Abstract
Mutations accumulated by novel Severe Acute Respiratory Syndrome Coronavirus 2 Omicron sublineages contribute to evasion of previously effective monoclonal antibodies for treatment or prevention of Coronavirus Disease 2019 (COVID-19). Other authorized or approved antiviral drugs such as nirmatrelvir/ritonavir, remdesivir, and molnupiravir are, however, predicted to maintain activity against these sublineages and are key tools to reduce severe COVID-19 outcomes in vulnerable populations. A stepwise approach may be taken to target the appropriate antiviral drug to the appropriate patient, beginning with identifying whether a patient is at high risk for hospitalization or other complications of COVID-19. Among higher risk individuals, patient profile (including factors such as age, organ function, and comedications) and antiviral drug access inform suitable antiviral drug selection. When applied in targeted fashion, these therapies serve as a complement to vital ongoing nonpharmaceutical interventions and vaccination strategies that reduce morbidity and maximize protection against COVID-19.
Keywords: COVID-19, antivirals, risk stratification, monoclonal antibody
Emerging Severe Acute Respiratory Syndrome Coronavirus 2 variants demonstrate evasion of monoclonal antibodies, leaving fewer therapeutic options for patients at increased risk for adverse Coronavirus Disease 2019 outcomes. Other antivirals retain activity, yet have unique advantages and disadvantages; thus, a stepwise approach can guide rational drug utilization.
Given increasing prevalence of variants that evade monoclonal antibodies for prevention and treatment of coronavirus disease 2019 (COVID-19), as well as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine-associated immunoprotection, providers should be aware of other available treatment options for individuals who are at higher risk for severe COVID-19 outcomes. These therapies, when administered to the appropriate patient, have an important role in reducing COVID-19 morbidity, including the need for hospitalization. In this viewpoint, we present an approach that may be used to tailor locally available therapeutics to an individual with COVID-19 in winter 2023. In the United States, drug distribution may be visualized using the Administration for Strategic Preparedness and Response Locator (available at COVID.gov), which includes 1-stop “test-to-treat” locations.
Illustrative Case
A 71-year-old man contacts your clinic to report 3 days of sore throat and malaise, with a subsequent positive at-home SARS-CoV-2 antigen test. He has a history of diabetes complicated by end-stage renal disease and underwent kidney transplantation 6 years ago (recent creatinine clearance, 48 mL/min/1.73 m2). His current medications include aspirin, atorvastatin, insulin, losartan, mycophenolate mofetil, and tacrolimus. He has received 3 monovalent mRNA-based SARS-CoV-2 vaccines, with the last dose 6 months ago, as well as preexposure prophylaxis with the monoclonal antibody combination tixagevimab and cilgavimab (EVUSHELD). He asks for your recommendation regarding COVID-19 treatment.
Approach to Therapeutic Decision-making
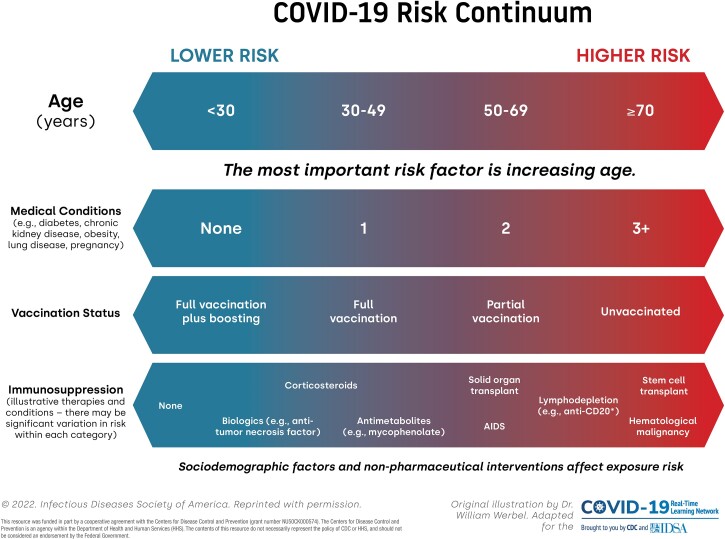
Assess risk for severe COVID-19. Initial clinical decision-making includes an assessment of risk for COVID-19 outcomes such as hospitalization, critical illness, and death, which is driven by key several demographic and immunologic factors (Figure 1). The most important nonmodifiable risk factor for severe outcomes is older age, particularly age ≥65 years [1, 2]. Accumulating medical comorbidities such as obesity, diabetes, and chronic organ dysfunction (renal, lung, or liver disease) also increase risk. The key modifiable factor to decrease COVID-19 risk is SARS-CoV-2 vaccination, in particular booster vaccination, which dramatically reduces incidence of severe disease in older individuals [3]. Importantly, increasing degree of immunocompromise contributes not only to an impaired ability to fend off viral infections [4] but may attenuate the response to SARS-CoV-2 vaccination [5].
