Abstract
Background
Heart failure (HF) is associated with a high bleeding risk after percutaneous coronary intervention (PCI). Additionally, major bleeding events increase the risk of subsequent major adverse cardiac events (MACE). However, whether brain natriuretic peptide (BNP) levels and major bleeding events following PCI are associated with MACE and all-cause death remains unknown. This study aimed to investigate the impact of HF severity or bleeding on subsequent MACE and all-cause death.
Methods
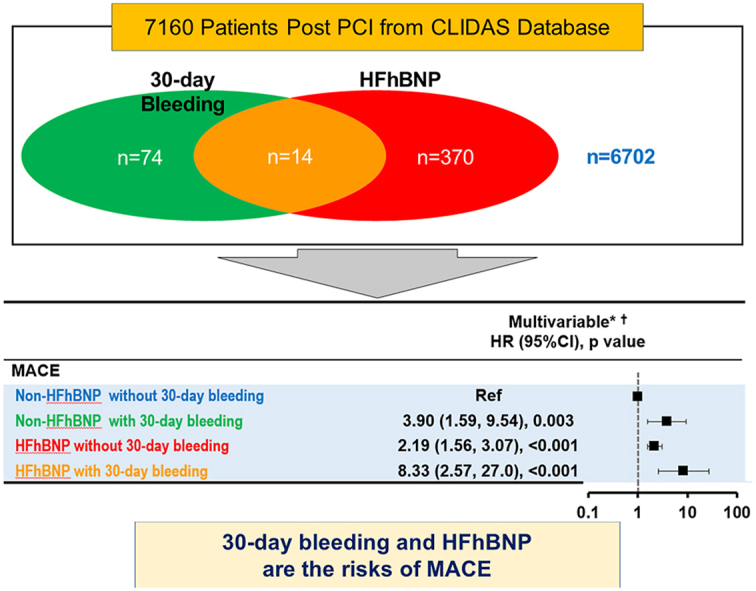
The Clinical Deep Data Accumulation System (CLIDAS), a multicenter database involving seven hospitals in Japan, was developed to collect data from electronic medical records. This retrospective analysis included 7160 patients who underwent PCI between April 2014 and March 2020 and completed a three-year follow-up. Patients were divided according to the presence of HF with high BNP (HFhBNP) (>100 pg/ml) and major bleeding events within 30 days post-PCI (30-day bleeding): HFhBNP with bleeding (n = 14), HFhBNP without bleeding (n = 370), non-HFhBNP with bleeding (n = 74), and non-HFhBNP without bleeding (n = 6702).
Results
In patients without 30-day bleeding, HFhBNP was a risk factor for MACE (hazard ratio, 2.19; 95% confidence interval, 1.56–3.07) and all-cause death (hazard ratio, 1.60; 95% confidence interval, 1.60–2.23). Among HFhBNP patients, MACE incidence was higher in patients with 30-day bleeding than in those without bleeding, but the difference was not significant (p = 0.075). The incidence of all-cause death was higher in patients with bleeding (p = 0.001).
Conclusions
HF with high BNP and bleeding events in the early stage after PCI might be associated with subsequent MACE and all-cause death.
Keywords: Acute coronary syndrome, Bleeding, Heart failure, High BNP, Major adverse cardiac event, Percutaneous coronary intervention
Graphical abstract
HFhBNP, patients with previous heart failure hospitalization with high BNP levels (>100 pg/ml); non-HFhBNP, patients with previous heart failure hospitalization with low BNP levels (≤100 pg/ml) and patients without previous heart failure hospitalization.
Highlights
-
•
HF with high BNP levels might increase MACE and all-cause death after PCI.
-
•
Coexistence of 30-day bleeding might increase MACE and all-cause death furthermore.
-
•
Management of HF severity could be important to prevent those adverse events.
-
•
Preventing bleeding complications in the early stages could be also important.
1. Introduction
Antiplatelet therapy is necessary for the primary and secondary prevention of coronary artery disease [1,2]. However, antiplatelet therapy often causes bleeding after a percutaneous coronary intervention (PCI). Moreover, it is known that major bleeding events after PCIs increase the incidence of major adverse cardiac events (MACE) and death [[3], [4], [5]]. Therefore, the prediction and prevention of bleeding events are important to improve prognosis. Scoring high bleeding risk (HBR) effectively predicts and prevents bleeding events and is advocated in Japan [6,7]. However, heart failure (HF) is a risk factor for HBR in Japan [8,9]; thus, we investigated the association between HF and adverse cardiac events after a PCI [10]. Our previous study showed that HF with high brain natriuretic peptide (BNP) levels (>100 pg/ml) increased the incidence of bleeding events, MACE, and all-cause death; however, there was no significant association between HF with low BNP levels (≤100 pg/ml) and these clinical events [10]. Moreover, the highest number of bleeding events occurred within 30 days after PCI [10]. Therefore, we hypothesized that a bleeding event in the early stage after PCIs would increase the risk of subsequent cardiovascular events, especially in patients with HF. Whether BNP levels and major bleeding events following PCI are associated with MACE and all-cause death is unclear; thus, we aimed to investigate this association. This study clarified whether HF with high BNP levels (>100 pg/ml) and major bleeding events within 30 days are associated with subsequent MACE and all-cause death.
