Summary
Background
Treatment options for patients with recurrent/metastatic nasopharyngeal carcinoma (RM-NPC) are not clear after progression on previous treatment with PD-(L)1 inhibitor; critical gaps in evidence remain for such cases. Immunotherapy combined with antiangiogenic therapy has been reported to have synergistic antitumor activity. Therefore, we evaluated the efficacy and safety of camrelizumab plus famitinib in patients with RM-NPC who failed treatment with PD-1 inhibitor-containing regimens.
Methods
This multicenter, adaptive Simon minimax two-stage, phase II study enrolled patients with RM-NPC refractory to at least one line of systemic platinum-containing chemotherapy and anti-PD-(L)1 immunotherapy. The patient received camrelizumab 200 mg every 3 weeks and famitinib 20 mg once per day. The primary endpoint was objective response rate (ORR), and the study could be stopped early as criterion for efficacy was met (>5 responses). Key secondary endpoints included time to response (TTR), disease control rate (DCR), progression-free survival (PFS), duration of response (DoR), overall survival (OS), and safety. This trial was registered with ClinicalTrials.gov, NCT04346381.
Findings
Between October 12, 2020, and December 6, 2021, a total of 18 patients were enrolled since six responses were observed. The ORR was 33.3% (90% CI, 15.6–55.4) and the DCR was 77.8% (90% CI, 56.1–92.0). The median TTR was 2.1 months, the median DoR was 4.2 months (90% CI, 3.0–not reach), and the median PFS was 7.2 months (90% CI, 4.4–13.3), with a median follow-up duration of 16.7 months. Treatment-related adverse events (TRAEs) of grade ≥3 were reported in eight (44.4%) patients, with the most common being decreased platelet count and/or neutropenia (n = 4, 22.2%). Treatment-related serious AEs occurred in six (33.3%) patients, and no deaths occurred due to TRAEs. Four patients developed grade ≥3 nasopharyngeal necrosis; two of them developed grade 3–4 major epistaxis, and they were cured by nasal packing and vascular embolization.
Interpretation
Camrelizumab plus famitinib exhibited encouraging efficacy and tolerable safety profiles in patients with RM-NPC who failed frontline immunotherapy. Further studies are needed to confirm and expand these findings.
Funding
Jiangsu Hengrui Pharmaceutical Co., Ltd.
Keywords: Camrelizumab, Famitinib, Nasopharyngeal carcinoma, PD-1 inhibitor, Retreatment, VEGFR
Research in context.
Evidence before this study
We searched for articles in PubMed up to February 1, 2023, using the terms PD-1, immune checkpoint inhibitor (ICI), retreatment, rechallenge, nasopharyngeal carcinoma (NPC), in title or abstract, and we did not find any relevant reports. Therefore, we decided to report this article and give some evidence for antitumor activity and safety of PD-1 inhibitor retreatment in recurrent or metastatic NPC.
Added value of this study
This is the first prospective study to evaluate the efficacy and safety of ICI retreatment in NPC. In this phase 2 study, camrelizumab in combination with famitinib exhibited promising antitumor activity in advanced NPC after platinum-based chemotherapy and immunotherapy, with an ORR of 33.3%, DCR of 77.8%, median PFS of 7.2 months.
Implications of all the available evidence
This study regimen may serve as a treatment option for the growing ICI-treated population with unmet medical needs. These data will be used when planning additional clinical trials with large sample size across the diverse ICI-treated set.
Introduction
Nasopharyngeal carcinoma (NPC) has the highest prevalence in Southeast Asian countries and China, with age-standardized rates ranging from 2.1 to 9.9 per 100,000.1,2 The incidence of local recurrence or distant metastases in the NPC endemic range is approximately 20%.3,4
Gemcitabine plus cisplatin and PD-1 inhibitor (camrelizumab or toripalimab) have been the standard first-line treatment for patients with metastatic or recurrent NPC for which local treatment is unsuitable, with an updated median progression-free survival (PFS) of 21.7 months.5,6 Under such conditions, the number of patients who receive PD-1 inhibitors as the first-line or early line therapy is dramatically increasing. Considering its long-term efficacy and low toxicity, PD-1 inhibitor retreatment could be an attractive option. Nonetheless, the efficacy and safety of immune checkpoint inhibitor (ICI) retreatment in patients with NPC remain unclear. Several studies on ICI resumption have been published for other solid cancers. However, the efficacy of ICI monotherapy retreatment has been shown to be poor compared with that of the initial treatment.
Angiogenesis is a validated target in the treatment of advanced NPC.7, 8, 9 Moreover, several preclinical studies have indicated that antiangiogenic therapy improves the efficacy of immunotherapy10; this treatment has been validated in several clinical trials and further approved by the FDA for certain advanced solid tumors, such as endometrial carcinoma,11 hepatocellular carcinoma,12 and renal carcinoma.13, 14, 15 Therefore, we hypothesized that the adding of antiangiogenic agents might improve the efficacy of ICI retreatment.
Camrelizumab is a fully humanized, high-affinity monoclonal antibody that binds PD-1. Famitinib is a tyrosine kinase inhibitor (TKI) targeting vascular endothelial growth factor receptor (VEGFR)−2 and −3, platelet-derived growth factor receptor β, stem-cell factor receptor, FMS-like tyrosine kinase-3 receptor, proto-oncogene tyrosine-protein kinase receptor, and the TAM family of kinases.16, 17, 18
This multicenter, basket phase 2 study addresses important gaps in the administration of ICI retreatment for NPC. We report the antitumor activity and safety of camrelizumab plus famitinib in patients with recurrent/metastatic (RM) NPC who failed at least one line of anti-PD-1-based therapy.
Methods
Ethics statement
Ethics committees of all participating study centers reviewed the study protocol and approved the study. This study was registered with Clinicaltrials.gov (NCT04346381) and conducted according to the declaration of Helsinki. All patients provided written informed consent before enrolment.
Study design and participants
The present study was an open-label, multicenter, basket phase 2 study of camrelizumab and famitinib as a combination therapy in patients with advanced solid cancers (see Protocol). Here, we report data from a cohort of patients with NPC (cohort 9).
