Abstract
Background
Old age is an important risk factor for developing cancer, but few data exist on this association in people with human immunodeficiency virus (HIV, PWH) in sub-Saharan Africa.
Methods
The South African HIV Cancer Match study is a nationwide cohort of PWH based on a linkage between HIV-related laboratory records from the National Health Laboratory Service and cancer diagnoses from the National Cancer Registry for 2004–2014. We included PWH who had HIV-related tests on separate days. Using natural splines, we modeled cancer incidence rates as a function of age.
Results
We included 5 222 827 PWH with 29 580 incident cancer diagnoses—most commonly cervical cancer (n = 7418), Kaposi sarcoma (n = 6380), and breast cancer (n = 2748). In young PWH, the incidence rates for infection-related cancers were substantially higher than for infection-unrelated cancers. At age 40 years, the most frequent cancer was cervical cancer in female and Kaposi sarcoma in male PWH. Thereafter, the rates of infection-unrelated cancers increased steeply, particularly among male PWH, where prostate cancer became the most frequent cancer type at older age. Whereas Kaposi sarcoma rates peaked at 34 years (101/100 000 person-years) in male PWH, cervical cancer remained the most frequent cancer among older female PWH.
Conclusions
Infection-related cancers are common in PWH in South Africa, but rates of infection-unrelated cancers overtook those of infection-related cancers after age 54 years in the overall study population. As PWH in South Africa live longer, prevention and early detection of infection-unrelated cancers becomes increasingly important. Meanwhile, control strategies for infection-related cancers, especially cervical cancer, remain essential.
Keywords: HIV, cancer, age, incidence, South Africa
We studied the association between age and the incidence of various cancers in people living with the human immunodeficiency virus in South Africa. The risk of most, but not all, cancer types increased with age.
Older age is an important, nonmodifiable risk factor for cancer with incidence rates increasing rapidly after age 50 years [1, 2]. The strong association between increasing age and cancer risk may be explained by the accumulation of somatic mutations, a weakening immune system, changes in the tissue environment, and longer exposure to carcinogens [3, 4]. However, not all cancer types follow this age pattern. Cancers that occur earlier in life are rare, associated with lifestyle factors or infections [4].
People with human immunodeficiency virus (HIV, PWH) have a higher cancer risk than HIV-negative individuals, potentially due to HIV-induced immunodeficiency, oncogenic coinfections, and, in certain settings, a higher prevalence of smoking and alcohol use among PWH [5, 6]. Antiretroviral therapy (ART) has reduced the incidence of certain cancers such as Kaposi sarcoma and non-Hodgkin lymphoma and improved life expectancy among PWH worldwide [7, 8]. As PWH live longer, the burden of cancers traditionally associated with older age has increased in this population [9–11]. Some studies reported that the risk of certain cancer types increased drastically with older age among PWH, independently of immunodeficiency and viral suppression status [12, 13]. However, studies of cancer risk in PWH often treat age as a factor of secondary interest. Large-scale studies specifically examining the relationship between age and individual cancer types in PWH are rare, especially in resource-limited settings. Yet a solid description of age-specific cancer incidence rates among PWH is essential for planning cancer prevention and care programs in this population. Therefore, we used data from the South African HIV Cancer Match (SAM) study, a nationwide cohort of PWH [14], to examine the association between age and cancer risk and estimate age-specific cancer rates in male and female PWH in South Africa.
METHODS
The SAM Study
The SAM study is based on a linkage between records from the National Health Laboratory Service (NHLS), the largest pathology service in South Africa, and the National Cancer Registry (NCR) for the years 2004–2014 [14]. The NHLS serves the public sector with laboratories in all provinces. Established in 1986, the NCR collects data on pathologically confirmed cancer diagnoses from public and private laboratories throughout South Africa [15]. Using a privacy-preserving probabilistic record linkage (P3RL) encryption tool [16], we first created a virtual cohort of PWH by identifying HIV-related laboratory records most likely belonging to the same individual. In a second step, we linked PWH to cancer diagnoses. HIV-related laboratory measurements included positive HIV tests, CD4 cell counts and percentages, and HIV RNA viral loads. We obtained ethical approval from the Human Research Ethics Committee of the University of the Witwatersrand (M190594), Johannesburg, South Africa, and the Cantonal Ethics committee (2016-00589) in Bern, Switzerland.
Inclusion Criteria and Definitions
We included PWH who had HIV-related laboratory tests on separate days and available information on sex and age. Individuals diagnosed with cancer before their first HIV-related laboratory result were excluded from the analysis of that specific cancer type. In a separate analysis, we restricted the study population to individuals with ≥1 CD4 count measurement and ≥1 year of follow-up after their first CD4 count measurement.
We divided cancers into infection-related and infection-unrelated. Infection-related cancers were categorized into Kaposi sarcoma, cervical cancer, non-Hodgkin lymphoma, human papillomavirus (HPV)-related cancers (non-AIDS-defining), Epstein-Barr virus-related cancers (non-AIDS-defining), conjunctival cancer, liver and bile duct cancer, stomach cancer, and bladder cancer. Infection-unrelated cancers included breast cancer, colorectal cancer, connective tissue cancer, leukemia, lung cancer, melanoma, esophageal cancer, prostate cancer, and other infection-unrelated cancers. Cancer types were identified using site and morphology codes from the International Classification of Diseases for Oncology, 3rd Edition (ICD-0-3), see Supplementary Table 1. Ill-defined cancers and cancers with unknown primary site were not considered. We excluded basal cell carcinoma (ICD-0-3 morphology codes 8090-8110) and squamous cell carcinoma of the skin (ICD-0-3 topography codes C44.0-9 in combination with morphology codes 8050-8084) from all analyses.
