Abstract
Pulmonary endarterectomy (PEA) may not achieve full clearance of vascular obstructions in patients with more distal chronic thromboembolic pulmonary hypertension (CTEPH). Balloon pulmonary angioplasty (BPA) may be indicated to treat these residual vascular lesions. We compared whether patients post‐PEA (PP) treated by BPA derived similar benefit to those who had inoperable CTEPH (IC), and assessed predictors of BPA response after surgery. We treated 109 patients with BPA—89 with IC and 20 PP. Serial right heart catheterization performed at baseline (immediately before BPA) and 3 months after completing BPA, compared pulmonary vascular resistance (PVR), mean pulmonary artery pressure (mPAP) as well as change in WHO functional class and 6‐minute walk distance. We also assessed the impact of total thrombus tail length (TTTL) from photographed PEA surgical specimens and PP computed tomography pulmonary angiography (CTPA)‐quantified residual disease burden on BPA response. PP and IC groups did not differ significantly in terms of demographics, baseline hemodynamics or procedural characteristics. However, IC derived greater hemodynamic benefit from BPA: ΔPVR (−27.9 ± 20.2% vs. −13.9 ± 23.9%, p < 0.05) and ΔmPAP (−17.1 ± 14.4% vs. −8.5 ± 18.0%, p < 0.05). There was a negative correlation between pre‐BPA PVR and TTTL (r = −0.47, p < 0.05) which persisted post‐BPA. PVR, mPAP, WHO FC and 6MWD were not improved significantly post‐BPA in PP patients. BPA response was not related to TTTL terciles or CTPA‐quantified residual disease burden. Patients PP experienced inferior response to BPA, despite similar baseline and procedural characteristics to IC. BPA does not abolish the relationship between TTTL and postsurgical PVR in PP patients, suggesting that BPA is less effective in treating residual PH after surgery in an experienced surgical center.
Keywords: Balloon pulmonary angioplasty (BPA), Chronic thromboembolic pulmonary hypertension (CTEPH), Mean pulmonary artery pressure (mPAP), Pulmonary endarterectomy (PEA), Pulmonary vascular resistance (PVR)
INTRODUCTION
Chronic thromboembolic pulmonary hypertension (CTEPH) is estimated to occur in approximately 2−6 adults per million population per year. 1 Pulmonary endarterectomy (PEA) is a highly effective surgical treatment for predominantly proximal disease distribution. When performed at an expert center, more distal subsegmental disease may also be treated that can provide symptomatic and prognostic improvement in eligible patients. If a full clearance is achieved pulmonary hemodynamics may be normalized, although long‐term oral anticoagulation is recommended. 2
However, approximately 40% patients with CTEPH are ineligible for PEA surgical intervention, 1 , 2 , 3 due to distal inaccessible lesions, and/or unfavorable risk/benefit‐relationship for surgery due to comorbidity being common reasons to forgo PEA. 2 Furthermore, in 17–51% of patients, symptomatic pulmonary hypertension persists despite PEA and requires further treatment. 3 , 4 Amongst these two cohorts—inoperable CTEPH (IC) and post‐PEA (PP), balloon pulmonary angioplasty (BPA) and medical therapy have emerged as a viable treatments to improve CTEPH management. 5 , 6
The nature of the relationship between the volume of obstructive material and pulmonary hypertension is complex and the degree of secondary distal microvasculopathy affects the correlation. 7 In some patients, despite adequate PEA clearance and/or BPA intervention that addresses the obstructive material, there is inadequate improvement in hemodynamics and poor perfusion persists. 6 , 7 , 8 Very distal subsegmental fibrotic obstructions with microvasculopathy are not amenable to PEA treatment or BPA, and medical therapy remains the treatment of choice for this group of patients. 6 , 7 , 8
The clinical and hemodynamic response to BPA in IC is well established but less is known in postsurgical CTEPH cohorts. In this study we compared the hemodynamic and clinical benefit of BPA of patients PP and those with IC treated at an experienced surgical center. We hypothesized that patients PP would be poorer responders and explored the relationship between surgical clearance, quantified by surgical specimen retrieved and PP CT imaging, and subsequent BPA response.
METHODS
Consecutive patients with IC or residual PH PP surgery on stable PH‐targeted medical therapy who were deemed eligible for BPA at a multi‐disciplinary meeting, guided by sub‐selective pulmonary angiography and cross sectional imaging, and who then underwent BPA at the UK National BPA Center, Royal Papworth Hospital, Cambridge, between October 2015 and January 2021 were included in this observational cohort study. Data were collected prospectively as standard of care for clinical service provision and were analyzed retrospectively. No ethical approvals were therefore necessary.
Measures of hemodynamic outcomes
Patients were maintained on PH‐targeted medical therapy for a minimum of 3 months before their first BPA procedure, throughout the periprocedure period and at 3‐month follow‐up (FU). Patients underwent serial right heart catheter (RHC) at baseline, before each BPA procedure and at 3‐month FU. Mean pulmonary artery pressure (mPAP), pulmonary vascular resistance (PVR), cardiac output (CO, via thermodilution) and right atrial pressure (RAP) were assessed. NT‐proBNP, 6‐min walk distance (6MWD), World Health Organization Functional Class (WHO FC) and Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR)—an internationally validated patient‐reported outcome measure designed in a cohort including CTEPH patients that assesses three domains: activity, quality of life (QoL) and symptoms 9 were assessed at baseline and 3‐month FU.
