Abstract
Background
The World Health Organization recommends vaccines for prevention and control of typhoid fever, especially where antimicrobial-resistant typhoid circulates. In 2018, the Navi Mumbai Municipal Corporation (NMMC) implemented a typhoid conjugate vaccine (TCV) campaign. The campaign targeted all children aged 9 months through 14 years within NMMC boundaries (approximately 320 000 children) over 2 vaccination phases. The phase 1 campaign occurred from 14 July 2018 through 25 August 2018 (71% coverage, approximately 113 420 children). We evaluated the phase 1 campaign's programmatic effectiveness in reducing typhoid cases at the community level.
Methods
We established prospective, blood culture–based surveillance at 6 hospitals in Navi Mumbai and offered blood cultures to children who presented with fever ≥3 days. We used a cluster-randomized (by administrative boundary) test-negative design to estimate the effectiveness of the vaccination campaign on pediatric typhoid cases. We matched test-positive, culture-confirmed typhoid cases with up to 3 test-negative, culture-negative controls by age and date of blood culture and assessed community vaccine campaign phase as an exposure using conditional logistic regression.
Results
Between 1 September 2018 and 31 March 2021, we identified 81 typhoid cases and matched these with 238 controls. Cases were 0.44 times as likely to live in vaccine campaign communities (programmatic effectiveness, 56%; 95% confidence interval [CI], 25% to 74%; P = .002). Cases aged ≥5 years were 0.37 times as likely (95% CI, .19 to .70; P = .002) and cases during the first year of surveillance were 0.30 times as likely (95% CI, .14 to .64; P = .002) to live in vaccine campaign communities.
Conclusions
Our findings support the use of TCV mass vaccination campaigns as effective population-based tools to combat typhoid fever.
Keywords: typhoid fever, Salmonella Typhi, vaccines, global health, India
In 2018, the Navi Mumbai Municipal Corporation conducted a typhoid conjugate vaccine campaign in half of its communities. Using a test-negative design, we estimate the campaign reduced typhoid risk by 56% (25%–74%) in vaccinated communities compared with noncampaign communities.
(See the Editorial Commentary by Carey on pages 145–7.)
Typhoid fever, caused by Salmonella enterica subspecies enterica serovar Typhi (S. Typhi), remains the cause of a significant burden of disease, with more than 11 million cases and 116 000 deaths in 2017, the majority of which occurred in children aged <15 years [1]. In the mid-20th century, typhoid fever mortality dropped significantly, from upward of 12% in the pre-antibiotic era [2] to approximately 1% in most areas after the introduction of effective antibiotics [3, 4]. Unfortunately, antimicrobial-resistant S. Typhi is on the rise, especially in Asia [5, 6], and is associated with increased severity of disease [7–9].
In conjunction with water, sanitation, and hygiene interventions, the World Health Organization (WHO) recommends vaccines as an important tool in typhoid prevention and control [10]. The WHO prequalified the first typhoid conjugate vaccine (TCV), Typbar-TCV (Bharat Biotech International Ltd, India), which comprises the Vi polysaccharide conjugated to tetanus toxoid, in December 2017 for use in children as young as 6 months [10]. A second TCV (TYPHIBEV, Biological E. Ltd, India) was prequalified in December 2020 [11]. Earlier typhoid vaccines, Ty21a and the unconjugated Vi polysaccharide (ViPS) vaccine, are not recommended for infants, and the ViPS vaccine is not as immunogenic as TCVs [10]. In multiple studies, including randomized, controlled trials, ranging from infants to adults, TCVs have shown 79%–95% efficacy in preventing typhoid fever [12–18], and earlier analysis from this project measured a vaccine effectiveness of 80.2% in our sampled population [19]. Additionally, TCV can safely be coadministered with other routine vaccines without immune interference or increased adverse events [20], making it ideal for inclusion in a country's routine immunization schedule.
Navi Mumbai, India, is an urban municipality outside of Mumbai administered by the Navi Mumbai Municipal Corporation (NMMC). Prior observations suggested a high burden of typhoid in children [21]. In 2018, the NMMC implemented the first phase of a public sector pediatric TCV campaign [22]. The campaign targeted all children aged 9 months through 14 years living within NMMC boundaries (approximately 320 000 children) over the course of 2 vaccination phases (each approximately160 000 children). The selection of areas to include in the 2 phases of the campaign were initially developed as a single-step, wedge-design, randomized, control trial in collaboration with NMMC. The first phase of the TCV mass vaccination campaign occurred over the course of 6 weeks between 14 July 2018 and 25 August 2018 and reached an estimated 113 420 children (71% of the target population) [22]; each child received 1 dose of Typbar-TCV. At the time of vaccination, each family received a TCV vaccination card to keep as a part of their medical record. The second delayed vaccination campaign was planned for 2020 but was postponed due to the coronavirus disease 2019 (COVID-19) pandemic and has not yet been conducted. The first confirmed case of COVID-19 was reported in India on 30 January 2020 [23], and a subsequent countrywide lockdown started on 25 March 2020.
