Abstract
A State of the Art lecture titled “Megakaryocytes and different thrombopoietic environments” was presented at the ISTH Congress in 2022. Circulating platelets are specialized cells produced by megakaryocytes. Leading studies point to the bone marrow niche as the core of hematopoietic stem cell differentiation, revealing interesting and complex environmental factors for consideration. Megakaryocytes take cues from the physiochemical bone marrow microenvironment, which includes cell-cell interactions, contact with extracellular matrix components, and flow generated by blood circulation in the sinusoidal lumen. Germinal and acquired mutations in hematopoietic stem cells may manifest in altered megakaryocyte maturation, proliferation, and platelet production. Diseased megakaryopoiesis may also cause modifications of the entire hematopoietic niche, highlighting the central role of megakaryocytes in the control of physiologic bone marrow homeostasis. Tissue-engineering approaches have been developed to translate knowledge from in vivo (inside) to functional mimics of native tissue ex vivo (outside). Reproducing the thrombopoietic environment is instrumental to gain new insight into its activity and answering the growing demand for human platelets for fundamental studies and clinical applications. In this review, we discuss the major achievements on this topic, and finally, we summarize relevant new data presented during the 2022 ISTH Congress that pave the road to the future of megakaryopoiesis.
Keywords: bone marrow, megakaryocytes, personalized therapy, platelets, thrombocytopenia
Essentials
-
•
Megakaryocytes take cues from the extracellular environment to produce platelets.
-
•
Modeling megakaryopoiesis is instrumental to understand the mechanisms of platelet formation.
-
•
Bioengineering approaches aim at guiding ex vivo collection of human platelets.
-
•
Finally, we summarize the related new research presented at the ISTH Congress 2022 in London.
1. The Thrombopoietic Bone Marrow Microenvironment in Vivo
Circulating platelets are specialized cells produced by megakaryocytes in the bone marrow, a soft and viscous tissue located in the hollow space of the bones (Figure 1) [1]. Bone marrow is composed of a mesh of vasculature and extracellular matrix (ECM) components with bound cytokines and growth factors.
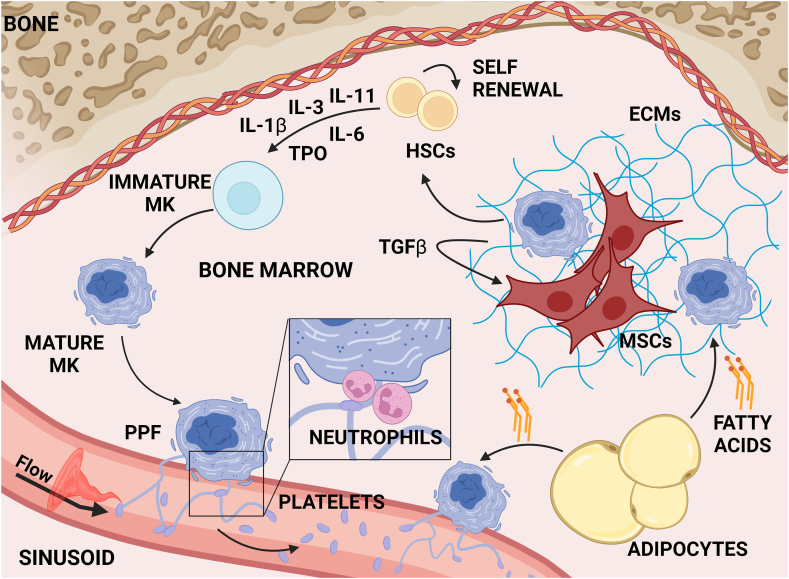
Figure 1.
Megakaryopoiesis in the bone marrow. Schematic representation of the bone marrow microenvironment, showing key steps of megakaryopoiesis. Megakaryocyte differentiation from hematopoietic stem cells occurs under the control of thrombopoietin, which acts in synergy with other cytokines to promote an increase in cell size and ploidy. Mature megakaryocytes extend long pseudopods, called proplatelets, into the lumen of sinusoids, where the flow of bloodstream, together with plucking neutrophils and other immune cells, allow platelet detachment. Nonhematopoietic bone marrow cells (eg, mesenchymal stromal cells and adipocytes) and the extracellular matrix interact with maturing megakaryocytes to support their differentiation and functions. ECM, extracellular matrix; HSC, hematopoietic stem cell; IL, interleukin; MK, megakaryocyte; MSC, mesenchymal stromal cell; PPF, proplatelet formation; TGF-β, transforming growth factor-β; TPO, thrombopoietin.
Megakaryocyte differentiation from hematopoietic stem cells (HSCs) is controlled mainly by thrombopoietin (TPO), which regulates highly specific intracellular pathways, including activation of biochemical signals, control of mitochondrial activity, modulation of calcium flow, and expression of lineage-specific genes, which act in concert to promote a unique program of endomitosis that drives nuclear polyploidization and cellular enlargement [[2], [3], [4], [5]]. TPO synergizes with additional cytokines, including stem cell factor and interleukins (ILs), to induce megakaryocyte commitment and maturation [[6], [7], [8]]. Final lineage consolidation consists of the upregulation of megakaryocytic surface markers, the development of cytoplasmic granules, and the formation of the demarcation membrane system [[9], [10], [11]].
Megakaryopoiesis occurs within subregions of the bone marrow, termed niches, revealing interesting and complex environmental factors for consideration [12]. Local cell populations vary across the marrow with (i) osteoblasts, osteoclasts, and chondrocytes concentrating close to bones, where immature megakaryocytes reside; and (ii) mesenchymal stromal cells (MSCs) and endothelial cells lining the perivascular space and vasculature, where mature megakaryocytes enlarge and produce platelets. Depending on their localization, megakaryocytes take cues from the surrounding microenvironment, which provides distinct biophysical and biochemical signals, including cell-cell interaction; gradients of biomolecular signals; and stiffness, composition, and structure of ECM [13].
The endosteum plays a pivotal role in remodeling the surface of the bones and provides an anatomic location for HSC maintenance [14], and interaction with MSCs, endothelial cells, and adipocytes dictates the fate of mature progenitors [15]. MSCs, mainly located in the marrow space surrounding the vasculature, secrete ECM proteins and cytokines that support megakaryopoiesis, including TPO, IL-6, IL-11, and leukemia inhibitory factor [16,17]. Adipocytes also support megakaryocyte maturation by secreting fatty acids that are taken up by megakaryocytes via CD36 translocase to enhance ploidy, membrane structure, and proplatelet formation [18].
Two distinct vascular niches have been described: the arteriolar niche, mainly associated with the endosteal region and composed of sympathetic nerve fibers and nestin+ cells, and the sinusoidal niche, where maturing blood cells interact with CXCL12-abundant reticular cells. Arterioles exhibit lower permeability than sinusoids, representing the preferential site of megakaryocyte maturation and platelet production. Periarteriolar nestin+ cells are primarily involved in regulating HSC dormancy [19], while CXCL12-abundant reticular cells surrounding sinusoids secrete CXCL12 [20], leading to the recruitment of mature megakaryocytes, which elongate proplatelets into the vessel lumen, where platelet detachment occurs [21]. Other chemokines released by the endothelial cells, such as fibroblast growth factor-4, vascular endothelial growth factor, and vascular cell adhesion protein-1, contribute to supporting megakaryocyte proliferation and maturation [22].
Mechanisms underlying the regulation of megakaryopoiesis also involve ECM scaffolding, a dynamic structure that confers bone marrow tissue tensegrity and compactness [23,24]. Depending on the unique composition and distribution of the ECM components, the bone marrow shows a regionally heterogeneous topography and very low stiffness [25,26]. The protein mesh shaping the ECM is mainly composed of glycosaminoglycans (GAGs), different collagen types (I, II, III, V, and XI), fibronectin, and laminins, continuously deposited and then remodeled by specific proteases, such as matrix metalloproteinases (MMPs). In addition to its structural function, this protein meshwork can model cell function via mechanical and biochemical signals through integrins and mechanosensitive receptors that integrate these messages into the cells.
Knowledge about the expression and role of GAGs in the bone marrow and their relevance for the regulation of megakaryocyte functions is rapidly growing, as recently reviewed by Falet et al. [27] Several studies reported that heparin, heparan sulfate, dermatan sulfate, chondroitin sulfate, and hyaluronic acid stimulate megakaryopoiesis [27]. Perlecan, a heparan sulfate proteoglycan, is abundantly expressed in the bone marrow, where it regulates megakaryocyte maturation and guides proplatelet formation in the lumen of sinusoidal blood vessels through binding to the G6b-B receptor [28]. For most of the other GAGs, the precise role remains unclear. Further insights are needed to clarify whether specific signals and cytokines, including TPO, depend on GAG expression to regulate platelet production.
