Abstract
Objectives
We evaluated the clinical characteristics and outcomes of patients with COVID-19 who received three-drug combination regimens for treatment of carbapenem-resistant Acinetobacter baumannii (CRAB) infections during a single-centre outbreak. Our objective was to describe the clinical outcomes and molecular characteristics and in vitro synergy of antibiotics against CRAB isolates.
Materials and methods
Patients with severe COVID-19 admitted between April and July 2020 with CRAB infections were retrospectively evaluated. Clinical success was defined as resolution of signs/symptoms of infection without need for additional antibiotics. Representative isolates underwent whole-genome sequencing (WGS) and in vitro synergy of two- or three-drug combinations was assessed by checkerboard and time-kill assays, respectively.
Results
Eighteen patients with CRAB pneumonia or bacteraemia were included. Treatment regimens included high-dose ampicillin-sulbactam, meropenem, plus polymyxin B (SUL/MEM/PMB; 72%), SUL/PMB plus minocycline (MIN; 17%) or other combinations (12%). Clinical resolution was achieved in 50% of patients and 30-day mortality was 22% (4/18). Seven patients had recurrent infections, during which further antimicrobial resistance to SUL or PMB was not evident. PMB/SUL was the most active two-drug combination by checkerboard. Paired isolates collected before and after treatment with SUL/MEM/PMB did not demonstrate new gene mutations or differences in the activity of two- or three-drug combinations.
Conclusions
Use of three-drug regimens for severe CRAB infections among COVID-19 resulted in high rates of clinical response and low mortality relative to previous studies. The emergence of further antibiotic resistance was not detected phenotypically or through WGS analysis. Additional studies are needed to elucidate preferred antibiotic combinations linked to the molecular characteristics of infecting strains.
Introduction
Acinetobacter baumannii is a difficult to treat nosocomial pathogen with a propensity for acquiring resistance against commonly used antibiotics. A significant proportion of isolates are carbapenem-resistant A. baumannii (CRAB) that are often missed empirically, and receipt of inactive therapy is a strong predictor of patient mortality.1,2 Current treatment options include polymyxins, tetracycline derivatives, such as tigecycline and eravacycline, and aminoglycosides, although none of these are ideal options due to their pharmacokinetic limitations and toxicity.3 Newer antimicrobial agents such as cefiderocol offer an alternative option; however, early clinical data did not demonstrate improved outcomes for patients with CRAB infections compared to best available therapy.4 As concerning, two randomized clinical trials have not shown any benefit for the combination of colistin plus meropenem when compared to colistin alone for treatment of CRAB infections.5,6 Thus, designing treatment regimens that are both safe and effective for CRAB infections remains a major challenge. In vitro data indicate that combination therapies including high-dose ampicillin/sulbactam with a carbapenem and a polymyxin demonstrate potent activity, but clinical data are limited to small case series including patients infected with colistin-resistant A. baumannii.5,6 Expert guidance from the Infectious Diseases Society of America now recommends ampicillin-sulbactam as a preferred single agent for mild infections. For moderate to severe CRAB infections, combination therapy with at least two in vitro active agents is suggested; however, which agents should be preferred over others is still unknown.7
We encountered a single-centre outbreak of CRAB infections among patients with severe COVID-19. Our locally developed institutional guidance prioritized an initial three-drug combination regimen given the limitations of the aforementioned treatment options. In this study, we aimed to evaluate the efficacy of this standardized approach to gain new insights into the treatment of CRAB infections. The specific objectives were to (i) define the clinical characteristics and outcomes of patients with infections due to CRAB treated with three-drug combination antibiotic therapy, (ii) determine the molecular epidemiology of infecting strains and (iii) explore in vitro synergy of two- and three-drug combination regimens using isolates from treated patients.
Materials and methods
Patients and bacterial isolates
Adult patients with severe COVID-19 admitted to the University of Maryland Medical Center between April and July 2020 with positive blood or respiratory cultures growing CRAB were identified. Only patients treated with initial three-drug antibiotic combinations were included. Optimized antibiotic regimens were used, which included high-dose ampicillin/sulbactam 9 g every 8 hours as a prolonged infusion over 4 hours, meropenem 2 g every 8 hours over 3 hours, minocycline 200 mg every 12 hours over a 1 hour and a polymyxin B 25 000 IU/kg loading dose followed by 15 000 IU/kg every 12 hours given over a 2-hour infusion. All agents were renally dose adjusted when applicable.6,8,9 Treatment regimens were selected by the patient care team and continued throughout the treatment course unless modifications were made due to adverse effects (e.g. discontinuation of polymyxin B secondary to renal toxicity). Isolates collected before (initial) and after (recurrent) treatment were stored at −80°C until analysis.
Ethics
The study was approved by the institutional review board at the University of Maryland, Baltimore (HP-00092949).
