Abstract
Advances in molecular pharmacology and an improved understanding of the mechanism of most diseases have created the need to specifically target the cells involved in the initiation and progression of diseases. This is especially true for most life-threatening diseases requiring therapeutic agents which have numerous side effects, thus requiring accurate tissue targeting to minimize systemic exposure. Recent drug delivery systems (DDS) are formulated using advanced technology to accelerate systemic drug delivery to the specific target site, maximizing therapeutic efficacy and minimizing off-target accumulation in the body. As a result, they play an important role in disease management and treatment. Recent DDS offer greater advantages when compared to conventional drug delivery systems due to their enhanced performance, automation, precision, and efficacy. They are made of nanomaterials or miniaturized devices with multifunctional components that are biocompatible, biodegradable, and have high viscoelasticity with an extended circulating half-life. This review, therefore, provides a comprehensive insight into the history and technological advancement of drug delivery systems. It updates the most recent drug delivery systems, their therapeutic applications, challenges associated with their use, and future directions for improved performance and use.
Keywords: Drug delivery system, Nanoparticles, Nanocarriers, Nanosheet, Tumour, Pharmacokinetics, Chemotherapy
1. Introduction
Drug delivery systems are technological systems that formulate and store drug molecules into suitable forms like tablets or solutions for administration. They hasten the reach of drugs to the specific targeted site in the body, thereby maximizing therapeutic efficacy and minimizing off-target accumulation in the body [1,2]. Drugs have various routes through which they can be introduced into the body, they include but are not limited to the oral route of administration [3,4], buccal and sublingual routes of administration [5], nasal and ophthalmic [6,7], transdermal and subcutaneous [8,9], anal and transvaginal [10,11] and intravesical [12,13]. The components of the drug account for its physiochemical properties and are responsible for the changes it influences in the body system when taken.
Over the past few decades, DDS have been applied effectively in the treatment of diseases and improvement of health due to increased systemic circulation and control of the pharmacological effect of the drug. The advancement of pharmacology and pharmacokinetics showed the importance of drug release in determining therapeutic effectiveness, giving rise to the concept of controlled release [14]. The controlled-release formulation of a drug was first approved in the 1950s, and it has since attracted considerable attention due to its significant advantages over conventional drugs. It releases drugs at a predetermined rate and for a specific period of time. In addition, controlled drug delivery systems are not affected by physiological conditions and can thus last for days to years. It also provides spatial control over drug release, with constant or variable release rates [15]. Furthermore, it improves drug solubility, target site accumulation, efficacy, pharmacological activity, pharmacokinetic properties, patient acceptance, and compliance, and reduces drug toxicity [2].
Recently, several drug delivery systems (NDDS) have been developed using advanced systems for more convenient, controlled, and targeted delivery. Each drug delivery system has its own peculiarities that determine its release rate and mechanism. This is largely due to the differences in the physical, chemical, and morphological characteristics which will ultimately affect their affinities for various drug substances [16]. Studies on these have identified diffusion, chemical reaction, solvent reaction, and stimuli control as major release mechanisms [17,18]. For instance, since most cancer cells can proliferate the porous blood vessels and lymphatic system, the drug can easily permeate through this opening to reach the target tissues. This is referred to as Enhanced Permeability and Retention (EPR) [19]. EPR is a passive diffusion mechanism well researched and applied in the delivery of many chemotherapeutic agents. Although EPR is a controversial concept, this effect has been observed by many researchers in various types of human tumors and is the basis for the use of nanomedicine in cancer treatment. Though it has a drawback of lack of selectivity and increased toxicity [20]. Active targeting overcomes the lack of specificity and selectivity found in passive targeting. It involves attaching to the carriers, certain ligands, and molecules that can actively bind to the surface of target tissues. This prevents uptake by non-target cells thereby reducing side effects and toxicity [21,22]. Selectivity of ligands to target cells, immunogenicity, and chances of lysosomal degradation after macrophage endocytosis still pose solid challenges to the full development of actively targeting Drugs [23]. These delivery systems can also reach the target cells through the control of one or more physical or chemical properties in the process of responsive stimuli targeting [24,25]. These physical properties include pH, temperature, ultrasound, magnetic and electric field.
2. The early period of drug delivery systems
In the ancient period, people depended on medicinal plants. Although they were beneficial, they lacked consistency, homogeneity, and specificity in drug delivery [26]. Before the use of controlled drug delivery, all pharmaceuticals were produced and stored in pill or capsule formulations. It is dissolved when it comes in contact with gastrointestinal fluids, permeates the gut wall, and is then absorbed into the bloodstream through blood capillaries. There was no capacity to control the drug release kinetics. With the aim to hide the bitter taste of drugs, Rhazes and Avicenna, introduced coated technology. This coating method altered the rate of release of the drug itself. It was adopted in the 10th century however in the form of gold, silver, and pearl-coated tablets.
In the 20th century, advanced coating technology with keratin, shellac, sugar, enteric coating, and pearl coating, was also introduced, but keratin and shellac were ineffective due to storage instability and high pH for adequate dissolution in the small intestine. Malm et al. [27] introduced an enteric-coating material with polymeric cellulose acetate phthalate that is dissolved at a very weak alkaline pH, like that of the small intestine, which made it highly suitable to be applied for enteric controlled release.
The first generation was extremely productive, it focused on the development of numerous oral and transdermal controlled-release formulations for clinical use and the establishment of controlled drug-release mechanisms. In 1951, Lipowski first introduced a patent oral sustained-release formulation, when he coated pills with enteric polymers (like beads) such that the drug and coat were layered alternatively, resulting in slow release of the drug, regularly, and periodically [28]. This was further developed by Smith, Klein Beecham, and French (SKF) in 1952, they developed Spansule technology, an oral predetermined-release formulation that sustains and controls the kinetic release of a drug gradually [29]. This formula is composed of hundreds of micro pellets drug loaded beads with variable layers of natural water-soluble wax with dissimilar thicknesses on individual pellets. On ingestion, the outer capsule rapidly disintegrates, the waxy coating around the beads gradually dissolves as they transit down the GI tract, and liberates the drug-loaded beads. This improved patient compliance and convenience by reducing the dosing schedule, resulting in great popularity [30]. This technology was further developed by replacing the wax with more reproducible synthetic polymers [31].
In 1955, the first nanoparticle therapeutic was reported by Jatzkewitz when he prepared the first polymer-drug conjugate. In the 1960s, the first nanotechnology known as liposome (lipid vesicles) was discovered [32,33]. Polymer-drug conjugates and liposomes mark the birth of nanocarriers. In this decade, the ALZA Corporation did not create drugs they specialized in targeting and controlling the release of drugs at the right place and time [34]. In 1972, Scheffel and his colleague prepared the first protein-based microspheres. In 1976, “micelle” and “emulsion” polymerization techniques were used to prepare drug-loaded nanoparticles and microcapsules by Peter Paul Speiser's research group [35]. In 1977, Couvreur et al. [36] reported the lysosomotropic effects of the nanoparticles, and they produced the first rapidly biodegradable acrylic nanoparticles.
The drug delivery formulations developed during the second generation (2G) were impressive, but they did not produce the expected clinical results [37]. The researchers were interested in developing drug delivery systems with constant drug release rates, self-regulating, long-term depot formulations, and nanotechnology-based formulations, particularly nanoparticle formulations. In this era, long-term depot-sustained drug-release formulations of peptide/protein drugs were developed [38]. In addition, smart polymers and hydrogels were developed to stabilise drug delivery systems that are affected by physiological changes such as pH, temperature, electric field, and glucose. Furthermore, efforts were made to develop targeted nanotechnology DDS for tumors and gene delivery using biodegradable polymers in nanoparticle structures such as polymeric micelles, chitosan, lipids, and dendrimers. The idea was to modify the nanoparticles so that they could be administered directly into the body for increased drug accumulation at the site of action. Although this nanotechnology-based DDS demonstrated high efficacy in controlling tumor growth in animal models, the FDA only approved a few drugs [31,39].
The third generation of drug delivery systems is the modern era of controlled release technology. For it to be beneficial and successful, it has to overcome the hurdles of both physicochemical and biological barriers, associated with the earlier drug delivery systems. The physiochemical challenges are caused by poor water solubility, the high molecular weight of therapeutic proteins and peptides, and the difficulty in achieving targeted and controlled drug release, whereas the biological barrier challenges are associated with systemic drug distribution issues [31,40]. Many new drug delivery systems must be developed during this time period to meet the challenges associated with earlier forms of drug delivery in order to improve performance and sustainability. However, designing a suitable carrier system is often very difficult due to the challenges associated with targeting a drug to a specific site and continuous release over a specified period of time.
3. Recent drug delivery systems and applications
Significant progress has been made in recent years toward the successful development of drug delivery systems based on organic, inorganic, and hybrid nanoparticles as drug carriers for active targeting, particularly in chemotherapy. Recent drug delivery systems (DDS) are formulated with improved properties such as smaller particle size, increased permeability, increased solubility, efficacy, specific site targeting, stability, toxicity, and sustained delivery. They can significantly improve therapeutic agent performance over conventional dosage forms [19,41].
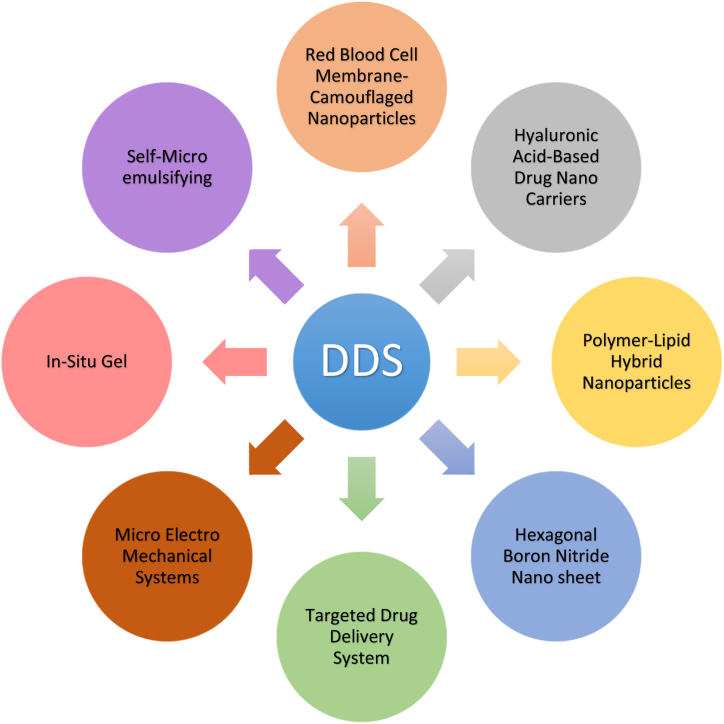
In the development of an optimal drug delivery system, recent drug delivery systems are recognized to be the newest developments and innovative understanding of the pharmacokinetic and pharmacodynamic behavior of pharmaceuticals. Because these DDS are transporters, they can keep medication concentrations in the therapeutic range for a long time while also delivering material to the site of action. The adoption of the delivery mechanism is directly tied to the commercial and therapeutic success of the innovation. This would entail involving patients early in the development process, recognizing any problems, and ensuring they receive the most out of the device. Improving delivery systems that reduce toxicity while increasing efficacy. The different types of drug delivery systems are depicted in Fig. 1.
Fig. 1.
Several types of recent drug delivery systems for different therapeutic purposes.
