 81, 83, 84, and CP6108.
High-risk HPV types include 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82, and IS39.
Similar patterns were observed when stratifying HPV infections by low or high risk (Table 2). We found a significant reduction in the likelihood of HPV clearance comparing infections in HIV-infected women to those in HIV-uninfected women among incident LR-HPV infections (adjusted HR = 0.55; 95% CI, .33–.93), but a nonsignificant positive association among prevalent LR-HPV infections (adjusted HR = 1.45; 95% CI, .82–2.58). For HR-HPV infections, the associations for incident versus prevalent infections showed a similar pattern but were attenuated, and the association among incident infections was not statistically significant (adjusted HR = 0.64; 95% CI, .36–1.13 for incident infections and adjusted HR = 1.01; 95% CI, .67–1.53 for prevalent infections).
Association of HIV Type, CD4 Cell Count, and HPV Clearance
Among incident HPV infections, the 2-year cumulative probability of HPV clearance was 73.5% (95% CI, 65.4%–81.0%) for HIV-1–infected women, 80.9% (95% CI, 60.3%–94.9%) for HIV-2–infected women, and 77.3% (95% CI, 48.9%–96.2%) for HIV-1/2 dually infected women. Among prevalent HPV infections, the 2-year cumulative probability of HPV clearance was 54.0% (95% CI, 46.3%–62.1%) for HIV-1–infected women and 21.4% (95% CI, 7.0%–55.1%) for HIV-1/2 dually infected women (we were unable to estimate 2-year cumulative probability for HIV-2–infected women with prevalent HPV infections due to sparse data) (Figure 2). Among HIV-positive women (Table 3), incident HPV infections in HIV-2–infected women and HIV-1/2 dually infected women were more likely to clear than those in HIV-1–infected women, but the relationship was not significant for HIV-2–infected women (adjusted HR = 1.66; 95% CI, .95–2.92 for HIV-2–infected women and adjusted HR = 2.17; 95% CI, 1.12–4.22 for HIV-1/2 dually infected women). No significant associations between HIV type and clearance of HPV were observed among prevalent HPV infections (adjusted HR = 0.77; 95% CI, .22–2.63 for HIV-2–infected women and adjusted HR = 0.69; 95% CI, .36–1.35 for HIV-1/2 dually infected women).
Figure 2.
Cumulative probability of clearing human papillomavirus (HPV) infection by human immunodeficiency virus (HIV) type. Data are for 99 HIV-positive Senegalese women with HPV infections, in 2006–2010. Cumulative probability of clearing HPV was estimated by Kaplan-Meier methods. A, In total, 207 type-specific incident HPV infections were analyzed, including 174 from HIV-1–infected women, 22 from HIV-2–infected women, and 11 from HIV-1/2 dually infected women. A total of 249 person-years were at risk of clearance. We observed 140 HPV infections were cleared, including 115 from HIV-1–infected women, 17 from HIV-2–infected women, and 8 from HIV-1/2 dually infected women. The result of log-rank test indicated that the distribution of time until clearance of incident HPV infection is the same for women infected by different HIV types at the .05 significance level (P = .1). B, In total, 274 type-specific prevalent HPV infections were analyzed, including 221 from HIV-1–infected women, 32 from HIV-2–infected women, and 21 from HIV-1/2 dually infected women. A total of 344 person-years were at risk of clearance. We observed 126 HPV infections were cleared, including 113 from HIV-1–infected women, 6 from HIV-2–infected women, and 7 from HIV-1/2 dually infected women. The result of log-rank test indicated that the distribution of time until clearance of prevalent HPV infection is the same for women infected by different HIV types at the .05 significance level (P = .5).
Table 3.
Hazard Ratios for the Association of HIV Type, CD4 Cell Count, and Clearance of HPV Infection Among 99 HIV-Positive Senegalese Women With HPV Infections, in 2006–2010
| Incident HPV Infectionsa | Prevalent HPV Infectionsa | P Valued | |||||
|---|---|---|---|---|---|---|---|
| Eventsb | Person-Years | HR (95% CI)c | Eventsb | Person-Years | HR (95% CI)c | ||
| HIV type | |||||||
| ȃHIV-1 | 115 | 214 | Ref. | 113 | 290 | Ref. | … |
| ȃHIV-2 | 17 | 25 | 1.66 (.95–2.92) | 6 | 28 | 0.77 (.22–2.63) | .21 |
| ȃDually infected | 8 | 10 | 2.17 (1.12–4.22) | 7 | 27 | 0.69 (.36–1.35) | <.01 |
| CD4 cell count | |||||||
| ȃ> 500 | 53 | 74 | Ref. | 19 | 55 | Ref. | … |
| ȃ≤ 500 | 81 | 156 | 0.65 (.42–1.01) | 104 | 280 | 0.86 (.46–1.60) | .45 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; HPV, human papillomavirus; HR, hazard ratio; Ref., reference category.