The most abundant bone marrow ECM components, namely fibronectin and type IV collagen, are located in the medullar cavity and around sinusoids, the softest sites of the whole microenvironment, where they support proplatelet formation by activating phosphoinositide-3-kinase/Akt (PI3K/Akt) and mitogen-activated protein kinases/extracellular signal-regulated kinase (MAPK/ERK) signaling pathways via integrins and intracellular calcium flow via ion channels, such as transient receptor potential cation channel subfamily V member 4 [29,30]. The surface of the endosteum is asymmetrically enriched with the stiffest ECM component, namely type I collagen, which prevents platelet production through the activation of integrin α2β1 and the Rho-ROCK pathway [23,24,31]. ECMs also possess topographic features ranging from nanometers to a hundred micrometers that influence megakaryocyte behavior, with rough surfaces, such as those of the complex microscale fibers of type I collagen, negatively influencing platelet production, and basement membrane proteins that exhibit a 3-dimensional (3D) texture in the range of nanometers, sustaining platelet formation [32].
There is a reciprocal interaction between the niche and megakaryocytes. Not only does the niche modify megakaryopoiesis but megakaryocytes also aid in modifying the niche [13]. For instance, we have demonstrated that megakaryocytes themselves secrete diverse ECM components, such as fibronectin, laminin, and type IV collagen, and profibrotic cytokines that contribute to building their protective niche [23,33]. More recently, single-cell RNA sequencing expanded the knowledge of megakaryocyte heterogeneity [[34], [35], [36]]. Particularly, it has been shown that aside from platelet-forming megakaryocytes, at least 5 additional megakaryocyte subpopulations exist, including “immune” megakaryocytes and “niche-supporting” megakaryocytes, the latter expressing high levels of genes associated with the ECM and its remodeling. The bone marrow microenvironment undergoes continuous adaptation by the action of proteolytic enzymes, belonging to the metzincin family, which can degrade ECM components and affect the biomechanical characteristics of the bone marrow. The metzincin family comprises secreted and membrane-bound MMPs as well as membrane-bound and secreted disintegrin and metalloproteases. Megakaryocytes express several MMPs, including MMP-2, MMP-9, MMP-14, MMP-24, and MMP-25, which confer collagenase activity to allow elongation of proplatelets and podosomes through the basement membrane of the bone marrow sinusoids. In addition, megakaryocytes can rearrange the ECM through proteolytic activity and by exhorting active tensile forces [[37], [38], [39]].
2. Dynamics of Platelet Formation in the Bloodstream
Platelets are produced by mature megakaryocytes through the formation of proplatelets, long, branched pseudopods that assemble nascent platelets at their terminal ends. However, in the past decade, whole-organ imaging techniques opened new scenarios to the understanding of the mechanisms of thrombopoiesis using mice.
Junt et al. [21] first observed in vivo thrombopoiesis using intravital 2-photon microscopy (2P-IVM) in mouse skull bone marrow. They demonstrated that mature megakaryocytes, located close to bone marrow sinusoids, extend proplatelets into the vessel lumen, where platelets are released. The same group clarified that a transendothelial gradient of the bioactive sphingolipid sphingosine 1-phosphate navigates proplatelet extension into sinusoids [40]. By combining 2P-IVM and light-sheet fluorescence microscopy, Stegner et al. [41] observed that megakaryocytes and their progenitors are widely distributed in the bone marrow, with a minimal migratory capacity and prominent localization along sinusoids, indicating that they are replenished by progenitors in close spatial proximity.
Here, platelet detachment from the proplatelet shaft can be attributed primarily to blood hydrodynamics and fluid shear. Measuring blood flow in vivo in human bone marrow vessels is challenging. Observation of bone marrow scalps from enhanced green fluorescent protein mice using 2P-IVM and particle image velocimetry demonstrated the presence of high turbulence in mouse vessels around moving proplatelet shafts and released platelet particles but not near resting megakaryocytes that were preferentially exposed to continuous laminar flow patterns, thus demonstrating that the fluid parameters are critical features of platelet production [42].
Intravital visualization of mouse thrombopoiesis recently revealed a previously unexplored path to platelet biogenesis. By plucking on proplatelets, neutrophils accelerate their growth and release into circulation. Particularly, following CXCR4-CXCL12–dependent migration toward perisinusoidal megakaryocytes, neutrophils actively pull on proplatelet shafts and trigger myosin light chain and ERK activation through reactive oxygen species [43]. Following myocardial infarction, the “plucking” neutrophils can drive the release of reticulated platelets, which boost the risk of ischemia. Aside from neutrophils, the group of Prof Hoffmeister recently demonstrated that plasmacytoid dendritic cell–like cells sense the desialylated Thomsen-Friedenreich antigen on megakaryocytes, which induces interferon-1 secretion and inhibits platelet release, thus paving the way to understand the molecular mechanisms of immune-mediated thrombocytopenia [44].
Alternative to proplatelet formation, megakaryocyte rupture, namely cytoplasmic fragmentation and rapid release of high platelet numbers, may occur to answer acute platelet needs during inflammation under the control of IL-1α [45]. Membrane budding has also been proposed to sustain the physiologic release of platelets directly into the peripheral circulation to supply the platelet biomass in mice [46].
In vivo approaches also opened perspectives for the study of thrombopoiesis in new and unexpected anatomic locations. Evidence that platelet biogenesis occurs in mouse lungs drew interest [47]. Similarities between bone marrow and lung microenvironments can be observed. The spongy lung parenchyma is sustained by type I and type III collagen and elastic fibers, composed of elastin and microfibrils, which are mostly fibrillin and fibulin, embedded in a matrix of GAGs [48]. In addition, normal human lung parenchyma shows an elastic modulus in the range of soft bone marrow tissue [49]. However, lung megakaryocytes display key immune regulatory roles dictated by the tissue environment distinct from their bone marrow counterparts. Particularly, lung-associated immune molecules skew the megakaryocyte profile toward roles in immunity and inflammation, such as processing of antigenic proteins and bacterial pathogens to induced CD4+ T-cell activation [50].
All recent findings on new mechanisms and sites of platelet production in mice open new avenues of investigations but need confirmation in humans, for whom proplatelet formation in the bone marrow is still considered the major trigger of physiologic thrombopoiesis. Limitations arise from technical challenges in investigating intact human tissues using live-cell imaging. The best approach to try to gain insights into the mechanisms of human thrombopoiesis mainly relies on the possibility of translating knowledge from in vivo (inside) to ex vivo exploitation of bone marrow–like tissues (outside).
3. Bone Marrow Tissue Engineering Toward Functional Mimics of Human Megakaryopoiesis
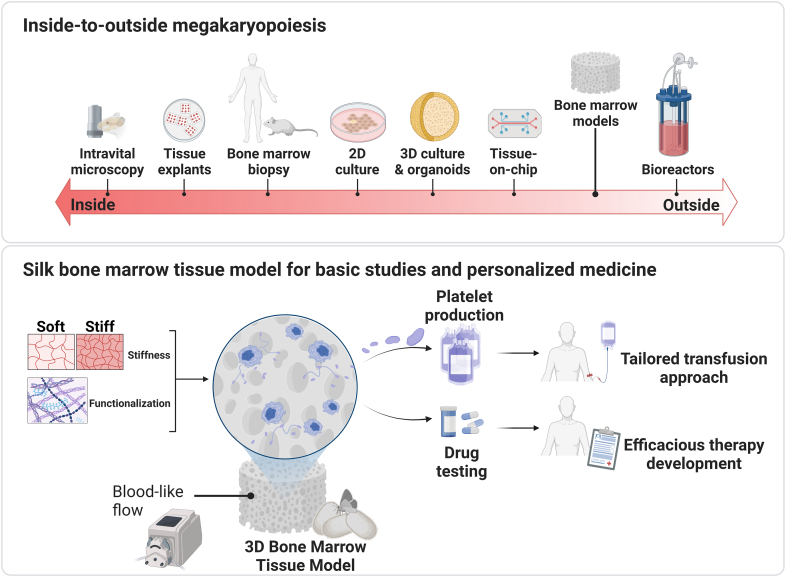
Reproducing a real 3D human bone marrow niche is instrumental to answering the growing demand for human blood cell production ex vivo to foster either mechanistic studies of normal and malignant hematopoiesis and for validation of novel pharmacologic therapies or clinical applications in the field of transfusion and regenerative medicine (Figure 2).
Figure 2.