The original study design involved a continuous community survey to gather healthcare utilization measurements, which would be used to adjust crude typhoid fever incidence. Because of the COVID-19 pandemic, the survey was halted; however, community-level blood culture surveillance at 6 affiliated hospitals continued. We used these data to assess the campaign's community-level programmatic effectiveness in reducing typhoid cases.
While previous randomized clinical trial data provide safety and efficacy data, only in Pakistan has TCV effectiveness been evaluated in a public sector outbreak setting [15]. In evaluating the campaign's programmatic effectiveness in reducing typhoid cases at the community level, our study provides an evaluation of a public sector mass vaccination campaign using TCV in a nonoutbreak setting. This information may be useful to key stakeholders and policymakers as decisions are made on whether to introduce TCV into routine immunization programs through immunization campaigns in S. Typhi–endemic countries.
METHODS
Setting
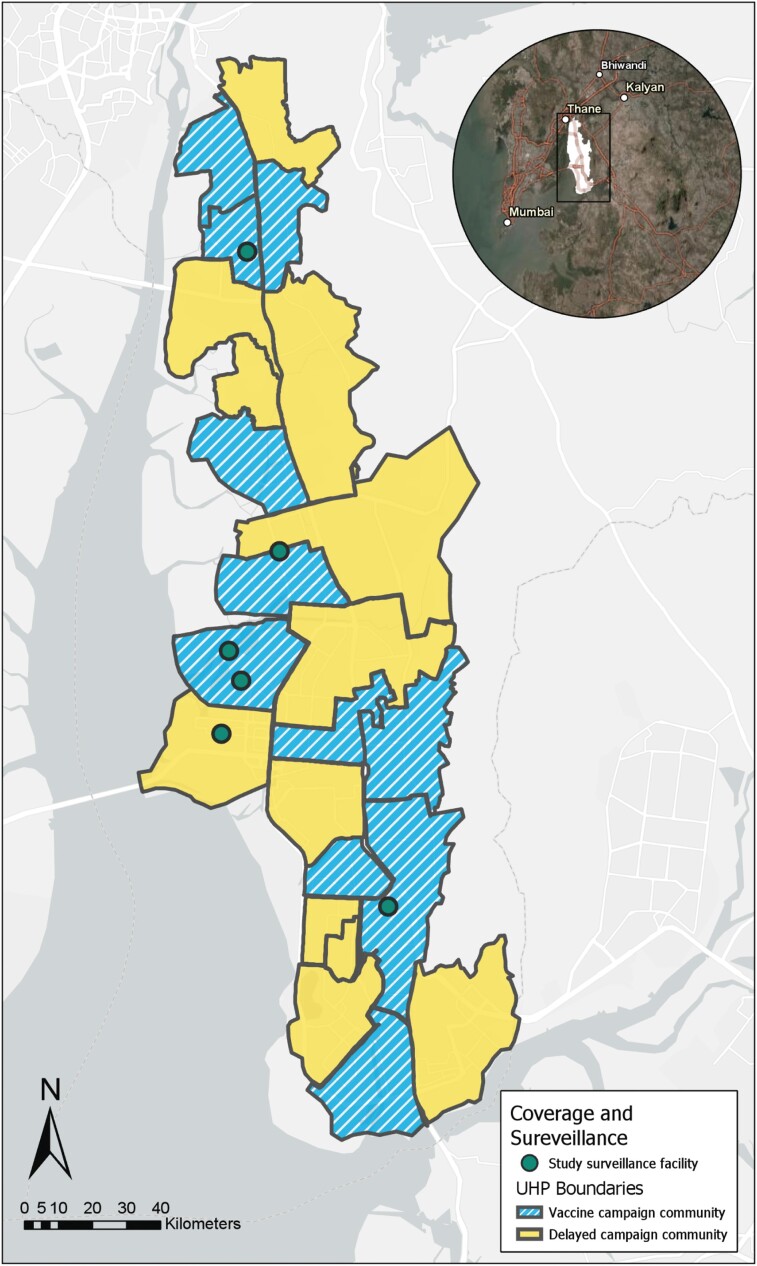
NMMC's administrative boundaries are divided into 22 areas of approximately even population size, demarcated by the catchment areas of the city's 22 urban health posts. In conjunction with NMMC, we stratified areas based on proportion of the population living in slums (stratum 1, <10%; stratum 2, 10%–70%; and stratum 3, >70%) [22] and randomly assigned areas to either vaccine campaign communities, which underwent the mass vaccination campaign in 2018, or delayed vaccine campaign communities, which were to be included in the second phase of the vaccination campaign (Figure 1). Children aged 9 months through 14 years were eligible for the vaccination campaign, amounting to an estimated 320 000 children within the NMMC administrative boundaries and an estimated 160 000 children targeted for immunization in the first phase of the campaign (baseline demographic characteristics provided by the NMMC in conjunction with the decennial Census of India; more details previously reported [22]).
Figure 1.