The study enrolled patients who were histologically or cytologically confirmed to have moderately differentiated or undifferentiated non-keratinized metastatic NPC or recurrent NPC unfit for radiotherapy and surgery and those who underwent radiological progression after at least one line of systemic platinum-containing chemotherapy and anti-PD-(L)1 immunotherapy (combined or sequential). Disease progression within 6 months after induction or adjuvant chemoradiotherapy was considered first-line treatment. Other eligibility criteria included age of 18–75 years; an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; at least one measurable lesion assessed using the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1; ability to swallow tablets; a life expectancy of at least 12 weeks; and adequate hematological, hepatic, and renal function. Exclusion criteria included prior therapy with any agent targeting VEGF(R), uncontrolled hypertension, hemorrhages of any grade ≥2 within 4 weeks before screening, high possibility of major vascular invasions that may cause fatal bleeding during treatment, ulceration of the skin or pharyngeal mucosa with tumor involvement, arteriovenous thrombosis occurring within 6 months before screening, significant bleeding symptoms or clear bleeding tendency within 3 months prior to enrollment, history of immune-related myocarditis, immune-related pneumonia of grade ≥2 or other immune-related adverse events (except for hypothyroidism) of grade ≥3, active central nervous system metastases, or other malignant tumors within 5 years (except for cured skin basal cell carcinoma or cervical carcinoma in situ). The full eligibility criteria are listed in protocol.
Study treatment
Famitinib 20 mg was administered orally once daily and camrelizumab 200 mg was administered intravenously on day 1 once every 3-week treatment cycle,16, 17, 18, 19 until confirmed disease progression (except quick radiological progression and clinical progression), death, unacceptable toxicities, patient decision or withdrawal of consent, withdrawal by the investigator, or loss to follow-up, whichever occurred first (see Protocol 1.2.4 for posology). Off-protocol anticancer drugs were not allowed before the occurrence of protocol-defined disease progression. Treatment beyond confirmed disease progression was allowed only if the patients were in a clinically stable condition according to the investigator's discretion. The total camrelizumab exposure did not exceed 2 years.
Dose modifications of camrelizumab were not permitted, and camrelizumab was discontinued if adverse events did not resolve to grade 0–1 within 12 weeks of the last infusion or if the patient had severe or life-threatening toxic effects. Dose interruptions and reductions (up to two reductions) of famitinib were permitted for toxicities that were not relieved by supportive care, with a first dose reduction to 15 mg once daily and a second reduction to 15 mg for 14 days and 7 days off. In case of hypertensive crisis, cerebral hemorrhage, hemorrhage of grade ≥3, arterial thrombosis, grade 4 venous thrombosis, gastrointestinal perforation, or high-risk major bleeding (such as nasopharyngeal necrosis invading the internal carotid artery [ICA]), as assessed by the investigator, famitinib administration was discontinued. Dose re-escalation was not allowed upon resolution of toxicity. Details of dose modifications and supportive measures are provided in the protocol.
Assessments
Responses were assessed by independent radiologists according to RECIST v1.1 using CT, MRI, or bone scan at baseline, every 3 cycles in the first 15 cycles, and every 4 cycles thereafter. Complete responses (CRs) or partial responses (PRs) were confirmed with a repeat scan at least 4 weeks after the initial response. After treatment discontinuation, the patients were followed up for survival status every 2 months.
Safety evaluation was performed in every cycle; this included physical examination (especially reactive capillary hyperplasia and hypertension), adverse events (AEs) reported by the subjects and investigators, blood routine, urine routine, serum biochemistry routine (including electrolytes, liver, and kidney function), and electrocardiograms. Thyroid function tests, cardiac enzyme tests, and coagulation tests were performed every 3 cycles.
AEs were recorded up to 30 days after treatment discontinuation, immune-related AEs (irAEs) were recorded up to 90 days after the last dose of PD-1 inhibitors, and severity was graded by investigators using the National Cancer Institute-Common Terminology Criteria for AEs v5.0. Serious AEs (SAEs) and treatment-related AEs (TRAEs) were recorded for 90 days after the last dose. Investigators indicated whether an adverse event could be attributed to either study medication.
PD-L1 was centrally tested using archival or fresh tumor tissues by PD-L1 IHC 22C3 pharmDx test (Agilent Technologies, CA, USA). PD-L1 expression was calculated as the Combined Positive Score (CPS), defined as the number of PD-L1 stained cells (tumor cells, lymphocytes, and macrophages) out of the total number of tumor cells, multiplied by 100.
Endpoints
The primary endpoint was the objective response rate (ORR), defined as the proportion of patients with a CR or PR according to RECIST v1.1, as assessed by independent radiologists.
Secondary endpoints included duration of response (DoR), defined as the time from the first evidence of response to disease progression or death; time to response (TTR), defined as the time to first response; disease control rate (DCR), defined as the proportion of patients who achieved CR, PR, or stable disease (SD) and safety evaluation; PFS, defined as the time from treatment initiation to disease progression according to RECIST v1.1 or death from any cause; 6/9/12-month overall survival (OS), defined as the time from treatment initiation until death; and safety. Exploratory endpoints included the association between efficacy and expression level of PD-L1.
Sample size
A Simon (minimax) two-stage design was used for this cohort (cohort 9; power, 70%; one-sided α, 0.05). An ORR less than 10% was considered ineffective, and 25% was considered the minimum effective bound. Nineteen patients were enrolled in stage one. Among the 19 patients, if there was more than 1 responder, the study treatment was considered effective, and the cohort would expand to 27 cases. The result would be positive if more than 5 of 27 patients achieved response in this trial. Notably, this trial could be terminated early if efficacy endpoints (>5 responses) had been achieved (see Appendix Note S1).
Statistical analysis
An efficacy analysis was performed in the intention-to-treat (ITT) population. Safety analyses were performed for all patients who received at least one dose of the study treatment.
The ORR and 90% CIs were calculated using the Clopper–Pearson method. The DoR, PFS, and OS were analyzed using the Kaplan–Meier method. Other clinical outcomes, demographic characteristics, and AEs were summarized using descriptive statistics, including proportions and frequencies, medians, and interquartile ranges (IQRs). Subgroup analysis to explore the tumor PD-L1 expression on efficacy was specified post hoc, and the statistical methods used were as described earlier. No adjustments were made for multiplicity in the analysis of the secondary outcomes.
All statistical tests were performed using the SPSS v25.0 and R package stats.
Role of the funding source
This study was designed by the principal investigator (Ming-Yuan Chen) and the sponsor (Jiangsu Hengrui Pharmaceuticals). The sponsor provided the study drugs and participated in data collection and review of the report. Ming-Yuan Chen, Xi Ding, Yi-Jun Hua, Xiong Zou, and Shu-Ni Wang have accessed and verified the data, and all authors were responsible for the decision to submit the manuscript.
Results
Considering that the study reached the positive target requirement (>5 responses) in advance, it was terminated early when enrollment reached 18 people.
Between October 12, 2020, and December 6, 2021, 18 patients with recurrent or metastatic NPC from four study sites in China were enrolled and all received at least one dose of the study regimen (ITT set and safety set; Fig. 1). Before the first scheduled post-baseline tumor assessment, one patient terminated the study therapy and refused further follow-up because of grade 2 pneumonitis. Treatment responses were evaluable in only 17 patients (Fig. 2).
