Abstract
Background
N-terminal pro-B-type natriuretic peptide (NT-proBNP) is a cardiac biomarker used in the clinical management of heart failure. We sought to create updated reference intervals for NT-proBNP for healthy US children, adolescents, and adults.
Methods
We identified a population of healthy individuals using the 1999 to 2004 cycles of the National Health and Nutrition Examination Survey (NHANES). We measured serum NT-proBNP in 12 346 adults and 15 752 children and adolescents with the Elecsys NT-proBNP assay on the Roche e601 autoanalyzer. We compared 4 methods for reference interval calculation, and presented the final reference intervals using the robust method partitioned by age and sex categories.
Results
NT-proBNP values were available for 1949 healthy adults and 5250 healthy children and adolescents. NT-proBNP concentrations in males and females varied according to age, being higher in early childhood, relatively lower in late adolescence, and highest through middle age and older age. Females tended to have higher NT-proBNP concentrations compared to men from late adolescence until middle age. The upper reference limit, or 97.5th percentile, for 50 to 59 year-old men was 225 ng/L (90% CI: 158 to 236), and for 50 to 59 year-old women, 292 ng/L (90% CI: 242 to 348).
Conclusions
Among healthy individuals, NT-proBNP concentrations varied greatly according age and sex. The reference intervals presented here should inform future clinical decision limits and suggest that age- and sex-specific intervals may be necessary to more precisely characterize risk.
Impact Statement
NT-proBNP is used in the clinical management of heart failure. Individuals at risk for heart failure across the entire life span stand to benefit from accurate and tailored reference intervals that distinguish between normal and abnormally elevated NT-proBNP. We found that the upper limit of NT-proBNP in healthy individuals, the 97.5th percentile, varied tremendously according to age and sex. Our results indicate that the commonly used cutoff of 125 ng/L is not suitable for all population subgroups.
Introduction
N-terminal pro B-type natriuretic peptide (NT-proBNP) is a cardiac biomarker used in the clinical management of heart failure (1). Though cardiovascular society guidelines endorse NT-proBNP testing, there are no universally accepted cut-points that delineate abnormal from normal (1–5). Establishing a singular cutoff is difficult because natriuretic peptides are used in many settings both to rule in and rule out disease (6). Defining the empirical distribution of NT-proBNP in healthy individuals can aid in distinguishing typical values from extreme values, thus informing optimal cutoffs for clinical practice.
The Clinical and Laboratory Standards Institute (CLSI) defines a reference interval as the range between lower and upper reference limits in a healthy population (7). The lower and upper reference limits are conventionally set to the 2.5th and 97.5th percentiles to capture the central 95% of reference values. Prior NT-proBNP reference intervals have been calculated, but have varied substantially due to differences in laboratory methods, statistical methodology, and characteristics of the underlying populations in which these intervals were derived (8–15).
We measured NT-proBNP concentrations in a large, nationally representative sample of US adults, adolescents, and children, and used demographic, medical questionnaire, laboratory, and physical examination data to rigorously identify a healthy subset of the population without disease or major cardiovascular risk factors. We partitioned the reference population by age and sex, compared multiple methods for constructing reference intervals, and present the calculated upper reference limits.
Materials and Methods
Study Population
The National Health and Nutrition Examination Survey (NHANES) is a continuously operating cross-sectional study of community dwelling children and adults in the United States (16). Approximately 5000 individuals are selected each year from multiple counties across the country to obtain a diverse and representative sample. Participants provide demographic, socioeconomic, medical history, and prescription medication information during the interview component and contribute body measurement and clinical data during the examination component. Participants who provided consent and were 12 months or older at the time of examination underwent a blood draw. For the 1999–2004 survey cycles, as part of the NHANES Biospecimen Program, surplus serum was collected and transferred into cryovials for long-term storage at −80°C in the CDC and Agency for Toxic Substances and Disease Registry Specimen Packaging, Inventory, and Repository (17). The measurement of NT-proBNP in stored serum was approved by the ethics review board of the National Center for Health Statistics.
Measurement of NT-proBNP
We obtained aliquots of all available stored serum samples from participants in NHANES 1999–2004. NT-proBNP was measured on the Roche e601 autoanalyzer using the Elecsys NT-proBNP assay at the University of Maryland School of Medicine Clinical Pathology Laboratory from 2018 to 2020. The coefficient of variation was 3.1% for low concentrations of NT-proBNP (mean, 46 ng/L) and 2.7% for high concentrations (mean, 32 805 ng/L). The lower limit of detection for this assay was 5 ng/L and 723 measurements below the lower limit of detection were replaced with 3.54 ng/L (), and 3 measurements above the limit of detection were replaced with 35 000 ng/L. Of the 21 206 serum samples tested, 20 063 (95%) were pristine, meaning they had never undergone a freeze–thaw cycle.
Statistical Analysis
Selection of healthy reference individuals
We used NT-proBNP measured in a healthy subsample of individuals to determine the reference interval. We defined this subsample by excluding individuals if they reported taking any hematologic or cardiovascular medications, were underweight, overweight, obese, were missing information on body mass index (BMI), had a history of hypertension, had a history of diabetes, or had hemoglobin A1c ≥6.5%, were treated for anemia in the past 3 months, or had an estimated glomerular filtration rate of <60 mL/min/1.73 m2.
Adults 20 years and older were excluded if they reported having a history of congestive heart failure, coronary heart disease, angina, myocardial infarction, stroke, emphysema, bronchitis, liver disease, hypercholesterolemia, or prescription for a cholesterol lowering medication, kidney disease, dialysis, or cancer. BMI was calculated for individuals younger than 24 months using World Health Organization z-scores for individuals aged 2 to 19 years, using CDC z-scores, and for adults 20 years and older, the weight in kilograms divided by the square of height in meters (18, 19). Children were considered to be in the normal BMI category if their z-score was between −2 and +1, and for adults, if their BMI was between 18.5 and 25 kg/m2 (normal weight). The estimated glomerular filtration rate was calculated for adults aged 20 years and older with the 2021 CKD-EPI creatinine–cystatin C equation, and for individuals younger than 20 years, with the 2021 CKiD U25 equations (20, 21).
