Abstract
No effective drug treatment is available for Alzheimer disease, thus the need arise to develop efficient drugs for its treatment. Natural products have pronounced capability in treating Alzheimer disease therefore current study aimed to evaluate the neuro-protective capability of folicitin against scopolamine-induced Alzheimer disease neuropathology in mice. Experimental mice were divided into four groups i.e. control (single dose of 250 μL saline), scopolamine-administered group (1 mg/kg administered for three weeks), scopolamine plus folicitin-administered group (scopolamine 1 mg/kg administration for three weeks followed by folicitin administration for last two weeks) and folicitin-administered group (20 mg/kg administered for 5 alternate days). Results of behavioral tests and Western blot indicated that folicitin has the capability of recovering the memory against scopolamine-induced memory impairment by reducing the oxidative stress through up-regulating the endogenous antioxidant system like nuclear factor erythroid 2-related factor and Heme oxygenase-1 while prohibiting phosphorylated c-Jun N-terminal kinase. Similarly, folicitin also improved the synaptic dysfunction by up-regulating SYP and PSD95. Scopolamine-induced hyperglycemia and hyperlipidemia were abolished by folicitin as evidenced through random blood glucose test, glucose tolerance test and lipid profile test. All these results revealed that folicitin being a potent anti-oxidant is capable of improving synaptic dysfunction and reducing oxidative stress through Nrf-2/HO-1 pathway, thus plays a key role in treating Alzheimer disease as well as possess hyperglycemic and hyperlipidemic effect. Furthermore, a detailed study is suggested.
Keywords: HO-1, Nrf-2, Phosphorylated c-Jun N- Kinase, Folicitin, Scopolamine, Y- maze, Morris water maze
Graphical abstract
Nomenclature
Abbreviations
- SYP
Synaptophysin
- PSD95
Postsynaptic density protein 95
- Nrf2
Nuclear factor-erythroid factor 2-related factor 2
- HO-1
Heme oxygenase-1
- p-JNK
phosphorylated c-Jun N-terminal kinase
1. Introduction
Alzheimer disease is an irreversible, progressive neurodegenerative disease, marked by the loss of memory, spatial disorientation and deterioration of intellectual ability. It is the most common form of neurodegenerative disease which is responsible for 80% diagnosis of all demential cases and is becoming the most exorbitant, fatal and burdening disease [1,2]. Important pathological hallmarks for the diagnosis of Alzheimer disease include the disposition of plaques of amyloid beta, glial responses, neurofibrillary tangles of Tau protein and neuronal and synaptic loss [3,4,5,6]. Risk factor for Alzheimer disease includes age, genetic mutations, stress, metabolic disorders and cardiovascular disease etc. [7]. Along with these, another key factor which initiates the pathology of Alzheimer disease is the oxidative stress [8]. Oxidative stress is described as disparity between formation and detoxification of reactive oxygen species [9,10]. Generation of these species is induced by amyloid beta plaques, age, tissue injury, and increased metal level or ischemia [11]. Evidences have shown the oxidative events happening early in the onset of disorder and before the formation of pathology, thus support a key role of oxidative stress in Alzheimer disease [12]. Brain has less antioxidant enzymes and is rich in polyunsaturated fatty acid, causing very prone to the oxidative stress [9,13,14,15].
Synaptic loss is the powerful correlate for the cognitive decline and is the early pathological event in Alzheimer disease [16,17,18]. It is well known that synapses integrate synaptic activity all over the nervous system and without its regulation, synaptic protein activity is lost, leading to stored memory degradation [19]. Mechanism which leads to synaptic loss and memory impairment in Alzheimer disease are not well understood but it is reported that due to soluble oligomers of amyloid beta, synaptic toxicity develops which leads to the development of Alzheimer disease [10,20,21,22]. Tau protein also performs a significant role in the regulation of synaptic function and synaptic plasticity [23]. When formation of neurofibrillary tangles occurs within neurons it causes microtubule destabilizations, neuronal and synaptic injury [24,25]. Loss of presynaptic and post synaptic protein i.e. PSD95 and Synaptophysin in Alzheimer disease shows the synaptic impairment [26]. Synaptophysin is found in all the terminals of neurons and perform a role in the exocytosis and endocytosis of synaptic vesicles [27,28]. PSD95 protein participates in memory and learning by the calcium ions influx to the neurons. It attaches with NMDAR –terminal receptors and its higher expression correlates with the increase synaptic transmission [29,30]. Plasticity homeostasis of synapses is controlled by PSD95 protein and performs a significant role in the synapses maturation [31].
Neurodegenerative disease and metabolic dysfunction are associated disease. Both these disease shows high occurrence in elderly and middle aged people [32]. Glucose is the main energy source for brain, its metabolism is not only used for energy substrate but also provides important compounds for neurons such as neurotransmitters (acetylcholine and glutamate) [33] while its high level leads to hyperglycemia which if persist for long time leads to the occurrence of Diabetes mellitus [34].
Hyperglycemia plays a significant part in the development of Alzheimer disease and can lead to slowly progressive structural and functional problems in brain and leads to cognitive decline in people who have abnormal glucose metabolism [33,35]. It initiates three mechanisms by which aging of the brain occurs which include accumulation of advance glycation end product, increase production of reactive oxygen species and micro vascular pathology [36]. Neuronal apoptosis and cell proliferation suppression are observed in the diabetic rodent's hippocampus [37]. Insulin resistance of brain and amyloidogenesis are the major mechanisms for cognitive function disability by hyperglycemia and it can lead to neuronal apoptosis even before the development of Diabetes mellitus [38,39]. Diabetes mellitus induced by high fat diet, can induce the amyloid beta peptide 40 and 42 amyloidogenesis in the brain of Alzheimer disease transgenic mouse model [40,41]. Risk factors for diabetes and dementia tends to overlap. These overlapping mechanisms include oxidative stress, inflammation, and dysfunction of mitochondria [39].
Another risk factor for neurodegenerative disease and Alzheimer disease is hyperlipidemia [42]. Hyperlipidemia relates to an increase in oxidative stress which results in the significant increase of oxygen free radicals. These radicals then initiate oxidative modifications in low density lipoprotein and leads to the development of atherosclerosis and other cardiovascular diseases [43]. Many studies reported that disorders in homeostasis of lipids are common risk factors for the cardiovascular diseases which are linked to the Alzheimer disease. According to a review high content of high density lipoprotein was associated with a decreased risk for Alzheimer disease and modulates the cognitive functions in neurodegenerative disease while elevated amount of low density lipoprotein can cause plaques buildup in the arteries and increases the risk of cardiovascular diseases and it is also an independent risk factor for the development of Alzheimer disease [44,45,46,47]. An antioxidant defense system consisting of enzymes and non-enzymatic compounds prevents oxidative damage of lipoproteins in the plasma. When the activity of this system decreases or the reactive oxygen species production increases, an oxidative stress may occur. Since fatty acids and triglyceride-rich emulsions can stimulate leukocytes to produce reactive oxygen species, it is conceivable that raised plasma triglyceride-rich lipoproteins such as very low density lipoprotein may overload the antioxidant system [48].
In current study, scopolamine is used as a drug for inducing Alzheimer disease via generating oxidative stress and synaptic loss. Scopolamine is capable of mediating amyloid beta deposition, synaptic dysfunction, and oxidative stress and impairs memory and learning. It has been used in experimental models of animals to evaluate the effects of drugs and its therapeutic potential against neurodegenerative disease [28].
A number of flavonoids have been described to attenuate the Alzheimer disease pathology development and make the cognitive deficit better in various experimental models because theses have the potency to bind with different proteins and make alterations in enzymes, hormones and remove the free radicals [49]. It also has many beneficial effects on metabolic disorders such as diabetes, cancer, obesity and cardiovascular diseases. Flavonoids possess anti diabetic activity which is consist of insulin secretion, carbohydrate digestion, insulin signaling and glucose uptake and play role in regulating the metabolism of serum lipid [12,50]. Hypericum oblongifolium is a beneficial medicinal plant, utilized traditionally for the cure of hepatitis, gastric ulcer, gastrointestinal disorders and external wounds. The phytochemical analysis of Hypericum oblongifolium shows that it contains saponin, tannins and flavonoids. A new antioxidant from Hypericum oblongifolium known as folicitin has been extracted [51]. It possesses anti-amyloidogenic effect and is potent antidepressant and anticholinesterase. The most important characteristic of flavonoids is that they have the ability to cross the blood brain barrier and that's why its use for the treatment of Alzheimer disease will give substantial results [52]. Therefore current study focused on the neuroprotective ability of antioxidant flavonoid, folicitin, against Alzheimer disease as it is known to possess neuroprotective, anti-inflammatory, anti-oxidant and neurotropic roles [53]. This study also focused on evaluating the hypoglycemic and hypolipidemic capability of folicitin against scopolamine induced hyperglycemia and hyperlipidemia as to the best of our knowledge no study has been reported up till now regarding hypoglycemic and hypolipidemic effect of folicitin.