Map of vaccine campaign communities (blue stripes) and delayed campaign communities (yellow solid) as demarcated by the borders of Navi Mumbai Municipal Corporation's 22 UHPs. Hospitals that participated in healthcare facility blood culture surveillance marked by green circles. Abbreviation: UHP, urban health post. The map was generated using data collected from ArcGIS Collector (Mobile application) and compiled using the desktop application and ArcGIS World Imagery and Light Canvas basemaps (Professional version, ESRI). Source credits for satellite imagery: Esri, DigitalGlobe, GeoEye, i-cubed, USDA FSA, USGS, AEX, Getmapping, Aerogrid, IGN, IGP, swisstopo, and the GIS User Community, DeLorme, HERE, MapmyIndia.
Design
Original Design and Statistical Analysis Plan
We planned to estimate the incidence of blood culture–confirmed typhoid among children eligible to receive the vaccine in the vaccine campaign communities and compare this to the delayed campaign communities [24], following a single-step wedge design. We intended to deploy hybrid surveillance that adjusted the crude typhoid fever incidence for healthcare utilization [25], using prospective, blood culture–based surveillance at 6 hospitals and a continuous community survey. The COVID-19 pandemic radically altered healthcare-seeking behavior in Navi, Mumbai, and necessitated the suspension of the continuous community survey to minimize risk to field workers. However, the healthcare facility blood culture surveillance continued (1 September 2018 through 31 March 2021), providing community-level blood culture data.
Revised Design and Statistical Analysis Plan
To evaluate the effectiveness of the mass vaccination campaign in the setting of radically altered and reduced healthcare seeking, we implemented a test-negative case-control design [26, 27]. Among pediatric patients who presented with a febrile illness to affiliated healthcare sites, we identified “test-positive” cases as being blood culture–positive for S. Typhi and “test-negative” controls as being blood culture–negative for S. Typhi. We then examined home address in a vaccine campaign community or a delayed campaign community as the exposure of interest.
Inclusion Criteria
We included children within the age range of eligibility for the vaccination campaign (9 months through 14 years) and through 16 years to account for aging of participants during the follow-up period who presented or were sent to a hospital sentinel site with a febrile illness (>3 days of fever within the past 7 days without upper respiratory tract symptoms and/or vesicular rash). The hospital sites were D.Y. Patil Medical College and Hospital, Nerul; Dr. Yewale Multispecialty Hospital for Children, Vashi; Mahatma Gandhi Memorial (MGM) New Bombay Hospital, Vashi; Mathadi Hospital Trust, Koparkharaine; NMMC General Hospital, First Referral Unit, Vashi; and Rajmata Jijau Hospital, NMMC, Airoli. We offered free blood culture to all participants who met the inclusion criteria. Participants were included for 33 months (1 September 2018 through 31 March 2021).
Exclusion Criteria
Participants were excluded if no result was recorded for their blood culture or if they did not have a place of residence listed or listed a location outside of NMMC boundaries.
Data Analyses
We matched each case with up to 3 controls on age (±12 months) and time of enrollment (±28 days) to account for age-related differences in typhoid incidence [1, 28, 29] and the seasonality of typhoid fever in the region [30]. We attempted to match on shorter time intervals of enrollment, but these failed to match all cases (R MatchIt Package, R version 4.0.4).
We performed conditional logistic regression (clogit function from R “survival” package, R version 4.0.4) to estimate the odds that a blood culture–positive S. Typhi case resided in the vaccine campaign communities vs the delayed vaccine campaign communities. We adjusted the model for participant sex and education group (whether respondent has more than primary school education or not). The campaign's programmatic effectiveness was calculated as (1 – odds ratio) × 100.
We performed stratified analyses by participant age group (<5 years vs ≥5 years), to assess age-specific effectiveness of the vaccine and by time since vaccine campaign (≤365 days [1 September 2018 through 31 August 2019] vs >365 days [1 September 2019 through 31 March 2021] since phase 1 of the campaign) to evaluate any differences in effectiveness between the first and second–third years after the campaign. These stratified analyses were both tested for the significance of the difference by interaction, as calculated using an analysis of variance test for the null vs an interaction model, where the interaction terms were “(study community) × (age strata)” in the age stratified analysis and “(study community) × (time since vaccine campaign strata)” in the time since vaccine campaign stratified analysis, where study community is defined by vaccine campaign communities vs delayed vaccine campaign communities.
Ethics Statement
We obtained written informed consent from adult caregivers and verbal assent from pediatric participants aged >12 years. The study protocol was approved by the MGM New Bombay Hospital Institutional Review Board, Vashi, India; Institutional Ethics Committee, Indian Council of Medical Research–National Institute of Cholera and Enteric Diseases; WHO Research Ethics Review Committee; and Stanford University Institutional Review Board.