Clinical data
Patient demographics, underlying medical conditions, sequential organ failure assessment (SOFA) score at the time of CRAB infection (culture collection), antibiotic therapy received before and after isolation of CRAB, and characteristics of the infection were collected. Pneumonia was defined by the isolation of CRAB in a pulmonary specimen, radiographic evidence of pneumonia, and signs and symptoms of infection. Diagnoses were confirmed by Infectious Diseases (ID) consultants caring for the patient. Clinical success was adjudicated by three independent investigators and defined as complete resolution of signs and symptoms of infection without a change or need for additional antibiotics at the end of the intended treatment course. Indeterminate outcomes were defined as either persistence of symptoms without evidence of infection or death due to severe COVID-19 infection that precluded classification as resolution or failure. Microbiologic failure was defined as subsequent isolation of CRAB from the respiratory tract or bloodstream after completion of the initial antibiotic course, or after >14 days of treatment if the initial treatment course was prolonged during the index admission. Recurrent pneumonia was defined as positive respiratory cultures with CRAB necessitating a repeat course of antibiotics within the same admission. All patients in the study were followed by ID consult services, and the diagnosis of both initial and recurrent pneumonia was confirmed by the ID consult team in the electronic medical record. Thirty-day all-cause mortality was assessed from the date of the index CRAB culture.
Organism identification and susceptibility testing
A. baumannii isolates were identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry on the Vitek MS (bioMérieux, Durham, NC, USA). For clinical care, antibiotic susceptibility was determined using Kirby–Bauer disc diffusion for meropenem, ampicillin/sulbactam, tigecycline, minocycline and amikacin in the microbiology laboratory at the University of Maryland Medical Center. MICs for amikacin were determined by gradient diffusion Etest strips (bioMerieux, Durham, NC, USA). Minimum inhibitory concentrations (MICs) of sulbactam, cefiderocol, colistin, eravacycline, meropenem, minocycline and tigeycline were determined by broth microdilution methods in post hoc analysis, and were not available to clinicians at the time of treatment. Cefiderocol MICs were determined in iron-depleted, cation-adjusted Mueller–Hinton broth. All MICs were determined in duplicate. If results did not agree, a third test was performed and the modal MIC reported. All MICs were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) susceptibility breakpoints.10Pseudomonas aeruginosa ATCC 27853 was used for quality control.
Whole-genome sequencing
Whole-genome sequencing (WGS) was performed as described previously.11 Raw sequences were assembled using SPAdes v.3.14.1.12 Core genome single-nucleotide polymorphism (cgSNP) differences between genome pairs were identified using Snippy v.4.4.5 (https://github.com/tseemann/snippy). Paired initial and recurrent isolates obtained from the same patient were compared using breseq.13 β-lactamase genes were identified through CARD.14 Raw sequence reads and draft genome assemblies have been deposited in the NCBI database under BioProject number PRJNA852776.
Synergy testing
In vitro synergy of two- or three-drug combinations was assessed by checkerboard and time-kill assays, respectively. Eight initial CRAB isolates from unique, representative patients were selected for checkerboard analysis of two-drug synergy between all possible combinations of meropenem, minocycline, polymyxin B and sulbactam. Synergistic and additive activity was defined as a fractional inhibitory concentration (FIC) ≤ 0.5 and 0.51–1 mg/L, respectively.15
Time-kill assays were performed as previously described using clinically achievable steady-state concentrations of meropenem (8 mg/L), polymyxin B (2 mg/L) and sulbactam (4 mg/L).16 Tests were conducted using a starting concentration of 1 × 106 cfu/mL for each isolate. The log kills were calculated at 24 hours as the difference in log cfu/mL from the starting inoculum. Synergy was defined as a ≥2 log greater kill in combination compared to the most active single agent.
Results
Eighteen patients with CRAB ventilator-associated pneumonia (VAP; 13/18, 72%), bacteraemia (3/18, 17%), or hospital-acquired pneumonia (2/18, 11%) were included. At infection onset, the median SOFA score was 11 (range 4–17), 39% (7/18) were receiving extracorporeal membrane oxygenation (ECMO) and 89% (16/18) were in an intensive care unit (ICU) (Table 1). The median time from initial positive SARS-CoV-2 test to index CRAB culture was 16 days (range 4–41 days). Fifty-six percent (10/18), 72% (13/18) and 83% (15/18) of patients received three-drug combination therapy within 24-, 48- and 72-hours of initial CRAB cultures, respectively. Treatment regimens included high-dose ampicillin/sulbactam, meropenem, plus polymyxin B (72%), ampicillin/sulbactam, minocycline, plus polymyxin B (17%) or other combinations (meropenem, minocycline, polymyxin b and ampicillin/sulbactam, meropenem, minocycline; 6% each). The median treatment duration was 10 days (range 2–35). Clinical success was achieved in 50% (9/18) of patients and did not differ across treatment regimens (Table 1). Microbiologic failures occurred in 56% (10/18) of patients, including 50% (4/8) of those with initial clinical success. Fifty-four percent (7/13) of patients with VAP experienced recurrent pneumonia. The overall 30-day mortality rate was 22% (4/18). COVID-19 was listed as the cause of death in 100% (4/4) of cases. Antibiotic-associated acute kidney injury occurred in 39% (7/18) patients.