2. Methods
2.1. Study population and protocol
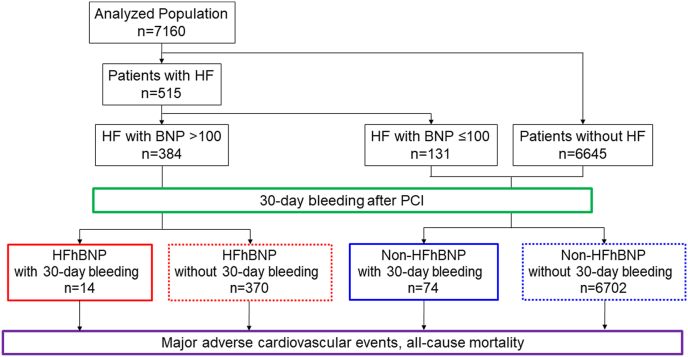
The Clinical Deep Data Accumulation System (CLIDAS), a multicenter real-world database involving seven tertiary medical hospitals in Japan, was developed to collect data in electronic medical records. Data on PCI date, age, sex, physiological tests (body mass index [BMI], blood pressure, and pulse rate), laboratory tests (creatinine, estimated glomerular filtration rate [eGFR], and BNP), medications (antiplatelet, anticoagulant, and proton-pomp inhibitor), echocardiogram parameters (left ventricular ejection fraction [LVEF]), cardiac catheterization, and PCI treatment (target lesion and multivessel) were all directly collected and analyzed based on the Standardized Structured Medical Information eXchange Extended Storage. Additionally, the following data were collected: urgency (acute cardiac syndrome), past history (hypertension, diabetes mellitus [DM], dyslipidemia, hemodialysis, previous PCI, previous coronary artery bypass grafting [CABG], previous myocardial infarction [MI], previous stroke, atrial fibrillation [AF], malignancy, smoking, and peripheral artery disease [PAD]), and long-term prognosis (bleeding events, MACE, and all-cause death) [11,12]. This retrospective study included a total of 9690 consecutive patients who underwent PCI for acute coronary syndrome (ACS) or stable coronary artery disease between April 2014 and March 2020 in the participating hospitals, were registered in the CLIDAS database, and followed up for three years. After excluding 2530 patients who underwent PCI without valid data for prior HF hospitalization, plasma BNP levels within 30 days of index PCI, or effective follow-up, the remaining 7160 patients were further analyzed. HF refers to a previous hospitalization for HF diagnosed by experienced cardiologists based on the Framingham criteria [13]. Patients were divided into two groups: HF with high BNP levels (HFhBNP) (>100 pg/ml) (n = 384) and non-HFhBNP (HF with low BNP levels [≤100 pg/ml] and patients without HF) (n = 6776). Each of the two groups was further divided into two subgroups based on the presence or absence of major bleeding events within 30 days after PCI (30-day bleeding) (Fig. 1). Major bleeding events were defined as moderate or severe bleeding according to the GUSTO bleeding criteria [14]. Furthermore, moderate bleeding events are those requiring blood transfusion but not resulting in hemodynamic compromise. Severe bleeding events are intracerebral hemorrhage or those resulting in substantial hemodynamic compromise requiring treatment.
Fig. 1.
Study flowchart. Patients were divided into four groups according to the presence of heart failure (HF) with high BNP levels (HFhBNP) (>100 pg/ml) and major bleeding events within 30 days after PCI. HF, heart failure; BNP, brain natriuretic peptide; PCI, percutaneous coronary intervention.
2.2. Ethical consideration
This study was approved by the Institutional Review Board of Kumamoto University Hospital (Senshin-No. 2406) and each institutional ethics committee and was conducted in accordance with the Declaration of Helsinki. This study was waived from the requirement for individual informed consent because all data were anonymized by the participating institutions and were then collected in the CLIDAS database.
2.3. Variables
In the CLIDAS database, data for BNP values were obtained from the lowest value measured 30 days before and after the index PCI. Other laboratory data were calculated as average values from 60 days before the index PCI to 30 days after the intervention. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg [15]. Moreover, patients who were prescribed medical treatment for hypertension were included. DM was defined as hemoglobin A1C level ≥6.5%, casual blood glucose level ≥200 mg/dL, or fasting blood glucose level ≥126 mg/dL [16]. Moreover, patients undergoing medical treatment for DM were included. Dyslipidemia was defined as a medical treatment for dyslipidemia at the index PCI. We calculated eGFR using the serum creatinine level, age, weight, and sex. The formula is as follows: eGFR = 194 × Cr−1.094 × age−0.287 (man); eGFR = 194 × Cr−1.094 × age−0.287 × 0.739 (woman) [17,18]. We defined chronic kidney disease (CKD) as eGFR <60 ml/min per 1.73 m2 [18]. We used echocardiogram measurements closest to the index PCI, between 100 and 0 days before the index PCI. LVEF was calculated using the modified Simpson's rule or the Teichholz method if the data for the modified Simpson's rule were missing [19,20]. The number of diseased vessels was defined as the number of coronary segments with severe stenosis (≥75%) in the major epicardial coronary arteries: right coronary artery, left anterior descending artery, and left circumflex artery. The presence of a diseased left main coronary trunk (LMT), defined as ≥75% stenosis, was considered separately. Patients were categorized according to the number of diseased vessels and the presence of an LMT.
2.4. Clinical outcomes
We defined the primary outcome as MACE between 30 days after a PCI and the three-year follow-up. MACE included cardiac death, MI, and stroke. The secondary outcome was defined as all-cause death between 30 days after a PCI and the three-year follow-up.
2.5. Statistical analysis
Normally distributed continuous variables are expressed as mean ± standard deviation (SD) and data with a skewed distribution as median values (interquartile range, IQR). Categorical variables are described as frequencies and percentages. Group comparisons were analyzed using the unpaired t-test or Mann-Whitney U test for continuous variables between two groups. Continuous variables were compared among three groups by one-way analysis of variance or the Kruskal-Wallis test, followed by multiple comparisons with the Bonferroni method. The chi-square or Fisher's exact test was used to compare categorical variables. A log-rank test was performed for MACE and all-cause death. Cox proportional hazards regression analysis was performed to compute hazard ratios (HRs) and 95% confidence intervals (CI) as estimates of MACE and all-cause death. Multivariate Cox proportional hazard regression analysis was adjusted for age, sex, BMI, ACS, hypertension, DM, dyslipidemia, CKD, hemodialysis, previous PCI, previous CABG, prior MI, prior stroke, prior AF, prior PAD, LMT, multivessel disease (MVD), anticoagulants, dual antiplatelet therapy, and proton pump inhibitors. For sensitivity analysis, multiple imputation analysis was performed with 20 imputed datasets generated by the fully conditional specification method. The results across the 20 imputed datasets were combined using Rubin's rules. A two-tailed p value of <0.05 denoted a statistically significant difference. All statistical analyses were performed using Statistical Package for the Social Sciences software version 23 (IBM Corporation, Armonk, NY, USA).