Platforms for studying megakaryopoiesis and platelet production. The knowledge from studies of the native bone marrow niche of mice and humans helps in developing methods for reproducing megakaryopoiesis ex vivo. A versatile silk bone marrow tissue model has been used to tailor conditions for producing human platelets and testing the efficacy of thrombopoietic drugs in thrombocytopenic patients. Approaches to efficiently culture human megakaryocytes ex vivo may translate into new possibilities for personalized approaches to cure megakaryocyte/bone marrow pathologies. 2D, 2-dimensional; 3D, 3-dimensional.
3.1. Moving from 2-dimensional (2D) to 3D culture
In vitro, culture conditions lack the bone marrow physical microenvironment. In classical 2D culture, cells are seeded in liquid media containing cytokines and growth factors but lose the complexity of 3D tissues. In a dish with liquid medium, the physical environmental components are very poor and far from the native ones. There is no confinement, no physical constraints, and no stiffness, except those deriving from the contact with the plastic bottom of the dish, which can create artificial cell polarization. Some strategies have been tested to complexify the 2D culture, such as the use of coculture systems and/or coating dishes with ECM components. Human megakaryocytes cocultured with endothelial cells and/or MSCs or seeded onto a thin layer of soft ECM components, such as fibrinogen, fibronectin, and/or type IV collagen, demonstrate increased proplatelet production. [32,51,52]
For a long time, the use of 3D hematopoietic cell culture was restricted to clonogenic assays [53]. Culture in organoids has been recently proposed to recreate a bone marrow–like 3D microenvironment. Organoids were generated from induced pluripotent stem cells committed to mesenchymal, endothelial, and hematopoietic lineages [54]. These 3D structures were able to mimic key features of human bone marrow, including stroma, lumen-forming sinusoids, and differentiation of proplatelet-forming megakaryocytes, either under physiologic or pathologic conditions. Interestingly, fibrosis of organoids occurred following transforming growth factor (TGF)-β stimulation and engraftment with cells from myelofibrosis but not healthy donor–derived cells, thus demonstrating possibilities for studying bone marrow malignancies. Nevertheless, organoids can face challenges such as breakthroughs in harvesting and usage of cells for clinical application, automation, reproducibility, and time efficacy [55].
As an alternative, bioengineering approaches have been proposed to provide 3D scaffolding for bone marrow mimics using natural and synthetic polymers having different compositions and complexity. Reactive hydrogel made of hyaluronan and ECM components demonstrated effective support of thrombopoiesis in a soft 3D microenvironment [56]. Instead, nonreactive hydrogels composed of pullulan-dextran or methylcellulose have been used [57,58] to test the impact of physical constraints and environmental stiffness on megakaryopoiesis regardless of their biochemical composition. Of note, megakaryocyte size, ploidy, and expression of lineage-specific transcription factors were increased in the 3D cultures. This was accompanied by larger proplatelet production, with the softest microenvironments better supporting thrombopoiesis. A key limitation of these models is the lack of sinusoidal-like vessels to reproduce the key flow hydrodynamics of highly vascularized native bone marrow tissue.
3.2. Microfluidic insights into thrombopoiesis
Microfluid chips represented the first approaches to generate a perfusable bone marrow–like system [59,60]. The first organ on chip for specifically making platelets was established by Thon et al. [61] The device was made of transparent poly(dimethylsiloxane) to support live imaging of platelet production. The device consisted of upper and lower microfluidic channels separated by a fenestrated barrier. In vitro–derived megakaryocytes, either from mice or from human induced pluripotent stem cells (hiPSCs), were seeded in the upper channel and extended proplatelets through the slits. This channel was filled with Matrigel or alginate hydrogel, and the lower channel was coated with endothelial cells to mimic vasculature. The perfusion was performed through a syringe pump and controllable shear rates across the chip to collect an average of 30 platelets per megakaryocyte. Later on, Avanzi et al. [62] created a flow chamber containing biocompatible membranes to allow human megakaryocytes to extend proplatelet processes into a flow through generated by hydrodynamic shear forces that can promote the harvesting of up to 100 platelets per input megakaryocyte. The microfluidic device created by Blin et al. [63] consisted of microchannels textured with organized micropillar arrays coated with von Willebrand factor to favor human megakaryocyte anchor and trapping. Channels were disposed in a serpentine shape to fit the dimensions of a single glass slide, thus allowing microscope observation of the whole system. The elongation and fragmentation of proplatelets were achieved by high hydrodynamic shear to produce approximately 4 platelets per megakaryocyte. A uniform-shear–rate bioreactor was also established, consisting of a microfluidic system with well-defined flow patterns and uniform shear profiles [64]. The device allowed real-time visualization of the proplatelet formation process and rapid release of individual platelet-like particles from human megakaryocytes.
Despite their valuable approach to recording platelet production ex vivo under physiologically relevant hydrodynamic shear stress, these systems do not mimic key features of the 3D bone marrow environment as a whole.
3.3. Three-dimensional bone marrow mimics toward manufacturing of functional niches of megakaryopoiesis
Combining knowledge from human bone marrow biopsies, mouse models, 3D cultures, and organs on chip has greatly advanced the field of biomaterial development and tissue engineering to develop perfusable 3D tissue systems reproducing the key features of thrombopoiesis.
Shepherd et al. [65] developed a flow bioreactor based on a 2-layer collagen scaffold capable of retaining hiPSC-derived megakaryocytes, while promoting the release of platelets. The porous, structurally graduated scaffold was meant to provide a structure to support megakaryocyte functions by a bone marrow–like structure. To modulate the pore size of the collagen scaffold, they used a 2-stage freezing technique to obtain a variety of pore sizes having a sieving capacity to collect platelets. The bioreactor was conceived as a twin-chamber system, whereby 1 chamber allowed the seeding of hiPSC-derived megakaryocytes, whereas cross-flow generated shear forces that induced platelet release into the other. To provide a high peak flow rate while keeping the collection volume to a minimum, the bioreactor was perfused by gravity with a sequential pulsed flow. In this condition, each megakaryocyte was capable of releasing ∼2 metabolically active intact platelets. By using the soft-gel lithography technique, Kotha et al. [66] generated collagen hydrogels as a scaffold for creating a microvascular network. Endothelial cells were seeded in the system to form vessels with the lumen, whereas megakaryocytes were encapsulated directly in surrounding hydrogels made of collagen. Megakaryocytes were able to migrate to the microvascular network to extend proplatelets. Interestingly, the interaction with endothelial cells increased vessel permeability, which allowed the transmigration of megakaryocytes through the endothelial barrier. One limitation was the big sizes of the vessel, which did not recapitulate those of native sinusoids.
In the last 10 years, the search for a biomaterial that could be chemically and mechanically tailored to entrap growth factors and ECM components, while retaining bioactivity, led our group’s research to the use of silk fibroin as scaffolds. Silk fibroin from Bombyx mori silkworm cocoons is a strong but elastic biocompatible protein having low immunogenicity and nonthrombogenicity [67,68]. Silk fibroin has been extensively used in clinics in biomedical applications and bioengineering approaches to create films, gels, sponges, or tubes in bone regeneration and vessel engineering [[69], [70], [71]]. Our first-generation 3D bioreactor was made of a silk tube functionalized with ECM proteins (fibronectin, type IV collagen, and laminin) and CXCL12, surrounded by a 3D silk sponge resembling the medullar topography to enhance the migration of cells within the structure [72,73]. In the vascular compartment, the presence of CXCL12 directed the migration of mature megakaryocytes toward the silk tube. The ECM composition improved proplatelets elongation on the luminal side, while the luminal flow through the silk tube facilitated the release of functional platelets. It was also shown that coculture with a monolayer of endothelial cells or functionalization of silk tubes with vascular endothelial growth factor and vascular cell adhesion protein increased the extent of platelet production [73]. The second generation of silk bone marrow models consisted of a scaled-up version intended to house a larger number of in vitro–derived megakaryocytes producing millions of platelets for functional studies. The flow chambers were made of research-grade silicon or biomaterial, holding silk sponges functionalized with extracellular matrix components such as fibronectin and collagen IV [74,75]. These sponges were cultured within modular flow chambers, allowing the flow to pass through the different channels/pores and proplatelets in direct contact with medium. This enhanced the capacity of platelet production because of a soft microenvironment and the perfusable area, which allowed us to increase the cell concentration.