2. Methodology
2.1. Chemicals
All the chemicals used in the current study were of analytical grade. Primary antibodies (Nrf-2, HO-1, PSD95, Phosphorylated c-Jun N- Kinase, SYP, β-actin) Sodium dodecyl sulfate (SDS), Scopolamine (Santa Cruz, CA, USA), Tetra methylene diamine (Temed), Ammonium per sulfate (APS) (Daejung Chemicals and Metals Co. Ltd, Korea), Tissue protein extraction reagent (T-PER), Methanol (Sigma Aldrich, Burlington, MA, United States), Skim Milk Powder (Oxoid Ltd, Basingstoke, UK), RNA wait (Beijing Solar bio Science and Technology Co. Ltd), Enhanced Chemiluminescence (ECL) solution A (Luminol) and solution B (Peroxide solution) and secondary antibody (anti-mouse) (Bio-Rad, United States) were bought while folicitin was gifted by Dr. Umar Farooq.
2.2. Animal model
Experiment was carried out on adult male mice of strain BALB/C (between 25 and 30 g) acquired from Veterinary Research Institute of Peshawar, Pakistan. Mice were brought to the animal section of the Neuro Molecular Medicine Research Centre (NMMRC), Ring Road, Peshawar, KPK, Pakistan and placed separately in cages (Biobase, China), a dark-light cycle of 12/12-h was maintained along with a steady room temperature of 25 ± °C and 60–65% humidity. Water and food was provided to them.
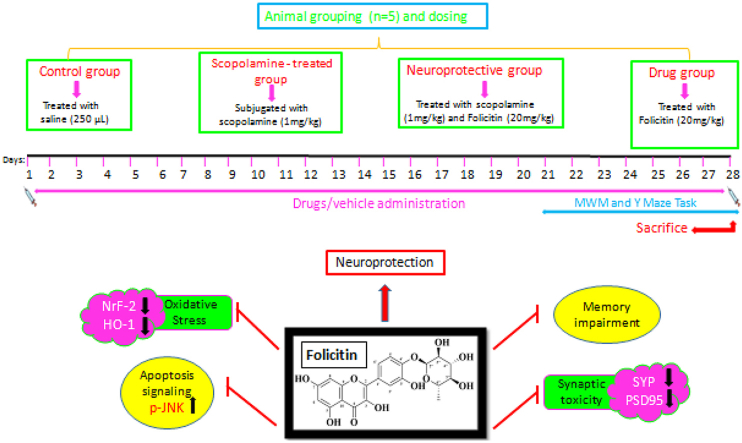
2.3. Experimental groups and their drug treatment
Mice were randomly placed into four groups i.e. n=5.
-
1.
Control group was given single saline dose (250 μL);
-
2.
Scopolamine treated group: Scopolamine (1 mg/kg) injected every day for 3 weeks
-
3.
Scopolamine (1 mg/kg) + Folicitin (20 mg/kg) treated group where scopolamine was given every day while folicitin for last 2 weeks
-
4.
Folicitin treated group: Folicitin injected to the drug group for 5 days.
Animals were injected intra peritoneally with great care. According to the considerations of the Act 1986 conducted for UK animals, care and treatment of the mice was carried out. Experimental procedures carried out on mice were approved (Ref. No. NMMRC/20/2021) on 1st July 2021 by the ethics committee of NMMRC, Peshawar.
2.4. Behavioral tests
After giving injection for two weeks, behavioral tests of mice were conducted to know the memory of mice and to find out neuroprotective capability of folicitin on the mice memory.
2.4.1. Morris water maize
This test was conducted for finding out the memory and spatial learning of the experimental groups. The equipment used for this test contained of a round water tank of around 100 cm in diameter and 40 cm in height. Mice were bought about 30 min before performing the test for acclimatization. Training was given to mice for first three days and escape latencies of the animals were recorded for 60sec to locate the immersed platform. In case of failing to find the platform, mice were directed towards the platform where they were rested for 10 s. This test was carried out for 5 days and the time was noted for all mice in search of platform. After resting for two days, the mice were allowed for probe test in which the mice have to search for the hidden platform and the time spent in the respective quadrant by all mice was noted down.
2.4.2. Y-maze test
The equipment utilized for the conduction of Y-maze test consisted of three arms of 50 cm in length, 10 cm in width and 20 cm in height, placed at 120° angle from each other. This test was performed for assessing the innate ability of mice to analyze new surroundings. To progressively analyze the memory of mice, they should carry a successful record of recently explored arm and successively restores the record. Mice which have disturb memory shows decreased spontaneous alterations. For exploring and adapting to new environment, mice were trained for 2 days for 10 min at first. Then mice were permitted for 8 min to analyze the Y maze. The mice were kept at the junction of Y-maze to analyze its arms. The total arm entries were noted and percentage of the alternations was calculated by using the formula:
2.5. Random blood glucose detection
Blood glucose levels (mg/dl) of all experimental groups were checked randomly with (On Call Plus) glucometer.
2.6. Glucose tolerance test (GTT)
To measure glucose tolerance test, Mice were kept on fastening for 6 to 7 h. Glucose levels of blood were checked considered at 0 time of all groups. 200 mg/kg dextrose was administered intra peritoneally to mice. The glucose levels of the mice were checked after the interval of 15 min, 30 min, 60 min, 120 min and 180 min.
2.7. Mice sacrifice and collection of brain proteins
After drug administration and performance of behavioral and blood glucose tests, mice were subjected to decapitation and their brains were collected with great caution and placed in a 1:1 solution of phosphate buffer saline and RNA wait for 60 s to restrict modifications in protein. Samples were subjected to homogenization through homogenizer machine by putting them in T-PER solution after which, centrifugation in Eppendorf centrifuge machine was carried out for 25 min on 14000 rpm speed and 4 °C. Supernatants were collected as a result of centrifugation whose absorbance was measured at 595 nm by use of UV Spectrometer as to examine proteins for SDS-PAGE.
2.8. Sample collection for lipid profile test
Amount of lipids of each experimental group was examined via the collection of blood samples which were transferred to eppendorf tubes at the time of decapitation during sacrifice. For serum extraction, these samples were centrifuged at 14000 rpm speed and 4 °C for 20 min.
2.9. Serum biochemical analysis
Hyperlipidemia was caused by scopolamine which caused serum lipid level to rise hence the effect of folicitin to lower the serum lipid level could be evaluated. Four major types of lipids were found in the serum i.e. total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) which were analyzed by the use of Easy Tech kit (Clinical Chemistry Analyzer) keeping in view its protocol.
2.10. Western blot analysis
Protein samples’ loading was done in order to perform 12–15% SDS PAGE. For the identification of the molecular weight of proteins, a pre-stained protein marker (Gang Nam-STAIN™, iNtRon Biotechnology, Inc., Seongnam, Korea) was utilized as a guide during the study. This protein marker included a wider range of molecular weights i.e. 10 –245 kDa. Then, transfer of proteins to PVDF membrane (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was done through Semi Dry Trans-Blot (Bio Rad). Next to it, primary antibodies including HO-1, Nrf-2, PSD95, SYP, p-JNK and β-actin (Table 1) were applied at 4 °C overnight for the detection of various proteins after which, 5% (w/v) skim milk for blocking the membranes from the attachment of non-specific proteins was used. It was followed by rinsing the blots and incubating them at room temperature with anti-mouse secondary antibody for 2 h. Visualization of membranes was done using ECL and the achieved results were developed on X-ray films that were scanned later.
Table 1.
List of primary and secondary antibodies with catalog numbers.
| S.# | Antibodies Name | Catalogue # |
|---|---|---|
| 1 | Anti–HO–1 | sc-136960 |
| 2 | Anti-Nrf-2 | sc-365949 |
| 3 | Anti-PSD95 | sc-71933 |
| 4 | Anti-SYP | sc-17750 |
| 5 | Anti-p-JNK | sc-6254 |
| 6 | Anti-β actin | sc-47778 |
| 7 | Goat anti-mouse (IgG-HRPs) secondary antibodies | W4028 |
2.11. Statistical analysis
Scanned X-rays were undergone through computer based software's for carrying out statistical analysis. For performing student t-test and one-way ANOVA, Prism 6 (Graph Pad Software, San Diego, CA, USA) was applied. Densitometry analysis for the scanned X-rays was done to know the optical densities of Western blot and immunofluorescence images via Image J program. Proteins densities were shown as the mean ± S.E.M in arbitrary units (A.U.s). # showed significant difference between control and scopolamine while * showed significant difference between scopolamine and scopolamine plus folicitin-treated group. *, #P < 0.05, **, ##P < 0.01, ***, ###P < 0.001. P < 0.05 was considered a statistically significant value.
