Abstract
Objective
Calcification of vascular grafts, including polyethylene terephthalate (PET) and expanded polytetrafluoroethylene (ePTFE) grafts may contribute to graft failure, but is under reported. The aim of this study was to review the literature to assess whether vascular graft calcification is deleterious to vascular graft outcomes.
Data sources
The Medline and Embase databases were searched.
Review methods
A systematic literature search according to PRISMA Guidelines was performed using a combined search strategy of MeSH terms. The MeSH terms used were “calcification, physiologic”, “calcinosis”, “vascular grafting”, “blood vessel prosthesis”, “polyethylene terephthalates”, and “polytetrafluoroethylene”.
Results
The systematic search identified 17 cases of PET graft calcification and 73 cases of ePTFE graft calcification over a 35 year period. All cases of PET graft calcification were reported in grafts explanted for graft failure. The majority of cases of ePTFE graft calcification were unexpectedly noted in grafts used during cardiovascular procedures and subsequently removed.
Conclusion
Calcification of synthetic vascular grafts is under reported but can compromise the long term performance of the grafts. More data, including specific analysis of radiological findings as well as explant analysis are needed to obtain a more sensitive and specific analysis of the prevalence and incidence of vascular graft calcification and the impact of calcification on synthetic graft outcomes.
Keywords: Blood vessel prosthesis, Calcification, Polyethylene terephthalates, Polytetrafluoroethylene, Vascular grafting
Highlights
-
•
Calcification of vascular grafts may compromise long term performance.
-
•
Over a 35 year period, 17 PET and 73 ePTFE graft calcifications were identified.
-
•
Calcification mechanism is a regulated process similar to skeletal bone formation.
-
•
Novel strategies are developed to combat graft calcification.
Introduction
Polyethylene terephthalate (PET) and expanded polytetrafluoroethylene (ePTFE) grafts are frequently used in clinical settings on account of their excellent durability and biocompatibility.1 PET is made of synthetic fibres of round cross section, bundled into multifilament yarns, which can be woven or knitted. The structure, either woven or knitted is finally compacted by a chemical treatment to reduce the porosity of the wall creating a swelling of the yarns and is embossed by a thermal treatment to provide longitudinal and radial compliance. ePTFE is an expanded polymer which is manufactured by a heating, stretching, and extruding process resulting in a non-textile porous tube composed of nodes and fibres. The fibre length is called internodal distance.1
Calcification of PET or ePTFE graft and adjacent tissues (vascular adventitia, media, and neointima) may contribute to graft failure, as calcification reduces compliance, increases stiffness, and generates a compliance mismatch.2 Vascular graft failure due to calcification has been described, but is a much less reported complication than usual complications such as thrombosis or neointimal hyperplasia.2, 3, 4, 5, 6 Unexpected calcifications on vascular grafts have been noted in grafts used during cardiovascular procedures and subsequently removed.7 These data prompted a review of the literature to make the point as to whether vascular graft calcification is indeed deleterious to vascular graft outcomes.
Methods
A systematic literature search was performed according to PRISMA Guidelines.8 The Medline and Embase databases were searched by a combined search strategy of MeSH terms. The search strategy was “calcification, physiologic” OR “calcinosis” AND “vascular grafting” OR “blood vessel prosthesis” AND “polyethylene terephthalates” AND “polytetrafluoroethylene”. Two investigators (A.L., B.B.) screened all titles and abstracts collected from the search strategy for relevance. The first 20 related items of all relevant studies were scanned for other potentially relevant studies. The full texts of all relevant articles were obtained and reviewed for suitability independently by both reviewers. The reference lists of each article were scanned for other potentially relevant studies. Any disagreement in study inclusion was resolved by consensus.
Eligibility criteria
All English studies reporting on calcification of PET or ePTFE vascular graft were included. Experimental studies were excluded.
Data items
Both reviewers independently extracted data using a standardised form. This was done in duplicate. Any disagreement in data collection was resolved by consensus. Data extracted were study characteristics (year of publication, study design, number of cases), graft description (type, location), duration of implantation, and graft analysis (in vivo and ex vivo data).