Statistical Analysis
We used descriptive statistics to assess age, calendar year, and CD4 count of PWH at their first HIV-related laboratory test (baseline). For baseline CD4 counts we considered measurements within 14 days of the first HIV-related laboratory test. Additionally, for PWH who developed cancer we assessed the age and calendar year at cancer diagnosis.
Individuals were considered at risk from their first HIV-related laboratory measurement until 6 months after their last HIV-related laboratory measurement, database closure (1 January 2015), or the first diagnosis of the cancer(s) under consideration, whichever came first. We estimated cancer incidence rates per 100 000 person-years (py) as a continuous function of age using Royston-Parmar parametric survival models [17]. We fit separate unadjusted models for the incidence of infection-related and infection-unrelated cancers. We estimated incidence rates for specific cancers including either sex, CD4 count (0–199, 200–349, ≥ 350 cells/µL), or baseline calendar year (2004–2009, 2010–2014) as an independent variable. The CD4 counts were time-updated and lagged by 1 year to minimize the risk of our results being affected by reverse causality. Thus, for analyses involving CD4 counts the time-at-risk started 1 year after an individual's first CD4 count measurement. For each cancer type, we considered 3–6 degrees of freedom for the natural spline basis for the incidence rate, choosing the models based on the lowest Akaike Information Criteria (AIC). We modeled interactions between age and either sex, CD4 count, or baseline calendar year using natural splines with 1–3 degrees of freedom. For each cancer, we compared the interaction model with lowest AIC to the corresponding model without the interaction using likelihood ratio tests. We retained the interaction if the P value was <.05. We summarize the choice of the number of degrees of freedom in Supplementary Table 2. Data management and analyses were performed in R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study Population
We included 5 222 827 PWH with 15 376 297 py of follow-up (Supplementary Figure 1). The median time-at-risk was 2.4 years (interquartile range [IQR] 1.1–4.2). Over two-thirds of the study population were female (N = 3 593 738; 69%). The median age at the first HIV-related test was 33 years (IQR 26–41), and the majority had their first HIV-related test between 2008 and 2011 (Table 1). A total of 29 580 individuals developed cancer, including 19 749 with an infection-related and 8764 with an infection-unrelated cancer. The most common cancers were cervical cancer (N = 7418), Kaposi sarcoma (N = 6380), and breast cancer (N = 2748). The characteristics of PWH with an incident cancer are shown in Table 2 (infection-related cancers, any cancer) and Table 3 (infection-unrelated cancers). The median age at cancer diagnosis varied from 35 years (IQR 29–43) for EBV-related non-AIDS-defining cancers and 35 years (IQR 30–42) for Kaposi sarcoma to 59 years (IQR 54–64) for prostate cancer.
Table 1.
Characteristics of Eligible Individuals From the South African HIV Cancer Match (SAM) Study at the Time of Their First HIV-related Laboratory Test
| Characteristic | N (%) |
|---|---|
| Total | 5 222 827 |
| Female | 3 593 738 (68.8) |
| Median age [IQR], y | 32.91 [26.13, 40.83] |
| Age group, y | |
| ȃ0–14 | 330 280 (6.3) |
| ȃ15–29 | 1 712 924 (32.8) |
| ȃ30–44 | 2 329 666 (44.6) |
| ȃ45–59 | 753 029 (14.4) |
| ȃ60–74 | 90 816 (1.7) |
| ≥ 75 | 6112 (0.1) |
| Calendar period | |
| ȃ2004–2007 | 1 131 399 (21.7) |
| ȃ2008–2011 | 2 679 987 (51.3) |
| ȃ2012–2014l | 1 411 441 (27.0) |
| Median CD4 cell count [IQR], cells/µL | 286 [151, 459] |
| CD4 cell count, cells/µL | |
| ȃ0–49 | 373 788 (7.2) |
| ȃ50–99 | 367 990 (7.0) |
| ȃ100–199 | 819 033 (15.7) |
| ȃ200–349 | 1 230 564 (23.6) |
| ȃ350–499 | 854 581 (16.4) |
| ȃ≥ 500 | 959 814 (18.4) |
| ȃMissing | 617 057 (11.8) |
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range.
Table 2.