BPA procedure
The BPA procedural details have previously been described. 10 BPA was performed via the right femoral vein using a 6 French sheath under local anesthesia by the same experienced team of two interventional cardiologists. Unfractionated heparin (70−100 IU/kg) was administered. The type, location and burden of disease treated was at operator discretion but in a single session, multiple lesions were targeted but only a single lung was treated. Selective angiography of all segmental vessels was commonly performed to find treatable disease not apparent on nonselective angiography. Cessation of BPA treatment course was also at operator discretion, often determined when all lesions had been treated or when the risk: benefit ratio of continuing was likely to be prohibitive, particularly when mPAP was below the prognostically important threshold of 38 mmHg. 4 Serial BPA procedures were performed monthly with FU investigations performed 3‐months after the last BPA session.
PP computed tomography pulmonary angiography (CTPA) assessment
CTPA was routinely performed 3−6 months postoperatively as standard of care for patients PP to assess residual disease burden and response to surgery. The acquisition parameters and pulmonary vascular assessment have been previously described in detail. 11 The PP central residual disease burden was quantified using the modified Qanadli score (post‐op mQS). 11 , 12 For this, the 10 segmental arteries (three branches to both upper lobes, two branches to the middle lobe and lingula, and five branches to both lower lobes) on each side were ascribed a score from 2 (no disease) to 0 (occlusive or multifocal disease) based on the severity of disease in the segmental branch and its first order subsegmental distributaries.
A maximal score of 2 was assigned for a patent segmental artery and its first order subsegmental branches, giving a total possible score of 40. One point was subtracted if there was clear evidence of subsegmental disease, a segmental web stenosis, or segmental partial occlusion. If two or more of these findings were present, a score of 0 was assigned to that segment. Segmental total occlusion assigned a score of 0. In the presence of laminated thrombus of the main or lobar arteries that exceeded 50% stenosis, 1 point was subtracted from the scores for all segmental arterial branches distal to this point. These scores were summed at a patient, lung and lobe level, and divided by the maximum possible score for each of these (40 for patient, 20 for each lung, and between 4 and 10 for each lobe), and multiplied by 100 to give a value that expresses the residual large vessel pulmonary vascular supply as a percentage of normal. CT scans were read by two experienced cardiothoracic radiologists to reach consensus, blinded to clinical data including PEA results and RHC data.
Analysis of PEA specimens
PEA specimens were examined in three principal ways. First, thrombus tails were measured in millimeters based on scaled photographs that were taken after PEA surgery. A key measure of clearance following PEA surgery is total thrombus tail length (TTTL) retrieved and this predicts surgical hemodynamic response (post‐op PVR, r = −0.580, p < 0.0001). 13 The technique we used was based on the one developed by Skoro‐Sajer et al. organized small‐vessel thrombi qualified for measurement if they were greater than 2 mm in length and less than 3 mm in width. 13 The person analyzing the specimens was blinded to hemodynamic data. Second, a Jamieson classification was allocated to each specimen; this designation was the surgeon's decision and found in the contemporaneous surgical notes. Thirdly, a note was made of whether the specimen had broken during surgery; this information was also found in the surgeon's notes.
Statistical analysis
Statistical analyses were performed using statistical computing environment R version 4.0.3 and GraphPad Prism version 9. Continuous variables are expressed as mean ± SD, or by median and interquartile range in the event of skewed distribution. Data were analyzed using either an independent student's t test, for normally distributed data, or Mann−Whitney U test, if the data was not normally distributed. Pearson's correlation coefficient was used when the data was normally distributed and the relationship between the variables was linear; whereas Spearman's rank correlation was used if the variables were rank‐ordered and not normally distributed. Categorical variables were analyzed using χ² test. Statistical significance was set at p < 0.05 for all analyses undertaken.
RESULTS
Baseline characteristics and hemodynamics
A total of 109 patients were included in the retrospective study, 89 patients had IC and 20 patients were PP. PEA surgery had been completed between August 2007 and May 2019 with a mean of 1410.8 ± 1178.5 days between PEA surgery and BPA. During this period, a total of 1694 PEA surgeries were performed at our institution. The PP group saw an improvement in pulmonary haemodynamics after surgery (mPAP: 47.7 ± 7 to 38.4 ± 9.0 mmHg, p < 0.001, CO: 3.7 ± 1.1 to 4.4 ± 0.8 L/min, p < 0.001 and PVR: 915 ± 343 to 532.3 ± 171.8 dynes.s.cm−5, p < 0.001) although haemodynamic parameters were not normalized and patients remained symptomatic despite medical therapy.
Baseline characteristics and hemodynamic data of the whole cohort and IC and PP subgroups assessed immediately before first BPA are presented in Tables 1 and 2. PP and IC patients do not differ significantly although there was a trend for PP patients to have slightly less severe hemodynamic derangement at baseline, particularly for PVR, but this difference did not reach statistical significance.
Table 1.
