Abstract
Background
Understanding immunity against Omicron infection and severe outcomes conferred by coronavirus disease 2019 (Covid-19) vaccination, prior severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and monoclonal antibody therapy will inform intervention strategies.
Methods
We considered 295 691 patients tested for SARS-CoV-2 at Cleveland Clinic between 1 October 2021 and 31 January 2022. We used logistic regression to investigate the association of vaccination and prior infection with the risk of SARS-CoV-2 infection and used Cox regression to investigate the association of vaccination, prior infection, and monoclonal antibody therapy with the risks of intensive care unit (ICU) stay and death.
Results
Vaccination and prior infection were less effective against Omicron than Delta infection but provided strong protection against ICU admission and death. Boosting greatly increased vaccine effectiveness against Omicron infection and severe outcomes, although effectiveness waned rapidly over time. Monoclonal antibody therapy considerably reduced risks of ICU admission and death. The relatively low mortality of the Omicron variant was due to both reduced lethality of this variant and increased population immunity acquired from booster vaccination and previous infection.
Conclusions
Booster vaccination and prior SARS-CoV-2 infection provide strong protection against ICU admission and death from Omicron infection. Monoclonal antibody therapy is also beneficial.
Keywords: COVID-19, SARS-CoV-2, mRNA vaccines, monoclonal antibodies, vaccine boosters, waning immunity
Using data from 295 691 patients who were tested for SARS-CoV-2 at Cleveland Clinic, we found booster vaccination and prior SARS-CoV-2 infection provide strong protection against ICU admission and death from Omicron infection and that monoclonal antibody therapy is also beneficial.
The Omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), first detected in South Africa on 25 November 2021, has spread rapidly around the world and has been the predominant strain in the United States since mid-December 2021 [1]. The Omicron variant contains multiple mutations that are associated with higher transmissibility and greater potential to evade immunity from neutralizing antibodies than the Delta variant that preceded it [2, 3]. Thus, coronavirus disease 2019 (Covid-19) vaccination and SARS-CoV-2 infection with previous strains of the virus may not confer strong immunity against Omicron infection. However, the waning immunity of booster vaccination and prior SARS-CoV-2 infection against Omicron infection and severe outcomes (eg, hospitalization, death) has not yet been quantified. In addition, there has been no investigation of the effectiveness of monoclonal antibody therapy given after Omicron infection against severe outcomes in a real-world setting.
To address the above issues, we recently conducted a large study in the United States to compare the association of COVID-19 vaccination and prior SARS-CoV-2 infection with infection with the Omicron versus Delta variants; we also compared the association of vaccination, prior infection, and monoclonal antibody therapy with severe illness caused by the 2 variants. Although we were primarily interested in the Omicron variant, the Delta variant provided a useful benchmark for us to evaluate how well vaccination, prior infection, and therapy protect against Omicron infection and severe outcomes, as well as the lethality of the Omicron variant.
METHODS
We considered all patients who were tested for SARS-CoV-2 by polymerase chain reaction in the Cleveland Clinic Health System between 1 October 2021 and 31 January 2022, extracting each patient's medical record from the Covid-19 registry database (Supplementary Methods) [4]. We divided the study period into 2 phases: 1 October to 11 December (the Delta-predominant period) and 19 December to 31 January (the Omicron-predominant period), omitting the week ending on 18 December, when the Omicron variant was emerging. A total of 171 249 and 124 442 patients were tested, with 28 998 and 47 352 positive results, during the Delta- and Omicron-predominant periods, respectively. The patients were followed for clinical outcomes, including hospitalization, admission to intensive care unit (ICU), and death, until 10 June 2022. The information on death was obtained from our electronic health records, which were linked to the social security death master file maintained by the US Social Security Administration and were also linked to the vital records maintained by the Ohio Department of Health. The patients’ demographic and clinical characteristics are shown in Table 1.
Table 1.