3.1. Red blood cell membrane-camouflaged nanoparticles drug delivery system
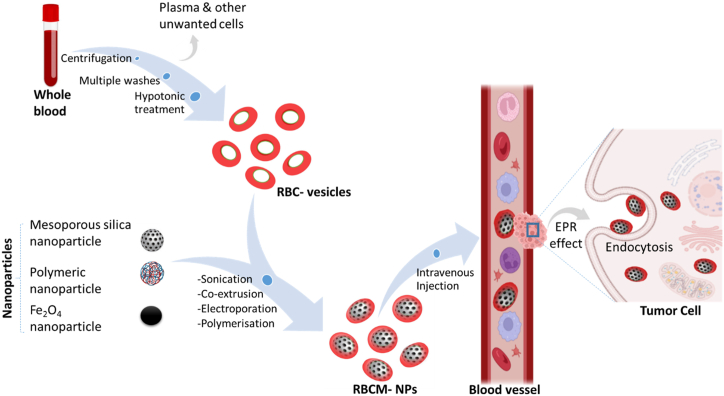
Researchers have recognized the potential benefits of nanotechnology in vastly improving medicine delivery methods throughout time. Red blood cell membrane-camouflaged nanoparticles are a new class of drug delivery systems. The nature and biological significance of red blood cells (RBCs) allow for their use as an efficient system as a nanoparticle camouflaging material [42]. Because red blood cells (RBCs) are the most abundant circulating cells in the body, their biocompatibility (non-immunogenic), biodegradability, and extended circulating half-life, making them an ideal vehicle for drug delivery [43]. Engineered RBCs have been investigated and found to be an excellent carriers for a variety of bioactive chemicals, including enzymes, medications, proteins, and macromolecules [44]. Because of their abundance, red blood cell membranes serve as a “camouflage,” allowing nanoparticles to combine the benefits of native red blood cell membranes with those of the nanomaterial. Several strategies have been developed to load therapeutic agents onto RBCs without comprising the structure and the physiological function of RBCs. The coated nanoparticles will mimic RBCs and interact with the environment to establish long systemic circulation when injected. Sonication is the most common method for creating RBC camouflaged nanoparticles. Other methods of RBC fusion with nanoparticles include in-situ polymerization, microfluidic electroporation, and extrusion. However, each has advantages and disadvantages in terms of synthesis, scale-up challenges, reproducibility, and the nature of the final product [42]. Prior to the fusion, the RBC membrane-derived vesicle is obtained through hypotonic treatment (dialysis, hemolysis or dilutions) of fresh whole blood from an organism. The hypotonic treatment will help to remove unwanted cells and plasma (Fig. 2).
Fig. 2.
Synthetic strategies for RBCM-NPs preparation for antitumor activity. Whole fresh blood is centrifuged and washed multiple times to remove the plasma and other unwanted cells. The resulting pure red blood cells are subjected to hypotonic hemolysis and are used to coat selected nanoparticles which are intravenously injected into the blood to maintain long systemic circulation. The RBCM-NPs permeate the tumor tissue via the EPR effect and finally enter into the tumor cells by endocytosis for therapeutic effect. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The use of RBCM-NPs drug delivery systems is extremely promising and offers numerous benefits due to their low immunogenicity and ability to maintain long systemic circulation (a lifespan of 120 days). Furthermore, because of the large number of cell membranes, RBC vesicles are inherently biocompatible and biodegradable, and can easily achieve high load capacity, resulting in higher accumulation at the target site. Remarkably, erythrocyte membrane-coated nano-formulations have been extensively applied in anticancer research to substantial accomplishment [45,46], cardiovascular diseases [47], and encephalopathy [48].
3.2. Hyaluronic acid-based drug nanocarriers drug delivery systems
The usage of hyaluronic acid is one of the drug delivery techniques. Hyaluronic acid is a novel polymer that can be used to make medication delivery systems [49]. It has a linear macromolecular mucopolysaccharide made up of proportionately connected glucuronic acid and N-acetylglucosamine saccharide units [50]. It exhibits biocompatibility, biodegradability, and high viscoelasticity, and it can be coupled with a specific cell surface receptor [51]. Because Hyaluronic acid is a natural component of eye tissue and plays an important function in wound healing, it makes sense to use it as a carrier for ocular drug delivery as long as the integrated pharmaceuticals are released consistently. They aid in the thickening, sustained release, and transdermal absorption of drugs, as well as improving drug targeting. Drug distribution to cancer cells was significantly improved using active targeted HA-based drug nanocarriers. In addition, lipid nanoparticles with an appropriate HA coating have been developed as biocompatible drug carriers with a great potential for targeted drug delivery to the target tissue while minimizing side effects and harming other tissues. Benefits of utilizing HA-based nanocarriers for cancers with elevated expression of the CD44 receptor include improved drug delivery, increased therapeutic efficacy, higher cytotoxicity, and considerable reduction of tumor development, as well as a high potential for targeted chemotherapy [52].
Another application is when an HA-based nanocarrier is combined with doxorubicin (DOX) and cisplatin (CDDP) and manufactured as a CD44-targeting anti-cancer drug delivery system, as well as its tumor inhibition activities in vitro and in vivo against CD44+ breast cancer cells. In 4T1 (CD44+) breast cancer cells, these dual drug-loaded HA micelles (HA-DOX-CDDP) showed significantly improved drug release under acidic circumstances, as well as higher cellular uptake and stronger cellular growth suppression than free medicines. HA-DOX-CDDP micelles are a potential drug delivery system with acid-sensitive drug release, CD44-targeted delivery, and high biocompatibility and biodegradability. These characteristics resulted in excellent tumor accumulation and less side effects, indicating that HA-DOX-CDDP micelles could be useful in breast cancer chemotherapy [53,54].
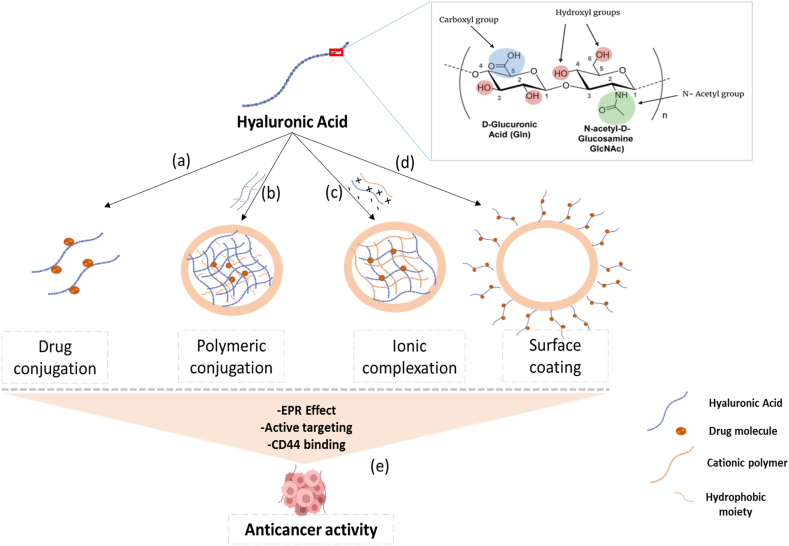
Hyaluronic acid and its derivatives are incorporated into various drug delivery systems (DDS) such as nanoparticle DDS, cationic polymer DDS, and gel DDS for active targeting of cancer cell CD44 receptors (Fig. 3). According to studies, the use of HA and drug conjugates after administration aggregates at the tumor site, where it maintains sustained drug release. The surface of HA-based nanocarriers is generally negative, which helps in blocking the systemic clearance of nanocarriers by the reticuloendothelial system (RES). The Hyaluronic acid-based drug nanocarriers NDDs selectively enter into the cancer cells through the EPR effect and active targeting of CD44 receptors [55,56].
Fig. 3.
The application of hyaluronic acid-based nanocarriers in cancer treatment. (a) and (b) Direct conjugation of cytotoxic drug with HA or hydrophobic moiety results in self-assembly of nanoparticles (NPs) that can be administered intravenously for cancer cell targeting; (b) HA hydrogel formation using a cationic polymer; (c) Surface coating of NP with HA; (e) Hyaluronic acid-based drug nanocarriers permeate cancerous tissues via EPR effect and binds to the CD44 receptor site to elicit anticancer activity.
3.3. Hexagonal Boron Nitride nanosheet drug delivery system
As technology continues to advance and science evolves, more and more materials are being researched and studied to help improve drug delivery. Among these materials is Boron nitride (BN) which is a crystalline material with a balanced stoichiometry of nitrogen (N) and boron (B) atoms. This material occurs in various forms such as cubic BN (c-BN), hexagonal BN (h-BN), wurtzite BN (w-BN), and rhombohedral BN (r-BN). Hexagonal Boron Nitride is a two-dimensional (2D) layered-dense structure with sp2 hybridized B–N bonds. It can also be called white graphene sometimes regarded as an analogue of graphite [57]. The B–N atoms substitute the carbon atoms and are held together by a strong covalent bond forming interlocking rings. The layers of the compound are held together by van der Waals forces with a bond length of 1.466 Å and an interlayer space of 3.331 Å. This compound is partially ionic and as a result of this unique characteristic has its B–N bonds to be polar. H-BN is an insulator that has gained wide applications in various fields of life such as cosmetics, dental, cement, ceramics, and most especially in medicine as a drug carrier similar to graphene or graphene oxide [58].
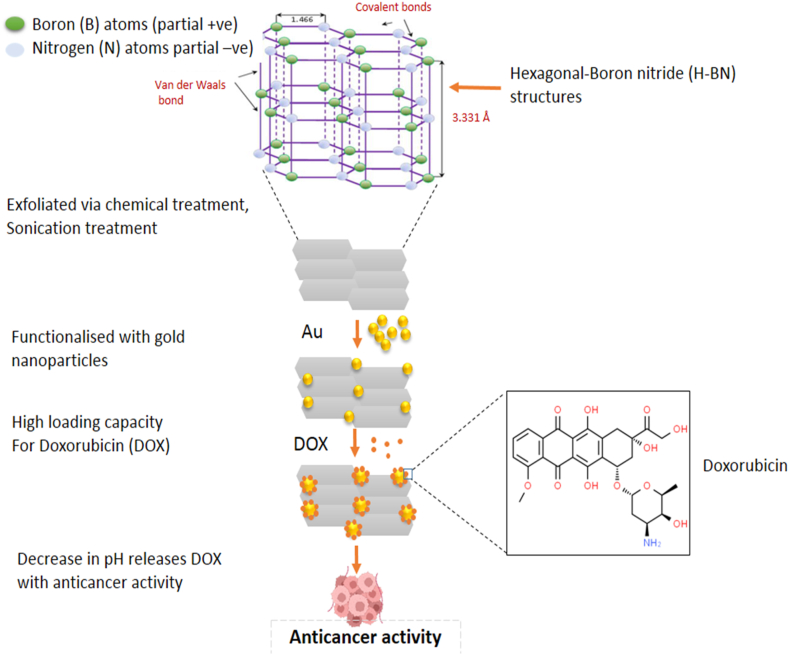
Hexagonal Boron Nitride has been proven to be useful in drug research and delivery systems (Fig. 4). The research by Jedrzejczak-Silicka and her colleagues reported a reduction in the proliferation of MCF-7 cell line cultures when compared with the normal L929 cell lines upon exposure to H-BN loaded with gold particles. H-BN was exfoliated via chemical treatment using modified Hummers' method, sonication treatment, and was finally functionalized with gold particles for the studies and analyzed using Neutral Red (NR) uptake assay [59]. In another study, H-BN nanosheets were conferred photothermal properties as a result of the in-situ deposition of Pd on its surface. This made the compound have a high loading capacity for doxorubicin which is an anticancer drug as well as functions effectively as a drug delivery carrier. The study reported a remarkable inhibition of tumor growth as the drug was administered in mice for two weeks. This was possible as a result of the decrease in pH which led to the release of doxorubicin from the nanohybrids and a concomitant increase in glutathione concentration as well as near infrared radiation (NIR) [60]. Another successful study showed that H-BN conjugated with DNA oligonucleotide and copper (II) phthalocyanine (CuPc) was effective as a therapeutic agent in photodynamic therapy (PDT) and in situ monitoring and miR-21 imaging [57]. Boron compound is being recognized today as an effective chemotherapeutic agent.
Fig. 4.
Schematic explanation of H-BN nanosheet drug delivery system. H-BN was exfoliated via chemical treatment and functionalized with Au particles resulting in high loading capacity of DOX, a chemotherapy drug which is released in tumor cells by decrease in pH.