Characteristics at the Time of Cancer Diagnosis for Individuals With an Infection-related Cancer or Any Cancer
| AIDS-defining Cancers | Non-AIDS-defining Cancers | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cervical cancer | Kaposi Sarcoma | Non-Hodgkin lymphoma | Human papillomavirus-related | Conjunctival cancer |
Epstein-Barr virus-related | Stomach cancer | Bladder cancer | Liver and bile duct cancer | Any infection-related cancer | Any Cancer | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Total | 7418 | 6380 | 2590 | 1094 | 1080 | 609 | 284 | 195 | 189 | 19 749 | 29 580 |
| Female | 7418 (100.0) | 3103 (48.6) | 1387 (53.6) | 765 (69.9) | 705 (65.3) | 300 (49.3) | 123 (43.3) | 87 (44.6) | 84 (44.4) | 13 890 (70.3) | 19 873 (67.2) |
| Median age [IQR], y | 42.57 [36.40, 49.69] |
35.49 [30.05, 42.02] |
38.62 [32.20, 45.79] |
40.62 [33.81, 48.80] |
38.00 [33.35, 43.67] |
35.18 [29.21, 42.71] |
49.17 [41.33, 57.06] |
51.63 [41.80, 58.37] |
44.84 [36.73, 53.82] |
39.21 [33.03, 46.75] |
41.60 [34.37, 50.20] |
| Age group, y | |||||||||||
| ȃ0–14 | 0 (0.0) | 105 (1.6) | 96 (3.7) | 2 (0.2) | 1 (0.1) | 30 (4.9) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 250 (1.3) | 363 (1.2) |
| ȃ15–29 | 454 (6.1) | 1470 (23.0) | 361 (13.9) | 119 (10.9) | 134 (12.4) | 138 (22.7) | 18 (6.3) | 4 (2.1) | 19 (10.1) | 3973 (20.1) | 3301 (11.2) |
| ȃ30–44 | 3943 (53.2) | 3718 (58.3) | 1426 (55.1) | 592 (54.1) | 725 (67.1) | 326 (53.5) | 86 (30.3) | 59 (30.3) | 75 (39.7) | 10 731 (54.3) | 14 344 (48.5) |
| ȃ45–59 | 2603 (35.1) | 961 (15.1) | 622 (24.0) | 329 (30.1) | 199 (18.4) | 109 (17.9) | 131 (46.1) | 92 (47.2) | 71 (37.6) | 4193 (21.2) | 9453 (32.0) |
| ȃ60–74 | 395 (5.3) | 123 (1.9) | 83 (3.2) | 49 (4.5) | 18 (1.7) | 6 (1.0) | 46 (16.2) | 38 (19.5) | 21 (11.1) | 570 (2.9) | 2028 (6.9) |
| ȃ≥ 75 | 23 (0.3) | 3 (0.0) | 2 (0.1) | 3 (0.3) | 3 (0.3) | 0 (0.0) | 3 (1.1) | 2 (1.0) | 2 (1.1) | 32 (0.2) | 91 (0.3) |
| Calendar period | |||||||||||
| ȃ2004–2007 | 962 (13.0) | 1517 (23.8) | 452 (17.5) | 95 (8.7) | 166 (15.4) | 105 (17.2) | 35 (12.3) | 19 (9.7) | 19 (10.1) | 7878 (39.9) | 4563 (15.4) |
| ȃ2008–2011 | 3083 (41.6) | 2960 (46.4) | 1147 (44.3) | 406 (37.1) | 510 (47.2) | 237 (38.9) | 116 (40.8) | 81 (41.5) | 89 (47.1) | 9153 (46.3) | 12 639 (42.7) |
| ȃ2012–2014 | 3373 (45.5) | 1903 (29.8) | 991 (38.3) | 593 (54.2) | 404 (37.4) | 267 (43.8) | 133 (46.8) | 95 (48.7) | 81 (42.9) | 2718 (13.8) | 12 378 (41.8) |
Abbreviation: IQR, interquartile range.
Table 3.
Characteristics at the Time of Cancer Diagnosis for Individuals With an Infection-unrelated Cancer
| Breast cancer | Colorectal cancer | Connective tissue cancer | Leukemia | Lung Cancer | Melanoma | Esophagus cancer | Prostate cancer | Other infection-unrelated cancers | Any infection-unrelated cancer | |
|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Total | 2748 | 655 | 243 | 359 | 734 | 235 | 589 | 652 | 2685 | 8764 |
| Female | 2707 (98.5) | 362 (55.3) | 130 (53.5) | 187 (52.1) | 208 (28.3) | 137 (58.3) | 262 (44.5) | 0 (0.0) | 1558 (58.0) | 5489 (62.6) |
| Median age [IQR], y | 44.77 [38.02, 52.16] |
48.02 [38.67, 56.19] |
40.01 [31.61, 49.86] |
36.51 [28.30, 46.33] |
52.59 [46.65, 58.34] |
47.57 [39.19, 55.08] |
52.47 [46.43, 58.02] |
59.00 [53.67, 64.46] |
45.86 [36.91, 54.87] |
47.46 [38.66, 55.55] |
| Age group, y | ||||||||||
| ȃ0–14 | 1 (0.0) | 0 (0.0) | 16 (6.6) | 38 (10.6) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 70 (2.6) | 126 (1.4) |
| ȃ15–29 | 118 (4.3) | 39 (6.0) | 39 (16.0) | 70 (19.5) | 7 (1.0) | 13 (5.5) | 9 (1.5) | 1 (0.2) | 238 (8.9) | 532 (6.1) |
| ȃ30–44 | 1274 (46.4) | 226 (34.5) | 99 (40.7) | 150 (41.8) | 134 (18.3) | 86 (36.6) | 109 (18.5) | 28 (4.3) | 970 (36.1) | 3063 (34.9) |
| ȃ45–59 | 1126 (41.0) | 283 (43.2) | 71 (29.2) | 80 (22.3) | 444 (60.5) | 110 (46.8) | 364 (61.8) | 324 (49.7) | 1080 (40.2) | 3819 (43.6) |
| ȃ60–74 | 222 (8.1) | 104 (15.9) | 18 (7.4) | 21 (5.8) | 147 (20.0) | 25 (10.6) | 102 (17.3) | 284 (43.6) | 308 (11.5) | 1175 (13.4) |
| ȃ≥ 75 | 7 (0.3) | 3 (0.5) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 1 (0.4) | 5 (0.8) | 15 (2.3) | 19 (0.7) | 49 (0.6) |
| Calendar period | ||||||||||
| ȃ2004–2007 | 278 (10.1) | 60 (9.2) | 51 (21.0) | 69 (19.2) | 90 (12.3) | 42 (17.9) | 65 (11.0) | 47 (7.2) | 351 (13.1) | 1041 (11.9) |
| ȃ2008–2011 | 1148 (41.8) | 244 (37.3) | 117 (48.1) | 167 (46.5) | 276 (37.6) | 84 (35.7) | 240 (40.7) | 212 (32.5) | 1202 (44.8) | 3645 (41.6) |
| ȃ2012–2014 | 1322 (48.1) | 351 (53.6) | 75 (30.9) | 123 (34.3) | 368 (50.1) | 109 (46.4) | 284 (48.2) | 393 (60.3) | 1132 (42.2) | 4078 (46.5) |
| ȃMissing | 375 (13.6) | 91 (13.9) | 41 (16.9) | 63 (17.5) | 105 (14.3) | 30 (12.8) | 78 (13.2) | 79 (12.1) | 433 (16.1) | 1275 (14.5) |
Abbreviation: IQR, interquartile range.