4. Addressing Challenges in the Diagnosis and Treatment of Megakaryocyte and Platelet Disorders
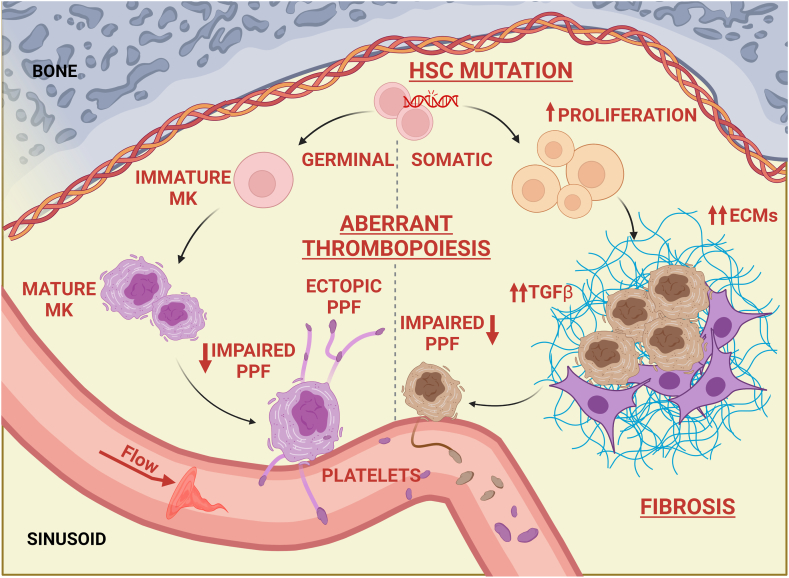
A platelet count that falls recurrently below the limit of 100 × 103/μL is defined as thrombocytopenia. The relevance of thrombocytopenia in each patient is extremely variable. Some patients can experience hemorrhages and/or excessive bleeding provoked by hemostatic challenges such as trauma or surgery. Clinically significant spontaneous bleeding does not usually occur until the platelet count is <20 × 103/μL. The main causes of thrombocytopenia can be subdivided into decreased platelet production, increased platelet destruction, and increased splenic sequestration [76]. Approximately 3 million people worldwide are affected by intrinsic genetic disorders such as inherited thrombocytopenia, a diverse group of disorders characterized by mutations in many different genes involved in the physiologic regulation of megakaryopoiesis and/or platelet production [77,78]. Alterations of megakaryopoiesis also occur in Philadelphia-negative myeloproliferative neoplasms (MPNs), a heterogeneous group of HSC diseases characterized by acquired mutations in JAK2, MPL, and CALR genes, converging in constitutive activation of TPO signaling and consequent proliferation of megakaryocytes having hypolobated nuclei and structural defects in proplatelets (Figure 3) [[79], [80], [81]]. Additionally, many MPNs experience progression to bone marrow fibrosis due to increased deposition of reticulin fibers within the bone marrow under the stimulus of profibrotic cytokines released by pathologic megakaryocytes [33,82]. Fibrosis contributes to ineffective hematopoiesis, leading to transfusion-dependent thrombocytopenia/anemia, splenomegaly, increased white blood cell counts or severe pancytopenia, and end-stage acute leukemia.
Figure 3.
Mechanisms of pathologic megakaryopoiesis. Inherited or acquired mutations of physiologically relevant genes for megakaryopoiesis may result in the differentiation and function of pathologic megakaryocytes. Pathogenic mechanisms include proliferation of megakaryocyte progenitors; increased secretion of extracellular matrix components and profibrotic cytokines; impaired migration; and/or altered/ectopic proplatelet formation, elongation, and branching. All these may result in the release of platelets having abnormal morphology, function, and/or viability. ECM, extracellular matrix; HSC, hematopoietic stem cell; MK, megakaryocyte; PPF, proplatelet formation; TGF-β, transforming growth factor-β.
For all these diseases, the pathogenesis is still unclear, and thus, optimal targeted therapies remain unknown. For patients with severe inherited thrombocytopenia, which is usually fatal at young ages, the treatment of choice is HSC transplantation [83,84]. However, the risks may outweigh the benefits. A significant advancement in the treatment of inherited thrombocytopenia is the use of drugs that stimulate platelet production by mimicking the effects of TPO. The TPO receptor agonists eltrombopag, romiplostim, avatrombopag, and lusutrombopag are promising [85,86]. However, the extent of platelet response is highly variable not only among different forms of inherited thrombocytopenia but also among patients affected by the same mutation. Furthermore, concerns exist about the safety of these treatments, especially for patients harboring mutations that predispose them to leukemic transformation.
Janus kinase 2 (JAK2) inhibitors, including ruxolitinib and fedratinib, have held promise for therapeutic targeting of MPNs [87]. Patients may benefit from reduced splenomegaly and symptoms; however, their efficacy in restraining disease-driving effects, such as clone expansion, is still poor. Mechanisms for MPN clone resistance to treatments are still a matter of study. Compensatory signaling and the pathologic process may likely contribute to the lack of response. The rate of negative prognosis is still high, making it an urgent need for novel approaches targeting new molecular pathways.
Validation of novel therapeutics usually passes through testing in animal models, which can mimic the physiologic environment of bone marrow, but sometimes generates results that can be poorly translated to humans, likely, in part, due to differences between species. The call for solutions to improve the efficacy of tailored therapeutic strategies for individual patients is still open.
Building on knowledge derived from patients, we have recently developed an ex vivo miniaturized 3D bone marrow tissue model that reproduces ex vivo platelet biogenesis of different forms of inherited thrombocytopenia [88]. This device could predict the in vivo clinical platelet response to eltrombopag at the individual scale using small amounts of peripheral blood–derived megakaryocytes. In addition, our bioengineered bone marrow tissue model, mimicking the 3D architecture, composition, and hydrodynamic environment of native tissue, could reproduce the pathologic features of megakaryopoiesis in MPNs, including massive proliferation and the extent of platelet formation defects [73]. Overall, our data suggest that the more closely we reproduce the bone marrow microenvironment, the better its applicability for gaining new insights into the mechanisms of diseases and performing a satisfactory evaluation of the effects of compounds that may impact platelet production. The system may represent a benchmark for preclinical testing of new therapies for thrombocytopenia-related disorders or other bone marrow pathologies and testing the effects of toxic agents for the whole hematopoietic niche.
In the future, our approach may serve as a foundation for generating solutions for ex vivo production of platelets for transfusion, which may benefit these and other diseases. The group of Ito et al. [42] identified turbulent energy as a crucial regulator in platelet biogenesis in big tank bioreactors. The system was a vertical liquid culture bioreactor that included 2 oval-shaped mixing blades fixed at a horizontal angle to the power axis and at right angles to each other to generate tailored turbulent energy to induce efficient platelet production by immortalized hiPSC-derived megakaryocytes. The scaled-up version allowed to reach clinical-scale manufacturing to obtain billions of autologous platelets for a patient who did not have a compatible platelet donor [89]. This first-in-human clinical trial assured the safety of the product through the dose-escalation study. Transfusion was well tolerated, with no significant complications, at 1 year of follow-up. The absence of side effects represents a step closer to successful transfusion of ex vivo–produced platelets. However, hiPSCs might not have the ability to produce fully mature megakaryocytes and/or proplatelets. This is reflected in the generation of hiPSC platelets with a short half-life and uncertain functionality, thus making it necessary to further refine ex vivo cultures to reach a cost-effective product that successfully and safely returns to patients.
5. Isth 2022 London Congress Report
Several abstracts presented at the 2022 ISTH meeting provided new mechanistic and clinical insights into megakaryocyte and platelet production. We selected some of those tracing the path toward successful mimicking of physiologic and pathologic megakaryopoiesis ex vivo. We apologize to those whose work was not included in this review due to space limitations.
As discussed above, the microenvironment next to bone marrow vasculature is crucial to support platelet production in vivo. Asquith et al. [90] developed a 3D self-forming coculture of endothelial cells and human hematopoietic progenitors that supports improved megakaryocyte differentiation ex vivo and the release of platelets and/or extracellular vesicles into a microfluidic chamber. As a further demonstration of the importance of the endothelium in promoting platelet production, Foster et al. [91], in collaboration with our group, functionalized the 3D silk bone marrow model with different endothelial-derived recombinant proteins, selected from a library of 259, that were able to significantly enhance platelet production by hiPSC-derived and human primary megakaryocytes. The fundamental role of vascular niche composition in sustaining platelet production was also proved by Mott et al. [92], who analyzed hematopoiesis after total body irradiation in mice and revealed that total body irradiation conditioning is associated with prolonged thrombocytopenia compared with chemotherapy due to alterations of the vascular niche. It is still unclear whether the spatial organization of megakaryocytes influences gene expression and consequent cell function. Tilburg et al. [93] mapped the molecular, cellular, and spatial composition of murine bone marrow megakaryocytes by combining transcriptomics with in situ spatial orientation and demonstrated transcriptional heterogeneity in compartments previously thought to be homogeneous, such as the proximal and distal axes of the bone. No association with adjacency to the vasculature was observed. In the future, we can expect that a combination of spatial proteomics and glycomics will help in further elucidating the full proteome and glycome of the bone marrow niche to understand their influence on megakaryopoiesis and ameliorate approaches for reproducing these features ex vivo.