3. Results
3.1. Folicitin improved the scopolamine induced memory impairment in adult male albino mice
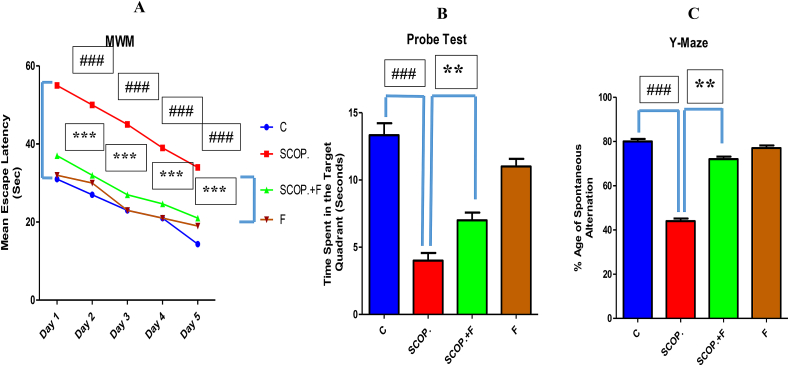
MWM test results have illustrated that the control group mice arrived to the platform on day 1st till day 5th in a very short time hence their performance increased from day 1 to day 5 and their mean escape latencies were decreasing. In contrast to control group, the scopolamine-injected group reached the platform on day 1st while on the following days, they took more time than control group hence their day by day there performance was decreasing meaning that their memory was impaired. The scopolamine plus folicitin-injected group took smaller time in contrast to alone scopolamine-injected group and their day by day performance was also increasing hence there was decrease in their mean escape latency time showing the recovery of memory impairment induced by scopolamine while only folicitin-injected group have also demonstrated good performance and their mean latency time was decreasing (Fig. 1-A).
Fig. 1.
1-A showed the mean escape latency (seconds) of all the experimental groups of Morris Water Maze test. 1-B showed the probe test results of all the experimental groups. 1-C showed results of the Y-maze test for spontaneous alterations percentage of each group.
In the probe test, it was found that the control group mice spend more time in target quadrant due to good condition of their memory The scopolamine-injected mice have impaired memory that's why they spent lesser time in target quadrant. The scopolamine plus folicitin-treated mice also spent more time in the target quadrant indicating the reversal of the memory deficits caused by scopolamine. Only folicitin-treated group have also demonstrated good results by consuming more time in target quadrant (Fig. 1-B).
In Y-maze test, the highest spontaneous alternation percentage was found in control group. In comparison to control group, the scopolamine-injected group has shown least spontaneous alteration percentage while in contrast to this group, the scopolamine plus folicitin-injected group has demonstrated high percentage of spontaneous alteration. The folicitin injected group has shown high spontaneous alteration percentage (Fig. 1-C).
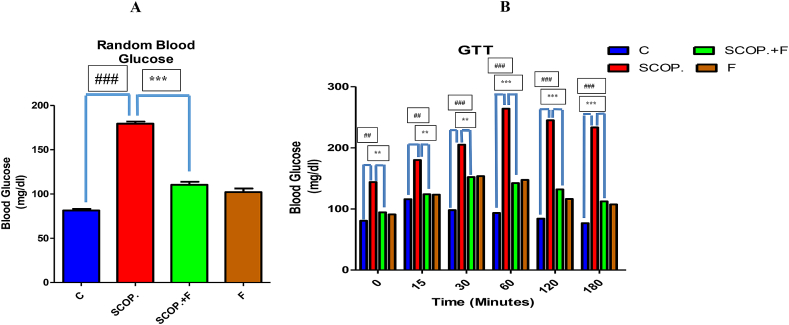
3.2. Folicitin reduced hyperglycemia induced by scopolamine in adult male albino mice
According to the random blood glucose test results, control group has shown lowest amount of glucose while highest concentration of glucose was seen in scopolamine-treated group. Folicitin plus scopolamine has shown reduced glucose level in the blood when compared with scopolamine-treated group. Only folicitin-injected group has also expressed decreased glucose level in the mice blood (Fig. 2-A).
Fig. 2.
2-A showed the depiction of random blood glucose levels of all the experimental mice models. 2-B illustrated the glucose tolerance test results of all experimental groups over time i.e. from 0 level to 180 min. Scopolamine-treated group in comparison to all other groups expressed high level of glucose in blood while scopolamine plus folicitin-injected group showed less concentration of blood glucose elucidating the neuroprotective capability of folicitin.
These results were further confirmed by performing glucose tolerance test. Between 0 level and 180 min, the blood glucose level of scopolamine-treated group have shown highest level of glucose among all the groups while scopolamine plus folicitin-treated group has shown a reduced amount of glucose compared with scopolamine-treated group. Same appeared in case of only folicitin-injected group which have also illustrated low concentration of glucose in the mice blood. Therapeutic efficacy of folicitin has been confirmed from these results against hyperglycemia induced by scopolamine in mice (Fig. 2-B).
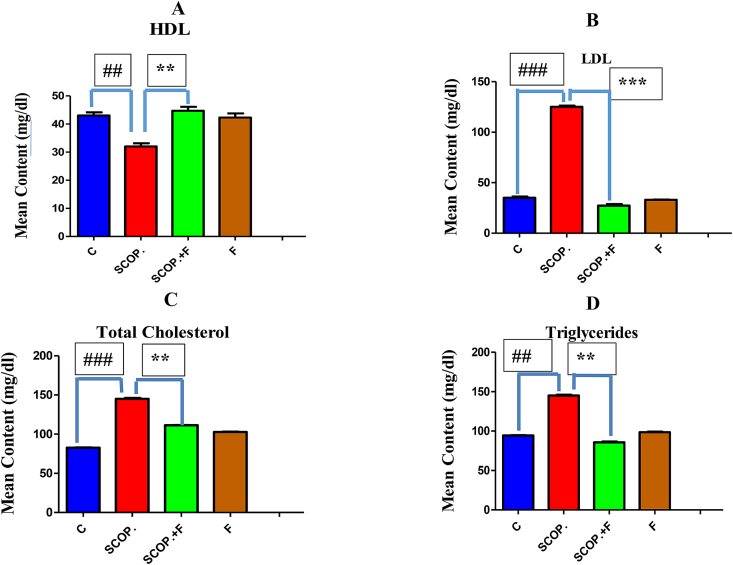
3.3. Decrease of serum lipid content by folicitin in adult male albino mice
For the evaluation of hypolipidemic capability of folicitin, lipid profile test was carried out. HDL content of serum was high in control group while lowest in scopolamine-treated group whose effect was reversed by folicitin as noticed in scopolamine plus folicitin-injected group by increasing the serum HDL level (Fig. 3-A). The LDL content of serum was greatly increased by scopolamine as seen in scopolamine-injected group in comparison to control group which was lowered by folicitin as seen in scopolamine plus folicitin injected group (Fig. 3-B). Total cholesterol level was also elevated by scopolamine as seen in scopolamine injected group compared to control group which was reversed by folicitin as shown in scopolamine plus folicitin injected group by decreasing this increase when compared to scopolamine-treated group (Fig. 3-C). Serum content of Triglycerides was also elevated in scopolamine-treated group compared to all other groups which were reversed by folicitin as seen in scopolamine plus folicitin-treated group (Fig. 3-D). These results concluded that folicitin possess hypolipidemic effects.
Fig. 3.
High serum lipid content i.e. HDL, LDL, total cholesterol and Triglycerides caused by scopolamine was reduced by folicitin as seen in scopolamine and scopolamine plus folicitin-treated groups while their levels were statistically compared among control, scopolamine and scopolamine plus folicitin-treated groups.
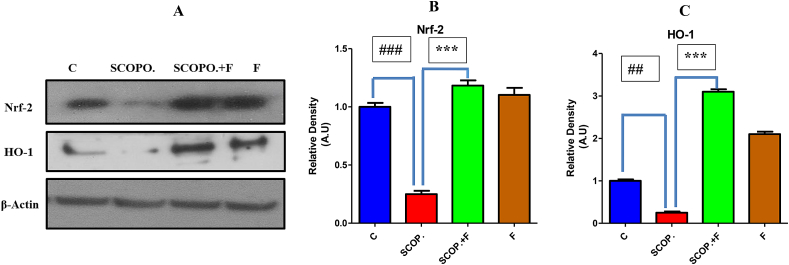
3.4. Oxidative stress induced by scopolamine is reduced by folicitin via up-regulating Nrf-2/HO-1 pathway in adult male albino mice
To prevent nerve cells from the oxidative stress, Nrf-2/HO-1 pathway is described to play a critical role. Reactive oxygen species inhibition by Nrf-2 occurs by introducing phase-2 detoxifying enzymes including HO-1 [54]. Therefore, to explore potential anti-oxidant effect of folicitin against oxidative stress induced by scopolamine, the current study was carried out in which, oxidative stress was generated in mice by through scopolamine treatment for three weeks. The immune blot results showed that scopolamine caused inhibition of Nrf-2/HO-1 while folicitin increased the expression of these proteins as seen in scopolamine, and scopolamine plus folicitin-injected groups, thus reduced the oxidative stress (Fig. 4-A–C). The expression of Nrf-2 and HO-1 in only folicitin-injected group indicates that this drug is safe and possesses anti-oxidant potential.
Fig. 4.
4-A represented the western blot results of both antioxidant enzymes i.e. Nrf-2 and HO-1. Beta actin was used as standard. 4-B showed the expression of Nrf-2 in the four experimental groups. The Nrf-2 level was lowered in scopolamine-injected group compared to control group while in scopolamine plus folicitin-treated its expression was up-regulated. 4-C showed the HO-1 expression in all the groups of mice. In comparison to control group, HO-1 expression was also suppressed in scopolamine-treated group which was up-regulated in scopolamine plus folicitin-treated group.
