Abstract
Dengue virus infection results in a broad spectrum of diseases ranging from mild dengue fever (DF) to severe dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS). Hitherto, there is no consensus biomarker for the prediction of severe dengue disease in patients. Yet, early identification of patients who progress to severe dengue is pivotal for better clinical management. We have recently reported that an increased frequency of classical (CD14 ++CD16−) monocytes with sustained high TLR2 expression in acutely infected dengue patients correlates with severe dengue development. Here, we hypothesized that the relatively lower TLR2 and CD14 expression in mild dengue patients is due to the shedding of their soluble forms (sTLR2 and sCD14) and that these could be used as indicators of disease progression. Therefore, using commercial sandwich ELISAs, we evaluated the release of sTLR2 and sCD14 by peripheral blood mononuclear cells (PBMCs) in response to in vitro dengue virus (DENV) infection and assessed their levels in acute-phase plasma of 109 dengue patients. We show that while both sTLR2 and sCD14 are released by PBMCs in response to DENV infection in vitro, their co-circulation in an acute phase of the disease is not always apparent. In fact, sTLR2 was found only in 20% of patients irrespective of disease status. In contrast, sCD14 levels were detected in all patients and were significantly elevated in DF patients when compared to DHF patients and age-matched healthy donors. Altogether, our results suggest that sCD14 may help in identifying patients at risk of severe dengue at hospital admittance.
Keywords: Dengue virus, PBMCs, sTLR2, sCD14, Biomarkers
1. Introduction
The four serotypes of dengue virus (DENV1-4) co-circulate in over 100 countries and are responsible for an estimated 390 million infections annually [[1], [2], [3]]. DENV infections can be inapparent or result in a relatively mild dengue fever (DF). However, in some cases the disease progresses to a severe form called dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) [4]. Infection with any of the serotypes confers a life-long protection against that serotype. However, it increases the risk of severe disease following secondary infection with another serotype. The acute and viremic phase of DENV infection is associated with increased systemic host responses which contribute to the resolution of infection. Dysregulation of these responses results in increased vascular permeability, plasma leakage and decreased platelet concentrations that are hallmarks of DHF/DSS [4,5]. Early identification of dengue patients who are at increased risk of developing severe dengue is essential for better clinical management of these individuals, especially in resource-constricted settings. Currently, there is no single immune-related consensus marker readily available that would allow prediction of severe disease pathogenesis upon hospital admittance. Consequently, early diagnosis relies on multi-parameter interpretation of clinical and biological markers by the health care provider [[6], [7], [8], [9], [10]].
The initiation of the innate immune response and ultimately containment of any infection depends on the recognition of pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors (PRRs) [11,12]. Several of such PRRs expressed on the surface of blood monocytes, including TLR2 and CD14, are capable of sensing both bacterial and viral ligands, such as DENV [[13], [14], [15], [16], [17], [18], [19]]. An efficient TLR2-mediated response requires the activity of the co-receptor CD14 [20] and CD14 is known to amplify TLR-mediated pro-inflammatory responses [21,22]. Importantly, for the inflammation to be favorable to the host, its extent and duration needs to be tightly regulated [23,24]. One of such mechanisms relies on the reduction of the plasma membrane-expressed PRRs, such as TLR2 or CD14, through the release of their extracellular, soluble portions. Indeed, the levels of circulating sTLR2 and sCD14 increase in the course of inflammatory conditions [[23], [24], [25], [26], [27], [28], [29], [30], [31]] and their levels have been associated with higher mortality during sepsis [[32], [33], [34], [35], [36], [37], [38]].
Notably, while sTLR2 is incapable of signal transduction and functions as decoy receptor that by sequestering PAMPs and DAMPs prevents excessive immune activation, the function of sCD14 seem to depend on its concentration in body fluids. In the context of DENV infection, studies evaluating the concentrations of sCD14 during the acute phase of infection have yielded conflicting results in adult and paediatric dengue patients. In children, sCD14 concentrations were lower in patients with severe dengue and higher plasma leakage score compared to those with mild dengue and healthy controls suggesting that sCD14 may have a protective role in paediatric patients [39]. However, in adults, the opposite was reported with severe dengue patients producing more sCD14 [40,41]. Notably however, in a recent study, we noted that the expression of TLR2 on monocytes was reduced in acutely infected DENV paediatric patients when compared to healthy donors [14]. In addition, a significant correlation was observed between the expression of TLR2 on classical (CD14++CD16−) monocytes (CM) and the development of severe disease [14]. A decrease in TLR2 expression correlated with mild disease, while sustained, higher expression of TLR2 on CM was associated with the development of severe dengue. However, whether the decrease of TLR2 expression in the acute phase of dengue is due to the shedding of its soluble form remains unknown.
Here, we sought to assess if sTLR2 and sCD14 are released upon DENV infection and if their detection could be useful for patient management at hospital admittance. To that end, we evaluated the plasma concentrations of sTLR2 and sCD14 in a cohort of healthy donors and paediatric dengue patients in the acute phase of infection. Notably, only a few dengue patients (20%) produced detectable quantities of sTLR2 indicating that it likely has a limited role in regulating DENV-induced inflammation and cannot be utilized as a biomarker. In contrast, sCD14 concentrations were detectable in all children tested and were higher in DF patients compared to DHF/DSS patients and healthy donors. Thus, sCD14 may have a protective role on paediatric dengue patients and can be used as a biomarker for dengue severity at hospital admittance.
2. Methodology
2.1. Ethics statement
The clinical study was approved by the National Ethics Committee of Health Research of Cambodia. Written informed consent was obtained from the guardians of participants prior to their inclusion in the study.