Baseline characteristics.
| Overall Population (n = 109) | IC Group (n = 89) | PP group (n = 20) | p Value | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, years | 64.7 ± 11.1 | 65.1 ± 11.4 | 63.3 ± 9.5 | 0.51 |
| BMI, kg/m2 | 26.1 ± 4.8 | 26.1 ± 5.0 | 26.3 ± 4.3 | 0.83 |
| Female, n (%) | 56 (51.4) | 43 (48.3) | 13 (65.0) | 0.18 |
| Medical therapy for PH, n (%) | 104 (95.4) | 84 (94.4) | 20 (100.0) | 0.62 |
| Underlying medial conditions, n (%) | ||||
| Previous PE | 57 (52.3) | 46 (51.7) | 11 (55.0) | 0.79 |
| VC filter | 4 (3.7) | 1 (1.1) | 3 (15.0) | 0.02 |
| Hypertension | 29 (26.6) | 24 (27.0) | 5 (25.0) | 0.86 |
| Dyslipidaemia | 4 (3.7) | 4 (4.5) | 0 | 0.76 |
| Thrombophilia disorder | 11 (10.1) | 10 (11.2) | 1 (5.0) | 0.67 |
| Gynaecological disorder | 13 (11.9) | 11 (12.4) | 2 (10.0) | 0.93 |
| Thyroid disorder | 17 (15.6) | 12 (13.5) | 5 (25.0) | 0.35 |
| Psychiatric disorder | 13 (11.9) | 9 (10.1) | 4 (20.0) | 0.39 |
| Cancer | 17 (15.6) | 13 (14.6) | 4 (20.0) | 0.80 |
| Splenectomy | 17 (15.6) | 15 (16.9) | 2 (10.0) | 0.67 |
| Exercise and QoL at baseline | ||||
| 6‐MWD, m | 350.9 ± 116.3 | 350.3 ± 115.2 | 353.3 ± 125.3 | 0.93 |
| WHO FC (I/II/III/IV), n | 0/35/73/1 | 0/30/58/1 | 0/5/15/0 | 0.62 |
| CAMPHOR symptom score | 10.3 ± 5.7 | 10.7 ± 5.6 | 8.5 ± 5.6 | 0.12 |
Note: Continuous data are presented as mean ± SD, whilst discrete data are presented as data counts (%).
Abbreviations: 6‐MWD, 6 min walk distance; BMI, body mass index; CAMPHOR, Cambridge Pulmonary Hypertension Outcome Review; PE, pulmonary embolism; VC, vena cava; WHO FC, World Health Organization Functional Class.
Table 2.
Baseline hemodynamic data immediately before BPA intervention.
| Overall Population (n = 109) | IC Group (n = 89) | PP group (n = 20) | p Value | |
|---|---|---|---|---|
| Hemodynamic data at baseline | ||||
| PVR, dynes.s.cm−5 | 609.0 ± 256.5 | 628.5 ± 271.8 | 532.3 ± 171.8 | 0.09 |
| mPAP, mmHg | 42.3 ± 10.4 | 43.0 ± 10.6 | 38.4 ± 9.0 | 0.14 |
| COT, L/min | 4.5 ± 1.0 | 4.5 ± 1.0 | 4.4 ± 0.8 | 0.82 |
| RAP | 8.0 ± 4.1 | 7.9 ± 3.6 | 8.3 ± 5.9 | 0.69 |
| RVEDP | 10.6 ± 5.0 | 10.7 ± 4.8 | 9.9 ± 6.0 | 0.52 |
| PCWP | 10.1 ± 3.2 | 9.9 ± 2.8 | 11.1 ± 4.4 | 0.12 |
Abbreviations: COT, cardiac output by thermodilution; mPAP, mean pulmonary artery pressure; PA Sat, pulmonary artery O2 saturation; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RVEDP, right ventricular end diastolic pressure.
Procedural data and hemodynamic changes after BPA
The hemodynamic outcomes for IC and PP patient groups is presented in Table 3. Neither the total number of BPA procedures undertaken nor the total number of segments treated differed between the IC and PP groups. The degree of distal disease treated (quantified by the use of smaller balloon size) was also not different between groups. There was also no significant difference in the number of occlusive type lesions successfully treated by BPA, a lesion subset which is associated with larger hemodynamic impact, 14 between the two groups. Nevertheless, IC patients appeared to derive greater hemodynamic benefit from BPA; the percentage decrease in PVR and mPAP in the IC group was greater than the decrease observed in the PP group (PVR: −27.9 ± 20.2% vs. −12.7 ± 29.1%, p < 0.05; mPAP: −17.1 ± 14.4% vs. −7.3 ± 21.2%, p < 0.05; Figure 1).
Table 3.
Procedural and hemodynamic data at 3 months post‐BPA follow‐up.
| Overall population (n = 109) | IC group (n = 89) | PP group (n = 20) | p Value | |
|---|---|---|---|---|
| Procedural data | ||||
| Total number of BPA sessions | 2.57 ± 1.0 | 2.64 ± 1.1 | 2.25 ± 0.6 | 0.12 |
| Total segments treated | 5.96 ± 2.1 | 6.09 ± 2.2 | 5.40 ± 1.9 | 0.19 |
| Total occlusive type lesions, n (%) | 76 (28.3) | 62 (27.6) | 14 (31.8) | 0.97 |
| Proportion <4 mm balloons per patient | 0.24 ± 0.3 | 0.25 ± 0.3 | 0.24 ± 0.3 | 0.91 |
| Number of lesions where <4 mm balloon used, n (% total) | 66 (24.3) | 55 (24.3) | 11 (23.9) | 0.95 |
| Hemodynamic data at 3 m FU | ||||
| PVR, dyn s cm−5 | 434.1 ± 177.7 | 429.1 ± 177.5 | 454.4 ± 182.3 | 0.55 |
| mPAP, mmHg | 35.0 ± 8.3 | 35.1 ± 8.3 | 35.2 ± 9.2 | 0.96 |
| CO, L/min | 4.8 ± 1.0 | 4.8 ± 1.1 | 4.7 ± 0.9 | 0.95 |
| Exercise and symptom data at 3 m FU | ||||
| 6‐MWD, m | 395.4 ± 110.8 | 401.3 ± 110.7 | 363.4 ± 110.8 | 0.30 |
| WHO FC (I/II/III/IV), n | 13/59/35/2 | 13/53/23/0 | 0/6/12/2 | <0.01 |
| CAMPHOR symptom score | 6.9 ± 6.7 | 6.4 ± 6.7 | 9.1 ± 6.6 | 0.18 |
Abbreviations: 6‐MWD, 6 min walk distance; CAMPHOR, Cambridge Pulmonary Hypertension Outcome Review; COT, cardiac output by thermodilution; mPAP, mean pulmonary artery pressure; PVR, pulmonary vascular resistance; WHO FC, World Health Organization Functional Class.