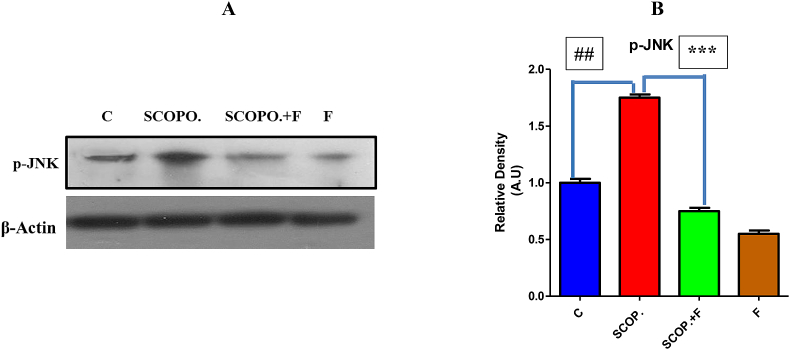
3.5. Phosphorylated c-jun N- kinase (p-JNK) inhibition by folicitin in male albino mice
Main factor for the regulation of apoptosis signaling is the activation of phosphorylated JNK pathway that's why it is crucial for pathological cell death related with neurodegenerative disease pathologies such as Alzheimer disease. JNK's particularly JNK3 elevates amyloid beta production neurodegenerative disease as well as neurofibrillary tangles maturation and growth. The higher expression of p-JNK decreases the expression of Nrf-2 and HO-1 which results in the impairment of memory and oxidative stress which leads to Alzheimer disease [55].
In current study, scopolamine administration increased the p-JNK expression while decreased HO-1 and Nrf-2 in contrast to control group. In comparison with scopolamine, folicitin significantly inhibited p-JNK and up-regulated HO-1 and Nrf-2 to reduce the oxidative stress and associated memory impairment caused by scopolamine as seen in folicitin plus scopolamine-injected group while only folicitin-treated group also reduced the expression of p-JNK pathway leading to reversal of the memory impairment (Fig. 5-A, -B).
Fig. 5.
Protein immune blot results (5-A) of p-JNK, and beta actin and respective bar graph of p-JNK (5-B) for the treatment groups. P-JNK pathway was up-regulated in scopolamine-treated group compared to the control group which was down-regulated by folicitin as seen in scopolamine plus folicitin-injected group and folicitin-treated group.
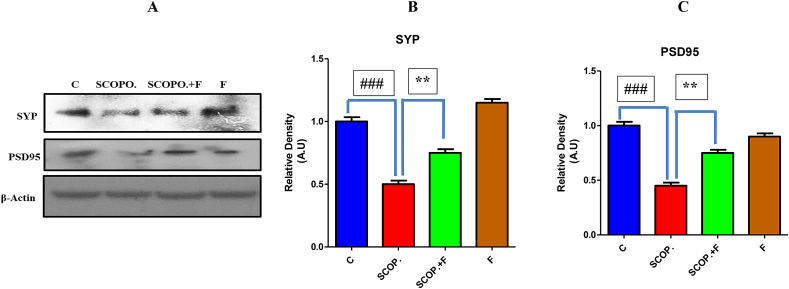
3.6. Folicitin improved pre-synapse and post synapse in adult male albino mice
Scopolamine is known to cause synaptic toxicity [28]. To illuminate the synaptic improvement potential of folicitin against scopolamine-induced synaptic toxicity, the expression of pre and post-synapse protein was evaluated in brain homogenates of all the four groups of mice. The results of Western blot Fig. (6-A) as well as the bar graphs (6-B, 6-C) showed that folicitin reduced synaptic toxicity significantly by increasing the pre and post-synapse proteins expression. Control group has shown higher expression of pre and post-synaptic proteins i.e. SYP and PSD95 which was reduced by scopolamine in scopolamine-injected group while the folicitin treatment reversed the toxic effect of scopolamine by high expression of pre and post-synapse proteins as seen in scopolamine plus folicitin-injected group.
Fig. 6.
Folicitin improved the pre-synaptic and post-synaptic proteins expression. Above are the results of immune blots (6-A) of SYP and PSD95 accompanied by the bar graphs (6-B) and (6-C) for all the three experimental groups. The standard used was beta actin. The protein bands were quantified with image J software.
4. Discussion
In current study the therapeutic capability of folicitin, one of the major compounds of the hypericum oblongifolium has been evaluated against scopolamine induced Alzheimer disease, hyperglycemia and hyperlipidemia. In present study it has been confirmed that folicitin abrogated synaptic loss and memory impairment via up regulating the expression of Synaptophysin and PSD95. It also eliminated scopolamine induced oxidative stress mediated AD via up regulating Nrf-2 and HO-1 while lowering the expression of pJNK. Besides this, current study also proved that folicitin has hypoglycemic and hypolipidemic capability against scopolamine induced hyperglycemia and hyperlipidemia. All these results revealed that folicitin might be a promising lead in therapeutic drugs designing for various disorders as it is a potential antioxidant, hormone modifier and have neuroprotective ability [49,51,56].
Scopolamine was used in current study to generate oxidative stress and synaptic loss in mice as it has been used for evaluating therapeutic potential of drugs in the experimental model of neurodegenerative disease to find anti demential drugs [57,58]. In current study, after giving drug treatment to the mice their memory and learning was assessed by conducting Y-maze and Morris water maze test. Scopolamine reduced the spontaneous alteration score of mice compared to control group while it was increased in folicitin plus scopolamine injected and in only folicitin injected group. The folicitin administration reduced the escape latency time day by day while in the case of scopolamine injected mice this difference was very little. From day to day reduction in escape latency represents reference memory in the first trail, while working memory is represented from first trial to second trial [57,59,60,61]. These findings showed that folicitin has the ability to effectively prevent the memory impairment induced by cholinergic and muscarinic receptors blockade.
Current study also revealed the hypoglycemic potency of folicitin which was confirmed by the blood glucose level and by the results of glucose tolerance test. Blood glucose level of scopolamine treated group was high compared to control group while it was decreased in scopolamine plus folicitin and only folicitin treated group. Extracts from Bauhinia forficate, kaempferol, has reduced glucose level of plasma in diabetic rats, enhanced glucose uptake and lowered hyperglycemia, mimicking the action of insulin, it's another extract N-butanol also lowered blood glucose level in alloxan induced diabetic rats [62,63]. Quercetin is also involved in homeostasis of glucose [64]. Another phytochemical drug i.e. rutin significantly lowered fasting blood glucose in streptozotocin induced diabetic models of rats [65].
Intraperitoneal administration of folicitin to scopolamine induced hyperlipidemic mice has been shown to be capable of reducing serum lipid content. Research work of You et al. [66], reported that administration of flavonoids to the hyperlipidemic mice reduced LDL, triglycerides, total cholesterol and increases HDL. In accordance with the results of current study, research of Feng et al. [67], reported that the administration of flavonoids to hyperlipidemic rats were greatly effective in showing hypolipidemic effect. Work done by Sun et al. [68], also reported similar results. Results of another study reported that HDL function is improved by flavonoids through their effects on cellular antioxidant status and inflammation as they might initiate antioxidant defense of the cell through Nrf-2/HO-1 pathway and these antioxidant properties of flavonoids show great HDL content improving and functioning potential [69].
The neuroprotective capability of folicitin against scopolamine induced oxidative stress was further evaluated by the Nrf-2, HO-1 and pJNK proteins expression. Many studies have reported that oxidative stress play role in the pathological characteristics of neurodegenerative disease including Alzheimer disease [58,70,71]. Degradation of heme is catalyzed by HO-1, a key antioxidant enzyme which then leads to the production of carbon monoxide and biliverdin, which abrogates the oxidative stress induced neurodegenerative disease [72]. Inducing of antioxidants and phase 2 detoxification enzymes such HO-1 is done by Nrf-2, a redox sensitive transcription factor [73,74,75,76].
In scopolamine induced Alzheimer disease mice, level of HO-1 and Nrf-2 was significantly reduced. Several other studies have reported reduced expression of these antioxidant enzymes in scopolamine induced neuropathology [77,78]. Nrf-2 and HO-1 expression was up regulated in scopolamine plus folicitin and only folicitin injected mice. Results of the current study are consistent with the study of Prosman et al. [79] who confirmed that Pinocembrin can abrogate oxidative stress via Nrf-2/HO-1 induction in rats. Naringenin, a potential antioxidant also reported to possess neuroprotective effect through Nrf-2/HO-1 pathway [80]. According to the results of another study neuroprotective ability of k compound (obtained from red ginseng) was finished in Nrf-2 knockout mice [81]. Other natural flavonoids such as Icariin, Sophora flavanone and Tiliroside displayed neuroprotective properties by ameliorating oxidative stress via the up regulation of Nrf-2/HO-1 pathway [82,83,84]. Overall, findings of our study showed that folicitin possess a powerful neuroprotective effect and have potency for the treatment of Alzheimer disease as a drug in future.
pJNK is a family of serine-threonine kinase proteins whose phosphorylation is involved in neuronal death and play key roles in the pathology of Alzheimer disease such as it accumulates in the structure of NFT's and then play an important role in the maturation of these tangles in Alzheimer disease indirectly [85,86,87,88,89,90,91]. The pJNK expression was elevated in scopolamine treated group in comparison to control group while it was down regulated in the folicitin plus scopolamine and only folicitin injected group. Many studies reported that there was an elevated pJNK expression in the brains of Alzheimer disease patients and showed positive co-localization with amyloid beta disposition [92,93]. Minogue et al. [94], reported that those neurons will be protected from apoptosis inducing stress which lack the expression of pJNK. Research work of Qureshi et al. [95], also reported that activation of Jnk3 cause neuronal death which leads to the pathology of Alzheimer disease. Phosphorylated JNKs inhibition is an engaging therapeutic strategy which is investigated with extensive current efforts from pharmaceutical industry. Several flavonoids have shown to obstruct JNK activity [96]. Administration of epicatechin demonstrated a clear inhibition of LDL-induced JNK [97]. Flavonoid Luteolin also inhibited LPS-mediated phosphorylation of JNK [98]. The complete JNK phosphorylation inhibition by flavonoids suggests that these polyphenolic compounds may have powerful anti-apoptotic actions in neurons.
