Abstract
Objectives
Immunocompromised patients have an increased risk of severe or prolonged COVID-19. Currently available drugs are registered to treat COVID-19 during the first 5 to 7 days after symptom onset. Data on the effectivity in immunocompromised patients with chronic non-resolving COVID-19 are urgently needed. Here, we report the outcome of patients treated with nirmatrelvir/ritonavir together with high-titer convalescent plasma (CP) in six immunocompromised patients with non-resolving COVID-19.
Methods
Immunocompromised patients with persisting COVID-19 (positive PCR with Ct values <30 for ≥20 days) received off-label therapy with nirmatrelvir/ritonavir. It was combined with CP containing BA.5 neutralizing titers of ≥1/640 whenever available. Follow-up was done by PCR and sequencing on nasopharyngeal swabs on a weekly basis until viral genome was undetectable consecutively.
Results
Five immunocompromised patients were treated with high-titer CP and 5 days of nirmatrelvir/ritonavir. One patient received nirmatrelvir/ritonavir monotherapy. Median duration of SARS-CoV-2 PCR positivity was 70 (range 20–231) days before nirmatrelvir/ritonavir treatment. In four patients receiving combination therapy, no viral genome of SARS-CoV-2 was detected on day 7 and 14 after treatment while the patient receiving nirmatrelvir/ritonavir monotherapy, the day 7 Ct value increased to 34 and viral genome was undetectable thereafter. Treatment was unsuccessful in one patient. In this patient, sequencing after nirmatrelvir/ritonavir treatment did not show protease gene mutations.
Conclusions
In immunocompromised patients with non-resolving COVID-19, the combination of nirmatrelvir/ritonavir and CP may be an effective treatment. Larger prospective studies are needed to confirm these preliminary results and should compare different treatment durations.
Introduction
Since the global spread of the SARS-CoV-2 Omicron variant, a significant decrease in severe COVID-19 has been observed.1 However, severely immunocompromised patients (ICPs) still have an increased risk of a more severe or prolonged course of the disease.2
Vaccination remains the most important step in prevention against severe COVID-19, but a subgroup of ICPs may lack an adequate immune response.3 For high-risk patients, monoclonal antibodies, direct antiviral agents (e.g. remdesivir and nirmatrelvir/ritonavir) and convalescent plasma (CP), can prevent progression to severe disease provided that these are administered early during infection.4–6
Unfortunately, none of the available monoclonal antibodies have retained activity against the Omicron variants that are now circulating worldwide. Additionally, persistent COVID-19 is sometimes seen in ICPs even after early treatment with monoclonal antibody therapy.7 Two recent case reports suggest that combination therapy with multiple antivirals (remdesivir and nirmatrelvir/ritonavir) may be successful in ICPs.8,9 In this brief report, we present the successful outcome in five out of six consecutive ICPs with a prolonged COVID-19 infection treated with nirmatrelvir/ritonavir and CP.
Methods
In the Netherlands, nirmatrelvir/ritonavir became available in November 2022 for patients with a high risk for severe COVID-19 and is provided as standard of care within 5 days of symptom onset. In this report, nirmatrelvir/ritonavir was used off-label in ICPs with no other treatment options who had: (i) prolonged COVID-19 defined as persistent high viral loads [cycle thresholds (Ct) values <30] after 20 days and (ii) clinical indication for rapid viral clearance due to persisting symptoms or discontinuation of therapy for an underlying disease (e.g. malignancy).
The treatment was given after informed consent about the off-label use and was provided free of charge by Pfizer in the Netherlands on personal request. Since ICPs who are unable to clear SARS-CoV-2 often lack an adequate immune response after vaccination and/or during infection, we combined the treatment with nirmatrelvir/ritonavir with 2 units of 300 mL of high-titer CP. In the Netherlands, more than 95% of regular plasma donors received at least three anti-SARS-CoV-2 vaccinations, and most had also recovered from natural infection. Therefore, a large pool of high-titer CP donors is available. Donors were selected on the basis of their history of recent infection and/or results of an antibody test (Abbott Architect RBD-based COV-2IgGII). Donors with high concentrations of antibodies measured with the Abbott-test, were selected for further testing with the virus neutralization (PRNT50) assay. Only CP was used with a BA.5 neutralizing titer of 1/640 or higher as confirmed by PRTN50 assay.10 All patients consented to the use of their data and the consent was registered in the electronic patient file.