Assess current syndrome (severity and duration). Individuals with evidence of severe and progressive COVID-19, as with other causes of pneumonia, warrant urgent evaluation in the appropriate care setting. Signs and symptoms may include the presence of lower respiratory complaints such as severe dyspnea, especially when accompanied by hypoxia, ongoing high fevers, and/or lethargy. For those with milder symptoms appropriate for management in the outpatient setting, symptom duration impacts the appropriateness of available antiviral medications [6, 7]. Specifically, oral drugs such as nirmatrelvir/ritonavir (Paxlovid) and molnupiravir (Lagevrio) must be prescribed within 5 days of symptom onset per current Emergency Use Authorizations (EUA) issued by the US Food and Drug Administration (FDA) [8]. The intravenous antiviral remdesivir (Veklury) must be prescribed within the first 7 days of symptom onset. The day of the first positive antigen or molecular (polymerase chain reaction-based) test may be used as the reference for timing considerations if syndrome onset is unclear.
Selection of the appropriate antiviral drug for high-risk patients.
Figure 1.
Risk for severe COVID-19 outcomes, represented along continuua of several key modfiable and nonmodifiable risk factors. COVID-19, Coronavirus Disease 2019.
Impact of SARS-CoV-2 variants on antiviral therapeutics
The in vitro activity of antibody therapies is heavily impacted by mutations in the spike protein of novel viral variants such as BQ.1 sublineages, which have demonstrated convergent evolution surrounding several key epitopes [9]. The majority of circulating Omicron subvariants in the United States as of December 2022 (per the Centers for Disease Control and Prevention [CDC]; https://COVID.cdc.gov/COVID-data-tracker/#variant-proportions) evade tixagevimab and cilgavimab (a monoclonal antibody combination used for preexposure prophylaxis) and bebtelovimab (an intravenous monoclonal antibody therapy with recent FDA EUA revocation because of loss of neutralizing activity) [10]. Development of novel antibody therapies targeting epitopes that may be more robust to variant evolution (eg, ClinicalTrials.gov NCT05675943, NCT05648110) as well as evaluation of the role of nonneutralizing Fc effector function against Omicron sublineages [11] are ongoing. Fortunately, based on mechanisms of action [12] and in vitro data, other available antiviral drugs are predicted to maintain activity against current and emerging Omicron sublineages [13].
Considerations for antiviral drug selection
There are key differences in drug effectiveness, profile of adverse effects, and other factors among the 3 authorized or approved antiviral medications (nirmatrelvir/ritonavir, remdesivir, molnupiravir) that inform tailored drug selection [14]. Several clinical considerations are outlined in Table 1. Of note, much of the antiviral effectiveness data were generated from studies of unvaccinated populations and/or preceded the contemporary SARS-CoV-2 variant era; real-world data continue to accrue.
Table 1.