Table 1.
Clinical characteristics, treatment regimens and outcomes of COVID-19 patients with invasive CRAB infections
| Patient | Age | Sex | Type of infection | ICU | Charlson co-morbidity index | SOFA score | ECMO | Initial three-drug treatment regimen (treatment duration in days) | Clinical outcome | Microbiologic failurea | Recurrent pneumonia | Antibiotic-associated AKIb | 30-day mortality | Inpatient mortality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BCU-1 | 20 | M | BSI | Yes | 0 | 17 | Yes | SUL + MEM + PMB (14) | Resolution | Yes | — | No | No | No |
| MICU-1 | 26 | M | VAP | Yes | 0 | 15 | No | SUL + MEM + PMB (21) | Failure | Yes | Yes | No | No | No |
| BCU-4 | 58 | M | VAP | Yes | 2 | 12 | Yes | SUL + MEM + PMB (5) | Indeterminate | Yes | Yes | Yes | Yes | Yes |
| MICU-2 | 68 | F | VAP | Yes | 5 | 11 | No | SUL + MEM + PMB (3) | Indeterminate | N/A | No | No | Yes | Yes |
| MICU-7 | 35 | M | VAP | Yes | 0 | 9 | No | SUL + MEM + PMB (10) | Resolution | No | No | Yes | No | No |
| BCU-5 | 36 | M | VAP | Yes | 0 | 8 | Yes | SUL + MEM + PMB (8) | Failure | Yes | Yes | No | No | No |
| MICU-9 | 33 | F | VAP | Yes | 1 | 10 | No | SUL + MEM + PMB (8) | Resolution | N/A | No | No | No | No |
| BCU-6 | 50 | M | VAP | Yes | 1 | 12 | Yes | SUL + MEM + PMB (8) | Indeterminate | Yes | No | No | No | No |
| MICU-11 | 57 | M | VAP | Yes | 2 | 7 | Yes | SUL + MEM + PMB (14) | Resolution | Yes | Yes | No | No | No |
| MICU-13 | 79 | F | BSI | Yes | 6 | 8 | No | SUL + MEM + PMB (14) | Resolution | No | — | No | No | No |
| BCU-8 | 51 | F | VAP | Yes | 3 | 4 | Yes | SUL + MEM + PMB (10) | Resolution | Yes | Yes | Yes | No | No |
| BCU-9 | 52 | M | VAP | Yes | 1 | 7 | Yes | SUL + MEM + PMB (10) | Failure | Yes | Yes | Yes | No | Yes |
| Other2 | 64 | M | HAP | No | 5 | 5 | No | SUL + MEM + PMB (2) | Indeterminate | N/A | No | No | Yes | Yes |
| MICU-3 | 68 | M | VAP | Yes | 3 | 11 | No | SUL + MIN + PMB (3) | Failure | N/A | No | Yes | Yes | Yes |
| MICU-8 | 77 | F | VAP | Yes | 4 | 11 | No | SUL + MIN + PMB (8) | Resolution | No | No | Yes | No | No |
| MICU-12 | 61 | M | VAP | Yes | 7 | 12 | No | SUL + MIN + PMB (10) | Resolution | Yes | Yes | No | No | Yes |
| Other1 | 47 | F | HAP | No | 3 | 4 | No | MEM + MIN + PMB (14) | Resolution | N/A | No | Yes | No | No |
| MICU-10 | 43 | F | BSI | Yes | 1 | 16 | No | SUL + MEM + MIN (35) | Failure | Yes | −c | No | No | Yes |
BSI, bloodstream infection; ECMO, extracorporeal membrane oxygenation; F, female; HAP, hospital-acquired pneumonia; ICU, intensive care unit; M, male; MEM, meropenem; MIN, minocycline; PMB, polymyxin b; SUL, ampicillin-sulbactam; VAP, ventilator-associated pneumonia.
Yes: repeat cultures with CRAB from the respiratory or bloodstream after completion of the initial antibiotic course; No: repeat cultures without CRAB; N/A: repeat cultures not obtained.
Antibiotic associated acute kidney injury defined as a minimum of two increases in serum creatinine of at least 0.5 mg/dL or 50% or greater increase from baseline after several days of antibiotic therapy.