Age and Cancer Incidence
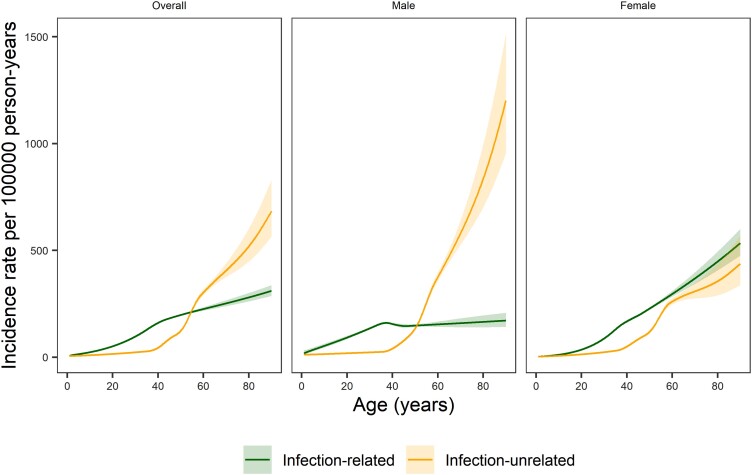
In young PWH, both male and female, the incidence rates for infection-related cancers were substantially higher than for infection-unrelated cancers (Figure 1). However, from the age of 40 years, the incidence rate of infection-unrelated cancers increased steeply. The rate of infection-unrelated cancers overtook those of infection-related cancers by age 55 years in the overall study population and by age 51 years in male PWH. In female PWH, the rate of infection-related cancers was higher than that of infection-unrelated cancers across all age groups.
Figure 1.
Incidence rates per 100 000 person-years (solid lines) as a function of age for any infection-related cancer and any infection-unrelated cancer, overall, for men, and for women. The shaded areas represent 95% confidence intervals. We included an interaction between sex and age in the sex-adjusted model for infection-related cancers (P value for interaction <.001) and infection-unrelated cancers (P value for interaction <.001).
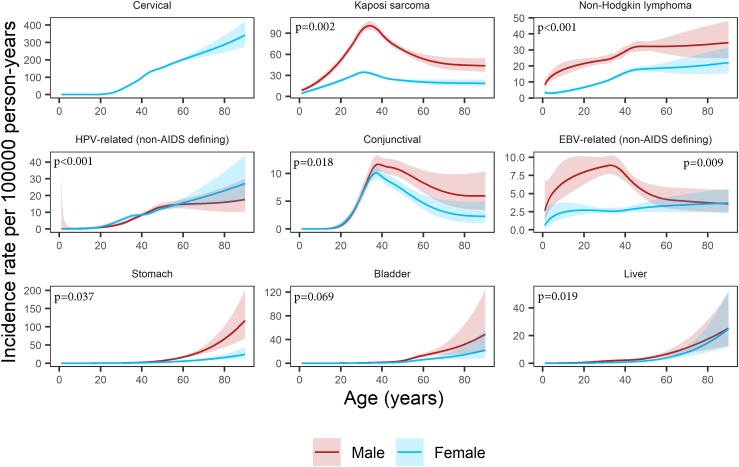
We found significant interactions between age and sex for all infection-related cancers except bladder cancer and for none of the infection-unrelated cancers. Still, for most cancer types and at most ages, the incidence rates in male PWH were higher than or similar to those in female PWH (Figure 2). Some of the infection-related cancers showed a distinct age-specific incidence pattern with a peak among middle-aged PWH. The Kaposi sarcoma rate peaked at age 31 years in female (34.4/100 000 py, 95% confidence interval [CI]: 32.7–36.2) and 34 years in male PWH (100.8/100 000 py, 95% CI: 94.6–107.5). The conjunctival cancer rate peaked at age 37 years in female (10.1/100 000 py, 95% CI: 8.9–11.4) and 38 years in male PWH (11.6/100 000 py, 95% CI: 10.0–13.2). For EBV-related non-AIDS-defining cancers we found an incidence rate peak at age 33 years in male PWH (8.9/100 000 py, 95% CI: 7.7–10.3), but no such peak was evident for female PWH. Incidence rates for other infection-related cancers were highest among older PWH (Figure 2). For example, the incidence rates of liver, stomach, and bladder cancer remained very low until age 40 years in both male and female PWH (<2.5/100 000 py) but increased at older age (Supplementary Table 3). In female PWH, the incidence rates of cervical and other HPV-related cancers increased after age 20 years. For example, between age 20 and 40 years, cervical cancer rates increased from 1.5/100 000 py (95% CI: 1.0–2.2) to 98.5/100 000 py (95% CI: 94.5–102.8). Of note, the incidence rate of non-AIDS defining HPV-related cancers was higher in male than female PWH between the ages of 41 and 56 years. In both male and female PWH, non-Hodgkin lymphoma rates increased steadily until age 45 years before stabilizing.