Figure 1.
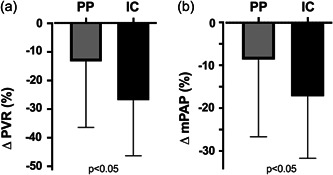
Comparison of the percentage change in (a) PVR and (b) mPAP from baseline to the 3 month post‐BPA follow‐up in inoperable CTEPH (IC) and post‐PEA (PP) patient groups. CTEPH, chronic thromboembolic pulmonary hypertension; mPAP, mean pulmonary artery pressure; PEA, pulmonary endarterectomy; PVR, pulmonary vascular resistance.
In addition, PP patients had a less improvement in WHO FC post‐BPA compared to IC patients (Figure 2) and indeed two deteriorated to FC IV in the PP group despite BPA. Also, whereas the CAMPHOR symptom scores improved from baseline to FU in IC patients (10.7 ± 5.6 vs. 6.4 ± 6.7, p < 0.05), in the PP group, there was no change in CAMPHOR symptom scores at FU (9.3 ± 5.0 vs. 9.1 ± 6.6, p = 0.92; Figure 3). There was a trend to less improvement in 6MWD among PP patients compared to IC patients (Δ6MWD [m]: IC +35.9 ± 68.7 vs. PP +12.3 ± 25.0, p = 0.32).
Figure 2.
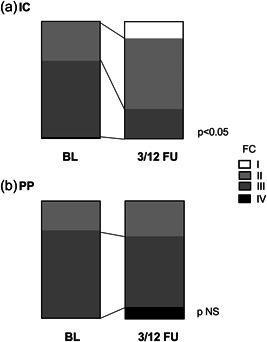
WHO Functional Class (FC) at baseline and follow‐up between (a) inoperable CTEPH (IC) and (b) post‐PEA (PP) patient groups. CTEPH, chronic thromboembolic pulmonary hypertension; mPAP, mean pulmonary artery pressure; PEA, pulmonary endarterectomy; PVR, pulmonary vascular resistance.
Figure 3.
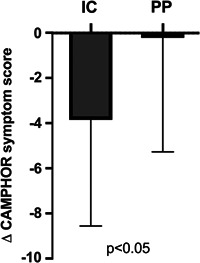
Comparison of the change in CAMPHOR symptom score from baseline to the 3 month post‐BPA follow‐up in inoperable CTEPH (IC) and post‐PEA (PP) patient groups. CAMPHOR, Cambridge Pulmonary Hypertension Outcome Review; CTEPH, chronic thromboembolic pulmonary hypertension; mPAP, mean pulmonary artery pressure; PEA, pulmonary endarterectomy; PVR, pulmonary vascular resistance.
Predictors of BPA hemodynamic outcomes in PP patients
A comparison of hemodynamics after PEA surgery (immediately before BPA) and 3 months after BPA confirmed modest, nonsignificant differences in PP patients (PVR: 532.3 ± 171.8 vs. 454.4 ± 182.3, p = 0.17; mPAP: 38.4 ± 9.0 vs. 35.2 ± 9.2, p = 0.27 and CO: 4.4 ± 0.8 vs. 4.7 ± 0.9, p = 0.32). There was no relationship with time interval between PEA and BPA, and BPA response (ΔPVR vs. days between PEA to BPA: r = −0.08, p = 0.70).
The mean TTTL retrieved surgically during PEA was 131.4 ± 60.6 mm and the mean Jamieson classification recorded was 2.6 ± 0.6 for the PP patient cohort. A quarter of specimens broke during surgery but this occurrence was not inversely related to TTTL (p = 0.39) or Jamieson Class (p = 0.90). The mean post‐op mQS in the PP patients selected for BPA was 52.6 ± 18.3%. There was no relationship between post‐op mQS before BPA and TTTL (r = 0.06, p = 0.81).
We confirmed that there was a negative correlation between PP (pre‐BPA) PVR and TTTL (r = −0.47, p < 0.05) which persisted post‐BPA (Figure 4a,c). Although there was no discernible association between pre‐BPA mPAP and TTTL, a negative correlation was apparent after BPA (Figure 4b,d). We also demonstrated that BPA hemodynamic response was not greater in those within the lower TTTL tercile (range 42−96 mm) compared to higher TTTL tercile (range 173−220 mm): ΔPVR −89.0 ± 144.6 versus −28.1 ± 127.5 dynes.s.cm−5, p = 0.42; ΔmPAP −5.1 ± 13.8 versus −4.7 ± 5.6 mmHg, p = 0.94. The middle tercile (range 119−156 mm) showed the greatest decrease in both PVR (−173.2 ± 140.1 dynes.s.cm−5) and mPAP (−8.2 ± 8.9 mmHg).