Patient developed CRAB VAP on day 8 of BSI treatment course.
Antibiotic MICs did not vary across initial isolates [Table 2, Table S1 (available as Supplementary data at JAC Online)]. The modal MICs for meropenem, minocycline, polymyxin b and ampicillin/sulbactam were >64, 8, 0.25 and 8 mg/L, respectively. Modal cefiderocol, eravacycline and tigecycline MICs were 0.12, 0.5 and 1 mg/L, respectively. Repeat susceptibility testing of recurrent isolates from patients who experienced microbiologic failures did not reveal further resistance following treatment (Table S1).
Table 2.
In vitro susceptibility of representative baseline CRAB isolates from eight patients
| Patient-isolate | MIC (mg/L) | Fractional inhibitory concentration (interpretation) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FDC | ERV | TGC | MIN | MEM | PMB | SUL | MEM + MIN | MEM + PMB | MEM + SUL | MIN + PMB | MIN + SUL | PMB + SUL | |
| BCU1-1 | 0.25 | 0.5 | 1 | 4 | >64 | ≤0.25 | 16 | 1.5 (I) | 2 (I) | 1 (I) | 2 (I) | 1 (I) | 1 (I) |
| MICU13-1 | 0.12 | 0.5 | 1 | 4 | >64 | ≤0.25 | 16 | 0.375 (S) | 2 (I) | 1.5 (I) | 2 (I) | 1 (I) | 1 (I) |
| BCU4-0 | 0.12 | 0.5 | 1 | 4 | >64 | 0.5 | 16 | 1.5 (I) | 1.5 (I) | 0.625 (A) | 0.501 (A) | 1 (I) | 0.625 (A) |
| MICU10-1 | 0.25 | 0.5 | 1 | 4 | >64 | 0.5 | 16 | 1.5 (I) | 1.5 (I) | 1.5 (I) | 2 (I) | 2 (I) | 1 (I) |
| BCU9-1 | 0.12 | 0.5 | 1 | 4 | >64 | 0.5 | 16 | 1.5 (I) | 2 (I) | 0.625 (A) | 0.565 (A) | 2 (I) | 1 (I) |
| MICU-12-1 | 0.25 | 0.5 | 1 | 8 | >64 | ≤0.25 | 8 | 1 (I) | 2 (I) | 1.25 (I) | 2 (I) | 1 (I) | 0.75 (A) |
| MICU7-1 | 0.12 | 0.5 | 2 | 4 | >64 | ≤0.25 | 8 | 1.5 (I) | 2 (I) | 2 (I) | 2 (I) | 1 (I) | 0.625 (A) |
| MICU11-0 | 0.12 | 0.5 | 2 | 4 | >64 | 0.5 | 8 | 1.5 (I) | 2 (I) | 1.5 (I) | 1.5 (I) | 1.5 (I) | 0.56 (A) |
FDC, Cefiderocol; ERV, Eravacycline; TGC, Tigecycline; MIN, Minocycline; MEM, Meropenem; PMB, Polymyxin B; SUL, Sulbactam.
Note. The following criteria was used to interpret fractional inhibitory concentration values: <0.5 = Synergy (S), 0.5–1 = Additive (A), >1 = Indifferent (I).
Thirteen representative CRAB isolates from 10 patients underwent WGS. Twelve isolates were collected prior to initial treatment, and one isolate was obtained at the time of recurrent VAP for comparison. All isolates were sequence type (ST) 2 as defined by the Pasteur Institute scheme (Pas) and ST208 by the Oxford scheme (Ox). Isolates varied by ≤2 core genome SNPs suggesting a clonal outbreak at the hospital (Table S2). Each isolate harboured blaOXA-66, blaOXA-24/40 and blaADC-30, and shared the same resistance gene content with one exception (Table S2). No additional mutations in resistance genes were noted, including in pmrCAB and lpxACD genes that mediate polymyxin resistance.
