Abstract
Aims
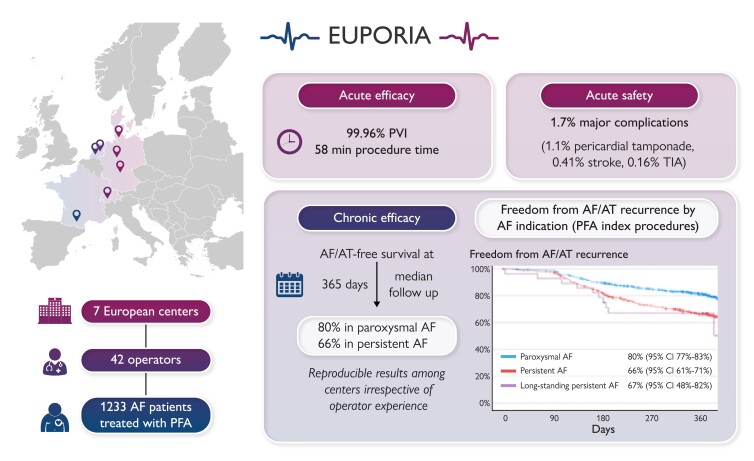
Pulsed field ablation (PFA) is a new, non-thermal ablation modality for pulmonary vein (PV) isolation in patients with atrial fibrillation (AF). The multi-centre EUropean Real World Outcomes with Pulsed Field AblatiOn in Patients with Symptomatic AtRIAl Fibrillation (EU-PORIA) registry sought to determine the safety, efficacy, and learning curve characteristics for the pentaspline, multi-electrode PFA catheter.
Methods and results
All-comer AF patients from seven high-volume centres were consecutively enrolled. Procedural and follow-up data were collected. Learning curve effects were analysed by operator ablation experience and primary ablation modality. In total, 1233 patients (61% male, mean age 66 ± 11years, 60% paroxysmal AF) were treated by 42 operators. In 169 patients (14%), additional lesions outside the PVs were performed, most commonly at the posterior wall (n = 127). Median procedure and fluoroscopy times were 58 (interquartile range: 40–87) and 14 (9–21) min, respectively, with no differences due to operator experience. Major complications occurred in 21/1233 procedures (1.7%) including pericardial tamponade (14; 1.1%) and transient ischaemic attack or stroke (n = 7; 0.6%), of which one was fatal. Prior cryoballoon users had less complication. At a median follow-up of 365 (323–386) days, the Kaplan–Meier estimate of arrhythmia-free survival was 74% (80% for paroxysmal and 66% for persistent AF). Freedom from arrhythmia was not influenced by operator experience. In 149 (12%) patients, a repeat procedure was performed due to AF recurrence and 418/584 (72%) PVs were durably isolated.
Conclusion
The EU-PORIA registry demonstrates a high single-procedure success rate with an excellent safety profile and short procedure times in a real-world, all-comer AF patient population.
Keywords: Ablation, Atrial fibrillation, Pulsed field ablation, Electroporation
Graphical Abstract
Graphical abstract.
What’s new?
European multi-centre registry data on outcomes after pulsed field ablation (PFA) atrial fibrillation ablation detailed per operator experience and operator primary ablation technology.
Freedom from atrial arrhythmia recurrence is not influenced by operator experience.
Operators with single shot device ablation experience seem to adopt PFA ablation quicker and experience less major complications during the learning phase.
Introduction
Atrial fibrillation (AF) is a growing global epidemic with substantial health economic burden. Increased awareness, advanced detection, and life expectancy contribute to the growing number of AF patients. An estimated 14–17 million Europeans will suffer from AF by 2030, and the expected number of new cases of AF per year will be 120 000–215 000.1 Patients with AF have an increased risk of stroke, morbidity, and hospitalization, which places significant strain on an already overburdened healthcare system,2 demonstrating the need for effective, safe, and readily available therapies.
Recent pivotal studies demonstrated catheter ablation as an effective first-line therapy in AF treatment3–5 and as a means to slow AF progression.6 With growing AF prevalence, the increased demand on electrophysiology labs necessitates continuous advancements in safe, effective, and efficient AF treatment strategies that allow for seamless adoption in clinical practice.7
Pulsed field ablation (PFA) is a new ablation modality for cardiac arrhythmias. Myocardium is characterized by a high susceptibility towards PFA in comparison to surrounding tissue.8–10 This opens a broad therapeutic window composed of high efficacy (myocardial damage) with little to no collateral damage. Pulsed field ablation ‘tissue selectivity’ was confirmed in pre-clinical and clinical studies showing low vulnerability of nerves, vasculature, and oesophageal tissue to PFA.11–16
A dedicated ‘single shot’ pulmonary vein isolation (PVI) device that obtained CE mark in Europe in January 2021 was the Farapulse™ PFA ablation system (Boston Scientific, Menlo Park, CA, USA). Since its commercial release, the pentaspline, multi-electrode PFA catheter has shown encouraging acute efficacy and safety profiles.13,17–22 Feasibility studies and early single-centre experiences have demonstrated lesion durability, safety, and initial long-term outcomes.13,17,19–25 However, real-world outcomes in large patient populations are still scarce.18 Chronic data are needed to further evaluate the use of this novel technology in a real-world setting and understand the learning curve. The aim of this registry is to describe real-world adoption, workflow, and acute and long-term outcomes after PFA in AF patients in high-volume European centres.
Methods
The study was approved by the Frankfurt ethics committee (2023-3251-evBO) and complies with the declaration of Helsinki. It was registered at clinical trials.gov (NCT05823818). The study device is CE marked.
Centres
All centres involved in this study are high-volume AF ablation centres in Europe (400–1400 AF ablations per year) that participated in the early market release for the Farapulse PFA technology in Europe. This ensures a high number of patients per centre as well as adequate follow-up time. All cases, including the initial use of the PFA catheter for each operator, were included in this study. Individual data on AF ablation experience were also collected. Operators were divided into three groups with <2, 2–5, and >5 years of AF ablation experience. Moreover, operators were classified as primarily single shot cryoballoon operators, primarily point-by-point ablation radiofrequency (RF) operators, or both.
Patients
All patients who underwent a catheter ablation procedure for symptomatic AF using the Farapulse PFA system from 25 March 2021 until 31 May 2022 were consecutively included in the analysis. No specific inclusion and exclusion criteria were defined.
Data collection
An electronic database was designed to retrospectively collect patient data in a pseudo-anonymized fashion at each participating centre. Data were then transferred to the leading investigational centre for data assembly, data cleaning, and statistical analysis. In the case of missing data, queries were sent to the study centres.
Ablation procedure
The ablation procedures were carried out per each centre’s standard of care. Procedures were performed either under general anaesthesia or deep sedation using a continuous propofol infusion. Procedural guidance varied between centres, with some using 3D electroanatomical mapping (EAM) while others used the pentaspline catheter with only fluoroscopic guidance.