Demographic and Clinical Characteristics of Patients Who Were Tested for SARS-CoV-2 in the Cleveland Clinic Health System During the Delta- and Omicron-Predominant Periods
| Variable | Total | Dose 2 | Dose 3 | Other Vaccination |
SARS-CoV-2 Positive | SARS-CoV-2 Positive Death | ||
|---|---|---|---|---|---|---|---|---|
| <180 d | ≥180 d | <180 d | ≥180 d | |||||
| All | 295 691 | 27 861 | 75 545 | 51 479 | 6834 | 17 593 | 76 350 | 1765 |
| Time period | ||||||||
| ȃDelta predominant | 171 249 (58) | 19 590 (70) | 46 741 (62) | 16 008 (31) | 3676 (54) | 9655 (55) | 28 998 (38) | 879 (50) |
| ȃOmicron predominant | 124 442 (42) | 8271 (30) | 28 804 (38) | 35 471 (69) | 3158 (46) | 7938 (45) | 47 352 (62) | 886 (50) |
| Location | ||||||||
| ȃOhio | 255 714 (86) | 24 706 (89) | 66 321 (88) | 44 145 (86) | 5600 (82) | 15 074 (86) | 69 130 (91) | 1613 (91) |
| ȃOther | 39 977 (14) | 3155 (11) | 9224 (12) | 7334 (14) | 1234 (18) | 2519 (14) | 7220 (9) | 152 (9) |
| Age, y | ||||||||
| ȃ <65 | 220 908 (75) | 24 291 (87) | 52 319 (69) | 26 653 (52) | 3034 (44) | 13 246 (75) | 61 927 (81) | 1613 (91) |
| ȃ ≥65 | 74 783 (25) | 3570 (13) | 23 226 (31) | 24 826 (48) | 3800 (56) | 4347 (25) | 14 423 (19) | 152 (8.6) |
| Sex | ||||||||
| ȃFemale | 173 286 (59) | 17 400 (62) | 46 665 (62) | 30 950 (60) | 3917 (57) | 10 216 (58) | 44 859 (59) | 788 (45) |
| ȃMale | 122 405 (41) | 10 461 (38) | 28 880 (38) | 20 529 (40) | 2917 (43) | 7377 (42) | 31 491 (41) | 977 (55) |
| Race and ethnicity | ||||||||
| ȃWhite, non-Hispanic | 201 158 (68) | 17 833 (64) | 55 920 (74) | 40 526 (79) | 5595 (82) | 11 820 (67) | 50 949 (67) | 1223 (70) |
| ȃBlack, non-Hispanic | 44 319 (15) | 5209 (19) | 8807 (12) | 4558 (9) | 564 (8) | 3085 (18) | 12 456 (16) | 355 (20) |
| ȃHispanic | 18 628 (6) | 2418 (9) | 4471 (6) | 2343 (4) | 263 (4) | 1161 (6) | 4750 (6) | 59 (3) |
| ȃOther/unknown | 31 586 (11) | 2401 (8) | 6347 (8) | 4052 (8) | 412 (6) | 1527 (9) | 8195 (11) | 128 (7) |
| Prior infection | ||||||||
| ȃNo | 272 759 (92) | 25 056 (90) | 69 631 (92) | 48 145 (94) | 6412 (94) | 15 881 (90) | 72 421 (95) | 1726 (98) |
| ȃYes | 22 932 (8) | 2805 (10) | 5914 (8) | 3334 (6) | 422 (6) | 1712 (10) | 3929 (5) | 39 (2) |
| Smoking | ||||||||
| ȃNever | 159 878 (54) | 15 981 (57) | 42 854 (57) | 29 586 (57) | 3678 (54) | 9019 (51) | 41 224 (54) | 592 (34) |
| ȃCurrent | 35 257 (12) | 4304 (16) | 7854 (10) | 3481 (7) | 622 (9) | 3030 (17) | 8551 (11) | 199 (11) |
| ȃUnknown | 100 556 (34) | 7576 (27) | 24 837 (33) | 18 412 (36) | 2534 (37) | 5544 (32) | 26 575 (35) | 974 (55) |
| Respiratory conditionsa | ||||||||
| ȃNo | 226 889 (77) | 20 639 (74) | 56 619 (75) | 37 435 (73) | 4768 (70) | 12 869 (73) | 60 375 (79) | 1143 (65) |
| ȃYes | 68 802 (23) | 7222 (26) | 18 926 (25) | 14 044 (27) | 2066 (30) | 4724 (27) | 15 975 (21) | 622 (35) |
| Nonrespiratory conditionsa | ||||||||
| ȃNo | 115 716 (39) | 10 310 (37) | 22 577 (30) | 11 670 (23) | 1198 (18) | 5615 (32) | 32 886 (43) | 180 (10) |
| ȃYes | 179 975 (61) | 17 551 (63) | 52 968 (70) | 39 809 (77) | 5636 (82) | 11 978 (68) | 43 464 (57) | 1585 (90) |
| Immunocompromised condition | ||||||||