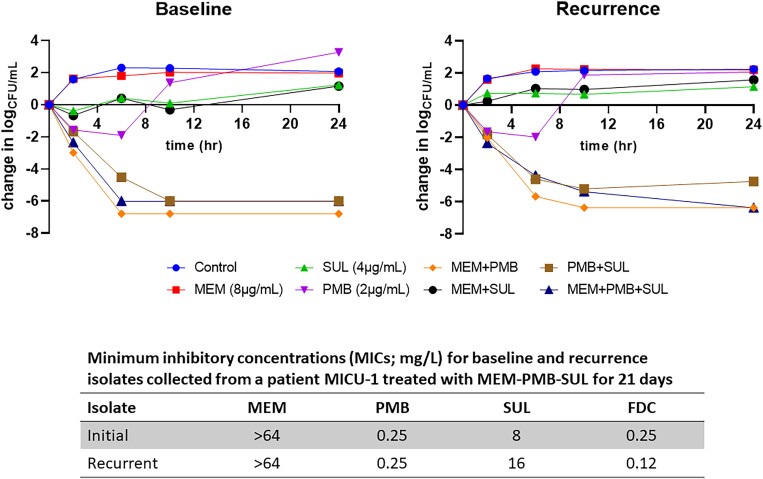
Eight representative initial CRAB isolates were tested for in vitro synergy. Rates of additive or synergistic activity were highest for polymyxin, ampicillin/sulbactam (50%); meropenem, ampicillin/sulbactam (25%); and minocycline, polymyxin b (25%); and <6% for all other combinations (Table 2). In time-kill analyses, initial isolates from three patients were rapidly killed by two-drug combinations of polymyxin b plus meropenem (mean log-kill = −6.29), polymyxin b plus ampicillin/sulbactam (mean log-kill = −5.77), and the three-drug combination of polymyxin b, meropenem and ampicillin/sulbactam (mean log-kill = −5.66). Paired initial and recurrent isolates from one patient (MICU-1) collected before and after 21 days of ampicillin/sulbactam, meropenem, polymyxin b treatment did not demonstrate the emergence of new resistance gene mutations or differences in the killing activity of two- or three-drug combinations (Figure 1).
Figure 1.
Time-kill assays performed for paired initial and recurrent isolates from one patient (MICU-1) collected before and after 21 days of ampicillin-sulbactam, meropenem, and polymyxin b treatment did not demonstrate the emergence of new resistance gene mutations or differences in the killing activity of 2- or 3-drug combinations. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Discussion
The management of CRAB infections remains a foremost challenge due to limited treatment options and difficulty determining whether poor clinical outcomes are attributable to suboptimal antibiotic therapy or underlying host factors. This paradigm is consistent with the organism’s predilection for causing hospital-acquired infections in vulnerable hosts.7,17 Previous pathogen-focused treatment studies of Acinetobacter spp. infections have demonstrated all-cause mortality rates >40%.4,18,19 Here, we showed the use of potentially synergistic three-drug regimens for severe CRAB infections among critically ill COVID-19 patients that resulted in lower rates of clinical failure and death than those previously reported among non-COVID-19 patients.18,20
The most commonly used three-drug regimen at our centre was ampicillin/sulbactam, meropenem and polymyxin b, which demonstrates potent bactericidal activity in dynamic hollow-fibre infection models against CRAB isolates.6,21 Clinical data, however, are limited to seven patients infected with colistin-resistant CRAB who all survived 30-days post-treatment.5 Our data, therefore, corroborate and extend prior findings, particularly in support of early initial treatment for severe CRAB infections. Among patients treated with ampicillin/sulbactam, meropenem and polymyxin b, 46% experienced complete resolution of signs and symptoms of infection and the 30-day mortality rate was 23% (3/13). Alternative combinations that have been studied include minocycline, continuous infusion sulbactam and polymyxin B, which also shows rapid killing and minimal development of resistance in a pharmacodynamic model.8 In our patients, minocycline (200 mg every 12 hours) was used instead of meropenem in three patients resulting in clinical success in two. All but one patient in our study received polymyxin B as part of combination therapy for which associated outcomes data remain sparse given that most studies have used colistin rather than polymyxin B. Polymyxin B demonstrates pharmacokinetic and safety advantages when compared to colistin, and thus warrants further investigation as part of combination regimens for CRAB infections.22 Such data may support or refute the frequent use of either colistin or polymyxin B for treatment of carbapenem-resistant Gram-negative bacterial infections among surveyed institutions in the USA and Europe.23 Motivation for colistin in combination with other agents stems from high rates of in vitro synergy; however, in vitro synergism against CRAB has not always been associated with improved clinical outcomes.18,24 A randomized controlled trial of 406 patients with infections due to carbapenem-resistant Gram-negative bacteria, most of which (77%) were caused by A. baumannii, did not find a difference in clinical failure rates among patients treated with the combination of meropenem plus colistin versus colistin alone.18 These findings were corroborated in a second randomized controlled trial of 425 patients where treatment with meropenem plus colistin did not result in improved outcomes compared to colistin alone for patients with CRAB infections.20
Patients may have fared better in our study because they received optimized dosing of all antimicrobial agents in combination. For instance, we administered 9 g of ampicillin/sulbactam every 8 hours as a prolonged infusion over 4 hours (equivalent to 3 g every 8 hours of sulbactam), based on pharmacodynamic modelling that shows this regimen achieves a high probability of target attainment for A. baumannii.9,25,26 Other antibiotic regimens were optimized wherever possible, including high-dose, extended infusion meropenem, high-dose minocycline and pharmacokinetically optimized doses of polymyxin B.6,8 Although we cannot draw definitive conclusions, the ampicillin-sulbactam doses employed here likely achieved pharmacokinetic-pharmacodynamic targets of at least 25% fT > MIC given that sulbactam MICs ranged from 8 to 16 mg/L across all isolates tested.27,28 These complex regimens are not without risk, however, as 39% of patients experienced antibiotic-associated acute kidney injury probably secondary to polymyxin B. No patients experienced neurotoxicity with the high-dose combinations of beta-lactams and polymyxin B, but our sample size was small.