Figure 4.
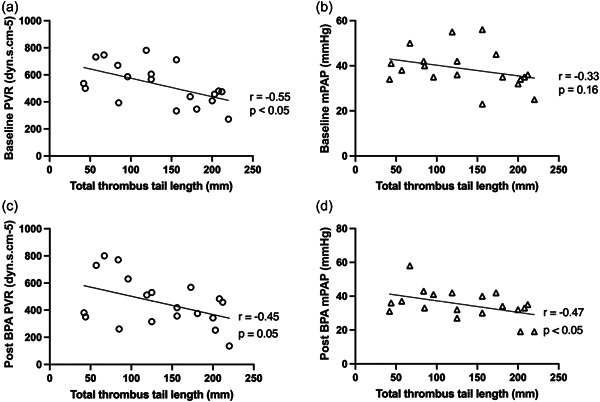
Correlation between total thrombus tail length (TTTL) and (a) pre‐BPA PVR, (b) pre‐BPA mPAP, (c) 3‐month post‐BPA PVR and (d) 3‐month post‐BPA mPAP in post‐PEA (PP) patients (one PP patient TTTL specimen lost). CTEPH, chronic thromboembolic pulmonary hypertension; mPAP, mean pulmonary artery pressure; PEA, pulmonary endarterectomy; PVR, pulmonary vascular resistance.
There was a weak but nonsignificant inverse correlation between the post‐op mQS and PP (immediate pre‐BPA) PVR (r = −0.27, p = 0.23) and mPAP (r = −0.25, p = 0.28). Those with poorer clearance (post‐op mQS 0−49.9%) did not derive greater hemodynamic response from BPA than those with good clearance (post‐op mQS ≥ 50%): ΔPVR −76.4 ± 140.0 versus −79.3 ± 163.6 dynes.s.cm−5, p = 0.97; ΔmPAP −2.8 ± 7.1 versus −4.2 ± 8.5 mmHg, p = 0.70. There was also no difference in BPA response in the PP group between those with high TTTL (>100 mm) and low post‐op mQS (<50%)—defined as possible recurrence (n = 7) and those with low TTTL (<100 mm) and low post‐op mQS (<50%)—defined as residual disease (n = 4): ΔPVR −73.6 ± 155.0 versus −81.3 ± 129.6 dynes.s.cm−5, p = 0.94; ΔmPAP −4.6 ± 7.1 versus −7.5 ± 18.4 mmHg, p = 0.71.
DISCUSSION
Our study has demonstrated that: (1) IC patients achieved greater PVR, mPAP and symptomatic improvement following BPA than PP patients; (2) there was no significant interval hemodynamic or symptomatic improvement in PP patients after BPA; (3) TTTL correlated with postoperative PVR but the TTTL/PVR relationship was not abolished by BPA; (4) we were unable to identify any PP patient subgroups that responded more favorably to BPA. Together, our data indicate that BPA as currently performed was less effective in treating residual CTEPH PP in patients from an experienced PEA surgical center.
Targeting intervention to patient subgroups most likely to benefit makes intuitive sense, particularly for procedures like BPA, which expose patients to risk and have associated healthcare costs. BPA for inoperable patients with CTEPH has an established evidence base 10 , 15 , 16 and planned hybrid procedures combining PEA and BPA to different lungs, where BPA targets naïve lesions that have not been endarterectomized, have also reported good results. 17 , 18 , 19 However, BPA for residual PH after PEA, targeting residual material “left behind” after PEA or recurrent disease has reported mixed results, with some groups demonstrating a step wise further reduction in PVR after BPA of up to 25−40%, whereas others describe negligible change or even worsening hemodynamics, as well as being technically more challenging with an increased risk of complications. 14 , 20 , 21 , 22 , 23 This study heterogeneity may be explained by differences in the degree of surgical clearance, the proportion of residual verses recurrent disease treated, differences in lesion type treated with the very distal and often occlusive residual “tails” following experienced surgical clearance perhaps responding less favorably to BPA, differences in the chronicity of the disease and a variable contribution of microvasculopathy, between the reported studies.
Our group has previously noted that 37.5% of nonresponders to BPA (defined as no hemodynamic improvement) had previously undergone PEA. 14 This initial cohort of 6 patients had fewer BPA sessions and segments treated, perhaps indicating a relative paucity of residual treatable disease, compared to other groups who have reported better response to BPA PP. To further our work, we now confirm in a larger PP cohort compared to IC that despite similar demographics, baseline hemodynamics and procedural characteristics, PP patients derive less hemodynamic and symptomatic benefit from BPA which translates to inferior exercise capacity, QoL and WHO FC improvements.