Results
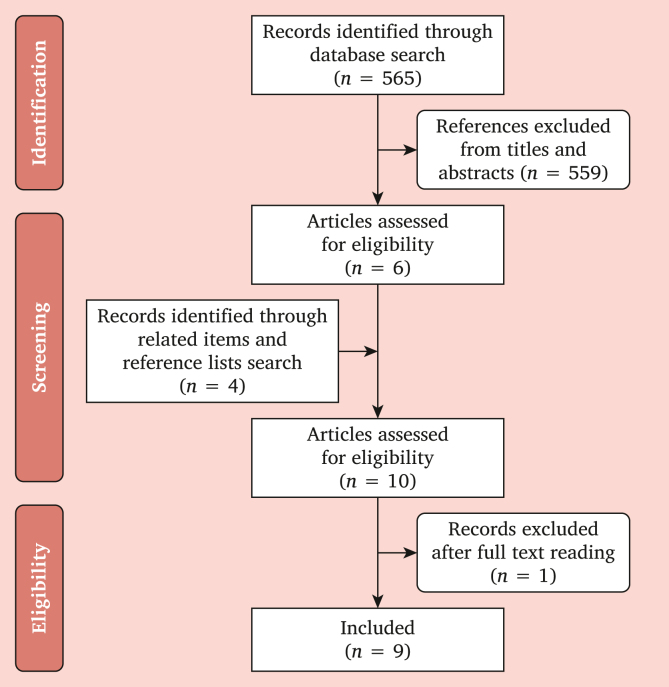
A flowchart showing study selection is provided in Fig. 1. Data extraction allowed the evaluation of 565 publications, and six of those were selected according to the inclusion criteria and four additional studies were found through reference lists and related items, with one study excluded after full text reading. There was no disagreement between reviewers (Fig. 1).
Figure 1.
Flowchart showing study selection to review the literature assessing whether vascular graft calcification is deleterious to vascular graft outcomes.
Among the nine remaining publications, four studies addressed PET graft calcifications2, 3, 4,9 and five studies reported ePTFE graft calcifications.5, 6, 7,10,11 A descriptive presentation of the data was performed.
Calcification of polyethylene terephthalate grafts
Three case reports and one case series addressed calcification on PET grafts.2, 3, 4,9 Accordingly, 17 cases of calcification of PET grafts have been reported (Table 1). These 17 PET grafts were explanted for graft failure.2, 3, 4,9
Table 1.
Calcifications described on vascular grafts.
| Author Journal Year |
Study design | Number of grafts | Graft (type, location) | Mean duration of engraftment | In vivo analysis | Ex vivo analysis |
|---|---|---|---|---|---|---|
| Padmakumar3 Ann R Coll Surg Engl 2004 |
Case report | 1 | Left subclavian to descending aorta PET bypass | 18 y | Conventional radiography: calcification throughout the graft with eccentric plaque in the middle portion of the graft | Not performed |
| Chong2 EJVES Short Rep 2016 |
Case report | 1 | Bifurcated supracoeliac aorta to renal arteries PET bypass | 9 y | Computed tomography: stenosis in the left limb of the bifurcated bypass | Histological evaluation: marked calcification, macrophages and multinuclear giant cells |
| Jayaswal4 SAGE Open Med Case Rep 2021 |
Case report | 1 | Ascending to thoracic aorta extra-anatomical PET bypass | 37 y | Computed tomography: 7.5 cm occlusion of the bypass with a residual patent lumen measuring 4 mm just above the distal anastomosis Per-operative examination: the tubular capsule of the graft was calcified, the graft was extrinsically compressed by the calcified thrombotic content |
Macroscopic examination: PET inner surface showed ulcerations and a lumen filled with thrombosed blood Histological evaluation: hyalinisation of the vessel wall with chunks of calcification |
| Walton9 Atherosclerosis 1986 |
Case series | 32 | PET grafts: Axillobrachial or femoral: 6 Aorto-iliac: 3 Aortofemoral/bifemoral: 8 Iliofemoral: 4 Femorofemoral or popliteal: 11 |
3.9 y (range 2 mo–12 y) | Not performed | Histological evaluation of all grafts: calcium deposits in 14 grafts (34%), in association with severe lipid/lipoproteins infiltration In one graft, extensive calcification as concentric involvement of both the neointima and neoadventitia |
| Barendregt5 Eur J Surg 1993 |
Case report | 1 | ePTFE haemodialysis graft | 5 y | Per-operative examination: large amount of calcified, thickened, intimal tissue in the graft | Histological evaluation: extensive calcification of the graft, resembling true atherosclerotic changes |
| Janssens de Varebeke6 Acta Chir Belg 1994 |
Case report | 1 | ePTFE femoropopliteal bypass | 9 y | Per-operative examination: the graft was rigid and felt bony hard | Histological evaluation: regions of heavy calcium salt deposits in the outer surface tissue and within the wall interstices, layer of dense connective tissue containing osteocytes |
| Tomizawa12 ASAIO J 1998 |
Case series | 10 | ePTFE grafts: modified Blalock Taussig shunt procedures: 10 | 2.5 y (range: 11 mo–5.5 y) | Not performed | Histological evaluation of all grafts: wall calcifications in three grafts (30%), with macrophages in the graft wall |