3.4. Polymer-lipid hybrid nanoparticles drug delivery system
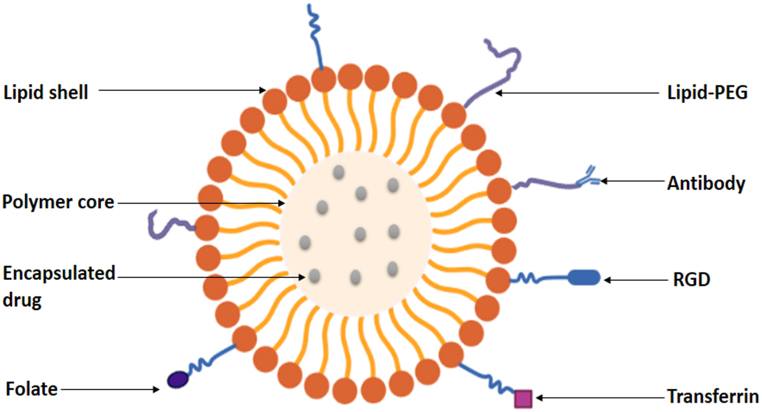
Nanocarriers are gaining wide usage as drug delivery systems because of their increased stability in storage, improved targeting ability on disease cells, sustained drug release, and higher encapsulation ability [61]. Amongst the widely accepted nanoparticles being used today for drug delivery, liposomes and polymeric nanoparticles are the most widely accepted. Liposomes which is a lipid-based nanoparticles though showed excellent biocompatibility still suffered drug leakage and instability upon storage while the polymeric nanoparticle which is a polymer-based nanoparticle was able to curb this limitation by exhibiting high encapsulation/drug loading ability as well as stability. However, it had its own shortfalls in that it showed lower biocompatibility [62,63]. In order to overcome these shortcomings and obtain an effective nanomaterial, researchers sought and developed a hybrid system that will combine the unique properties of the two classes of nanoparticles, which is known as Polymer-lipid hybrid nanoparticles (plhnps). This hybrid system was able to satisfy the requirements of biocompatibility, high storage stability, sustained drug release, minimal drug leakage, small particle size, and high encapsulation [64]. As a result of its efficacy, this system is being used today for different therapeutic purposes as well as diagnostic applications. Plhnps is made up of three distinct components which include: A polymeric core that encapsulates both hydrophilic and hydrophobic drugs effectively. This is possible as a result of the hydrophilic and hydrophobic nature of the core and results in a high sustained release, a lipid shell that provides biocompatibility and high stability and a lipid-polyethylene glycol (PEG) that is found in the outer part and covered by a lipid layer to provide increased steric stability, prevent immune recognition and increase time for circulation (Fig. 5). Plhnps has wide applications such as in the delivery of various chemotherapeutic agents, in gene transfer (sirna, DNA) and in photothermal, photodynamic therapy and ultrasound. Studies have shown that they can be used in the delivery of vaccines and immune activation as well as in imaging and alternative magnetic field (AMF). Hence its wide application in the fast-growing medical environment [65].
Fig. 5.
The formation of a Polymeric-Lipid Hybrid Nanoparticle. The Hybrid contains three distinct components which include: A polymeric core that encapsulates both hydrophilic and hydrophobic drugs effectively. A lipid shell that provides biocompatibility and high stability and a lipid-polyethylene glycol (PEG) that is found in the outer part and covered by a lipid layer to provide increased steric stability, prevent immune recognition and increase time for circulation.
3.5. Self-microemulsifying drug-delivery system
Recently, lipid-based drug preparations have received a lot of interest, with a specific focus on self-microemulsifying drug-delivery systems (SMEDDS) [66]. Inadequate bioavailability is one of the most difficult aspects of developing oral dosage forms of drugs [67]. Accordingly, minimal hydrophilicity is an essential factor for bioavailability in this context, because drugs cannot be absorbed via the gastrointestinal tract (GIT) except it exists in solution forms [68]. Aqueous solubility is a problem for many chemical compounds having notable and favorable pharmacological effects [69]. Furthermore, almost 30% of widely marketed medicinal entities and nearly 50% of innovative drug compounds accessible for product manufacture are hydrophobic in nature, meaning they have low water solubility [70]. The utilization of a lipid-based carrier system to boost the bioavailability of less water-soluble medications has grown in popularity in recent years [71]. The main rationale behind this formulation is to sustain the hydrophobic components in solution all through the digestive system [70].
Lipid-based carriers come in a variety of forms, including suspensions, dry emulsions, microemulsions, and self-emulsifying drug-delivery systems (SEDDS) [72]. SEDDS' ability to incorporate hydrophobic drugs was previously reported. SEDDS has also been revised as self-microemulsifying drug-delivery systems (SMEDDS) and self-nanoemulsifying drug-delivery systems (SNEDDS). Emulsions, on the other hand, are created by dispersing a liquid phase containing macroscopic particles in a different liquid phase composed of surfactant [73]. They are also thermodynamically unstable solution that is semitransparent (occasionally hazy) and has properties that resemble viscous liquids [74]. Emulsions are of three types, viz; water-in-oil, oil-in-water, and multiple emulsions. Additionally, conventional micro- or nanoemulsions differ from SMEDDS, in that following oral ingestion, they self-emulsify [75].
Surfactants (S) and Co-surfactants (CoSs) are the two types of emulsifying agents used in microemulsions (Fig. 6). However, surfactant is predominantly soluble in water, while CoS is mostly soluble in the oil phase. CoSs are essential for lowering the tension that exists between the two liquid phases to the optimal level required for the formation of a microemulsion [76,77]. The production of nanoemulsions with droplet sizes smaller than 100 nm, on the other hand, requires either mechanical or chemical energy [78]. Although nanoemulsions are classified as kinetically stable due to their extremely low destabilization rate, their long-duration stability (in months) is notable [79]. Consequently, nanoemulsion globules are shown to be stable across a variety of situations, including varied dilutions and temperatures, while microemulsions are mostly impacted by factors including dilutions and temperature [74,80]. The key distinctions between SMEDDS, SNEDDS, and SEDDS are shown in Table 1.
Fig. 6.
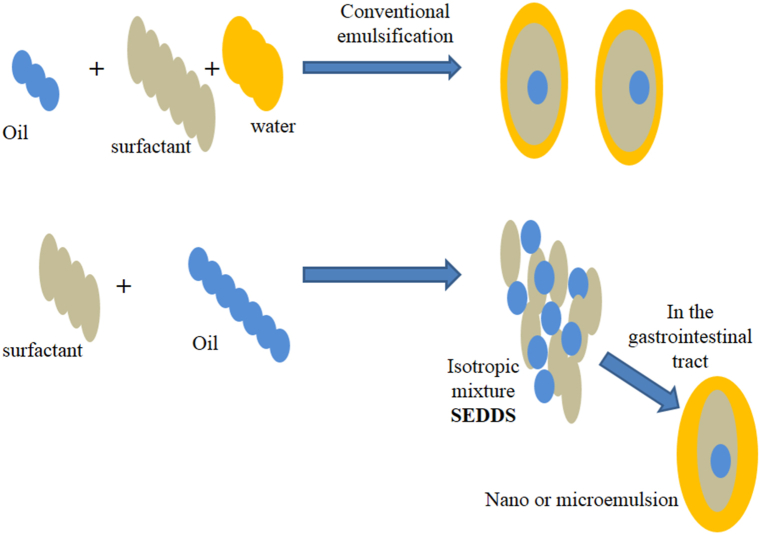
Mechanism of Self-emulsification in aqueous environment. SEDDS comprise a mixture drugs, surfactants, oil, stabilizers, and cosolvents. Like conventional emulsification, SEDDS (ionotropic mixture) form (o/w) nano or microemulsion within the gastrointestinal tract with very small energy input.
Table 1.
Four basic types of lipid-based drug-delivery systems (LBDDS) with their merits and demerits.
| Characteristics | SMEDDS | SNEDDS | SEDDS |
|---|---|---|---|
| Size of the globule | <250 nm | <100 nm | >300 nm |
| The system appearance | High Optical clarity | High Optical clarity | Cloudy |
| The surfactant HLB value | >12 | >12 | <12 |
| LFCS Classification | Type IIIB | Type IIIB | Type II |
| Oil phase | >20% | >20% | 40–80% |
| Surfactants Concentration | 40–80% | 40–80% | 30–40% |
HLB=Hydrophilic/lipophilic balance; LFCS = lipid formulation classification system; SEDDS = self-emulsifying drug-delivery systems; SMEDDS = self-microemulsifying drug-delivery systems; SNEDDS = self-nanoemulsifying drug-delivery system [70] (Rajpoot, Kuldeep 2020).
3.6. In-situ gel drug delivery system
The foremost goal of any drug delivery system is to change the drug's pharmacokinetic characteristics and tissue distribution in a meaningful way [81]. Over the last 60 years, there has been a lot of focus on developing controlled and well reliable drug delivery systems [82]. In-situ gel medication administration has emerged as one of the most innovative drug delivery systems. By virtue of its unique property of transitioning from Sol to Gel, the in-situ gel drug delivery system aids in the prolonged and regulated release of medications, as well as increased patient compliance and comfort [83]. In most case, formulations in solution form become transformed into gel form under certain physiological conditions before it enters the body [84]. A variety of stimuli, such as pH change, temperature modulation, and solvent exchange, combine to transform a solution into a gel form [85]. Oral, nasal, injectable, vaginal, rectal ocular, intraperitoneal, and parenteral routes have all been used in various research. Many polymeric methods capable of delivering pharmaceuticals have been created [86]. When these polymers come into touch with physiological stimuli, they go through a sol-gel transition. In situ gel drug delivery systems are made from a variety of natural and synthetic polymers [83]. Four processes are known to produce the formation of in-situ gel biomaterials, viz; (1) temperature and pH variations, (2) variations of the physical properties of the biomaterials such as solvent exchange and swelling, (3) Biochemical modification such as enzymatic and chemical reactions, and (4) photo-polymerization [87].
3.6.1. Applications of in-situ gel delivery system
3.6.1.1. Oral in-situ gel delivery systems
The application of pH-sensitive hydrogels to particular parts of the gastrointestinal tract for site-specific medicine delivery is the focus of this approach. With different amounts of Polyacrylic acid (PAA) derivatives and cross-linked Polyethylene glycol (PEG), silicone microsphere hydrogels that liberate prednisolone into the stomach media or exhibit gastroprotective properties have been produced. A possible colon-specific medicine delivery approach for decreasing edema at high pH has been developed using dextran hydrogels cross-linked with polysaccharides such as guar gum, inulin, and amidade pectin. Researchers produced gellan gum and sodium alginate formulations that utilized calcium ions as complexing agents and were gelatinized by releasing these ions into the stomach's acidic medium. The oral in situ gel distribution procedures employ natural polymers such as xyloglucan, pectin, and gellan gum. The pectin-based formulation was created to ensure that Metformin loaded pectin (PCM) was distributed continuously. Because pectin is water soluble, an organic solvent is not required [88,89].
3.6.1.2. Opthalmic in-situ gelling systems
Gellanic gum, alginic acid, and xyloglucan are examples of natural polymers employed in the ocular delivery system. Several combinations of anti-inflammatory, antibacterial, and autonomic medicines are utilized to reduce intraocular glaucoma stress in the local ophthalmic administration approach. Tear fluid has been devised to overcome the ocular in-situ gel bioavailability problem due to its rapid turnover and dynamics. Traditional delivery methods also result in limited availability and therapeutic response, making it easier to remove the medication from the eye. In ocular preparations, viscosity enhancers such as Carboxymethyl Cellulose, Polyvinyl alcohol, Carbomers, and Hydroxypropylmethyl cellulose are deployed to improve the viscosity of the drug formulations, resulting in enhanced bioavailability and precorneal residence duration. Chelating agents' penetration enhancers are utilized to promote the infiltration of corneal substances such as surfactants and preservers [90,91].