2.2. Patient and healthy subject recruitment
Venous blood (∼3–5 ml) was drawn in one EDTA tube from 109 paediatric patients (DF: 63; DHF/DSS: 46) more than 2 years of age who presented with dengue-like symptoms at the Kantha Bopha Hospital in Phnom Penh, Cambodia between 2016 and 2019. All patients’ blood samples were collected within 120 h of appearance of fever. A second blood sample was collected at the time of discharge from the hospital. All hospitalized patients were classified according to the WHO 1997 classification scheme in dengue fever (DF), dengue hemorrhagic fever (DHF) or dengue shock syndrome (DSS) [4]. Blood samples of 30 healthy donors were obtained from a household-based study in Kampong Cham province in 2018. These healthy donors belonged to the same age group and demographics as the pediatric dengue patients. They were classified as healthy based on the lack of dengue-like clinical symptoms at time of sample collection and by being negative for dengue RT-PCR at time of sample collection and during follow-up 10 days afterwards. The demographics of the patient and healthy donor cohorts are summarized in Table 1.
Table 1.
Demographics and clinical parameters of healthy donors (n = 30) and dengue patients (n = 109) included in the study. * DHF/DSS cases were classified according to the WHO 1997 classification criteria. Final diagnosis of DHF/DSS was performed at hospital discharge. $ A rise in hematocrit equal to or greater than 20% above the age-sex and population adjusted average. % ≤ 100 × 109/L.
| Dengue patients |
Healthy donors | |||
|---|---|---|---|---|
| Total | DF* | DHF/DSS* | ||
| No. of individuals | 109 | 63 | 46 | 30 |
| Age (years) | 9.2 ± 3.1 | 9.0 ± 2.9 | 9.5 ± 3.3 | 8.1 ± 4.1 |
| M/F ratio | 1.1 | 1.3 | 0.9 | 1.5 |
| Temperature at hospital admission (°C) | 37.6 ± 0.6 | 37.6 ± 0.6 | 37.8 ± 0.9 | NA |
| Hematocrit at hospital admission (%) | 39.6 ± 6.4 | 39.6 ± 5.2 | 39.4 ± 6.7 | |
| Evidence of plasma leakage at admission (%)$ | 17 | 5 | 33 | |
| Thrombocytopenia (at admission)% | 51 | 16 | 100 | |
| Day of fever (mean, range) | 3.8 (2–5) | 3.5 (2–5) | 4.1 (3–5) | |
| DENV1 | 58 | 43 | 15 | |
| DENV2 | 43 | 14 | 27 | |
| DENV3 | 1 | 1 | 0 | |
| DENV4 | 7 | 5 | 2 | |
| NS1+ | 75 | 48 | 27 | |
| PCR | 107 | 63 | 44 | |
| Viral load (RNA copies/ml) (median, IQR) | 430 (3.2–7290) | 210 (1.2–4320) | 1043 (4.2–9955) | |
| Viral load (RNA copies/ml) (geometric mean, SD) | 182.2 ± 226.2 | 104.3 ± 298.2 | 390.7 ± 145.1 | |
| Secondary infection (%) | 78 | 62 | 100 | |
2.3. Laboratory diagnosis
Plasma was obtained by centrifugation and aliquoted. One aliquot was used for dengue diagnosis and characterization. DENV infection was detected by DENV-specific qRT-PCR [42]. For establishing the real-time PCR used in this study, DENV RNA was reverse transcribed, amplified using the mentioned primers and cloned into DNA plasmids. These plasmids were used as DNA standards for the PCR. The LOD for each set of recombinant plasmids was 5 copies/reaction for DENV-1, 1 copy/reaction for DENV-2, 5 copies/reaction for DENV-3 and 10 copies/reaction for DENV-4. Serum samples were tested for presence of DENV NS1 antigen using a rapid antigen test (SD Bioline Dengue Duo kits, Standard Diagnostics, Kyonggi-do, Korea) as per manufacturer's instructions. Anti-DENV IgM in patient serum was measured using an IgM-capture ELISA as described [43]. The criteria for classification of patients as having acute DENV infection were the presence of a positive qRT-PCR or NS1 at the time of hospital admittance, or seroconversion of anti-DENV IgM during hospitalization. Patients were identified as having primary/secondary DENV infection using a hemagglutination inhibition (HI) test in line with the WHO 2009 criteria [44].
2.4. Virus
DENV2 (strain 16681) was produced by transfection of the RNA transcripts derived from the cDNA pD2/IC-30P clone plasmid into BHK-15 cells as described previously [45]. The produced virus was passaged maximum of 2 times in of C6/36 cells by infecting the cells at a multiplicity of infection (MOI) of 0.1. After 72–96 h of infection, the supernatant was harvested and centrifuged to remove the cells. The cell-free supernatant was aliquoted and stored at −80 °C. The infectious titer and number of genome-equivalent RNA copies in the DENV2 preparation were determined using plaque assay and real-time quantitative PCR respectively, as described previously [46,47].
2.5. In vitro infection with DENV
In a separate part of this study, buffy coats were obtained from adult, healthy, anonymous volunteers (n = 3) presenting at the Sanquin Bloodbank in the Netherlands according to the ethical guidelines presented in the declaration of Helsinki (Sanquin Bloodbank, Groningen, The Netherlands) and used for isolation of peripheral blood mononuclear cells (PBMCs). Isolated PBMCs were cryopreserved in liquid nitrogen. After thawing, the PBMCs were resuspended in RPMI 1640, counted, and seeded at a concentration of 1x106 cell/ml in the respective wells. The cells were infected with a standard DENV2 preparation (DENV2 16681 strain) at MOI 10 and incubated at 37 °C, 5% CO2. Supernatants were collected at 24, 48 & 72 h after infection, snap-frozen in liquid nitrogen and preserved at −80 °C.
2.6. Detection of soluble TLR2 and CD14
The concentrations of soluble TLR2 and soluble CD14 were determined in supernatants harvested at different time-points from the in vitro-infected PBMCs or patient plasma samples using the Human TLR2 DuoSet ELISA (R&D systems, catalogue no: DY2616) and Human TLR2 DuoSet ELISA (R&D systems, catalogue no: DY383) according to the manufacturer's instructions.
2.7. Statistical analysis
All statistical analyses in this study were performed using GraphPad Prism 9.0 (GraphPad). Non-parametric Mann-Whitney U test or Kolmogorov-Smirnov was used to compare data between two groups as the data was not in accordance with the D'Agostino-Pearson criteria for normality. All values are shown as median and interquartile range (IQR). Receiver-operating characteristic (ROC) curve analysis was done using Graphpad Prism version 9.0.