| Hayabuchi10 Am Heart J 2007 |
Case series | 76 | ePTFE grafts: Ventricular septal defect patches: 29 Right ventricular outflow tract grafts: 32 Atrial septal patches: 8 Extracardiac grafts: 7 |
5.5 years (range: 6 mo–17 y) | Computed tomography: calcification in 41 grafts (54%) | Histological evaluation of four grafts: two grafts were covered in calcified fibrocollagenous tissue. Bone formation with dystrophic calcification in one patient |
| Mehta7 Cardiovasc Pathol 2011 |
Case series | 40 | ePTFE grafts: Arteriovenous grafts: 16 Arterial grafts: 11 Cardiac grafts: 13 |
5.3 y (range: 1–21.2 y) | Not performed | Histological evaluation of all grafts: 27 grafts (68%) showed evidence of calcification either within or adjacent to ePTFE, or both: interstitial calcification of the graft material itself in 15 grafts, calcification of the neointimal or luminal content in 14 grafts, and calcification of the adventitia in five grafts |
PET = polyethylene terephthalate; ePTFE = expanded polytetrafluoroethylene.
The first report described the case of a 16 year old girl with post-subclavian coarctation and cranial hypertension, requiring open revascularisation using a PET graft from left subclavian artery to descending aorta.3 She presented 18 years later with cranial hypertension, clinical re-coarctation, and pressure gradient of 60 mmHg across the graft. Conventional radiography and angiography showed a calcified graft with an eccentric plaque in the middle portion of the graft. Aortic valve replacement and revision of the coarctation repair was planned. No analysis of the explanted graft was performed.
The second report described the case of a seven year old boy with mid aortic syndrome with bilateral renal artery stenosis due to post-surgical resection of a retroperitoneal neuroblastoma when he was six months old.2 He underwent an open revascularisation using a bifurcated PET graft from the supracoeliac aorta to both renal arteries. A stenosis at the junction of the proximal and middle thirds of the right limb of the graft with a pressure gradient of 44 mmHg across the limb of the graft was diagnosed seven years later due to recurrent hypertension. The stenosis was refractory to cutting balloon angioplasties and resection of the right limb and replacement with a PET graft was performed when he was 16 years old. Histological examination with haematoxylin eosin staining of the explanted graft confirmed the presence of marked calcification responsible for the stenosis. Macrophages and multinuclear giant cells were visualised around the PET graft material.
The third report described the case of thrombotic occlusion of an ascending to thoracic extra-anatomical aortic PET bypass performed 37 years ago in a 46 year old man with Takayasu arteritis.4 The patient presented with uncontrolled hypertension and lower limb claudication. Blood pressure was 160/110 in the upper limbs and 70/30 in the lower limbs. Conventional radiography showed calcifications of the extra-anatomical graft. The bypass was occluded for 7.5 cm with a residual patent lumen measuring 4 mm just above the distal anastomosis on computed tomography angiography. The bypass was excised and a new extra-anatomical bypass was performed. On inspection of the excised graft, the tubular capsule of tissue around the graft was calcified, and the graft was extrinsically compressed by the calcified thrombotic content. The inner surface of the graft showed ulcerations and a lumen filled with thrombosed blood. Histopathological examination of the graft showed hyalinisation of the vessel wall with chunks of calcification.