Fig. 1.
Trial profile. ITT, Intention-to-treat; irAE, Immune-related adverse event.
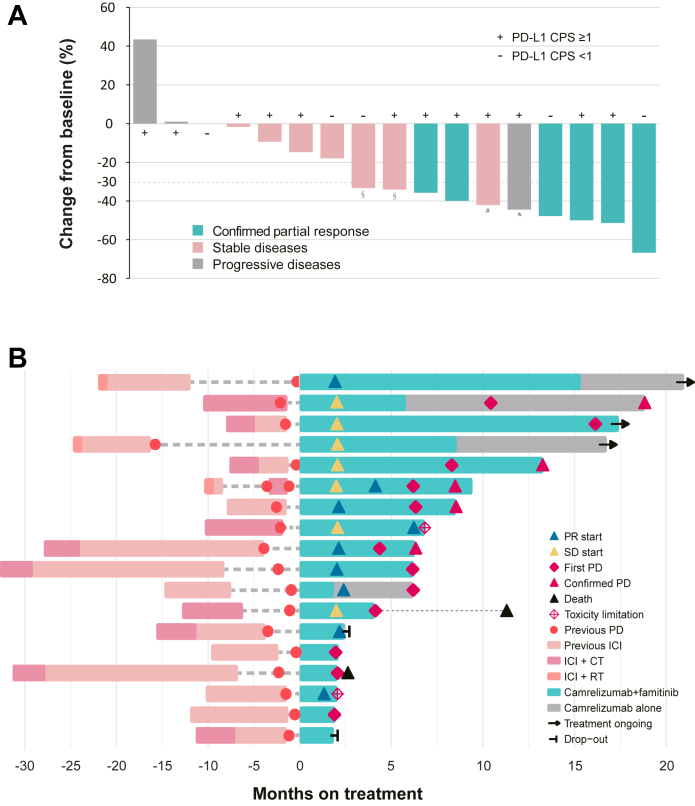
Fig. 2.
Antitumor activity. (A) Best percentage change from baseline in target lesion (n = 17) (B) Treatment exposure and response duration (n = 18). CPS, Combined positive score; PR, Partial response; SD, Stable disease; PD, Progressive disease; ICI, Immune checkpoint inhibitor; CT, Chemotherapy; RT, Radiotherapy; ∗ new lesion and the first PR occurred simultaneously; # the first PR followed by PD; § no imagings available for review after the first PR.
The baseline patient characteristics are summarized in Table 1. Seventeen patients had undifferentiated non-keratinizing carcinoma. Five (27.8%) patients had locoregional recurrence only, seven (38.9%) had distant metastases at initial diagnosis, and six (33.3%) had secondary distant metastases after radical treatment. All patients received at least one line of prior systemic therapy for advanced NPC, with five (27.8%) receiving 2–3 lines. Fourteen patients had received PD-1 inhibitor monotherapy for 2.9–21.4 months (median, 7.5) before enrollment, and the median time between the last PD-1 inhibitor administration and enrollment was 2.9 months (IQR, 1.4–7.5). The previous chemotherapy regimens received by each patient are listed in Appendix Table S1, and the most common chemotherapy regimens before enrollment were gemcitabine plus cisplatin and PD-1 inhibitor (10 [55.6%] of 18 patients).
Table 1.
Baseline demographics and disease characteristics in the intention-to-treat population.
| Characteristic | Patients N = 18 (%) |
|---|---|
| Sex | |
| Female | 3 (16.7) |
| Male | 15 (83.3) |
| Age, years | |
| Median (IQR) | 47 (40–53) |
| Ethnicity | |
| Han chinese | 18 (100) |
| Karnofsky performance status score | |
| 90–100 | 16 (88.9) |
| 80 | 2 (11.1) |
| History of cigarette use | |
| Current or former | 5 (27.8) |
| Never | 13 (72.2) |
| Histology | |
| Undifferentiated nonkeratinizing | 17 (94.4) |
| Poorly differentiated nonkeratinizing | 1 (5.6) |
| Stage | |
| Distant metastases at initial diagnosis | 7 (38.9) |
| Secondary distant metastases without local-regional recurrence | 4 (22.2) |
| Secondary distant metastases with local-regional recurrence | 2 (11.1) |
| Local-regional recurrence only | 5 (27.8) |
| Stage of locoregional recurrence | |
| T4N1M0 | 1 (5.6) |
| T4N0M0 | 2 (11.1) |
| T0N3M0 | 1 (5.6) |
| T0N1M0 | 1 (5.6) |
| Metastases sites | |
| Liver | 5 (27.8) |
| Bone | 5 (27.8) |
| Distant lymph nodes | 5 (27.8) |
| Lung | 4 (22.2) |
| Others | 2 (11.1) |
| Number of metastatic foci | |
| 1–5 | 3 (16.7) |
| >5 | 10 (55.6) |
| Previous local-regional radiotherapy | |
| None | 5 (27.8) |
| One course | 11 (61.1) |
| Two courses | 2 (11.1) |
| Previous lines of therapy for advanced disease | |
| 1 | 13 (72.2) |
| 2 | 4 (22.2) |
| 3 | 1 (5.6) |
| Last PD-1 inhibitor treatment model | |
| Combination with full-course chemotherapy | 4 (22.2) |
| Post-chemotherapy single-agent maintenance | 11 (61.1) |
| Single-agent neoadjuvant and (or) adjuvant therapy | 3 (16.7) |
| Duration of last PD-1 inhibitor monotherapya | |
| Median (IQR), months | 7.5 (5.4–13.2) |
| Interval between last PD-1 inhibitor therapy and study therapy | |
| Median (IQR), months | 2.9 (1.4–7.5) |
| Previous treatment for advanced diseaseb | |
| PD-1 inhibitor | 18 (100.0) |
| Toripalimab | 12 (66.7) |
| Tislelizumab | 4 (22.2) |
| Palivizumab | 1 (5.6) |
| Sintilimab | 1 (5.6) |
| Cisplatin | 12 (66.7) |
| Gemcitabine | 11 (61.1) |
| Capecitabine | 3 (16.7) |
| Docetaxel | 3 (16.7) |
| Paclitaxel | 3 (16.7) |
| Lobaplatin | 2 (11.1) |
| Nimotuzumab | 2 (11.1) |
| Nedaplatin | 1 (5.6) |
| Tegafur | 1 (5.6) |
| 5-fluorouracil | 1 (5.6) |
| Cetuximab | 1 (5.6) |
IQR, Interquartile range; PD-1, Programmed cell death 1.