Major Considerations and Comparison Among United States FDA Authorized or Approved Antivirals to Treat COVID-19 in the Outpatient Setting
| … | Timing | Age and Weight | Route and Course | Effectiveness Dataa | Hepatic Considerations | Renal Considerations | Major Concerns | Other Notes |
|---|---|---|---|---|---|---|---|---|
| Remdesivir (Veklury) |
≤7 d of symptoms | ≥28 d and ≥3 kg | IV 3 d, daily | Strong | Mild-moderate transaminase ↑ | GFR < 30: not recommendedb | Logistics | FDA-approved Data for use in pregnancy |
| Nirmatrelvir/ritonavir (Paxlovid) | ≤5 d of symptoms | ≥12 y and ≥40 kg | Oral 5 d, twice daily | Strong | Child C disease: not recommended | GFR 30–59: ½ dose nirmatrelvir GFR < 30: not recommended |
Drug–drug interactions | GI adverse effects Rebound syndromesc |
| Molnupiravir (Lagevrio) | ≤5 d of symptoms | ≥18 y and ≥40 kg | Oral 5 d, twice daily | Weak | None | None | Lower effectiveness Mutagenicity/teratogenicity |
Avoid in pregnancy Contraception counseling |
Abbreviations: COVID-19, Coronavirus Disease 2019; FDA, Food and Drug Administration; GFR, glomerular filtration rate; GI, gastrointestinal; IV, intravenous.
Qualitative assessment of effectiveness data refers to accumulated evidence for benefit against severe COVID-19 outcomes in high-risk populations.
Several real-world studies have demonstrated safe use of remdesivir in populations with renal impairment.
Rebound has been reported following nirmatrelvir/ritonavir treatment yet may also occur with other antiviral therapies.
Nirmatrelvir/ritonavir has shown high effectiveness against severe COVID-19 outcomes in older individuals and those with incomplete vaccination [15, 16]. A key consideration in prescribing nirmatrelvir/ritonavir is ensuring careful assessment of possible drug–drug interactions because ritonavir potently affects the activity of multiple cytochrome P450 enzymes; the effect may also endure after drug discontinuation during enzyme regeneration. Several excellent resources are available for point-of-care reference to avoid and reduce clinically important interactions including those stewarded by the University of Liverpool (https://www.COVID19-druginteractions.org/checker) and National Institutes of Health treatment guidelines (https://www.COVID19treatmentguidelines.nih.gov/); notably, in the United States, licensed pharmacists are permitted to prescribe nirmatrelvir/ritonavir after medication review. Important drug classes to consider include immunosuppressants (eg, tacrolimus), antiarrhythmics (eg, amiodarone), antiepileptics (eg, phenytoin), anticoagulants (eg, direct-acting oral anticoagulants, such as rivaroxaban), and lipid-lowering medications (eg, atorvastatin). Additionally, nirmatrelvir/ritonavir is not recommended for individuals with severe decompensated liver disease or renal impairment because of limited safety and efficacy data in these patient populations; there are dose adjustments for persons with moderate chronic kidney disease. In sum, primary considerations for use of nirmatrelvir/ritonavir for a high-risk individual include manageability of drug–drug interactions and assessment of end-organ function. The phenomenon of “rebound” syndromes after antiviral discontinuation is later discussed in “Additional Considerations.”
The intravenous antiviral remdesivir has also shown good efficacy against severe disease in high-risk individuals, as well as real-world evidence for improved outcomes and safety in some immunocompromised individuals, pregnant persons, and children [17–20]. The major consideration in use is the lack of oral formulation (ie, requires an infrastructure where patients can receive 3 once-daily outpatient infusions). Although mild-to-moderate transaminase elevations have been described in certain series using 5- or 10-day courses of remdesivir [21], severe injury is very uncommon, and in the controlled trial setting there has not been a signal for clinical hepatic injury [17, 22, 23]. FDA prescribing information [22] does not recommend remdesivir for individuals with glomerular filtration rate < 30 mL/min/1.73 m2 because of potential accumulation of its renally cleared carrier (sulfobutylether beta-cyclodextrin sodium [SBECD]). Real-world study of remdesivir, however, as well as experience with intravenous voriconazole that is also formulated with SBECD, have not demonstrated adverse renal outcomes across multiple populations with renal impairment [24–26]. Use of the lyophilized powder formulation of remdesivir (containing less SBECD) is one approach for patients with known severe renal impairment [6]. Overall, in settings in which access to outpatient infusion can be arranged in timely fashion, remdesivir is an evidence-based option for medically complex, high-risk patients given absence of major drug–drug interactions.