Taken together, these data bring to the forefront the central challenges in managing CRAB infections, which are frequent microbiologic failures, differentiating recurrent infection versus colonization and the development of further antibiotic resistance after treatment. CRAB typically affects critically ill patients whose prognosis is influenced by underlying diseases, comorbid conditions, severity of illness and in this report, COVID-19 pneumonia.29 In our experience with three-drug regimens, microbiologic failures were still common, particularly among patients with VAP. However, increased resistance was not noted among patients with recurrent infection or in circulating isolates associated with the outbreak at our centre. While our in vitro findings did not indicate that the three-drug combinations offered superior in vitro killing over two-drug combinations, we hypothesize that the addition of a third agent helped to mitigate the emergence of further resistance. By comparison, rates of treatment-emergent colistin resistance ranges from 8% to 36% among patients treated with colistin–meropenem combinations.20,30 It is unclear whether the propensity for treatment-emergent resistance to colistin differs from polymyxin B.
Another important factor that may have contributed to positive patient outcomes in our study was the early initiation of treatment. Given the nature of the CRAB outbreak, we were able to initiate three-drug combination regimens in 72% of patients within 48 hours of culture collection. This strategy is particularly notable given that delayed time to appropriate antimicrobial therapy is associated with increased mortality in Acinetobacter spp. infections.31,32 It is also possible that treatment responses vary by geographic region and/or CRAB sequence type, which may have impacted findings in this study. Indeed, mortality rates have been shown to vary by CRAB clonal group in previous studies.33 Here, all patients were infected with closely related ST2Pas/ST208Ox isolates and received standardized three-drug regimens. Accordingly, our findings are specific to the clone infecting patients at our centre and underscore the need for future studies that link treatment response to the underlying molecular characteristics of CRAB isolates. These data are particularly important given that the clone causing an outbreak at our institution was universally susceptible to polymyxin B, but minocycline MICs were at or above the susceptibility breakpoint in all cases. Thus, ideal combinations should be tailored to the predominant strain at each institution.
Finally, it should be noted that evaluation of clinical outcomes in this study was limited by the fact that all patients had severe COVID-19 pneumonia. This was problematic in the patients with VAP given the extensive underlying lung damage. For this reason, we conservatively identified those with clinical resolution, and classified other patients as indeterminant if treatment responses could not be clearly ascertained. In addition, we adjudicated outcomes through the independent review of three investigators, which was consistent with the interpretations of treating ID providers. Using this approach, we have shown the potential utility of three-drug regimens for severe CRAB infections among COVID-19 patients that resulted in reasonable rates of clinical response and lower rates of 30-day mortality relative to previous studies. Importantly, the emergence of further antibiotic resistance was not detected phenotypically or through WGS analysis. Given our sample size, we have not correlated individual patient outcomes to infecting isolates that did or did not demonstrate antibiotic synergy. Further studies are needed to link molecular, phenotypic and clinical characteristics with responses to CRAB treatment to elucidate preferred antibiotic combinations, particularly with newer antimicrobial agents such as cefiderocol and with polymyxin-sparing combinations.
Supplementary Material
Contributor Information
Emily L Heil, Department of Practice, Science, and Health Outcomes Research, University of Maryland School of Pharmacy, 20 North Pine Street, Baltimore, MD, USA.
Kimberly C Claeys, Department of Practice, Science, and Health Outcomes Research, University of Maryland School of Pharmacy, 20 North Pine Street, Baltimore, MD, USA.
Ellen G Kline, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, USA.
Tara M Rogers, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, USA.
Kevin M Squires, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, USA.
Alina Iovleva, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, USA.
Yohei Doi, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, USA.
Mary Banoub, Department of Pharmacy, University of Maryland Medical Center, Baltimore, MD, USA.
Mandee M Noval, Department of Pharmacy, University of Maryland Medical Center, Baltimore, MD, USA.
Paul M Luethy, Department of Pathology, University of Maryland School of Medicine, Baltimore, MD, USA.
Ryan K Shields, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, USA.
Funding
This work was supported by The National Center for Advancing Translational Sciences of the National Institutes of Health under award number 2KL2TR001856-06 (A.I.) and by research grants from the NIH, including grant numbers R01AI104895 and R21AI151362 awarded to Y.D. and R03AI144636 and R21AI151363 awarded to R.K.S.