The Farawave™ ablation catheter was introduced into the left atrium (LA) via a steerable sheath (13.0 F inner diameter; Faradrive™) and was navigated over-the-wire to the desired ablation area. For ablation, PFA applications were delivered using the generator (Farastar™) with a voltage output of 1.8–2.0 kV. Energy applications were delivered as a biphasic waveform on a microsecond scale, unsynchronized to cardiac rhythm.26 A group of five consecutive pulse trains was delivered, accounting for a total of 2.5 s ablation time per PFA application. Pulsed field ablation lesion sets were performed based on institution standard of care. During conduct of this study, the use of the Farapulse PFA System for the treatment of non-paroxysmal AF and for extra-PVI ablation is outside of the labelled indication.
Follow-up
Follow-up for subjects was based on each institution’s standard practice; generally, outpatient visits including 24–120 h Holter monitoring were performed at 3, 6, and 12 month follow-up. Data on individual patient follow-up schedule were not recorded. Any episode of atrial tachycardia (AT) or AF lasting more than 30 s was considered an arrhythmia recurrence.
Major adverse clinical events including tamponade, air embolism, stroke, transient ischaemic attack, atrio-oesophageal fistula, and death were captured. The relevance of each adverse event to the device and/or procedure was determined by the participating centre. Moreover, information on antiarrhythmic drugs (AADs) and oral anticoagulation status was collected.
Repeat ablation
Patients with symptomatic AT/AF recurrences underwent a repeat mapping and ablation procedure. Procedures were performed using a 3D EAM system, and PVI durability was assessed using multipolar mapping catheters. Moreover, the durability of extra-pulmonary vein (PV) ablation lesion sets was investigated (i.e. conduction block of linear lesions or durable posterior wall isolation). Subsequently, the AT mechanism was analysed and categorized as either lesion-associated AT (e.g. the critical AT isthmus or AT focus was adjacent to the PFA lesion set) or substrate-associated AT (e.g. the critical AT isthmus or AT focus was located within pre-existing low-voltage areas). Repeat ablation was carried out using commercially available irrigated RF ablation catheters.
Statistical analysis
All categorical variables, such as patient and procedural characteristics, are reported as absolute and relative frequencies and were compared using Fisher’s exact test. The continuous variables were tested for normal distribution using the Shapiro–Wilk test. They were reported as mean ± standard deviation in case of normal distribution and as median and interquartile range (first quartile, third quartile) otherwise. The continuous variables were compared using the non-paired Student’s t-test when normally distributed and the corresponding non-parametric test (Mann–Whitney U test) otherwise. The procedure time comparisons were performed using a Kruskal–Wallis test.
The association between variables and arrhythmia recurrence was assessed using binary logistic regression and was reported as odds ratio (OR) and 95% confidence intervals (CIs). The variables with a P < 0.05 in the univariable model were included in a multivariable binary logistic regression model. Parameters with perfect collinearity were excluded from the logistic regression analysis and were reported descriptively.
Multivariate analysis modelled on the probability of a recurrence was performed using a Cox regression model.
All P-values are two sided. A P-value of <0.05 was considered significant. All statistical analyses were performed using SPSS version 28.0 (IBM SPSS Statistics).
Results
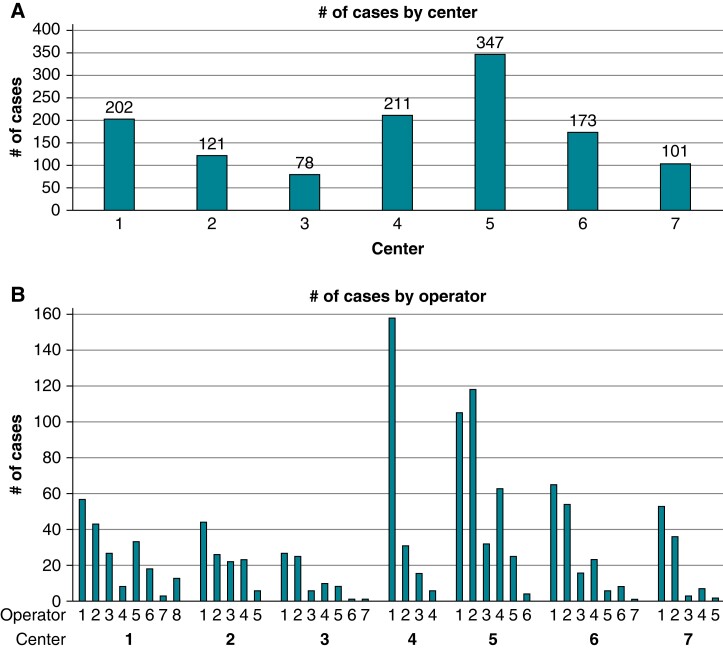
At seven participating centres, 1233 patients were treated by 42 operators. The number of patients treated ranged from 78 to 347 per centre and from 1 to 158 per operator (Figure1A and B). The median number of ablation procedures per operator was 23 (6–35). Of the 42 operators, 3 (7%), 13 (31%), and 26 (62%) reported <2, 2–5, and >5 years of experience in AF ablation, respectively. The primary ablation modality of the operators was point-by-point RF ablation in 11 (26%) operators, cryoballoon in 13 (31%) operators, and both in 18 (43%) operators.
Figure 1.
Overview of EUropean Real World Outcomes with Pulsed Field AblatiOn in Patients with Symptomatic AtRIAl Fibrillation (EU-PORIA) patients and distribution per centre and per operator. (A) Enrolment per centre. (B) Catheter ablation procedures per operator. (C) Operator experience and primary ablation modality.
Details of the patient characteristics are given in Table 1. In brief, mean age was 66 ± 11 years, and 478/1233 (39%) patients were female. The mean CHA2DS2-VASc score was 2.3 ± 1.6. Patients presented with paroxysmal, persistent, and long-standing persistent AF in 60%, 37%, and 3% of cases, respectively. For 65 patients, information on prior AAD use was unavailable, but in 647/1168 (55%) patients, ablations were carried out without current or previous use of membrane active AADs. Of the 1233 PFA ablation procedures performed, 1184 (96%) were index procedures and 49 (4%) were repeat procedures after an initial thermal ablation.
Table 1.
Patient demographics
| Parameter | N = 1233 |
|---|---|
| Female sex, n (%) | 478 (39) |
| Age (years) | 66 ± 11 |
| Hypertension, n (%) | 668 (54) |
| Diabetes, n (%) | 138 (11) |
| History of stroke/TIA, n (%) | 80 (6) |
| Heart failure, n (%) | 204 (17) |
| Coronary artery disease, n (%) | 154 (12) |
| CHA2DS2-VASc score | 2.3 ± 1.6 |
| BMI (kg/m2) | 28 ± 5 |
| Type of AF, n (%) | |
| Paroxysmal AF | 742 (60) |
| Persistent AF | 457 (37) |
| Long-standing persistent AF | 34 (3) |
| Prior use of Class I or III AAD, n (%) | 521/1168 (45) |
| Left ventricular ejection fraction (%) | 57 ± 10%a |
Data are given as absolute number and frequencies in parenthesis. Mean ± standard deviation are reported.