RESULTS
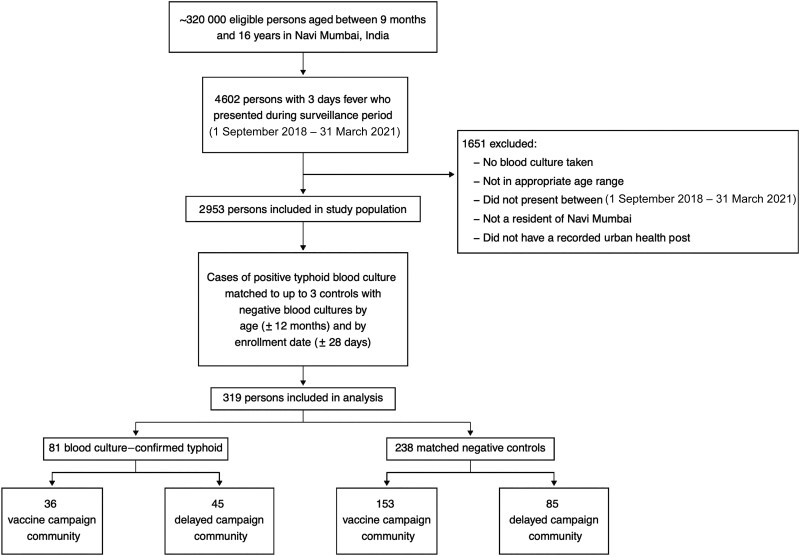
From 1 September 2018 through 31 March 2021 (33 months), we enrolled 2953 participants who met the fever definition (Figure 2). There were 81 blood culture–positive cases of typhoid. After matching on date of test (±28 days) and age (±12 months), we included 319 participants in our analysis (Table 1, Figure 1). Among the 81 blood culture–positive cases of S. Typhi, 36 were from the vaccine campaign communities and 45 were from the delayed vaccine campaign communities. There were 238 blood culture–negative controls, 153 from the vaccine campaign communities and 85 from the delayed vaccine campaign communities. The mean age of the cases was 6.9 years, and the mean age of matched controls was 6.8 years. Females represented 40% of cases and 47% of matched controls.
Figure 2.
Flow diagram depicting total estimated eligible participant population in Navi Mumbai (approximately 320 000), number of participants reported by healthcare facility blood culture surveillance (4602), number of participants with complete records (2953), number of participants included in analysis after matching (319), and blood culture–positives and blood culture–negatives included in analysis.
Table 1.
Demographic Characteristics of Child Participants and Adult Respondents
| Characteristic | Blood Culture–Positive Cases (N = 81) | Blood Culture–Negative Controls (N = 238) | P Value |
|---|---|---|---|
| Child age, y | … | … | … |
| Mean (SD) | 6.85 (3.54) | 6.77 (3.54) | .9 |
| Median [min, max] | 7.00 [1.00, 15.0] | 7.00 [1.00, 15.0] | … |
| Child gender | … | … | … |
| Male | 49 (60.5%) | 127 (53.4%) | .3 |
| Female | 32 (39.5%) | 111 (46.6%) | … |
| Adult respondent gender | … | … | … |
| Male | 38 (46.9%) | 122 (51.3%) | .5 |
| Female | 42 (51.9%) | 113 (47.5%) | … |
| Missing | 1 (1.2%) | 3 (1.3%) | … |
| Adult respondent age, y | … | … | … |
| Mean (SD) | 34.9 (7.43) | 34.6 (8.66) | .7 |
| Median [min, max] | 35.0 [19.0, 59.0] | 34.0 [18.0, 85.0] | … |
| Missing | 0 (0%) | 1 (0.4%) | … |
| Adult respondent education level | … | … | … |
| Primary school certificate (through grade 4) or less | 6 (7.4%) | 32 (13.4%) | .1 |
| Middle school certificate (grade 5–10) | 35 (43.2%) | 112 (47.1%) | … |
| High school certificate (grade 11–12) | 14 (17.3%) | 45 (18.9%) | … |
| Bachelor’s degree or greater | 25 (30.9%) | 49 (20.6%) | … |
| Don't know or refused | 1 (1.2%) | 0 (0%) | … |
Abbreviation: SD, standard deviation.
The unadjusted odds ratio that culture-confirmed typhoid cases resided in the vaccine campaign communities vs in the delayed vaccine campaign communities was 0.44 (95% confidence interval [CI], .27 to .74; P = .002). The adjusted odds ratio was 0.44 (95% CI, .26 to .75; P = .003), indicating that the effectiveness of the mass vaccination campaign was 56% (95% CI, 25% to 74%) in the vaccine campaign communities (Table 2).
Table 2.
Overall and Stratified Campaign Effectiveness
| Campaign Effectiveness | Odds Ratio (95% CI) | Campaign Effectiveness, % (95% CI) | P Value |
|---|---|---|---|
| Overall campaign effectiveness | 0.44 (.26 to .75) | 56% (25 to 74) | .003 |
| Stratified by age | … | … | |
| <5 years (case n = 21, control n = 64) | 0.70 (.24 to 2.10) | 30% (–110 to 76) | .5 |
| ≥5 years (case n = 60, control n = 174) | 0.37 (.19 to .70) | 63% (30 to 81) | .002 |
| Stratified by time since vaccination campaign | … | … | |
| ≤365 days (case n = 45, control n = 135) | 0.30 (.14 to .64) | 70% (36 to 86) | .002 |
| >365 days (case n = 36, control n = 103) | 0.75 (.33 to 1.70) | 25% (–70 to 67) | .5 |
When stratified by participant age group (<5 years vs ≥5 years), among participants aged <5 years, cases were 0.70 times as likely to live in vaccine campaign communities compared with delayed vaccine campaign communities (programmatic effectiveness, 30%; 95% CI, −110 to 76; P = .5), while participants aged ≥5 years were 0.37 times as likely to live in vaccine campaign communities (programmatic effectiveness, 63%; 95% CI, 30 to 81; P = .002). When stratified by time since campaign (≤365 days vs >365 days), cases that occurred within the first year after the campaign were 0.30 times as likely to live in vaccine campaign communities (programmatic effectiveness, 70%; 95% CI, 36 to 86; P = .002), while cases identified during the second–third year after the campaign were 0.75 times as likely to live in vaccine campaign communities (programmatic effectiveness, 25%; 95% CI, −70 to 67; P = .5).