| ȃNo | 273 933 (93) | 25 891 (93) | 69 813 (92) | 45 774 (89) | 5938 (87) | 15 762 (90) | 71 822 (94) | 1127 (64) |
| ȃYes | 21 758 (7) | 1970 (7) | 5732 (8) | 5705 (11) | 896 (13) | 1831 (10) | 4528 (6) | 638 (36) |
Data are No. (%).
Respiratory conditions are chronic pulmonary disease and pulmonary circulation disease. Nonrespiratory conditions are congestive heart failure, valvular disease, peripheral vascular disease, paralysis, other neurological disorders, diabetes, hypothyroidism, renal failure, liver disease, peptic ulcer disease and bleeding, lymphoma, metastatic cancer, solid tumor without metastasis, rheumatoid arthritis/collagen vascular diseases, coagulopathy, obesity, fluid and electrolyte disorders, chronic blood loss anemia, deficiency anemias, alcohol abuse, drug abuse, psychoses, and depression. Elixhauser Comorbidity Software version 3.7 was used to extract the data. The definitions and ICD-10 codes can be found at: https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp.
We used the case-control design [5] to evaluate the association of vaccination and prior infection with SARS-CoV-2 infection, separately for the Delta- and Omicron-predominant periods. We performed multivariable logistic regression of SARS-CoV-2 infection on vaccination status, prior infection, age, sex, race/ethnicity, smoking status, comorbidities, week of testing, and geographic location. We classified vaccination status into 6 categories: 14–179 days after dose 3 of mRNA vaccine (BNT162b2 or mRNA-1273); ≥ 180 days after dose 3 of mRNA vaccine; 14–179 days after dose 2 of mRNA vaccine; ≥ 180 days after dose 2 of mRNA vaccine; any other COVID-19 vaccination (including 1 dose of mRNA or Ad26.COV2.S vaccine); and unvaccinated. We defined prior infection as any infection more than 90 days before the current infection. We estimated the odds ratio of SARS-CoV-2 infection for each exposure and defined the effectiveness against SARS-CoV-2 infection as 1 minus the odds ratio.
We used the cohort design to evaluate the association of COVID-19 vaccination, prior SARS-CoV-2 infection, and monoclonal antibody therapy with progression to hospitalization, ICU admission, or death among those who had tested positive for SARS-CoV-2, separately for the Delta- and Omicron-predominant periods. Of note, monoclonal antibodies were infused in adults 18 years and older who had symptoms within 7 days of a positive test for the SARS-CoV-2 virus. We used the Kaplan-Meier method to compare the survival probabilities among the patients who tested positive for SARS-CoV-2 infection during the Delta- versus Omicron-predominant periods. In addition, we used multivariable Cox regression to relate the hazard of hospitalization, ICU admission, or death to vaccination status, prior infection, monoclonal antibody therapy, and other risk factors, separately for the Delta- and Omicron-predominant periods. We estimated the hazard ratio of each outcome with each exposure and defined the effectiveness against each outcome as 1 minus the hazard ratio. Finally, we estimated the overall relative risk of acquiring COVID-19 and then dying for each exposure by multiplying the odds ratio of SARS-CoV-2 infection and the hazard ratio of death.