Incident HPV infections are type-specific HPV infections initially detected during follow-up; prevalent HPV infections are type-specific HPV infections present at baseline.
Events are number of clearances of type-specific HPV infection.
Adjusted for the following covariates: age at enrollment, lifetime number of sex partners at enrollment, and concurrent infection with ≥1 additional HPV type at time of initial detection of type-specific HPV infection.
P value for difference in HRs between incident and prevalent HPV infection.
Separately (Table 3), incident HPV infections in HIV-positive women with CD4 cell count ≤500 cells/μL were borderline statistically significantly less likely to clear compared with those in HIV-positive women with CD4 cell count >500 cells/μL (adjusted HR = 0.65; 95% CI, .42–1.01). No significant association was observed for prevalent HPV infections (adjusted HR = 0.86; 95% CI, .46–1.60).
DISCUSSION
We observed that the effect of HIV on clearance of type-specific HPV infection among women in Senegal, Africa was different for incident versus prevalent HPV infections. Among incident HPV infections, those in HIV-positive women were less likely to clear than those in HIV-negative women, those in HIV-2–infected women and HIV-1/2 dually infected women were more likely to clear than those in HIV-1–infected women, and those in HIV-infected women with CD4 cell count ≤500 cells/μL were less likely to clear than those in HIV-infected women with CD4 cell count >500 cells/μL. However, these associations were not observed among prevalent HPV infections.
A number of cross-sectional studies of HPV infection among sub-Saharan African women have been conducted, indicating HIV-positive women are more likely to harbor HPV than HIV-negative women [10, 14, 25]. Importantly, however, because persistent infection with HPV is a necessary cause of cervical cancer, research into associations between HIV and HPV persistence and clearance can be used to inform strategies for identifying and monitoring populations that may be at high risk for CIN and ICC [26], especially in Africa where resources for implementing cervical cancer screening and HPV vaccination programs are limited [17].
Although we did not observe any association between HIV status and clearance of prevalent HPV infection, HIV-positive women in our study had a significant reduction in the likelihood of clearance of incident HPV infection, compared with HIV-negative women. The finding for incident HPV infections is consistent with most other research [6–8]. For example, Whitham et al [6] used data obtained from 6 studies in Senegal that included some of the same women assessed in our analysis. They observed that HIV-positive women had a 0.46-times (95% CI, .39–.54) lower probability of HPV clearance than HIV-negative women. When analyzing prevalent cases of HPV infection, results from several studies were inconsistent [8, 27–29]. Although some studies [8, 27] provided evidence of a reduction in the likelihood of HPV clearance among HIV-positive women, compared with HIV-negative women, other studies [28, 29] reported that HIV status did not have an impact on clearance of any HPV, HR-HPV, or LR-HPV. The duration of prevalent HPV infection is likely to be underestimated due to left censoring. The association between HIV infection and HPV clearance is potentially attenuated for prevalent HPV infections if the impact of HIV is more significant at the beginning of an HPV infection. Incident infection is probably more informative for assessing the effect of HIV infection on clearance of HPV infection.
Our analyses demonstrated that the associations of HIV status and HPV clearance were similar between LR-HPV and HR-HPV infections. The conclusion was consistent with previous studies [7, 8]. Rowhani-Rahbar et al [8] reported that the strength of the association between HIV status and HPV clearance did not significantly differ between LR-HPV and HR-HPV infections among Senegalese women (P = .18). The result may suggest a simplified model in which we can combine HR-HPV and LR-HPV infections when analyzing the effect of HIV on HPV.