Fourteen patients received PD-1 inhibitor monotherapy.
The previous chemotherapy regimens are listed in Appendix Table S1.
Antitumor activity
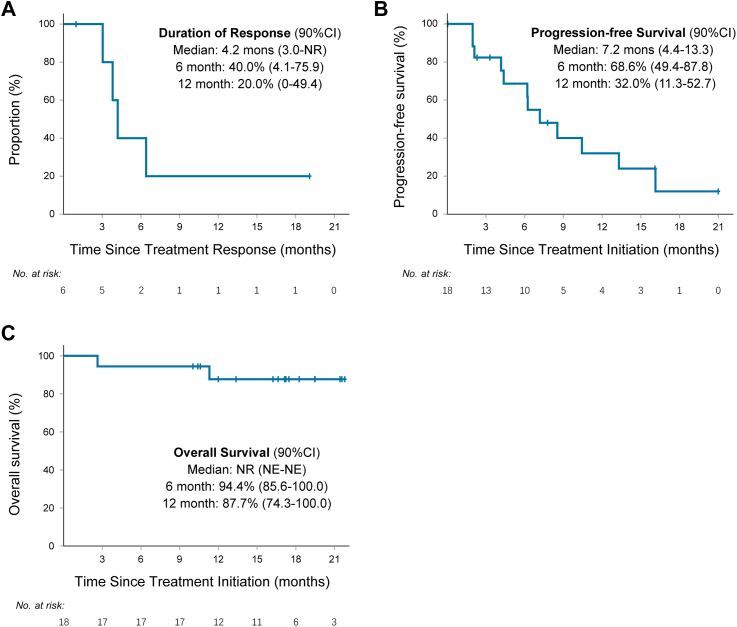
Six patients (33.3%; 90% CI, 15.6–55.4) achieved a confirmed objective response; however, none achieved CR (Fig. 2A), and the DCR was 77.8% (90% CI, 56.1–92.0; Table 2). The median TTR was 2.1 months (IQR, 1.9–2.4; Fig. 2B), and the median DoR was 4.2 months (90% CI, 3.0–not reach [NR]; Fig. 3A). One (5.6%) response lasted more than 19 months, and it was still ongoing (Appendix Fig. S1). Two patients (11.1%) who achieved a best of response of SD experienced prolonged stable disease for more than 12 months.
Table 2.
Antitumor activity in the intention-to-treat population.
| Response evaluation | N = 18 |
|---|---|
| Objective response rate, n (%) | 6 (33.3) |
| 90% CI | 15.6–55.4 |
| Disease control rate, n (%) | 14 (77.8) |
| 90% CI | 56.1–92.0 |
| Best overall response, n (%, [90% CI]) | |
| Complete response | 0 (0, 0.0–15.3) |
| Partial response | 6 (33.3, 15.6–55.4) |
| Stable disease | 8 (44.4, 24.4–65.9) |
| Progressive disease | 3 (16.7, 4.7–37.7) |
| Not evaluable | 1 (5.6, 0.3–23.8) |
Fig. 3.
Kaplan–Meier curves of duration of response, progression-free survival, and overall survival. (A) Duration of response was assessed in responders (n = 6) (B) Progression-free survival and (C) overall survival were assessed in the intention-to-treat population (n = 18). NR, Not reached; NE, Not evaluable.
Regarding the data cutoff (October 30, 2022), the median follow-up was 16.7 months (IQR, 11.1–19.5). Fifteen patients (83.3%) discontinued the study treatment because 11 developed disease progression, two declined further study treatment, one had unacceptable AEs (grade 3 left-sided ventricular systolic dysfunction) and one received bevacizumab for radiation encephalopathy (protocol violation). The median PFS in this study was 7.2 months (90% CI, 4.4–13.3), and the PFS rate at 12 months was 32.0% (90% CI, 11.3–52.7; Fig. 3B). Six patients continued the study regimen after the first radiographic PD, and two of them remained stable for 7 and 11 cycles. The median OS was not reached, the 6-, 9-month OS was 94.4% (90% CI, 85.6–100.0), and the 12-month OS was 87.7% (90% CI, 74.3–100.0; Fig. 3C).
In relation to the prior use of PD-1 inhibitors, in particular, two patients had an interval between the last PD-1 inhibitor medication and enrollment of more than one year, and they had not progressed at the time of the data cutoff with a median PFS of 16.1 and 21.0 months. Moreover, three patients maintained their previous PD-1 inhibitor treatment for 24 months, and two achieved PR in this study; however, they progressed at 2–6 months (Fig. 2B).
Safety
TRAEs of different grades occurred in all 18 patients at an incidence greater than 50% (Table 3): neutropenia (n = 12, 66.7%), albuminuria (n = 11, 61.1%), anemia (n = 11, 61.1%), leukopenia (n = 11, 61.1%), reactive cutaneous capillary endothelial cell proliferation (RCCEP; n = 10, 55.6%), and hand-foot syndrome (n = 9, 50%). The most common irAEs were RCCEP (n = 10, 55.6%), hypothyroidism (n = 7, 38.9%), diarrhea (n = 7, 38.9%), and fatigue (n = 5, 27.8%). Eight patients (44.4%) experienced TRAEs of grade ≥3, and the most frequent AEs were decreased platelet count and/or neutropenia (n = 4, 22.2%). The incidence of irAEs of grade ≥3 was 5.6% (n = 1; Table 3). Treatment-related SAEs occurred in six (33.3%) patients, including decreased platelet count (n = 1, 5.6%), tuberculosis (n = 1, 5.6%; undiagnosed), upper respiratory infection and rash (n = 1, 5.6%), nasopharyngeal necrosis (n = 1, 5.6%), and major nasopharyngeal bleeding (n = 2, 11.1%; Appendix Fig. S1). Other SAEs included aspiration pneumonia, radiation encephalopathy, and death due to disease progression. No AEs of special interest was reported.
Table 3.