3. Results
3.1. Baseline characteristics
The number of patients without previous HF hospitalization was 6,645, and their BNP level was 51 (21, 134) pg/ml. In addition, the BNP levels of patients with previous HF hospitalization were 231 (98, 504) pg/ml.
Furthermore, we categorized the patients into four groups based on the presence or absence of 30-day bleeding: HFhBNP with 30-day bleeding (n = 14), HFhBNP without 30-day bleeding (n = 370), non-HFhBNP with 30-day bleeding (n = 74), and non-HFhBNP without 30-day bleeding (n = 6702) (Fig. 1 and Table 1).
Table 1.
Baseline characteristics.
| HFhBNP with 30-day bleeding (n = 14) | HFhBNP without 30-day bleeding (n = 370) | Non-HFhBNP with 30-day bleeding (n = 74) | Non-HFhBNP without 30-day bleeding (n = 6702) | p value | |
|---|---|---|---|---|---|
| Age, years | 74.5 (65.5, 84.25) | 75 (67, 81) | 70 (65.25, 78.75) | 71 (64, 78) | <0.001 |
| Male | 8 (42.9%) | 259 (70.0%) | 40 (54.1%) | 5243 (78.2%) | <0.001 |
| BMI, kg/m2 | 26.0 (23.3, 28.1) | 22.5 (20.2, 25.1) | 21.1 (19.8, 24.7) | 24.0 (21.9, 26.3) | <0.001 |
| missing data | 0 (0%) | 5 (1.35%) | 0 (0%) | 134 (2.00%) | |
| SBP at discharge | 115 (105.5, 132) | 114 (99, 127) | 107 (98, 122.5) | 118 (106, 130) | <0.001 |
| missing data | 3 (21.4%) | 41 (11.1%) | 36 (48.6%) | 945 (14.1%) | |
| DBP at discharge | 58 (52, 66.5) | 60 (54, 68) | 63 (56, 67.75) | 64 (58, 72) | <0.001 |
| missing data | 3 (21.4%) | 41 (11.1%) | 36 (48.6%) | 946 (14.1%) | |
| PR at discharge | 73 (56, 92) | 70 (63, 78) | 70 (65, 78) | 67 (60, 75) | <0.001 |
| missing data | 5 (35.7%) | 45 (12.2%) | 37 (50%) | 982 (14.7%) | |
| ACS | 8 (57.1%) | 110 (29.7%) | 45 (60.8%) | 3066 (45.7%) | <0.001 |
| Hypertension | 10 (71.4%) | 336 (90.8%) | 50 (67.6%) | 5569 (83.1%) | <0.001 |
| missing data | 0 (0%) | 0 (0%) | 1 (1.4%) | 15 (0.2%) | |
| Dyslipidemia | 10 (71.4%) | 274 (74.1%) | 46 (62.2%) | 5277 (78.7%) | <0.001 |
| missing data | 0 (0%) | 0 (0%) | 0 (0%) | 10 (0.1%) | |
| Diabetes | 10 (71.4%) | 211 (57.0) | 28 (37.8%) | 2851 (42.5%) | <0.001 |
| missing data | 0 (0%) | 2 (0.5%) | 0 (0%) | 38 (0.6%) | |
| Smoking | 1 (7.1%) | 72 (19.5%) | 11 (14.9%) | 1431 (21.4%) | 0.009 |
| missing data | 5 (35.7%) | 168 (45.4%) | 21 (28.4%) | 3046 (45.4%) | |
| CKD (eGFR<60) | 14 (100%) | 282 (76.2%) | 42 (56.8%) | 2939 (43.4%) | <0.001 |
| missing data | 0 (0%) | 10 (2.7%) | 8 (10.8%) | 426 (6.4%) | |
| eGFR | 22.6 (11.4, 37.8) | 40.2 (16.2, 57.2) | 50.4 (21.7, 71.7) | 61.6 (47.9, 73.8) | <0.001 |
| Hemodialysis | 3 (21.4%) | 67 (18.1%) | 11 (14.9%) | 367 (5.5%) | <0.001 |
| missing data | 0 (0%) | 1 (0.3%) | 0 (0%) | 6 (0.01%) | |
| Family history of CVD | 1 (7.1%) | 66 (17.8%) | 16 (21.6%) | 1418 (21.2%) | 0.207 |
| missing data | 2 (14.3%) | 73 (19.7%) | 1 (1.4%) | 1294 (12.3%) | |
| Malignancy | 1 (7.1%) | 49 (13.2%) | 6 (8.1%) | 627 (9.4%) | 0.081 |
| missing data | 1 (7.1%) | 21 (5.7%) | 3 (4.1%) | 297 (4.4%) | |
| Previous MI | 3 (21.4%) | 106 (28.6%) | 9 (12.2%) | 976 (14.6%) | <0.001 |
| missing data | 1 (7.1%) | 1 (0.3%) | 0 (0%) | 6 (0.01%) | |
| Previous CABG | 3 (21.4%) | 65 (17.6%) | 7 (9.5%) | 338 (5.0%) | <0.001 |
| missing data | 0 (0%) | 0 (0%) | 0 (0%) | 5 (0.01%) | |
| Previous stroke | 2 (14.3%) | 61 (16.5%) | 6 (8.1%) | 770 (11.5%) | 0.0239 |
| missing data | 0 (0%) | 1 (0.3%) | 0 (0%) | 8 (0.01%) | |
| Atrial fibrillation | 2 (14.3%) | 52 (14.1%) | 2 (2.7%) | 284 (4.2%) | <0.001 |
| missing data | 0 (0%) | 1 (0.3%) | 0 (0%) | 9 (0.01%) | |
| Peripheral arterial disease | 2 (14.3%) | 55 (14.9%) | 9 (12.2%) | 522 (7.8%) | <0.001 |
| missing data | 4 (28.6%) | 22 (5.9%) | 5 (6.8%) | 723 (10.8%) | |
| Culprit lesion | |||||