Because rebound viremia has been reported after treatment with nirmatrelvir/ritonavir and may be more frequent in ICPs, we followed the patients with PCR until no viral genome was found during two consecutive PCR tests.11 When a PCR test was positive, an attempt was made to sequence the RNA to detect mutations in the spike protein or protease gene as previously described.12
We collected data on patient characteristics: age, sex, cause of immunocompromised state, vaccination status, antibody status (measured with the Liaison SARS-CoV-2 TrimericS IgG assay, DiaSorin) and previous SARS-CoV-2 treatment. Virological data were also collected: Ct values of PCR and sequences.
Results
Between December 2022 and January 2023, six ICPs with a prolonged SARS-CoV-2 infection were treated with nirmatrelvir/ritonavir. Four patients were female and the median age was 58 years (range 44–70 years). Four patients had an underlying B-cell malignancy, one patient a T-cell malignancy and one patient a common variable immune deficiency. All patients had received at least two vaccinations. Three patients had SARS-CoV-2 antibodies (646, 1660 and 5660 BAU/mL) around the time of nirmatrelvir/ritonavir initiation following previous unsuccessful treatment with CP or tixagevimab/cilgavimab, two were antibody negative and in one patient, SARS-CoV-2 antibodies were not measured before treatment. One patient was treated while hospitalized for worsening of respiratory symptoms and all other patients were treated as outpatients.
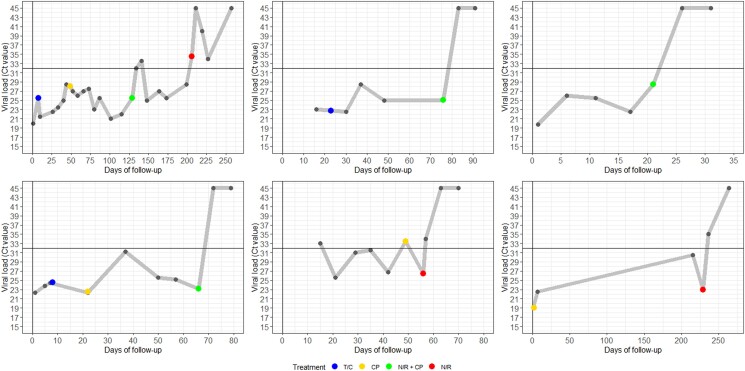
Table 1 and Figure 1 provide an overview on the treatment and follow-up for all patients. Three patients received tixagevimab/cilgavimab before and two of them also received CP monotherapy preceding the treatment with nirmatrelvir/ritonavir. The median time between first positive SARS-CoV-2 test and start of nirmatrelvir/ritonavir was 70 days (range 20–231 days). In four patients, two units of high-titer CP were given on day 1 of nirmatrelvir/ritonavir therapy while one patient had received one unit of CP 7 days before the start of nirmatrelvir/ritonavir. One patient did not receive CP around the time of nirmatrelvir/ritonavir. SARS-CoV-2 genome was undetectable within 7 days after start of nirmatrelvir/ritonavir in four patients. In one patient, the Ct value was 34 after 7 days, no viral genome was found 32 days after therapy. One patient was not treated successfully. However, he eventually cleared the virus 257 days after the first SARS-CoV-2 test, 128 days after treatment with nirmatrelvir/ritonavir plus CP and 51 days after a second course of 5-day nirmatrelvir/ritonavir monotherapy. This patient developed two mutations in the spike protein (K444R and A942Y) around time of treatment with tixagevimab/cilgavimab on day 8, and sequencing was successful multiple times after treatment with nirmatrelvir/ritonavir, however, only the K444R could be found repetitively while no mutations in the protease gene were found. No serious adverse drug reactions were reported, and nirmatrelvir/ritonavir was well-tolerated. As discussed in the short case summaries of each patient (Supplementary Data 1, available at JAC Online), in one of the three patients who had been treated with tixagevimab/cilgavimab previously, mutations known to confer resistance to cilgavimab (K444R) were detected at the start of nirmatrelvir/ritonavir therapy.
Table 1.