Adverse events.
| Adverse event | N = 18 (%) |
||
|---|---|---|---|
| Grades 1-2 | Grade 3 | Grade 4 | |
| Any treatment-related adverse event | |||
| Neutropenia | 10 (55.6) | 2 (11.1) | 0 |
| Albuminuria | 10 (55.6) | 1 (5.6) | 0 |
| Leukopenia | 11 (61.1) | 0 | 0 |
| Anemia | 11 (61.1) | 0 | 0 |
| Reactive capillary hyperplasia | 10 (55.6) | 0 | 0 |
| Hand-foot syndrome | 7 (38.9) | 2 (11.1) | 0 |
| Hypertension | 7 (38.9) | 1 (5.6) | 0 |
| Blood triglyceride elevation | 7 (38.9) | 1 (5.6) | 0 |
| Fatigue | 7 (38.9) | 1 (5.6) | 0 |
| Thrombocytopenia | 3 (16.7) | 4 (22.2) | 0 |
| Hypothyroidism | 7 (38.9) | 0 | 0 |
| Diarrhea | 7 (38.9) | 0 | 0 |
| Lymphopenia | 5 (27.8) | 1 (5.6) | 0 |
| Pharyngolaryngeal pain | 6 (33.3) | 0 | 0 |
| Headache | 6 (33.3) | 0 | 0 |
| Blood cholesterol elevation | 6 (33.3) | 0 | 0 |
| Mucositis oral | 6 (33.3) | 0 | 0 |
| Hematuria | 6 (33.3) | 0 | 0 |
| AST elevation | 4 (22.2) | 1 (5.6) | 0 |
| ALT elevation | 5 (27.8) | 0 | 0 |
| Rash | 3 (16.7) | 1 (5.6) | 0 |
| Nasopharyngeal necrosis | 0 | 2 (11.1) | 2 (11.1) |
| Epistaxis | 1 (5.6) | 1 (5.6) | 1 (5.6) |
| Nausea | 3 (16.7) | 0 | 0 |
| Hyperuricemia | 3 (16.7) | 0 | 0 |
| Serum creatinine elevation | 3 (16.7) | 0 | 0 |
| Total bilirubin elevation | 3 (16.7) | 0 | 0 |
| Electrocardiogram QTc interval prolonged | 3 (16.7) | 0 | 0 |
| Upper respiratory infection | 1 (5.6) | 1 (5.6) | 0 |
| Cough | 2 (11.1) | 0 | 0 |
| Periodontal disease | 2 (11.1) | 0 | 0 |
| Anorexia | 2 (11.1) | 0 | 0 |
| Vomiting | 2 (11.1) | 0 | 0 |
| Chest pain | 2 (11.1) | 0 | 0 |
| Left-sided ventricular systolic dysfunction | 0 | 1 (5.6) | 0 |
| Fever | 0 | 1 (5.6) | 0 |
| Creatine phosphokinase elevation | 1 (5.6) | 0 | 0 |
| Creatine phosphokinase MB soenzyme | 1 (5.6) | 0 | 0 |
| Hyponatraemia | 1 (5.6) | 0 | 0 |
| Expectoration | 1 (5.6) | 0 | 0 |
| Constipation | 1 (5.6) | 0 | 0 |
| Tuberculosis | 1 (5.6) | 0 | 0 |
| Abdominal pain | 1 (5.6) | 0 | 0 |
| Hemoptysis | 1 (5.6) | 0 | 0 |
| Cardiac troponin I increased | 1 (5.6) | 0 | 0 |
| Atrioventricular block first degree | 1 (5.6) | 0 | 0 |
| Atrial tachyarrhythmias | 1 (5.6) | 0 | 0 |
| Oral hemorrhage | 1 (5.6) | 0 | 0 |
| Dizziness | 1 (5.6) | 0 | 0 |
| Facial pain | 1 (5.6) | 0 | 0 |
| Atrioventricular block first degree | 1 (5.6) | 0 | 0 |
| Premature ventricular contraction | 1 (5.6) | 0 | 0 |
| Pneumonia | 1 (5.6) | 0 | 0 |
| Potential immune-related adverse events | |||
| Reactive capillary proliferation | 10 (55.6) | 0 | 0 |
| Hypothyroidism | 7 (38.9) | 0 | 0 |
| Diarrhoea | 7 (38.9) | 0 | 0 |
| Fatigue | 5 (27.8) | 0 | 0 |
| Rash | 1 (5.6) | 1 (5.6) | 0 |
| Anemia | 2 (11.1) | 0 | 0 |
| Anorexia | 2 (11.1) | 0 | 0 |
| Cough | 2 (11.1) | 0 | 0 |
| Fever | 0 | 1 (5.6) | 0 |
| Upper respiratory infection | 0 | 1 (5.6) | 0 |
| Albuminuria | 1 (5.6) | 0 | 0 |
| Troponin I increased | 1 (5.6) | 0 | 0 |
| Blood creatine phosphokinase increased | 1 (5.6) | 0 | 0 |
| Tuberculosis | 1 (5.6) | 0 | 0 |
| Pneumonia | 1 (5.6) | 0 | 0 |
| Nausea | 1 (5.6) | 0 | 0 |
| Vomiting | 1 (5.6) | 0 | 0 |
| Dysgeusia | 1 (5.6) | 0 | 0 |
| Hair color changes | 1 (5.6) | 0 | 0 |
| Atrioventricular block first degree | 1 (5.6) | 0 | 0 |
| Premature ventricular contraction | 1 (5.6) | 0 | 0 |
| Adverse events of special interest | none | ||
AST, Aspartate aminotransferase; ALT, Alanine aminotransferase; QTc, QT corrected; MB, Myocardial band.
Eleven patients (61.1%) required one or more pauses for famitinib. Dose reductions, observed in five patients (27.8%), was due to thrombocytopenia (n = 3), hand-foot syndrome (n = 2), and albuminuria (n = 1). Five patients (27.8%) terminated famitinib before radiographic PD as a result of TRAE, including nasopharyngeal necrosis (n = 4) with or without major epistaxis (n = 2) and grade 3 left-sided ventricular systolic dysfunction (n = 1).
A total of four patients developed nasopharyngeal necrosis of grade ≥3, and two patients underwent internal carotid artery embolization when invasion of necrotic foci occurred (Grade 4; Appendix Fig. S2). Two exhibited grade 1–2 epistaxis, whereas the other two developed grade 3–4 major epistaxis and were cured by nasal packing and vascular embolization (Appendix Fig. S1). Regarding clinical characteristics, two of the four patients underwent two courses of locoregional radiotherapy; three patients had a recurrent T4 lesion and the remaining one patient received naso-radiotherapy within one year before enrollment.
Efficacy in subgroup by PD-L1 expression
The tumor biospecimens of all 18 patients were analyzed for PD-L1 expression. Thirteen (72.2%) patients had positive expression (CPS ≥1) with an ORR being 30.8% (4/13; 90% CI, 11.3–57.3). The PD-L1-negative patients (CPS <1) had an ORR of 40.0% (2/5; 90% CI, 7.6–81.1; Fig. 2A). The median PFS for patients with positive and negative PD-L1 expression was 6.2 months (90% CI, 2.1–16.1) and 7.9 months (90% CI, 4.2-NR), respectively (Appendix Fig. S3).