RESULTS
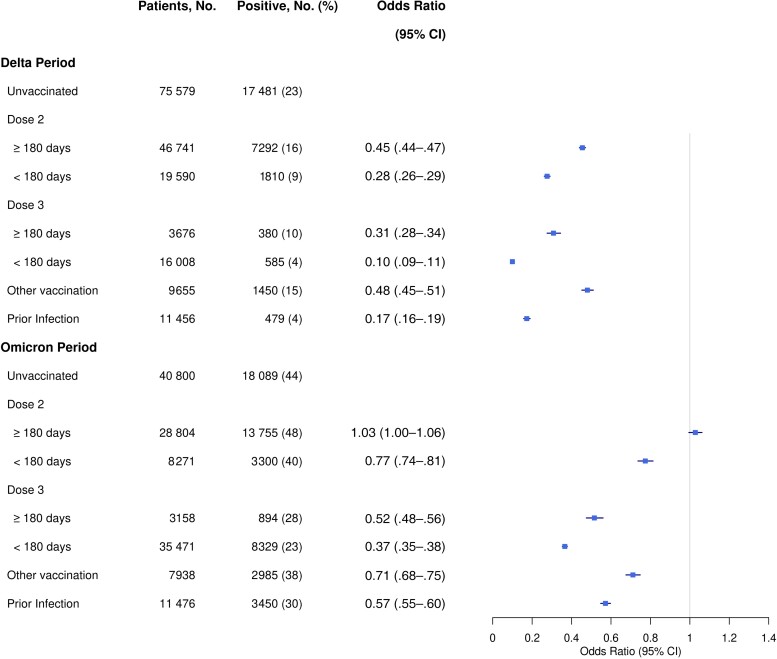
Figure 1 shows the estimated odds ratios of SARS-CoV-2 infection with vaccination status and prior infection adjusted for other risk factors. The 3-dose series was found to be much more effective than the 2-dose series against SARS-CoV-2 infection, especially with the Omicron variant. The effectiveness of both the 2-dose and 3-dose series waned markedly over time, and both series were less effective against the Omicron variant than against the Delta variant. These results extend existing knowledge about vaccine effectiveness [6–9]. Specifically, the odds ratio of infection with the Delta variant associated with having dose 3 less than 180 days ago (compared to the unvaccinated group) was estimated at 0.10 (95% confidence interval [CI], .09–.11), whereas the odds ratio of infection with the Omicron variant associated with having dose 2 more than 180 days ago was estimated at 1.03 (95% CI, 1.00–1.06). In addition, the odds ratios of infection with the Delta and Omicron variants associated with prior infection were estimated at 0.17 (95% CI, .16–.19) and 0.57 (95% CI, .55–.60), respectively, suggesting that prior infection provided less protection against the Omicron variant than the Delta variant.
Figure 1.
Association of COVID-19 vaccination and prior SARS-CoV-2 infection with the risk of infection with the Delta and Omicron variants. The odds ratio estimates are indicated by square symbols and the 95% confidence intervals (CI) are shown by horizontal lines.