3.6.1.3. Nasal in-situ gelling systems
In nasal in-situ production, polymers such as gum gelan and gum xanthan are employed. The efficacy of Momethasone furoate as an in situ gel in the management of allergic rhinitis has been studied. The impact of in-situ gel on antigen-induced nasal symptoms was shown in vivo using sensitized rats as a model of allergic rhinitis. In situ gel was proven to resist an increase in nasal complications when compared to the commercialized nosonexex preparation [92,93].
3.6.1.4. Rectal in-situ gelling systems
This method can be used to prescribe a variety of pharmaceuticals that are packed as liquid, semi-solid (liniments, emulsions, and froths), or suppositories in solid administration formulations. During penetration, traditional suppositories can cause discomfort. Furthermore, because suppositories cannot be effectively kept at a single rectum site, they can migrate upward into the gut, allowing the drug's first-pass impact to be experienced. Indomethacin-loaded xyloglucan-based device administration to rabbits revealed a substantial medication immersion and a prolonged drug residence period when compared to commercial suppository administration [94].
3.6.1.5. Vaginal in-situ gelling systems
The vaginal canal is a possible route for medication delivery. A delivery system based on a thermoplastic graft copolymer undergoing in situ gelation has been formulated for the continuous liberation of active substances such as estrogens, peptides, progestins, and proteins. In a recent study, clotrimazole medication antifungal efficiency was boosted and sustained using a mix of poloxamers and polycarbophils mucoadhesive thermosensitive gels in contrast to conformist polyethylene glycol-based formulations [95].
3.6.1.6. Injectable in-situ gelling system
Formulating dose forms like injectable or implanted delivery systems is one of the only obvious ways to give drugs for prolonged release. Thermo-reversible gels, typically made of poloxamers, are the most commonly utilized. These dosage forms might be useful in the manufacture of controlled drug delivery for systemic absorption. Pluronic F127 gels with insulin or insulin-PLGA nanoparticles have been tested. Poloxamer gels have also been used to subcutaneously and intramuscularly deliver human growth hormone as well as to generate a single-dose lidocaine injectable with a longer duration of action. Pluronics recently developed a new type of depot protein injectables controlled release formulation made up of blends of poly (D, l-lactide)/1-methyl-2-pyrrolidone solutions. At a temperature of roughly 45 °C, the hydrogel develops into a gel after being injected subcutaneously, and fast body temperature cooling was achieved by employing biodegradable polymers of poly (ethylene oxide) and poly (propylene oxide) (l-lactic acid). The injectable drug delivery method is utilized to cross-link pluronic acid-modified hydrazide with aldehyde-modified cellulose derivatives such as hydroxypropylmethyl cellulose, carboxymethyl cellulose, and methylcellulose, among others. In order to avoid postoperative peritoneal adhesion, this in-situ forming gel has been used to reduce pelvic discomfort, bowel blockage, and infertility [96,97].
3.7. Micro electro mechanical systems (MEMS) for drug delivery
MEMS technology has vast applications in fields such as actuators, drug delivery, motion sensing, accelerometers, and inkjet printing [98]. The devices produced through this technology incorporate microfabrication techniques to produce micro/nano-sized electromechanical and mechanical devices or implants [99]. Interestingly, the use of these techniques enhances the efficacy of these devices by allowing considerable control over their topography, microarchitecture, and size of the resulting devices [100].
Among the numerous materials and processes available for designing these MEMS-based devices, the most commonly used, incorporate creative blends of varying micromachining techniques such as; deposition (an addictive process), etching (a subtractive process), lithography (a patterning process), ink jetting, ion implantation, oxidation, and micromolding [[101], [102], [103], [104]]. As drug delivery systems, MEMS technology fabricates miniaturized systems comprising of various materials such as silicon, glass, metals, and nitrides, as well as polymers, micropumps, sensors, microvalves, reservoirs, actuators, and high-performance processors [100,103,105,106]. These distinct components function synergistically, to provide the broadly reported multi-functionality and precision of MEMS devices, relative to other conventional drug delivery systems. Each of these features works strategically, for instance; actuators mostly play a vital role in the drug release process, as it pressurizes the drug reservoir to facilitate drug release [103]. Reservoirs provide port(s) to house the drug(s) and can be singular or multiple [107]. Single reservoir architecture comprises a relatively large port that can contain a single drug. It is thus able to contain a relatively larger amount of drug and is also suited for long-term usage as it can be refilled. On the other hand, multi-reservoirs comprise different ports (within the same substrate) that separately store drugs, and as such different drugs can be incorporated into these. However, they are less suitable for long-term usage as they would require repetitive replacement surgeries, due to a lack of refilling methods. Moreover, microvalves are employed to control fluid flow rate, sealing, as well as switching on/off of the delivery device [108]. Silicon is popularly used as a substrate or structural material during fabrication, due to its favorable mechanical and electrical properties [109]. Sensors, on their part, utilize electrical radiation, mechanical, thermal, magnetic, or biochemical mechanisms to monitor the flow measurements of fluid or gas being delivered [[110], [111], [112]]. As such, the choice of each feature during the design process is crucial to the overall functionality of the MEMS-based delivery device.
MEMS-based devices play essential roles in targeted and precise drug delivery by facilitating controlled and pulsatile release of enclosed pharmaceuticals [113]. For this purpose, these devices could be designed either as electric-powered or non-electric powered. The electric-powered devices allow selective drug release from the reservoir(s) by electric potential, while the non-powered devices utilize diffusion, and osmotic environmental stimulus mechanisms to enable drug release [105]. The most popular type of MEMS technology applicable in drug delivery is microchips, followed by microfluidic devices, majorly micropumps. These microchips are reservoir-based implantable devices, which are capable of delivering pharmaceutics in solid, gel, or liquid forms through trans/intradermal delivery [109]. On the other hand, micropumps which are either classified as mechanical or non-mechanical, depending on the presence of moving parts, are distinctly limited to the delivery of drug suspensions or solutions [106]. The major components of MEMs are shown in Fig. 7.
Fig. 7.
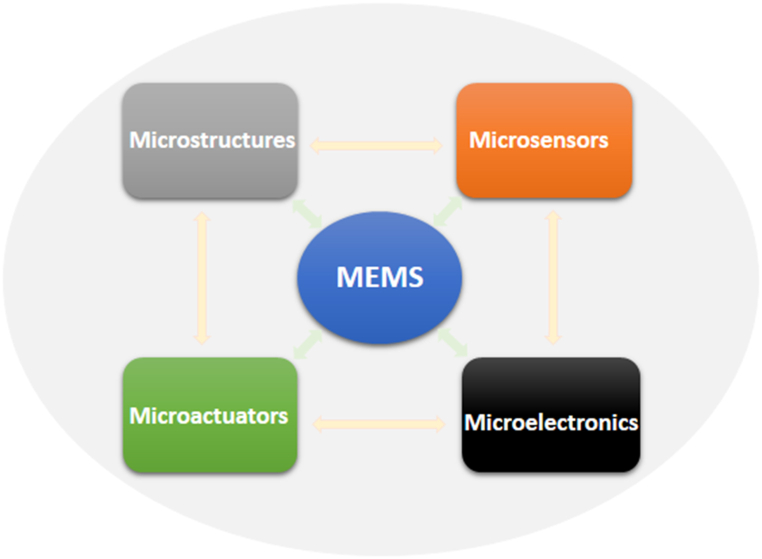
Schematic representation of MEMS components. Generally, MEMS is made up of mechanical microstructures, microactuators, microsensors, and microelectronics all integrated onto a single silicon chip. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Drug delivery devices fabricated by MEMS technologies offer several advantages over conventional delivery methods, such as; enhanced performance, automation, precision, and efficacy due to the integration of their miniaturized sizes with multi-functional components. It also contributes to less painful and invasive attributes of the devices [105]. In addition, MEMS-based devices have the ability to maintain drug stability during encapsulation, adjustable and continuous delivery, and also facilitate the automated release of multiple drugs from reservoirs [114]. Furthermore, it enhances bioavailability and localized release of medication [115], sustainability over a long period of time for medications requiring complex dosing, as well as personalized dosing profile [109], and has the ability to function following sustained zero-order kinetics. However, MEMS-drug delivery devices may pose technical challenges; because the incorporation of wireless electronics to remotely control and track the device's operation, as well as patient's response, has also been projected to be capable of increasing device security risks, medical packaging, and regulatory complexities [105]. Moreover, surgeries would be required for the implantation and removal of these devices; highly stable products would be needed for long-term uses; and the technologies required for their fabrication are relatively expensive [114].
3.8. Combined drug delivery approach
Resistance to drugs has been a recurrent issue in medical therapy. Today, combination therapy proves more viable as a result of wider target specificity and complementarity in enhancing treatment efficiency and increasing clinical results. Combined drug delivery approach has been widely adopted in cancer research and therapy as a means to overcome multidrug resistance. It has been reported that combination drug delivery approach reduces therapeutic dosage as well as adverse reactions while efficiency and decrease in drug resistance are maintained [116].
A study conducted by Zamora-Mera et al. reported positive results in the use of combination therapy for magnetic hyperthermia therapy. They crosslinked chitosan nanoparticles (CSNPs) ionically with tripolyphosphate salts (TPP). The magnetic CSNPs were obtained by encapsulation with three different ferrofluid concentrations and a constant 5-Fluorouracil (5-FU) concentration. They used normal cells, fibroblasts (FHB) and cancer cells, human glioblastoma A-172 cells [117]. The CSNPs showed dose-dependent cytotoxicity and were successfully up-taken in both cell lines. The study reported that the MH-treatment in the A-172 cells produced a 67–75% cell viability whereas no cell viability was noticed in FHB. The study equally reported a 4-h regrowth of the population upon MH treatment with CSNPs loaded only with ferrofluid but a decreased amount of released 5-FU upon combination with the MH treatment and 5-FU demonstrating a positive result using a combination approach [117].
In a study conducted by Silva et al., they reported an increase in the application of combination approach in drug research and in therapeutic studies. They investigated the use of a combined method to remove endotoxins from protein nanocages for drug delivery approaches. They combined an affinity purification with Endotrap-HD resin and treatment with Triton X-144. The study yielded good results that showed combination treatment as a good potential in chemotherapy [118].
A review article conducted by Pang et al. on a particular combination drug delivery approach that focused on exploiting cells in combination with nanoparticles reported that nanoparticles loaded in cells were more effective than the nanoparticle drug delivery system. The cell-based therapy showed improved drug efficacy, extended half-lives, sustained drug release, and limited immunogenicity and cytotoxicity [119]. Combining nanoparticles with exploit cells did not affect its migration or chemotaxic ability. As a result, combination drug delivery approach is viewed as a promising approach in drug research and medical therapy [119].
3.9. Targeted drug delivery system
This approach is an advanced technique employed recently due to its efficiency and reduced side effects. It is a system that delivers drugs in a targeted sequence which in turn leads to an increase in the drug concentration as it is being delivered to its target site [120]. The dosage of the drug is reduced to minimize side effects but its efficacy and strength remain untouched. This approach employs other drug carriers such as soluble polymers, biodegradable microsphere polymers, neutrophils, liposomes, micelles, and artificial cells amongst others [120]. This technique is gaining wide acceptance as it proves useful, especially in the fight against cancer.
A study conducted by Murugun showed effectiveness in the use of this drug delivery system. Topotecan (TPT) and quercetin (QT) were delivered using polyacrylic acid chitosan surface-modified mesoporous silica nanoparticle (MSN) to target negative breast cancer cells (TNBC) (MDA-MB-231) and multidrug-resistant breast cancer cells (MCF-7) [121]. The surface of the nanoparticles was grafted with RGD-peptides which is an amino acid made up of Arg-Gly-Asp sequences. This was done to effectively target αvβ3 integrin. The RGD peptide led to an effective release of encapsulated drugs as well as cellular uptakes by the cancer cells. Both cell lines showed cell death, molecular and structural changes of cellular nucleus, endoplasmic reticulum, and mitochondria. A synergistic antiproliferative effect was also observed [121].