AAD, antiarrhythmic drug; AF, atrial fibrillation; BMI, body mass index; TIA, transient ischaemic attack.
Left ventricular ejection fraction reported in 886 subjects.
Procedural metrics and ablation results
Procedural characteristics are summarized in Table 2. The ablation procedure was carried out under deep sedation or general anaesthesia in 983 (80%) and 250 (20%) of cases, respectively. In 412/1233 (33%) cases, complimentary 3D EAM was used. For ablation, the 31 and the 35 mm device were selected in 947 (77%) and 286 (23%) procedures. A total of 4870/4872 PVs (99.96%) were successfully isolated exclusively using the PFA catheter. In only two PVs (0.04%), irrigated RF touch-up ablation at residual conduction gaps was performed.
Table 2.
Procedural characteristics
| Parameter | N = 1233 |
|---|---|
| First AF ablation, n (%) | 1184 (96) |
| Sedation technique | |
| General anaesthesia, n (%) | 250 (20) |
| Deep sedation, n (%) | 983 (80) |
| Use of 3D mapping, n (%) | 412 (33) |
| No. of PVs isolated/attempted, n (%) | 4870/4872 (99.96) |
| Skin-to-skin procedure time (min) | 58 (40–87) |
| Fluoroscopy time (min) | 14 (9–21) |
| Ablation device useda | |
| 31 mm, n (%) | 947 (77) |
| 35 mm, n (%) | 285 (23) |
| PVI only ablation, n (%) | 1064 (86) |
| Extra-PV ablation | 169 (14) |
| Posterior wall isolation, n (%) | 127 (10) |
| LA isthmus ablation, n (%) | 62 (5) |
| Cavo-tricuspid isthmus ablation, n (%) | 6 (0.5) |
Data are given as number of patients and frequencies in parenthesis. Times are given as median (interquartile range).
AF, atrial fibrillation; LA, left atrium; PV, pulmonary vein; PVI, pulmonary vein isolation.
Ablation device size recorded in 1230 subjects.
In 169 patients (14%), additional lesions were performed, most commonly at the LA posterior wall (n = 127). During the index PFA procedure, ablation beyond PVI was performed in 41/723 (5.7%), 82/433 (18.9%), and 5/28 (17.9%) patients with paroxysmal, persistent, and long-standing persistent AF, respectively. In cases, where PFA was used for the repeat ablation after a prior thermal ablation, lesion sets beyond PVI were used in 12/19 (63.2%), 23/24 (95.8%), and 6/6 (100%) patients with paroxysmal, persistent, and long-standing persistent AF, respectively. The median skin-to-skin procedure time was 58 (40–87) min including a fluoroscopy time of 14 (9–21) min. In uncomplicated PVI-only cases, the median procedure and fluoroscopy times were 52 (38–78) and 13 (8–19) min, respectively. Use of 3D mapping significantly prolonged the median procedure time from 45 (35–60) to 94 (74–120) min (P < 0.0001) and the fluoroscopy time from 11 (7–17) to 20 (15–27) min (P < 0.0001).
Procedural safety
In total, 45 peri-procedural complications were noted (3.6%; Table 3). This included 21 major and 24 minor complications. Pericardial tamponade occurred in 14 cases (1.1%). Of the 14 reported cases, two patients (0.16%) underwent cardiac surgery. All remaining pericardial effusions were drained percutaneously. Root cause analysis of all pericardial tamponade events was performed, and the perforation was attributed to the straight tip guidewire (n = 7; 50%), the diagnostic catheter (n = 3; 21%), the transseptal puncture (n = 3; 21%), and the sheath (n = 1; 7%), respectively. During surgery in one patient, right ventricular perforation by the diagnostic pacing catheter was confirmed, and in one patient, a laceration at the junction of the right superior PV with the LA roof caused by the unprotected sheath was found. Pericardial tamponade occurred in cases performed by 6/42 (14.3%) operators (5 with >5 years AF ablation experience) after a median of 28 (17–78) PFA cases (Table 4). The rate of pericardial tamponade was significantly different between operators based on previous ablation modality. In 9/14 (64%) pericardial tamponades, the operator’s primary ablation modality was point-by-point RF ablation (Table 5).
Table 3.
Procedural complications
| Complications | N = 1233 |
|---|---|
| Major complications, n (%) | 21 (1.7) |
| Pericardial tamponade, n (%) | 14 (1.1) |
| Stroke, n (%) | 5 (0.41)a |
| TIA, n (%) | 2 (0.16) |
| Minor complications, n (%) | 24 (1.9) |
| Vascular access site complication | 12 (0.97) |
| Phrenic nerve dysfunction | 4 (0.32)b |
| Air embolism | 3 (0.24) |
| Coronary spasm | 1 (0.08) |
| Haemoptysis | 1 (0.08) |
| Pericarditis | 2 (0.16) |
| Pneumonia | 1 (0.08) |
Data are given as absolute number of events and frequency.
TIA, transient ischaemic attack.
Including one fatal stroke.
Phrenic nerve function did not recover in one patient by the end of the follow-up.
Table 4.
Outcomes by operator experience
| Year of experience | <2 years 3 operators 11 procedures |
2–5 years 13 operators 281 procedures |
>5 years 26 operators 941 procedures |
P-value |
|---|---|---|---|---|
| Procedural characteristics | ||||
| PVI only, n (%) | 8 (72.7) | 262 (93.2) | 794 (84.3) | <0.0001 |
| 3D mapping, n (%) | 1 (9.1) | 78 (27.8) | 333 (35.4) | 0.0114 |
| General anaesthesia, n (%) | 0 | 33 (11.7) | 217 (23.1) | <0.0001 |
| Index PFA procedure | 10 (90.9) | 276 (98.2) | 898 (95.4) | 0.0400 |
| Type of AF | ||||
| Paroxysmal AF, n (%) | 7 (63.6) | 175 (62.3) | 560 (59.5) | n.s. |
| Persistent AF, n (%) | 4 (36.3) | 100 (35.6) | 353 (37.5) | n.s. |
| Long-standing persistent AF, n (%) | 0 | 6 (2.1) | 28 (3.0) | n.s. |
| Procedure times | ||||
| Skin-to-skin procedure time, min | 51 (46–77) | 50 (38–78) | 60 (40–88) | 0.0878 |
| Fluoroscopy time, min | 19 (14–20) | 12 (7–19) | 15 (9–21) | 0.0011 |
| Safety | ||||
| Complications, n (%) | 0 | 6 (2.1) | 39 (4.1) | 0.2566 |
| Stroke/TIA, n (%) | 0 | 1 (0.4) | 6 (0.6) | 1.0000 |
| Pericardial tamponade, n (%) | 0 | 2 (0.7) | 12 (1.3) | 0.7786 |
| Efficacy | ||||
| PV reconnection rate, n (%) | 0/8 (0) | 38/128 (29.7) | 128/448 (28.6) | 0.2056 |
| Freedom from AF/AT at 12 months, n (%) | 8/11 (72.7) | 200/281 (71.2) | 698/941 (74.2) |
Data are given as absolute number of events and frequency. Times are given as median (interquartile range).