Discussion
To our knowledge, this is the first prospective study to evaluate the antitumor activity and safety of ICI retreatment in RM-NPC. In this phase 2 study, camrelizumab in combination with famitinib exhibited promising antitumor activity in advanced NPC after platinum-based chemotherapy and immunotherapy, with an ORR of 33.3%, DCR of 77.8%, median PFS of 7.2 months, and PFS rate at 12 months of 32.0%.
Treatment options for patients with RM-NPC are not clear after progression on previous treatment with PD-1/PD-L1 inhibitors; critical gaps in evidence remain for such cases. In other cancers, when disease progression occurs after ICI-based treatment, the common resuming regimen at present involves combining the original treatment with a targeted drug (such as PD-1 inhibitor plus antiangiogenic agent),20, 21, 22 changing the type of ICI (such as switching from CTLA-4 inhibitor to PD-1 inhibitor),23,24 or combining with other ICIs simultaneously (such as PD-L1 inhibitor plus CTLA-4 inhibitor).25
Increasing evidence has demonstrated that the appropriate administration of antiangiogenic agents can normalize the tumor vascular network, which could directly or indirectly alleviate hypoxia, promote T cell infiltration, induce M1 macrophage polarization,26 decrease the recruitment of Tregs and myeloid suppressor cells,27 and downregulate inhibitory immune checkpoints such as PD-L1,28 thus converting the tumor immune environment from immune-suppressive to immune-supportive.10,29 Therefore, the addition of famitinib may enhance immunogenicity, thereby increasing the therapeutic efficacy of ICIs. We previously reported on the combination of apatinib, an antiangiogenic TKI, and camrelizumab in patients with RM-NPC.30 In patients progressing after first-line platinum-containing treatment, this regimen exhibited a favorable efficacy with a median PFS of 10.4 months and an ORR of 65.5%. When this regimen was combined with gemcitabine as first-line therapy, the median PFS was more than 25.8 months and the ORR reached 90.2%,31 whereas for a regimen of gemcitabine and platinum plus PD-1 inhibitor, these indicators were 9.6–21.4 months5,6,32,33 and 77.4–87.3%, respectively. This implies that the efficacy of antiangiogenic agents plus immunotherapy is promising for NPC and that it is possible to re-sensitize immunotherapy-resistant NPC to subsequent ICI treatment, thus rescuing responses in patients with NPC who progressed on anti-PD-(L)1-based therapy. For example, in the LEAP-004 trial, 21.4% of patients with melanoma responded to lenvatinib plus pembrolizumab, and the DCR was 66.0% under the premise of using a strict definition of progressive disease on previous ICI-based therapy that required confirmed PD to occur within 12 weeks of the last dose of anti-PD-1/L1 therapy.20 In our study, the ORR was 33.3% and median PFS was 7.2 months, which was lower than that with the initial regimen of the same type (65.5% and 10.4 months). Indeed, ICI retreatment was less effective than the initial ICI administration, and the switch from PD-1/PD-L1 to PD-L1/PD-1 revealed limited clinical efficacy.34
In this study, we did not change the type of ICI used when resuming it. Theoretically, a switch from anti-PD-(L)1 to anti-CTLA-4 therapy may be reasonable because of the nonoverlapping mechanisms. However, treatment with nivolumab or pembrolizumab in patients with melanoma refractory to ipilimumab was found to result in an ORR of 21–32%,23,24 which seems still lower than that with prior anti-PD-1 antibody monotherapy (36–45%).35,36 In particular, patients who exhibited disease progression after stopping a full-course ICI treatment (35 cycles or 2 years) responded well to the same ICIs with a PR rate of 36.4–42.9% and DCR of 78.6–90.9%,37,38 which supports the rechallenge with the same ICIs. Three patients experienced the same situation before enrollment in our study, and two of them achieved PR; however, the long-term outcome was not optimistic even with the addition of a targeted drug, with the median PFS ranging from 2.1 to 6.2 months. Notably, two other patients who restarted ICI-based therapy more than 12 months after the last dose of PD-1 inhibitor showed no progression at the data cutoff (PFS, 16.1 and 21.0 months). Our data suggest that restarting ICIs after long-term use is not effective; however, patients may regain sensitivity to immunotherapy after prolonged suspension of ICIs.
The efficacy and safety profiles of camrelizumab and famitinib were consistent with that of the same type of medication for NPC or other cancers. Regarding efficacy, camrelizumab + famitinib exhibited an ORR of 84.6% and a DCR of 100% for treatment-naïve advanced or metastatic RCC,17 which is similar to that for other frequently used combinations of immunotherapies and antiangiogenic therapies, such as avelumab + axitinib, pembrolizumab + axitinib, nivolumab + cabozantinib, atezolizumab + bevacizumab, and pembrolizumab + lenvatinib (ORR, 32–73%; DCR, 73–92%).13, 14, 15,39, 40, 41, 42 In this study, grade ≥3 TRAEs were reported in eight patients (44.4%), which is in line with the apatinib plus camrelizumab regimen for subsequent-line NPC (58.6%).30 In addition, the safety profile in this paper is not inferior to that of the other common regimens described above, with the incidence of grade ≥3 TRAEs being 44.4% versus 45.6–87.5%.13, 14, 15,39, 40, 41, 42 Referring to clinical trials that also used camrelizumab plus famitinib to treat other solid tumors,16, 17, 18, 19 we found that the toxicity profile reported in this study was similar to that observed in those trials (grade ≥3 TRAEs, 61.1–81.1%). The main toxicities observed, which included leukopenia/neutropenia, proteinuria, anemia, hypertension, thrombocytopenia, and hand-foot syndrome, were also consistent.
Notably, this study was associated with a high incidence of nasopharyngeal necrosis, with 4 cases (22.2%) showing a grade ≥3; 2 of them were followed by grade 3–4 major epistaxis. Nasopharyngeal necrosis is not uncommon when an antiangiogenic TKI is used for treating NPC, and it can be avoided by excluding high-risk patients based on our recent researches, such as those who experienced two courses of locoregional radiotherapy, had a recurrent T3-4 lesion, or received naso-radiotherapy within one year before enrollment.30,31 These possible factors are also reflected in this article.
The predictive value of PD-L1 expression for NPC remains unclear. In our study, we did not observe any significant correlation between PD-L1 expression and efficacy, which could be attributed to the small sample size. However, we did notice a small subset of PD-L1-positive patients showing prolonged clinical benefits, as evident from the K-M curve of PFS. In another clinical trial published by our team, investigating the combination of anti-angiogenic agent and camrelizumab, we found that high expression of PD-L1 significantly predicted a favorable DoR,30 indicating the potential predictive value of PD-L1 expression for efficacy if the sample size is expanded.