Flavonoids, compounds of natural origin actively support neuroplasticity, synaptogenesis and neurogenesis [99,100]. Therefore present study also evaluated the reversal of synaptic loss in terms of the expression of presynaptic protein synaptophysin and post synaptic protein, PSD95 in scopolamine induced Alzheimer disease mice by folicitin administration. In Alzheimer disease the loss of synapse is related with the cognition memory and impairment but the molecular mechanism is not known for this loss yet fully [20,101]. Immunohistochemicle studies and electron microscopy have shown that in Alzheimer disease, the synaptic pathology initiates from presynaptic terminal and then progresses to postsynaptic terminals. It is evident from the progressive damage in the presynaptic protein synaptophysin and is believed the best brain correlate of cognitive decline in human Alzheimer disease [55,102,103]. The level of synaptophysin and PSD95 decreased in scopolamine induced Alzheimer disease mice in comparison to control group while it was up regulated in the scopolamine plus folicitin and only folicitin injected mice. Studies have shown that the level of synaptophysin decreased with amyloid beta disposition, progressive aphasia and dementia [104,105,106,107]. PSD95 is an important post synaptic protein which is required for the structure and function of brain cells [29]. Studies reported a great loss of postsynaptic marker PSD95 compared to the synaptophysin protein [108,109]. Many other studies reported reduced level of PSD95 protein expression in Alzheimer disease mouse model and patients [9,30,104,110,111,112]. In contrast, other two studies showed that the level of PSD95 was high during Alzheimer disease [113]. A study reported that Icariin up regulated the expression of PSD95 and synaptophysin in mouse model of traumatic brain injury [114]. Quercetin-3-O-galactoside also up regulates the expression of presynaptic protein synaptophysin [115]. Hesperidin promotes synaptogenesis and remarkably up regulated synaptophysin and PSD95 protein [116].
5. Conclusion
Oxidative stress induced by scopolamine is reduced by folicitin via up regulating Nrf-2/pJNK pathway and improved the memory in mice. Current study also reported that folicitin abrogates scopolamine induced synaptic loss by upregulating the expression of synaptophysin and PSD95 protein. It is also reported for the first time that folicitin possess hypoglycemic and hypolipidemic capability against scopolamine induced hyperglycemia and hyperlipidemia. These findings suggest that folicitin is a potential candidate for mitigating scopolamine induced Alzheimer disease, hyperglycemia and hyperlipidemia.
6. Recommendations
Although neuro protective and anti-oxidative effect of folicitin has analyzed against oxidative stress induced by scopolamine in adult male mice, the study is complete in a sense but we recommend for further investigation that there should be some transgenic mice in which Nrf-2 or pJNK are mutated. We also recommend cell culture to check the exact pathway of folicitin against scopolamine induced oxidative stress and synaptic loss which leads to memory impairments. Further in detailed investigation should be carried out regarding the hypoglycemic effect of folicitin and its possible mechanism pathway in a mice model over a time sufficient for the conversion of hyperglycemic condition to diabetes.
Author contribution statement
Seema Gul: Performed the experiments; Wrote the paper.
Sobia Attaullah; Mujeeb Ullah: Analyzed and interpreted the data; Wrote the paper.
Habib Ullah Khan, Mahdi H Alsugoor: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Shahid Ali Shah: Conceived and designed the experiments; Analyzed and interpreted the data.
Sanaullah Khan: Analyzed and interpreted the data.
Hafiza Sara Salahuddin: Analyzed and interpreted the data
Funding statement
Internal grant IRCC-2021-010, Qatar National Library, Doha Qatar and Qatar University, Qatar.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are grateful to Dr. Umar Farooq, Department of Pharmacy, Abbottabad Campus, COMSATS University Islamabad, Pakistan for generously providing us the folicitin compound which enabled us to explore the potential applications of folicitin.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e16930.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Scheltens P., Blennow K., Breteler M.M., de Strooper B., Frisoni G.B., Salloway S., Van der Flier W.M. Alzheimer's disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 2.Lopez O.L., Kuller L.H. Epidemiology of aging and associated cognitive disorders: prevalence and incidence of Alzheimer's disease and other dementias. Handb. Clin. Neurol. 2019;167:139–148. doi: 10.1016/B978-0-12-804766-8.00009-1. [DOI] [PubMed] [Google Scholar]
- 3.Amor S., Puentes F., Baker D., Van Der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129(2):154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shukla V., Mishra S.K., Pant H.C. Oxidative stress in neurodegeneration. Adv. Pharm. Sci. 2011;2011 doi: 10.1155/2011/572634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantzavinos V., Alexiou A. Biomarkers for alzheimer's disease diagnosis. Curr. Alzheimer Res. 2017;14(11):1149–1154. doi: 10.2174/1567205014666170203125942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Congdon E.E., Sigurdsson E.M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018;14(7):399–415. doi: 10.1038/s41582-018-0013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards G.A., Iii, Gamez N., Escobedo G., Jr., Calderon O., Moreno-Gonzalez I. Modifiable risk factors for alzheimer's disease. Front. Aging Neurosci. 2019;11:146. doi: 10.3389/fnagi.2019.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y., Zhao B. Oxidative stress and the pathogenesis of Alzheimer's disease. Oxid. Med. Cell. Long. 2013;2013 doi: 10.1155/2013/316523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tönnies E., Trushina E. Oxidative stress, synaptic dysfunction, and Alzheimer's disease. J. Alzheim. Dis. 2017;57(4):1105–1121. doi: 10.3233/JAD-161088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swerdlow R.H. Mitochondria and mitochondrial cascades in alzheimer's disease. J. Alzheim. Dis.: JAD. 2018;62(3):1403–1416. doi: 10.3233/JAD-170585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma P., Jha A.B., Dubey R.S., Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Botany. 2012;2012 [Google Scholar]
- 12.Wang J., Wang Y., Xu C., Wang B., Yu J., Kang X.…Zhang Y. Effects of total flavonoids extracted from Polygonum perfoliatum L. on hypolipidemic and antioxidant in hyperlipidemia rats induced by high-fat diet. Int. J. Clin. Exp. Med. 2018;11(7):6758–6766. [Google Scholar]
- 13.Markesbery W.R. The role of oxidative stress in alzheimer disease. Arch. Neurol. 1999;56(12):1449–1452. doi: 10.1001/archneur.56.12.1449. [DOI] [PubMed] [Google Scholar]
- 14.Perry G., Nunomura A., Hirai K., Zhu X., Prez M., Avila J., Smith M.A. Is oxidative damage the fundamental pathogenic mechanism of Alzheimer's and other neurodegenerative diseases? Free Radic. Biol. Med. 2002;33(11):1475–1479. doi: 10.1016/s0891-5849(02)01113-9. [DOI] [PubMed] [Google Scholar]
- 15.Huang W.J., Zhang X., Chen W.W. Role of oxidative stress in Alzheimer's disease. Biomed. Rep. 2016;4(5):519–522. doi: 10.3892/br.2016.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serrano-Pozo A., Frosch M.P., Masliah E., Hyman B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Persp. 2011;1(1) doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai Q., Tammineni P. Mitochondrial aspects of synaptic dysfunction in Alzheimer's disease. J. Alzheim. Dis. 2017;57(4):1087–1103. doi: 10.3233/JAD-160726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skaper S.D., Facci L., Zusso M., Giusti P. Synaptic plasticity, dementia and alzheimer disease. CNS Neurol. Disord. - Drug Targets. 2017;16(3):220–233. doi: 10.2174/1871527316666170113120853. [DOI] [PubMed] [Google Scholar]