The reference population was partitioned by age and sex due to prior evidence of sex and age-based differences in natriuretic peptide concentration among healthy individuals (22–28). We partitioned adults by decade, and used the NHANES analytic guidelines to determine age categories for children and adolescents (29).
Reference interval calculation
We examined the natural distribution of NT-proBNP as a continuous function of age in the healthy subsample using survey weighted quantile regression, with a natural cubic spline of age, stratified by sex, to estimate the 97.5th quantile for males and females separately. We used the age, in months, at the time of the NHANES examination, and age at the time of the interview if examination age was unavailable. Because the distribution of NT-proBNP was right-skewed, reference interval calculations were performed on log-transformed NT-proBNP, and the final estimates with confidence intervals were exponentiated. We assessed the normality assumption of log-transformed NT-proBNP with Kolmogorov–Smirnov and Shapiro–Wilk tests.
We compared quantile regression, parametric, nonparametric, and robust methods to construct the reference intervals (7, 30, 31). Because the sample size for healthy individuals was limited in the oldest age categories, we grouped all adults aged 70 years or older, and used the robust method, which has been shown to have sufficient power even in very small samples (32). We did not remove extreme values within the set of reference values for healthy individuals under the assumption that these values reflect true variability in the full distribution of NT-proBNP (33). For all 4 methods, we reported the 90% confidence interval for the 2.5th and 97.5th percentile reference limits.
Survey weighting
The sample data in NHANES is weighted to account for survey sampling and nonresponse bias to match as close as possible to the US Census population (16). NHANES survey weights were used for quantile regressions and calculating the proportions of individuals with a NT-proBNP measurement ≥125 and 450 ng/L, age-based and FDA-approved used cutoffs for NT-proBNP in the ambulatory setting to detect heart failure (3, 5). The baseline characteristics tables and the parametric, nonparametric, and robust calculations were created without using survey weights.
Statistical software
All analyses were performed in R (version 4.2.2). The reference intervals were calculated with the referenceIntervals (version 1.3.0) package, and the survey package was used to account for the NHANES sample design (version 4.1–1) (34, 35). Data files used for this analysis are available online from the National Center for Health Statistics and the statistical code to fully reproduce these analyses is available as a Quarto document (Supplemental File 2).
Results
Study Sample
NT-proBNP values were available for 12 346 of the 15 376 adult participants (≥ 20 years) and 8860 of the 15 752 pediatric participants (<20 years of age) in the 1999–2004 NHANES survey cycles. Overall, 15.8% of adult participants were apparently healthy (1949 individuals), and 59.3% of participants between age 1 and 19 were apparently healthy (5250 individuals) (Tables 1 and 2). The number of apparently healthy reference individuals within each sex/age group was >200 for all age groups between 2 and 39 years, but for other age groups, the number of reference individuals ranged between 43 and 157 (Table 3).
Table 1.
Baseline characteristics of NHANES adults, 1999–2004.
| Characteristic | Healthy participants, n = 1949a | Excluded participants, n = 10 397a | Overall, N = 12 346a |
|---|---|---|---|
| Age at examination (years) | 34 (25, 45) | 52 (36, 68) | 48 (33, 66) |
| Gender | |||
| Male | 944 (48%) | 4955 (48%) | 5899 (48%) |
| Female | 1005 (52%) | 5442 (52%) | 6447 (52%) |
| Race/ethnicity | |||
| Mexican American | 426 (22%) | 2365 (23%) | 2791 (23%) |
| Other Hispanic | 88 (4.5%) | 478 (4.6%) | 566 (4.6%) |
| Non-Hispanic White | 1012 (52%) | 5339 (51%) | 6351 (51%) |
| Non-Hispanic Black | 322 (17%) | 1881 (18%) | 2203 (18%) |
| Other race—including multiracial | 101 (5.2%) | 334 (3.2%) | 435 (3.5%) |
| NT-proBNP (ng/L) | 38 (19, 71) | 55 (25, 135) | 51 (24, 119) |
| BMI category | |||
| Underweight | — | 179 (1.7%) | 179 (1.4%) |
| Normal weight | 1949 (100%) | 1675 (16%) | 3624 (29%) |
| Overweight | — | 4332 (42%) | 4332 (35%) |
| Obese | — | 3871 (37%) | 3871 (31%) |
| Missing | — | 340 (3.3%) | 340 (2.8%) |
| Reported any hematologic or cardiovascular medication | — | 3587 (35%) | 3587 (29%) |
| History of hypertension | — | 3899 (38%) | 3899 (32%) |
| Prescribed antihypertensive medication | — | 3171 (30%) | 3171 (26%) |
| History of hypercholesterolemia | — | 3344 (32%) | 3344 (27%) |
| Prescribed hypercholesterolemia medication | — | 1789 (17%) | 1789 (14%) |
| History of diabetes | — | 1207 (12%) | 1207 (9.8%) |
| Hemoglobin A1c ≥6.5 | — | 1102 (11%) | 1102 (8.9%) |
| History of kidney disease | — | 344 (3.3%) | 344 (2.8%) |
| eGFR <60 mL/min/1.73 m2 | — | 757 (7.3%) | 757 (6.1%) |
| Receiving dialysis | — | 13 (0.1%) | 13 (0.1%) |
| Receiving anemia treatment | — | 423 (4.1%) | 423 (3.4%) |
| History of congestive heart failure | — | 389 (3.7%) | 389 (3.2%) |
| History of coronary heart disease | — | 555 (5.3%) | 555 (4.5%) |
| History of angina | — | 459 (4.4%) | 459 (3.7%) |
| History of myocardial infarction | — | 575 (5.5%) | 575 (4.7%) |
| History of stroke | — | 419 (4.0%) | 419 (3.4%) |
| History of emphysema or bronchitis | — | 913 (8.8%) | 913 (7.4%) |
| History of liver disease | — | 390 (3.8%) | 390 (3.2%) |
| History of cancer | — | 1060 (10%) | 1060 (8.6%) |
This table displays the baseline characteristics of 12 346 adults who participated in the combined NHANES cycles from 1999–2004, divided into apparently healthy and excluded participants. The proportion of individuals belonging to each characteristic are given in parentheses. The median age and NT-proBNP concentrations are displayed, along with the interquartile interval in parentheses (25th, 75th).