Walton et al. examined 32 explanted PET grafts implanted for peripheral artery disease by histology and immunohistology to follow the development of the tissue response to synthetic grafts and to compare lipid containing lesions with atherosclerotic lesions in arteries.9 Explanted grafts were occluded in 17 cases and infected in four cases, presented aneurysmal degeneration in seven cases, and were obtained during autopsy in four cases. Mean duration of implantation was 3.9 years. The grafts were initially permeated by thrombus containing platelet antigens and this became organised and converted to granulation and then to fibrous tissue. The newly formed tissue contained foreign body giant cells in contact with the plastic prosthesis and showed evidence of permeation by plasma proteins. In grafts of over two years of implantation, this reactive tissue no longer contained platelet antigens but invariably revealed bound lipid, identifiable as apolipoprotein B containing lipoproteins and fibrinogen related antigens, in a distribution resembling that seen in atherosclerotic arteries. Calcification occurred as microscopic deposits in 14 grafts (grafts of longest duration of implantation), in association with severe lipid and or lipoprotein infiltration. In one graft (PET graft of six years and three months of implantation), much more extensive calcification was seen as concentric involvement of both the neointima and neoadventitia.
Calcification of expanded polytetrafluoroethylene grafts
Calcification of ePTFE grafts was described in five publications (two case reports and three case series).5, 6, 7,10,12 Thus, 73 cases of calcification on PTFE grafts have been reported (Table 1). Graft failure was described in two cases, but not reported in other series, as grafts were removed during a planned corrective procedure and or systematically analysed.5,6
The first report described the case of a 28 year old woman with end stage renal disease.5 She had a ePTFE graft implanted for haemodialysis. The graft occluded two years later as a result of neointimal thickening at the venous anastomosis. A thrombectomy was performed, after which the anastomosis was widened with a venous patch. The graft occluded again three years later. It was explored and found to contain thrombus and a large amount of calcified, thickened, intimal tissue. Histological examination of the intimal tissue showed extensive calcification, resembling true atherosclerotic intimal changes.
The second report described the case of a 58 year old man presenting with disabling claudication due to right superficial femoral artery occlusion.6 An above knee ePTFE femoropopliteal bypass was performed. The patient presented nine years later with acute right limb ischaemia. He underwent urgent vascular exploration. On palpation the whole graft was rigid and felt bony hard. A new above the knee ePTFE femoropopliteal bypass was performed. Histological analysis of the explanted ePTFE graft revealed regions of heavy calcium salt deposits in the outer surface tissue and within the wall interstices, the graft was enveloped with a layer of dense connective tissue containing osteocytes.
Tomizawa et al. investigated the healing characteristics of small calibre ePTFE grafts implanted in patients with congenital heart disease as modified Blalock Taussig shunt procedures.12 At the time of total corrective procedures, 10 ePTFE grafts were removed and evaluated. The mean duration of implantation was 2.5 years. Microscopically, the walls of three ePTFE grafts were calcified, and macrophages were immunohistologically observed in the graft wall.
Hayabuchi et al. evaluated 76 implanted ePTFE grafts in patients with surgically repaired congenital heart disease.10 The mean duration of implantation was 5.5 years. Calcification was evaluated with computed tomography (CT): a calcified lesion was defined as an area >3 connected pixels with a CT attenuation >130 Hounsfield units (HU) applying 3D connectivity criteria. ePTFE calcification was detected in 41 cases: five of 29 (17%) for ventricular septal defect patches, 26 of 32 (81%) for right ventricular outflow tract grafts, two of eight (25%) for atrial septal patches, and seven of seven (100%) for extracardiac grafts of total cavopulmonary connection. The CT attenuation of PTFE revealed statistically significantly different values for ventricular septal defect patches (114 ± 61 HU), right ventricular outflow tract grafts (243 ± 132 HU), atrial septal patches (163 ± 161 HU), and extracardiac grafts (230 ± 29 HU) (p < .0001). The CT density value of ventricular septal defect patches was statistically significantly lower than those of right ventricular outflow tract and extracardiac grafts (p < .050). Explanted ePTFE grafts (two right ventricular outflow tract grafts and two atrial septal patches) were evaluated histologically as four patients underwent re-intervention. In two patients whose CT images revealed calcified ePTFE grafts, histological analysis showed the ePTFE grafts to be covered in calcified fibrocollagenous tissue. Bone formation with dystrophic calcification was present in one patient. For two patients without calcification on CT, histological findings revealed the ePTFE to be covered in fibroblast and collagen fibres without calcification.