Figure 2.
Incidence rates per 100 000 person-years (solid lines) as a function of age and sex for infection-related cancers, with P values from the likelihood ratio test comparing the model with an interaction between age and sex to the model without. The interaction was included in all analyses except that of bladder cancer. The shaded areas represent 95% confidence intervals. Abbreviations: EBV, Epstein-Barr virus; HPV, human papillomavirus.
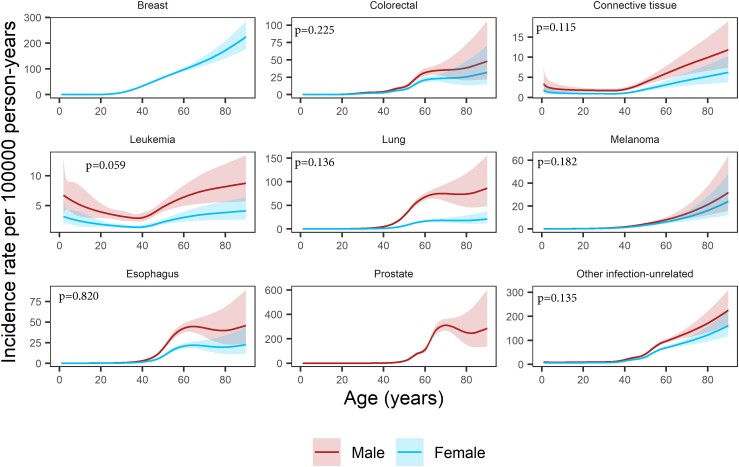
For most infection-unrelated cancers, the steep incidence rate increase started after the age of 40 years (Figure 3). Breast cancer incidence rates increased substantially from age 30 years already, reaching 33.4/100 000 py (95% CI: 31.6–35.2) at age 40 years and 171.6/100 000 py (95% CI: 140.5–209.6) at age 80 years. For colorectal, lung, esophageal, and prostate cancer, incidence rates stabilized in older PWH. In contrast, incidence rates for leukemia showed a bimodal pattern with highest rates in young and old PWH. The lowest leukemia rates occurred at age 37 years in both female (1.3/100 000 py, 95% CI: 1.1–1.6) and male PWH (2.9/100 000 py, 95% CI: 2.3–3.5).
Figure 3.
Incidence rates per 100 000 person-years (solid lines) as a function of age and sex for infection-unrelated cancers, with P values from the likelihood ratio test comparing the model with an interaction between age and sex to the model without. We did not include an interaction between age and sex in any of the analyses. The shaded areas represent 95% confidence intervals.
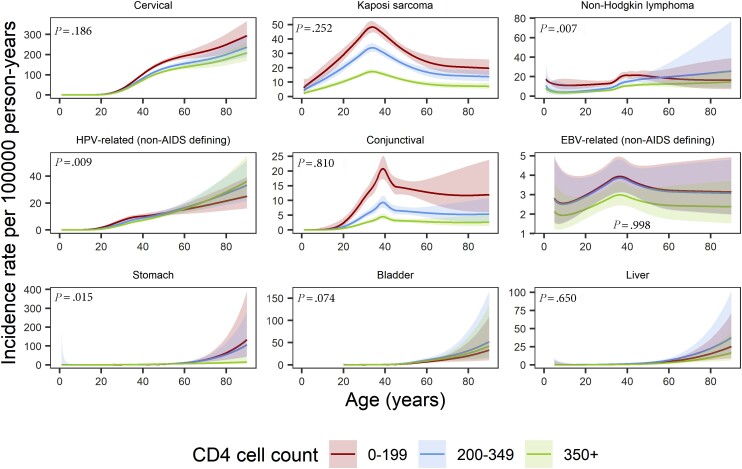
There were significant interactions between age and CD4 cell count for some of the infection-related cancers, that is, non-Hodgkin lymphoma, non-AIDS defining HPV-related cancers, and stomach cancer but for none of the infection-unrelated cancers. Incidence rates of most infection-related cancers were higher at lower CD4 cell counts (Figure 4 and Supplementary Table 4). The non-Hodgkin lymphoma rate peaked between ages 40 and 45 years (23.3/100 000 py, 95% CI: 20.2–26.9) in PWH with CD4 cell counts <200 cells/µL, whereas for PWH with ≥200 CD4 cells/µL incidence rates increased from middle-age onward. There were negligible differences by CD4 cell counts in the incidence rates of infection-unrelated cancers (Supplementary Figure 2, Supplementary Table 4). We found an interaction between age and baseline calendar period for non-AIDS defining HPV-related cancers and conjunctival cancer, but not for other types of cancers (Supplementary Figures 3 and 4, Supplementary Table 5).
Figure 4.