Median (25th, 75th percentiles); n (%).
Table 2.
Baseline characteristics of NHANES children and adolescents, 1999–2004.
| Characteristic | Healthy participants, n = 5250a | Excluded participants, n = 3610a | Overall, N = 8860a |
|---|---|---|---|
| Age at examination (years) | 13.0 (9.0, 16.0) | 13.0 (10.0, 17.0) | 13.0 (9.0, 16.0) |
| Gender | |||
| Male | 2639 (50%) | 1831 (51%) | 4470 (50%) |
| Female | 2611 (50%) | 1779 (49%) | 4390 (50%) |
| Race/Ethnicity | |||
| Mexican American | 1737 (33%) | 1335 (37%) | 3072 (35%) |
| Other Hispanic | 219 (4.2%) | 154 (4.3%) | 373 (4.2%) |
| Non-Hispanic White | 1501 (29%) | 831 (23%) | 2332 (26%) |
| Non-Hispanic Black | 1575 (30%) | 1157 (32%) | 2732 (31%) |
| Other Race—Including Multiracial | 218 (4.2%) | 133 (3.7%) | 351 (4.0%) |
| NT-proBNP (ng/L) | 36 (18, 65) | 32 (16, 56) | 34 (17, 61) |
| BMI category | |||
| Underweight | — | 273 (7.6%) | 273 (3.1%) |
| Normal weight | 5250 (100%) | 179 (5.0%) | 5429 (61%) |
| Overweight | — | 1439 (40%) | 1439 (16%) |
| Obese | — | 1590 (44%) | 1590 (18%) |
| Missing | — | 129 (3.6%) | 129 (1.5%) |
| Reported any hematologic or cardiovascular medication | — | 55 (1.5%) | 55 (0.6%) |
| History of hypertension | — | 120 (3.3%) | 120 (1.4%) |
| Prescribed antihypertensive medication | — | 18 (0.5%) | 18 (0.2%) |
| History of diabetes | — | 27 (0.7%) | 27 (0.3%) |
| Hemoglobin A1c ≥6.5 | — | 25 (0.7%) | 25 (0.3%) |
| eGFR <60 mL/min/1.73 m2 | — | 2 (<0.1%) | 2 (<0.1%) |
| Receiving anemia treatment | — | 136 (3.8%) | 136 (1.5%) |
This table displays the baseline characteristics of 8860 children and adolescents who participated in the combined NHANES cycles from 1999–2004, divided into healthy and excluded participants. The proportion of individuals belonging to each characteristic are given in parentheses. The median age and NT-proBNP concentrations are displayed, along with the interquartile interval in parentheses (25th, 75th).
Median (25th, 75th percentiles); n (%)
Table 3.
NT-proBNP reference intervals (robust method).
| Age category (years) | Sex | Sample size | 2.5th percentile (90% CI) | 50th percentile (90% CI) | 97.5th percentile (90% CI) |
|---|---|---|---|---|---|
| 1 | Male | 60 | 25 (20, 493) | 97 (83, 116) | 379 (303, 493) |
| Female | 57 | 23 (18, 475) | 93 (79, 108) | 367 (290, 475) | |
| 2 to 5 | Male | 254 | 12 (10, 351) | 60 (55, 66) | 304 (264, 351) |
| Female | 241 | 14 (12, 326) | 61 (57, 66) | 277 (235, 326) | |
| 6 to 11 | Male | 634 | 11 (10, 296) | 54 (51, 57) | 267 (241, 296) |
| Female | 633 | 12 (11, 279) | 55 (52, 58) | 254 (233, 279) | |
| 12 to 15 | Male | 810 | 4 (4, 193) | 28 (26, 30) | 177 (163, 193) |
| Female | 883 | 7 (6, 179) | 34 (33, 36) | 165 (154, 179) | |
| 16 to 19 | Male | 881 | 3 (3, 91) | 16 (15, 17) | 84 (77, 91) |
| Female | 797 | 7 (6, 162) | 32 (30, 33) | 149 (137, 162) | |
| 20 to 29 | Male | 374 | 3 (3, 103) | 16 (15, 18) | 91 (80, 103) |
| Female | 395 | 10 (9, 223) | 44 (41, 47) | 199 (179, 223) | |
| 30 to 39 | Male | 229 | 4 (4, 117) | 21 (19, 23) | 100 (86, 117) |
| Female | 272 | 14 (12, 211) | 50 (47, 54) | 186 (164, 211) | |
| 40 to 49 | Male | 155 | 4 (3, 170) | 24 (21, 27) | 140 (118, 170) |
| Female | 157 | 15 (12, 287) | 59 (54, 65) | 238 (196, 287) | |
| 50 to 59 | Male | 77 | 7 (5, 326) | 41 (35, 46) | 225 (158, 326) |
| Female | 86 | 17 (13, 348) | 71 (61, 81) | 292 (242, 348) | |
| 60 to 69 | Male | 48 | 8 (5, 757) | 61 (49, 75) | 456 (292, 757) |
| Female | 52 | 28 (22, 369) | 90 (79, 105) | 290 (233, 369) | |
| 70+ | Male | 61 | 19 (12, 1930) | 155 (123, 193) | 1286 (849, 1930) |
| Female | 43 | 32 (22, 1510) | 177 (141, 222) | 976 (664, 1510) |
The lower reference limit (2.5th percentile), median, and upper reference limit (97.5th percentile) of NT-proBNP in healthy NHANES participants from survey cycles 1999–2004, partitioned by sex and age category, using the robust method are displayed here. This iterative method determines a measure of spread around the median, downweighting outliers in successive iterations.