Molnupiravir is an oral antiviral that has demonstrated efficacy against severe COVID-19 in the trial setting [27], albeit weak and mixed evidence for real-world effectiveness in vaccinated populations [28–30] despite evident antiviral activity [31]; this equipoise has led some regulators such as the European Medicines Agency to recommend against authorization of this therapy [32]. Although the drug does not require dose adjustment for renal or liver disease and does not have major drug–drug interactions, a major consideration is the potential for mutagenicity and/or teratogenicity based primarily on in vitro data. Thus, this drug is restricted to patients aged ≥18 years and its use is not recommended in pregnant persons; the FDA EUA for molnupiravir provides recommendations regarding contraception and counseling when prescribing this agent [33]. Per National Institutes of Health and IDSA guidance, molnupiravir may be considered an alternative COVID-19 therapy in high-risk, nonpregnant adults without access to nirmatrelvir/ritonavir or remdesivir or with contraindications to their use (ie, unmanageable drug–drug interactions or severe end-organ dysfunction).
OTHER AUTHORIZED THERAPIES
High-titer Convalescent Plasma
Plasma collected from persons with prior SARS-CoV-2 antigen exposure (infection and/or vaccination) may contain neutralizing antibody and other immunological components with antiviral activity [34]. Plasma confirmed as “high-titer,” particularly if collected from individuals who experienced infection during the Omicron era (ideally reflecting “hybrid” immune responses resulting from previous vaccination plus infection) [6], is currently authorized as an early outpatient COVID-19 therapy only for individuals with “immunosuppressive disease or receiving immunosuppressive treatment” [35, 36]. Considerations for use include a need to confirm antibody titer commensurate with neutralizing activity against immune evasive Omicron sublineages, appropriate blood type matching, and counseling about potential transfusion-associated complications (volume or immune-mediated). High-titer plasma is therefore a potential therapy for immunosuppressed individuals with confirmed COVID-19 who have access to outpatient infusion.
ADDITIONAL CONSIDERATIONS
COVID-19 “Rebound” Syndromes
Recurrence of COVID-19 symptoms after initial improvement (ie, “rebound”) may occur during SARS-CoV-2 infection [37], at times accompanied by repeat positive molecular testing, high viral loads, and isolation of cultivable live virus [38]. The precise frequency of rebound syndromes and impact of antiviral therapy (such as nirmatrelvir/ritonavir) is unclear, particularly among vaccinated individuals in the Omicron variant era. Although underlying mechanisms remain uncertain, rebound syndromes do not appear to be primarily driven by acquired drug resistance [39]. Notably, early clinical trials of nirmatrelvir/ritonavir reported low and similar rates of virological rebound across placebo and treatment groups (2%–7% vs 2%–4%, varying by rebound definition [40]). In contrast, more contemporary, less controlled observational reports have reported higher incidence with nirmatrelvir/ritonavir use (>10%) [41–43]. Across studies, however, rebound syndromes occurring following antiviral therapy do not appear associated with severe illness and/or need for hospitalization. This in part underlies CDC recommendations to isolate if symptoms consistent with COVID-19 return following antiviral treatment [44], though no additional therapeutic interventions are currently recommended.
Clinical Case Revisited: Therapeutic Decision-making for a 71-year-old Male Kidney Transplant Recipient With Confirmed COVID-19
Assess risk for severe COVID-19. The patient has many risk factors for severe COVID-19 including older age, multiple comorbidities (obesity, diabetes, renal dysfunction), and transplant immunosuppression. Although the patient has received CDC-recommended 3-dose primary mRNA vaccine series, he has not received a booster, and the combination of potential blunted initial immune coupled with waning immunity places him at elevated risk for COVID-19. Prior receipt of tixagevimab and cilgavimab prophylaxis should not affect treatment decisions given this combination is not active against contemporary Omicron sublineages. Overall, guidelines recommend prompt antiviral therapy.