Incidence rates per 100 000 person-years (solid lines) as a function of age and CD4 cell count (cells/µL, time-updated, lagged 1 year) for infection-related cancers, with P values from the likelihood ratio test comparing the model with an interaction between age and CD4 cell count to the model without. We included the interaction in the analysis of non-Hodgkin lymphoma, HPV-related (non-AIDS defining) cancers, and stomach cancer. The estimates for bladder cancer are left-truncated at age 20 y and the estimates for the EBV-related cancers and liver cancer are left-truncated at age 5 y. The shaded areas represent 95% confidence intervals. Abbreviations: EBV, Epstein-Barr virus; HPV, human papillomavirus.
DISCUSSION
In this study of over 5 million PWH in South Africa, we found that below the age of 40 years, infection-related cancers were the predominant incident cancer types in both male and female PWH. After that age, incidence rates of infection-unrelated cancers sharply increased. The rates of infection-unrelated cancers surpassed those of infection-related cancers by the age of 55 years in the overall study population and by age 51 years in male PWH. In female PWH, infection-related cancers remained the dominant incident cancer type throughout the life span. The rates of most cancer types increased continuously with older age. In contrast, incidence rates of Kaposi sarcoma and conjunctival cancer—2 cancer types strongly associated with immunodeficiency—peaked in middle-aged PWH.
This is 1 of the first large-scale studies to focus on age as an important risk factor for developing cancer in PWH in Sub-Saharan Africa. The large cohort size allowed the assessment of individual cancer types. We used flexible parametric survival models with splines to estimate cancer rates across the age continuum and avoided applying arbitrary age category cutoffs. All cancers were laboratory confirmed and ICD-0-3 coded. However, our results must be interpreted considering some limitations. The cancer incidence rates in our study are likely underestimated because diagnoses were pathology-based and clinically diagnosed cases were missed. A Dutch study found that individuals with clinically diagnosed cancer were older than those with pathology-confirmed diagnoses [18]. If the same pattern applies to South Africa, we may have underestimated cancer risk in older PWH more than in young PWH. We defined incident cancers as diagnoses that occurred at any time after the first HIV-related laboratory measurement. Other studies used clinic enrolment or ART initiation as starting points for time-at-risk. Therefore, incidence estimates across studies may not be readily comparable. The SAM study does not currently include death or migration data; thus, we censored time-at-risk 6 months after an individual's last HIV-related laboratory measurement. Furthermore, we did not have access to socioeconomic, behavioral, or ART information and could not assess whether these factors modified the association between age and cancer incidence.
Few studies specifically examined age as a risk factor for developing cancer among PWH, and data from Sub-Saharan Africa are particularly scarce. Furthermore, most studies did not assess age as a continuous variable, and comparisons across studies are hampered by the varying age categories chosen in the individual studies. Nevertheless, in line with our results, a European study reported that old age was a strong risk factor for infection-related and infection-unrelated cancers in PWH with the association being stronger for infection-unrelated cancers [10]. Because of the relatively low number of cancers (n = 643), cancer types could not be assessed individually [10]. In contrast to our results, this study did not find significant interactions between CD4 cell count and age for the risk of developing infection-related cancers whereas for infection-unrelated cancers the association of lower CD4 counts with incident cancer seemed to be stronger among PWH aged <50 years [10]. However, findings between the 2 studies are difficult to compare as the European study did not assess individual cancers and used only two broad age categories (<50 years and ≥50 years) to assess interactions between age and CD4 counts. Interaction between age and sex was not assessed. A larger US-based study restricted to PWH aged ≥50 years found that incidence rates for different infection-unrelated cancers such as lung, prostate, and colon cancer increased substantially with older age [19]. In contrast, Kaposi sarcoma rates were lower in PWH aged >69 years compared to those aged 50–59 years [19]. The association between age and Kaposi sarcoma remains unclear with some studies reporting highest incidence rates in young [20–22] or middle-aged PWH [23] and others in older PWH [12, 24]. These conflicting results may be partly explained by the large geographic variation in the age-specific prevalence of human herpesvirus 8, the infectious cause of Kaposi sarcoma [25], and differences in ART coverage between the study populations.
In the South African general population, 85% of incident cancers occur in people aged ≥40 years [26]. Aging may increase cancer risk through prolonged carcinogenic exposure, accumulation of somatic mutations, a deteriorating immune system, and changes in the tissue environment [3, 4]. Several studies have suggested that the ageing process is accelerated in PWH, and thus, age-related comorbidities such as cancer may occur earlier [27, 28], but some controversy around this hypothesis remains [29, 30]. In our analysis, incidence rates in young PWH were higher for infection-related than infection-unrelated cancers. The most frequent cancer type at the age of 40 years was Kaposi sarcoma in male and cervical cancer in female PWH. Interestingly, incidence rates of Kaposi sarcoma and conjunctival cancer—the cancer types most strongly associated with low CD4 counts in a previous analysis of the SAM study [31]—peaked in middle-aged PWH, whereas cervical cancer rates started increasing substantially and steadily after the age of 20 years. Although the HIV-attributable fraction of cervical cancer is particularly high (86%) among young women (≤35 years) in Southern Africa [32], we found that cervical cancer was also the most frequent cancer type in older women with HIV. In contrast, the most frequent cancer type in older men with HIV was prostate cancer.