Recent single-cell analysis data identified megakaryocytes as cells with multiple functions in the bone marrow, beyond platelet production [36]. It is known that megakaryocytes express and release different growth factors that contribute to regulating their function, bone marrow niche homeostasis, and are also involved in the pathogenesis of some diseases. Among these, TGF-β1 released from megakaryocytes is responsible for promoting myelofibrosis. Becker et al. [94] showed that TGF-β1 trafficking is dependent on the Ras homolog family member A (RhoA) and ADP-ribosylation factor 6 (Arf6) signaling pathways, which regulate secretory autophagy. These are interesting findings and are consistent with our data that revealed that defective autophagy is responsible for altered platelet production and function in myopathies caused by collagen VI mutations [95]. Unexpected soluble factors have also been proven to promote megakaryocyte function and platelet production such as thyroid hormones and glucocorticoids [96]. These data offer interesting new angles to interpret diseases and therapeutic opportunities [97]. We are still far from understanding the complete mechanism of platelet production, and new data are emerging from studying diseases. Marin-Quilez et al. [98,99] demonstrated that severe externalization of glycoprotein Ib-alpha (GPIbα) defects is responsible for the altered platelet production in patients with thrombocytopenia associated with variants of the GALE gene, which encodes UDP-galactose-4-epimerase, an enzyme of galactose metabolism and glycosylation pathways critical to megakaryocyte development [100]. These data further emphasize the critical role of glycosylation in regulating both megakaryocyte and platelet functions [27]. Another interesting result from the clinic was presented by Bhoopalan et al. [101], showing increased platelet count in mice and humans treated with the ribosomal biogenesis inhibitor CX-5461, a candidate anticancer therapeutic that has completed phase I/II clinical trials. To date, proplatelet formation has been considered as a cytoskeleton-dependent process; however, new data were provided as this ISTH was regarding the role of lipids in platelet formation. As described above, Valet et al. [18] already demonstrated the importance of the crosstalk between adipocytes and megakaryocytes in the regulation of platelet formation in the bone marrow. Barrachina et al. [102] proposed the use of targeted mass spectrometry to obtain a lipidomic profile of murine bone marrow megakaryocytes, demonstrating that fatty acid metabolism and synthesis are critical for megakaryocyte differentiation, thus pointing out the importance of finely controlled megakaryocyte feeding to support proper cell maturation and functionality.
As flow is also a fundamental trigger of platelet production in vivo, systems created to produce platelets outside the body should take this into account. In line with this evidence, Boiron et al. [103] used a combination of computational fluid dynamics studies and simulations into a platelet-producing device to demonstrate that shear stress accumulation must be properly tailored to obtain efficient platelet release. In an attempt to mimic the lung vasculature, Tarassova et al. [104] investigated platelet production through multiple passages of mouse megakaryocytes into a new lung ex vivo culture system. They demonstrated that the platelets generated in this system were functional, although they showed some signs of preactivation due to multiple passages into the lung-like system.
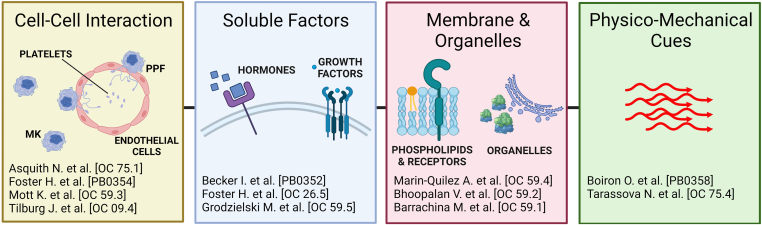
Overall, these data trace 4 major paths toward successful mimicking of megakaryopoiesis ex vivo: (i) recapitulation of cell-cell interaction to support megakaryocyte functions, (ii) selection of humoral and growth factors that physiologically stimulate platelet production, (iii) appropriate cell feeding to improve membrane and metabolic activities, and (iv) precise physicomechanical stimulation (Figure 4).
Figure 4.
Illustrated ISTH 2022 London congress summary. Major paths traced to the future of megakaryopoiesis included the importance of studying the role of cell-cell interaction to support megakaryocyte functions, the need to select proper humoral and growth factors to physiologically stimulate platelet production, the necessity for appropriate cell feeding to improve membrane and metabolic activities, and the role of physicomechanical stimulation. MK, megakaryocyte; PPF, proplatelet formation.
6. Future Research Directions
The need for human platelets for fundamental studies and clinical applications remains high due to the uncertainty of the mechanisms of platelet-related diseases and the clinical need for platelet transfusions for patients. The bone marrow represents a challenging human organ to study due to its structure and complexity within the bone cavity. Because of this, the mechanisms underlying the relationships between HSCs and their environment remain poorly understood, especially in humans, in whom invasive approaches are not possible. Megakaryocytes take cues from the physiochemical environment in the bone marrow, which includes contact with ECM components (such as fibronectin, collagens, and GAGs), contact with other cell types (including vascular cells), and the shear stress generated by blood circulation in blood vessels. ECM components shape the structure of the bone marrow niche and play an active role in regulating hematopoiesis and platelet production. In this context, cytokines, growth factors, and ions (eg, calcium and iron) also determine HSC proliferation or differentiation by directing interactions between cells and their niche environment. Alterations in platelet production or function are the outcome of several human pathologies. Because many of the pathogeneses and specific target therapies are still unknown, these conditions are treated with palliative therapies. As demonstrated at this ISTH meeting, studying diseases and their therapeutic targets may help in understanding triggers of platelet production to be used in vivo and in vitro. It was demonstrated that we can learn from both diseases directly correlated with platelet production defects and other diseases such as collagenopathies, thyroid dysfunction, and Cushing syndrome. We also developed a bone marrow model with enough features to support human platelet production and predict patient response to eltrombopag in vivo [88]. These are important steps forward for comprehensive knowledge on platelet production and new possibilities to cure human diseases. The data support the hypothesis that observing and modeling human physiology and diseases will be instrumental to understand the mechanisms of platelet production and potential triggers to replicate this process in vitro for clinical applications. We have produced many results from different angles, and now, to be even more efficient, we need a systematic approach and common agreement on different aspects of the process, such as the following: (i) how to define a megakaryocyte making platelets, (ii) the exact contribution of the bone marrow niche in human physiology and pathologies, and (iii) the difference between models and systems for platelet production in terms of features to replicate. To add to that, we need to understand the impact of the source of hematopoietic stem and progenitor cells on the regulation of platelet production and function.
Acknowledgments
The figures were made using BioRender (https://www.biorender.com/).
Funding
This work was supported by the European Innovation Council (EIC Transition SilkPlatelet ID: 101058349), Associazione Italiana per la Ricerca sul Cancro (IG 2016 18700), European Hematology Association (RG-202012-00212), and Progetti Di Ricerca Di Rilevante Interesse Nazionale (PRIN 2017Z5LR5Z). The funders had no role in the preparation of the manuscript.
Author contributions
C.A.D.B. and A.B. conceived the review. C.A.D.B and C.P.M. prepared the figures. C.A.D.B., C.P.M., and A.B. wrote the review.
Relationship Disclosure
There are no competing interests to disclose.