Reference Intervals
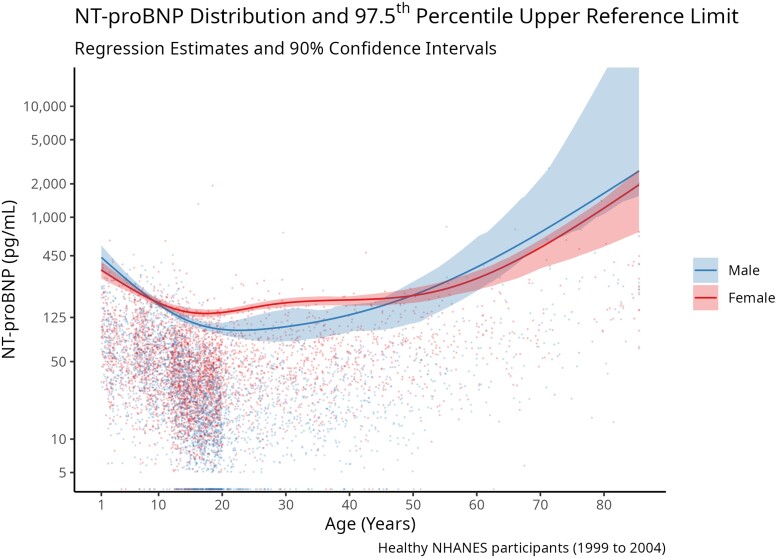
We regressed the 97.5th percentile of NT-proBNP on the natural cubic spline of age with 3 degrees of freedom, stratified by sex (Fig. 1). The 97.5th percentile upper reference limit for natural distribution of NT-proBNP varied according to age and sex (Fig. 2). The upper reference limit for NT-proBNP was 379 ng/L (90% CI: 303 to 493) in 1-year-old boys, and 367 ng/L (90% CI: 290 to 475) in 1 year-old girls (Table 3). This upper limit was lower through childhood, adolescence, and early adulthood, reaching a nadir of 84 (90% CI: 77 to 91) in 16 to 19 year-old boys, and 149 ng/mL (90% CI: 137 to 162) in 16 to 19 year-old girls. The reference limit was higher with each decade of life after young adulthood and was much higher in older adults. Men aged 70 and older had an upper reference limit of 1286 ng/L (90% CI: 849 to 1930), and women aged 70 and older had an upper reference limit of 976 ng/L (90% CI: 664 to 1510). Females had similar NT-proBNP concentrations compared to males in childhood and late adulthood, but had higher NT-proBNP concentrations than males starting from adolescence until approximately age 50. Reference intervals for children and adolescents partitioned by year are included in Supplemental Table 1 and reference intervals with separate partitions for adults aged 70 to 79 and 80 or older are included in Supplemental Table 2.
Fig. 1.
NT-proBNP distribution and 97.5th percentile upper reference limit. The 97.5th percentile of NT-proBNP was estimated using a natural cubic spline with 3 df for healthy males and females. Individual NT-proBNP values are also plotted, with each point representing a single measurement of NT-proBNP. NT-proBNP concentration is on the logarithmic scale, and the regression line gives the 97.5th percentile estimate and corresponding 90% confidence interval. The gap in the distribution of NT-proBNP at low values is an artifact of the limit of detection of the NT-proBNP assay, where all values below 5 ng/L are replaced with 3.54 ng/L.
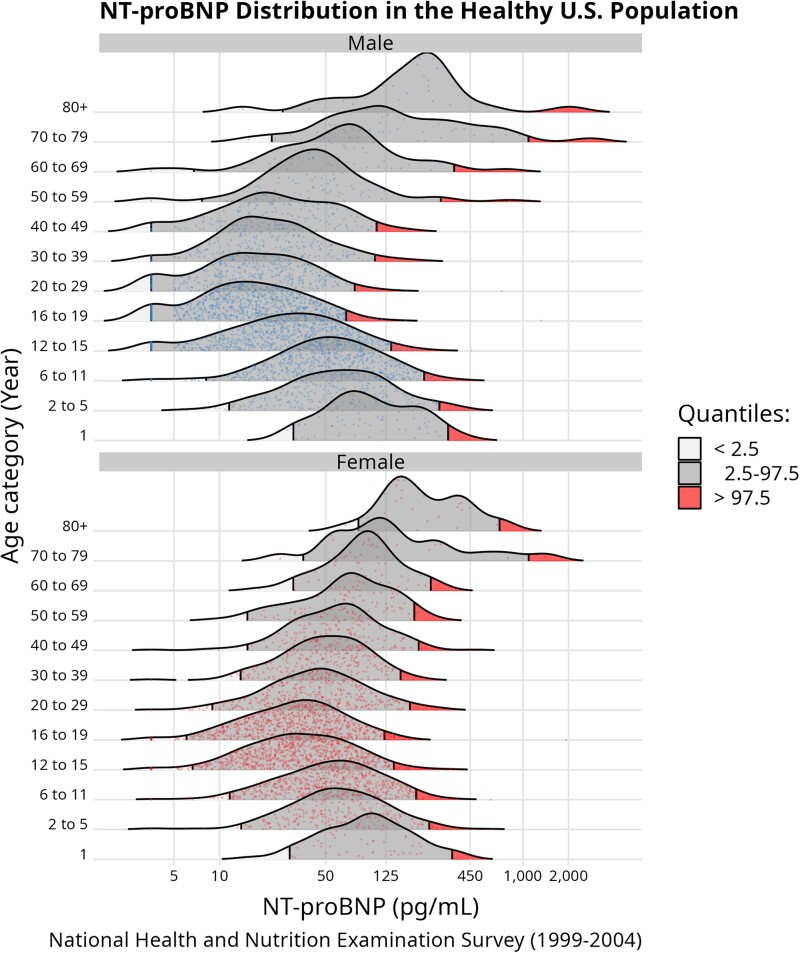
Fig. 2.