Few prior studies evaluated evidence regarding the impact of HIV type on HPV infection [6, 8, 14]. Our previous research [8] indicated that HIV-2 is associated with lower risk of HPV detection and persistent HPV infection compared with HIV-1, but the associations were attenuated by adjustment for CD4 cell count. This suggests that HIV type affects HPV infection, at least to some degree, through the differential rates of CD4 cell depletion. Compared with HIV-1, lower pathogenicity of HIV-2 may permit the host to mount more effective, sustained T-cell immunity to control HPV infection [30]. However, the study was conducted during the pre-ART era, with inconsistent HPV detection methods that did not allow for individual typing of specific HPV types, and the analyses only evaluated prevalent HPV infections and not incident infections. The present analysis found that women with HIV-2 infection or HIV-1/2 dual infection had higher likelihood of clearance of HPV infection than women with HIV-1 infection, although the results may be unreliable with wide confidence intervals. In the era of ART, different therapies for HIV-1, HIV-2, and dual infection, and different proportions of treated patients across HIV types may impact the association between HIV types and clearance of HPV infection. CD4 cell count and HIV load may have a stronger relationship with HPV clearance than HIV type. In the current analysis, we observed the association between lower CD4 cell count and lower likelihood of clearance of HPV infection among incident HPV infections, consistent with most other studies [6–8, 14, 29, 31]. CD4 cell count may be on the causal pathway of the relationship between HIV type and HPV clearance. A systematic review [32] also suggested that CD4 cell count may have a more instrumental role in HPV persistence and cervical oncogenesis than either HIV type or ART use.
This study has several strengths, most notably a longitudinal sample to compare HPV clearance in HIV-positive and HIV-negative women. It allows us to better identify the sequence of HIV infections and HPV infections, understand their potential causal relationships, and provide insight into the natural history of HPV infection over time. Furthermore, we were able to assess the effect of HIV type, as both HIV-1 and HIV-2 are endemic to West Africa. Another strength is we evaluated the effect of HIV infection on the natural history of HPV infection at the infection level, instead of at the individual level. It takes advantage of the richness of the data collection, which included detection of 38 HPV types assessed over numerous study visits. Finally, we examined the difference in effect of HIV infection between prevalent and incident HPV infections, and reported the results separately for prevalent and incident HPV infections to reduce the bias caused by underestimated duration of prevalent infections.
Several limitations in our study should be noted. First, it is still possible that the estimated effects were confounded by unmeasured factors, although we took multiple potential confounders into account. For example, information on HIV load and ART use was unavailable in the study. They may be important factors confounding the relationship between HIV types and HPV clearance. However, we examined the impact of CD4 cell count to capture some of HIV load and the treatment effect indirectly. The second limitation is a modest number of women recruited, although each participant was sampled repeatedly. The small sample size may have limited our ability to detect statistical significance when stratifying analyses by prevalent versus incident HPV infection. In particular, the infections were stratified into 4 cross-strata by HPV infection status and HPV DNA risk type to assess the relationship between HIV status and clearance of HR versus LR-HPV infection separately. Finally, some variables may be time-varying, but we did not consider the variability. For example, CD4 cell counts were not constant over follow-up and CD4 cell count at the most recent visit may be more relevant to HPV clearance than baseline CD4 cell count. However, it may not be a main concern because the data showed CD4 cell counts over time were generally concentrated near the baseline value.
In summary, we found that HIV-positive women were less likely than HIV-negative women to clear incident HPV infection in Senegal, West Africa. Among HIV-positive women, we observed low CD4 cell count and dual HIV infection were each associated with reduced likelihood of clearance. In the limited resource setting of sub-Saharan Africa, targeted cervical cancer screening programs for the high-risk population of HIV-positive women, especially populations with low CD4 cell count, are needed. In addition, HPV vaccination programs should be aimed at populations that are at high risk for HIV infection because HIV is associated with reduced ability to clear HPV infections. Further studies assessing the potential long-term impact of ART and HIV load suppression on persistence of HPV infection at the population level and investigating its mechanisms at the individual level are needed.
Notes
Acknowledgments. We thank the research teams from the University of Washington and Senegal for undertaking this research, as well as the many women who participated in this study.
Financial support. This work was supported by the National Institutes of Health, National Cancer Institute (grant number CA111187 to N. B. K., support for the study in which the data were collected). This article reports a secondary data analysis of previously collected data and did not have specific support.
Contributor Information
Zhuochen Li, Department of Epidemiology, University of Washington, Seattle, Washington, USA.
Rachel L Winer, Department of Epidemiology, University of Washington, Seattle, Washington, USA.
Selly Ba, Service des Maladies Infectieuses Centre Hospitalier National Universitaire (CHNU) de Fann, Dakar, Sénégal.