- 19.Shankar G.M., Walsh D.M. Alzheimer's disease: synaptic dysfunction and Aβ. Mol. Neurodegener. 2009;4(1):1–13. doi: 10.1186/1750-1326-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsui C., Inoue E., Kakita A., Arita K., Deguchi-Tawarada M., Togawa A., Yamada A., Takai Y., Takahashi H. Involvement of the γ-secretase-mediated EphA4 signaling pathway in synaptic pathogenesis of Alzheimer's disease. Brain Pathol. 2012;22(6):776–787. doi: 10.1111/j.1750-3639.2012.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tiwari S., Atluri V., Kaushik A., Yndart A., Nair M. Alzheimer's disease: pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019;14:5541–5554. doi: 10.2147/IJN.S200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bakota L., Brandt R. Tau biology and tau-directed therapies for Alzheimer's disease. Drugs. 2016;76(3):301–313. doi: 10.1007/s40265-015-0529-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mondragón-Rodríguez S., Perry G., Zhu X., Moreira P.I., Acevedo-Aquino M.C., Williams S. Phosphorylation of tau protein as the link between oxidative stress, mitochondrial dysfunction, and connectivity failure: implications for Alzheimer's disease. Oxid. Med. Cell. Long., 2013;2013 doi: 10.1155/2013/940603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rusek M., Pluta R., Ułamek-Kozioł M., Czuczwar S.J. Ketogenic diet in Alzheimer's disease. Int. J. Mol. Sci. 2019;20(16):3892. doi: 10.3390/ijms20163892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breijyeh Z., Karaman R. Comprehensive review on alzheimer's disease: causes and treatment. Molecules. 2020;25(24):5789. doi: 10.3390/molecules25245789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuki D., Sugiura Y., Zaima N., Akatsu H., Takei S., Yao I.…Setou M. DHA-PC and PSD-95 decrease after loss of synaptophysin and before neuronal loss in patients with Alzheimer's disease. Sci. Rep. 2014;4(1):1–9. doi: 10.1038/srep07130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillessen S., Templeton A., Marra G., Kuo Y.F., Valtorta E., Shahinian V.B. Risk of colorectal cancer in men on long-term androgen deprivation therapy for prostate cancer. J. Natl. Cancer Inst. 2010;102(23):1760–1770. doi: 10.1093/jnci/djq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen C., Li X.H., Zhang S., Tu Y., Wang Y.M., Sun H.T. 7, 8-dihydroxyflavone ameliorates scopolamine-induced Alzheimer-like pathologic dysfunction. Rejuvenation Res. 2014;17(3):249–254. doi: 10.1089/rej.2013.1519. [DOI] [PubMed] [Google Scholar]
- 29.Sultana R., Banks W.A., Butterfield D.A. Decreased levels of PSD95 and two associated proteins and increased levels of BCl2 and caspase 3 in hippocampus from subjects with amnestic mild cognitive impairment: insights into their potential roles for loss of synapses and memory, accumulation of Aβ, and neurodegeneration in a prodromal stage of Alzheimer's disease. J. Neurosci. Res. 2010;88(3):469–477. doi: 10.1002/jnr.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dore K., Carrico Z., Alfonso S., Marino M., Koymans K., Kessels H.W., Malinow R. PSD-95 protects synapses from β-amyloid. Cell Rep. 2021;35(9) doi: 10.1016/j.celrep.2021.109194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong Y., Lippa C.F. Disruption of the postsynaptic density in Alzheimer's disease and other neurodegenerative dementias. Am. J. Alzheimer's Dis. Other Dementias. 2010;25(7):547–555. doi: 10.1177/1533317510382893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lupaescu A.V., Iavorschi M., Covasa M. The use of bioactive compounds in hyperglycemia- and amyloid fibrils-induced toxicity in type 2 diabetes and alzheimer's disease. Pharmaceutics. 2022;14(2):235. doi: 10.3390/pharmaceutics14020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mergenthaler P., Lindauer U., Dienel G.A., Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 2013;36(10):587–597. doi: 10.1016/j.tins.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Capiotti K.M., Antonioli R., Jr., Kist L.W., Bogo M.R., Bonan C.D., Da Silva R.S. Persistent impaired glucose metabolism in a zebrafish hyperglycemia model. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2014 May;171:58–65. doi: 10.1016/j.cbpb.2014.03.005. Epub 2014 Apr 3. PMID: 24704522. [DOI] [PubMed] [Google Scholar]
- 35.Chakrabarty R., Yousuf S., Singh M.P. Contributive role of hyperglycemia and hypoglycemia towards the development of alzheimer's disease. Mol. Neurobiol. 2022 Jul;59(7):4274–4291. doi: 10.1007/s12035-022-02846-y. Epub 2022 May 3. PMID: 35503159. [DOI] [PubMed] [Google Scholar]
- 36.Kodl C.T., Seaquist E.R. Cognitive dysfunction and diabetes mellitus. Endocr. Rev. 2008;29(4):494–511. doi: 10.1210/er.2007-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roriz Filho J.S., Sá-Roriz T.M., Rosset I., Camozzato A.L., Santos A.C., Chaves M.L.…Roriz-Cruz M. (Pre) diabetes, brain aging, and cognition. Biochimica et biophysica acta (BBA)-Mol. Basi. Dis. 2009;1792(5):432–443. doi: 10.1016/j.bbadis.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Potenza M.A., Sgarra L., Desantis V., Nacci C., Montagnani M. Diabetes and alzheimer's disease: might mitochondrial dysfunction help deciphering the common path? Antioxidants. 2021;10(8):1257. doi: 10.3390/antiox10081257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee H.J., Seo H.I., Cha H.Y., Yang Y.J., Kwon S.H., Yang S.J. Diabetes and alzheimer's disease: mechanisms and nutritional aspects. Clin. Nutr. Res. 2018;7(4):229–240. doi: 10.7762/cnr.2018.7.4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ho L., Qin W., Pompl P.N., Xiang Z., Wang J., Zhao Z.…Pasinetti G.M. Diet‐induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer's disease. Faseb. J. 2004;18(7):902–904. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- 41.Mehla J., Chauhan B.C., Chauhan N.B. Experimental induction of type 2 diabetes in aging-accelerated mice triggered Alzheimer-like pathology and memory deficits. J. Alzheim. Dis. 2014;39(1):145–162. doi: 10.3233/JAD-131238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao X., Wu Q., Peng J., Pan L., Ren Z., Liu H., Liu L. Hyperlipidemia-induced apoptosis of hippocampal neurons in apoE(-/-) mice may be associated with increased PCSK9 expression. Mol. Med. Rep. 2017;15:712–718. doi: 10.3892/mmr.2016.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shattat G.F. A review article on hyperlipidemia: types, treatments and new drug targets. Biomed Pharm. J. 2014;7(2) [Google Scholar]
- 44.Hottman D.A., Chernick D., Cheng S., Wang Z., Li L. HDL and cognition in neurodegenerative disorders. Neurobiol. Dis. 2014;72 Pt:22–36. doi: 10.1016/j.nbd.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y., Chen X., Xue J., et al. Flavonoids furom Coreopsis tinctoria adjust lipid metabolism in hyperlipidemia animals by down-regulating adipose differentiation-related protein. Lipids Health Dis. 2014;13:193. doi: 10.1186/1476-511X-13-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karr S. Epidemiology and management of hyperlipidemia. Am. J. Manag. Care. 2017;23(9 Suppl):S139–S148. [PubMed] [Google Scholar]
- 47.Sáiz-Vazquez O., Puente-Martínez A., Ubillos-Landa S., Pacheco-Bonrostro J., Santabárbara J. Cholesterol and alzheimer's disease risk: a meta-meta-analysis. Brain Sci. 2020;10(6):386. doi: 10.3390/brainsci10060386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Araujo F.B., Barbosa D.S., Hsin C.Y., Maranhão R.C., Abdalla D.S. Evaluation of oxidative stress in patients with hyperlipidemia. Atherosclerosis. 1995;117(1):61–71. doi: 10.1016/0021-9150(94)05558-z. [DOI] [PubMed] [Google Scholar]
- 49.Ayaz M., Sadiq A., Junaid M., Ullah F., Ovais M., Ullah I., Ahmed J., Shahid M. Flavonoids as prospective neuroprotectants and their therapeutic propensity in aging associated neurological disorders. Front. Aging Neurosci. 2019;11:155. doi: 10.3389/fnagi.2019.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Al-Ishaq R.K., Abotaleb M., Kubatka P., Kajo K., Büsselberg D. Flavonoids and their anti-diabetic effects: cellular mechanisms and effects to improve blood sugar levels. Biomolecules. 2019;9(9):430. doi: 10.3390/biom9090430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raziq N., Saeed M., Ali M.S., Zafar S., Ali M.I. In vitro anti-oxidant potential of new metabolites from Hypericum oblongifolium (Guttiferae) Nat. Prod. Res. 2015;29(24):2265–2270. doi: 10.1080/14786419.2015.1009064. [DOI] [PubMed] [Google Scholar]
- 52.de Andrade Teles R.B., Diniz T.C., Costa Pinto T.C., de Oliveira Junior R.G., Gama e Silva M., de Lavor É.M.…da Silva Almeida J.R.G. Flavonoids as therapeutic agents in Alzheimer's and Parkinson's diseases: a systematic review of preclinical evidences. Oxid. Med. Cell. Long. 2018;2018 doi: 10.1155/2018/7043213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gomes A., Pimpão R.C., Fortalezas S., Figueira I., Miguel C., Aguiar C.…Santos C.N. Chemical characterization and bioactivity of phytochemicals from Iberian endemic Santolina semidentata and strategies for ex situ propagation. Ind. Crop. Prod. 2015;74:505–513. [Google Scholar]