3. Results
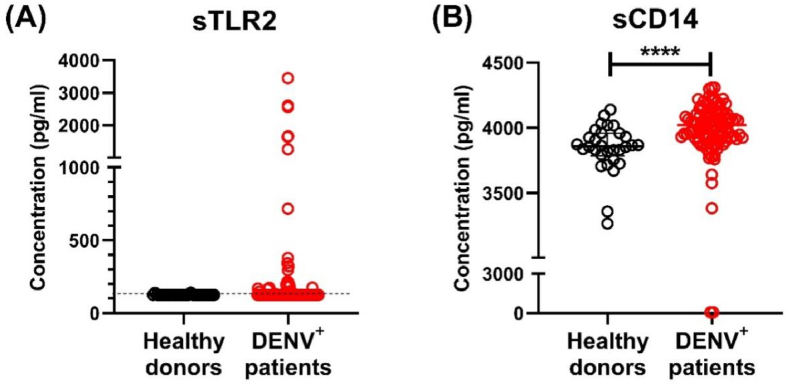
Before assessing if sTLR2 and sCD14 detection could be useful for patient management at hospital admittance, we first, we tested whether DENV infection leads to the production of soluble forms of TLR2 and CD14 in the established in vitro model of acute phase of the infection. To this end, PBMCs from healthy donors were infected with DENV2 and sTLR2 and sCD14 were quantified in cell supernatants at 24, 48 and 72 h post infection (hpi). Mock-treated PBMCs produced little or no TLR2 (values below the limit of detection of 125 pg/ml were set to 0). In contrast, DENV2 infection triggered the release of considerable levels of sTLR2 (median: 1753 pg/ml), which remain similar at all time-points sTLR2 (Supplementary Fig. 1A). At the same time, DENV2 infection of PBMCs also induced a massive increase in the production of sCD14 (median: 6198 pg/ml) (Supplementary Fig. 1B) while mock treated PBMCs did not produce detectable levels of sCD14 (limit of detection = 62.5 pg/ml). Thus, infection of PBMCs with DENV2 in vitro triggers the release of both sTLR2 and sCD14. Whether in vitro infection with other DENV serotypes can elicit similar quantities of sTLR2 and sCD14 was not investigated. However, based on the previously noted changes in TLR2 and CD14 expression on monocytes during DENV infection [14,15], we felt encouraged to test whether plasma levels sTLR2 and sCD14 have potential as biomarkers for severe disease during the acute phase of infection. Accordingly, we used a cohort 109 paediatric dengue patients in the acute phase of infection and 30 healthy donors (Table 1). The concentrations of sTLR2 and sCD14 were measured in plasma samples obtained from these healthy donors and dengue patients using a sandwich ELISA. Only 26 (2 healthy donors and 24 dengue patients) out of the 139 individuals tested had detectable quantities of plasma sTLR2 (Fig. 1A). The sTLR2 levels found in DENV patients were similar to those in healthy donors (Fig. 1A). In contrast to sTLR2, sCD14 was detectable in all healthy donors and in all but one dengue patient. Dengue patients had significantly higher concentrations of sCD14 compared to healthy donors (p < 0.0001) (Fig. 1B).
Fig. 1.
Detection of sTLR2 and sCD14 in plasma from patients undergoing acute dengue infection. Concentrations of (A) sTLR2 and (B) sCD14 were determined in healthy donors (n = 30) and dengue patients (n = 109) using sandwich ELISA. P values were determined using Kolmogorov-Smirnov test. Error bars represent median ± interquartile range (IQR). *p < 0.05, ****p < 0.0001.
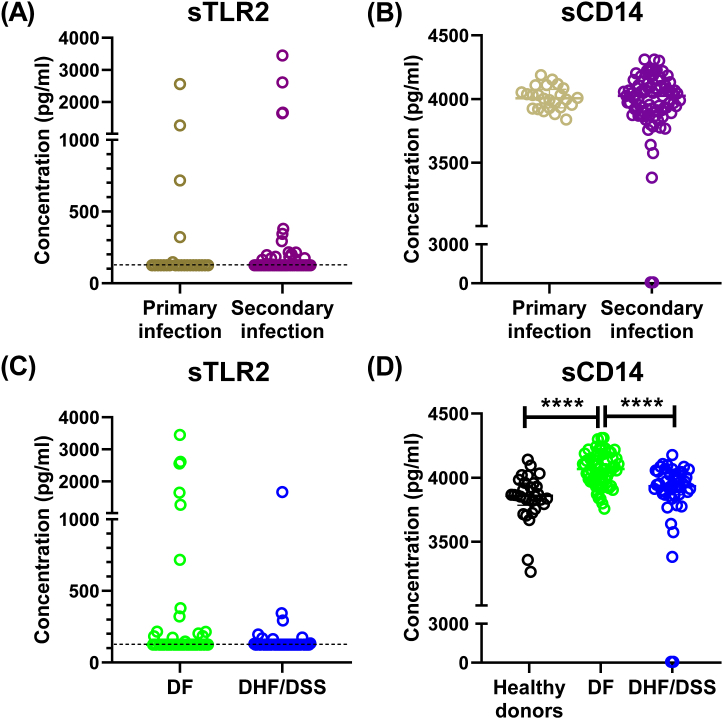
To further investigate the role of sTLR2 and sCD14 as a biomarker for disease severity, we analyzed whether their concentrations correlated with the immune history and development of severe disease in acute dengue patients. However, no difference was observed in the concentrations of sTLR2 and sCD14 in dengue patients based on immune history (Fig. 2A). Based on the clinical presentation of dengue patients at time of admission and during the course of stay in hospital, the dengue patients were divided into DF and DHF/DSS, according to WHO1997 classification criteria. However, we also did not find a difference in sTLR2 concentrations in DF patients compared to patients who progress to severe DHF/DSS (Fig. 2C). Interestingly, the concentrations of sCD14 were significantly higher in DF patients compared to those in DHF/DSS patients, which were similar to those observed in healthy donors (Fig. 2D). Furthermore, no differences were observed in sTLR2 and sCD14 production when patients were stratified based on the infecting DENV serotype (Supplementary Fig. 2A). Previously, Chen et al., had suggested that the DENV NS1 protein activates TLR2-mediated responses, although later studies showed that NS1 protein interacted with TLR4 and not TLR2 [48,49]. When dengue patients were classified based on positivity to DENV NS1 protein rapid diagnostic test, no differences were observed in the concentrations of sTLR2 and sCD14, further indicating that NS1 protein alone might not have a role in regulating TLR2 responses to DENV infection (Supplementary Fig. 2B). We also then investigated whether DENV viremia correlated with the production of sTLR2 and sCD14 in patient plasma. However, no such correlation was observed (Supplementary Figs. 3A and 3B).