Another study conducted by Wu et al. showed an enhanced release of methrotrexate (MTX) from Fe3O4MgAl-LDH (layered double hydroxide) nanoparticles of ∼230 nm [122]. They reported 84.94% release in the tumor with a pH of 3.5 within 48 h. Their study showed higher antitumor activities across the cell lines that were investigated. Lin et al. used this approach to target HeLa cells. Mitomycin C (MMC) and 10-hydroxycamptothecin (HCPT) were co-delivered using a folate-functionalized soybean phosphatidylcholine micellar Nano formulation to determine their therapeutic effect on the HeLa Cells [123]. The study reported enhanced cellular uptake both in vitro and in vivo and an enhanced decrease in tumor burden compared to free drugs. These findings and much more suggest that a targeted drug delivery system is an area researchers’ ought to also pay more attention to. A comprehensive summary of recent drug delivery systems including their therapeutic uses, advantages, and disadvantages were presented in Table 2.
Table 2.
The summary of recent drug delivery systems, their uses and advantages, and disadvantages.
| Recent Drug Delivery System | Advantages | Disadvantages | Therapeutic Use | References |
|---|---|---|---|---|
| Red Blood Cell Membrane-Camouflaged Nanoparticles Drug Delivery System | Avoids triggering the immune system and achieves long-term circulation. Inherent biocompatibility and biodegradability which avoid accumulation related toxicity. |
Being camouflaged as part of the biological system, complex issues of regulation may arise, also, protein identification, purification, and conjugation may be bypassed. | erythrocyte membrane-coated Nano-formulations have been applied in antitumor and anticancer research to substantial accomplishment | [45,46]. [124] |
| Hyaluronic Acid-Based Drug Nano Carriers Drug Delivery Systems | Good biocompatibility, biodegradable and non-immunogenicity, it has the ability to recognize over-expressed cells and kill them | They may increase cell proliferation when they interact with certain protein receptors, potentially hazardous to cancer patients | Cancer chemotherapy | [56,125] |
| Hexagonal Boron Nitride Nano sheet Drug Delivery System | Biocompatibility, very little to no level of toxicity, high drug-loading ability | Hydrophobicity makes it challenging to function in a biological system | Used in tumor labeling and sensing | [57] |
| Polymer-Lipid Hybrid Nanoparticles Drug Delivery System | Physical stability in encapsulation and biocompatibility, in vivo cell delivery efficacy | Poor drug entrapment and loading capacity, Inflammation and tissue damage can affect or inactivate lipids in serum. | Targeted anticancer therapy | [65,126,127] |
| Self-Micro emulsifying Drug-Delivery System | Bioavailability of poor solubility drugs, long duration stability, protection of drug-sensitive materials, availability of the liquid and solid forms of drugs | Interaction of drugs could potentially accelerate physical aging in patients due to interactions of glyceride and oxidation of vegetable oil. | Used in the treatment of pulmonary infections | [128,129] |
| In-Situ Gel Drug Delivery System | Increased patient compliance, reduced number of administrations, increased bioavailability, controlled and sustained release | Only drugs with a low dosage can be given, eating and drinking may be restricted after administration for a few hours, low stability due to chemical degradation, require more fluid | Employed in rhinitis, used to reduce ocular intolerance in glaucoma, and insulin permeation interactions. | [83,130] |
| Micro Electro Mechanical Systems (Mems) For Drug Delivery | Large drug loading capacity, bioavailability, precise drug delivery, efficient and less painful | Requires repeated surgeries for refilling | Heart-related conditions, contraceptives in women | [105,108,115] |
| Combination drug delivery approach | More loading capacity and increased efficacy, sustained drug release, limited cytotoxicity, and immunogenicity. | There could be low patient compliance, inflexible fixed dose ratio, incompatible pharmacokinetics, increased toxicity | Chemotherapy, hypothermia therapy | [117,119,131] |
| Targeted Drug Delivery System | Reduced side effects and increased efficacy, materials can be metabolized by the liver and kidney thus lower the level of toxicity | High cost of productivity, immunogenicity, and non-specificity of the targeting ligand, can be easily clearance from the blood | Cancer therapy, tumor-related diseases. | [120,132,133] |
4. Challenges associated with current drug delivery systems
Many delivery systems have been used successfully recently, there has been a great development in the quest to deliver drugs from various plant sources to their target sites for treatment in the body, however there are numerous limitations and challenges to what these systems can achieve in treatment, some of which are discussed below.
A major challenge facing the advancement of drug delivery systems is the limited amount of literature and variation in the available literature. Literature provides important information for the advancement of any research and in this case, nanomedicine approaches to treatment. The variation in published studies in relation to recorded characterization of reported experimental details is seen to also be a major difficulty in the progression of nanotechnology application in medicine [134]. The limited and varied information that should be a guide for industries could impede future breakthroughs of nanomedicines and delay the transformation from research and experimentation to clinical application [135]. Many researchers agree that nanoparticles can be either good or bad, the benefits of nanoparticles are more widely known and recognized but there is a scarcity of information when it comes to how safe these particles are, their level of interactions with proteins that are not specific to them and their movement and interaction with other organs that are not their target organs [136].
Some of these delivery systems make use of large particles as carriers which are not particularly favorable for treatment because they can constitute challenges such as poor absorption and solubility, in vivo instability, poor bioavailability, target-specific delivery complications, and several adverse side effects upon administration [23], the use of much smaller particles for delivery to the human biological system is a way out that solves the issues that come with using much larger particles.
Target-specific delivery complication is a challenge that faces all delivery systems. Even though target-specific delivery has been found to reduce toxicity and shows more effective treatment, its efficacy cannot be assured until it is able to reach the targeted site in sufficient amount, this is seen when siRNA is given systemically, rarely do they get to their target cell/organ because they are easily degraded by body enzymes, and when administered in large quantity their negative charge becomes an obstacle to absorption by the cells [137] resulting in little or no absorption by the body. Micelles and liposomes which are lipid nanoparticles are being studied for target drug delivery, but the downside to this is the lowering of their efficiency by their reaction with the body, these reactions include phagocytic absorption and hepatic filtration, which could lead to failure in target delivery, also the nanoparticles could show signs of toxicity [138]. The challenge to targeted delivery is that a patient that is unconscious cannot take a dose, there is low solubility and permeability at the target site, they could interact with food and may be degraded by gastro intestinal flora [139].
The toxicity of particles used in delivery is another great challenge that faces drug delivery systems in general, some of the nanomaterials used can be harmful to human health and also the environment [140]. In vivo and in vitro experimentation has shown the harmful effect of silver, gold, silica, and titanium used as nanoparticles for coupling and delivering drugs [136]. Carbon nanotubes (CNTs) have become widely used in gene therapy, bio-imaging, and drug delivery [141]. because they have been found to have the ability to cross cell membranes even when they are used as carriers for biomolecules [142] but the properties of carbon nanotubes have raised concerns among researchers especially in its use in drug delivery because experiments show that they can cause harm to embryos, genes, liver, heart, neurons and immune system [136]. Although carbon nanotubes have shown favorable results in their use, it is important to carry out crucial toxicity tests to ensure their safety before a widespread application in treatment [141]. In application, their effects have become an obstacle in their use for cancer treatment [143].
Scientists have successfully produced drugs that can serve as carriers and drugs as well, one of the major challenges facing drug delivery systems is biocompatibility (their ability to function with the body in specific situations) and acceptability (being received by the body without triggering the immune system), this is a problem because the way the body reacts to biological materials are very different from the way they react to synthetic materials [140]. In addition, because of the complex structure of the human system, there could be natural barriers to the functions of these delivery systems for example the blood brain-barrier (BBB) has a selectively permeable feature that makes it difficult in achieving therapeutic drug concentration in the brain tissues, the BBB prevents the entry of carrier particles into the brain and entire central nervous system causing ineffectiveness of therapeutic agents in the treatment of cerebral diseases due to inability to deliver and sustain intended drugs within the brain efficiently [144]. Also, one of the most abundant carriers in the body are the monoclonal antibodies (mAb), because they form immunoliposomes by binding to liposome surfaces however the functions of these immunoliposomes are limited because they can trigger an immune response and low levels of absorption, distribution, metabolism, and elimination by the body, this presents a challenge in the use of liposomes to achieve efficacy as a site-specific drug carrier [145].
The kidney and liver also have a natural ability to detox the body, these organs can treat the nanoparticles as potential waste products. Their function can constitute obstruction in drug delivery and lead to the accumulation of nanoparticles in these organs. In the liver, nanomaterials accumulate primarily in the Kupffer cells, macrophages in the liver, sinusoidal endothelial cells, hepatic stellate cells, and a little in the hepatocytes. Meanwhile the size, charge and shape of the kidney decide the fate of the nanomaterials once they get into the renal system [146].