Footnotes
Handling Editor: Prof Cihan Ay
References
- 1.Stone A.P., Nascimento T.F., Barrachina M.N. The bone marrow niche from the inside out: how megakaryocytes are shaped by and shape hematopoiesis. Blood. 2022;139:483–491. doi: 10.1182/blood.2021012827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sangkhae V., Etheridge S.L., Kaushansky K., Hitchcock I.S. The thrombopoietin receptor, MPL, is critical for development of a JAK2V617F-induced myeloproliferative neoplasm. Blood. 2014;124:3956–3963. doi: 10.1182/blood-2014-07-587238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakamura-Ishizu A., Matsumura T., Stumpf P.S., Umemoto T., Takizawa H., Takihara Y., et al. Thrombopoietin metabolically primes hematopoietic stem cells to megakaryocyte-lineage differentiation. Cell Rep. 2018;25:1772–1785. doi: 10.1016/j.celrep.2018.10.059. [DOI] [PubMed] [Google Scholar]
- 4.Di Buduo C.A., Abbonante V., Marty C., Moccia F., Rumi E., Pietra D., et al. Defective interaction of mutant calreticulin and SOCE in megakaryocytes from patients with myeloproliferative neoplasms. Blood. 2020;135:133–144. doi: 10.1182/blood.2019001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhat F.A., Advani J., Khan A.A., Mohan S., Pal A., Gowda H., et al. A network map of thrombopoietin signaling. J Cell Commun Signal. 2018;12:737–743. doi: 10.1007/s12079-018-0480-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaushansky K. Historical review: megakaryopoiesis and thrombopoiesis. Blood. 2008;111:981–986. doi: 10.1182/blood-2007-05-088500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu M., Cantor A.B. Megakaryopoiesis and thrombopoiesis: an update on cytokines and lineage surface markers. Methods Mol Biol. 2012;788:291–303. doi: 10.1007/978-1-61779-307-3_20. [DOI] [PubMed] [Google Scholar]
- 8.Di Buduo C.A., Aguilar A., Soprano P.M., Bocconi A., Miguel C.P., Mantica G., et al. Latest culture techniques: cracking the secrets of bone marrow to mass-produce erythrocytes and platelets ex vivo. Haematologica. 2021;106:947–957. doi: 10.3324/haematol.2020.262485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckly A., Heijnen H., Pertuy F., Geerts W., Proamer F., Rinckel J.Y., et al. Biogenesis of the demarcation membrane system (DMS) in megakaryocytes. Blood. 2014;123:921–930. doi: 10.1182/blood-2013-03-492330. [DOI] [PubMed] [Google Scholar]
- 10.Antkowiak A., Viaud J., Severin S., Zanoun M., Ceccato L., Chicanne G., et al. Cdc42-dependent F-actin dynamics drive structuration of the demarcation membrane system in megakaryocytes. J Thromb Haemost. 2016;14:1268–1284. doi: 10.1111/jth.13318. [DOI] [PubMed] [Google Scholar]
- 11.Machlus K.R., Italiano J.E. The incredible journey: from megakaryocyte development to platelet formation. J Cell Biol. 2013;201:785–796. doi: 10.1083/jcb.201304054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrison S.J., Scadden D.T. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malara A., Abbonante V., Di Buduo C.A., Tozzi L., Currao M., Balduini A. The secret life of a megakaryocyte: emerging roles in bone marrow homeostasis control. Cell Mol Life Sci. 2015;72:1517–1536. doi: 10.1007/s00018-014-1813-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le P.M., Andreeff M., Battula V.L. Osteogenic niche in the regulation of normal hematopoiesis and leukemogenesis. Haematologica. 2018;103:1945–1955. doi: 10.3324/haematol.2018.197004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinho S., Frenette P.S. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol. 2019;20:303–320. doi: 10.1038/s41580-019-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbonante V., Gruppi C., Catarsi P., Avanzini M.A., Tira M.E., Barosi G., et al. Altered fibronectin expression and deposition by myeloproliferative neoplasm-derived mesenchymal stromal cells. Br J Haematol. 2016;172:140–144. doi: 10.1111/bjh.13471. [DOI] [PubMed] [Google Scholar]
- 17.Cheng L., Qasba P., Vanguri P., Thiede M.A. Human mesenchymal stem cells support megakaryocyte and pro-platelet formation from CD34(+) hematopoietic progenitor cells. J Cell Physiol. 2000;184:58–69. doi: 10.1002/(SICI)1097-4652(200007)184:1<58::AID-JCP6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 18.Valet C., Batut A., Vauclard A., Dortignac A., Bellio M., Payrastre B., et al. Adipocyte fatty acid transfer supports megakaryocyte maturation. Cell Rep. 2020;32 doi: 10.1016/j.celrep.2020.107875. [DOI] [PubMed] [Google Scholar]
- 19.Kunisaki Y., Bruns I., Scheiermann C., Ahmed J., Pinho S., Zhang D., et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugiyama T., Kohara H., Noda M., Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Junt T., Schulze H., Chen Z., Massberg S., Goerge T., Krueger A., et al. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317:1767–1770. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 22.Avecilla S.T., Hattori K., Heissig B., Tejada R., Liao F., Shido K., et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 23.Malara A., Currao M., Gruppi C., Celesti G., Viarengo G., Buracchi C., et al. Megakaryocytes contribute to the bone marrow-matrix environment by expressing fibronectin, type IV collagen, and laminin. Stem Cells. 2014;32:926–937. doi: 10.1002/stem.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malara A., Gruppi C., Pallotta I., Spedden E., Tenni R., Raspanti M., et al. Extracellular matrix structure and nano-mechanics determine megakaryocyte function. Blood. 2011;118:4449–4453. doi: 10.1182/blood-2011-04-345876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivanovska I.L., Shin J.W., Swift J., Discher D.E. Stem cell mechanobiology: diverse lessons from bone marrow. Trends Cell Biol. 2015;25:523–532. doi: 10.1016/j.tcb.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leiva O., Leon C., Kah Ng S., Mangin P., Gachet C., Ravid K. The role of extracellular matrix stiffness in megakaryocyte and platelet development and function. Am J Hematol. 2018;93:430–441. doi: 10.1002/ajh.25008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falet H., Rivadeneyra L., Hoffmeister K.M. Clinical impact of glycans in platelet and megakaryocyte biology. Blood. 2022;139:3255–3263. doi: 10.1182/blood.2020009303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vögtle T., Sharma S., Mori J., Nagy Z., Semeniak D., Scandola C., et al. Heparan sulfates are critical regulators of the inhibitory megakaryocyte-platelet receptor G6b-B. eLife. 2019;8 doi: 10.7554/eLife.46840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abbonante V., Di Buduo C.A., Gruppi C., De Maria C., Spedden E., De Acutis A., et al. A new path to platelet production through matrix sensing. Haematologica. 2017;102:1150–1160. doi: 10.3324/haematol.2016.161562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malara A., Gruppi C., Rebuzzini P., Visai L., Perotti C., Moratti R., et al. Megakaryocyte-matrix interaction within bone marrow: new roles for fibronectin and factor XIII-A. Blood. 2011;117:2476–2483. doi: 10.1182/blood-2010-06-288795. [DOI] [PubMed] [Google Scholar]
- 31.Chen Z., Naveiras O., Balduini A., Mammoto A., Conti M.A., Adelstein R.S., et al. The May-Hegglin anomaly gene MYH9 is a negative regulator of platelet biogenesis modulated by the Rho-ROCK pathway. Blood. 2007;110:171–179. doi: 10.1182/blood-2007-02-071589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balduini A., Pallotta I., Malara A., Lova P., Pecci A., Viarengo G., et al. Adhesive receptors, extracellular proteins and myosin IIA orchestrate proplatelet formation by human megakaryocytes. J Thromb Haemost. 2008;6:1900–1907. doi: 10.1111/j.1538-7836.2008.03132.x. [DOI] [PubMed] [Google Scholar]
- 33.Abbonante V., Di Buduo C.A., Gruppi C., Malara A., Gianelli U., Celesti G., et al. Thrombopoietin/TGF-β1 loop regulates megakaryocyte extracellular matrix component synthesis. Stem Cells. 2016;34:1123–1133. doi: 10.1002/stem.2285. [DOI] [PubMed] [Google Scholar]