Fig. 2.
Comparison of sTLR2 and sCD14 in healthy donors and dengue patients. Concentrations of sTLR2 and sCD14 in dengue patients (n = 109) classified based on infection history (A & B), disease severity (C & D). Error bars show median ± interquartile range (IQR). ****p < 0.0001.
Next, we stratified the dengue patients based on day of fever at time of admission to the hospital to understand the day-wise dynamic changes of sTLR2 and sCD14 concentrations. However, no differences were observed based on day of fever suggesting that the concentrations of sTLR2 and sCD14 remain consistent during the early acute phase of infection (Supplementary Fig. 4A). Next, we investigated whether the detection and day-wise changes in concentrations of sTLR2 and sCD14 at hospital admission could be used to identify severe dengue patients. As limited number of patients produced sTLR2, there were no significant differences observed upon stratification of patients for disease severity (Supplementary Fig. 4B). However, DHF/DSS patients were found to produce significantly lower quantities of sCD14 between days 3–5 of fever compared to DF patients (Supplementary Fig. 4C).
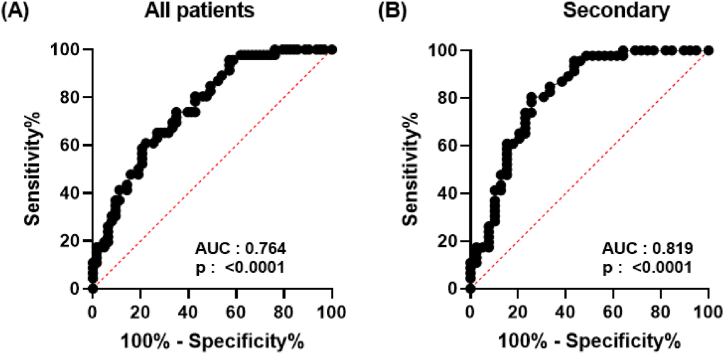
Since we observed that sCD14 concentrations were significantly lower in severe dengue patients compared to those with dengue fever, we next evaluated its potential as a biomarker using a receiver operating characteristic (ROC) analysis. ROC curve was generated by plotting sensitivity against specificity for sCD14 concentrations in dengue patients classified based on the WHO 1997 classification. The area under the ROC curve (AUC) for patients was 0.764 (95% CI: 0.6764–0.8512; p < 0.0001) (Fig. 3A). However, when the ROC curve was generated for dengue patients with secondary infection, which account for ∼78% of patients admitted in the hospital, the AUC improved to 0.819 (95% CI: 0.7261–0.9127; p < 0.0001) (Fig. 3B). Altogether, these results suggest that sCD14 alone is not a robust biomarker associated with severe dengue, yet it could be utilized as an extra indicator to identify patients at risk of severe dengue at hospital admittance.
Fig. 3.
Predictive analysis of soluble CD14 as biomarker for severe dengue disease. The ROC curve of sCD14 concentrations of (A) all acute dengue patients and (B) secondary dengue infected patients stratified into DF and DHF/DSS groups was calculated using GraphPad Prism 9. The values of sensitivity and specificity for each sample are represented on a graph.
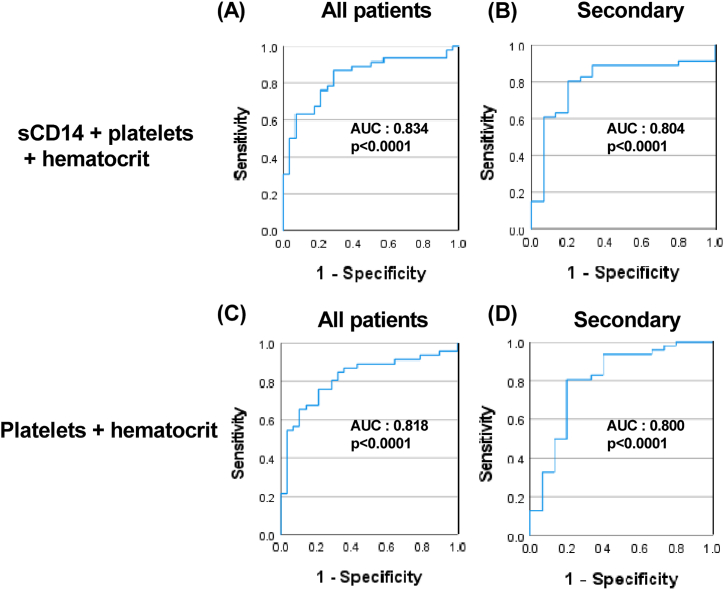
Based on previous literature, platelet counts and hematocrit, two important clinical parameters used in the WHO classification for distinguishing patients with DF and DHF/DSS, were chosen as representative markers for severe dengue. Indeed, at the time of hospital admission, platelet counts were lower, and hematocrit was higher in patients who subsequently developed DHF/DSS compared to those with DF (Supplementary Figs. 5A–D). Hematocrit and platelet count modestly correlated with sCD14 concentrations in patients with secondary dengue infections (Supplementary Fig. 6B). However, no such correlation was observed in all dengue patients or patients stratified based on DF and DHF/DSS (Supplementary Fig. 6A, 6C-D). Next, a combinatorial biomarker ROC analysis was done to ascertain whether sCD14 can be used along with platelet counts and hematocrit to identify patients who may develop severe dengue at hospital admission. We observed that the addition of sCD14 to platelets and hematocrit modestly improved the AUC from 0.818 to 0.834 in all patients and a similar increase was observed in when comparing dengue patients with secondary infections, which account for more than 75% of clinical cases in our cohort (Fig. 4). These results suggest that sCD14 may have some value as a marker for severe dengue when used as a part of a biomarker panel.