Assess symptoms and severity. The patient currently notes upper respiratory symptoms and malaise, without evidence of a severe syndrome or decompensation that would indicate need for urgent inpatient evaluation and management. Additionally, he reports 3 days of symptoms, which places him in an early window to receive any of the authorized or approved antiviral therapies; US FDA EUA restrictions continue to require treatment initiation within 5 days of symptoms for oral antivirals.
Tailor drug selection. Among available medications, remdesivir and nirmatrelvir/ritonavir have shown highest effectiveness against severe COVID-19 and are thus preferred therapies for this high-risk outpatient. If remdesivir infusion is available, this may be given over the next several days, without need for major dose adjustment or medication changes. In contrast, evaluation of the patient's chronic medications indicates major interactions with nirmatrelvir/ritonavir (particularly tacrolimus), requiring significant adjustment and expert consultation prior to starting this antiviral. Several groups, for example, propose discontinuing tacrolimus before administrating nirmatrelvir/ritonavir and using drug-level monitoring to guide timing of restart [45, 46]. Nirmatrelvir dose reduction is also necessary because of chronic kidney disease. If remdesivir and nirmatrelvir/ritonavir are not available or deemed unable to be safely administrated, then molnupiravir may be used as a third-line drug without dose or comedication adjustment. Because of the patient's immunocompromised status, convalescent plasma, if available, may also be administered.
SUMMARY
The evolving COVID-19 pandemic continues to have the highest impact on vulnerable populations, such as the elderly, persons with immunocompromising conditions, and incompletely vaccinated patients. Although SARS-CoV-2 mutations have resulted in evasion of previously effective monoclonal antibody therapies, available oral and intravenous antiviral therapies maintain activity and serve as key tools to reduce COVID-19 morbidity. Among high-risk individuals, antiviral drug access and patient profile determine optimal antiviral selection. These therapies serve as a complement to key ongoing nonpharmaceutical interventions to reduce viral circulation in the community and vaccination strategies that maximize immunoprotection.
Contributor Information
William A Werbel, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Ethel D Weld, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Sonali D Advani, Department of Medicine, Duke University School of Medicine, Durham, North Carolina, USA.
Payal K Patel, Division of Infectious Diseases and Clinical Epidemiology, Intermountain Healthcare, Murray, Utah, USA.
Maria E Sundaram, Center for Clinical Epidemiology and Population Health, Marshfield Clinical Research Institute, Marshfield, Wisconsin, USA.
Varun K Phadke, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Notes
Financial support. Dr Werbel reports grant funding from the National Institutes of Health (K23AI157893). Dr Advani reports grant funding from the Centers for Disease Control and Prevention, National Institutes of Health (NIDDK K12DK100024), and Duke Claude D. Pepper Older Americans Independence Center (NIA P30AG028716). Dr Sundaram reports grant funding from GlaxoSmithKline.
References
- 1. Centers for Disease Control and Prevention . COVID-19 data review: update on COVID-19–related mortality. Available at: https://www.cdc.gov/coronavirus/2019-ncov/science/data-review/index.html. Accessed 14 December 2022.
- 2. Vo AD, La J, Wu JT, et al. Factors associated with severe COVID-19 among vaccinated adults treated in US Veterans Affairs hospitals. JAMA Netw Open 2022; 5:e2240037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McConeghy KW, White EM, Blackman C, et al. Effectiveness of a second COVID-19 vaccine booster dose against infection, hospitalization, or death among nursing home residents—19 states, March 29–July 25, 2022. MMWR Morb Mortal Wkly Rep 2022; 71:1235–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roberts MB, Fishman JA. Immunosuppressive agents and infectious risk in transplantation: managing the “net state of immunosuppression”. Clin Infect Dis 2021; 73:e1302–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 2021; 325:2204–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. COVID-19 Treatment Guidelines Panel . Coronavirus disease 2019 (COVID-19) treatment guidelines. Therapeutic management of nonhospitalized adults with COVID-19. National Institutes of Health. Available athttps://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults–therapeutic-management/. Updated 26 September 2022. Accessed 15 December 2022.