Overview of patients
| Patient | ICP state | SARS-CoV-2 variant1 | Previous therapy | Antibody status (BAU/mL)2 | Timing3 N/R | Concomitant therapy4 | COVID-19 severity5 | PCR Ct value D1 | Ct value D5–7 | Ct value D10–20 | Ct value D21–30 | Ct > D30 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | HM + CAR-T | BA.2 | Day 8: T/C Day 46: CP |
Day 129: 1660 Day 134: 2220 Day 141: 1880 Day 148: 1560 |
Day 129 | CP | WHO—2 | Day 129: 25.5 | Day 134: 32 | Day 141: 33.5 Day 148: 25 |
NA | Day 164: 27 Day 173: 25.5 Day 199: 28.5 Day 206: 34.56 |
Day 211: 45 Day 219: 40 Day 227: 34 Day 257: 45 |
| 2 | HM + anti-CD19 therapy | BA.5.1 | Day 23: T/C | Day 77: 5660 Day 83: 9120 Day 91: 6680 |
Day 77 | CP | WHO—2 | Day 76: 25.1 | Negative | Negative | Stop FU | NA | |
| 3 | HM | BE.1.1 | None | Day 21: <33.8 Day 26: 1400 Day 31: 592 |
Day 21 | CP | WHO—2 | Day 21: 28.5 | Negative | Negative | Stop FU | NA | |
| 4 | HM + anti-CD20 therapy | Unknown | Day 8: T/C Day 23: CP |
NA | Day 65 | CP | WHO—1 | Day 65: 23.1 | Negative | Negative | Stop FU | NA | |
| 5 | HM + anti-CD20 therapy | Failed | Day 49: CP | Day 49: <33.8 Day 57: 366 Day 63: 334 |
Day 56 | CP | WHO—2 | Day 56: 26.4 | Negative | Negative | Stop FU | NA | |
| 6 | CVID | BA.2.12.1 | Day 2: CP | Day 215: 646 | Day 232 | NA | WHO—4 | Day 229: 23 | Day 236: 35 | NA | NA | Negative | |
Column names 9 to 13 on PCR values represent the time in days after N/R treatment. The days represented in each cell are the time since the first positive SARS-CoV-2 test. CP = convalescent plasma. This CP came from donors with documented BA.5 neutralizing antibody titers of at least 1/640; CVID = common variable immune deficiency; FU = follow-up; HM = haematological malignancy; ICP = immunocompromised patient; NA = not available; N/R = nirmatrelvir/ritonavir; T/C = tixagevimab/cilgavimab. 1. All Omicron variants. 2. Antibody status is reported if measured within 30 days before N/R treatment. Antibodies were measured with the LIAISON® SARS-CoV-2 TrimericS IgG assay (DiaSorin), this test is reported negative if the value is less than 33.8 BAU/mL. 3. Follow-up starts from the first positive SARS-CoV-2 test (antigen or PCR test). 4. Patients 1 to 4 received 2 units of CP (600 mL) on the first day of N/R, and these were administered without interval. Patient 5 received 1 unit of CP 7 days before the administration of nirmatrelvir/ritonavir. 5. COVID-19 severity according to the WHO-scale at time of nirmatrelvir/ritonavir treatment. 6. Patient 1 was treated with N/R for a second time between days 206 and 210 (since the start of follow-up).
Figure 1.
Timeline on viral load and treatment in immunocompromised patients with persisting COVID-19. From left to right and from above to below, this represents patients 1 to 6 as described in Table 1 and Supplemental Data S1. Day 0 is the first positive SARS-CoV-2 test: if this test was not performed in the hospital (e.g. antigen test) than no Ct value was reported; however, these days also count as the time of follow-up. The grey dots represent the Ct values of the PCR test performed on a nasopharyngeal swab. The blue dot represents the timing of treatment with tixagevimab/cilgamab. The yellow dot represents the timing of treatment with CP. The green dot represents the timing of nirmatrelvir/ritonavir and CP combination therapy and the red dot represents treatment with nirmatrelvir/ritonavir monotherapy. The vertical black line represents the start of follow-up (day 0) and is also the Ct value scale that ranges from 15 to 45 (no lower Ct values were reported during follow-up). The horizontal black line represents the threshold for which patients had a Ct value above 32 and for which isolation may be halted. According to the local protocol, virological follow-up was halted if the Ct value exceeded 32; however, since viral rebound is reported after treatment with nirmatrelvir/ritonavir, we advise to perform follow-up until viral genome was undetectable twice.