Transparency declarations
Y.D. has served as a consultant for Shionogi, Gilead Sciences, MSD, GSK, Meiji Seika Pharma, Chugai, bioMerieux and has received investigator-initiated funding from Entasis, Shionogi and Asahi Kasei. P.M.L. has served as a consultant for bioMerieux. R.K.S. has served as a consultant for Allergan, Cidara, Shionogi, Menarini, Melinta, Merck, Entasis, Utility and Venatorx and has received investigator-initiated funding from Merck, Melinta, Shionogi and Venatorx. All other authors report no potential conflicts of interest.
Supplementary data
Tables S1 and S2 are available as Supplementary data at JAC Online.
References
- 1. Wong D, Nielsen TB, Bonomo Ret al. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Reviews 2017; 30: 409–47. 10.1128/CMR.00058-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Esterly JS, Griffith M, Qi Cet al. Impact of carbapenem resistance and receipt of active antimicrobial therapy on clinical outcomes of Acinetobacter baumannii bloodstream infections. Antimicrob Agents Chemother 2011; 55: 4844–9. 10.1128/AAC.01728-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Isler B, Doi Y, Bonomo RAet al. New treatment options against carbapenem-resistant Acinetobacter baumannii infections. Antimicrob Agents Chemother 2019; 63: e01110–18. 10.1128/AAC.01110-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bassetti M, Echols R, Matsunaga Yet al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis 2021; 21: 226–40. 10.1016/S1473-3099(20)30796-9 [DOI] [PubMed] [Google Scholar]
- 5. Qureshi ZA, Hittle LE, O’Hara JAet al. Colistin-resistant Acinetobacter baumannii: beyond carbapenem resistance. Clin Infect Dis 2015; 60: 1295–303. 10.1093/cid/civ048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lenhard JR, Smith NM, Bulman ZP, et al. High-dose ampicillin-sulbactam combinations combat polymyxin-resistant Acinetobacter baumannii in a hollow-fiber infection model. Antimicrob Agents Chemother 2017; 61: e01268–16. 10.1128/AAC.01268-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tamma PD, Aitken SL, Bonomo RAet al. Infectious Diseases Society of America guidance on the treatment of AmpC β-lactamase-producing enterobacterales, carbapenem-resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia infections. Clin Infect Dis 2022; 74: 2089–114. [DOI] [PubMed] [Google Scholar]
- 8. Beganovic M, Daffinee KE, Luther MKet al. Minocycline alone and in combination with polymyxin B, meropenem, and sulbactam against carbapenem-susceptible and -resistant Acinetobacter baumannii in an in vitro pharmacodynamic model. Antimicrob Agents Chemother 2021; 65: e01680–20. 10.1128/AAC.01680-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jaruratanasirikul S, Wongpoowarak W, Wattanavijitkul Tet al. Population pharmacokinetics and pharmacodynamics modeling to optimize dosage regimens of sulbactam in critically ill patients with severe sepsis caused by Acinetobacter baumannii. Antimicrob Agents Chemother 2016; 60: 7236–44. 10.1128/AAC.01669-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. CLSI . Performance Standards for Antimicrobial Susceptibility Testing—Thirtieth Edition. 2020. [Google Scholar]
- 11. Iovleva A, Mustapha MM, Griffith MPet al. Carbapenem-resistant Acinetobacter baumannii in U.S. hospitals: diversification of circulating lineages and antimicrobial resistance. mBio 2022; 13: e0275921. 10.1128/mbio.02759-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bankevich A, Nurk S, Antipov Det al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 2012; 19: 455–77. 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deatherage DE, Barrick JE. Identification of mutations in laboratory-evolved microbes from next-generation sequencing data using breseq. Methods Mol Biol 2014; 1151: 165–88. 10.1007/978-1-4939-0554-6_12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alcock BP, Raphenya AR, Lau TTYet al. CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res 2020; 48: D517–525. 10.1093/nar/gkz935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shields RK, Kwak EJ, Potoski BAet al. High mortality rates among solid organ transplant recipients infected with extensively drug-resistant Acinetobacter baumannii: using in vitro antibiotic combination testing to identify the combination of a carbapenem and colistin as an effective treatment regimen. Diagn Microbiol Infect Dis 2011; 70: 246–52. 10.1016/j.diagmicrobio.2010.12.023 [DOI] [PubMed] [Google Scholar]