Fig. 4.
Predictive analysis of a combination of soluble CD14, platelet counts and hematocrit as biomarkers for severe dengue disease. The ROC curve of combination of two or three biomarkers of all acute dengue patients (A, C) and with secondary dengue infection (B, D) stratified based on WHO 1997 classification into DF and DHF/DSS groups was calculated using SPSS. The values of sensitivity and specificity for each sample are represented on a graph.
4. Discussion
Here, we assessed the prognostic value of sTLR2 and sCD14 in the course of DENV infection in a paediatric cohort of dengue patients in Cambodia. Analysis of plasma samples from 109 dengue patients and 30 age-matched controls revealed that sTLR2 is present in merely one-fifth of tested dengue patients and healthy donors. In contrast, sCD14 was present in all of the plasma samples from dengue patients and healthy donors, yet no differences were found in the measured concentrations. However, when dengue patients were classified based on disease severity, we observed that DF patients had elevated levels of sCD14 compared to DHF/DSS patients.
To the best of our knowledge, thus far, only few studies have addressed sTLR2-mediated immune regulation during viral infections. Previously, in vitro studies have shown that sTLR2 interacts with the PAMPs from HIV leading to reduced NF-κB activation and HIV-infection [[50], [51], [52]]. Because we have previously [14] observed significantly reduced TLR2 expression on monocytes of dengue patients compared to those of healthy donors, we hypothesized that dengue infection leads to the release of sTLR2 which aids regulation of inflammation and thus its levels would be increased in dengue patients. Indeed, in vitro infection of PBMC triggered the release of sTLR2 and thereby supported the premise of our hypothesis. Surprisingly however, plasma levels of sTLR2 were detected in only a subset (20%) of dengue patients. The reasons for that apparent mismatch are not clear. The low abundance of measured plasma sTLR2 in dengue patients, despite its relatively potent release from the in vitro infected PBMCs, could be due to the high turnover of soluble factors in patients during acute DENV infection. Alternatively, it could be caused by the inability to detect ligand-bound sTLR2 in the ELISA. It is also important to note that while our in vitro assay evaluated the release of sTLR2 following DENV2 infection, in our cohort, patients infected with DENV1 seemed to have higher plasma levels of sTLR2 compared to patients infected with DENV2 or DENV4. Altogether these results suggest that infection with any of DENV serotype can induce the release of sTLR2 by PBMCs. However, further studies are needed to elucidate the mechanism and function of sTLR2 in course of dengue.
We observed that concentrations of sCD14 were significantly lower in patients with DHF/DSS compared to DF patients in the acute phase of DENV infection. Based on ROC curve analysis, sCD14 seems to have limited value as a sole biomarker at hospital admittance associated with severe dengue. This suggests that early monitoring of sCD14 concentrations, in combination with other routine diagnostic methods such as measurement of lymphocyte and platelet counts, concentrations of liver enzymes ALT and AST and other biomarkers under consideration such as IL-10 and endothelial cell activation markers, may have prognostic value as a biomarker for disease severity. Recently, a comprehensive study by Vuong et al., which utilized patient samples from multiple cohorts, has demonstrated that a set of cytokines and vasculatory biomarkers was reliably associated with the development of severe dengue in both children and adults [53]. In this regard, our preliminary results show that adding sCD14 to a combination of platelet counts and hematocrit may improve the identification of patients progressing to severe dengue. However, further investigation is necessary to show that sCD14 can also be an important biomarker for the early identification of patients progressing to severe dengue and form a part of such diagnostic panels. This group of biomarkers could then be developed into cost-effective multiplex rapid tests or lateral flow assays and put to use in dengue-endemic settings.
The immunoregulatory function of sCD14, which is shed primarily by monocytes seems to depend on its concentration. In vitro high concentrations of sCD14 (3–4 μg/ml) downregulate LPS-mediated responses by transferring LPS to lipoproteins for clearance [27]. At low concentrations (<1 μg/ml) however, sCD14 can confer LPS-responsiveness to cells not expressing CD14 such as endothelial and epithelial cells and thus result in increased production of pro-inflammatory cytokines [27,54,55]. How these concentrations tested in vitro translate to in vivo situation remains unclear. In our paediatric cohort, plasma concentrations of sCD14 were in the order of 4–5 ng/ml. Yet, higher levels of sCD14 in DF than DHF/DSS patients may also indicate a role for sCD14 in protection from severe disease. This is in line with the findings of a previous study where sCD14 concentrations correlated negatively with plasma leakage score in children [39]. Notably, the baseline concentrations of sCD14 observed in children were significantly lower compared to those reported in studies with adult patients. Moreover, in contrast to data obtained from children, in adult dengue patients elevated plasma concentrations of sCD14 (>2 μg/ml) associate with poor disease prognosis [40,41]. Similar observations have also been made with regards to sCD14 concentrations in adult patients with HIV or COVID-19 infections [56,57]. To further investigate the role of sCD14 as a biomarker for severity during acute dengue infection, studies involving larger cohorts spanning different age groups are needed to confirm the findings. Whether the opposite functions of sCD14 in DENV vs HIV and SARS-CoV-2 are age or concentration dependent or rely on other, yet unknown mechanisms remain to be elucidated.
In this study, we aimed to test the use of sTLR2 and/or sCD14 concentrations for distinguishing mild and severe dengue patients, not to use it as a biomarker for distinguishing dengue infection from other febrile illnesses. Indeed, changes in the concentrations of sCD14 itself is not specific to patients with acute dengue infection and has been reported in patients with bacterial sepsis, HIV and COVID-19 [56,57]. To determine if sCD14 levels can be used to help distinguish dengue infection or other febrile diseases, future studies will need to include early acute phase samples from patients infected with other flaviviruses such as West Nile virus or Zika virus and other fevers of viral and bacterial origin.