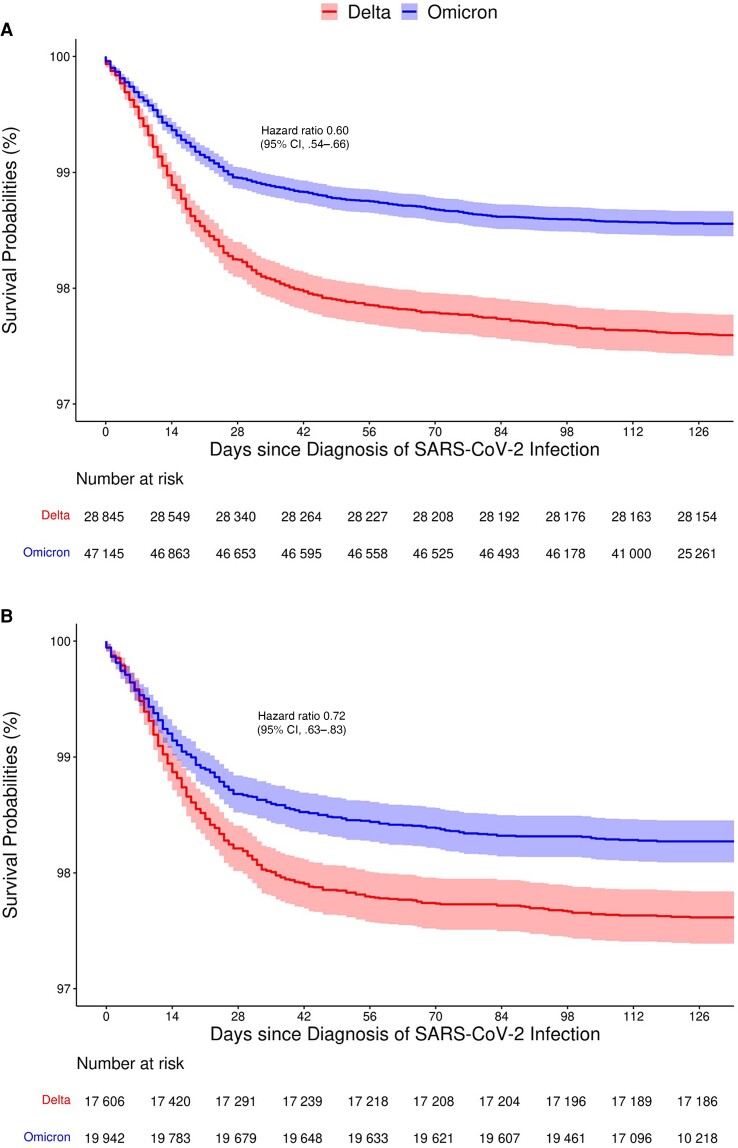
As of 10 June 2022, a total of 879 and 886 patients had died out of the 28 998 and 47 352 patients who had tested positive for SARS-CoV-2 during the Delta- and Omicron-predominant periods, respectively (Table 1). The Kaplan-Meier estimates of the survival probabilities among the patients who had tested positive for SARS-CoV-2 infection during the Omicron-predominant period were much higher than those of the Delta-predominant period, with the 28-day mortality being 1.00% (95% CI, .99%–1.10%) and 1.80% (95% CI, 1.60%–1.90%), respectively, and with the hazard ratio of dying of the Omicron versus Delta variants being 0.60 (95% CI, .54–.66) (Figure 2A ). To account for the fact that the rates of vaccination and prior infection were higher during the Omicron-predominant period than during the Delta-predominant period, we further considered the subset of patients who had not been vaccinated or previously infected. (Of note, 55.9% and 67.2% of the patients were vaccinated and 6.7% and 9.2% were previously infected in the Delta- and Omicron-predominant periods, respectively). Then the survival curves between the 2 time periods were much closer to each other, and the hazard ratio was 0.72 (95% CI, .63–.83) (Figure 2B ), indicating that the Omicron variant is intrinsically less lethal than the Delta variant.
Figure 2.
Kaplan-Meier estimates of the survival probabilities for patients who had tested positive for SARS-CoV-2 during the Delta- and Omicron-predominant periods: (A) all patients; (B) patients not vaccinated or previously infected.