- 54.Zhang Q., Liu J., Duan H., Li R., Peng W., Wu C. Activation of Nrf2/HO-1 signaling: an important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021;34:43–63. doi: 10.1016/j.jare.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gourmaud S., Paquet C., Dumurgier J., Pace C., Bouras C., Gray F., Laplanche J.L., Meurs E.F., Mouton-Liger F., Hugon J. Increased levels of cerebrospinal fluid JNK3 associated with amyloid pathology: links to cognitive decline. J. Psychiatr. Neurosci. : JPN (J. Psychiatry Neurosci.) 2015;40(3):151–161. doi: 10.1503/jpn.140062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farooq U., Khan T., Shah S.A., Hossain M.S., Ali Y., Ullah R.…Capasso R. Isolation, characterization and neuroprotective activity of folecitin: an in vivo study. Life. 2021;11(8):825. doi: 10.3390/life11080825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolf A., Bauer B., Abner E.L., Ashkenazy-Frolinger T., Hartz A.M. A comprehensive behavioral test battery to assess learning and memory in 129S6/tg2576 mice. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0147733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee G.Y., Lee C., Park G.H., Jang J.H. Amelioration of scopolamine-induced learning and memory impairment by α-pinene in C57BL/6 mice. Evid. base Compl. Alternative Med. : eCAM. 2017;2017 doi: 10.1155/2017/4926815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vorhees C.V., Williams M.T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lenz R.A., Baker J.D., Locke C., Rueter L.E., Mohler pharmacodynamic E.G., Wesnes K.…Saltarelli M.D. The scopolamine model as a marker in early drug development. Psychopharmacology. 2012;220(1):97–107. doi: 10.1007/s00213-011-2456-4. [DOI] [PubMed] [Google Scholar]
- 61.Park H.S., Hwang E.S., Choi G.Y., Kim H.B., Park K.S., Sul J.Y.…Park J.H. Sulforaphane enhances long-term potentiation and ameliorate scopolamine-induced memory impairment. Physiol. Behav. 2021;238 doi: 10.1016/j.physbeh.2021.113467. [DOI] [PubMed] [Google Scholar]
- 62.Silva F.R.M.B., Szpoganicz B., Pizzolatti M.G., Willrich M.A.V., de Sousa E. Acute effect of Bauhinia forficata on serum glucose levels in normal and alloxan-induced diabetic rats. J. Ethnopharmacol. 2002;83(1–2):33–37. doi: 10.1016/s0378-8741(02)00193-9. [DOI] [PubMed] [Google Scholar]
- 63.Gomes L.O., Lívero F.A.R., Stolf A.M., Souza C.E.A., Werneck M.P., Ghizoni C.V.C.…Acco A. Ethanolic extract of Bauhinia forficata metabolic effects in diabetic and normoglycemic rats. Int. J. Diab. Clin. Res. 2016;3:1–8. [Google Scholar]
- 64.M Eid H., S Haddad P. The antidiabetic potential of quercetin: underlying mechanisms. Curr. Med. Chem. 2017;24(4):355–364. doi: 10.2174/0929867323666160909153707. [DOI] [PubMed] [Google Scholar]
- 65.Prince P.S.M., Kannan N.K. Protective effect of rutin on lipids, lipoproteins, lipid metabolizing enzymes and glycoproteins in streptozotocin‐induced diabetic rats. J. Pharm. Pharmacol. 2006;58(10):1373–1383. doi: 10.1211/jpp.58.10.0011. [DOI] [PubMed] [Google Scholar]
- 66.You C.L., Su P.Q., Zhou X.X. Study on effect and mechanism of Scutellaria baicalensis stem-leaf total flavonoid in regulating lipid metabolism. Zhongguo Zhong yao za zhi= Zhongguo zhongyao zazhi= China J. Chin. materia medica. 2008;33(9):1064–1066. [PubMed] [Google Scholar]
- 67.Feng L.J., Yu C.H., Ying K.J., Hua J., Dai X.Y. Hypolipidemic and antioxidant effects of total flavonoids of Perilla Frutescens leaves in hyperlipidemia rats induced by high-fat diet. Food Res. Int. 2011;44(1):404–409. [Google Scholar]
- 68.Sun J., Wang Z., Chen L., Sun G. Hypolipidemic effects and preliminary mechanism of Chrysanthemum flavonoids, its main components luteolin and luteoloside in hyperlipidemia rats. Antioxidants. 2021;10(8):1309. doi: 10.3390/antiox10081309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Millar C.L., Duclos Q., Blesso C.N. Effects of dietary flavonoids on reverse cholesterol transport, HDL metabolism, and HDL function. Adv. Nutr.(Bethesda, Md. 2017;8(2):226–239. doi: 10.3945/an.116.014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barnham K.J., Masters C.L., Bush A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004;3(3):205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 71.Singh A., Kukreti R., Saso L., Kukreti S. Oxidative stress: a key modulator in neurodegenerative diseases. Molecules. 2019;24(8):1583. doi: 10.3390/molecules24081583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jazwa A., Cuadrado A. Targeting heme oxygenase-1 for neuroprotection and neuroinflammation in neurodegenerative diseases. Curr. Drug Targets. 2010;11(12):1517–1531. doi: 10.2174/1389450111009011517. [DOI] [PubMed] [Google Scholar]
- 73.Ramsey C.P., Glass C.A., Montgomery M.B., Lindl K.A., Ritson G.P., Chia L.A., Hamilton R.L., Chu C.T., Jordan-Sciutto K.L. Expression of Nrf2 in neurodegenerative diseases. J. Neuropathol. Exp. Neurol. 2007;66(1):75–85. doi: 10.1097/nen.0b013e31802d6da9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang M., An C., Gao Y., Leak R.K., Chen J., Zhang F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog. Neurobiol. 2013;100:30–47. doi: 10.1016/j.pneurobio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davies D.A., Adlimoghaddam A., Albensi B.C. Role of Nrf2 in synaptic plasticity and memory in alzheimer's disease. Cells. 2021;10(8):1884. doi: 10.3390/cells10081884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saha S., Buttari B., Profumo E., Tucci P., Saso L. A perspective on Nrf2 signaling pathway for neuroinflammation: a potential therapeutic target in alzheimer's and Parkinson's diseases. Front. Cell. Neurosci. 2022;15 doi: 10.3389/fncel.2021.787258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ohno M., Watanabe S. D-cycloserine, a glycine site agonist, reverses working memory failure by hippocampal muscarinic receptor blockade in rats. Eur. J. Pharmacol. 1996;318(2–3):267–271. doi: 10.1016/s0014-2999(96)00907-7. [DOI] [PubMed] [Google Scholar]
- 78.Venkatesan R., Subedi L., Yeo E.J., Kim S.Y. Lactucopicrin ameliorates oxidative stress mediated by scopolamine-induced neurotoxicity through activation of the NRF2 pathway. Neurochem. Int. 2016;99:133–146. doi: 10.1016/j.neuint.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 79.Promsan S., Jaikumkao K., Pongchaidecha A., Chattipakorn N., Chatsudthipong V., Arjinajarn P.…Lungkaphin A. Pinocembrin attenuates gentamicin-induced nephrotoxicity in rats. Can. J. Physiol. Pharmacol. 2016;94(8):808–818. doi: 10.1139/cjpp-2015-0468. [DOI] [PubMed] [Google Scholar]
- 80.Habtemariam S. 2019. The Nrf2/HO-1 axis as Targets for Flavanones: Neuroprotection by Pinocembrin, Naringenin, and Eriodictyol. Oxidative Medicine and Cellular Longevity, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seo J.Y., Ju S.H., Oh J., Lee S.K., Kim J.S. Neuroprotective and cognition-enhancing effects of compound K isolated from red ginseng. J. Agric. Food Chem. 2016;64(14):2855–2864. doi: 10.1021/acs.jafc.5b05789. [DOI] [PubMed] [Google Scholar]
- 82.Li X.A., Ho Y.S., Chen L., Hsiao W.W. The protective effects of icariin against the homocysteine-induced neurotoxicity in the primary embryonic cultures of rat cortical neurons. Molecules. 2016;21(11):1557. doi: 10.3390/molecules21111557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Velagapudi R., El-Bakoush A., Olajide O.A. Activation of Nrf2 pathway contributes to neuroprotection by the dietary flavonoid tiliroside. Mol. Neurobiol. 2018;55(10):8103–8123. doi: 10.1007/s12035-018-0975-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singh S., Nagalakshmi D., Sharma K.K., Ravichandiran V. Natural antioxidants for neuroinflammatory disorders and possible involvement of Nrf2 pathway: a review. Heliyon. 2021;7(2) doi: 10.1016/j.heliyon.2021.e06216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morishima Y., Gotoh Y., Zieg J., Barrett T., Takano H., Flavell R., Davis R.J., Shirasaki Y., Greenberg M.E. Beta-amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J. Neurosci. : Offi. J. Soci. Neurosci. 2001;21(19):7551–7560. doi: 10.1523/JNEUROSCI.21-19-07551.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Okazawa H., Estus S. The JNK/c-Jun cascade and Alzheimer's disease. Am. J. Alzheimer's Dis. Other Dementias. 2002;17(2):79–88. doi: 10.1177/153331750201700209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Waetzig V., Czeloth K., Hidding U., Mielke K., Kanzow M., Brecht S.…Hanisch U.K. c‐Jun N‐terminal kinases (JNKs) mediate pro‐inflammatory actions of microglia. Glia. 2005;50(3):235–246. doi: 10.1002/glia.20173. [DOI] [PubMed] [Google Scholar]