The failure to effect as good an hemodynamic improvement in PP patients despite a similar disease burden, number of sessions, segments and type and location of lesion treated as IC patients who did respond, may have a number of explanations: (i) the amount of untreated surgical disease in our cohort (quantified by post‐op mQS) was modest—centers selecting patients with lower post‐op mQS may achieve different results; (ii) a third of our surgical cohort had presumed recurrence/reocclusion where the clearance measured by TTTL was good but the post‐op mQS was low—this lesion subset may respond to BPA differently from residual disease; and (iii) distal microvasculopathy may be particularly important in PP patients treated in an experienced surgical center, where reocclusion dominates and the residual disease is often very distal. This is supported by Thistlethwaite et al who reported on 202 patients with PVR ranging from 194 to 2950 dynes.s.cm−5 who underwent PEA. Patients with type 3 or 4 (distal) disease, had higher postoperative pulmonary artery systolic pressure (p < 0.0001), and postoperative PVR (p < 0.0001) compared with patients with type 1 or 2 (proximal) disease. 24 The more distal the disease, the more limited the hemodynamic response to PEA, due to technical limitations and distal microvasculopathy. Nevertheless, we found that TTTL and to a lesser extent the post‐op mQS was still inversely correlated with PP PVR, indicating that surgical clearance is important in determining hemodynamic response in PP patients.
Furthermore, Tsuji et al studied predictive factors of BPA outcomes in 35 IC patients with mPAP > 30 mmHg who underwent BPA (average 4.2 ± 1.4 sessions/patient); eight of these patients were non‐responders to BPA. The duration from symptom onset to BPA together with diastolic PAP at baseline, were determined to predict hemodynamic response to BPA. 23 Tsuji et al hypothesized that long‐standing pulmonary hypertension might exacerbate secondary microvasculopathy, and that higher diastolic PAP reflected more extensive small vessel remodeling and in agreement with this, Taniguchi et al. demonstrated that poor subpleural perfusion (PSP) is a key predictor BPA hemodynamic response. 6 , 25 BPA may therefore also have reduced efficacy in patients with prolonged PH and microvascular arteriopathy and although we did not confirm a temporal relationship between interval between PEA and BPA and BPA response in PP patients, this too should be considered.
Although we confirm a clear relationship with TTTL, a measure of surgical clearance proposed by Skoro‐Sajer et al. 13 measured from the postoperative specimen and PVR PP, this relationship persists post‐BPA and is re‐established for mPAP. If BPA was effective PP in those with poorer clearance, this relationship should be attenuated or abolished. The futility of BPA in the PP patients in our cohort is further supported by evidence that post‐BPA PVR and mPAP values were not significantly different from PVR and mPAP measurements taken immediately before BPA and nor did PP patients feel appreciably better after BPA, indeed two deteriorated to FC IV. Finally, we did not discern a better BPA response in specific PP patient subgroups with residual or recurrent disease, defined by the TTTL yield in those with low post‐op mQS, as would be expected if BPA were effective. Although we did not observe a difference between recurrent and residual disease response to BPA in the PP group, based on Virchow's triad, that those with more severe microvasculopathy should be at higher risk of recurrent thrombosis and therefore recurrent disease may be particularly futile to retreat by BPA. Medical therapy is now licensed and may be more effective than BPA in postsurgical patients, particularly when microvasculopathy is believed to be the dominant cause of residual PH. 1
Limitations
This study was a single‐center study with a modest sample size of PP patients, although relative to other published studies of BPA PP, the cohort size was comparable. This in particular will affect any PP subgroup analysis and any conclusions should be cautiously drawn and confirmed in larger cohorts. Interaction between variables also cannot be excluded; multivariate analysis was not possible due to the small sample size. TTTL was measured from scaled photographs and not wet specimens. Some of the thrombus tails were not displayed perfectly straight before being photographed and estimates had to be made which may have led to inaccuracy. We attributed poor clearance to shorter TTTL, as PEA surgery is infrequently offered for low burden (<6 segments) of disease. However, TTTL measurement may not fully represent the surgical clearance and may underestimate the clearance component from removal of bulky disease or opening of pouch occlusions. TTTL was not related to CT evidence of clearance measured by post‐op mQS and although recurrence after PEA may explain this discrepancy, both post‐op mQS and TTTL are not universally accepted as validated tools for surgical clearance, although no other quantitative tools exist. CT was performed at 3 months PP, thus residual versus recurrent disease cannot be accurately discerned. We speculate that microvasculopathy may be a cause of poor BPA response in PP patients. Although we did not quantify microvasculopathy by pressure‐wire or PSP measured by digital subtraction angiography or dual energy CT, PSP is a predictor of poor outcomes PP 26 and remains the subject of future studies. FU data were obtained from standard clinical FU rather than from a dedicated research study, and as a consequence, complete data were not available in all cases. The treating interventionist was also not blinded to the hemodynamic data at the time of BPA and this is a potential confounder, although PP and IC groups received the same number of BPA sessions and segments treated and therefore bias is unlikely to have influenced our data. The number of sessions and subsegmental lesions treated were fewer in our cohort than other series. It is possible that with a more aggressive treatment strategy, a greater treatment effect may have been seen in the PP group, although, as has also been published, at a cost of more complications.
CONCLUSION
We demonstrate that the response to BPA in PP patients is inferior to IC patients in patients treated in an experienced surgical center. We did not detect any subgroup of PP patients where BPA was effective. The justification of BPA in PP patients should be considered carefully as BPA efficacy may be compromised in this group.