In summary, our data show that while sTLR2 and sCD14 were significantly elevated upon infection of PBMCs with DENV in vitro, sCD14 but not sTLR2 was detectable in plasma of children in acute phase of dengue. Furthermore, we demonstrated that the levels of sCD14 measured were significantly higher in DF patients than in DHF/DSS patients and healthy donors. Based on currently available data, CD14 may have a potential use as a biomarker for predicting patients who will progress to severe dengue. However, this finding needs to be further validated and tested in additional dengue cohorts.
Author contributions
Conceived and designed the experiments: Izabela Rodenhuis-Zybert, Tineke Cantaert, Vinit Upasani.
Performed the experiments: Vinit Upasani, Bram M. ter Ellen, Sotheary Sann, Sokchea Lay.
Analyzed and interpreted the data: Vinit Upasani, Izabela Rodenhuis-Zybert, Tineke Cantaert, Bram M. ter Ellen.
Contributed reagents, materials, analysis tools or data: Izabela Rodenhuis-Zybert, Tineke Cantaert, Jolanda M. Smit, Denis Laurent, Sothy Heng, Sowath Ly, Philippe Dussart, Veasna Duong.
Wrote the paper: Vinit Upasani, Izabela Rodenhuis-Zybert, Tineke Cantaert, Bram M. ter Ellen, Jolanda M. Smit, Sotheary Sann, Sokchea Lay, Denis Laurent, Sothy Heng, Sowath Ly, Philippe Dussart, Veasna Duong.
Funding
This study has been funded by a Research Grant 2019 from the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) to IR-Z. TC was funded by the Institute Pasteur International Network and is an HHMI-Wellcome Trust International Research Scholar (208710/Z/17/Z). VU was funded by the Institute Pasteur International Network Calmette and Yersin Ph.D. scholarship and UMCG. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank all patients and legal guardians who participated in the study. We would like to thank all the doctors and staff at Kantha Bopha Children's Hospital who were involved in recruitment and inclusion of patients.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e17265.
Contributor Information
Tineke Cantaert, Email: tineke.cantaert@pasteur.fr.
Izabela A. Rodenhuis-Zybert, Email: i.a.rodenhuis-zybert@umcg.nl.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Guzman M.G., Kouri G. Dengue and dengue hemorrhagic fever in the Americas: lessons and challenges. J. Clin. Virol. 2003;27(1):1–13. doi: 10.1016/s1386-6532(03)00010-6. [DOI] [PubMed] [Google Scholar]
- 2.Simmons C.P., Farrar J.J., Nguyen, van V.C., Wills B. Dengue. N Engl J Med. 2012;366(15):1423–1432. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 3.Green S., Rothman A. Immunopathological mechanisms in dengue and dengue hemorrhagic fever. Curr. Opin. Infect. Dis. 2006;19(5):429–436. doi: 10.1097/01.qco.0000244047.31135.fa. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . World Health Organization Press; Geneva: 1997. Dengue Guidelines for Diagnosis, Treatment, Prevention and Control. [Google Scholar]
- 5.Costa V.V., Fagundes C.T., Souza D.G., Teixeira M.M. Inflammatory and innate immune responses in dengue infection: protection versus disease induction. Am. J. Pathol. 2013;182(6):1950–1961. doi: 10.1016/j.ajpath.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 6.da Silva M.M.C., Gil L.H.V.G., Marques ET de A Jr, Calzavara-Silva C.E. Potential biomarkers for the clinical prognosis of severe dengue. Mem. Inst. Oswaldo Cruz. 2013;108(6):755–762. doi: 10.1590/0074-0276108062013012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson M., Einav S. Towards predicting progression to severe dengue. Trends Microbiol. 2020;28(6):478–486. doi: 10.1016/j.tim.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Dussart P., Duong V., Bleakley K., Fortas C., Lorn Try P., Kim K.S., et al. Comparison of dengue case classification schemes and evaluation of biological changes in different dengue clinical patterns in a longitudinal follow-up of hospitalized children in Cambodia. PLoS Neglected Trop. Dis. 2020;14(9) doi: 10.1371/journal.pntd.0008603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikolayeva I., Bost P., Casademont I., Duong V., Koeth F., Prot M., et al. A blood RNA signature detecting severe disease in young dengue patients at hospital arrival. J. Infect. Dis. 2018;217(11):1690–1698. doi: 10.1093/infdis/jiy086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bournazos S., Vo H.T.M., Duong V., Auerswald H., Ly S., Sakuntabhai A., et al. Antibody fucosylation predicts disease severity in secondary dengue infection. Science. 2021;372(6546):1102–1105. doi: 10.1126/science.abc7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar H., Kawai T., Akira S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011;30(1):16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 12.Mogensen T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009;22(2):240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliveira-Nascimento L., Massari P., Wetzler L.M. The role of TLR2 in infection and immunity. Front. Immunol. 2012;3:79. doi: 10.3389/fimmu.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aguilar-Briseño J.A., Upasani V., Ellen B.M.T., Moser J., Pauzuolis M., Ruiz-Silva M., et al. TLR2 on blood monocytes senses dengue virus infection and its expression correlates with disease pathogenesis. Nat. Commun. 2020;11(1):3177. doi: 10.1038/s41467-020-16849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azeredo E.L., Neves-Souza P.C., Alvarenga A.R., Reis S.R.N.I., Torrentes-Carvalho A., Zagne S.-M.O., et al. Differential regulation of toll-like receptor-2, toll-like receptor-4, CD16 and human leucocyte antigen-DR on peripheral blood monocytes during mild and severe dengue fever: monocyte activation after dengue infection. Immunology. 2010;130(2):202–216. doi: 10.1111/j.1365-2567.2009.03224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nightingale Z.D., Patkar C., Rothman A.L. Viral replication and paracrine effects result in distinct, functional responses of dendritic cells following infection with dengue 2 virus. J. Leukoc. Biol. 2008;84(4):1028–1038. doi: 10.1189/jlb.0208105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raby A.-C., Holst B., Le Bouder E., Diaz C., Ferran E., Conraux L., et al. Targeting the TLR co-receptor CD14 with TLR2-derived peptides modulates immune responses to pathogens. Sci. Transl. Med. 2013;5(185) doi: 10.1126/scitranslmed.3005544. [DOI] [PubMed] [Google Scholar]