Table 2 shows the estimated hazard ratios of death with vaccination status, prior SARS-CoV-2 infection, monoclonal antibody therapy, and other risk factors, separately for the Omicron and Delta variants. Older adults, males, smokers, and patients with comorbidities or immunocompromised were at elevated risk of death from both variants. The effectiveness of the 2 mRNA vaccines on the progression to death decreased over time during the Omicron-predominant period. Receiving the 2-dose or 3-dose series less than 6 months ago reduced the risk of death from the Omicron variant much more than from the Delta variant. Specifically, the hazard ratios of death from the Delta and Omicron variants associated with having dose 3 less than 180 days ago (compared with the unvaccinated group) were estimated at 0.43 (95% CI, .29–.64) and 0.28 (95% CI, .22–.35), respectively. The hazard ratios of death from the Delta and Omicron variants associated with prior infection were estimated at 0.22 (95% CI, .08–.59) and 0.47 (95% CI, .30–.73), respectively. The hazard ratio of death from the Delta and Omicron variants associated with monoclonal antibody treatments were estimated at 0.45 (95% CI, .32–.62) and 0.50 (95% CI, .31–.79), respectively.
Table 2.
Association of COVID-19 Vaccination, Prior SARS-CoV-2 Infection, and Monoclonal Antibody Therapy With the Risk of Death Caused by the Delta or Omicron Variant
| Variable | Delta Variant, HR (95% CI) | Omicron Variant, HR (95% CI) |
|---|---|---|
| Vaccination status | ||
| ȃUnvaccinated | Reference | Reference |
| ȃDose 2 ≥180 d | 0.45 (.38–.54) | 0.53 (.44–.64) |
| ȃDose 2 <180 d | 0.45 (.30–.66) | 0.26 (.14–.46) |
| ȃDose 3 ≥180 d | 0.27 (.15–.49) | 0.33 (.20–.55) |
| ȃDose 3 <180 d | 0.43 (.29–.64) | 0.28 (.22–.35) |
| ȃOther vaccination | 0.92 (.70–1.21) | 0.77 (.58–1.00) |
| Prior infection | 0.22 (.08–.59) | 0.47 (.30–.73) |
| Monoclonal antibodies | 0.45 (.32–.62) | 0.50 (.31–.79) |
| Age ≥65 y (vs <65 y) | 8.57 (7.17–10.3) | 9.92 (8.13–12.1) |
| Male (vs female) | 1.51 (1.30–1.75) | 1.41 (1.21–1.64) |
| Race and ethnicity | ||
| ȃWhite, non-Hispanic | Reference | Reference |
| ȃBlack, non-Hispanic | 1.06 (.88–1.29) | 1.13 (.94–1.36) |
| ȃHispanic | 0.99 (.65–1.51) | 0.94 (.62–1.41) |
| ȃOther/unknown | 1.42 (1.07–1.89) | 1.28 (.95–1.72) |
| Smoking status | ||
| ȃNever | Reference | Reference |
| ȃCurrent | 1.20 (.91–1.57) | 1.32 (1.03–1.70) |
| ȃUnknown | 1.92 (1.63–2.26) | 1.64 (1.38–1.94) |
| Respiratory conditions | 0.97 (.83–1.14) | 1.18 (1.00–1.39) |
| Nonrespiratory conditions | 3.36 (2.61–4.32) | 3.78 (2.77–5.17) |
| Immunocompromised conditions | 4.18 (3.55–4.93) | 4.56 (3.87–5.36) |
| Other location (vs Ohio) | 0.64 (.42–.97) | 0.52 (.39–.71) |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Table 3 shows the overall relative risk of acquiring COVID-19 and then dying of COVID-19 for each exposure. Specifically, the overall relative risk associated with receiving dose 3 less than 180 days ago (compared to the unvaccinated group) was estimated at 0.04 (95% CI, .02–.06) and 0.10 (95% CI, .08–.13) for the Delta and Omicron variants, respectively. By contrast, the overall relative risk associated with receiving dose 2 more than 180 days ago (compared to the unvaccinated group) was estimated at 0.21 (95% CI, .17–.25) and 0.54 (95% CI, .41–.65) for the Delta and Omicron variants, respectively. The overall relative risk associated with prior infection was estimated at 0.04 (95% CI, .00–.08) and 0.27 (95% CI, .11–.37) for the Delta and Omicron variants, respectively.