With ART widely available, the distribution of PWH in South Africa is shifting toward older ages [33, 34]. We found that in PWH aged ≥55 years, incidence rates for infection-unrelated cancers were higher than for infection-related cancers. Over time, the prevention and early detection of infection-unrelated cancers will become increasingly important to reduce cancer-related morbidity and mortality among PWH in South Africa. Prevention strategies for infection-unrelated cancers include smoking cessation interventions, promotion of a healthy lifestyle, avoidance of excessive sun exposure, and adherence to recommended routine screenings [35, 36]. Nevertheless, prevention of infection-related cancers remains important throughout the lifespan of PWH but especially among young PWH where infection-related cancers are the predominant incident cancer types. Prevention strategies to reduce the burden of infection-related cancers include HPV vaccination, screening for and treatment of pre-cancerous cervical lesions, hepatitis B virus (HBV) vaccination, and treatment of HBV and hepatitis C virus infection [35]. Furthermore, early ART initiation may reduce the risk of developing infection-related and potentially also infection-unrelated cancers [37].
In conclusion, incidence rates of most cancers increased with older age among PWH in South Africa, but rates of Kaposi sarcoma and conjunctival cancer peaked among middle-aged PWH. In young PWH infection-related cancers were the dominant cancer types, whereas most cancer diagnoses in PWH above the age of 54 years were infection-unrelated. As PWH in South Africa become older, prevention and early detection of infection-unrelated cancers will become increasingly important. However, promotion of prevention strategies for infection-related cancers will remain essential to reduce the cancer burden among young PWH in South Africa.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Yann Ruffieux, Institute of Social and Preventive Medicine (ISPM), University of Bern, Bern, Switzerland.
Mazvita Muchengeti, National Cancer Registry, National Health Laboratory Service, Johannesburg, South Africa; School of Public Health, University of the Witwatersrand, Johannesburg, South Africa.
Victor Olago, National Cancer Registry, National Health Laboratory Service, Johannesburg, South Africa.
Tafadzwa Dhokotera, Institute of Social and Preventive Medicine (ISPM), University of Bern, Bern, Switzerland; National Cancer Registry, National Health Laboratory Service, Johannesburg, South Africa; Swiss Tropical and Public Health Institute, Allschwil, Switzerland; University of Basel, Basel, Switzerland; Graduate School for Cellular and Biomedical Sciences, University of Bern, Bern, Switzerland.
Julia Bohlius, Institute of Social and Preventive Medicine (ISPM), University of Bern, Bern, Switzerland; Swiss Tropical and Public Health Institute, Allschwil, Switzerland; University of Basel, Basel, Switzerland.
Matthias Egger, Institute of Social and Preventive Medicine (ISPM), University of Bern, Bern, Switzerland; Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, United Kingdom; Centre for Infectious Disease Epidemiology and Research (CIDER), School of Public Health and Family Medicine, University of Cape Town, Cape Town, South Africa.
Eliane Rohner, Institute of Social and Preventive Medicine (ISPM), University of Bern, Bern, Switzerland.
Notes
Acknowledgments. The authors would like to express our deep gratitude to the late Elvira Singh, (acting) head of the South African NCR from 2013 to 2022, for her invaluable role in making the SAM study possible. They thank Lina Bartels for performing the record linkage and Yannick Turdo for his help with reviewing the literature. Calculations were performed on UBELIX (http://www.id.unibe.ch/hpc), the HPC cluster at the University of Bern.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH) and by the National Cancer Institute (NCI) under award number U01AI069924 to the IeDEA-Southern Africa (ongoing sub-award), an NIH administrative supplement (grant number U01AI069924-09 to M. E. and J. B.), PEPFAR supplement (to M. E.), the Swiss National Science Foundation (SNSF, grant number 320030_169967 to J. B. and M. E.), and the US CRDF Global (grant number HIV_DAA3-16-62705-1 to M. M.). M. E. was supported by special project funding (grant number 207285) from the SNSF, and T. D. by the European Union’s Horizon 2020 research and innovation programme (Marie Skłodowska-Curie grant agreement number 801076), through the SSPH+Global PhD Fellowship Programme. M. M. also reports support for this work from the U.S. CRDF Global BIG Cat award ($50 000 USD catalytic grant).
References
- 1. Risk Factors: Age - National Cancer Institute. 2022. Available at: https://www.cancer.gov/about-cancer/causes-prevention/risk/age . Accessed 5 September 2022.
- 2. Ervik M, Lam F, Laversanne M, Ferlay J, Bray F. Global Cancer Observatory: Cancer Over Time. Lyon, France: International Agency for Research on Cancer. 2021. Available at:https://gco.iarc.fr/overtime/en/dataviz/cohorts?populations=3600&sexes=1&cohort_type=age&cohort=cohort&cancers=0&scale=linear&min_zero=1. Accessed 5 September 2022.