- 18.Leifer C.A., Medvedev A.E. Molecular mechanisms of regulation of Toll-like receptor signaling. J. Leukoc. Biol. 2016;100(5):927–941. doi: 10.1189/jlb.2MR0316-117RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raby A.-C., Le Bouder E., Colmont C., Davies J., Richards P., Coles B., et al. Soluble TLR2 reduces inflammation without compromising bacterial clearance by disrupting TLR2 triggering. J. Immunol. 2009;183(1):506–517. doi: 10.4049/jimmunol.0802909. [DOI] [PubMed] [Google Scholar]
- 20.Schütt C. CD14. Int. J. Biochem. Cell Biol. 1999;31(5):545–549. doi: 10.1016/s1357-2725(98)00153-8. [DOI] [PubMed] [Google Scholar]
- 21.Lee C.C., Avalos A.M., Ploegh H.L. Accessory molecules for Toll-like receptors and their function. Nat. Rev. Immunol. 2012 Mar;12(3):168–179. doi: 10.1038/nri3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baumann C.L., Aspalter I.M., Sharif O., Pichlmair A., Blüml S., Grebien F., et al. CD14 is a coreceptor of Toll-like receptors 7 and 9. J. Exp. Med. 2010;207(12):2689–2701. doi: 10.1084/jem.20101111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henrick B.M., Yao X.-D., Taha A.Y., German J.B., Rosenthal K.L. Insights into soluble Toll-like receptor 2 as a downregulator of virally induced inflammation. Front. Immunol. 2016;7:291. doi: 10.3389/fimmu.2016.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeBouder E., Rey-Nores J.E., Rushmere N.K., Grigorov M., Lawn S.D., Affolter M., et al. Soluble forms of Toll-like receptor (TLR)2 capable of modulating TLR2 signaling are present in human plasma and breast milk. J. Immunol. 2003;171(12):6680–6689. doi: 10.4049/jimmunol.171.12.6680. [DOI] [PubMed] [Google Scholar]
- 25.Gegner J.A., Ulevitch R.J., Tobias P.S. Lipopolysaccharide (LPS) signal transduction and clearance: dual roles for lps binding protein and membrane cd14. J. Biol. Chem. 1995;270(10):5320–5325. doi: 10.1074/jbc.270.10.5320. [DOI] [PubMed] [Google Scholar]
- 26.Pugin J., Heumann I.D., Tomasz A., Kravchenko V.V., Akamatsu Y., Nishijima M., et al. CD14 is a pattern recognition receptor. Immunity. 1994;1(6):509–516. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 27.Kitchens R.L., Thompson P.A. Modulatory effects of sCD14 and LBP on LPS-host cell interactions. J. Endotoxin Res. 2005;11(4):225–229. doi: 10.1179/096805105X46565. [DOI] [PubMed] [Google Scholar]
- 28.Shive C.L., Jiang W., Anthony D.D., Lederman M.M. Soluble CD14 is a nonspecific marker of monocyte activation. AIDS. 2015;29(10):1263–1265. doi: 10.1097/QAD.0000000000000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bazil V., Strominger J.L. Shedding as a mechanism of down-modulation of CD14 on stimulated human monocytes. J. Immunol. 1991;147(5):1567–1574. [PubMed] [Google Scholar]
- 30.Bufler P., Stiegler G., Schuchmann M., Hess S., Krüger C., Stelter F., et al. Soluble lipopolysaccharide receptor (CD14) is released via two different mechanisms from human monocytes and CD14 transfectants. Eur. J. Immunol. 1995;25(2):604–610. doi: 10.1002/eji.1830250244. [DOI] [PubMed] [Google Scholar]
- 31.Lien E., Aukrust P., Sundan A., Müller F., Frøland S.S., Espevik T. Elevated levels of serum-Soluble CD14 in human immunodeficiency virus type 1 (HIV-1) infection: correlation to disease progression and clinical events. Blood. 1998;92(6):2084–2092. doi: 10.1182/blood.V92.6.2084. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J., Hu Z.-D., Song J., Shao J. Diagnostic value of presepsin for sepsis: a systematic review and meta-analysis. Medicine (Baltim.) 2015;94(47) doi: 10.1097/MD.0000000000002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zdolsek H.A., Jenmalm M.C. Reduced levels of soluble CD14 in atopic children. Clin. Exp. Allergy. 2004;34(4):532–539. doi: 10.1111/j.1365-2222.2004.1921.x. [DOI] [PubMed] [Google Scholar]
- 34.Prester L., Protrka N., Macan J., Katunarić M. Salivary sCD14 as a potential biomarker of dental caries activity in adults. Arh. Hig. Rada. Toksikol. 2017;68(4):315–321. doi: 10.1515/aiht-2017-68-2974. [DOI] [PubMed] [Google Scholar]
- 35.Holst B., Szakmany T., Raby A.-C., Hamlyn V., Durno K., Hall J.E., et al. Soluble Toll-like receptor 2 is a biomarker for sepsis in critically ill patients with multi-organ failure within 12 h of ICU admission. Intensive Care Med Exp. 2017;5(1):2. doi: 10.1186/s40635-016-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landmann R., Zimmerli W., Sansano S., Link S., Hahn A., Glauser M.P., et al. Increased circulating soluble CD14 is associated with high mortality in gram-negative septic shock. J. Infect. Dis. 1995;171(3):639–644. doi: 10.1093/infdis/171.3.639. [DOI] [PubMed] [Google Scholar]
- 37.Burgmann H., Winkler S., Locker G.J., Presterl E., Laczika K., Staudinger T., et al. Increased serum concentration of soluble CD14 is a prognostic marker in gram-positive sepsis. Clin. Immunol. Immunopathol. 1996;80(3):307–310. doi: 10.1006/clin.1996.0128. [DOI] [PubMed] [Google Scholar]