AF, atrial fibrillation; AT, atrial tachycardia; n.s., not specified; PFA, pulsed field ablation; PV, pulmonary vein; PVI, PV isolation; TIA, transient ischaemic attack.
Table 5.
Outcomes by operator primary ablation modality
| Primary ablation technology | Cryoballoon 13 operators 217 procedures |
RF 11 operators 334 procedures |
Both 18 operators 682 procedures |
P-value |
|---|---|---|---|---|
| Procedural characteristics | ||||
| PVI only, n (%) | 187 (86.2) | 288 (86.2) | 589 (86.4) | 1.0000 |
| 3D mapping, n (%) | 9 (4.2) | 120 (35.9) | 283 (41.5) | <0.0001 |
| General anaesthesia, n (%) | 60 (27.6) | 44 (13.2) | 146 (21.4) | <0.0001 |
| Index PFA procedure | 192 (88.4) | 326 (97.6) | 666 (97.7) | <0.0001 |
| Type of AF | ||||
| Paroxysmal AF, n (%) | 134 (61.8) | 221 (66.2) | 387 (56.7) | 0.0136 |
| Persistent AF, n (%) | 72 (33.2) | 109 (32.6) | 276 (40.5) | 0.0224 |
| Long-standing persistent AF, n (%) | 11 (5.1) | 4 (1.2) | 19 (2.8) | 0.0246 |
| Procedure times | ||||
| Skin-to-skin procedure time, min | 59 (50–75) | 71 (45–106) | 51 (34–80) | <0.0001 |
| Fluoroscopy time, min | 15 (11–21) | 18 (13–25) | 11 (7–18) | <0.0001 |
| Safety | ||||
| Complications, n (%) | 4 (1.8) | 15 (4.5) | 26 (3.8) | 0.2409 |
| Stroke/TIA, n (%) | 1 (0.5) | 3 (0.9) | 3 (0.4) | 0.7685 |
| Pericardial tamponade, n (%) | 0 | 9 (2.7) | 5 (0.7) | 0.0058 |
| Efficacy | ||||
| PV reconnection rate, n (%) | 33/98 (33.7) | 62/162 (38.3) | 71/324 (21.9) | 0.0004 |
| Freedom from AF/AT at 12 months, n (%) | 155/217 (71.4) | 252/334 (75.4) | 499/682 (73.2) |
Data are given as absolute number of events and frequency. Times are given as median (interquartile range).
AF, atrial fibrillation; AT, atrial tachycardia; PFA, pulsed field ablation; PV, pulmonary vein; PVI, PV isolation; RF, radiofreuqency; TIA, transient ischaemic attack.
In addition, TIA and stroke were noted in two (0.16%) and five (0.41%) patients, respectively. Of the latter, one patient died 4 days after the ablation procedure despite successful thrombectomy.
Minor complications included access site complications in 12 (0.97%) patients. Phrenic nerve palsy, defined as an absent or weakening of the diaphragmatic contraction, was observed in four (0.3%) patients, only one of which had not resolved by the end of the follow-up. In a single patient, a coronary spasm with ST-segment elevation was noted after ablation at the right superior PV, which completely resolved after intracoronary nitroglycerin injection.
PFA for repeat ablation procedures
In three centres, the pentaspline PFA catheter was used for repeat ablation of patients with recurrent tachyarrhythmias after an index thermal ablation. Data on 49 repeat procedures (4% of total cohort) were collected and analysed. This included 19, 24, and 6 patients with paroxysmal, persistent, and long-standing persistent AF, respectively. In 8/49 (16%) patients, a PVI-only ablation strategy was performed. Extra-PV ablation was carried out in the majority of patients (41/49; 84%). Peri-procedural complications occurred in two (4%) patients, including TIA (n = 1) and vascular access site complication (n = 1), respectively.
Follow-up
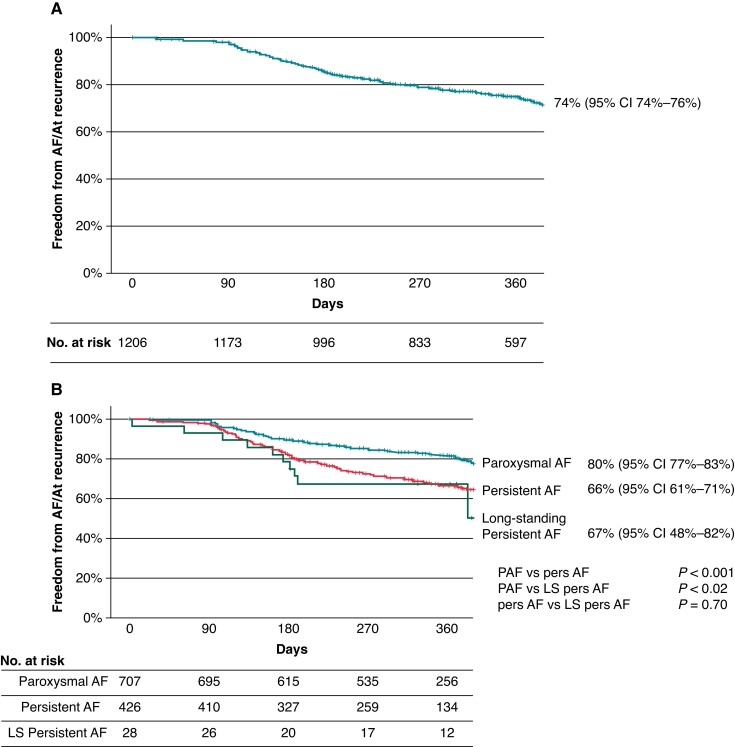
At a median follow-up time of 365 (323–386) days, the Kaplan–Meier estimate of AF/AT-free survival was 74% for the total cohort (Figure 2A). At 12 months follow-up, 70 patients were still on Class I or III AADs, including 54 because of a documented AF/AT recurrence (e.g. a primary endpoint event). The Kaplan–Meier estimate for AF-free survival for patients with an index PFA procedure for paroxysmal, persistent, and long-standing persistent AF was 80, 66, and 67%, respectively (Figure 2B).