AUTHOR CONTRIBUTIONS
Stephen P. Hoole conceived the study. The data were collected by Louise C. Kirkby, Matthew S. Rodgers and Liliana Amaral‐Almeida. Louise C. Kirkby performed the analysis with the assistance of Stephen P. Hoole. All authors (Louise C. Kirkby, Matthew S. Rodgers, Liliana Amaral‐Almeida, Karen Sheares, Mark Toshner, Katherine Bunclark, Aleksandra Bartnik, Dolores Taboada, Choo Ng, Fouad J. Taghavi, Steven Tsui, John E. Cannon, Jonathan R. Weir‐McCall, John G. Coghlan, David P. Jenkins, Joanna Pepke‐Zaba, Stephen P. Hoole) have reviewed and agree with the published manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
ACKNOWLEDGMENTS
The authors would like to acknowledge their patients and the care provided by the catheter laboratory and ward staff at Royal Papworth Hospital.
Kirkby LC, Rodgers MS, Amaral‐Almeida L, Sheares K, Toshner M, Bunclark K, Bartnik A, Taboada D, Ng C, Taghavi FJ, Tsui S, Cannon JE, Weir‐McCall JR, Coghlan JG, Jenkins DP, Pepke‐Zaba J, Hoole SP. Balloon pulmonary angioplasty outcomes in patients previously treated by pulmonary endarterectomy surgery are inferior to those of inoperable patients. Pulm Circ. 2023;13:e12265. 10.1002/pul2.12265
REFERENCES
- 1. Humbert M, Kovacs G, Hoeper MM. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2022;43(38):3618–3731. 10.1183/13993003.00879-2022 [DOI] [PubMed] [Google Scholar]
- 2. Jenkins D, Madani M, Fadel E, D'Armini AM, Mayer E. Pulmonary endarterectomy in the management of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26(143):160111. 10.1183/16000617.0111-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lang I, Meyer BC, Ogo T, Matsubara H, Kurzyna M, Ghofrani HA, Mayer E, Brenot P. Balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26(143):160119. 10.1183/16000617.0119-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cannon JE, Su L, Kiely DG, Page K, Toshner M, Swietlik E, Treacy C, Ponnaberanam A, Condliffe R, Sheares K, Taboada D, Dunning J, Tsui S, Ng C, Gopalan D, Screaton N, Elliot C, Gibbs S, Howard L, Corris P, Lordan J, Johnson M, Peacock A, MacKenzie‐Ross R, Schreiber B, Coghlan G, Dimopoulos K, Wort SJ, Gaine S, Moledina S, Jenkins DP, Pepke‐Zaba J. Dynamic risk stratification of patient long‐term outcome after pulmonary endarterectomy: results from the United Kingdom national cohort. Circulation. 2016;133(18):1761–1771. 10.1161/CIRCULATIONAHA.115.019470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang L, Bai Y, Yan P, He T, Liu B, Wu S, Qian Z, Li C, Cao Y, Zhang M. Balloon pulmonary angioplasty vs. pulmonary endarterectomy in patients with chronic thromboembolic pulmonary hypertension: a systematic review and meta‐analysis. Heart Fail Rev. 2021;26(4):897–917. 10.1007/s10741-020-10070-w [DOI] [PubMed] [Google Scholar]
- 6. Taniguchi Y, Brenot P, Jais X, Garcia C, Weatherald J, Planche O, Fadel E, Humbert M, Simonneau G. Poor subpleural perfusion predicts failure after balloon pulmonary angioplasty for nonoperable chronic thromboembolic pulmonary hypertension. Chest. 2018;154(3):521–531. 10.1016/j.chest.2018.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coghlan JG, Rothman AMK, Hoole SP. Balloon pulmonary angioplasty: state of the art. Intervent Cardiol. 2021;16:e02. 10.15420/ICR.2020.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fedullo P, Kerr KM, Kim NH, Auger WR. Chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2011;183(12):1605–1613. 10.1164/rccm.201011-1854CI [DOI] [PubMed] [Google Scholar]
- 9. Newnham M, Bunclark K, Abraham N, Ali S, Amaral‐Almeida L, Cannon JE, Doughty N, Ng C, Ponnaberanam A, Sheares K, Speed N, Taboada D, Toshner M, Tsui S, Jenkins DP, Pepke‐Zaba J. CAMPHOR score: patient‐reported outcomes are improved by pulmonary endarterectomy in chronic thromboembolic pulmonary hypertension. Eur Respir J. 2020;56(4):1902096. 10.1183/13993003.02096-2019 [DOI] [PubMed] [Google Scholar]
- 10. Hoole SP, Coghlan JG, Cannon JE, Taboada D, Toshner M, Sheares K, Fletcher AJ, Martinez G, Ruggiero A, Screaton N, Jenkins D, Pepke‐Zaba J. Balloon pulmonary angioplasty for inoperable chronic thromboembolic pulmonary hypertension: the UK experience. Open Heart. 2020;7(1):e001144. 10.1136/openhrt-2019-001144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoey ETD, Mirsadraee S, Pepke‐Zaba J, Jenkins DP, Gopalan D, Screaton NJ. Dual‐energy CT angiography for assessment of regional pulmonary perfusion in patients with chronic thromboembolic pulmonary hypertension: initial experience. Am J Roentgenol. 2011;196(3):524–532. 10.2214/AJR.10.4842 [DOI] [PubMed] [Google Scholar]
- 12. Qanadli SD, Hajjam M el, Vieillard‐Baron A. New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography; 2001. www.ajronline.org [DOI] [PubMed]