This study had several limitations. First, on the basis of the definition of resistance from the Society for Immunotherapy of Cancer, all enrolled patients could not be considered resistant to ICIs, since their prior regimens were not ICI monotherapy. Therefore, our study does not reflect practical situations in which patients are resistant to ICIs (ruling out the interference of other therapies). Second, this study was terminated prematurely before reaching the target enrollment for the first phase. However, it is still statistically meaningful based on the Simon two-stage design. Out of 18 patients enrolled in this study, 6 achieved a PR, meeting the preset condition that if more than 5 patients achieved PR out of 27 participants (total sample size), the result is considered positive. Moreover, the ORR is 33.3%, exceeding the pre-defined minimum effective bound of 25%; the lower bound of the 90% CI is 15.6%, which is also above the pre-defined ineffective bound of 10%. Based on the current data, further clinical trials are justifiable. Third, this was a an unblinded, single-arm study with small sample size, thus we cannot determine whether the promising efficacy was due to the synergistic effect of famitinib and camrelizumab or the effect of famitinib alone. Moreover, we did not perform in-depth analyses of the possible molecular biomarkers of response. Further studies, with an improved clinical trial design, are warranted for identifying the clinical and molecular features associated with the benefits in this setting.
Our findings indicate that camrelizumab plus famitinib showed encouraging efficacy and tolerable overall safety profile in patients with RM-NPC who failed frontline immunotherapy (combinations). This regimen may serve as a treatment option for the growing ICI-treated population with unmet medical needs. Additional clinical trials with large sample size across the diverse ICI-treated set and molecular studies are needed to explore these results further.
Contributors
Conceptualization: Ming-Yuan Chen, Jia Fan; Sample collection and selection: Ming-Yuan Chen, Yi-Jun Hua, Xiong Zou, Xiao-Zhong Chen, Xi-Mei Zhang, Bei Xu, Xi Ding, Zi-Wei Tu, Yan-Feng Ouyang, Yong-Long Liu, Hui-Feng Li, You-Ping Liu; Methodology: Xi Ding, Rui You, Wei-Jing Zhang, Shu-Ni Wang; Formal analysis: Xi Ding, Yi-Jun Hua, Xiong Zou, Chong-Yang Duan; Writing-original draft: Xi Ding, Yi-Jun Hua, Xiong Zou, Ming-Yuan Chen; Writing-review and editing: All authors; All authors read and approved the final manuscript and take responsibility for its content; Supervision: Ming-Yuan Chen and Jia Fan.
Data sharing statement
Our raw database will be deposited on the Research Data Deposit public platform (www.researchdata.org.cn). Data may be requested 24 months after publication. Qualified researchers should submit a proposal to the corresponding author outlining the reasons for requiring the data. The leading clinical site and sponsor will check whether the request is subject to any intellectual property restriction. Use of data must also comply with the requirements of Human Genetics Resources Administration of China and other country or region-specific regulations. A signed data access agreement with the sponsor is required before accessing shared data.
Declaration of interests
Shu-Ni Wang is employed Jiangsu Hengrui Pharmaceuticals. All other authors declare no competing interests.
Acknowledgements
We are grateful to all patients and their families who participated in the study. We appreciate the support from Jiangsu Hengrui Pharmaceuticals.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102043.
Contributor Information
Jia Fan, Email: fan.jia@zs-hospital.sh.cn.
Ming-Yuan Chen, Email: chenmy@sysucc.org.cn.
Appendix A. Supplementary data
References
- 1.Ferlay J., Ervik M., Lam F., et al. Global cancer observatory: cancer today. International Agency for Research on Cancer; Lyon, France: 2020. https://gco.iarc.fr/today [Google Scholar]
- 2.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Lee A.W.M., Ng W.T., Chan J.Y.W., et al. Management of locally recurrent nasopharyngeal carcinoma. Cancer Treat Rev. 2019;79 doi: 10.1016/j.ctrv.2019.101890. [DOI] [PubMed] [Google Scholar]
- 4.Li Y.Q., Tian Y.M., Tan S.H., et al. Prognostic model for stratification of radioresistant nasopharynx carcinoma to curative salvage radiotherapy. J Clin Oncol. 2018;36(9):891–899. doi: 10.1200/jco.2017.75.5165. [DOI] [PubMed] [Google Scholar]
- 5.Mai H.Q., Chen Q.Y., Chen D., et al. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial. Nat Med. 2021;27(9):1536–1543. doi: 10.1038/s41591-021-01444-0. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y., Qu S., Li J., et al. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2021;22(8):1162–1174. doi: 10.1016/s1470-2045(21)00302-8. [DOI] [PubMed] [Google Scholar]
- 7.Xue C., Huang Y., Huang P.Y., et al. Phase II study of sorafenib in combination with cisplatin and 5-fluorouracil to treat recurrent or metastatic nasopharyngeal carcinoma. Ann Oncol. 2013;24(4):1055–1061. doi: 10.1093/annonc/mds581. [DOI] [PubMed] [Google Scholar]
- 8.Elser C., Siu L.L., Winquist E., et al. Phase II trial of sorafenib in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or nasopharyngeal carcinoma. J Clin Oncol. 2007;25(24):3766–3773. doi: 10.1200/JCO.2006.10.2871. [DOI] [PubMed] [Google Scholar]
- 9.Lim W.T., Ng Q.S., Ivy P., et al. A Phase II study of pazopanib in Asian patients with recurrent/metastatic nasopharyngeal carcinoma. Clin Cancer Res. 2011;17(16):5481–5489. doi: 10.1158/1078-0432.Ccr-10-3409. [DOI] [PubMed] [Google Scholar]
- 10.Fukumura D., Kloepper J., Amoozgar Z., Duda D.G., Jain R.K. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15(5):325–340. doi: 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makker V., Colombo N., Casado Herráez A., et al. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med. 2022;386(5):437–448. doi: 10.1056/NEJMoa2108330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finn R., Qin S., Ikeda M., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 13.Motzer R.J., Penkov K., Haanen J., et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rini B.I., Plimack E.R., Stus V., et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 15.McDermott D.F., Lee J.L., Ziobro M., et al. Open-label, single-arm, phase II study of pembrolizumab monotherapy as first-line therapy in patients with advanced non-clear cell renal cell carcinoma. J Clin Oncol. 2021;39(9):1029–1039. doi: 10.1200/jco.20.02365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qu Y.Y., Sun Z., Han W., et al. Camrelizumab plus famitinib for advanced or metastatic urothelial carcinoma after platinum-based therapy: data from a multicohort phase 2 study. J Immunother Cancer. 2022;10(5) doi: 10.1136/jitc-2021-004427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qu Y.Y., Zhang H.L., Guo H., et al. Camrelizumab plus famitinib in patients with advanced or metastatic renal cell carcinoma: data from an open-label, multicenter phase II basket study. Clin Cancer Res. 2021;27(21):5838–5846. doi: 10.1158/1078-0432.Ccr-21-1698. [DOI] [PubMed] [Google Scholar]