Figure 2.
Kaplan–Meier curve of atrial fibrillation (AF)/atrial tachycardia (AT)-free survival for (A) all patients and (B) patients who underwent an index pulsed field ablation (PFA) procedure.
Twenty-seven patients were lost to follow-up. During follow-up, 13 (1.1%) patients died. Three patients (0.2%) experienced a stroke, and one patient (0.08%) experienced a myocardial infarction. No further procedure-related or device-related events occurred, in particular no atrial oesophageal fistulas were noted.
Predictors of arrhythmia recurrence
Multivariate analysis identified CHA2DS2-VaSc score (OR 1.034; CI 1.008–1061; P = 0.01) and body mass index (OR 1.154, CI 1.062–1.255; P = 0.0008) as independent predictors for arrhythmia recurrence (see Supplementary material online, Table S1). As expected, the presence of paroxysmal AF was associated with a favourable outcome (OR 0.573; CI 0.44–0.746; P < 0.001).
Findings during repeat procedures after an index PFA ablation
In 149 (12%) patients, a repeat ablation was performed a median of 226 (157–292) days after the index PFA procedure. The mean age of the patients was 67 ± 10 years, and 57 (38%) were female. The index arrhythmia was paroxysmal AF in 78 (52%) patients, persistent AF in 66 (44%) patients, and long-standing persistent AF in 5 (3%) patients. Of the 149 patients undergoing repeat ablation, 52 (35%) had EAM performed during the index procedure. The 31 or the 35 mm Farawave catheter had been used in 102 (68%) and 47 (32%) procedures, respectively. The ablation strategy was PVI-only in 121 (81%) and PVI plus extra-PV ablation in 28 (19%) patients. In the latter group, the LA posterior wall had been ablated in 22/28 (79%) patients.
The indication for the repeat procedure was AT in 50 (34%) patients and recurrent AF in the remaining 99 (66%) patients. Of the latter, 60 were in sinus rhythm during the repeat ablation procedure. During remapping, 418/584 (72%) of PVs (in one patient, PVs were not mapped due to right-sided AT) were found to be durably isolated. Complete durable PVI (i.e. all PVs in an individual patient) was found in 54/148 (36%) patients. In patients with reconnected PVs, a median of one PV demonstrated a gap in the lesion set. For patients with paroxysmal, persistent, and long-standing persistent AF, PV durability per PV was 232/305 (76%), 174/258 (67%), and 10/20 (50%), respectively. Complete durable PVI was observed in 34/78, 22/66, (33%) and 0/5 patients, respectively.
Of the 50 patients with recurrent AT, 14 (28%) had posterior wall-associated AT, peri-mitral AT occurred in 17 (34%) patients, the others were right AT (n = 2; 4%), left focal AT (n = 7; 14%), or remained unclassified (n = 10; 20%).
Effects of operator experience
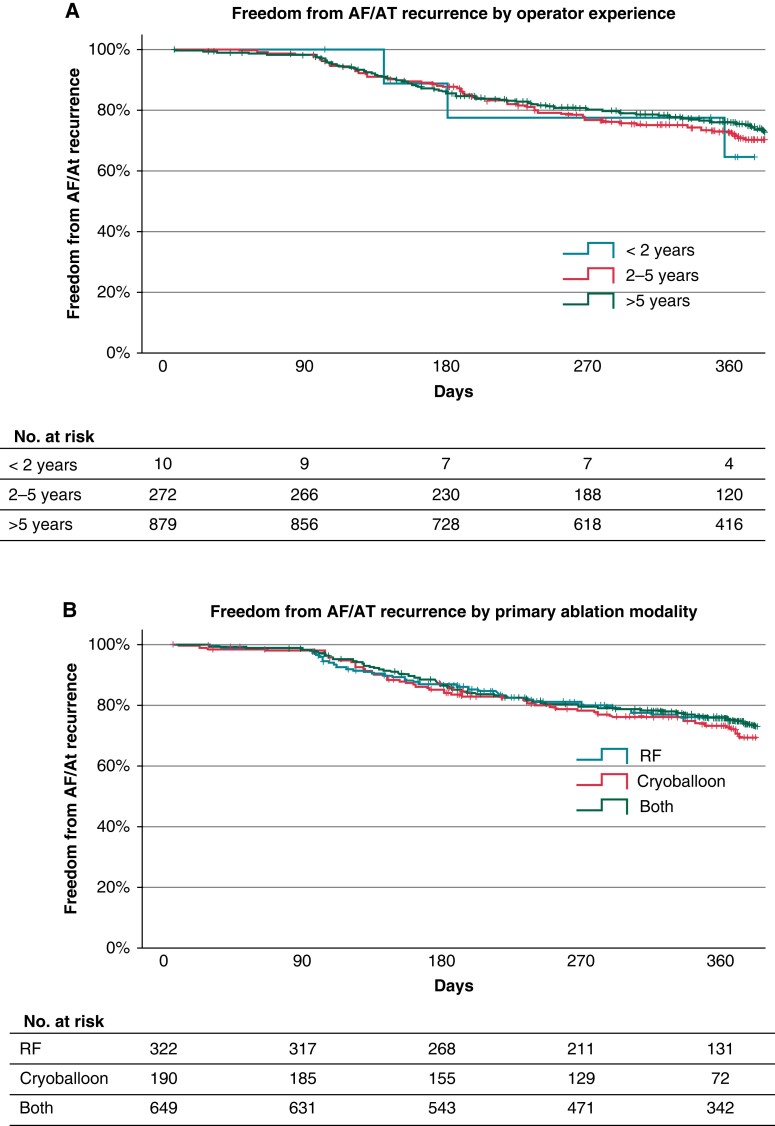
Procedural and outcome data were analysed according to the operator experience with AF ablation. No significant differences were found for procedural metrics or procedural complications (Table 4). When stratifying for the primary ablation modality previously used by each operator, prior cryoballoon users had shorter procedure times and fewer cardiac perforations (Table 5). Neither ablation centre, operator’s AF ablation experience, nor the operator’s previous primary ablation modality had an influence on the AF/AT-free survival during follow-up for patients undergoing an index PFA procedure (Figure 3).
Figure 3.
Kaplan–Meier curves of atrial fibrillation (AF)/atrial tachycardia (AT)-free survival in patients who underwent an index pulsed field ablation (PFA) procedure by (A) operator experience and (B) operator ablation primary modality.
Discussion
The EUropean Real World Outcomes with Pulsed Field AblatiOn in Patients with Symptomatic AtRIAl Fibrillation (EU-PORIA) registry provided real-world outcomes from seven high-volume European AF ablation centres on the early adoption of the novel Farapulse PFA technology. The results demonstrated consistent, short procedure times despite a large number of operators with varied experience. In EU-PORIA, the pentaspline PFA catheter was shown to be a safe and effective treatment strategy in a large spectrum of patients, including paroxysmal and non-paroxysmal AF patients with an overall atrial arrhythmia recurrence free rate of 74% and a safety event rate of 3.6%. A subset of 149 patients (12%) returned for repeat ablation during follow-up. In these patients, EAM revealed a high rate of PVI with 72% of PVs durably isolated.