- 13. Skoro‐Sajer N, Marta G, Gerges C, Hlavin G, Nierlich P, Taghavi S, Sadushi‐Kolici R, Klepetko W, Lang IM. Surgical specimens, haemodynamics and long‐term outcomes after pulmonary endarterectomy. Thorax. 2014;69(2):116–122. 10.1136/thoraxjnl-2013-203746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hug KP, Gerry Coghlan J, Cannon J, Taboada D, Toshner M, Sheares K, Ruggiero A, Screaton N, Jenkins D, Pepke‐Zaba J, Hoole SP. Serial right heart catheter assessment between balloon pulmonary angioplasty sessions identify procedural factors that influence response to treatment. J Heart Lung Transplant. 2021;40(10):1223–1234. 10.1016/j.healun.2021.06.011 [DOI] [PubMed] [Google Scholar]
- 15. Andreassen AK, Ragnarsson A, Gude E, Geiran O, Andersen R. Balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Heart. 2013;99(19):1415–1420. 10.1136/heartjnl-2012-303549 [DOI] [PubMed] [Google Scholar]
- 16. Tanabe N, Kawakami T, Satoh T, Matsubara H, Nakanishi N, Ogino H, Tamura Y, Tsujino I, Ogawa A, Sakao S, Nishizaki M, Ishida K, Ichimura Y, Yoshida M, Tatsumi K. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: A systematic review. Respirat Investigat. 2018;56(4):332–341. 10.1016/j.resinv.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 17. Wiedenroth CB, Liebetrau C, Breithecker A, Guth S, Lautze HJF, Ortmann E, Arlt M, Krombach GA, Bandorski D, Hamm CW, Möllmann H, Mayer E. Combined pulmonary endarterectomy and balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant. 2016;35(5):591–596. 10.1016/j.healun.2015.10.030 [DOI] [PubMed] [Google Scholar]
- 18. Shimura N, Kataoka M, Inami T, Yanagisawa R, Ishiguro H, Kawakami T, Higuchi Y, Ando M, Fukuda K, Yoshino H, Satoh T. Additional percutaneous transluminal pulmonary angioplasty for residual or recurrent pulmonary hypertension after pulmonary endarterectomy. Int J Cardiol. 2015;183:138–142. 10.1016/j.ijcard.2015.01.034 [DOI] [PubMed] [Google Scholar]
- 19. Yanaka K, Nakayama K, Shinke T, Shinkura Y, Taniguchi Y, Kinutani H, Tamada N, Onishi H, Tsuboi Y, Satomi‐Kobayashi S, Otake H, Tanaka H, Okita Y, Emoto N, Hirata K. Sequential hybrid therapy with pulmonary endarterectomy and additional balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. J Am Heart Assoc. 2018;7(13):e008838. 10.1161/JAHA.118.008838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Araszkiewicz A, Darocha S, Pietrasik A, Pietura R, Jankiewicz S, Banaszkiewicz M, Sławek‐Szmyt S, Biederman A, Mularek‐Kubzdela T, Lesiak M, Torbicki A, Kurzyna M. Balloon pulmonary angioplasty for the treatment of residual or recurrent pulmonary hypertension after pulmonary endarterectomy. Int J Cardiol. 2019;278:232–237. 10.1016/j.ijcard.2018.10.066 [DOI] [PubMed] [Google Scholar]
- 21. Kurzyna M, Darocha S, Koteja A, Pietura R, Torbicki A. Editorial balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Advances in Intervent Cardiol. 2015;1:1–4. 10.5114/pwki.2015.49176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maneiro Melon NM, Velazquez Martin M, Huertas Nieto S, Albarran Gonzalez‐Trevilla A, Sarnago Cebada F, Cruz Utrilla A, Hinojosa Camargo W, Aguilar Colindres R, Melendo Viu M, Lopez Gude MJ, Morales Ruiz R, Perez Nuñez M, Arribas Ynsaurriaga F, Escribano Subias P. Effectiveness and safety of balloon pulmonary angioplasty for the treatment of patients with persistent pulmonary hypertension after pulmonary endarterectomy. J Clin Med. 2023;12(3):905. 10.3390/jcm12030905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ito R, Yamashita J, Sasaki Y, Ikeda S, Suzuki S, Murata N, Ogino H, Chikamori T. Efficacy and safety of balloon pulmonary angioplasty for residual pulmonary hypertension after pulmonary endarterectomy. Int J Cardiol. 2021;334:105–109. 10.1016/j.ijcard.2021.04.013 [DOI] [PubMed] [Google Scholar]
- 24. Thistlethwaite PA, Mo M, Madani MM, Deutsch R, Blanchard D, Kapelanski DP, Jamieson SW. Operative classification of thromboembolic disease determines outcome after pulmonary endarterectomy. J Thorac Cardiovasc Surg. 2002;124(6):1203–1211. 10.1067/mtc.2002.127313 [DOI] [PubMed] [Google Scholar]
- 25. Tsuji A, Ogo T, Ueda J, Fukui S, Morita Y, Fukuda T, Nakanishi N, Ogawa H, Yasuda S. Predictors of residual pulmonary hypertension after balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. Int J Cardiol. 2017;226:118–120. 10.1016/j.ijcard.2016.09.132 [DOI] [PubMed] [Google Scholar]
- 26. Tanabe N, Sugiura T, Jujo T, Sakao S, Kasahara Y, Kato H, Masuda M, Tatsumi K. Subpleural perfusion as a predictor for a poor surgical outcome in chronic thromboembolic pulmonary hypertension. Chest. 2012;141:929–934. [DOI] [PubMed] [Google Scholar]