Table 3.
Overall Relative Risks of Infection With the Delta or Omicron Variant and Progression to Death Associated with COVID-19 Vaccination, Prior SARS-CoV-2 Infection, and Monoclonal Antibody Therapy
| Variable | Delta Variant | Omicron Variant |
|---|---|---|
| Vaccination status | ||
| ȃUnvaccinated | Reference | Reference |
| ȃDose 2 ≥180 d | 0.21 (.17–.25) | 0.54 (.41–.65) |
| ȃDose 2 <180 d | 0.12 (.05–.16) | 0.20 (.05–.32) |
| ȃDose 3 ≥180 d | 0.08 (.02–.13) | 0.17 (.08–.25) |
| ȃDose 3 <180 d | 0.04 (.02–.06) | 0.10 (.08–.13) |
| ȃOther vaccination | 0.44 (.29–.57) | 0.54 (.38–.67) |
| Prior infection | 0.04 (.00–.08) | 0.27 (.11–.37) |
| Monoclonal antibodies | 0.45 (.29–.57) | 0.50 (.28–.74) |
Shown in parentheses are 95% confidence intervals based on 200 bootstrap samples.
Of the 28 998 and 47 352 patients who tested positive for SARS-CoV-2 during the Delta- and Omicron-predominant periods, 3141 and 4174 were hospitalized, and 915 and 837 were admitted to the ICU, respectively. Supplementary Tables 1 and 2 show the estimated hazard ratios of hospitalization and ICU admission with vaccination status, prior SARS-CoV-2 infection, monoclonal antibody therapy, and other risk factors, separately for the Omicron- and Delta- predominant periods. In general, the association with hospitalization was weaker than the association with death, and the association with ICU admission was similar to the association with death.
DISCUSSION
In summary, vaccination and prior infection were less effective against infection with the Omicron variant than with the Delta variant. However, vaccination and prior infection still provided strong protection against ICU admission and death caused by Omicron infection. Boosting greatly increased the effectiveness of the 2 mRNA vaccines against infection, ICU admission, and death, although its effectiveness waned markedly over time. In addition, monoclonal antibody therapy given after Omicron infection considerably reduced the risk of ICU admission and death. Finally, the relatively low mortality caused by the Omicron variant, as compared to the Delta variant, was due to both the reduced lethality of the Omicron variant and the increased population immunity acquired from booster vaccination and previous infection.
We found that having dose 2 more than 180 days ago provided little protection against infection with the Omicron variant. This finding reflects the fact that the Omicron variant spike protein can almost completely escape from neutralizing antibodies elicited in recipients of only 2 mRNA vaccine doses [10].
We also found that prior infection provided less protection against the Omicron variant than the Delta variant. There were 2 contributing factors to this phenomenon. First, the prior infections during the Omicron-predominant period were primarily with the Delta variant, and SARS-CoV-2 infection might induce weaker immunity against a new variant than the same variant. Second, the prior infections tended to be more recent (relative to current infections) in the Delta-predominant period than in the Omicron-predominant period, the median times between prior and current infections being 322 days in the Delta-predominant period and 369 days in the Omicron-predominant period, and the immunity acquired via SARS-CoV-2 infection wanes over time.
Several other studies have evaluated the immunity conferred by booster vaccination or SARS-CoV-2 infection against the Omicron variant [7–9, 11–13]. However, the follow-up periods of previous studies were short, and severe outcomes, such as ICU admission and death, were not considered. By leveraging a large database with granular, high-quality clinical information, we were able to provide clear answers to several open questions, including (1) waning effectiveness of vaccine boosters; (2) impact of prior infection on Omicron infection; (3) protection of booster vaccination, prior infection, and monoclonal antibody therapy against ICU admission and death from Omicron infection; and (4) lethality of the Omicron variant.