Workflow and procedural efficiency
The median procedure time for all PFA cases, inclusive of varied indications and institutional workflow, included in this registry was 58 (40–87) min. This procedure time falls within the range of previously published procedure times in a real-world setting for the pentaspline PFA catheter.18,19,22 These procedure times are considerably shorter than typically reported for thermal ablation, which averages 82–128 min for cryoballoon27–29 and 140–162 min point-by-point RF ablation.27,28,30 A most recent single-centre comparison between CB and PFA ablation confirmed a 30% reduction in procedure times with PFA.31 Further, procedure times in the present study were independent of operator’s prior AF ablation experience. This may increase the availability of ablation to symptomatic AF patients outside highly specialized ablation centres, thereby reducing waiting times. However, future randomized studies will have to investigate the non-inferiority of PFA in comparison to established thermal ablation modalities.
Current European Society of Cardiology guidelines for the diagnosis and management of AF provide a Class IA recommendation for first-line ablation therapy only to select patients with heart failure.32 EU-PORIA data suggest that current clinical practice has already changed with 55% of patients with paroxysmal and persistent AF receiving interventional treatment without prior AAD use (specifically Class I or III AAD). This progressive approach to symptomatic AF patients is also reflected by current European surveys, where 42% of operators would favour first-line ablation in PAF patients.33 Scientifically, this is supported by the findings of several randomized studies favouring ablation outcomes over medical therapy.3–5,34 Future growing demand may even prolong the already existing, extensive waiting times for an AF ablation in some geographies.35 Nonetheless, during decision-making with a symptomatic AF patient, the merits and demerits of all treatment options should be carefully considered for an optimal individual counselling. Further studies are needed to answer questions with regards to ablation timing, lesion sets, and workflow to ensure patient safety.
Safety
In this registry, the overall safety event rate was 3.6% with 45 events reported in 1233 subjects. This event rate is similar to those previously reported for real-world experiences with thermal ablation modalities.28–30 In EU-PORIA, the rate of cardiac tamponade was 1.1%. Most of the events were attributable to the learning curve and were mitigated by workflow changes (no pacing catheter in the right ventricle, introduction of J-tip guidewire) during the course of the study. Overall, one patient died from a peri-procedural stroke in the early phase of the study. Aside from uninterrupted anticoagulation strategies, careful sheath management to avoid air embolism is critical. In this context, repeated catheter exchanges through the steerable sheath should be avoided, in particular since 3D mapping did not translate to improved procedural outcomes. Several ongoing studies will directly compare PFA to thermal ablation and will provide further insights into the safety profiles.
Efficacy
Although this registry reflects the very early European experience, including learning curves for all operators, the observed arrhythmia free survival rates may be comparable to thermal ablation technologies.28–30 The reported observations from this registry are preliminary, and PFA needs to prove non-inferiority towards standard of care, thermal ablation. Randomized studies are keys to further investigate its role in the landscape of ablation technologies. This holds also true for studies on different ablation strategies including PVI vs. PVI plus extra-PV ablation. Most recently, a pilot study investigating a new multipolar circular PFA catheter demonstrated similar effectiveness rates and most importantly a very low adverse event rate of 0.7%.36
Lesion durability
In EU-PORIA, 149 repeat ablation procedures were performed and subject to analysis. One key performance parameter of an ablation modality is durable PV isolation. For the Farapulse PFA system, PVI durability rates of 96% were reported in patients with planned re-mapping regardless of arrhythmia recurrences.13 In a recent single-centre study, patients who were re-mapped due to clinical arrhythmia recurrences had a PVI durability rate of 91%.37 In EU-PORIA, 72% of all re-mapped PVs remained durably isolated. In comparison, a recently published pilot study using a variable loop circular PFA catheter had 13 patients returning for repeat ablation procedures and had a PV durability rate of 27% (13/49 PVs).38 With thermal ablation in the CIRCA-DOSE trial, 112/201 (56%) of PVs exhibited durable PV isolation.39 This real-world dataset provides additional evidence supporting the PVI ablation workflow with the pentaspline PFA catheter. Future studies systematically evaluating the lesion durability will be needed to directly compare across modalities.
In this clinical experience, the use of 3D mapping with the pentaspline catheter did not improve lesion durability. However, future full integration allowing for simulation of the electrical field within the acquired 3D map may be beneficial.
Learning curve
Several studies have shown a close relationship between centre volume and safety. An annual procedural volume of <74 ablation procedures per year was significantly associated with an increase in adverse outcomes.40 In the present registry, no difference in complication rate between experienced (>5 years) and less experienced (<5 years) operators was found. Similarly, operator experience had no influence on efficacy in terms of arrhythmia-free survival. This may partly be explained by a technically less demanding procedure without the need for PV occlusion as during cryoballoon ablation or achieving pre-defined contact force values for longer periods of time at several locations during a point-by-point RF ablation.
In contrast, the primary ablation modality seems to exert an influence on the adoption speed of the PFA pentaspline catheter. No cardiac tamponade was observed in previous primary cryoballoon operators who may be more used to navigating an over-the-wire device through a large bore steerable sheath. Pulmonary vein isolation durability was also improved which may be a result of better single-shot device positioning at the respective PV ostium.
Limitations
EU-PORIA was designed to evaluate the real-world use and adoption of a novel PFA technology for an all-comer AF patient population. No specified inclusion or exclusion criteria were considered. This was a retrospective, observational study, where AF ablation and patient management were all performed according to standard-of-care at each centre. In particular, follow-up and arrhythmia recurrence monitoring were performed based on each centre’s standard practice and were not recorded for each patient. No data monitoring was applied. Several operators utilized 3D mapping for lesion visualization, but at this time, the current PFA system is not fully integrated into a 3D mapping system. Comparison to prospective studies with rigorous monitoring strategies in regard to effectiveness should therefore be carried out with caution since monitoring strategies may differ substantially. In contrast, most recently, the standard AF recurrence definition of 30 s episode duration has been challenged since clinically relevant increases in healthcare utilization occur only with episodes > 1 h and AF burden > 0.1%.6 Therefore, healthcare utilization parameters like the number of electrical cardioversions, repeat ablations, or hospitalizations should also be taken into account to assess the effectiveness of an ablation modality.
It needs to be highlighted that operators used the PFA device for extra PV ablation in a subset of patients. This is (i) currently outside of the labelled indication for the pentaspline PFA catheter and (ii) current guidelines recommend to reserve extra-PV ablation to select patients only (Class II b).