Mehta et al. examined the tissue responses occurring within and adjacent to ePTFE grafts that were used during cardiovascular procedures and subsequently removed.7 The authors evaluated 40 surgical specimens corresponding to arteriovenous grafts (16 cases), arterial grafts (11 cases), and cardiac grafts (13 cases). The mean implantation duration was 5.3 years. Among the 40 specimens, 27 (68%) showed evidence of calcification either within or adjacent to ePTFE, or both: 15 specimens showed interstitial calcification of the graft material itself (punctate, lamellar, or transmural), 14 specimens showed calcification of the neointimal or luminal content, and five specimens showed calcification of the adventitia.
Discussion
This systematic review allowed identification of 17 cases of PET graft calcification and 73 cases of ePTFE graft calcification over a 35 year period. The 17 calcified PET grafts were explanted for graft failure. Most of the ePTFE graft calcifications were not linked to graft failure, as case series reported systematic analysis of grafts identifying unexpected calcification. Accordingly, graft calcification can possibly compromise the long term performance of the graft, but correlating graft calcification with deleterious graft outcome is however, questionable.
Graft calcification is probably under reported, but grafts that might have calcification but are not compromised are probably not reported either. Accordingly, having a real understanding of graft calcification pathophysiology is mandatory, as this helps in understanding when or why graft calcification can contribute to graft failure.
By definition, pathological calcification refers to the deposition of calcium phosphates or other calcific salts at sites that would not normally have become mineralised. Calcification of vascular grafts closely resembles vascular calcification in atherosclerotic arteries. An unresolved issue is, however, the directionality of the process: calcification could come from the native vessel and creep back into the graft, but this process could also be linked to cellular ingrowth from the anastomoses then resulting in heterotopic calcification, or even be the result of biological activity in thrombus lining the grafts resulting in calcification. Moreover, calcification can also develop outside the graft. However, none of the published reports investigated the directionality of the process and the difference between PET and ePTFE grafts. Accordingly, the specific mechanism of calcification pathogenes remains elusive.11 Calcification of vascular grafts was first considered as a passive process of mineral precipitation, due to the formation of a biological lining on the vascular graft lumen surface, known as a neointimal lining, facilitating the increased permeation of calcium and phosphate ions via an increase in the immediate calcium ion influx. However, it is now clear that vascular calcification is a consequence of a tightly regulated process that resembles skeletal bone formation.13 During the process, vascular smooth muscle cells undergo osteochondrogenic differentiation, developing ectopic mineralisation.14 A variety of factors have been linked to the pathogenesis of calcification, including matrix remodelling, endoplasmic reticulum stress, apoptosis, inflammation, and reactive oxygen species.15 Accordingly, calcium deposition onto a biomaterial surface occurs as calcium and phosphate ions combine to form hydroxyapatite crystals.16 Vascular smooth muscle cells differentiate into osteogenic cells and secrete extracellular vesicles that can bind calcium directly or stimulate further osteogenic differentiation.15 Phenotype changes are also driven by high levels of reactive oxygen species (from dysfunctional mitochondria) and stiffness of extracellular matrix.17 Endoplasmic reticulum stress in vascular cells mediates apoptosis, while persistent inflammation of the vessel involves cytokine release and upregulation of inflammatory vs. anti-inflammatory macrophages.18
There are currently no satisfactory preventive measures or therapies to reverse calcification of vascular grafts, and no disruptive innovations have taken hold in the vascular graft market for the last 40 years. However, major research efforts have been made to combat vascular graft calcification. Novel strategies include the development of biomaterial design that favours cell recruitment and matrix remodelling, the development of vascular grafts with immunomodulatory capability, and the development of vascular grafts with antioxidant activity.15 The development of biomaterial design that favours cell recruitment and matrix remodelling is based on prompt remodelling of prosthetic vascular grafts from biomaterials to neo-arteries reducing the duration of host exposure to foreign materials, thus reducing the risk of