DISCUSSION
The TCV campaign successfully vaccinated an estimated 113 420 children in 6 weeks. Typhoid fever cases were 56% less likely to reside in vaccine campaign communities than in delayed vaccine campaign communities. The overall programmatic effectiveness of the vaccination campaign on the reduction of typhoid in the intervention community was consistent with 71% estimated vaccine coverage of an 80.2% effective vaccine. Our crude and adjusted overall campaign effect odds ratios were similar, indicating that adjustment for participant sex and respondent education had little impact and that these variables were unlikely to be strong confounders.
The decreased programmatic effectiveness in participants aged <5 years and cases identified during the second–third year after the campaign may be due to children who aged into inclusion for surveillance during the study surveillance period. When we repeated our analysis but removed all participants who aged into surveillance and were not eligible for the campaign (aged <9 months on 25 August 2018, the last day of the vaccine campaign), the estimated programmatic effectiveness improved (data not shown). This suggests the importance of continuing to vaccinate children to maintain programmatic effectiveness. The drop in programmatic effectiveness over time should not be misconstrued to attribute a drop in TCV efficacy or effectiveness, neither of which was evaluated in this analysis. Rather, this drop represents a statistically nonsignificant reduction in the immediate impact of a single vaccination campaign over time, for which we did not have the statistical power to make comparisons between our stratified groups of age and time since campaign.
To minimize risk to project field workers from the COVID-19 pandemic, we halted the continuous community survey. Since the original study design and statistical analysis plan did not allow for this significant impact on data collection and given the effect of the marked change in healthcare utilization due to the COVID-19 pandemic, we could not follow the prespecified study design. Instead, using the available blood culture data, we used a test-negative design to analyze our results [31, 32]. This allowed us to evaluate the overall impact of the vaccine campaign on the reduction of typhoid cases between the intervention communities vs the delayed vaccine campaign communities. This study design, modeled off of the popular test-negative design used in influenza [33], rotavirus [34], and COVID-19 [35, 36] vaccine effectiveness studies, has been shown to be concordant with vaccine effectiveness results from randomized, controlled trials [26, 34] and has recently been used in a typhoid vaccine effectiveness evaluation, using a gold standard clinical trial database, with parameters comparable to those in a randomized, controlled trial of TCV in Malawi [27]. Recently, the WHO issued guidance recommending use of the test-negative design for COVID-19 vaccine effectiveness studies due to logistical ease and reduction of health utilization bias [35, 36].
In addition to the unpredictable impact of the COVID-19 pandemic, this study was limited by use of S. Typhi blood culture as the outcome measure for test-positive cases vs test-negative controls. Blood culture sensitivity in detecting S. Typhi infection is estimated to be between 55% and 65% [1, 37] and is dependent on the blood volume collected [37], for which we do not have data. As such, blood culture positivity as an outcome measure may result in underreporting of test-positive cases and loss of statistical power. However, this would be expected to affect both vaccine campaign and delayed vaccine campaign communities and so would not bias the estimate of campaign effectiveness. For now, blood culture remains the standard outcome used in evaluations of interventions to combat S. Typhi infection in controlled trials and public health programs and in S. Typhi surveillance [1, 5, 6, 9, 13–15, 17, 18]. Due to the cessation of the continuous community survey, we are unable to report specific rates of in- and out-migration from the study communities. However, any in- and out-migration would be expected to bias our outcome toward the null rather than inflate our programmatic effectiveness.
There are a number of descriptions in the literature of programmatic or outbreak implementation of typhoid vaccines [15, 38–40]. However, formal assessment is limited, and many lack a systematically evaluated population control group, limiting generalizability [15, 38–40]. Our results represent a metropolitan introduction of typhoid vaccine in which we systematically evaluated the overall campaign effectiveness with control communities that had not yet undergone a TCV immunization campaign. Compared with typhoid vaccines from previous generations, unconjugated Vi polysaccharide and Ty21a, TCVs induce consistently higher levels of protective efficacy and are indicated for use in younger children [10]. TCVs provide more durable immune responses than do unconjugated Vi polysaccharide vaccine and are expected to have longer durations of protection than typhoid vaccines from prior generations [10, 16]. This study provides further evidence that TCVs are effective when rolled out in the form of a municipal program.