| RCA | 8 (57.1%) | 207 (55.9%) | 35 (47.3%) | 3418 (51.0%) | 0.131 |
| LAD | 11 (78.6%) | 264 (71.4%) | 43 (58.1%) | 4609 (68.8%) | 0.162 |
| LCX | 9 (64.3%) | 176 (47.6%) | 31 (41.9%) | 2759 (41.2%) | 0.013 |
| LMT | 3 (21.4%) | 40 (10.8%) | 9 (12.2%) | 492 (7.3%) | 0.004 |
| missing data | 0 (0%) | 30 (8.1%) | 9 (12.2%) | 416 (6.2%) | |
| MVD | 9 (64.3%) | 223 (60.3%) | 37 (50.0%) | 3408 (50.9%) | 0.003 |
| BNP | 269.3 (200.2, 745.1) | 350.4 (193.4, 663.6) | 83.3 (29.2, 368.2) | 51.0 (21.3, 130.0) | <0.001 |
| LVEF category | |||||
| <40 | 5 (35.7%) | 143 (38.6%) | 13 (17.6%) | 548 (8.2%) | |
| ≥50 | 3 (21.4%) | 127 (34.3%) | 38 (51.4%) | 4229 (63.1%) | |
| 40–49 | 5 (35.7%) | 71 (19.2%) | 7 (9.5%) | 753 (11.2%) | |
| missing data | 1 (7.1%) | 29 (7.8%) | 16 (21.6%) | 1172 (17.5%) | |
| Anticoagulant | 6 (42.9%) | 101 (27.3%) | 14 (18.9%) | 814 (12.1%) | <0.001 |
| DOAC | 0 (0%) | 42 (11.4%) | 1 (1.4%) | 325 (4.8%) | <0.001 |
| Warfarin | 6 (42.9%) | 62 (16.8%) | 13 (17.6%) | 507 (7.6%) | <0.001 |
| DAPT | 12 (85.7%) | 307 (83.0%) | 57 (77.0%) | 5743 (85.7%) | 0.085 |
| Aspirin | 13 (92.9%) | 339 (91.6%) | 67 (90.5%) | 6204 (92.6%) | |
| missing data | 1 (7.1%) | 31 (8.4%) | 7 (9.5%) | 498 (7.4%) | |
| P2Y12 inhibitor | 13 (92.9%) | 324 (87.6%) | 60 (81.1%) | 6103 (91.1%) | 0.005 |
| PPI | 13 (92.9%) | 308 (83.2%) | 63 (85.1%) | 5497 (82.0%) | 0.705 |
HF indicates heart failure; BNP, B-type natriuretic peptide; BMI, body mass index; SBP, systolic bood pressure; DBP, diastolic blood pressure; PR, pulse rate; ACS, acute coronary syndrome; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; CVD, cardio vascular disease; MI, myocardial infarction; CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention; RCA, right coronary artery; LAD, left anterior descending artery; LCX, left circumflex artery; LMT, left main coronary trunk; MVD, multi vessel disease; LVEF; left ventricular ejection fraction; DOAC, direct oral anticoagulant; DAPT, double anti platelet therapy; PPI, proton pump inhibitor.
The HFhBNP with 30-day bleeding group had a higher BMI and heart rate and significantly higher proportion of DM, CKD, hemodialysis, previous CABG, prior AF, and use of anticoagulants and P2Y12 inhibitors than the other groups. The HFhBNP without 30-day bleeding group had higher age and BNP levels and higher proportion of hypertension, previous MI, and PAD than the other groups. The non-HFhBNP with 30-day bleeding group had a higher proportion of ACS than the other groups. The non-HFhBNP without 30-day bleeding group had more men and a higher proportion of smoking but lower rates of PAD and anticoagulant use than the other groups.
3.2. Primary outcome
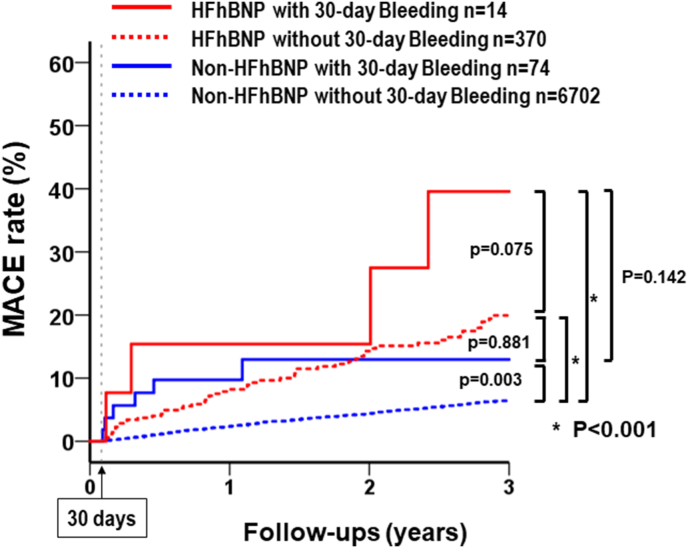
After PCIs, a total of 388 patients developed MACE during follow-up: 28.6% of those in the HFhBNP with 30-day bleeding group (n = 4), 14.9% of those in the HFhBNP without 30-day bleeding group (n = 55), 8.33% of those in the non-HFhBNP with 30-day bleeding group (n = 6), and 4.82% of those in the non-HFhBNP without 30-day bleeding group (n = 323). The Kaplan-Meier survival curves showed a higher rate of MACE in the HFhBNP without 30-day bleeding group than in the non-HFhBNP without 30-day bleeding group (log rank p < 0.001, Fig. 2). Among HFhBNP patients, the incidence of MACE tended to increase in those with 30-day bleeding, but the association was statistically non-significant (log rank p = 0.075). However, 30-day bleeding clearly increased the incidence of MACE in non-HFhBNP patients (log rank p = 0.003). Thus, among patients with 30-day bleeding, the incidence of MACE tended to be higher in the HFhBNP group than in the non-HFhBNP group (log rank p = 0.142).