- 34.Wang H., He J., Xu C., Chen X., Yang H., Shi S., et al. Decoding human megakaryocyte development. Cell Stem Cell. 2021;28:535–549. doi: 10.1016/j.stem.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Sun S., Jin C., Si J., Lei Y., Chen K., Cui Y., et al. Single-cell analysis of ploidy and the transcriptome reveals functional and spatial divergency in murine megakaryopoiesis. Blood. 2021;138:1211–1224. doi: 10.1182/blood.2021010697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu C., Huang B., Wang H., Zhou J. The heterogeneity of megakaryocytes and platelets and implications for ex vivo platelet generation. Stem Cells Transl Med. 2021;10:1614–1620. doi: 10.1002/sctm.21-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schachtner H., Calaminus S.D., Sinclair A., Monypenny J., Blundell M.P., Leon C., et al. Megakaryocytes assemble podosomes that degrade matrix and protrude through basement membrane. Blood. 2013;121:2542–2552. doi: 10.1182/blood-2012-07-443457. [DOI] [PubMed] [Google Scholar]
- 38.Eckly A., Scandola C., Oprescu A., Michel D., Rinckel J.Y., Proamer F., et al. Megakaryocytes use in vivo podosome-like structures working collectively to penetrate the endothelial barrier of bone marrow sinusoids. J Thromb Haemost. 2020;18:2987–3001. doi: 10.1111/jth.15024. [DOI] [PubMed] [Google Scholar]
- 39.Oprescu A., Michel D., Antkowiak A., Vega E., Viaud J., Courtneidge S.A., et al. Megakaryocytes form linear podosomes devoid of digestive properties to remodel medullar matrix. Sci Rep. 2022;12:6255. doi: 10.1038/s41598-022-10215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L., Orban M., Lorenz M., Barocke V., Braun D., Urtz N., et al. A novel role of sphingosine 1-phosphate receptor S1pr1 in mouse thrombopoiesis. J Exp Med. 2012;209:2165–2181. doi: 10.1084/jem.20121090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stegner D., vanEeuwijk J.M.M., Angay O., Gorelashvili M.G., Semeniak D., Pinnecker J., et al. Thrombopoiesis is spatially regulated by the bone marrow vasculature. Nat Commun. 2017;8:127. doi: 10.1038/s41467-017-00201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ito Y., Nakamura S., Sugimoto N., Shigemori T., Kato Y., Ohno M., et al. Turbulence activates platelet biogenesis to enable clinical scale ex vivo production. Cell. 2018;174:636–648. doi: 10.1016/j.cell.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 43.Petzold T., Zhang Z., Ballesteros I., Saleh I., Polzin A., Thienel M., et al. Neutrophil “plucking” on megakaryocytes drives platelet production and boosts cardiovascular disease. Immunity. 2022;55:2285–2299. doi: 10.1016/j.immuni.2022.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee-Sundlov M.M., Burns R.T., Kim T.O., Grozovsky R., Giannini S., Rivadeneyra L., et al. Immune cells surveil aberrantly sialylated O-glycans on megakaryocytes to regulate platelet count. Blood. 2021;138:2408–2424. doi: 10.1182/blood.2020008238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishimura S., Nagasaki M., Kunishima S., Sawaguchi A., Sakata A., Sakaguchi H., et al. IL-1α induces thrombopoiesis through megakaryocyte rupture in response to acute platelet needs. J Cell Biol. 2015;209:453–466. doi: 10.1083/jcb.201410052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Potts K.S., Farley A., Dawson C.A., Rimes J., Biben C., de Graaf C., et al. Membrane budding is a major mechanism of in vivo platelet biogenesis. J Exp Med. 2020;217 doi: 10.1084/jem.20191206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lefrançais E., Ortiz-Muñoz G., Caudrillier A., Mallavia B., Liu F., Sayah D.M., et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544:105–109. doi: 10.1038/nature21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burgstaller G., Oehrle B., Gerckens M., White E.S., Schiller H.B., Eickelberg O. The instructive extracellular matrix of the lung: basic composition and alterations in chronic lung disease. Eur Respir J. 2017;50 doi: 10.1183/13993003.01805-2016. [DOI] [PubMed] [Google Scholar]
- 49.Sicard D., Haak A.J., Choi K.M., Craig A.R., Fredenburgh L.E., Tschumperlin D.J. Aging and anatomical variations in lung tissue stiffness. Am J Physiol Lung Cell Mol Physiol. 2018;314:L946–L955. doi: 10.1152/ajplung.00415.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pariser D.N., Hilt Z.T., Ture S.K., Blick-Nitko S.K., Looney M.R., Cleary S.J., et al. Lung megakaryocytes are immune modulatory cells. J Clin Invest. 2021;131 doi: 10.1172/JCI137377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goette N.P., Borzone F.R., Lupi A.D., Chasseing N.A., Rubio M.F., Costas M.A., et al. Megakaryocyte-stromal cell interactions: effect on megakaryocyte proliferation, proplatelet production, and survival. Exp Hematol. 2022;107:24–37. doi: 10.1016/j.exphem.2022.01.002. [DOI] [PubMed] [Google Scholar]
- 52.Di Buduo C.A., Moccia F., Battiston M., De Marco L., Mazzucato M., Moratti R., et al. The importance of calcium in the regulation of megakaryocyte function. Haematologica. 2014;99:769–778. doi: 10.3324/haematol.2013.096859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bradley T.R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966;44:287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- 54.Khan A.O., Rodriguez-Romera A., Reyat J.S., Olijnik A.A., Colombo M., Wang G., et al. Human bone marrow organoids for disease modelling, discovery and validation of therapeutic targets in hematological malignancies. Cancer Discov. 2023;13:364–385. doi: 10.1158/2159-8290.CD-22-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bose S., Clevers H., Shen X. Promises and challenges of organoid-guided precision medicine. Med. 2021;2:1011–1026. doi: 10.1016/j.medj.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Currao M., Malara A., Di Buduo C.A., Abbonante V., Tozzi L., Balduini A. Hyaluronan based hydrogels provide an improved model to study megakaryocyte-matrix interactions. Exp Cell Res. 2016;346:1–8. doi: 10.1016/j.yexcr.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aguilar A., Pertuy F., Eckly A., Strassel C., Collin D., Gachet C., et al. Importance of environmental stiffness for megakaryocyte differentiation and proplatelet formation. Blood. 2016;128:2022–2032. doi: 10.1182/blood-2016-02-699959. [DOI] [PubMed] [Google Scholar]
- 58.Pietrzyk-Nivau A., Poirault-Chassac S., Gandrille S., Derkaoui S.M., Kauskot A., Letourneur D., et al. Three-dimensional environment sustains hematopoietic stem cell differentiation into platelet-producing megakaryocytes. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sieber S., Wirth L., Cavak N., Koenigsmark M., Marx U., Lauster R., et al. Bone marrow-on-a-chip: long-term culture of human haematopoietic stem cells in a three-dimensional microfluidic environment. J Tissue Eng Regen Med. 2018;12:479–489. doi: 10.1002/term.2507. [DOI] [PubMed] [Google Scholar]
- 60.Torisawa Y.S., Spina C.S., Mammoto T., Mammoto A., Weaver J.C., Tat T., et al. Bone marrow-on-a-chip replicates hematopoietic niche physiology in vitro. Nat Methods. 2014;11:663–669. doi: 10.1038/nmeth.2938. [DOI] [PubMed] [Google Scholar]
- 61.Thon J.N., Mazutis L., Wu S., Sylman J.L., Ehrlicher A., Machlus K.R., et al. Platelet bioreactor-on-a-chip. Blood. 2014;124:1857–1867. doi: 10.1182/blood-2014-05-574913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Avanzi M.P., Oluwadara O.E., Cushing M.M., Mitchell M.L., Fischer S., Mitchell W.B. A novel bioreactor and culture method drives high yields of platelets from stem cells. Transfusion. 2016;56:170–178. doi: 10.1111/trf.13375. [DOI] [PubMed] [Google Scholar]
- 63.Blin A., Le Goff A., Magniez A., Poirault-Chassac S., Teste B., Sicot G., et al. Microfluidic model of the platelet-generating organ: beyond bone marrow biomimetics. Sci Rep. 2016;6 doi: 10.1038/srep21700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martinez A.F., Miller W.M. Enabling large-scale ex vivo production of megakaryocytes from CD34+ cells using gas-permeable surfaces. Stem Cells Transl Med. 2019;8:658–670. doi: 10.1002/sctm.18-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shepherd J.H., Howard D., Waller A.K., Foster H.R., Mueller A., Moreau T., et al. Structurally graduated collagen scaffolds applied to the ex vivo generation of platelets from human pluripotent stem cell-derived megakaryocytes: enhancing production and purity. Biomaterials. 2018;182:135–144. doi: 10.1016/j.biomaterials.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 66.Kotha S., Sun S., Adams A., Hayes B., Phong K.T., Nagao R., et al. Microvasculature-directed thrombopoiesis in a 3D in vitro marrow microenvironment. PLoS One. 2018;13 doi: 10.1371/journal.pone.0195082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Di Buduo C.A., Abbonante V., Tozzi L., Kaplan D.L., Balduini A. Three-dimensional tissue models for studying ex vivo megakaryocytopoiesis and platelet production. Methods Mol Biol. 2018;1812:177–193. doi: 10.1007/978-1-4939-8585-2_11. [DOI] [PubMed] [Google Scholar]