Although the campaign did reduce typhoid, some children who lived in the intervention community still developed typhoid, even some of those children who were vaccinated [19]. Effective communication to stakeholders and policymakers should include the caveat that while programmatic implementation of TCV in children and adolescents will reduce typhoid incidence, it is not likely to interrupt all transmission and eliminate the disease for several reasons [14]. First, although highly effective, typhoid vaccines are not 100% effective, as has been reported from individually randomized trials [12–18]. Second, adults who are susceptible to typhoid are not usually included in typhoid immunization campaigns or programs. These adults sometimes develop typhoid fever and transmit S. Typhi. Even the targeted age groups are unlikely to be fully reached by vaccine campaigns; in this study, vaccine coverage was an estimated 71%. Finally, vaccination will not cure S. Typhi chronic carriers who will remain a reservoir for future transmission. Thus, public health planners should be prepared to communicate that TCV reduces but does not eliminate risk and that increased attention to providing food and drinking water not contaminated with feces will be important to reduce typhoid-associated morbidity and mortality.
Our findings support the use of TCV mass vaccination campaigns as an effective population-based tool to reduce the pediatric public health burden of typhoid fever, as part of the introduction of TCV into routine immunization programs. This evaluation adds to the body of research that supports TCV campaigns by demonstrating that these campaigns can be effective at the population level, while prior modeling suggests they may be highly cost-effective [41]. Additionally, our experience is in the setting of atypical population healthcare utilization or low reported typhoid incidence, the test-negative design is useful in estimating programmatic campaign effectiveness. Our data add to the growing evidence that stakeholders can use to support decisions on TCV introduction to reduce the burden of typhoid fever.
Contributor Information
Seth A Hoffman, Division of Infectious Diseases and Geographic Medicine, Department of Medicine, Stanford University School of Medicine, Stanford, California, USA.
Christopher LeBoa, Division of Infectious Diseases and Geographic Medicine, Department of Medicine, Stanford University School of Medicine, Stanford, California, USA.
Kashmira Date, Global Immunization Division, Center for Global Health, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Pradeep Haldar, Ministry of Health & Family Welfare, Government of India, New Delhi, India.
Pauline Harvey, World Health Organization-Country Office for India, National Public Health Surveillance Project, New Delhi, India.
Rahul Shimpi, World Health Organization-Country Office for India, National Public Health Surveillance Project, New Delhi, India.
Qian An, Global Immunization Division, Center for Global Health, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Chenhua Zhang, Global Immunization Division, Center for Global Health, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Niniya Jayaprasad, World Health Organization-Country Office for India, National Public Health Surveillance Project, New Delhi, India.
Lily Horng, Division of Infectious Diseases and Geographic Medicine, Department of Medicine, Stanford University School of Medicine, Stanford, California, USA.
Kirsten Fagerli, Global Immunization Division, Center for Global Health, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Priyanka Borhade, World Health Organization-Country Office for India, National Public Health Surveillance Project, New Delhi, India.
Savita Daruwalla, Department of Pediatrics, NMMC General Hospital, Navi Mumbai, India.
Dhanya Dharmapalan, Dr. Yewale Multispecialty Hospital for Children, Navi Mumbai, India.
Jeetendra Gavhane, Department of Pediatrics, MGM New Bombay Hospital, MGM Medical College, Navi Mumbai, India.
Shrikrishna Joshi, Dr. Joshi's Central Clinical Microbiology Laboratory, Navi Mumbai, India.
Rajesh Rai, Department of Pediatrics & Neonatology, Dr. D.Y. Patil Medical College and Hospital, Navi Mumbai, India.
Varsha Rathod, Rajmata Jijau Hospital, Airoli (NMMC), Navi Mumbai, India.
Keertana Shetty, Department of Microbiology, Dr. D.Y. Patil Medical College and Hospital, Navi Mumbai, India.
Divyalatha S Warrier, Department of Pediatrics, Mathadi Trust Hospital, Navi Mumbai, India.
Shalini Yadav, Department of Microbiology, MGM New Bombay Hospital, Navi Mumbai, India.
Debjit Chakraborty, National Institute of Cholera and Enteric Diseases, Indian Council of Medical Research, Kolkata, India.
Sunil Bahl, World Health Organization South-East Asia Regional Office, New Delhi, India.
Arun Katkar, World Health Organization-Country Office for India, National Public Health Surveillance Project, New Delhi, India.
Abhishek Kunwar, World Health Organization-Country Office for India, National Public Health Surveillance Project, New Delhi, India.
Vijay Yewale, Dr. Yewale Multispecialty Hospital for Children, Navi Mumbai, India.
Jason R Andrews, Division of Infectious Diseases and Geographic Medicine, Department of Medicine, Stanford University School of Medicine, Stanford, California, USA.
Pankaj Bhatnagar, World Health Organization-Country Office for India, National Public Health Surveillance Project, New Delhi, India.
Shanta Dutta, National Institute of Cholera and Enteric Diseases, Indian Council of Medical Research, Kolkata, India.
Stephen P Luby, Division of Infectious Diseases and Geographic Medicine, Department of Medicine, Stanford University School of Medicine, Stanford, California, USA.