Fig. 2.
Major adverse cardiac event (MACE) rates of the four groups between 30 days and three years. Kaplan-Meier survival curves show the rate of MACE in PCI patients stratified by HF with high BNP levels (>100 pg/ml) and 30-day bleeding. P-values were calculated using the log-rank test. MACE, major adverse cardiac events; HF, heart failure; BNP, brain natriuretic peptide; PCI, percutaneous coronary intervention.
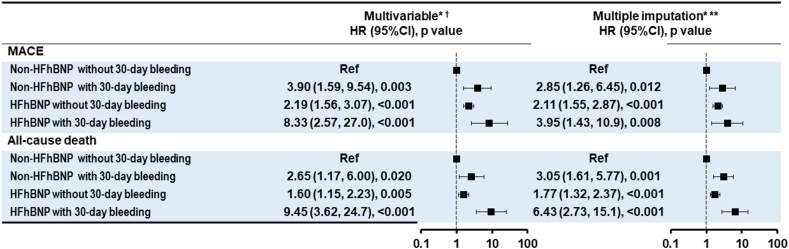
The multivariate Cox regression analysis showed significantly higher rates of bleeding events in all three groups than in the non-HFhBNP without 30-day bleeding group: non-HFhBNP with 30-day bleeding (HR, 3.90; 95% CI, 1.59–9.54, p = 0.003), HFhBNP without 30-day bleeding (HR, 2.19; 95% CI, 1.56–3.07, p < 0.001), and HFhBNP with 30-day bleeding (HR, 8.33; 95% CI, 2.57–27.0, p < 0.001) (Fig. 3).
Fig. 3.
Cox proportional hazard regression for MACE and all-cause death between 30 days and three years. *Adjusted for age, sex, body mass index, systolic blood pressure at admission, acute coronary syndrome or chronic coronary syndrome, hypertension, diabetes, dyslipidemia, chronic kidney disease, hemodialysis, previous PCI, previous coronary artery bypass grafting, prior MI, prior stroke, prior AF, prior PAD, LMT, MVD, anticoagulants, DAPT, and PPI. †Complete case analysis (n = 5330, 74.4%). ** A multiple imputation analysis was performed with 20 imputed datasets generated by the fully conditional specification method. The results across the 20 imputed datasets were combined using Rubin's rules. HR, hazard ratio, CI; confidence interval; HF, heart failure; BNP, brain natriuretic peptide; PCI, percutaneous coronary intervention; AF, atrial fibrillation; MVD, mitral valve disease; PAD, peripheral artery disease LMT, left main coronary trunk; DAPT, dual antiplatelet therapy; PPI, proton pump inhibitor.
3.3. Secondary outcome
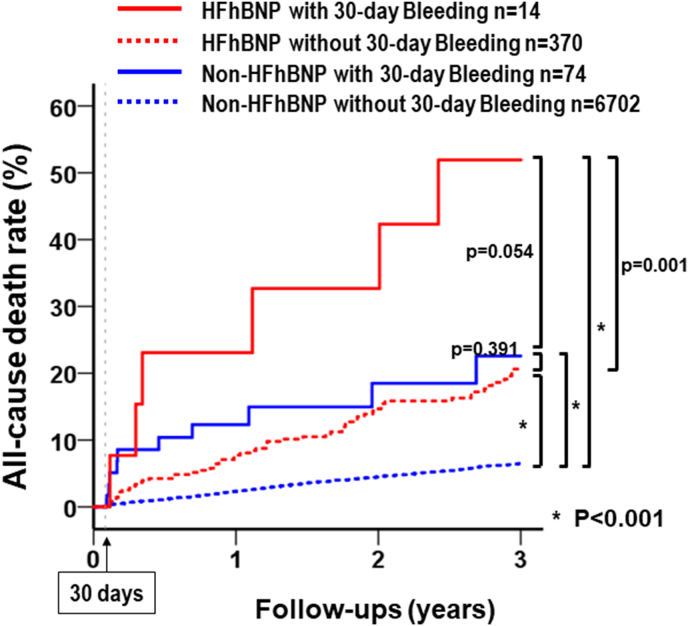
After PCI, a total of 409 patients had all-cause death during follow-up: 42.9% in the HFhBNP with 30-day bleeding group (n = 6), 15.7% in the HFhBNP without 30-day bleeding group (n = 58), 13.5% in the non-HFhBNP with 30-day bleeding group (n = 10), and 5.0% in the non-HFhBNP without 30-day bleeding group (n = 335). The Kaplan-Meier survival curves showed a higher rate of all-cause death in the HFhBNP without 30-day bleeding group than in the non-HFhBNP without 30-day bleeding group (log rank p < 0.001, Fig. 4). The rate of all-cause death increased with 30-day bleeding in both HFhBNP (log rank p = 0.001) and non-HFhBNP patients (log rank p < 0.001). Among patients with 30-day bleeding events, the all-cause death incidence tended to be high in non-HFhBNP patients (log rank p = 0.054).
Fig. 4.
All-cause death rates of the four groups between 30 days and three years. Kaplan-Meier survival curves show the all-cause death rate in PCI patients stratified by HF with high BNP levels (>100 pg/ml) and 30-day bleeding. P-values were calculated using the log-rank test. HF, heart failure; BNP, brain natriuretic peptide; PCI, percutaneous coronary intervention.