Following commercialization of a medical device, systematic data collection on safety and efficacy as well as clinical adoption provide important insights into real-world outcomes and may enhance our understanding of its value in everyday clinical practice. In this clinical experience, the observed characteristics of PFA-guided AF ablation, including short operator learning curves, fast procedure times, and favourable 1-year outcomes, may form a solid base for future prospective randomized trials.
Conclusion
The EU-PORIA registry demonstrates a favourable single procedure success rate along with short procedure times in a real-world, all-comer AF patient population. Future randomized, multi-centre trials will compare PFA-guided ablation to thermal ablation modalities to assess its true value for patients with AF.
Supplementary Material
Acknowledgements
We would like to acknowledge Jonathan D. Raybuck, PhD (Boston Scientific), for assistance with drafting and editing the manuscript and Scott Wehrenberg, MS (Boston Scientific), for assistance with data analysis.
Contributor Information
Boris Schmidt, Cardioangiologisches Centrum Bethanien, Wilhelm-Epstein Str. 4, 60431 Frankfurt, Germany; Universitätsklinikum Frankfurt, Medizinische Klinik 3- Klinik für Kardiologie, Theodor-Stern-Kai 7, Frankfurt, Germany.
Stefano Bordignon, Cardioangiologisches Centrum Bethanien, Wilhelm-Epstein Str. 4, 60431 Frankfurt, Germany.
Kars Neven, Department of Electrophysiology, Alfried Krupp Hospital, Essen, Germany; Department of Medicine, Witten/Herdecke University, Witten, Germany.
Tobias Reichlin, Inselspital—Bern University Hospital, University of Bern, Bern, Switzerland.
Yuri Blaauw, Department of Cardiology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Jim Hansen, Arrhythmia Unit, Department of Cardiology, Gentofte Hospital, Copenhagen, Denmark.
Raquel Adelino, Heart Rhythm Department, Clinique Pasteur, Toulouse, France.
Alexandre Ouss, Heart Center Catharina Hospital, Eindhoven, The Netherlands.
Anna Füting, Department of Electrophysiology, Alfried Krupp Hospital, Essen, Germany; Department of Medicine, Witten/Herdecke University, Witten, Germany.
Laurent Roten, Inselspital—Bern University Hospital, University of Bern, Bern, Switzerland.
Bart A Mulder, Department of Cardiology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Martin H Ruwald, Arrhythmia Unit, Department of Cardiology, Gentofte Hospital, Copenhagen, Denmark.
Roberto Mené, Heart Rhythm Department, Clinique Pasteur, Toulouse, France.
Pepijn van der Voort, Heart Center Catharina Hospital, Eindhoven, The Netherlands.
Nico Reinsch, Department of Electrophysiology, Alfried Krupp Hospital, Essen, Germany; Department of Medicine, Witten/Herdecke University, Witten, Germany.
Thomas Kueffer, Inselspital—Bern University Hospital, University of Bern, Bern, Switzerland.
Serge Boveda, Heart Rhythm Department, Clinique Pasteur, Toulouse, France.
Elizabeth M Albrecht, Boston Scientific Corporation, St. Paul, MN, USA.
Christopher W Schneider, Boston Scientific Corporation, St. Paul, MN, USA.
Kyoung Ryul Julian Chun, Cardioangiologisches Centrum Bethanien, Wilhelm-Epstein Str. 4, 60431 Frankfurt, Germany.
Supplementary material
Supplementary material is available at Europace online.
Funding
The EU-PORIA registry was supported by a research grant from Boston Scientific.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol 2014;6:213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meyre P, Blum S, Berger S, Aeschbacher S, Schoepfer H, Briel Met al. . Risk of hospital admissions in patients with atrial fibrillation: a systematic review and meta-analysis. Can J Cardiol 2019;35:1332–43. [DOI] [PubMed] [Google Scholar]
- 3. Andrade JG, Wells GA, Deyell MW, Bennett M, Essebag V, Champagne Jet al. . Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med 2021;384:305–15. [DOI] [PubMed] [Google Scholar]
- 4. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan Aet al. . Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–16. [DOI] [PubMed] [Google Scholar]
- 5. Wazni OM, Dandamudi G, Sood N, Hoyt R, Tyler J, Durrani Set al. . Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med 2021;384:316–24. [DOI] [PubMed] [Google Scholar]
- 6. Andrade JG, Deyell MW, Macle L, Wells GA, Bennett M, Essebag Vet al. . Progression of atrial fibrillation after cryoablation or drug therapy. N Engl J Med 2023;388:105–16. [DOI] [PubMed] [Google Scholar]
- 7. Metzner A, Straube F, Tilz RR, Kuniss M, Noelker G, Tebbenjohanns Jet al. . Electrophysiology lab efficiency comparison between cryoballoon and point-by-point radiofrequency ablation: a German sub-analysis of the FREEZE cohort study. BMC Cardiovasc Disord 2023;23:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moshkovits Y, Grynberg D, Heller E, Maizels L, Maor E. Differential effect of high-frequency electroporation on myocardium vs. non-myocardial tissues. Europace 2023;25:748–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sugrue A, Maor E, Del-Carpio Munoz F, Killu AM, Asirvatham SJ. Cardiac ablation with pulsed electric fields: principles and biophysics. Europace 2022;24:1213–22. [DOI] [PubMed] [Google Scholar]
- 10. Wittkampf FHM, van Es R, Neven K. Electroporation and its relevance for cardiac catheter ablation. JACC Clin Electrophysiol 2018;4:977–86. [DOI] [PubMed] [Google Scholar]
- 11. Koruth J, Kuroki K, Iwasawa J, Enomoto Y, Viswanathan R, Brose Ret al. . Preclinical evaluation of pulsed field ablation: electrophysiological and histological assessment of thoracic vein isolation. Circ Arrhythm Electrophysiol 2019;12:e007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koruth JS, Kuroki K, Kawamura I, Brose R, Viswanathan R, Buck EDet al. . Pulsed field ablation versus radiofrequency ablation: esophageal injury in a novel porcine model. Circ Arrhythm Electrophysiol 2020;13:e008303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reddy VY, Dukkipati SR, Neuzil P, Anic A, Petru J, Funasako Met al. . Pulsed field ablation of paroxysmal atrial fibrillation: 1-year outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin Electrophysiol 2021;7:614–27. [DOI] [PubMed] [Google Scholar]