Discussion
We showed successful treatment with nirmatrelvir/ritonavir in combination with CP in four out of five ICPs with a prolonged SARS-CoV-2 infection, and nirmatrelvir/ritonavir monotherapy was used successfully in the fifth patient. The sixth patient eventually cleared the virus, but this was only 128 days after treatment, the effect of nirmatrelvir/ritonavir plus CP will have been negligible at that time point.
Persistent COVID-19 may cause multiple problems on an individual and global scale. First, patients may have prolonged morbidity, and curative treatments for underlying diseases (e.g. malignancies) are frequently postponed that can compromise overall survival. Second, prolonged infection may lead to viral evolution, and new variants can arise and spread globally.9,13
Nirmatrelvir/ritonavir has already proven its value in the early treatment of high-risk patients by significantly reducing progression to severe COVID-19.6 Very few data on the effectivity of direct antivirals in ICPs with persistent COVID-19 are available.11 To the best of our knowledge, so far only two papers describe the successful treatment of a total of four ICPs with persisting COVID-19 with nirmatrelvir/ritonavir monotherapy.14,15 Two other publications report on the use of nirmatrelvir/ritonavir and remdesivir combination therapy.8,9 Our data are in line with these reports. Except for one patient, all patients made a clinical recovery within 7 days and in four patients, no viral genome was detected within 1 week.
Treatment-related resistance has been repeatedly reported after monoclonal antibody monotherapy.7,13 Mutations against nirmatrelvir/ritonavir have been described in in vitro studies and were also found sporadically in circulating strains before nirmatrelvir/ritonavir emergency use authorization (natural resistance).11 By using combination therapy, we hoped to decrease the risk of development of treatment-related resistance. None was demonstrated in this study, but sequencing was not possible in five of the six patients after nirmatrelvir/ritonavir treatment due to the prompt viral clearance. In one patient with persisting COVID-19, we were able to sequence multiple nasopharyngeal swabs. The patient already had acquired a K444R spike protein mutation after treatment with tixagevimab/cilgavimab. No viral evolution in the protease gene was detected even after nirmatrelvir/ritonavir treatment.
In conclusion, combination therapy of nirmatrelvir/ritonavir with CP may be a solution for difficult-to-treat COVID-19 infections in immunocompromised patients. However, prospective comparative trials are urgently needed to confirm our observation. These studies should compare different treatment durations (e.g. 5 versus 10 days) and ideally also combinations of therapies with drugs that have different modes of action.
Supplementary Material
Acknowledgements
Pfizer, the Netherlands, for providing nirmatrelvir/ritonavir.
Contributor Information
Sammy Huygens, Department of Internal Medicine, Section of Infectious Diseases and Department of Medical Microbiology and Infectious Diseases, Erasmus MC, University Medical Center, Dr. Molewaterplein 40 3015 GD, Rotterdam, The Netherlands.
Arvind Gharbharan, Department of Internal Medicine, Section of Infectious Diseases and Department of Medical Microbiology and Infectious Diseases, Erasmus MC, University Medical Center, Dr. Molewaterplein 40 3015 GD, Rotterdam, The Netherlands.
Yasmina Serroukh, Department of Hematology, Erasmus MC, University Medical Center, Rotterdam, The Netherlands.
Britt Snoek, Department of Hematology, Erasmus MC, University Medical Center, Rotterdam, The Netherlands.
Bas Franken, Department of Hematology, Medical Center Leeuwarden, Leeuwarden, The Netherlands.
Bas B Oude Munnink, Department of Viroscience, Erasmus MC, University Medical Center, Rotterdam, The Netherlands.
P Martin Van Hagen, Department of Internal Medicine and Immunology, Erasmus MC, University Medical Center, Rotterdam, The Netherlands.
Susanne Bogers, Department of Viroscience, Erasmus MC, University Medical Center, Rotterdam, The Netherlands.
Corine H Geurtsvankessel, Department of Viroscience, Erasmus MC, University Medical Center, Rotterdam, The Netherlands.