The multivariate Cox regression analysis revealed significantly higher rates of bleeding events in all the other three groups than in the non-HFhBNP without 30-day bleeding group: non-HFhBNP with 30-day bleeding (HR, 2.65; 95% CI, 1.17–6.00, p = 0.020), HFhBNP without 30-day bleeding (HR, 1.60; 95% CI, 1.15–2.23, p = 0.005), and HFhBNP with 30-day bleeding (HR, 9.45; 95% CI, 3.62–24.7, p < 0.001) (Fig. 3).
3.4. Sensitivity analysis
To assess the robustness of the association between HF severity, 30-day bleeding events, and clinical outcomes, sensitivity analyses were performed using the multiple imputation method (Fig. 3). The sensitivity analysis results have been recapitulated in the main analysis.
4. Discussion
Based on large-scale real-world data, this study revealed that HFhBNP was a risk factor for MACE and all-cause death among patients without 30-day bleeding. Moreover, 30-day bleeding increased the risk of MACE in both the HFhBNP and non-HFhBNP groups. Therefore, HF with high BNP levels (>100 pg/ml) and bleeding events within 30 days of PCI might increase the risk of MACE and all-cause death in patients undergoing PCI.
There is a strong association between HF and subsequent adverse events [8,9]. Specifically, Natsuaki et al. reported that HF was a strong risk factor for both thrombotic and bleeding events [8]. Moreover, it is known that BNP levels are predictors of future cardiovascular events after PCIs [21,22]. Our findings are consistent with those of these previous studies. Previously, we reported that HF with high BNP levels is a risk factor for bleeding events, MACE, and all-cause death after PCIs [10]. Additionally, we reported no significant difference in future bleeding events between HF patients with preserved ejection fraction and those with reduced ejection fraction [10]. Therefore, we did not include ejection fraction in this study. However, some prior studies reported that reduced LVEF was a risk factor for adverse cardiovascular events after PCIs, including MACE or all-cause death [23,24].
A strong association was observed between bleeding and increased risk of death and cardiovascular events in patients who underwent PCI [3,5,25,26]. Consistent with previous findings, we report that 30-day bleeding increased the rate of MACE and all-cause death. Rao SV et al. reported that in-hospital post-PCI bleeding complications were associated with recurrent bleeding, MACE, and all-cause death [3]. Kazi et al. revealed that spontaneous bleeding after PCI was independently associated with high long-term mortality [4]. Kaikita et al. reported that major bleeding was strongly associated with subsequent MACE in patients with AF [3]. The mechanism underlying the association between bleeding and mortality is thought to involve the activation of the coagulation cascade, increased prothrombotic cytokines, hypovolemia, anemia (compromised oxygen delivery), reflex tachycardia (increased myocardial oxygen demand), transfusion of blood products, and cessation of antiplatelet and anticoagulant therapy [4]. These events might lead to fatal cardiac events.
Among the patients enrolled in our study, 88 experienced a bleeding event within 30 days after PCI, accounting for 31.9% of the patients who experienced bleeding events within three years of follow-up. We employed a 30-day cut-off because bleeding events within 30 days accounted for a large percentage of the total bleeding events within three years. These results suggest that appropriate management to prevent bleeding events should be performed immediately after a PCI. However, the impact and outcome of bleeding events that occur from 30 days to three years, while the effect of those occurring after three years remains unclear.
Data analysis from the CLIDAS revealed that prior hospitalization due to HF with a high BNP level increases the risk of mortality in patients who undergo PCI. Notably, patients with ischemic cardiomyopathy often develop HF and require hospitalization [1,27]. Our findings showed the importance of HF management in patients with ischemic cardiomyopathy. Additionally, it might be important to evaluate the long-term risk to assess whether the scheduled PCI could be applicable, the extent to which HF was controllable, and the extent of bleeding risk. We could not confirm the association between bleeding risk and long-term prognosis because our database cannot include information on some bleeding risk factors, such as frailty, thrombotic disorders, and hepatic cirrhosis. Therefore, further studies adjusting for these factors are necessary.
4.1. Study limitations
This study has several limitations. First, bleeding events and HF are considered risk factors for adverse cardiac events. However, these factors may be associated with an overall poor prognosis. Second, this study did not account for MACE and all-cause death events within 30 days after PCI and excluded 123 and 96 patients who had MACE and all-cause death within 30 days after PCI, respectively. This might have caused a bias in the results. Moreover, the impact and importance of these acute events remain unclear. Third, in our study, the incidence of MACE and all-cause death observed among the patients are lower than those reported previously by Rao et al. and Kazi et al. [4,5]. This can be explained by the lack of traceability of the CLIDAS, which included data from the Japanese Diagnosis Procedure Combination system. In this case, a patient who was hospitalized in a different hospital after a PCI was lost to follow-up. Therefore, our database may potentially lack information on some adverse events. Fourth, as mentioned above, our database lacked records of some factors, including thrombotic disorders, frailty, and hepatic cirrhosis. These factors are included in Japanese HBR [8]. We could not clarify the association between those factors and long-term prognosis after PCIs.
5. Conclusions
Data analysis from the real-world database CLIDAS revealed that HF with high BNP levels and major bleeding events within 30 days after PCIs might be associated with MACE and all-cause death after PCIs. Therefore, managing HF and preventing bleeding complications in the early stages after PCIs could be important in preventing MACE and all-cause death.
Credit author statement
So Ikebe: Writing - Original Draft, Visualization, Conceptualization; Masanobu Ishii: Formal analysis, Writing - Review & Editing, Conceptualization, Methodology; Yasuhiro Otsuka: Investigation, Validation; Taishi Nakamura: Conceptualization, Methodology; Kenichi Tsujita: Conceptualization, Methodology; Tetsuya Matoba: Investigation, Takahide Kohro: Investigation, Yusuke Oba: Investigation, Tomoyuki Kabutoya: Data Curation, Software; Yasushi Imai: Data Curation, Software; Kazuomi Kario: Investigation, Arihiro Kiyosue: Investigation, Yoshiko Mizuno: Investigation, Kotaro Nochioka: Investigation, Masaharu Nakayama: Investigation, Takamasa Iwai: Investigation, Yoshihiro Miyamoto: Investigation, Hisahiko Sato: Project administration, Resources; Naoyuki Akashi: Investigation, Hideo Fujita: Supervision; Ryozo Nagai: Supervision, Project administration, Funding acquisition.