5. Future directions and conclusion
Drug delivery and nanomedicine have become a very fascinating area of research in modern science, in the past years, it has gotten a lot of attention in both research, experimentation and in a number of clinical trials [147]. Despite the setback that have limited the clinical application of these delivery systems, the recent drug delivery system holds great potential, it would require a collaboration across academic theory, laboratory experimentation, the knowledge of medicine, pharmaceuticals, and great research to help achieve the efficiency we need to take, findings from bench to bedside [148]. Vargason et al. [2] believe that the use of cell therapies can go a long way to solve the bio-acceptability issues that drug delivery systems face, they also think it will create a single dose that is effective that avoids high accumulation of drugs in the system. As a matter of fact, cell therapies promise a seeming sustained source of complex biologics, break down innate biological barriers and create responses that appear natural within the system. Adepu [149] have suggested the use of inorganic mesoporous nanoparticles, micro fluids, and molecular imprinting polymers as some of the ways to combat some of the challenges that face drug delivery. According to Khalid et al. [137] a way to improve drug delivery efficacy is by the use of priming agents that can influence the biological environment where they are administered, especially those that can change the form and function of tissues in a way that makes the administered drug favorable without harming the patient. Also, cell-based drug systems should be considered in the field of biomaterials, this means the use of cells coupled with nano biomaterials since cells are indigenous to the human system, this is a novel method and still theoretical but appears to be most creative, encourages drug delivery method with hopes to achieve maximum drug delivery pattern. A lot of research and clinical trials are still needed to foster the efficiency of these modern drug delivery systems and the challenges that face their usage.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
No data was used for the research described in the article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Rayaprolu B.M., Strawser J.J., Anyarambhatla G. Excipients in parenteral formulations: selection considerations and effective utilization with small molecules and biologics. Drug Dev. Ind. Pharm. 2018;44:1565–1571. doi: 10.1080/03639045.2018.1483392. [DOI] [PubMed] [Google Scholar]
- 2.Vargason A.M., Anselmo A.C., Mitragotri S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021;5:951–967. doi: 10.1038/s41551-021-00698-w. [DOI] [PubMed] [Google Scholar]
- 3.Alqahtani M.S., Kazi M., Alsenaidy M.A., Ahmad M.Z. Advances in oral drug delivery. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.618411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sahoo D., Bandaru R., Samal S.K., Naik R., Kumar P., Kesharwani P., Dandela R. In: Chapter 9 - Oral Drug Delivery of Nanomedicine. Kesharwani P., Taurin S., T.-T. K.B., A. of N.N. GreishGreish, editors. Academic Press; 2021. pp. 181–207. [DOI] [Google Scholar]
- 5.Morales J.O., Vuddanda P.R., Velaga S. Fundam. Drug Deliv. 2021. Controlled drug delivery via the buccal and sublingual routes; pp. 433–448. [DOI] [Google Scholar]
- 6.Hussein N.R., Omer H.K., Elhissi A.M.A., Ahmed W. In: Chapter 15 - Advances in Nasal Drug Delivery Systems. Ahmed W., Phoenix D.A., Jackson M.J., in M. C.P.B.T.-A., Charalambous S.E., editors. Academic Press; 2020. pp. 279–311. [DOI] [Google Scholar]
- 7.Chauhan A., Fitzhenry L., Serro A.P. Recent Advances in Ophthalmic Drug Delivery. 2022:1–5. doi: 10.3390/pharmaceutics14102075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thirunavukkarasu A., Nithya R., Jeyanthi J. Transdermal drug delivery systems for the effective management of type 2 diabetes mellitus: a review. Diabetes Res. Clin. Pract. 2022 doi: 10.1016/j.diabres.2022.109996. [DOI] [PubMed] [Google Scholar]
- 9.Sharma P., Gajula K., Dingari N.N., Gupta R., Gopal S., Rai B., Iacocca R.G. Subcutaneous drug delivery: a review of the state-of-the-art modelling and experimental techniques. J. Biomech. Eng. 2022 doi: 10.1115/1.4055758. [DOI] [PubMed] [Google Scholar]
- 10.Misbah Ul Haq M., Razzak M., Uddin M.A., Ahmed N., Shahidulla D. Rectal drug delivery system: an overview. Clin. Pharmacol. Biopharm. 2021;10 [Google Scholar]
- 11.Mahant S., Sharma A.K., Gandhi H., Wadhwa R., Dua K., Kapoor D.N. Emerging trends and potential prospects in vaginal drug delivery. Curr. Drug Deliv. 2022 doi: 10.2174/1567201819666220413131243. [DOI] [PubMed] [Google Scholar]
- 12.Cho M., Joo M., Kim K., Wook Y., Lee S., Mi Y., Ho I. Biochemical and Biophysical Research Communications the immunotherapeutic effects of recombinant Bacillus rin resistant to antimicrobial peptides on Calmette-Gu e bladder cancer cells. Biochem. Biophys. Res. Commun. 2018 doi: 10.1016/j.bbrc.2018.12.097. [DOI] [Google Scholar]
- 13.Palugan L., Cerea M., Cirilli M., Moutaharrik S., Maroni A., Zema L., Melocchi A., Uboldi M., Filippin I., Foppoli A., Gazzaniga A. International Journal of Pharmaceutics : X Intravesical drug delivery approaches for improved therapy of urinary bladder diseases. Int. J. Pharm. X. 2021;3 doi: 10.1016/j.ijpx.2021.100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verma R., Garg S. 2001. Current Status of Drug Delivery Technologies and Future Directions. [Google Scholar]
- 15.Keraliya R.A., Patel C., Patel P., Keraliya V., Soni T.G., Patel R.C., Patel M.M. Osmotic drug delivery system as a part of modified release dosage form. ISRN Pharm. 2012 doi: 10.5402/2012/528079. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattos B.D., Rojas O.J., Magalh W.L.E. Biogenic silica nanoparticles loaded with neem bark extract as green. slow-release biocide. 2017;142:4206–4213. doi: 10.1016/j.jclepro.2016.11.183. [DOI] [Google Scholar]
- 17.Ding C., Li Z. A review of drug release mechanisms from nanocarrier systems. Mater. Sci. Eng. C. 2017;76:1440–1453. doi: 10.1016/j.msec.2017.03.130. [DOI] [PubMed] [Google Scholar]
- 18.Chen K., Chen X. 2010. Design and Development of Molecular Imaging Probes; pp. 1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faheem A.M., Abdelkader D.H. Elsevier LTD.; 2020. Novel Drug Delivery Systems. 1. [DOI] [Google Scholar]
- 20.Pathak C., Vaidya F.U., Pandey S.M. Elsevier Inc.; 2019. Chapter 3 - Mechanism for Development of Nanobased Drug Delivery System. [DOI] [Google Scholar]
- 21.Danhier F., Feron O., Préat V. To exploit the tumor microenvironment : passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Contr. Release. 2015;148:135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 22.Agrahari V. 2018. Novel Drug Delivery Systems, Devices, and Fabrication Methods; pp. 303–306. [DOI] [PubMed] [Google Scholar]
- 23.Patra J.K., Das G., Fraceto L.F., Vangelie E., Campos R., Rodriguez P., Susana L., Torres A., Armando L., Torres D., Grillo R. Nano based drug delivery systems : recent developments and future prospects. J. Nanobiotechnol. 2018:1–33. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X., Malardier-jugroot C. 2016. Folic Acid-Conjugated Amphiphilic Alternating Copolymer as a New Active Tumor Targeting Drug Delivery Platform; pp. 4101–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torchilin V.P. Nat. Publ. Gr.; 2014. Nanoparticulate Systems for Drug Delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akala E.O. Theory Pract. Contemp. Pharm. CRC Press; Florida: 2004. Oral controlled release solid dosage forms; pp. 333–366. [Google Scholar]
- 27.Malm C.J., Emerson J., Hiait G.D. Cellulose acetate phthalate as an enteric coating material. J. Am. Pharm. Assoc. (Scientific Ed.). 1951;40:520–525. doi: 10.1002/jps.3030401014. [DOI] [PubMed] [Google Scholar]
- 28.Hillery A., Park K. CRC Press; 2016. Drug Delivery: Fundamentals and Applications. [Google Scholar]
- 29.Lee P.I., Li J.-X. In: Oral Control. Release Formul. Des. Drug Deliv. Theory to Pract. Wen H., Park K., editors. John Wiley & Sons, Inc.; Hoboken, NJ: 2010. Evolution of oral controlled release dosage forms; pp. 21–31. [Google Scholar]
- 30.Yun Y., Lee B.K., Park K. Controlled drug delivery systems: the next 30 years. Front. Chem. Sci. Eng. 2014;8:276–279. doi: 10.1007/s11705-014-1426-x. [DOI] [Google Scholar]
- 31.Zhang W., Zhao Q., Deng J., Hu Y., Wang Y., Ouyang D. Big data analysis of global advances in pharmaceutics and drug delivery 1980-2014. Drug Discov. Today. 2017;22:1201–1208. doi: 10.1016/j.drudis.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Bangham A.D., Horne R.W. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 1964;8 doi: 10.1016/S0022-2836(64)80115-7. 660-IN10. [DOI] [PubMed] [Google Scholar]
- 33.Bangham A.D., Standish M.M., Watkins J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965;13 doi: 10.1016/S0022-2836(65)80093-6. 238-IN27. [DOI] [PubMed] [Google Scholar]
- 34.Shen J., Burgess D. In: Drug Deliv. Fundam. Appl. Hillery A., Park K., editors. CRC Press; New York: 2012. Long acting injections and implants; pp. 73–91. [DOI] [Google Scholar]
- 35.Birrenbach G., Speiser P.P. Polymerized micelles and their use as adjuvants in immunology. J. Pharm. Sci. 1976;65:1763–1766. doi: 10.1002/jps.2600651217. [DOI] [PubMed] [Google Scholar]
- 36.Couvreur P., Tulkens P., Roland M., Trouet A., Speiser P. Nanocapsules: a new type of lysosomotropic carrier. FEBS Lett. 1977;84:323–326. doi: 10.1016/0014-5793(77)80717-5. [DOI] [PubMed] [Google Scholar]
- 37.Park K. Drug delivery of the future: chasing the invisible gorilla. J. Contr. Release. 2016;240:2–8. doi: 10.1016/j.jconrel.2015.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye M., Kim S., Park K. Issues in long-term protein delivery using biodegradable microparticles. J. Control. Release Off. J. Control. Release Soc. 2010;146:241–260. doi: 10.1016/j.jconrel.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 39.Park K. Controlled drug delivery systems: past forward and future back. J. Contr. Release. 2014;190:3–8. doi: 10.1016/j.jconrel.2014.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yun Y.H., Lee B.K., Park K. Controlled drug delivery: historical perspective for the next generation. J. Contr. Release. 2015;219:2–7. doi: 10.1016/j.jconrel.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rahman H.S., Othman H.H., Hammadi N.I., Yeap S.K., Amin K.M., Samad N.A., Alitheen N.B. Novel drug delivery systems for loading of natural plant extracts and their biomedical applications. Int. J. Nanomed. 2020;15:2439–2483. doi: 10.2147/IJN.S227805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vincy A., Mazumder S., Amrita, Banerjee I., Hwang K.C., Vankayala R. Recent progress in red blood cells-derived particles as novel bioinspired drug delivery systems: challenges and strategies for clinical translation. Front. Chem. 2022;10 doi: 10.3389/fchem.2022.905256. https://www.frontiersin.org/articles/10.3389/fchem.2022.905256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patil P.B., Datir S.K., Saudagar R.B. A review on topical gels as drug delivery system. J. Drug Deliv. Therapeut. 2019;9 doi: 10.22270/jddt.v9i3-s.2930. [DOI] [Google Scholar]
- 44.Hanley T., Vankayala R., Lee C.-H., Tang J.C., Burns J.M., Anvari B. Phototheranostics using erythrocyte-based particles. Biomolecules. 2021;11 doi: 10.3390/biom11050729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang C L.Z., Sun X., Cheng L., Yin S., Yang G., Li Y. Multifunctional theranostic red blood cells for magnetic-field-enhanced in vivo combination therapy of cancer. Adv. Mater. 2014;26:4794–4802. doi: 10.1002/adma.201400158. [DOI] [PubMed] [Google Scholar]
- 46.Jiawei Chen L.H. Nanoscale delivery system for nutraceuticals: preparation, application, characterization, safety, and future trends. Food Eng. Rev. 2020;12:14–31. doi: 10.1007/s12393-019-09208. [DOI] [Google Scholar]