- 16. Oleksiuk LM, Nguyen MH, Press EGet al. In vitro responses of Acinetobacter baumannii to two- and three-drug combinations following exposure to colistin and doripenem. Antimicrob Agents Chemother 2014; 58: 1195–9. 10.1128/AAC.01779-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weiner-Lastinger LM, Abner S, Edwards JRet al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: summary of data reported to the national healthcare safety network, 2015–2017. Infect Control Hosp Epidemiol 2020; 41: 1–18. 10.1017/ice.2019.296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paul M, Daikos GL, Durante-Mangoni Eet al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis 2018; 18: 391–400. 10.1016/S1473-3099(18)30099-9 [DOI] [PubMed] [Google Scholar]
- 19. Durante-Mangoni E, Signoriello G, Andini Ret al. Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: a multicenter, randomized clinical trial. Clin Infect Dis 2013; 57: 349–58. 10.1093/cid/cit253 [DOI] [PubMed] [Google Scholar]
- 20. Pogue JM, Rybak MJ, Stamper Ket al. 638. The impact of in vitro synergy between colistin and meropenem on clinical outcomes in invasive carbapenem-resistant gram-negative infections: a report from the OVERCOME trial. Open Forum Infect Dis 2021; 8: S421–2. 10.1093/ofid/ofab466.835 [DOI] [Google Scholar]
- 21. Lenhard JR, Thamlikitkul V, Silveira FPet al. Polymyxin-resistant, carbapenem-resistant Acinetobacter baumannii is eradicated by a triple combination of agents that lack individual activity. J Antimicrob Chemother 2017; 72: 1415–20. 10.1093/jac/dkx002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nation RL, Velkov T, Li J. Colistin and polymyxin B: peas in a pod, or chalk and cheese? Clin Infect Dis 2014; 59: 88–94. 10.1093/cid/ciu213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Papst L, Beović B, Pulcini Cet al. Antibiotic treatment of infections caused by carbapenem-resistant Gram-negative bacilli: an international ESCMID cross-sectional survey among infectious diseases specialists practicing in large hospitals. Clin Microbiol Infect 2018; 24: 1070–6. 10.1016/j.cmi.2018.01.015 [DOI] [PubMed] [Google Scholar]
- 24. Nutman A, Lellouche J, Temkin Eet al. Colistin plus meropenem for carbapenem-resistant gram-negative infections: in vitro synergism is not associated with better clinical outcomes. Clin Microbiol Infect 2020; 26: 1185–91. 10.1016/j.cmi.2020.03.035 [DOI] [PubMed] [Google Scholar]
- 25. Jaruratanasirikul S, Wongpoowarak W, Aeinlang Net al. Pharmacodynamics modeling to optimize dosage regimens of sulbactam. Antimicrob Agents Chemother 2013; 57: 3441–4. 10.1128/AAC.00342-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yokoyama Y, Matsumoto K, Ikawa Ket al. Population pharmacokinetic-pharmacodynamic target attainment analysis of sulbactam in patients with impaired renal function: dosing considerations for Acinetobacter baumannii infections. J Infect Chemother 2015; 21: 284–9. 10.1016/j.jiac.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 27. Jaruratanasirikul S, Nitchot W, Wongpoowarak Wet al. Population pharmacokinetics and Monte Carlo simulations of sulbactam to optimize dosage regimens in patients with ventilator-associated pneumonia caused by Acinetobacter baumannii. Eur J Pharm Sci 2019; 136: 104940. 10.1016/j.ejps.2019.05.018 [DOI] [PubMed] [Google Scholar]
- 28. Abouelhassan Y, Kuti J, Nicolau D, et al. Sulbactam against Acinetobacter baumannii pneumonia: pharmacokinetic/pharmacodynamic appraisal for current dosing recommendations. ID Week2022, Washington, DC, USA. [Google Scholar]
- 29. Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 2008; 21: 538–82. 10.1128/CMR.00058-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shields RK, Clancy CJ, Gillis LMet al. Epidemiology, clinical characteristics and outcomes of extensively drug-resistant Acinetobacter baumannii infections among solid organ transplant recipients. PLoS ONE 2012; 7: e52349. 10.1371/journal.pone.0052349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Erbay A, Idil A, Gözel MGet al. Impact of early appropriate antimicrobial therapy on survival in Acinetobacter baumannii bloodstream infections. Int J Antimicrob Agents 2009; 34: 575–9. 10.1016/j.ijantimicag.2009.07.006 [DOI] [PubMed] [Google Scholar]
- 32. Kwon KT, Oh WS, Song J-Het al. Impact of imipenem resistance on mortality in patients with Acinetobacter bacteraemia. J Antimicrob Chemother 2007; 59: 525–30. 10.1093/jac/dkl499 [DOI] [PubMed] [Google Scholar]
- 33. Nutman A, Glick R, Temkin Eet al. A case-control study to identify predictors of 14-day mortality following carbapenem-resistant Acinetobacter baumannii bacteraemia. Clin Microbiol Infect 2014; 20: O1028–34. 10.1111/1469-0691.12716 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.