- 38.Aalto H., Takala A., Kautiainen H., Siitonen S., Repo H. Monocyte CD14 and soluble CD14 in predicting mortality of patients with severe community acquired infection. Scand. J. Infect. Dis. 2007;39(6–7):596–603. doi: 10.1080/00365540701199808. [DOI] [PubMed] [Google Scholar]
- 39.van de Weg C.A., Koraka P., van Gorp E.C., Mairuhu A.T., Supriatna M., Soemantri A., et al. Lipopolysaccharide levels are elevated in dengue virus infected patients and correlate with disease severity. J. Clin. Virol. 2012 Jan;53(1):38–42. doi: 10.1016/j.jcv.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 40.Yong Y.K., Tan H.Y., Jen S.H., Shankar E.M., Natkunam S.K., Sathar J., et al. Aberrant monocyte responses predict and characterize dengue virus infection in individuals with severe disease. J. Transl. Med. 2017 May 31;15(1):121. doi: 10.1186/s12967-017-1226-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van de Weg C.A., Pannuti C.S., de Araújo E.S., van den Ham H.J., Andeweg A.C., Boas L.S., et al. Microbial translocation is associated with extensive immune activation in dengue virus infected patients with severe disease. PLoS Neglected Trop. Dis. 2013 May 23;7(5) doi: 10.1371/journal.pntd.0002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hue K.D., Tuan T.V., Thi H.T., Bich C.T., Anh H.H., Wills B.A., et al. Validation of an internally controlled one-step real-time multiplex RT-PCR assay for the detection and quantitation of dengue virus RNA in plasma. J. Virol. Methods. 2011;177(2):168–173. doi: 10.1016/j.jviromet.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duong V., Ly S., Lorn Try P., Tuiskunen A., Ong S., Chroeung N., et al. Clinical and virological factors influencing the performance of a NS1 antigen-capture assay and potential use as a marker of dengue disease severity. PLoS Neglected Trop. Dis. 2011;5(7):e1244. doi: 10.1371/journal.pntd.0001244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.World Health Organization . new edition. World Health Organization; 2009. Dengue Guidelines for Diagnosis, Treatment, Prevention and Control. [PubMed] [Google Scholar]
- 45.Rodenhuis-Zybert I.A., van der Schaar H.M., da Silva Voorham J.M., van der Ende-Metselaar H., Lei H.-Y., Wilschut J., et al. Immature dengue virus: a veiled pathogen? PLoS Pathog. 2010;6(1) doi: 10.1371/journal.ppat.1000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaar H.M. Characterization of the early events in dengue virus cell entry by biochemical assays and single-virus tracking. J. Virol. 2007;81:12019–12028. doi: 10.1128/JVI.00300-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zybert I.A., van der Ende-Metselaar H., Wilschut J., Smit J.M. Functional importance of dengue virus maturation: infectious properties of immature virions. J. Gen. Virol. 2008;89(Pt 12):3047–3051. doi: 10.1099/vir.0.2008/002535-0. [DOI] [PubMed] [Google Scholar]
- 48.Modhiran N., Watterson D., Muller D.A., Panetta A.K., Sester D.P., Liu L., et al. Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci. Transl. Med. 2015;7(304) doi: 10.1126/scitranslmed.aaa3863. [DOI] [PubMed] [Google Scholar]
- 49.Modhiran N., Watterson D., Blumenthal A., Baxter A.G., Young P.R., Stacey K.J. Dengue virus NS1 protein activates immune cells via TLR4 but not TLR2 or TLR6. Immunol. Cell Biol. 2017;95(5):491–495. doi: 10.1038/icb.2017.5. [DOI] [PubMed] [Google Scholar]
- 50.Henrick B.M., Nag K., Yao X.-D., Drannik A.G., Aldrovandi G.M., Rosenthal K.L. Milk matters: soluble Toll-like receptor 2 (sTLR2) in breast milk significantly inhibits HIV-1 infection and inflammation. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0040138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Henrick B.M., Yao X.-D., Drannik A.G., Abimiku A., Rosenthal K.L., INFANT Study Team Soluble toll-like receptor 2 is significantly elevated in HIV-1 infected breast milk and inhibits HIV-1 induced cellular activation, inflammation and infection. AIDS. 2014;28(14):2023–2032. doi: 10.1097/QAD.0000000000000381. [DOI] [PubMed] [Google Scholar]
- 52.Heggelund L., Flo T., Berg K., Lien E., Mollnes T.E., Ueland T., et al. Soluble toll-like receptor 2 in HIV infection: association with disease progression. AIDS. 2004;18(18):2437–2439. [PubMed] [Google Scholar]
- 53.Vuong N.L., Lam P.K., Ming D.K.Y., Duyen H.T.L., Nguyen N.M., Tam D.T.H., et al. Combination of inflammatory and vascular markers in the febrile phase of dengue is associated with more severe outcomes. Elife [Internet] 2021;10 doi: 10.7554/eLife.67460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Durieux J.J., Vita N., Popescu O., Guette F., Calzada-Wack J., Munker R., et al. The two soluble forms of the lipopolysaccharide receptor, CD14: characterization and release by normal human monocytes. Eur. J. Immunol. 1994;24(9):2006–2012. doi: 10.1002/eji.1830240911. [DOI] [PubMed] [Google Scholar]
- 55.Zanoni I., Granucci F. Role of CD14 in host protection against infections and in metabolism regulation. Front. Cell. Infect. Microbiol. 2013;3:32. doi: 10.3389/fcimb.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sandler N.G., Wand H., Roque A., Law M., Nason M.C., Nixon D.E., et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J. Infect. Dis. 2011;203(6):780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Messner C.B., Demichev V., Wendisch D., Michalick L., White M., Freiwald A., et al. Ultra-high-throughput clinical proteomics reveals classifiers of COVID-19 infection. Cell Syst. 2020;11(1):11–24.e4. doi: 10.1016/j.cels.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.