- 47.Zinger A., Cooke J.P., Taraballi F. Biomimetic nano drug delivery carriers for treating cardiovascular diseases. Nanomed. Nanotechnol. Biol. Med. 2021;33 doi: 10.1016/j.nano.2021.102360. [DOI] [PubMed] [Google Scholar]
- 48.Gao C., Chu X., Gong W., Zheng J., Xie X., Wang Y., Yang M., Li Z., Gao C., Yang Y. Neuron tau-targeting biomimetic nanoparticles for curcumin delivery to delay progression of Alzheimer's disease. J. Nanobiotechnol. 2020;18:71. doi: 10.1186/s12951-020-00626-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Mamo T., Moseman E.A., Kolishetti N., Salvador-Morales C., Shi J., Kuritzkes D.R., Langer R., Von Andrian U., Farokhzad O.C. Emerging nanotechnology approaches for HIV/AIDS treatment and prevention. Nanomedicine. 2010;5:269–285. doi: 10.2217/nnm.10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moss Joseph Anthony. HIV/AIDS review. Radiol. Technol. 2013;84:247–267. [PubMed] [Google Scholar]
- 51.Alaniz L., Cabrera P.V., Blanco G G., Ernst G., Rimoldi G., Alvarez E., Hajos S.E. Interaction of CD44 with different forms of hyaluronic acid. Its role in adhesion and migration of tumor cells. Cell Commun. Adhes. 2002;9:117–130. doi: 10.1080/15419060214522. [DOI] [PubMed] [Google Scholar]
- 52.Kang L., Gao Z., Huang W., Jin M., Wang Q. Nanocarrier-mediated co-delivery of chemotherapeutic drugs and gene agents for cancer treatment. Acta Pharm. Sin. B. 2015;5:169–175. doi: 10.1016/j.apsb.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rizvi S.A.A., Saleh A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharmaceut. J. 2018;26:64–70. doi: 10.1016/j.jsps.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu T., Li Y., Gu X., Li Q. Development of a hyaluronic acid-based nanocarrier incorporating doxorubicin and cisplatin as a pH-sensitive and CD44-targeted anti-breast cancer drug delivery system. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.532457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jia Y., Chen S., Wang C., Sun T., Yang L. Hyaluronic acid-based nano drug delivery systems for breast cancer treatment: recent advances. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.990145. https://www.frontiersin.org/articles/10.3389/fbioe.2022.990145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang G., Huang H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018;25:766–772. doi: 10.1080/10717544.2018.1450910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharker S.M. Hexagonal boron nitrides (white graphene): a promising method for cancer drug delivery. Int. J. Nanomed. 2019;14:9983–9993. doi: 10.2147/IJN.S205095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weng Q., Wang X., Wang X., Bando Y., Golberg D. Functionalized hexagonal boron nitride nanomaterials: emerging properties and applications. Chem. Soc. Rev. 2016;45:3989–4012. doi: 10.1039/C5CS00869G. [DOI] [PubMed] [Google Scholar]
- 59.Jedrzejczak-Silicka M., Trukawka M., Dudziak M., Piotrowska K., Mijowska E. Nanomater.; Basel, Switzerland: 2018. Hexagonal Boron Nitride Functionalized with Au Nanoparticles-Properties and Potential Biological Applications; p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu J., Zheng T., Tian Y. Functionalized h-BN nanosheets as a theranostic platform for SERS real-time monitoring of MicroRNA and photodynamic therapy. Angew. Chem. Int. Ed. Engl. 2019;58:7757–7761. doi: 10.1002/anie.201902776. [DOI] [PubMed] [Google Scholar]
- 61.Jose C., Amra K., Bhavsar C., Momin M., Omri A. Polymeric lipid hybrid nanoparticles: properties and therapeutic applications. Crit. Rev. Ther. Drug Carrier Syst. 2018;35:555–588. doi: 10.1615/CritRevTherDrugCarrierSyst.2018024751. [DOI] [PubMed] [Google Scholar]
- 62.Pérez-Herrero E., Fernández-Medarde A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Fur Pharm. Verfahrenstechnik e.V. 2015;93:52–79. doi: 10.1016/j.ejpb.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 63.Choudhury H., Gorain B., Pandey M., Khurana R.K., Kesharwani P. Strategizing biodegradable polymeric nanoparticles to cross the biological barriers for cancer targeting. Int. J. Pharm. 2019;565:509–522. doi: 10.1016/j.ijpharm.2019.05.042. [DOI] [PubMed] [Google Scholar]
- 64.Rizwanullah M., Alam M., Harshita, Mir S.R., Rizvi M.M.A., Amin S. Polymer-lipid hybrid nanoparticles: a next-generation nanocarrier for targeted treatment of solid tumors. Curr. Pharmaceut. Des. 2020;26:1206–1215. doi: 10.2174/1381612826666200116150426. [DOI] [PubMed] [Google Scholar]
- 65.Mohanty A., Uthaman S., Park I.-K. Utilization of polymer-lipid hybrid nanoparticles for targeted anti-cancer therapy. Molecules. 2020;25 doi: 10.3390/molecules25194377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pouton C.W. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2006;29:278–287. doi: 10.1016/j.ejps.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 67.Savjani K.T., Gajjar A.K., Savjani J.K. Drug solubility: importance and enhancement techniques. ISRN Pharm. 2012 doi: 10.5402/2012/195727. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vinarov Z., Abdallah M., Agundez J.A.G., Allegaert K., Basit A.W., Braeckmans M., Ceulemans J., Corsetti M., Griffin B.T., Grimm M., Keszthelyi D., Koziolek M., Madla C.M., Matthys C., McCoubrey L.E., Mitra A., Reppas C., Stappaerts J., Steenackers N., Trevaskis N.L., Vanuytsel T., Vertzoni M., Weitschies W., Wilson C., Augustijns P. Impact of gastrointestinal tract variability on oral drug absorption and pharmacokinetics: an UNGAP review. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2021;162 doi: 10.1016/j.ejps.2021.105812. [DOI] [PubMed] [Google Scholar]
- 69.Tang B., Cheng G., Gu J.-C., Xu C.-H. Development of solid self-emulsifying drug delivery systems: preparation techniques and dosage forms. Drug Discov. Today. 2008;13:606–612. doi: 10.1016/j.drudis.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 70.Rajpoot K., Tekade M., Pandey V., Nagaraja S., Youngren-Ortiz S., Tekade R. Self-microemulsifying drug-delivery system: ongoing challenges and future ahead, in. 2019:393–454. doi: 10.1016/B978-0-12-814487-9.00009-0. [DOI] [Google Scholar]
- 71.Gursoy R.N., Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed. Pharmacother. 2004;58:173–182. doi: 10.1016/j.biopha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 72.Mehanna M.M., Mneimneh A.T. Formulation and applications of lipid-based nanovehicles: spotlight on self-emulsifying systems. Adv. Pharmaceut. Bull. 2021;11:56–67. doi: 10.34172/apb.2021.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baloch J., Sohail M.F., Sarwar H.S., Kiani M.H., Khan G.M., Jahan S., Rafay M., Chaudhry M.T., Yasinzai M., Shahnaz G. Self-nanoemulsifying drug delivery system (SNEDDS) for improved oral bioavailability of chlorpromazine: in vitro and in vivo evaluation. Medicina (Kaunas) 2019:55. doi: 10.3390/medicina55050210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dokania S., Joshi A.K. Self-microemulsifying drug delivery system (SMEDDS)--challenges and road ahead. Drug Deliv. 2015;22:675–690. doi: 10.3109/10717544.2014.896058. [DOI] [PubMed] [Google Scholar]
- 75.Lokhande S.S., Phalke N.N., Raje V.N., More S.S. An update review on recent advancements in multiple emulsion. Int. J. Res. Sci. Innov. 2018;5:90–96. www.rsisinternational.org [Google Scholar]
- 76.Parmar N., Singla N., Amin S., Kohli K. Study of cosurfactant effect on nanoemulsifying area and development of lercanidipine loaded (SNEDDS) self nanoemulsifying drug delivery system. Colloids Surf. B Biointerfaces. 2011;86:327–338. doi: 10.1016/j.colsurfb.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 77.Rahman M.A., Hussain A., Hussain M.S., Mirza M.A., Iqbal Z. Role of excipients in successful development of self-emulsifying/microemulsifying drug delivery system (SEDDS/SMEDDS) Drug Dev. Ind. Pharm. 2013;39:1–19. doi: 10.3109/03639045.2012.660949. [DOI] [PubMed] [Google Scholar]
- 78.Azmi N.A., Elgharbawy A.A.M., Motlagh S.R., Samsudin N., Salleh H.M. Nanoemulsions: factory for food, pharmaceutical and cosmetics. Processes. 2019;7 doi: 10.3390/pr7090617. [DOI] [Google Scholar]
- 79.Rao J., Mcclements D. Food-grade microemulsions and nanoemulsions: role of oil phase composition on formation and stability. Food Hydrocolloids. 2012;29:326–334. doi: 10.1016/j.foodhyd.2012.04.008. [DOI] [Google Scholar]
- 80.Maphosa Y., Jideani V.A. In: Factors Affecting the Stability of Emulsions Stabilised by Biopolymers. Karakuş S., editor. IntechOpen; Rijeka: 2018. Ch. 5. [DOI] [Google Scholar]
- 81.Glassman P.M., Muzykantov V.R. Pharmacokinetic and pharmacodynamic properties of drug delivery systems. J. Pharmacol. Exp. Therapeut. 2019;370:570–580. doi: 10.1124/jpet.119.257113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tiwari G., Tiwari R., Sriwastawa B., Bhati L., Pandey S., Pandey P., Bannerjee S.K. Drug delivery systems: an updated review. Int. J. Pharm. Investig. 2012;2:2–11. doi: 10.4103/2230-973X.96920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vigani B., Rossi S., Sandri G., Bonferoni M.C., Caramella C.M., Ferrari F. Recent advances in the development of in situ gelling drug delivery systems for non-parenteral administration routes. Pharmaceutics. 2020;12 doi: 10.3390/pharmaceutics12090859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hua S. Physiological and pharmaceutical considerations for rectal drug formulations. Front. Pharmacol. 2019;10:1196. doi: 10.3389/fphar.2019.01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singh B., Khurana R.K., Garg B., Saini S., Kaur R. Stimuli-responsive systems with diverse drug delivery and biomedical applications: recent updates and mechanistic pathways. Crit. Rev. Ther. Drug Carrier Syst. 2017;34:209–255. doi: 10.1615/CritRevTherDrugCarrierSyst.2017017284. [DOI] [PubMed] [Google Scholar]
- 86.Khule M., Vyavahare S. A Review: in-situ Gel Drug Delivery System. 2021;9:2455–6211. [Google Scholar]
- 87.Bashir R., Maqbool M., Ara I., Zehravi M. An in sight into novel drug delivery system: in situ gels. TANG [HUMANITAS Med. 2021 doi: 10.5667/CellMed.2021.0006. [DOI] [Google Scholar]
- 88.Bashir R., Majeed A., Ali T., Farooq S., Khan N. Floating oral in-situ gel: a review. J. Drug Deliv. Therapeut. 2019;9:442–448. doi: 10.22270/jddt.v9i2.2372. [DOI] [Google Scholar]
- 89.Padhan A., Nanda B.K., Behera B.C. Floating oral in-situ gel, a comprehensive approach of gastro-retentive drug delivery system: a review. Int. J. Pharm. Sci. Res. 2019;10:4026–4039. doi: 10.13040/IJPSR.0975-8232.10(9).4026-39. [DOI] [Google Scholar]
- 90.Sheshala R., Kok Y.Y., Ng J.M., Thakur R.R.S., Dua K. In situ gelling ophthalmic drug delivery system: an overview and its applications., recent pat. Drug Deliv. Formul. 2015;9:237–248. doi: 10.2174/1872211309666150724101227. [DOI] [PubMed] [Google Scholar]
- 91.Wu Y., Liu Y., Li X., Kebebe D., Zhang B., Ren J., Lu J., Li J., Du S., Liu Z. Research progress of in-situ gelling ophthalmic drug delivery system. Asian J. Pharm. Sci. 2019;14:1–15. doi: 10.1016/j.ajps.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paul A., Fathima K.M., Nair S.C. Intra nasal in situ gelling system of lamotrigine using ion activated mucoadhesive polymer. Open Med. Chem. J. 2017;11:222–244. doi: 10.2174/1874104501711010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Agrawal M., Saraf S., Saraf S., Dubey S.K., Puri A., Gupta U., Kesharwani P., Ravichandiran V., Kumar P., Naidu V.G.M., Murty U.S., Ajazuddin, Alexander A. Stimuli-responsive in situ gelling system for nose-to-brain drug delivery. J. Contr. Release. 2020;327:235–265. doi: 10.1016/j.jconrel.2020.07.044. [DOI] [PubMed] [Google Scholar]
- 94.Bialik M., Kuras M., Sobczak M., Oledzka E. Achievements in thermosensitive gelling systems for rectal administration. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22115500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kouchak M. In situ gelling systems for drug delivery. Jundishapur J. Nat. Pharm. Prod. 2014;9 doi: 10.17795/jjnpp-20126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dash S., Singh S., Dash A.K. Injectable in situ gelling delivery system for the treatment of jawbone infections. Ther. Deliv. 2021;12:775–788. doi: 10.4155/tde-2021-0054. [DOI] [PubMed] [Google Scholar]