- 7. Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. 2022:ciac724. doi: 10.1093/cid/ciac724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. U.S. Food and Drug Administration. Emergency Use Authorization . Drugs and non-vaccine biological products. Updated November 15, 2022. Available at: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization#coviddrugs. Accessed December 15, 2022.
- 9. Wang Q, Iketani S, Li Z, et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 2022; 186:279–286.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. U.S. Food and Drug Administration . FDA announces bebtelovimab is not currently authorized in any US region. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-announces-bebtelovimab-not-currently-authorized-any-us-region. Updated 30 November 2022. Accessed 14 December 2022.
- 11. Hérate C, Marlin R, Touret F, et al. Sotrovimab retains activity against SARS-CoV-2 Omicron variant BQ.1.1 in a non-human primate model. bioRxiv 2023: 2023.02.15.528538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Atmar RL, Finch N. New perspectives on antimicrobial agents: molnupiravir and nirmatrelvir/ritonavir for treatment of COVID-19. Antimicrob Agents Chemother 2022; 66:e0240421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Imai M, Ito M, Kiso M, et al. Efficacy of antiviral agents against omicron subvariants BQ.1.1 and XBB. N Engl J Med 2022; 388:89–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saravolatz LD, Depcinski S, Sharma M. Molnupiravir and nirmatrelvir-ritonavir: oral COVID antiviral drugs. Clin Infect Dis 2022; 76:165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med 2022; 386:1397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shah MM, Joyce B, Plumb ID, et al. Paxlovid associated with decreased hospitalization rate among adults with COVID-19—United States, April–September 2022. MMWR Morb Mortal Wkly Rep 2022; 71:1531–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med 2022; 386:305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Solera JT, Arbol BG, Bahinskaya I, Marks N, Humar A, Kumar D. Short-course early outpatient remdesivir prevents severe disease due to COVID-19 in organ transplant recipients during the omicron BA.2 wave. Am J Transplant 2022; 23:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burwick RM, Yawetz S, Stephenson KE, et al. Compassionate use of remdesivir in pregnant women with severe coronavirus disease 2019. Clin Infect Dis 2021; 73:e3996–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goldman DL, Aldrich ML, Hagmann SHF, et al. Compassionate use of remdesivir in children with severe COVID-19. Pediatrics 2021; 147:e2020047803. [DOI] [PubMed] [Google Scholar]
- 21. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med 2020; 382:2327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Full Prescribing Information: Veklury (remdesivir) for injection, for intravenous use. Available at: https://www.gilead.com/-/media/files/pdfs/medicines/covid-19/veklury/veklury_pi.pdf. Revised December 2022. Accessed 16 December 2022.
- 23. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med 2020; 383:1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ackley TW, McManus D, Topal JE, Cicali B, Shah S. A valid warning or clinical lore: an evaluation of safety outcomes of remdesivir in patients with impaired renal function from a multicenter matched cohort. Antimicrob Agents Chemother 2021; 65:e02290–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thakare S, Gandhi C, Modi T, et al. Safety of remdesivir in patients with acute kidney injury or CKD. Kidney Int Rep 2021; 6:206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abel S, Allan R, Gandelman K, Tomaszewski K, Webb DJ, Wood ND. Pharmacokinetics, safety and tolerance of voriconazole in renally impaired subjects: two prospective, multicentre, open-label, parallel-group volunteer studies. Clin Drug Investig 2008; 28:409–20. [DOI] [PubMed] [Google Scholar]
- 27. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med 2022; 386:509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study. Lancet 2022; 400:1213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zheng B, Green ACA, Tazare J, et al. Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe covid-19 outcomes in patients in the community: observational cohort study with the OpenSAFELY platform. BMJ 2022; 379:e071932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Butler CC, Hobbs FDR, Gbinigie OA, et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. Lancet 2023; 401:281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khoo SH, FitzGerald R, Saunders G, et al. Molnupiravir versus placebo in unvaccinated and vaccinated patients with early SARS-CoV-2 infection in the UK (AGILE CST-2): a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Infect Dis 2022; 23:183–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. European Medicine Agency . Refusal of the marketing authorization for Lagevrio (molnupiravir). 24 February 2023. Available at: https://www.ema.europa.eu/en/medicines/human/summaries-opinion/lagevrio. Accessed 15 March 2023.