Notes
Acknowledgments. We thank the following organizations and individuals for their contributions to this evaluation: the Navi Mumbai Municipal Corporation leadership and staff, the Government of India Ministry of Health and Family Welfare Universal Immunization Program, State of Maharashtra Department of Public Health and Family Welfare, Indian Academy of Pediatrics Navi Mumbai Chapter, Bharat Biotech International Limited, the Indian Council of Medical Research, WHO-India National Public Health Surveillance Project, Grant Government Medical College (Dr. Nilma Hirani), and the Centers for Disease Control and Prevention, Atlanta, Georgia (Dr. Kathleen Wannemuehler, Benjamin Nygren, and Matt Mikoleit).
Disclaimer. The findings and conclusions presented here are those of the authors and do not necessarily represent the official position, policies, or views of the CDC or the WHO.
Financial support. This work was supported by Bill & Melinda Gates Foundation grant OPP1169264 (principal investigator S. P. L.). S.A.H. is supported in part by the National Institutes of Health under the National Institute of Allergy and Infectious Diseases grant T32AI007502, as well as by the Stanford Maternal and Child Health Research Institute.
References
- 1. GBD 2017 Typhoid and Paratyphoid Collaborators . The global burden of typhoid and paratyphoid fevers: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis 2019; 19:369–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Woodward TE, Smadel JE. Management of typhoid fever and its complications. Ann Intern Med 1964; 60:144–57. [DOI] [PubMed] [Google Scholar]
- 3. Marmion DE. The treatment of typhoid fever with chloramphenicol; a clinical study of 330 cases of enteric fever treated in Egypt. Trans R Soc Trop Med Hyg 1952; 46:619–38. [DOI] [PubMed] [Google Scholar]
- 4. Woodward TE, Smadel JE, Parker RT, WissemanCL, Jr. Treatment of typhoid fever with antibiotics. Ann N Y Acad Sci 1952; 55:1043–55. [DOI] [PubMed] [Google Scholar]
- 5. Qamar FN, Yousafzai MT, Dehraj IF, et al. . Antimicrobial resistance in typhoidal salmonella: surveillance for enteric fever in Asia project, 2016–2019. Clin Infect Dis 2020; 71(Suppl 3):S276–S84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Samajpati S, Pragasam AK, Mandal S, Balaji V, Dutta S. Emergence of ceftriaxone resistant Salmonella enterica serovar Typhi in Eastern India. Infect Genet Evol 2021; 96:105093. [DOI] [PubMed] [Google Scholar]
- 7. Bhutta ZA. Impact of age and drug resistance on mortality in typhoid fever. Arch Dis Child 1996; 75:214–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walia M, Gaind R, Mehta R, Paul P, Aggarwal P, Kalaivani M. Current perspectives of enteric fever: a hospital-based study from India. Ann Trop Paediatr 2005; 25:161–74. [DOI] [PubMed] [Google Scholar]
- 9. Longley AT, Hemlock C, Date K, et al. . Illness severity and outcomes among enteric fever cases from Bangladesh, Nepal, and Pakistan: data from the Surveillance for Enteric Fever in Asia project, 2016–2019. Clin Infect Dis 2020; 71(Suppl 3):S222–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization . Typhoid vaccines: WHO position paper, March 2018—recommendations. Vaccine 2019; 37:214–6. [DOI] [PubMed] [Google Scholar]
- 11. Birkhold M, Mwisongo A, Pollard AJ, Neuzil KM. Typhoid conjugate vaccines: advancing the research and public health agendas. J Infect Dis 2021; 224(12 Suppl 2):S781–S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin FY, Ho VA, Khiem HB, et al. . The efficacy of a Salmonella typhi Vi conjugate vaccine in two-to-five-year-old children. N Engl J Med 2001; 344:1263–9. [DOI] [PubMed] [Google Scholar]
- 13. Shakya M, Colin-Jones R, Theiss-Nyland K, et al. . Phase 3 efficacy analysis of a typhoid conjugate vaccine trial in Nepal. N Engl J Med 2019; 381:2209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qadri F, Khanam F, Liu X, et al. . Protection by vaccination of children against typhoid fever with a Vi-tetanus toxoid conjugate vaccine in urban Bangladesh: a cluster-randomised trial. Lancet 2021; 398:675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yousafzai MT, Karim S, Qureshi S, et al. . Effectiveness of typhoid conjugate vaccine against culture-confirmed Salmonella enterica serotype Typhi in an extensively drug-resistant outbreak setting of Hyderabad, Pakistan: a cohort study. Lancet Glob Health 2021; 9:e1154–e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vadrevu KM, Raju D, Rani S, et al. . Persisting antibody responses to Vi polysaccharide-tetanus toxoid conjugate (Typbar TCV®) vaccine up to 7 years following primary vaccination of children <2 years of age with, or without, a booster vaccination. Vaccine 2021; 39:6682–90. [DOI] [PubMed] [Google Scholar]
- 17. Patel PD, Patel P, Liang Y, et al. . Safety and efficacy of a typhoid conjugate vaccine in Malawian children. N Engl J Med 2021; 385:1104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shakya M, Voysey M, Theiss-Nyland K, et al. . Efficacy of typhoid conjugate vaccine in Nepal: final results of a phase 3, randomised, controlled trial. Lancet Glob Health 2021; 9:e1561–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Date K, Harvey P, Bhatnagar P, et al. Field effectiveness of a typhoid conjugate vaccine—Navi Mumbai (India), 2018–2020. American Society of Tropical Medicine and Hygiene 2020 National Meeting. Arlington, VA, 2020. Available at: https://www.abstractsonline.com/pp8/#!/9181/presentation/4739. Accessed 20 January 2022.