NT-proBNP distribution in the healthy US population. This ridgeline plot shows the NT-proBNP distribution in healthy NHANES participants from survey cycles 1999–2004, partitioned by age category and sex. Survey weight information was incorporated in the quantile calculation and individual measurements are plotted within the density curve. The portions of the distribution above the 97.5th percentile are shaded in red. The increased densities at the lower portions of the NT-proBNP distributions is an artifact of the limit of detection of the NT-proBNP assay, where all values below 5 ng/L are replaced with 3.54 ng/L.
The 4 different methods for estimating the upper reference limit produced similar results when the sample size for any particular group was approximately 100 or greater, but there was substantial variability between methods for groups with smaller sample sizes. In particular, the nonparametric method tended to produce estimates that were larger than the other methods when sample size was small. The methods were successful in producing estimates with confidence intervals except for the oldest age groups using the weighted quantile regression method because there were too few reference individuals (Supplemental Table 3).
Proportion of Healthy Individuals with Elevated NT-proBNP
Table 4 displays the proportion of healthy individuals within each sex and age category with a NT-proBNP ≥125 ng/L, and in older individuals, ≥ 450 ng/L. In healthy 70–79-year-olds, 49.9% (95% CI: 14.3% to 85.6%) of men and 52.6% (95% CI: 14.0 to 89.4%) of women had a NT-proBNP concentration ≥125 ng/L (Table 4). For adults, the likelihood of an elevated NT-proBNP ≥125 ng/L increased with age, and there was a consistent sex-based difference, as women were more likely to have elevated NT-proBNP compared to men.
Table 4.
Weighted proportion of healthy individuals with elevated NT-proBNP.
| Proportion with NT-proBNP at or above 125 or 450 ng/L (95% confidence interval) | |||
|---|---|---|---|
| Age category (years) | Sex | 125 ng/L | 450 ng/L |
| 1 | Male | 34.2% (17.5, 53.2) | — |
| Female | 28.8% (12.9, 48.0) | — | |
| 2 to 5 | Male | 15.7% (9.8, 22.7) | — |
| Female | 17.5% (11.8, 24.2) | — | |
| 6 to 11 | Male | 14.3% (10.3, 18.7) | — |
| Female | 12.5% (9.2, 16.1) | — | |
| 12 to 15 | Male | 3.3% (2.1, 4.8) | — |
| Female | 3.5% (2.1, 5.3) | — | |
| 16 to 19 | Male | 0.1% (0.0, 0.3) | — |
| Female | 2.5% (1.1, 4.3) | — | |
| 20 to 29 | Male | 1.1% (0.1, 3.1) | — |
| Female | 8.8% (5.9, 12.3) | — | |
| 30 to 39 | Male | 2.0% (0.4, 4.8) | — |
| Female | 5.9% (2.6, 10.4) | — | |
| 40 to 49 | Male | 3.1% (0.3, 8.6) | — |
| Female | 14.7% (8.6, 22.1) | — | |
| 50 to 59 | Male | 6.5% (1.0, 16.1) | — |
| Female | 22.7% (13.4, 33.8) | — | |
| 60 to 69 | Male | 15.1% (4.1, 31.4) | — |
| Female | 22.5% (7.5, 42.5) | — | |
| 70 to 79 | Male | 49.9% (14.3, 85.6) | 23.5% (0.3, 66.5) |
| Female | 52.6% (14.0, 89.4) | 19.5% (0.2, 67.3) | |
| 80+ | Male | 69.3% (34.6, 94.7) | 6.4% (0.3, 28.9) |
| Female | 83.6% (46.4, 100.0) | 8.8% (2.2, 46.8) | |
This table displays the survey weighted proportion of healthy individuals from NHANES survey cycles 1999–2004, partitioned by sex and age category, with an NT-proBNP measurement equal to or above the preselected values of 125 and 450 ng/L. 95% confidence intervals were calculated using the variance-stabilizing arcsine square root transformation for the binomial distribution, subsequently expressed on the probability scale.
Discussion
In this nationally representative sample of healthy US children and adults, we demonstrated that the natural distribution of NT-proBNP varied according to age and sex, with females having higher NT-proBNP concentrations from adolescence to the sixth decade of life. Also, many adults older than 50 years without apparent disease had NT-proBNP concentrations greater than the common clinical cutoff of 125 ng/L. Even considering an age-adjusted cutoff of 450 ng/L, in those aged 70 to 79, nearly one-quarter and one-fifth of healthy males and females, respectively, had an elevated NT-proBNP concentration (6).
Our study extends and updates previous reference interval calculations. We applied a consistent methodology and included individuals across the entire life span, beginning at 12 months of age. Our results are consistent with previous studies that showed a decrease in NT-proBNP from birth to early adolescence, with a sex-based difference appearing in adolescence (15, 28). In the Leipzig Research Center for Civilization Diseases cohort, the upper reference limit for 1-year-old children was 362.6 ng/L for males and 379.7 ng/L for females, decreasing to 76.2 ng/L and 135.9 ng/L, respectively, by age 17 (28).