Marie Pierre Sy, Service des Maladies Infectieuses Centre Hospitalier National Universitaire (CHNU) de Fann, Dakar, Sénégal.
John Lin, Department of Epidemiology, University of Washington, Seattle, Washington, USA; Department of Pathology, School of Medicine, University of Washington, Seattle, Washington, USA.
Qinghua Feng, Department of Pathology, School of Medicine, University of Washington, Seattle, Washington, USA.
Geoffrey S Gottlieb, Division of Allergy and Infectious Diseases, School of Medicine, University of Washington, Seattle, Washington, USA; Department of Global Health, University of Washington, Seattle, Washington, USA.
Papa Salif Sow, Service des Maladies Infectieuses Centre Hospitalier National Universitaire (CHNU) de Fann, Dakar, Sénégal.
Nancy B Kiviat, Department of Pathology, School of Medicine, University of Washington, Seattle, Washington, USA.
Stephen E Hawes, Department of Epidemiology, University of Washington, Seattle, Washington, USA; Department of Global Health, University of Washington, Seattle, Washington, USA; Department of Health Systems and Population Health, School of Public Health, University of Washington, Seattle, Washington, USA.
References
- 1. Franco EL, Rohan TE, Villa LL. Epidemiologic evidence and human papillomavirus infection as a necessary cause of cervical cancer. J Natl Cancer Inst 1999; 91:506–11. [DOI] [PubMed] [Google Scholar]
- 2. Koutsky LA, Holmes KK, Critchlow CW, et al. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med 1992; 327:1272–8. [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention . 2015 Sexually transmitted diseases treatment guidelines—human papillomavirus (HPV) infection, June2021. https://www.cdc.gov/std/treatment-guidelines/hpv.htm. Accessed15March2021. [Google Scholar]
- 4. Guan P, Howell-Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 2012; 131:2349–59. [DOI] [PubMed] [Google Scholar]
- 5. Moscicki AB, Schiffman M, Burchell A, et al. Updating the natural history of human papillomavirus and anogenital cancers. Vaccine 2012; 30:F24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Whitham HK, Hawes SE, Chu H, et al. A comparison of the natural history of HPV infection and cervical abnormalities among HIV-positive and HIV-negative women in Senegal, Africa. Cancer Epidemiol Biomarkers Prev 2017; 26:886–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tong Y, Tonui P, Ermel A, et al. Persistence of oncogenic and non-oncogenic human papillomavirus is associated with human immunodeficiency virus infection in Kenyan women. SAGE Open Med 2020; 8:2050312120945138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rowhani-Rahbar A, Hawes SE, Sow PS, et al. The impact of HIV status and type on the clearance of human papillomavirus infection among Senegalese women. J Infect Dis 2007; 196:887–94. [DOI] [PubMed] [Google Scholar]
- 9. Ahdieh L, Muñoz A, Vlahov D, Trimble CL, Timpson LA, Shah K. Cervical neoplasia and repeated positivity of human papillomavirus infection in human immunodeficiency virus-seropositive and -seronegative women. Am J Epidemiol 2000; 151:1148–57. [DOI] [PubMed] [Google Scholar]
- 10. Clifford GM, Tully S, Franceschi S. Carcinogenicity of human papillomavirus (HPV) types in HIV-positive women: a meta-analysis from HPV infection to cervical cancer. Clin Infect Dis 2017; 64:1228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu G, Sharma M, Tan N, Barnabas RV. HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. AIDS 2018; 32:795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gottlieb GS, Raugi DN, Smith RA. 90-90-90 For HIV-2? Ending the HIV-2 epidemic by enhancing care and clinical management of patients infected with HIV-2. Lancet HIV 2018; 5:e390–9. [DOI] [PubMed] [Google Scholar]
- 13. Nyamweya S, Hegedus A, Jaye A, Rowland-Jones S, Flanagan KL, Macallan DC. Comparing HIV-1 and HIV-2 infection: lessons for viral immunopathogenesis. Rev Med Virol 2013; 23:221–40. [DOI] [PubMed] [Google Scholar]