calcification due to chronic foreign body reaction. A variety of bioresorbable polymers (poly-L-lactic acid, polyglycolic acid, polydioxanone) have therefore been developed for vascular tissue engineering applications, and from several studies carried out by different research groups, vascular grafts with higher rates of cellular infiltration consistently led to a notable reduction in calcification development.19,20 The development of vascular grafts with immunomodulatory capability is based on inflammation and adverse foreign body reaction being another contributing factor for pathological calcification. Currently, PET and ePTFE vascular grafts are designed to be bioinert so as to not induce a strong immune response from the host, but these grafts are certainly not entirely bioinert as they generate at least a fibroblastic response. As more is understood about foreign body responses towards implanted biomaterials, bioactive materials and molecules have been incorporated into vascular grafts to reduce inflammation by modulating the phenotypic switching of macrophages, which have been categorised into non-activated (M0), pro-inflammatory (M1), or anti-inflammatory and anti-osteogenic (M2) phenotypes.21 A variety of strategies have been used to induce the M2 phenotype, including the incorporation of stem cells, their secretomes, or anti-inflammatory pharmacological agents. From several studies carried out by different research groups, vascular grafts that could modulate macrophages into the M2 phenotype could reduce inflammation associated calcification formation.21 Targeting oxidative stress and mitochondrial dysfunction is another strategy. Given the important role of radical oxygen species in vascular graft calcification, incorporation of antioxidant compounds such as gallic acid, chitosan, mitoquinone, or polyphenols into vascular grafts has been proposed. From several studies carried out by different research groups, incorporation of antioxidant compounds into vascular grafts could prevent graft calcification in pre-clinical models.22
However, there is currently still no clinically approved prosthetic vascular graft that can successfully prevent calcification in the long term. The performance of future vascular grafts will need to be carefully evaluated in clinically relevant animal models to provide valuable lessons on the promises and challenges of bio-engineered designs. However, graft calcification surveillance is also mandatory and plain Xray, computed tomography, and flow parameter assessment would probably help differentiating calcification responsible for graft dysfunction from calcified grafts that are not compromised. Finally, explant analysis is another key to improving the performance of future generations of devices, as current pre-market bench testing is essential but insufficient to predict the in vivo fate of implanted devices. Even if lessons have already been learned from explant analysis, data obtained from future observational, histological, mechanical, and chemical evaluations are mandatory to improve manufacturing of the next generation of vascular grafts.23
This study suffers from several limitations. First, no homogeneous diagnosis exists for assessing calcification with objective evaluation of calcification characteristics. Second, the number of publications is very low and does not allow for valid and robust conclusions. Finally, clinical data are missing from most of the published reports: patient clinical status, medication, and especially factors that may contribute to calcification are not reported and this prevents full understanding of the pathophysiological process of graft calcification.
Conclusion
Vascular graft calcification is probably under reported although it possibly compromises the long term performance of grafts. More data, including specific analysis of radiological findings as well as explant analysis are needed to obtain a more sensitive and specific analysis of the prevalence and incidence of vascular graft calcification, and the impact of calcification on synthetic graft outcomes. Recent insights into the pathogenesis of vascular graft calcification will also guide the design of future vascular grafts with greater potential for translational success.
Conflict of interest
None.
Funding
None.
References
- 1.Lejay A., Vento V., Kuntz S., Steinmetz L., Georg Y., Thaveau F., et al. Current status on vascular substitutes. J Cardiovasc Surg. 2020;61:538–543. doi: 10.23736/S0021-9509.20.11592-1. [DOI] [PubMed] [Google Scholar]