In middle-aged adults, previous studies have shown that NT-proBNP increases with age (8, 12). As an example, reference limits calculated from the Framingham Heart Study had an upper limit of 41.8 ng/L in 20–24 year-old men, which increased to 131.2 ng/L by age 50–59. In women, the corresponding reference limits were 103.5 ng/L (20–24 year olds) to 223.8 ng/L (50–54 year olds) (12).
In the oldest adults, upper limits for NT-proBNP reference intervals can often extend beyond the commonly used clinical cut-points of 125, 300, and 450 ng/L used in diagnosis and staging of heart failure. Reference intervals calculated using combined data from 2 German cohorts (Activity and Function in the Elderly Study and the Study of Health in Pomerania) showed that men older than 80 years had an upper reference limit of 697 ng/L, and for women, 1276 ng/L (36). In that analysis, 27.1% and 44.6% of healthy men and women aged 65 and above had an NT-proBNP concentration over 125 ng/L. In our study, we discovered that most healthy individuals aged 80 or older had an NT-proBNP concentration above 125 ng/L.
A strength of our study was the nationally representative sampling of NHANES, which included >5000 healthy children and nearly 2000 healthy adults. The standardized physical and clinical measurements allowed rigorous identification of a healthy subsample within the general population, and our statistical techniques were reproducible. The robust method of reference interval calculation theoretically performs well even if log-transformed NT-proBNP does not follow a normal distribution and also when sample sizes are limited (7, 30).
Our investigation had limitations that should be noted. First, the CLSI working group recommends the direct sampling technique, with clearly defined a priori exclusion criteria, but our reference values were obtained via indirect sampling from a pre-existing cross-sectional study. Direct sampling can alleviate the problem of an inadequate reference sample group size by enrolling a pre-specified number of reference individuals. Second, our definition of the healthy subsample partially relied on self-reported information, which may have resulted in misclassification. Incorrectly classifying unhealthy individuals as healthy inappropriately inflates the imprecision of the estimates. Third, the reference ranges were derived from a single sample, which discount the potential diurnal and rhythmic patterns of NT-proBNP secretion (37). Fourth, the sample size was limited for the oldest adults which led to imprecise estimates in those groups. The CLSI recommends a 90% confidence interval of “less than 0.2 times the width of the reference interval,” which was met or nearly met in the children and middle-aged partitions, but not for the oldest adults (7).
Last, another weakness of this study is that the transport, storage, and measurement of frozen serum is a departure from fresh plasma/blood collection and analysis in clinical practice. However, previous studies have demonstrated that the NT-proBNP assay is stable in long-term storage and robust to freeze–thaw cycles (38, 39). Most prior reference interval studies of NT-proBNP were conducted using stored samples and our results were similar (15, 36). Because concentrations tend to be slightly lower when measured in stored samples, it is possible that the reference intervals presented here may be slight underestimates of the true upper limit of normal had fresh specimens been analyzed. This reinforces our conclusion that the commonly used clinical decision limit of 125 ng/L is actually within the expected range of NT-proBNP, especially for older adults.
Our study has implications for both research and clinical practice. Future studies should extend this study by using contemporary measurements of NT-proBNP, larger sample sizes, especially in older adults, and the direct sampling technique. Our results underscore the need for additional investigation to understand the various factors that modulate the synthesis, secretion, or clearance of NT-proBNP over the lifespan. NT-proBNP reference intervals can inform more accurate clinical decision limits, which can then be prospectively validated. As NT-proBNP testing is indicated for acute diagnosis of heart failure, outpatient management of chronic heart failure, and increasingly being used for cardiovascular risk stratification, the cutoffs could be different for each of these indications. Our results suggest that incorporating age and sex information in future clinical decision limits may increase accuracy and usefulness.
Conclusion
We determined that the upper limit of the reference interval for NT-proBNP varied considerably according to age and sex. Clinical decision limits using NT-proBNP for heart failure and other conditions should take into account this heterogeneity and avoid using a single cut-point for more precise and tailored risk estimates.
Supplemental Material
Supplemental material is available at The Journal of Applied Laboratory Medicine online.
Supplementary Material
Acknowledgments
Scott Z. Mu would like to acknowledge Elizabeth Selvin and Josef Coresh for their tireless support, guidance, and mentorship.
Contributor Information
Scott Mu, Department of Epidemiology and the Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, United States.
Justin B Echouffo-Tcheugui, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine, Johns Hopkins University, Baltimore, MD, United States.
Chiadi E Ndumele, Division of Cardiology, Department of Medicine, Johns Hopkins University, Baltimore, MD, United States.
Josef Coresh, Department of Epidemiology and the Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, United States.
Stephen Juraschek, Division of General Internal Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States.
Tammy Brady, Division of Pediatric Nephrology, Department of Pediatrics, Johns Hopkins University, Baltimore, MD, United States.
John William McEvoy, Division of Cardiology and National Institute for Prevention and Cardiovascular Health, National University of Ireland, Galway, Ireland.
Bige Ozkan, Division of Cardiology, Department of Medicine, Johns Hopkins University, Baltimore, MD, United States.
Olive Tang, Department of Epidemiology and the Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins Bloomberg School of Public Health, and Johns Hopkins University School of Medicine, Baltimore, MD, United States.
Robert H Christenson, Department of Pathology, University of Maryland School of Medicine, Baltimore, MD, United States.
Elizabeth Selvin, Department of Epidemiology and the Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, United States.