- 14. Cochet H, Nakatani Y, Sridi-Cheniti S, Cheniti G, Ramirez FD, Nakashima Tet al. . Pulsed field ablation selectively spares the oesophagus during pulmonary vein isolation for atrial fibrillation. Europace 2021;23:1391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakatani Y, Sridi-Cheniti S, Cheniti G, Ramirez FD, Goujeau C, Andre Cet al. . Pulsed field ablation prevents chronic atrial fibrotic changes and restrictive mechanics after catheter ablation for atrial fibrillation. Europace 2021;23:1767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koruth J, Kawamura I, Dukkipati SR, Neuzil P, Reddy VY. Preclinical assessment of the feasibility, safety and lesion durability of a novel ‘single-shot’ pulsed field ablation catheter for pulmonary vein isolation. Europace 2023;25:1369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blockhaus C, Guelker JE, Feyen L, Bufe A, Seyfarth M, Shin DI. Pulsed field ablation for pulmonary vein isolation: real-world experience and characterization of the antral lesion size compared with cryoballoon ablation. J Interv Card Electrophysiol 2023;66:567–75. [DOI] [PubMed] [Google Scholar]
- 18. Ekanem E, Reddy VY, Schmidt B, Reichlin T, Neven K, Metzner Aet al. . Multi-national survey on the methods, efficacy, and safety on the post-approval clinical use of pulsed field ablation (MANIFEST-PF). Europace 2022;24:1256–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Magni FT, Mulder BA, Groenveld HF, Wiesfeld ACP, Tieleman RG, Cox MGet al. . Initial experience with pulsed field ablation for atrial fibrillation. Front Cardiovasc Med 2022;9:959186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reddy VY, Anic A, Koruth J, Petru J, Funasako M, Minami Ket al. . Pulsed field ablation in patients with persistent atrial fibrillation. J Am Coll Cardiol 2020;76:1068–80. [DOI] [PubMed] [Google Scholar]
- 21. Ruwald MH, Johannessen A, Hansen ML, Haugdal M, Worck R, Hansen J. Pulsed field ablation in real-world atrial fibrillation patients: clinical recurrence, operator learning curve and re-do procedural findings. J Interv Card Electrophysiol 2023. [DOI] [PubMed] [Google Scholar]
- 22. Schmidt B, Bordignon S, Tohoku S, Chen S, Bologna F, Urbanek Let al. . 5S Study: safe and simple single shot pulmonary vein isolation with pulsed field ablation using sedation. Circ Arrhythm Electrophysiol 2022;15:e010817. [DOI] [PubMed] [Google Scholar]
- 23. Bohnen M, Weber R, Minners J, Jadidi A, Eichenlaub M, Neumann FJet al. . Characterization of circumferential antral pulmonary vein isolation areas resulting from pulsed-field catheter ablation. Europace 2023;25:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Futing A, Reinsch N, Howel D, Brokkaar L, Rahe G, Neven K. First experience with pulsed field ablation as routine treatment for paroxysmal atrial fibrillation. Europace 2022;24:1084–92. [DOI] [PubMed] [Google Scholar]
- 25. Kueffer T, Baldinger SH, Servatius H, Madaffari A, Seiler J, Muhl Aet al. . Validation of a multipolar pulsed-field ablation catheter for endpoint assessment in pulmonary vein isolation procedures. Europace 2022;24:1248–55. [DOI] [PubMed] [Google Scholar]
- 26. Reddy VY, Neuzil P, Koruth JS, Petru J, Funosako M, Cochet Het al. . Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardiol 2019;74:315–26. [DOI] [PubMed] [Google Scholar]
- 27. Kuck KH, Brugada J, Furnkranz A, Metzner A, Ouyang F, Chun KRet al. . Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016;374:2235–45. [DOI] [PubMed] [Google Scholar]
- 28. Hoffmann E, Straube F, Wegscheider K, Kuniss M, Andresen D, Wu LQet al. . Outcomes of cryoballoon or radiofrequency ablation in symptomatic paroxysmal or persistent atrial fibrillation. Europace 2019;21:1313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chun KRJ, Okumura K, Scazzuso F, Keun On Y, Kueffer FJ, Braegelmann KMet al. . Safety and efficacy of cryoballoon ablation for the treatment of paroxysmal and persistent AF in a real-world global setting: results from the cryo AF global registry. J Arrhythm 2021;37:356–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arbelo E, Brugada J, Blomstrom-Lundqvist C, Laroche C, Kautzner J, Pokushalov Eet al. . Contemporary management of patients undergoing atrial fibrillation ablation: in-hospital and 1-year follow-up findings from the ESC-EHRA atrial fibrillation ablation long-term registry. Eur Heart J 2017;38:1303–16. [DOI] [PubMed] [Google Scholar]
- 31. Urbanek L, Bordignon S, Schaack D, Chen S, Tohoku S, Han Efe Tet al. . Pulsed field versus cryoballoon pulmonary vein isolation for atrial fibrillation: efficacy, safety and long-term follow-up in a 400 patient cohort. Circ Arrhythm Electrophysiol 2023:e011920. [DOI] [PubMed] [Google Scholar]
- 32. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist Cet al. . 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 33. Iliodromitis K, Lenarczyk R, Scherr D, Conte G, Farkowski MM, Marin Fet al. . Patient selection, peri-procedural management, and ablation techniques for catheter ablation of atrial fibrillation: an EHRA survey. Europace 2023;25:667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marrouche NF, Kheirkhahan M, Brachmann J. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;379:492. [DOI] [PubMed] [Google Scholar]
- 35. Qeska D, Singh SM, Qiu F, Manoragavan R, Cheung CC, Ko DTet al. . Variation and clinical consequences of wait-times for atrial fibrillation ablation: population level study in Ontario, Canada. Europace 2023;25:euad074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Verma A, Haines DE, Boersma LV, Sood N, Natale A, Marchlinski FEet al. . Pulsed field ablation for the treatment of atrial fibrillation: PULSED AF pivotal trial. Circulation 2023;147:1422–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tohoku S, Chun KRJ, Bordignon S, Chen S, Schaack D, Urbanek Let al. . Findings from repeat ablation using high-density mapping after pulmonary vein isolation with pulsed field ablation. Europace 2023;25:433–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Duytschaever M, De Potter T, Grimaldi M, Anic A, Vijgen J, Neuzil Pet al. . Paroxysmal atrial fibrillation ablation using a novel variable-loop biphasic pulsed field ablation catheter integrated with a 3-dimensional mapping system: 1-year outcomes of the multicenter inspIRE study. Circ Arrhythm Electrophysiol 2023;16:e011780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cheung CC, Deyell MW, Macle L, Verma A, Champagne J, Leong-Sit Pet al. . Repeat atrial fibrillation ablation procedures in the CIRCA-DOSE study. Circ Arrhythm Electrophysiol 2020;13:e008480. [DOI] [PubMed] [Google Scholar]
- 40. Vassilikos VP, Pagourelias ED, Laroche C, Blomstrom-Lundqvist C, Kautzner J, Maggioni APet al. . Impact of centre volume on atrial fibrillation ablation outcomes in Europe: a report from the ESC EHRA EORP atrial fibrillation ablation long-term (AFA LT) registry. Europace 2021;23:49–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.