- 3. Laconi E, Marongiu F, DeGregori J. Cancer as a disease of old age: changing mutational and microenvironmental landscapes. Br J Cancer 2020; 122:943–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rozhok A, DeGregori J. A generalized theory of age-dependent carcinogenesis. Elife 2019; 8:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol 2012; 24:506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neto MMS, Brites C, Borges ÁH. Cancer during HIV infection. APMIS 2020; 128:121–8. [DOI] [PubMed] [Google Scholar]
- 7. Engels EA, Pfeiffer RM, Goedert JJ, et al. . Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS 2006; 20:1645–54. [DOI] [PubMed] [Google Scholar]
- 8. Wandeler G, Johnson LF, Egger M. Trends in life expectancy of HIV-positive adults on antiretroviral therapy across the globe: comparisons with general population. Curr Opin HIV AIDS 2016; 11:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shiels MS, Pfeiffer RM, Gail MH, et al. . Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst 2011; 103:753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shepherd L, Borges Á, Ledergerber B, et al. . Infection-related and -unrelated malignancies, HIV and the aging population. HIV Med 2016; 17:590–600. [DOI] [PubMed] [Google Scholar]
- 11. Shiels MS, Islam JY, Rosenberg PS, Hall HI, Jacobson E, Engels EA. Projected cancer incidence rates and burden of incident cancer cases in HIV-infected adults in the United States through 2030. Ann Intern Med 2018; 168:866–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guiguet M, Boué F, Cadranel J, Lang JM, Rosenthal E, Costagliola D. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol 2009; 10:1152–9. [DOI] [PubMed] [Google Scholar]
- 13. Sigel K, Wisnivesky J, Crothers K, et al. . Immunological and infectious risk factors for lung cancer in US veterans with HIV: a longitudinal cohort study. Lancet HIV 2017; 4:e67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Muchengeti M, Bartels L, Olago V, et al. . Cohort profile: the South African HIV Cancer Match (SAM) Study, a national population-based cohort. BMJ Open 2022; 12:e053460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Singh E, Sengayi M, Urban M, Babb C, Kellett P, Ruff P. The South African national cancer registry: an update. Lancet Oncol 2014; 15:e363. [DOI] [PubMed] [Google Scholar]
- 16. Schmidlin K, Clough-Gorr KM, Spoerri A, Group for SNC study . Privacy preserving probabilistic record linkage (P3RL): a novel method for linking existing health-related data and maintaining participant confidentiality. BMC Med Res Methodol 2015; 15:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Royston P, Parmar MKB. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med 2002; 21:2175–97. [DOI] [PubMed] [Google Scholar]
- 18. van der Willik KD, Rojas-Saunero LP, Labrecque JA, et al. . Pathology-confirmed versus non pathology-confirmed cancer diagnoses: incidence, participant characteristics, and survival. Eur J Epidemiol 2020; 35:557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mahale P, Engels EA, Coghill AE, Kahn AR, Shiels MS. Cancer risk in older persons living with human immunodeficiency virus infection in the United States. Clin Infect Dis 2018; 67:50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luo Q, Satcher Johnson A, Hall HI, Cahoon EK, Shiels M. Kaposi sarcoma rates among persons living with human immunodeficiency virus in the United States: 2008—2016. Clin Infect Dis 2021; 73:e2226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wong IKJ, Grulich AE, Poynten IM, et al. . Time trends in cancer incidence in Australian people living with HIV between 1982 and 2012. HIV Med 2022; 23:134–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luu HN, Amirian ES, Chiao EY, Scheurer ME. Age patterns of Kaposi's sarcoma incidence in a cohort of HIV-infected men. Cancer Med 2014; 3:1635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Asasira J, Lee S, Tran TXM, et al. . Infection-related and lifestyle-related cancer burden in Kampala, Uganda: projection of the future cancer incidence up to 2030. BMJ Open 2022; 12:e056722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cancer Project Working Group for the Collaboration of Observational HIV Epidemiological Research Europe (COHERE) study in EuroCoord . Changing incidence and risk factors for Kaposi sarcoma by time since starting antiretroviral therapy: collaborative analysis of 21 European cohort studies. Clin Infect Dis 2016; 63:1373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rohner E, Wyss N, Heg Z, et al. . HIV and human herpesvirus 8 co-infection across the globe: systematic review and meta-analysis. Int J Cancer 2016; 138:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferlay J, Ervik M, Lam F, et al. . Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. 2020. Available at: https://gco.iarc.fr/today , accessed 5 September 2022.
- 27. Guaraldi G, Orlando G, Zona S, et al. . Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011; 53:1120–6. [DOI] [PubMed] [Google Scholar]
- 28. De Francesco D, Wit FW, Bürkle A, et al. . Do people living with HIV experience greater age advancement than their HIV-negative counterparts? AIDS 2019; 33:259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rasmussen LD, May MT, Kronborg G, et al. . Time trends for risk of severe age-related diseases in individuals with and without HIV infection in Denmark: a nationwide population-based cohort study. Lancet HIV 2015; 2:e288–98. [DOI] [PubMed] [Google Scholar]
- 30. Wing EJ. HIV And aging. Int J Infect Dis 2016; 53:61–8. [DOI] [PubMed] [Google Scholar]
- 31. Ruffieux Y, Muchengeti M, Egger M, et al. . Immunodeficiency and cancer in 3.5 million people living with human immunodeficiency virus (HIV): the South African HIV cancer match study. Clin Infect Dis 2021; 73:e735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khalil A I, Mpunga T, Wei F, et al. . Age-specific burden of cervical cancer associated with HIV: a global analysis with a focus on sub-Saharan Africa. Int J Cancer 2022; 150:761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hontelez JAC, Lurie MN, Newell M-L, et al. . Ageing with HIV in South Africa. AIDS 2011; 25:1665–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rishworth A, Aging KB. (Un)certainty and HIV management in South Africa. Popul Space Place 2022; 28:e2552. [Google Scholar]
- 35. Goncalves PH, Montezuma-Rusca JM, Yarchoan R, Uldrick TS. Cancer prevention in HIV-infected populations. Semin Oncol 2016; 43:173–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Department of Health, South Africa . National Cancer Strategic Framework for South Africa 2017–2022.
- 37. Borges ÁH, Neuhaus J, Babiker AG, et al. . Immediate antiretroviral therapy reduces risk of infection-related cancer during early HIV infection. Clin Infect Dis 2016; 63:1668–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.