Author Contributions: The corresponding author takes full responsibility that all authors on this publication have met the following required criteria of eligibility for authorship: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved. Nobody who qualifies for authorship has been omitted from the list.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest: Employment or Leadership: R.H. Christenson, The Journal of Applied Laboratory Medicine, AACC. E. Selvin is a Deputy Editor of Diabetes Care and a member of the Editorial Board of Diabetologia. Consultant or Advisory Role: R.H. Christenson has received consulting fees from Beckman Coulter, Roche Diagnostics, Siemens Healthineers, PixCell Medical, Becton Dickinson, Sphingotech, Quidel Medical. Stock Ownership: None declared. Honoraria: R.H. Christenson, Roche Diagnostics, Quidel Medical, Becton Dickinson, Siemens Healthineers, Beckman Coulter. Research Funding: This work was funded by a grant from the Foundation for the National Institutes of Health Biomarkers Consortium to the Johns Hopkins Bloomberg School of Public Health (Principal investigator: E. Selvin). The Foundation for the National Institutes of Health received support for this project from Abbott Laboratories, AstraZeneca, Johnson & Johnson, the National Dairy Council, Ortho Clinical Diagnostics, Roche Diagnostics, and Siemens Healthcare Diagnostics. Reagents for the NT-proBNP assays were donated by the Roche Diagnostics Corporation, and reagents for the cystatin C assay were donated by the Siemens Diagnostics Corporation. Reagents and funding for measurements were from Johns Hopkins University. S.Z. Mu was supported by NIH/NHLBI grant T32 HL007024; J.B. Echouffo-Tcheugui was supported by NIH/NHLBI grant K23 HL153774; J. Coresh was supported by grants from the NIH; S. Juraschek was supported by grants from the NIH; B. Ozkan was supported by American Heart Association grant 20SFRN35120152; E. Selvin was supported by NIH/NHLBI grant K24 HL152440, National Institutes of Health, and American Heart Association; O. Tang was supported by NIH/NHLBI grant T32 HL007024 and NIH/NIDDK F30 DK120160; R.H. Christenson was supported by grants from AACC, contracts from Becton Dickinson, Abbott Diagnostics, Siemens Healthineers, Ortho Diagnostics, Roche Diagnostics, Beckman Coulter, PixCell Medical, National Institute of Standards and Technology. Expert Testimony: None declared. Patents: None declared. Other Remuneration: E. Selvin receives royalties for authorship of sections of UpToDate (Wolters Kluwer Health) related to screening and diagnosis of diabetes and laboratory tests for diabetes. R.H. Christenson has received travel support from Quidel Medical, Roche Diagnostics, and Werfen.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, preparation of manuscript, or final approval of manuscript.
References
- 1. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure. J Am Coll Cardiol 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 2. McCullough PA, Kluger AY. Interpreting the wide range of NT-proBNP concentrations in clinical decision making. J Am Coll Cardiol 2018;71:1201–3. [DOI] [PubMed] [Google Scholar]
- 3. Maddox TM, Januzzi JL, Allen LA, Breathett K, Butler J, Davis LL, et al. 2021 Update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction. J Am Coll Cardiol 2021;77:772–810. [DOI] [PubMed] [Google Scholar]
- 4. Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, et al. Heart failure association of the European Society of Cardiology Practical Guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail 2019;21:715–31. [DOI] [PubMed] [Google Scholar]
- 5. Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail 2021;27:387–413. [DOI] [PubMed] [Google Scholar]
- 6. Hammerer-Lercher A, Gruson D, Stankovic S, Collinson P, Suvisaari J, Pulkki K, et al. Update on current practice in laboratory medicine in respect of natriuretic peptide testing for heart failure diagnosis and management in Europe. The CARdiac MArker guideline uptake in Europe (CARMAGUE) study. Clin Chim Acta 2020;511:59–66. [DOI] [PubMed] [Google Scholar]
- 7. CLSI . EP28-A3c: Defining, establishing, and verifying reference intervals in the clinical laboratory; Approved guideline. 3rd Ed. Wayne (PA): Clinical and Laboratory Standards Institute; 2010.
- 8. Hess G, Runkel S, Zdunek D, Hitzler WE. Reference interval determination for N-terminal-B-type natriuretic peptide (NT-proBNP): a study in blood donors. Clin Chim Acta 2005;360:187–93. [DOI] [PubMed] [Google Scholar]
- 9. Shi X, Xu G, Xia T, Song Y, Lin Q. N-terminal-pro-B-type natriuretic peptide (NT-proBNP): reference range for Chinese apparently healthy people and clinical performance in Chinese elderly patients with heart failure. Clin Chim Acta 2005;360:122–7. [DOI] [PubMed] [Google Scholar]
- 10. Alehagen U, Goetze JP, Dahlström U. Reference intervals and decision limits for B-type natriuretic peptide (BNP) and its precursor (nt-proBNP) in the elderly. Clin Chim Acta 2007;382:8–14. [DOI] [PubMed] [Google Scholar]
- 11. Lee K-H, Kim J-Y, Koh S-B, Lee S-H, Yoon J, Han S-W, et al. N-Terminal pro-B-type natriuretic peptide levels in the Korean general population. Korean Circ J 2010;40:645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fradley MG, Larson MG, Cheng S, McCabe E, Coglianese E, Shah RV, et al. Reference limits for N-terminal-pro-B-type natriuretic peptide in healthy individuals (from the Framingham Heart Study). Am J Cardiol 2011;108:1341–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gaggin HK, Dang PV, Do LD, deFilippi CR, Christenson RH, Lewandrowski EL, et al. Reference interval evaluation of high-sensitivity troponin T and N-terminal B-type natriuretic peptide in Vietnam and the US: the north south east west trial. Clin Chem 2014;60:758–64. [DOI] [PubMed] [Google Scholar]
- 14. Nir A, Nasser N. Clinical value of NT-ProBNP and BNP in pediatric cardiology. J Card Fail 2005;11:S76–80. [DOI] [PubMed] [Google Scholar]
- 15. Lam E, Higgins V, Zhang L, Chan MK, Bohn MK, Trajcevski K, et al. Normative values of high-sensitivity cardiac troponin T and B-terminal pro-B-type natriuretic peptide in children and adolescents: a study from the CALIPER cohort. J Appl Lab Med 2021;6:344–53. [DOI] [PubMed] [Google Scholar]
- 16. National Center for Health Statistics . The National Health and Nutrition Examination Survey: Sample design,1999–2006. Hyattsville (MD): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2012.