Disclosures
T.M. received research grants from Amgen and honoraria from Abbott Medical and Bayer. T.K. received scholarship funds from Abbott Medical. Y.I. received honoraria from Daiichi Sankyo and Toa Eiyo. K.K. received research grants and honoraria from Sanwa Kagaku Kenkyusho. A.K. received honoraria from AstraZeneca, Eli Lilly, and Sumitomo Pharma. Y.N. received research grants and consulting fees from Bayer. K.T. received research grants from PPD-Shin Nippon Biomedical Laboratories and Alexion Pharmaceuticals; and scholarship funds from Abbott Medical, Bayer, Boehringer Ingelheim, Daiichi Sankyo, ITI, Ono Pharmaceutical, Otsuka Pharmaceutical, and Takeda Pharmaceutical; affiliation with the endowed department from Abbott Medical, Boston Scientific, Cardinal Health, Fides-ONE, Fukuda Denshi, GM Medical, ITI, Japan Lifeline, Kaneka Medix, Medical Appliance, Medtronic, Nipro, and Terumo; and honoraria from Abbott Medical, Amgen, AstraZeneca, Bayer, Daiichi Sankyo, Medtronic, Kowa, Novartis Pharma, Otsuka Pharmaceutical, Pfizer, and Janssen Pharmaceutical. H.S. reports stock or stock options in Precision. H.F. received consulting fees from Mehergen Group Holdings; and honoraria from Novartis Pharma and Otsuka Pharmaceutical. R.N. received honoraria from Kowa, Takeda Pharmaceutical, Tanabe-Mitsubishi Pharmaceutical, and Boehringer-Ingelheim. All other authors have no conflicts of interest to declare.
Funding statement
This work was supported by Kowa Company, Ltd. Health Labour Sciences Research Grant (22FA1016).
Acknowledgement
The authors thank the Kowa Company for funding the development of CLIDAS. The authors appreciate the contributions of all CLIDAS research group members. We thank Yuri Matoba (Precision Inc., Tokyo, Japan) for helping us integrate the data.
Handling Editor: D Levy
References
- 1.Kimura K., Kimura T., Ishihara M., Nakagawa Y., Nakao K., Miyauchi K., Sakamoto T., Tsujita K., Hagiwara N., Miyazaki S., et al. JCS 2018 guideline on diagnosis and treatment of acute coronary syndrome. Circ. J. 2019;83:1085–1196. doi: 10.1253/circj.CJ-19-0133. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura M., Yaku H., Ako J., Arai H., Asai T., Chikamori T., Daida H., Doi K., Fukui T., Ito T., et al. JCS/JSCVS 2018 guideline on revascularization of stable coronary artery disease. Circ. J. 2022;86:477–588. doi: 10.1253/circj.CJ-20-1282. [DOI] [PubMed] [Google Scholar]
- 3.Kaikita K., Yasuda S., Akao M., Ako J., Matoba T., Nakamura M., Miyauchi K., Hagiwara N., Kimura K., Hisayama A., et al. Bleeding and subsequent cardiovascular events and death in atrial fibrillation with stable coronary artery disease. Circ. CardioVasc Int. 2021;14:1065–1072. doi: 10.1161/CIRCINTERVENTIONS.120.010476. [DOI] [PubMed] [Google Scholar]
- 4.Kazi D.S., Leong T.K., Chang T.I., Solomon M.D., Hlatky M.A., Go A.S. Association of spontaneous bleeding and myocardial infarction with long-term mortality after percutaneous coronary intervention. J. Am. Coll. Cardiol. 2015;65:1411–1420. doi: 10.1016/j.jacc.2015.01.047. [DOI] [PubMed] [Google Scholar]
- 5.Rao S.V., Dai D., Subherwal S., Weintraub W.S., Brindis R.S., Messenger J.C., Lopes R.D., Peterson E.D. Association between periprocedural bleeding and long-term outcomes following percutaneous coronary intervention in older patients. J Am Coll Cardiol Int., JACC Cardiovasc. Interv. 2012;5:958–965. doi: 10.1016/j.jcin.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao D., Mehran R., Dangas G., Baber U., Sartori S., Chandiramani R., Stefanini G.G., Angiolillo D.J., Capodanno D., Urban P., et al. Validation of the academic research consortium high bleeding risk definition in contemporary PCI patients. J. Am. Coll. Cardiol. 2020;75:2711–2722. doi: 10.1016/j.jacc.2020.03.070. [DOI] [PubMed] [Google Scholar]
- 7.Urban P., Gregson J., Owen R., Mehran R., Windecker S., Valgimigli M., Varenne O., Krucoff M., Saito S., Baber U., et al. Assessing the risks of bleeding vs thrombotic events in patients at high bleeding risk after coronary stent implantation: the ARC-High Bleeding Risk Trade-off Model. JAMA Cardiol. 2021;6:410–419. doi: 10.1001/jamacardio.2020.6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakamura M., Kimura K., Kimura T., Ishihara M., Otsuka F., Kozuma K., Kosuge M., Shinke T., Nakagawa Y., Natsuaki M., et al. JCS 2020 guideline focused update on antithrombotic therapy in patients with coronary artery disease. Circ. J. 2020;84:831–865. doi: 10.1253/circj.CJ-19-1109. [DOI] [PubMed] [Google Scholar]
- 9.Natsuaki M., Morimoto T., Yamaji K., Watanabe H., Yoshikawa Y., Shiomi H., Nakagawa Y., Furukawa Y., Kadota K., Ando K., et al. Prediction of thrombotic and bleeding events after percutaneous coronary intervention: CREDO-Kyoto thrombotic and bleeding risk scores. J. Am. Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.008708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otsuka Y., Ishii M., Nakamura T., Tsujita K., Fujita H., Matoba T., Kohro T., Kabutoya T., Kario K., Kiyosue A., et al. Impact of BNP level in patients with heart failure on Major bleeding events after percutaneous coronary intervention. Eur. Heart J. 2022;43 doi: 10.1093/eurheartj/ehac544.1148. [DOI] [Google Scholar]