- 33. Fact Sheet for Healthcare Providers: Emergency use authorization for Lagevrio (molnupiravir) capsules . Updated August 2022. Available at: https://www.fda.gov/media/155054/download. Accessed 5 April 2023.
- 34. Sullivan DJ, Franchini M, Joyner MJ, Casadevall A, Focosi D. Analysis of anti-SARS-CoV-2 Omicron-neutralizing antibody titers in different vaccinated and unvaccinated convalescent plasma sources. Nat Commun 2022; 13:6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. U.S. Food and Drug Administration . Convalescent Plasma EUA Letter of Authorization. December 28, 2021. Available at: https://www.fda.gov/media/141477/download. Accessed 5 April 2023.
- 36. Sullivan DJ, Gebo KA, Shoham S, et al. Randomized controlled trial of early outpatient COVID-19 treatment with high-titer convalescent plasma. medRxiv 2021. [Google Scholar]
- 37. Deo R, Choudhary MC, Moser C, et al. Viral and symptom rebound in untreated COVID-19 infection. medRxiv 2022: 2022.08.01.22278278. [Google Scholar]
- 38. Boucau J, Uddin R, Marino C, et al. Characterization of virologic rebound following nirmatrelvir-ritonavir treatment for coronavirus disease 2019 (COVID-19). Clin Infect Dis 2023; 76:e526–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Anderson AS, Caubel P, Rusnak JM, Investigators E-HT. Nirmatrelvir-ritonavir and viral load rebound in Covid-19. N Engl J Med 2022; 387:1047–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Soares H, Baniecki ML, Cardin R, et al. Viral load rebound in placebo and nirmatrelvir-ritonavir treated COVID-19 patients is not associated with recurrence of severe disease or mutations. Preprint (Research Square). 2022. [Google Scholar]
- 41. Charness ME, Gupta K, Stack G, et al. Rebound of SARS-CoV-2 infection after nirmatrelvir-ritonavir treatment. N Engl J Med 2022; 387:1045–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Epling BP, Rocco JM, Boswell KL, et al. Clinical, virologic, and immunologic evaluation of symptomatic coronavirus disease 2019 rebound following nirmatrelvir/ritonavir treatment. Clin Infect Dis 2022; 76:573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pandit JA, Radin JM, Chiang D, et al. The coronavirus disease 2019 rebound study: a prospective cohort study to evaluate viral and symptom rebound differences in participants treated with nirmatrelvir plus ritonavir versus untreated controls. Clin Infect Dis 2023; 77:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. CDC Health Alert Network . COVID-19 Rebound after Paxlovid treatment. 24 May 2022. Available at: https://emergency.cdc.gov/han/2022/pdf/CDC_HAN_467.pdf. Accessed 15 March 2023.
- 45. Lemaitre F, Budde K, van Gelder T, et al. Therapeutic drug monitoring and dosage adjustments of immunosuppressive drugs when combined with nirmatrelvir/ritonavir in patients with COVID-19. Ther Drug Monit 2022; 45:191–9. [DOI] [PubMed] [Google Scholar]
- 46. Lange NW, Salerno DM, Jennings DL, et al. Nirmatrelvir/ritonavir use: managing clinically significant drug-drug interactions with transplant immunosuppressants. Am J Transplant 2022; 22:1925–6. [DOI] [PubMed] [Google Scholar]