- 14. Hanisch RA, Sow PS, Toure M, et al. Influence of HIV-1 and/or HIV-2 infection and CD4 count on cervical HPV DNA detection in women from Senegal, West Africa. J Clin Virol 2013; 58:696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ngom NF, Faye MA, Ndiaye K, et al. ART Initiation in an outpatient treatment center in Dakar, Senegal: a retrospective cohort analysis (1998–2015). PLoS One 2018; 13:e0202984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kelly H, Weiss HA, Benavente Y, de Sanjose S, Mayaud P; ART and HPV Review Group . Association of antiretroviral therapy with high-risk human papillomavirus, cervical intraepithelial neoplasia, and invasive cervical cancer in women living with HIV: a systematic review and meta-analysis. Lancet HIV 2018; 5:e45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Black E, Richmond R. Prevention of cervical cancer in sub-Saharan Africa: the advantages and challenges of HPV vaccination. Vaccines (Basel) 2018; 6:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heitzinger K, Sow PS, Dia Badiane NM, et al. Trends of HIV-1, HIV-2 and dual infection in women attending outpatient clinics in Senegal, 1990–2009. Int J STD AIDS 2012; 23:710–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gottlieb GS, Sow PS, Hawes SE, et al. Equal plasma viral loads predict a similar rate of CD4+ T cell decline in human immunodeficiency virus (HIV) type 1- and HIV-2-infected individuals from Senegal, West Africa. J Infect Dis 2002; 185:905–14. [DOI] [PubMed] [Google Scholar]
- 20. Hawes SE, Critchlow CW, Sow PS, et al. Incident high-grade squamous intraepithelial lesions in Senegalese women with and without human immunodeficiency virus type 1 (HIV-1) and HIV-2. J Natl Cancer Inst 2006; 98:100–9. [DOI] [PubMed] [Google Scholar]
- 21. Kuypers JM, Critchlow CW, Gravitt PE, et al. Comparison of dot filter hybridization, southern transfer hybridization, and polymerase chain reaction amplification for diagnosis of anal human papillomavirus infection. J Clin Microbiol 1993; 31:1003–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bouvard V, Baan R, Straif K, et al. A review of human carcinogens–part B: biological agents. Lancet Oncol 2009; 10:321–2. [DOI] [PubMed] [Google Scholar]
- 23. Holmes RS, Hawes SE, Touré P, et al. HIV Infection as a risk factor for cervical cancer and cervical intraepithelial neoplasia in Senegal. Cancer Epidemiol Biomarkers Prev 2009; 18:2442–6. [DOI] [PubMed] [Google Scholar]
- 24. Winer RL, Hughes JP, Feng Q, Stern JE, Xi LF, Koutsky LA. Incident detection of high-risk human papillomavirus infections in a cohort of high-risk women aged 25–65 years. J Infect Dis 2016; 214:665–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taku O, Businge CB, Mdaka ML, et al. Human papillomavirus prevalence and risk factors among HIV-negative and HIV-positive women residing in rural Eastern Cape, South Africa. Int J Infect Dis 2020; 95:176–82. [DOI] [PubMed] [Google Scholar]
- 26. Sudenga SL, Shrestha S. Key considerations and current perspectives of epidemiological studies on human papillomavirus persistence, the intermediate phenotype to cervical cancer. Int J Infect Dis 2013; 17:e216–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Banura C, Sandin S, van Doorn LJ, et al. Type-specific incidence, clearance and predictors of cervical human papillomavirus infections (HPV) among young women: a prospective study in Uganda. Infect Agent Cancer 2010; 5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Adebamowo SN, Famooto A, Dareng EO, et al. Clearance of type-specific, low-risk, and high-risk cervical human papillomavirus infections in HIV-negative and HIV-positive women. J Glob Oncol 2018; 4:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koshiol JE, Schroeder JC, Jamieson DJ, et al. Time to clearance of human papillomavirus infection by type and human immunodeficiency virus serostatus. Int J Cancer 2006; 119:1623–9. [DOI] [PubMed] [Google Scholar]
- 30. Zheng NN, Kiviat NB, Sow PS, et al. Comparison of human immunodeficiency virus (HIV)-specific T-cell responses in HIV-1- and HIV-2-infected individuals in Senegal. J Virol 2004; 78:13934–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Travassos AG, Netto E, Xavier-Souza E, et al. Predictors of HPV incidence and clearance in a cohort of Brazilian HIV-infected women. PLoS One 2017; 12:e0185423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Menon S, Rossi R, Zdraveska N, et al. Associations between highly active antiretroviral therapy and the presence of HPV, premalignant and malignant cervical lesions in sub-Saharan Africa, a systematic review: current evidence and directions for future research. BMJ Open 2017; 7:e015123. [DOI] [PMC free article] [PubMed] [Google Scholar]