- 97.Singh K., Sl H. Injectable in-situ gelling controlled release drug delivery system. Int. J. Drug Dev. Res. 2012;4:56–69. [Google Scholar]
- 98.Khudiyev T., Clayton J., Levy E., Chocat N., Gumennik A., Stolyarov A.M., Joannopoulos J., Fink Y. Electrostrictive microelectromechanical fibres and textiles. Nat. Commun. 2017;8:1435. doi: 10.1038/s41467-017-01558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Christ R.D., Wernli R.L., E., editors. Chapter 17 - Navigational Sensors. Second. Butterworth-Heinemann; Oxford: 2014. pp. 453–475. [DOI] [Google Scholar]
- 100.Koch B., Rubino I., Quan F.-S., Yoo B., Choi H.-J. vol. 9. Mater.; Basel, Switzerland: 2016. (Microfabrication for Drug Delivery). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chirra H.D., Desai T.A. Multi-reservoir bioadhesive microdevices for independent rate-controlled delivery of multiple drugs. Small. 2012;8:3839–3846. doi: 10.1002/smll.201201367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ortigoza-Diaz J., Scholten K., Larson C., Cobo A., Hudson T., Yoo J., Baldwin A., Weltman Hirschberg A., Meng E. Techniques and considerations in the microfabrication of parylene C microelectromechanical systems. Micromachines. 2018;9 doi: 10.3390/mi9090422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zaghloul M.E. Mech. Eng. Handb.; 2015. Introduction to Microelectromechanical Systems (MEMS): Design and Application; pp. 1–12. [DOI] [Google Scholar]
- 104.Ziaie B., Baldi A., Lei M., Gu Y., Siegel R.A. Hard and soft micromachining for BioMEMS: review of techniques and examples of applications in microfluidics and drug delivery. Adv. Drug Deliv. Rev. 2004;56:145–172. doi: 10.1016/j.addr.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 105.Cobo A., Sheybani R., Meng E. MEMS: enabled drug delivery systems. Adv. Healthc. Mater. 2015;4:969–982. doi: 10.1002/adhm.201400772. [DOI] [PubMed] [Google Scholar]
- 106.Zhang H., Jackson J.K., Chiao M. Microfabricated drug delivery devices: design, fabrication, and applications. Adv. Funct. Mater. 2017;27 doi: 10.1002/adfm.201703606. [DOI] [Google Scholar]
- 107.Song P., Tng D.J.H., Hu R., Lin G., Meng E., Yong K.-T. An electrochemically actuated MEMS device for individualized drug delivery: an in vitro study. Adv. Healthc. Mater. 2013;2:1170–1178. doi: 10.1002/adhm.201200356. [DOI] [PubMed] [Google Scholar]
- 108.Oh K.W., Ahn C.H. A review of microvalves. J. Micromech. Microeng. 2006;16:R13. doi: 10.1088/0960-1317/16/5/R01. [DOI] [Google Scholar]
- 109.Sutradhar K.B., Sumi C.D. Implantable microchip: the futuristic controlled drug delivery system. Drug Deliv. 2016;23:1–11. doi: 10.3109/10717544.2014.903579. [DOI] [PubMed] [Google Scholar]
- 110.Kuo J.T.W., Yu L., Meng E. Micromachined thermal flow sensors—a review. Micromachines. 2012;3:550–573. doi: 10.3390/mi3030550. [DOI] [Google Scholar]
- 111.Nguyen N.T., Wereley S.T., Shaegh S.A.M. Artech House; 2019. Fundamentals and Applications of Microfluidics.https://books.google.com.ng/books?id=PCStvAEACAAJ [Google Scholar]
- 112.Saliterman S.S. first ed. SPIE Publications; Washington: 2006. Fundamentals of BioMEMS and Medical Microdevices. [Google Scholar]
- 113.Pons-Faudoa F.P., Ballerini A., Sakamoto J., Grattoni A. Advanced implantable drug delivery technologies: transforming the clinical landscape of therapeutics for chronic diseases. Biomed. Microdevices. 2019;21:47. doi: 10.1007/s10544-019-0389-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Staples M. Microchips and controlled-release drug reservoirs. WIREs Nanomedicine and Nanobiotechnology. 2010;2:400–417. doi: 10.1002/wnan.93. [DOI] [PubMed] [Google Scholar]
- 115.Hilt J.Z., Peppas N.A. Microfabricated drug delivery devices. Int. J. Pharm. 2005;306:15–23. doi: 10.1016/j.ijpharm.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 116.Wang H., Huang Y. Combination therapy based on nano codelivery for overcoming cancer drug resistance. Med. Drug Discov. 2020;6 doi: 10.1016/j.medidd.2020.100024. [DOI] [Google Scholar]
- 117.Zamora-Mora V., Fernández-Gutiérrez M., González-Gómez Á., Sanz B., Román J.S., Goya G.F., Hernández R., Mijangos C. Chitosan nanoparticles for combined drug delivery and magnetic hyperthermia: from preparation to in vitro studies, Carbohydr. Polym. 2017;157:361–370. doi: 10.1016/j.carbpol.2016.09.084. [DOI] [PubMed] [Google Scholar]
- 118.Silva F., Sitia L., Allevi R., Bonizzi A., Sevieri M., Morasso C., Truffi M., Corsi F., Mazzucchelli S. Combined method to remove endotoxins from protein nanocages for drug delivery applications: the case of human ferritin. Pharmaceutics. 2021;13 doi: 10.3390/pharmaceutics13020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pang L., Zhang C., Qin J., Han L., Li R., Hong C., He H., Wang J. A novel strategy to achieve effective drug delivery: exploit cells as carrier combined with nanoparticles. Drug Deliv. 2017;24:83–91. doi: 10.1080/10717544.2016.1230903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rani K., Paliwal S. A review on targeted drug delivery: its entire focus on advanced therapeutics and diagnostics. Scholars J. Appl. Med. Sci. 2014;2:328–331. [Google Scholar]
- 121.Murugan C., Rayappan K., Thangam R., Bhanumathi R., Shanthi K., Vivek R., Thirumurugan R., Bhattacharyya A., Sivasubramanian S., Gunasekaran P., Kannan S. Combinatorial nanocarrier based drug delivery approach for amalgamation of anti-tumor agents in breast cancer cells: an improved nanomedicine strategy. Sci. Rep. 2016;6 doi: 10.1038/srep34053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu J., Deng A., Jiang W., Tian R., Shen Y. Synthesis and in vitro evaluation of pH-sensitive magnetic nanocomposites as methotrexate delivery system for targeted cancer therapy. Mater. Sci. Eng. C. Mater. Biol. Appl. 2017;71:132–140. doi: 10.1016/j.msec.2016.09.084. [DOI] [PubMed] [Google Scholar]
- 123.Lin J., Li Y., Wu H., Yang X., Li Y., Ye S., Hou Z., Lin C. Tumor-targeted co-delivery of mitomycin C and 10-hydroxycamptothecin via micellar nanocarriers for enhanced anticancer efficacy. RSC Adv. 2015;5:23022–23033. doi: 10.1039/C4RA14602F. [DOI] [Google Scholar]
- 124.Xia Q., Zhang Y., Li Z., Hou X., Feng N. Red blood cell membrane-camouflaged nanoparticles: a novel drug delivery system for antitumor application. Acta Pharm. Sin. B. 2019;9:675–689. doi: 10.1016/j.apsb.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Simone P., Alberto M. Caution should be used in long-term treatment with oral compounds of hyaluronic acid in patients with a history of cancer. Clin. Drug Invest. 2015;35:689–692. doi: 10.1007/s40261-015-0339-x. [DOI] [PubMed] [Google Scholar]
- 126.Hadinoto K., Sundaresan A., Cheow W.S. Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Fur Pharm. Verfahrenstechnik e.V. 2013;85:427–443. doi: 10.1016/j.ejpb.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 127.Sivadasan D., Sultan M.H., Madkhali O., Almoshari Y., Thangavel N. Polymeric lipid hybrid nanoparticles (PLNs) as emerging drug delivery platform-A comprehensive review of their properties, preparation methods, and therapeutic applications. Pharmaceutics. 2021;13 doi: 10.3390/pharmaceutics13081291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kumar A., Sharma S., Kamble R. Self emulsifying drug delivery system (SEDDS) Future Aspects. 2010:2. [Google Scholar]
- 129.Mishra V., Nayak P., Yadav N., Singh M., Tambuwala M.M., Aljabali A.A.A. Orally administered self-emulsifying drug delivery system in disease management: advancement and patents. Expert Opin. Drug Deliv. 2021;18:315–332. doi: 10.1080/17425247.2021.1856073. [DOI] [PubMed] [Google Scholar]
- 130.Khule M.R., Vyavahare S.B. 2021. A Review : In-Situ Gel Drug Delivery System A Review : In-Situ Gel Drug Delivery System. [Google Scholar]
- 131.Shenfield G.M. Fixed combination drug therapy. Drugs. 1982;23:462–480. doi: 10.2165/00003495-198223060-00003. [DOI] [PubMed] [Google Scholar]
- 132.Zhang W., Zhang Z., Zhang Y. 2011. The Application of Carbon Nanotubes in Target Drug Delivery Systems for Cancer Therapies; pp. 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen W.C., Zhang A.X., Li S. Limitations and niches of the active targeting approach for nanoparticle drug delivery. 2012;4:89–93. doi: 10.1515/ejnm-2012-0010. [DOI] [Google Scholar]
- 134.Youn Y.S., Bae Y.H. Perspectives on the past, present, and future of cancer nanomedicine. Adv. Drug Deliv. Rev. 2018;130:3–11. doi: 10.1016/j.addr.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 135.Pasut G. Grand challenges in nano-based drug delivery. Front. Med. Technol. 2019;1:10–13. doi: 10.3389/fmedt.2019.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sharma S., Parveen R., Chatterji B.P. Toxicology of nanoparticles in drug delivery. Curr. Pathobiol. Rep. 2021;9:133–144. doi: 10.1007/s40139-021-00227-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Khalid A., Persano S., Shen H., Zhao Y., Blanco E., Wolfram J., Medicine-qatar W.C., Medicine W.C., Medicine W.C., Medicine W.C. HHS Public Access. 2018;14:865–877. doi: 10.1080/17425247.2017.1243527.Strategies. [DOI] [Google Scholar]
- 138.Zargar S.M., Hafshejani D.K., Eskandarinia A., Rafienia M., Kharazi A.Z. A review of controlled drug delivery systems based on cells and cell membranes. J. Med. Signals Sens. 2019;9:181–189. doi: 10.4103/jmss.JMSS_53_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gautami J. Research and Reviews: Journal of Pharmaceutics and Nanotechnology IMPLANTABLE DRUG DELIVERY SYSTEM. 2015;3:24–31. [Google Scholar]
- 140.Singh N., Joshi A., Toor A.P., Verma G. Elsevier Inc.; 2017. (Chapter 27) - Drug delivery: Advancements And Challenges. [DOI] [Google Scholar]
- 141.Zare H., Ahmadi S., Ghasemi A., Ghanbari M., Rabiee N., Bagherzadeh M., Karimi M., Webster T.J., Hamblin M.R., Mostafavi E. Carbon nanotubes: smart drug/gene delivery carriers. Int. J. Nanomed. 2021;16:1681–1706. doi: 10.2147/IJN.S299448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Perepelytsina O.M., Ugnivenko A.P., V Dobrydnev A., Bakalinska O.N., Marynin A.I., V Sydorenko M. Influence of carbon nanotubes and its derivatives on tumor cells in vitro and biochemical parameters, cellular blood composition in vivo. Nanoscale Res. Lett. 2018;13:286. doi: 10.1186/s11671-018-2689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hafeez V., Celia Muhammad Nadeem, Christian, Petrikaite Challenges towards targeted drug delivery in cancer nanomedicines. Processes. 2021;9:1527. doi: 10.3390/pr9091527. [DOI] [Google Scholar]
- 144.Danaei M., Dehghankhold M., Ataei S., Hasanzadeh Davarani F., Javanmard R., Dokhani A., Khorasani S., Mozafari M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10:1–17. doi: 10.3390/pharmaceutics10020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Spitler Ryan J.T., Zanganeh Saeid H.J.Q., Nasser Khakpash, Mohsen Erfanzadeh, Nastaran S. Biomater. Towar. Med. Serv. 2017. Drug delivery systems: possibilities and challenges; pp. 1–51. [DOI] [Google Scholar]
- 146.Bartucci R., Paramanandana A., Boersma Y.L., Olinga P., Salvati A. Comparative study of nanoparticle uptake and impact in murine lung, liver and kidney tissue slices. Nanotoxicology. 2020;14:847–865. doi: 10.1080/17435390.2020.1771785. [DOI] [PubMed] [Google Scholar]
- 147.Pandit A., Zeugolis D.I. Twenty-five years of nano-bio-materials: have we revolutionized healthcare? Nanomedicine (Lond). 2016;11:985–987. doi: 10.2217/nnm.16.42. [DOI] [PubMed] [Google Scholar]
- 148.Haider S. Nanoparticles : the Future of Drug Delivery. 2020;38:1–2. [Google Scholar]
- 149.Adepu S., Ramakrishna S. Controlled drug delivery systems: current status and future directions. Molecules. 2021;26 doi: 10.3390/molecules26195905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.