- 88.Zhao Y., Herdegen T. Cerebral ischemia provokes a profound exchange of activated JNK isoforms in brain mitochondria. Mol. Cell. Neurosci. 2009;41(2):186–195. doi: 10.1016/j.mcn.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 89.Killick R., Ribe E.M., Al-Shawi R., Malik B., Hooper C., Fernandes C., Dobson R., Nolan P.M., Lourdusamy A., Furney S., Lin K., Breen G., Wroe R., To A.W., Leroy K., Causevic M., Usardi A., Robinson M., Noble W., Williamson R.…Lovestone S. Clusterin regulates β-amyloid toxicity via Dickkopf-1-driven induction of the wnt-PCP-JNK pathway. Mol. Psychiatr. 2014;19(1):88–98. doi: 10.1038/mp.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sclip A., Tozzi A., Abaza A., Cardinetti D., Colombo I., Calabresi P.…Borsello T. c-Jun N-terminal kinase has a key role in Alzheimer disease synaptic dysfunction in vivo. Cell Death Dis. 2014;5(1):e1019. doi: 10.1038/cddis.2013.559. e1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de Los Reyes Corrales T., Losada-Pérez M., Casas-Tintó S. JNK pathway in CNS pathologies. Int. J. Mol. Sci. 2021;22(8):3883. doi: 10.3390/ijms22083883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yoon S.O., Park D.J., Ryu J.C., Ozer H.G., Tep C., Shin Y.J., Lim T.H., Pastorino L., Kunwar A.J., Walton J.C., Nagahara A.H., Lu K.P., Nelson R.J., Tuszynski M.H., Huang K. JNK3 perpetuates metabolic stress induced by Aβ peptides. Neuron. 2012;75(5):824–837. doi: 10.1016/j.neuron.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yarza R., Vela S., Solas M., Ramirez M.J. c-Jun N-terminal kinase (JNK) signaling as a therapeutic target for alzheimer's disease. Front. Pharmacol. 2016;6:321. doi: 10.3389/fphar.2015.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Minogue A.M., Schmid A.W., Fogarty M.P., Moore A.C., Campbell V.A., Herron C.E., Lynch M.A. Activation of the c-Jun N-terminal kinase signaling cascade mediates the effect of amyloid-β on long term potentiation and cell death in hippocampus: a role for interleukin-1β? J. Biol. Chem. 2003;278(30):27971–27980. doi: 10.1074/jbc.M302530200. [DOI] [PubMed] [Google Scholar]
- 95.Qureshi H.Y., Han D., MacDonald R., Paudel H.K. Overexpression of 14-3-3z promotes tau phosphorylation at Ser262 and accelerates proteosomal degradation of synaptophysin in rat primary hippocampal neurons. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0084615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hole K.L., Williams R.J. Flavonoids as an intervention for alzheimer's disease: progress and hurdles towards defining a mechanism of action. Brain Plast. 2021;6(2):167–192. doi: 10.3233/BPL-200098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schroeter H., Spencer J.P., Rice-Evans C., Williams R.J. Flavonoids protect neurons from oxidized low-density-lipoprotein-induced apoptosis involving c-Jun N-terminal kinase (JNK), c-Jun and caspase-3. Biochem. J. 2001;358(Pt 3):547–557. doi: 10.1042/0264-6021:3580547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jang S., Kelley K.W., Johnson R.W. Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc. Natl. Acad. Sci. U.S.A. 2008;105(21):7534–7539. doi: 10.1073/pnas.0802865105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rendeiro C., Guerreiro J.D., Williams C.M., Spencer J.P. Flavonoids as modulators of memory and learning: molecular interactions resulting in behavioural effects. Proc. Nutr. Soc. 2012;71(2):246–262. doi: 10.1017/S0029665112000146. [DOI] [PubMed] [Google Scholar]
- 100.Cichon N., Saluk-Bijak J., Gorniak L., Przyslo L., Bijak M. Flavonoids as a natural enhancer of neuroplasticity-an overview of the mechanism of neurorestorative action. Antioxidants. 2020;9(11):1035. doi: 10.3390/antiox9111035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Love S., Siew L.K., Dawbarn D., Wilcock G.K., Ben-Shlomo Y., Allen S.J. Premorbid effects of APOE on synaptic proteins in human temporal neocortex. Neurobiol. Aging. 2006;27(6):797–803. doi: 10.1016/j.neurobiolaging.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 102.Savage M.J., Lin Y.G., Ciallella J.R., Flood D.G., Scott R.W. Activation of c-Jun N-terminal kinase and p38 in an Alzheimer's disease model is associated with amyloid deposition. J. Neurosci. 2002;22(9):3376–3385. doi: 10.1523/JNEUROSCI.22-09-03376.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tampellini D., Capetillo-Zarate E., Dumont M., Huang Z., Yu F., Lin M.T., Gouras G.K. Effects of synaptic modulation on beta-amyloid, synaptophysin, and memory performance in Alzheimer's disease transgenic mice. J. Neurosci. : Offi. J. Soci. Neurosci. 2010;30(43):14299–14304. doi: 10.1523/JNEUROSCI.3383-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mucke L., Masliah E., Yu G.Q., Mallory M., Rockenstein E.M., Tatsuno G.…McConlogue L. High-level neuronal expression of Aβ1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J. Neurosci. 2000;20(11):4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lippa C.F., Rosso A.L. Loss of synaptophysin immunoexpression in primary progressive aphasia. Am. J. Alzheimer's Dis. Other Dementias. 2012;27(4):250–253. doi: 10.1177/1533317512446187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Robinson J.L., Molina-Porcel L., Corrada M.M., Raible K., Lee E.B., Lee V.M., Kawas C.H., Trojanowski J.Q. Perforant path synaptic loss correlates with cognitive impairment and Alzheimer's disease in the oldest-old. Brain : J. Neurol. 2014;137(Pt 9):2578–2587. doi: 10.1093/brain/awu190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang B., Jin X., Kuang X., Tian S. Chronic administration of parecoxib exerts anxiolytic-like and memory enhancing effects and modulates synaptophysin expression in mice. BMC Anesthesiol. 2017;17(1):1–8. doi: 10.1186/s12871-017-0443-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Calon F., Lim G.P., Yang F., Morihara T., Teter B., Ubeda O., Rostaing P., Triller A., Salem N., Jr., Ashe K.H., Frautschy S.A., Cole G.M. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer's disease mouse model. Neuron. 2004;43(5):633–645. doi: 10.1016/j.neuron.2004.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gylys K.H., Fein J.A., Yang F., Wiley D.J., Miller C.A., Cole G.M. Synaptic changes in Alzheimer's disease: increased amyloid-beta and gliosis in surviving terminals is accompanied by decreased PSD-95 fluorescence. Am. J. Pathol. 2004;165(5):1809–1817. doi: 10.1016/s0002-9440(10)63436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leuba G., Walzer C., Vernay A., Carnal B., Kraftsik R., Piotton F.…Savioz A. Postsynaptic density protein PSD-95 expression in Alzheimer's disease and okadaic acid induced neuritic retraction. Neurobiol. Dis. 2008;30(3):408–419. doi: 10.1016/j.nbd.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 111.Manczak M., Kandimalla R., Yin X., Reddy P.H. Hippocampal mutant APP and amyloid beta-induced cognitive decline, dendritic spine loss, defective autophagy, mitophagy and mitochondrial abnormalities in a mouse model of Alzheimer's disease. Hum. Mol. Genet. 2018;27(8):1332–1342. doi: 10.1093/hmg/ddy042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao Z., Ren Y., Jiang H., Huang Y. Dexmedetomidine inhibits the PSD95-NMDA receptor interaction to promote functional recovery following traumatic brain injury. Exp. Ther. Med. 2021;21(1):1. doi: 10.3892/etm.2020.9436. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Leuba G., Savioz A., Vernay A., Carnal B., Kraftsik R., Tardif E.…Riederer B.M. Differential changes in synaptic proteins in the Alzheimer frontal cortex with marked increase in PSD-95 postsynaptic protein. J. Alzheim. Dis. 2008;15(1):139–151. doi: 10.3233/jad-2008-15112. [DOI] [PubMed] [Google Scholar]
- 114.Joo H., Bae J., Lee J.S., Bang Y., Lee B.J., Park J.W.…Bu Y. Icariin improves functional behavior in a mouse model of traumatic brain injury and promotes synaptic plasticity markers. Planta Med. 2019;85(3):231–238. doi: 10.1055/a-0753-0400. [DOI] [PubMed] [Google Scholar]
- 115.Orbán-Gyapai O., Raghavan A., Vasas A., Forgo P., Hohmann J., A Shah Z. Flavonoids isolated from Rumex aquaticus exhibit neuroprotective and neurorestorative properties by enhancing neurite outgrowth and synaptophysin. CNS Neurol. Disord. - Drug Targets. 2014;13(8):1458–1464. doi: 10.2174/1871527313666141023154446. [DOI] [PubMed] [Google Scholar]
- 116.Matias I., Diniz L.P., Buosi A., Neves G., Stipursky J., Gomes F.C.A. Flavonoid hesperidin induces synapse formation and improves memory performance through the astrocytic TGF-β1. Front. Aging Neurosci. 2017;9:184. doi: 10.3389/fnagi.2017.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.