- 17. National Center for Health Statistics . National Health and Nutrition Examination Survey Biospecimen Program: NHANES III (1988–1994) and NHANES 1999-2014. Hyattsville (MD): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2015.
- 18. CDC . Growth charts. 2019. https://www.cdc.gov/growthcharts/index.htm(Accessed November 2022).
- 19. CDC . About adult BMI. 2022. https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html (Accessed November 2022).
- 20. Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New creatinine- and cystatin cbased equations to estimate GFR without race. N Engl J Med 2021;385:1737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pierce CB, Muñoz A, Ng DK, Warady BA, Furth SL, Schwartz GJ. Age- and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney Int 2021;99:948–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hildebrandt P, Collinson PO, Doughty RN, Fuat A, Gaze DC, Gustafsson F, et al. Age-dependent values of N-terminal pro-B-type natriuretic peptide are superior to a single cut-point for ruling out suspected systolic dysfunction in primary care. Eur Heart J 2010;31:1881–9. [DOI] [PubMed] [Google Scholar]
- 23. McKie PM, Cataliotti A, Lahr BD, Martin FL, Redfield MM, Bailey KR, et al. The prognostic value of N-terminal ProB-type natriuretic peptide for death and cardiovascular events in healthy normal and stage A/B heart failure subjects. J Am Coll Cardiol 2010;55:2140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bombelli M, Maloberti A, Rossi S, Rea F, Corrao G, Bonicelli Della Vite C, et al. Clinical value of NT-proBNP assay in the emergency department for the diagnosis of heart failure (HF) in very elderly people. Arch Gerontol Geriatr 2015;61:296–300. [DOI] [PubMed] [Google Scholar]
- 25. Myhre PL, Claggett B, Yu B, Skali H, Solomon SD, Røsjø H, et al. Sex and race differences in N-terminal ProB-type natriuretic peptide concentration and absolute risk of heart failure in the community. JAMA Cardiol 2022;7:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Suthahar N, Meijers WC, Ho JE, Gansevoort RT, Voors AA, van der Meer P, et al. Sex-specific associations of obesity and N-terminal pro-B-type natriuretic peptide levels in the general population. Eur J Heart Fail 2018;20:1205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Choi H-I, Lee MY, Oh BK, Lee SJ, Kang JG, Lee SH, et al. Effects of age, sex, and obesity on N-terminal pro B-type natriuretic peptide concentrations in the general population. Circ J 2021;85:647–54. [DOI] [PubMed] [Google Scholar]
- 28. Kiess A, Green J, Willenberg A, Ceglarek U, Dähnert I, Jurkutat A, et al. Age-dependent reference values for hs-troponin T and NT-proBNP and determining factors in a cohort of healthy children (the LIFE child study). Pediatr Cardiol 2022;43:1071–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. National Center for Health Statistics . National Health and Nutrition Examination Survey: analytic guidelines, 1999–2010. Hyattsville (MD): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2013.
- 30. Horn PS, Pesce AJ, Copeland BE. Reference interval computation using robust vs parametric and nonparametric analyses. Clin Chem 1999;45:2284–5. [PubMed] [Google Scholar]
- 31. Asgari S, Higgins V, McCudden C, Adeli K. Continuous reference intervals for 38 biochemical markers in healthy children and adolescents: comparisons to traditionally partitioned reference intervals. Clin Biochem 2019;73:82–9. [DOI] [PubMed] [Google Scholar]
- 32. Horn PS, Pesce AJ, Copeland BE. A robust approach to reference interval estimation and evaluation. Clin Chem 1998;44:622–31. [PubMed] [Google Scholar]
- 33. Hickman PE, Koerbin G, Potter JM, Glasgow N, Cavanaugh JA, Abhayaratna WP, et al. Choice of statistical tools for outlier removal causes substantial changes in analyte reference intervals in healthy populations. Clin Chem 2020;66:1558–61. [DOI] [PubMed] [Google Scholar]
- 34. Lumley T. Package ‘survey’. Analysis of complex survey samples. Version 4.1-1. Vienna (Austria): R Found. Stat. Comput; 2020.
- 35. Finnegan D. Package ‘referenceIntervals’. Reference Intervals. Version 1.3.0. Vienna (Austria): R Found. Stat. Comput; 2022.
- 36. Braisch U, Koenig W, Rothenbacher D, Denkinger M, Friedrich N, Felix SB, et al. N-terminal pro brain natriuretic peptide reference values in community-dwelling older adults. ESC Heart Fail 2022;9:1703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Parcha V, Patel N, Gutierrez OM, Li P, Gamble KL, Musunuru K, et al. Chronobiology of natriuretic peptides and blood pressure in lean and obese individuals. J Am Coll Cardiol 2021;77:2291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dallmeier D, Pencina MJ, Rajman I, Koenig W, Rothenbacher D, Brenner H. Serial measurements of N-terminal pro-brain natriuretic peptide in patients with coronary heart disease. PLoS ONE 2015;10:e0117143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cauliez B, Guignery J, Marinier S, Mariau I, Lavoinne A. Two-year stability of NT-proBNP in frozen samples using the Roche elecsys system. Ann Clin Biochem 2008;45:318–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.