- 20. Sirima SB, Ouedraogo A, Barry N, et al. . Safety and immunogenicity of co-administration of meningococcal type A and measles-rubella vaccines with typhoid conjugate vaccine in children aged 15–23 months in Burkina Faso. Int J Infect Dis 2021; 102:517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gavhane J, Yewale V, Weekey P, Dhanya, Warrior D. Enteric fever in children from Navi Mumbai—clinical profile, hematological features, sensitivity patterns and response to antimicrobials. Pediatr Infect Dis 2010; 2:5–9. [Google Scholar]
- 22. Date K, Shimpi R, Luby S, et al. . Decision making and implementation of the first public sector introduction of typhoid conjugate vaccine—Navi Mumbai, India, 2018. Clin Infect Dis 2020; 71(Suppl 2):S172–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Andrews MA, Areekal B, Rajesh KR, et al. . First confirmed case of COVID-19 infection in India: a case report. Indian J Med Res 2020; 151:490–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.https://clinicaltrials.gov/ct2/show/NCT03554213
- 25. Andrews JR, Barkume C, Yu AT, et al. . Integrating facility-based surveillance with healthcare utilization surveys to estimate enteric fever incidence: methods and challenges. J Infect Dis 2018; 218(Suppl 4):S268–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Serres G, Skowronski DM, Wu XW, Ambrose CS. The test-negative design: validity, accuracy and precision of vaccine efficacy estimates compared to the gold standard of randomised placebo-controlled clinical trials. Euro Surveill 2013; 18:20585. [DOI] [PubMed] [Google Scholar]
- 27. Liang Y, Driscoll AJ, Patel PD, et al. . Typhoid conjugate vaccine effectiveness in Malawi: evaluation of a test-negative design using randomised, controlled clinical trial data. Lancet Glob Health 2022;11:e136–e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sinha A, Sazawal S, Kumar R, et al. . Typhoid fever in children aged less than 5 years. Lancet 1999; 354:734–7. [DOI] [PubMed] [Google Scholar]
- 29. Walia M, Gaind R, Paul P, Mehta R, Aggarwal P, Kalaivani M. Age-related clinical and microbiological characteristics of enteric fever in India. Trans R Soc Trop Med Hyg 2006; 100:942–8. [DOI] [PubMed] [Google Scholar]
- 30. Saad NJ, Lynch VD, Antillón M, Yang C, Crump JA, Pitzer VE. Seasonal dynamics of typhoid and paratyphoid fever. Sci Rep 2018; 8:6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anders KL, Cutcher Z, Kleinschmidt I, et al. . Cluster-randomized test-negative design trials: a novel and efficient method to assess the efficacy of community-level dengue interventions. Am J Epidemiol 2018; 187:2021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jewell NP, Dufault S, Cutcher Z, Simmons CP, Anders KL. Analysis of cluster-randomized test-negative designs: cluster-level methods. Biostatistics 2019; 20:332–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jackson ML, Chung JR, Jackson LA, et al. . Influenza vaccine effectiveness in the United States during the 2015–2016 season. N Engl J Med 2017; 377:534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schwartz LM, Halloran ME, Rowhani-Rahbar A, Neuzil KM, Victor JC. Rotavirus vaccine effectiveness in low-income settings: an evaluation of the test-negative design. Vaccine 2017; 35:184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. World Health Organization . Evaluation of COVID-19 vaccine effectiveness: interim guidance, 17 March 2021. Geneva, Switzerland: World Health Organization, 2021. [Google Scholar]
- 36. Patel MK, Bergeri I, Bresee JS, et al. . Evaluation of post-introduction COVID-19 vaccine effectiveness: summary of interim guidance of the World Health Organization. Vaccine 2021; 39:4013–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mogasale V, Ramani E, Mogasale VV, Park J. What proportion of Salmonella typhi cases are detected by blood culture? A systematic literature review. Ann Clin Microbiol Antimicrob 2016; 15:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang HH, Kilgore PE, Yang LH, et al. . An outbreak of typhoid fever, Xing-An County, People's Republic of China, 1999: estimation of the field effectiveness of Vi polysaccharide typhoid vaccine. J Infect Dis 2001; 183:1775–80. [DOI] [PubMed] [Google Scholar]
- 39. Khan MI, Ochiai RL, Clemens JD. Population impact of Vi capsular polysaccharide vaccine. Expert Rev Vaccines 2010; 9:485–96. [DOI] [PubMed] [Google Scholar]
- 40. Nga TVT, Duy PT, Lan NPH, Chau NVV, Baker S. The control of typhoid fever in Vietnam. Am J Trop Med Hyg 2018; 99(3_Suppl):72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ryckman T, Karthikeyan AS, Kumar D, et al. . Comparison of strategies for typhoid conjugate vaccine introduction in India: a cost-effectiveness modeling study. J Infect Dis 2021; 224(Suppl 5):S612–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]