- 11.Oba Y., Kabutoya T., Kohro T., Imai Y., Kario K., Sato H., Nochioka K., Nakayama M., Fujita H., Mizuno Y., et al. Relationship among heart rate, β-blocker dosage, and prognosis in patients with coronary artery disease in a real-world database using a multimodal data acquisition system. Circ J. 2023;87(2):336–344. doi: 10.1253/circj.CJ-22-0314. [DOI] [PubMed] [Google Scholar]
- 12.Akashi N., Matoba T., Kohro T., Oba Y., Kabutoya T., Imai Y., Kario K., Kiyosue A., Mizuno Y., Nochioka K., Nakayama M. Sex differences in long-term outcomes in patients with chronic coronary syndrome after percutaneous coronary intervention: insights from A Japanese Real-World database using A Strage system. Circ J. 2023;87(6):775–782. doi: 10.1253/circj.CJ-22-0653. [DOI] [PubMed] [Google Scholar]
- 13.McKee P.A., Castelli W.P., McNamara P.M., Kannel W.B. The natural history of congestive heart failure: the Framingham study. N. Engl. J. Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 14.GUSTO investigators An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N. Engl. J. Med. 1993;329:673–682. doi: 10.1056/NEJM199309023291001. [DOI] [PubMed] [Google Scholar]
- 15.Umemura S., Arima H., Arima S., Asayama K., Dohi Y., Hirooka Y., Horio T., Hoshide S., Ikeda S., Ishimitsu T., et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2019) Hypertens. Res. 2019;42:1235–1481. doi: 10.1038/s41440-019-0284-9. [DOI] [PubMed] [Google Scholar]
- 16.Araki E., Goto A., Kondo T., Noda M., Noto H., Origasa H., Osawa H., Taguchi A., Tanizawa Y., Tobe K., Yoshioka N. Japanese clinical practice guideline for diabetes 2019. J. Diabetes Investig. 2020;11:1020–1076. doi: 10.1111/jdi.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuo S., Imai E., Horio M., Yasuda Y., Tomita K., Nitta K., Yamagata K., Tomino Y., Yokoyama H., Hishida A. Collaborators developing the Japanese equation for estimated GFR, Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 18.Imai E., Iseki K., Arata K., Fukagawa M., Yasuda Y., et al. Clinical practice guidebook for diagnosis and treatment of chronic kidney 2012. Nihon Jinzo gakkai shi. 2012;54:1034–1191. [PubMed] [Google Scholar]
- 19.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., Flachskampf F.A., Foster E., Goldstein S.A., Kuznetsova T., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015;28 doi: 10.1016/j.echo.2014.10.003. 1–39.e14. [DOI] [PubMed] [Google Scholar]
- 20.Ohte N., Ishizu T., Izumi C., Itoh H., Iwanaga S., Okura H., Otsuji Y., Sakata Y., Shibata T., Shinke T., et al. JCS 2021 guideline on the clinical application of echocardiography. Circ. J. 2022;86:2045–2119. doi: 10.1253/circj.CJ-22-0026. [DOI] [PubMed] [Google Scholar]
- 21.Rakesyu K., Alexis L.B., Rajesh J., Mathilda R., Alan H.B.W., Mary A.W. B-type natriuretic peptides for prediction of cardiovascular events in patients with stable coronary heart disease: the heart and soul study. J. Am. Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gong X., Zhang T., Feng S., Song D., Chen Y., Yao T., Han P., Liu Y., Li C., Song Z., et al. Association between N-terminal pro-BNP and 12 months major cardiac events among patients admitted with NSTEMI. Ann. Palliat. Med. 2021;10:5231–5243. doi: 10.21037/apm-20-2538. [DOI] [PubMed] [Google Scholar]
- 23.Ng V.G., Lansky A.J., Meller S., Witzenbichler B., Guagliumi G., Peruga J.Z., Brodie B., Shah R., Mehran R., Stone G.W. The prognostic importance of left ventricular function in patients with ST-segment elevation myocardial infarction: the HORIZONS-AMI trial. Eur. Heart J. Acute Cardiovasc. Care. 2014;3:67–77. doi: 10.1177/2048872613507149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chyrchel M., Gallina T., Januszek R., Szafrański O., Gębska M., Surdacki A. The reduction of left ventricle ejection fraction after multi-vessel PCI during acute myocardial infarction as a predictor of Major adverse cardiac events in long-term follow-up. Int. J. Environ. Res. Publ. Health. 2022;19 doi: 10.3390/ijerph192013160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manoukian S.V., Feit F., Mehran R., Voeltz M.D., Ebrahimi R., Hamon M., Dangas G.D., Lincoff A.M., White H.D., Moses J.W., et al. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY trial. J. Am. Coll. Cardiol. 2007;49:1362–1368. doi: 10.1016/j.jacc.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 26.Palmerini T., Bacchi Reggiani L., Della Riva D., Romanello M., Feres F., Abizaid A., Gilard M., Morice M.C., Valgimigli M., Hong M.K., et al. Bleeding-related deaths in relation to the duration of dual-antiplatelet therapy after coronary stenting. J. Am. Coll. Cardiol. 2017;69:2011–2022. doi: 10.1016/j.jacc.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 27.Tsutsui H., Isobe M., Ito H., Okumura K., Ono M., Kitakaze M., Kinugawa K., Kihara Y., Goto Y., et al. JCS 2017/JFCS 2017 guideline on diagnosis and treatment of acute and chronic heart failure - digest version. Circ. J. 2019;83:2084–2184. doi: 10.1253/circj.CJ-19-0342. [DOI] [PubMed] [Google Scholar]