- 18.Xia L., Zhou Q., Gao Y., et al. A multicenter phase 2 trial of camrelizumab plus famitinib for women with recurrent or metastatic cervical squamous cell carcinoma. Nat Commun. 2022;13(1):7581. doi: 10.1038/s41467-022-35133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia L., Peng J., Lou G., et al. Antitumor activity and safety of camrelizumab plus famitinib in patients with platinum-resistant recurrent ovarian cancer: results from an open-label, multicenter phase 2 basket study. J Immunother Cancer. 2022;10(1) doi: 10.1136/jitc-2021-003831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arance A., de la Cruz-Merino L., Petrella T.M., et al. Phase II LEAP-004 study of lenvatinib plus pembrolizumab for melanoma with confirmed progression on a programmed cell death protein-1 or programmed death ligand 1 inhibitor given as monotherapy or in combination. J Clin Oncol. 2022;41 doi: 10.1200/jco.22.00221. [DOI] [PubMed] [Google Scholar]
- 21.Kim R., Kwon M., An M., et al. Phase II study of ceralasertib (AZD6738) in combination with durvalumab in patients with advanced/metastatic melanoma who have failed prior anti-PD-1 therapy. Ann Oncol. 2022;33(2):193–203. doi: 10.1016/j.annonc.2021.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Powles T., Atkins M.B., Escudier B., et al. Efficacy and safety of atezolizumab plus bevacizumab following disease progression on atezolizumab or sunitinib monotherapy in patients with metastatic renal cell carcinoma in IMmotion150: a randomized phase 2 clinical trial. Eur Urol. 2021;79(5):665–673. doi: 10.1016/j.eururo.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ribas A., Puzanov I., Dummer R., et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908–918. doi: 10.1016/s1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber J.S., D'Angelo S.P., Minor D., et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi: 10.1016/s1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 25.Olson D.J., Eroglu Z., Brockstein B., et al. Pembrolizumab plus ipilimumab following anti-PD-1/L1 failure in melanoma. J Clin Oncol. 2021;39(24):2647–2655. doi: 10.1200/jco.21.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y., Yuan J., Righi E., et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A. 2012;109(43):17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du Four S., Maenhout S.K., Niclou S.P., Thielemans K., Neyns B., Aerts J.L. Combined VEGFR and CTLA-4 blockade increases the antigen-presenting function of intratumoral DCs and reduces the suppressive capacity of intratumoral MDSCs. Am J Cancer Res. 2016;6(11):2514–2531. [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao S., Ren S., Jiang T., et al. Low-dose apatinib optimizes tumor microenvironment and potentiates antitumor effect of PD-1/PD-L1 blockade in lung cancer. Cancer Immunol Res. 2019;7(4):630–643. doi: 10.1158/2326-6066.Cir-17-0640. [DOI] [PubMed] [Google Scholar]
- 29.Jain R.K. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26(5):605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding X., Zhang W.J., You R., et al. Camrelizumab plus apatinib in patients with recurrent or metastatic nasopharyngeal carcinoma: an open-label, single-arm, phase II study. J Clin Oncol. 2023;41 doi: 10.1200/jco.22.01450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.You R., Zou X., Ding X., et al. Gemcitabine combined with apatinib and toripalimab in recurrent or metastatic nasopharyngeal carcinoma. Med. 2022;3(10):664–681.e6. doi: 10.1016/j.medj.2022.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L., Yang Y., Pan J.J., et al. Session HEAD AND NECK CANCER; Chicago, Illinois, USA: 2022. RATIONALE-309: updated progression-free survival (PFS), PFS after next line of treatment, and overall survival from a phase 3 double-blind trial of tislelizumab versus placebo, plus chemotherapy, as first-line treatment for recurrent/metastatic nasopharyngeal cancer. presented at: ASCO Annual Meeting.https://ascopubs.org/doi/abs/10.1200/JCO.2022.40.36_suppl.384950 [Google Scholar]
- 33.Mai H.Q., Chen Q.Y., Chen D., et al. Final progression-free survival analysis of JUPITER-02, a randomized, double-blind, phase 3 study of toripalimab or placebo plus gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma New Orleans LA. Philadelphia (PA). presented at: Proceedings of the 113th annual meeting of the AACR. 2022. Session PO.CT03.01 - Phase III Clinical Trials. [DOI]
- 34.Xu S., Shukuya T., Tamura J., et al. Heterogeneous outcomes of immune checkpoint inhibitor rechallenge in patients with NSCLC: a systematic review and meta-analysis. JTO Clin Res Rep. 2022;3(4) doi: 10.1016/j.jtocrr.2022.100309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodi F.S., Chiarion-Sileni V., Gonzalez R., et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(11):1480–1492. doi: 10.1016/s1470-2045(18)30700-9. [DOI] [PubMed] [Google Scholar]
- 36.Schachter J., Ribas A., Long G.V., et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006) Lancet. 2017;390(10105):1853–1862. doi: 10.1016/s0140-6736(17)31601-x. [DOI] [PubMed] [Google Scholar]
- 37.Herbst R.S., Garon E.B., Kim D.W., et al. Long-term outcomes and retreatment among patients with previously treated, programmed death-ligand 1‒positive, advanced non‒small-cell lung cancer in the KEYNOTE-010 study. J Clin Oncol. 2020;38(14):1580–1590. doi: 10.1200/jco.19.02446. [DOI] [PubMed] [Google Scholar]
- 38.Reck M., Rodríguez-Abreu D., Robinson A.G., et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J Clin Oncol. 2021;39(21):2339–2349. doi: 10.1200/jco.21.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Motzer R., Alekseev B., Rha S.Y., et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289–1300. doi: 10.1056/NEJMoa2035716. [DOI] [PubMed] [Google Scholar]
- 40.Atkins M.B., Plimack E.R., Puzanov I., et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: a non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial. Lancet Oncol. 2018;19(3):405–415. doi: 10.1016/s1470-2045(18)30081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rini B.I., Powles T., Atkins M.B., et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393(10189):2404–2415. doi: 10.1016/s0140-6736(19)30723-8. [DOI] [PubMed] [Google Scholar]
- 42.Choueiri T.K., Powles T., Burotto M., et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829–841. doi: 10.1056/NEJMoa2026982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.