We did not determine COVID-19 variants by genomic sequencing on individual patients, but rather by the variant proportions produced by the Center for Disease Control's national genomic surveillance system. Thus, our findings pertained to the Delta-predominant versus Omicron-predominant periods, rather than the Delta versus Omicron variants. However, the vast majority of the patients tested positive in the Delta-predominant period were infected with the Delta variant, and the vast majority of the patients tested positive in the Omicron-predominant period were infected with the Omicron variant.
Monoclonal antibody injections are provided only in infusion centers that are staffed by experienced personnel, and only patients with underlying health conditions were given the treatments. In addition, prior infections were likely underreported. Thus, the effectiveness of prior infection and monoclonal antibody therapy might have been underestimated in our study.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the National Institutes of Health (grant number R01 AI029168 to D.-Y. L.).
Supplementary Material
Contributor Information
Xiaofeng Wang, Lerner Research Institute, Cleveland, Ohio, USA.
Joe Zein, Lerner Research Institute, Cleveland, Ohio, USA; Respiratory Institute, Cleveland Clinic, Cleveland, Ohio, USA.
Xinge Ji, Lerner Research Institute, Cleveland, Ohio, USA.
Dan-Yu Lin, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, North Carolina, USA.
References
- 1. World Health Organization . Enhancing response to Omicron SARS-CoV-2 variant.https://www.who.int/publications/m/item/enhancing-readiness-for-omicron-(b.1.1.529)-technical-brief-and-priority-actions-for-member-states. Accessed 21 January 2022. [PubMed]
- 2. European Centre for Disease Prevention and Control . Implications of the further emergence and spread of the SARS-CoV-2 B.1.1.529 variant of concern (Omicron) for the EU/EEA—first update.https://www.ecdc.europa.eu/sites/default/files/documents/threat-assessment-covid-19-emergence-sars-cov-2-variant-omicron-december-2021.pdf. Accessed 2 December 2021.
- 3. Chen J, Wang R, Gilby NB, Wei GW. Omicron variant (B.1.1.529): infectivity, vaccine breakthrough, and antibody resistance. J Chem Inf Model 2022; 62:412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang X, Jehi L, Ji X, Mazzone PJ. Phenotypes and subphenotypes of patients with COVID-19: a latent class modeling analysis. Chest 2021; 159:2191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dean NE, Hogan JW, Schnitzer ME. COVID-19 vaccine effectiveness and the test-negative design. N Engl J Med 2021; 385:1431–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin DY, Gu Y, Wheeler B, et al. . Effectiveness of COVID-19 vaccines over a 9 month period in North Carolina. N Engl J Med 2022; 386:933–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Accorsi EK, Britton A, Fleming-Dutra KE, et al. . Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 Omicron and Delta variants. J Am Med Ass 2022; 327:639–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andrews N, Stowe J, Kirsebom F, et al. . COVID-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med 2022; 386:1532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. . Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med 2022; 386:1804–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Evans JP, Zeng C, Carlin C, et al. . Neutralizing antibody responses elicited by SARS-CoV-2 mRNA vaccination wane over time and are boosted by breakthrough infection. Sci Transl Med 2022; 14:eabn8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goldberg Y, Mandel M, Bar-On YM, et al. . Protection and waning of natural and hybrid immunity to SARS-CoV-2. N Engl J Med 2022; 386:2201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hall V, Foulkes S, Insalata F, et al. . Protection against SARS-CoV-2 after COVID-19 vaccination and previous infection. N Engl J Med 2022; 386:1207–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Altarawneh HN, Chemaitelly H, Hasan MR, et al. . Protection against the Omicron variant from previous SARS-CoV-2 infection. N Engl J Med 2022; 386:1288–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.