Bart J A Rijnders, Department of Internal Medicine, Section of Infectious Diseases and Department of Medical Microbiology and Infectious Diseases, Erasmus MC, University Medical Center, Dr. Molewaterplein 40 3015 GD, Rotterdam, The Netherlands.
Funding
This study has been partly funded by EU Horizon 2020 projects RECoVer (grant number: 101003589) and VEO (grant number: 874735).
Supplementary data
Available as Supplementary data at JAC Online.
Conflicts of interest
Bas Oude Munnink has received funding by EU Horizon 2020 projects RECoVer (grant number: 101003589) and VEO (grant number: 874735). All other authors declare to have no conflicts of interest.
Transparency declaration
All authors have seen and approved the manuscript, contributed significantly to the work.
This manuscript has not been previously published and is not being considered for publication elsewhere.
References
- 1. Nyberg T, Ferguson NM, Nash SGet al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet 2022; 399: 1303–12. 10.1016/S0140-6736(22)00462-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Belsky JA, Tullius BP, Lamb MGet al. COVID-19 in immunocompromised patients: a systematic review of cancer, hematopoietic cell and solid organ transplant patients. J Infect 2021; 82: 329–38. 10.1016/j.jinf.2021.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boyarsky BJ, Werbel WA, Avery RKet al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 2021; 325: 2204–6. 10.1001/jama.2021.7489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Levine AC, Fukuta Y, Huaman MAet al. COVID-19 convalescent plasma outpatient therapy to prevent outpatient hospitalization: a meta-analysis of individual participant data from five randomized trials. Clin Infect Dis. 2023In press. 10.1093/cid/ciad088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weinreich DM, Sivapalasingam S, Norton Tet al. REGEN-COV antibody combination and outcomes in outpatients with COVID-19. N Engl J Med 2021; 385: e81. 10.1056/NEJMoa2108163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hammond J, Leister-Tebbe H, Gardner Aet al. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N Engl J Med 2022; 386: 1397–408. 10.1056/NEJMoa2118542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huygens S, Oude Munnink B, Gharbharan Aet al. Sotrovimab resistance and viral persistence after treatment of immunocompromised patients infected with the severe acute respiratory syndrome coronavirus 2 Omicron variant. Clin Infect Dis 2023; 76: e507–e9. 10.1093/cid/ciac601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blennow O, Vesterbacka J, Tovatt Tet al. Successful combination treatment for persistent SARS-CoV-2 infection. Clin Infect Dis. 2023In press. 10.1093/cid/ciad085 [DOI] [PubMed] [Google Scholar]
- 9. Trottier CA, Wong B, Kohli Ret al. Dual antiviral therapy for persistent coronavirus disease 2019 and associated organizing pneumonia in an immunocompromised host. Clin Infect Dis 2022; 76: 923–5. 10.1093/cid/ciac847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Geers D, Shamier MC, Bogers Set al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci Immunol. 2021; 6. 10.1126/sciimmunol.abj1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Focosi D, McConnell S, Shoham Set al. Nirmatrelvir and COVID-19: development, pharmacokinetics, clinical efficacy, resistance, relapse, and pharmacoeconomics. Int J Antimicrob Agents 2023; 61: 106708. 10.1016/j.ijantimicag.2022.106708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Munnink BB O, Nieuwenhuijse DF, Stein Met al. Rapid SARS-CoV-2 whole-genome sequencing and analysis for informed public health decision-making in The Netherlands. Nat Med 2020; 26: 1405–10. 10.1038/s41591-020-0997-y [DOI] [PubMed] [Google Scholar]
- 13. Casadevall A, Focosi D. SARS-CoV-2 variants resistant to monoclonal antibodies in immunocompromised patients constitute a public health concern. J Clin Invest 2023; 133: e168603. 10.1172/JCI168603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Graziani L, Gori L, Manciulli Tet al. Successful use of nirmatrelvir/ritonavir in immunocompromised patients with persistent and/or relapsing COVID-19. J Antimicrob Chemother 2023; 78: 555–8. 10.1093/jac/dkac433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pérez Catalán I, García Muñoz S, Roig Martí Cet al. Nirmatrelvir/ritonavir as a potential treatment for prolonged SARS-CoV-2 infection in immunocompromised patients. Rev Esp Quimioter 2022; 35: 589–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.