- 2.Chong D.S.T., Constantinou J., Davis M., Hamilton G. Calcification of a synthetic renovascular graft in a child. EJVES Short Rep. 2016;33:13–15. doi: 10.1016/j.ejvssr.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Padmakumar R., Krishnamoorthy K.M., Tharakan J.A. Calcific stenotic jump graft. Ann R Coll Surg Engl. 2004;86:36–37. doi: 10.1308/14787080492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jayaswal S.K., Makwana R., Mehra S., Shetty V. Unusual case of dystrophic calcification and thrombosis of an extra-anatomical graft in a patient with Takayasu's arteritis. SAGE Open Med Case Rep. 2021;9:1–5. doi: 10.1177/2050313X211056744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barendregt W.B., van Knippenberg L.A., Mravunac M. Calcification of a polytetrafluoroethylene haemodialysis graft. Eur J Surg. 1993;159:433–435. [PubMed] [Google Scholar]
- 6.Janssens de Varebeke B., Van Osselaer G. Late occlusion of a polytetrafluoroethylene femoropopliteal graft due to implant calcification and heterotopic ossification. Acta Chir Belg. 1994;94:288–290. [PubMed] [Google Scholar]
- 7.Mehta R.I., Mukherjee A.K., Patterson T.D., Fishbein M.C. Pathology of explanted polytetrafluoroethylene vascular grafts. Cardiovasc Pathol. 2011;20:213–221. doi: 10.1016/j.carpath.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Page M.J., McKenzie J.E., Bossuuyt P., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walton K.W., Slaney G., Ashton F. Atherosclerosis in vascular grafts for peripheral vascular disease. Part 2. Synthetic arterial prostheses. Atherosclerosis. 1986;61:155–167. doi: 10.1016/0021-9150(86)90076-6. [DOI] [PubMed] [Google Scholar]
- 10.Hayabuchi Y., Mori K., Kitagawa T., Sakata M., Kagami S. Polytetrafluoroethylene graft calcification in patients with surgically repaired congenital heart disease: evaluation using multidetector-row computed tomography. Am Heart J. 2007;153:806.e1–806.e8. doi: 10.1016/j.ahj.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 11.Levy R.J., Schoen F.J., Anderson H.C., Harasaki H., Koch T.H., Brown W., et al. Cardiovascular implant calcification: a survey and update. Biomaterials. 1991;12:707–714. doi: 10.1016/0142-9612(91)90017-5. [DOI] [PubMed] [Google Scholar]
- 12.Tomizawa Y., Takanashi Y., Noishiki Y., Nishida H., Endo M., Koyanagi H. Evaluation of small caliber vascular prostheses implanted in small children: activated angiogenesis and accelerated calcification. ASAIO J. 1998;44:496–500. doi: 10.1097/00002480-199809000-00035. [DOI] [PubMed] [Google Scholar]
- 13.Demer L.L., Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938–2948. doi: 10.1161/CIRCULATIONAHA.107.743161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson R.C., Leopold J.A., Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044–1059. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- 15.Brown T.K., Alharbi S., Jiang H.B. Prosthetic vascular grafts engineered to combat calcification: progress and future directions. Biotechnol Bioeng. 2023;120:953–969. doi: 10.1002/bit.28316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bäck M., Michel J.B. From organic and inorganic phosphates to valvular and vascular calcifications. Cardiovasc Res. 2021;117:2016–2029. doi: 10.1093/cvr/cvab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phadwal K., Vrahnas C., Ganley I.G., MacRae V.E. Mitochondrial dysfunction: cause or consequence of vascular calcification. Front Cel Devel Biol. 2021;9 doi: 10.3389/fcell.2021.611922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan X., Zhou Y., Teng X., Tang C., Qi Y. Endoplasmic reticulum stress-mediated apoptosis is activated in vascular calcification. Biochem Biophys Res Comm. 2009;387:694–699. doi: 10.1016/j.bbrc.2009.07.085. [DOI] [PubMed] [Google Scholar]
- 19.Toong D.W., Toh H.W., Ng J.C.K., Wong P.E.H., Leo H.L., Venkatraman S., et al. Bioresorbable polymeric scaffold in cardiovascular applications. Int J Mol Sci. 2020;21:3444. doi: 10.3390/ijms21103444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salacinski H.J., Punshon G., Krijgsman B., Hamilton G., Seigalian A.M. A hybrid compliant vascular graft seeded with microvascular endothelial cells extracted from human omentum. Artif Organs. 2001;25:974–982. doi: 10.1046/j.1525-1594.2001.06716.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang F., King M. Immunomodulation strategies for the successful regeneration of a tissue-engineered vascular graft. Adv Healthc Mater. 2022;11 doi: 10.1002/adhm.202200045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui L., Zhou Q., Zheng X., Sun B., Zhao S. Mitoquinone attenuates vascular calcification by suppressing oxidative stress and reducing apoptosis of vascular smooth muscle cells via the Keap1/Nrf2 pathway. Free Radic Biol Med. 2020;161:23–31. doi: 10.1016/j.freeradbiomed.2020.09.028. [DOI] [PubMed] [Google Scholar]
- 23.Lejay A., Colvard B., Magnus L., Dion D., Georg Y., Papillon J., et al. Explanted vascular and endovascular graft analysis: where do we stand and what should we do? Eur J Vasc Endovasc Surg. 2018;55:567–576. doi: 10.1016/j.ejvs.2018.01.022. [DOI] [PubMed] [Google Scholar]