- 68.Omenetto F.G., Kaplan D.L. New opportunities for an ancient material. Science. 2010;329:528–531. doi: 10.1126/science.1188936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rockwood D.N., Preda R.C., Yücel T., Wang X., Lovett M.L., Kaplan D.L. Materials fabrication from Bombyx mori silk fibroin. Nat Protoc. 2011;6:1612–1631. doi: 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thurber A.E., Omenetto F.G., Kaplan D.L. In vivo bioresponses to silk proteins. Biomaterials. 2015;71:145–157. doi: 10.1016/j.biomaterials.2015.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li A.B., Kluge J.A., Guziewicz N.A., Omenetto F.G., Kaplan D.L. Silk-based stabilization of biomacromolecules. J Control Release. 2015;219:416–430. doi: 10.1016/j.jconrel.2015.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pallotta I., Lovett M., Kaplan D.L., Balduini A. Three-dimensional system for the in vitro study of megakaryocytes and functional platelet production using silk-based vascular tubes. Tissue Eng Part C Methods. 2011;17:1223–1232. doi: 10.1089/ten.tec.2011.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Di Buduo C.A., Wray L.S., Tozzi L., Malara A., Chen Y., Ghezzi C.E., et al. Programmable 3D silk bone marrow niche for platelet generation ex vivo and modeling of megakaryopoiesis pathologies. Blood. 2015;125:2254–2264. doi: 10.1182/blood-2014-08-595561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tozzi L., Laurent P.A., Di Buduo C.A., Mu X., Massaro A., Bretherton R., et al. Multi-channel silk sponge mimicking bone marrow vascular niche for platelet production. Biomaterials. 2018;178:122–133. doi: 10.1016/j.biomaterials.2018.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Di Buduo C.A., Soprano P.M., Tozzi L., Marconi S., Auricchio F., Kaplan D.L., et al. Modular flow chamber for engineering bone marrow architecture and function. Biomaterials. 2017;146:60–71. doi: 10.1016/j.biomaterials.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smock K.J., Perkins S.L. Thrombocytopenia: an update. Int J Lab Hematol. 2014;36:269–278. doi: 10.1111/ijlh.12214. [DOI] [PubMed] [Google Scholar]
- 77.Pecci A., Balduini C.L. Inherited thrombocytopenias: an updated guide for clinicians. Blood Rev. 2021;48 doi: 10.1016/j.blre.2020.100784. [DOI] [PubMed] [Google Scholar]
- 78.Heremans J., Freson K. High-throughput sequencing for diagnosing platelet disorders: lessons learned from exploring the causes of bleeding disorders. Int J Lab Hematol. 2018;40:89–96. doi: 10.1111/ijlh.12812. [DOI] [PubMed] [Google Scholar]
- 79.Barbui T., Thiele J., Gisslinger H., Finazzi G., Vannucchi A.M., Tefferi A. The 2016 revision of WHO classification of myeloproliferative neoplasms: clinical and molecular advances. Blood Rev. 2016;30:453–459. doi: 10.1016/j.blre.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 80.Balduini A., Badalucco S., Pugliano M.T., Baev D., De Silvestri A., Cattaneo M., et al. In vitro megakaryocyte differentiation and proplatelet formation in Ph-negative classical myeloproliferative neoplasms: distinct patterns in the different clinical phenotypes. PLoS One. 2011;6 doi: 10.1371/journal.pone.0021015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Badalucco S., Di Buduo C.A., Campanelli R., Pallotta I., Catarsi P., Rosti V., et al. Involvement of TGFβ1 in autocrine regulation of proplatelet formation in healthy subjects and patients with primary myelofibrosis. Haematologica. 2013;98:514–517. doi: 10.3324/haematol.2012.076752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kuter D.J., Bain B., Mufti G., Bagg A., Hasserjian R.P. Bone marrow fibrosis: pathophysiology and clinical significance of increased bone marrow stromal fibres. Br J Haematol. 2007;139:351–362. doi: 10.1111/j.1365-2141.2007.06807.x. [DOI] [PubMed] [Google Scholar]
- 83.Locatelli F., Rossi G., Balduini C. Hematopoietic stem-cell transplantation for the Bernard-Soulier syndrome. Ann Intern Med. 2003;138:79. doi: 10.7326/0003-4819-138-1-200301070-00028. [DOI] [PubMed] [Google Scholar]
- 84.Balduini C.L., Pecci A., Noris P. Diagnosis and management of inherited thrombocytopenias. Semin Thromb Hemost. 2013;39:161–171. doi: 10.1055/s-0032-1333540. [DOI] [PubMed] [Google Scholar]
- 85.Deng J., Hu H., Huang F., Huang C., Huang Q., Wang L., et al. Comparative efficacy and safety of thrombopoietin receptor agonists in adults with thrombocytopenia: a systematic review and network meta-analysis of randomized controlled trial. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.704093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kuter D.J. The biology of thrombopoietin and thrombopoietin receptor agonists. Int J Hematol. 2013;98:10–23. doi: 10.1007/s12185-013-1382-0. [DOI] [PubMed] [Google Scholar]
- 87.Loscocco G.G., Vannucchi A.M. Role of JAK inhibitors in myeloproliferative neoplasms: current point of view and perspectives. Int J Hematol. 2022;115:626–644. doi: 10.1007/s12185-022-03335-7. [DOI] [PubMed] [Google Scholar]
- 88.Di Buduo C.A., Laurent P.A., Zaninetti C., Lordier L., Soprano P.M., Ntai A., et al. Miniaturized 3D bone marrow tissue model to assess response to thrombopoietin-receptor agonists in patients. eLife. 2021;10 doi: 10.7554/eLife.58775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sugimoto N., Kanda J., Nakamura S., Kitano T., Hishizawa M., Kondo T., et al. iPLAT1: the first-in-human clinical trial of iPSC-derived platelets as a phase 1 autologous transfusion study. Blood. 2022;140:2398–2402. doi: 10.1182/blood.2022017296. [DOI] [PubMed] [Google Scholar]
- 90.Asquith N., Freire D., Shelton S., Machlus K., Kamm R., Italiano J. A 3D microvasculature assay reveals novel sub-cellular dynamics of megakaryocyte endothelial barrier interactions, proplatelet formation and release. Res Pract Thromb Haemost. 2022;6 [Google Scholar]
- 91.Foster H., Colzani M., Bouet Chalon G., Howard D., Di Buduo C., Muller-Sienerth N., et al. Production of platelets in vitro in functionalised 3-dimensional scaffolds mimicking the bone marrow niche. Res Pract Thromb Haemost. 2022;6 [Google Scholar]
- 92.Mott K., Keß C., Frank A., Hölle S., Nagy Z., Schlegel N., et al. Megakaryocyte engraftment and altered platelet function in a murine model of hematopoietic stem cell transplantation. Res Pract Thromb Haemost. 2022;6 [Google Scholar]
- 93.Tilburg J., Stone A., Billingsley J., Scoville D., Italiano J., Machlus K. Spatial transcriptomics reveals megakaryocyte heterogeneity in mouse bone marrow. Res Pract Thromb Haemost. 2022;6 doi: 10.1016/j.rpth.2023.100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Becker I., Lykins J., Barrachina M., Roweth H.G., Nieswandt B., Whiteheart S.W., et al. Secretion of transforming growth factor β1 from megakaryocytes is mediated by autophagy signaling pathways. Res Pract Thromb Haemost. 2022;6 [Google Scholar]
- 95.Abbonante V., Malara A., Chrisam M., Metti S., Soprano P., Semplicini C., et al. Lack of COL6/collagen VI causes megakaryocyte dysfunction by impairing autophagy and inducing apoptosis. Autophagy. 2023;19:984–999. doi: 10.1080/15548627.2022.2100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Foster H., Herbert N., Waller A., Di Buduo C., Fang J., Schmidt A., et al. Thyroid hormones and analogues promote the acute release of platelets from megakaryocytes: from blood donor biology to the production of platelets in vitro. Res Pract Thromb Haemost. 2022;6 [Google Scholar]
- 97.Grodzielski M., Cidlowski J. Glucocorticoids stimulate thrombopoiesis in murine megakaryocytes. Res Pract Thromb Haemost. 2022;6 [Google Scholar]
- 98.Marin-Quilez A., Di Buduo C., Díaz-Ajenjo L., Abbonante V., Vuelta E., Soprano P.M., et al. Novel variants in GALE cause syndromic macrothrombocytopenia by disrupting thrombopoiesis and glycosylation. Res Pract Thromb Haemost. 2022;6 doi: 10.1182/blood.2022016995. [DOI] [PubMed] [Google Scholar]
- 99.Marín-Quilez A., Di Buduo C.A., Díaz-Ajenjo L., Abbonante V., Vuelta E., Soprano P.M., et al. Novel variants in GALE cause syndromic macrothrombocytopenia by disrupting glycosylation and thrombopoiesis. Blood. 2023;141:406–421. doi: 10.1182/blood.2022016995. [DOI] [PubMed] [Google Scholar]
- 100.Seo A., Gulsuner S., Pierce S., Ben-Harosh M., Shalev H., Walsh T., et al. Inherited thrombocytopenia associated with mutation of UDP-galactose-4-epimerase (GALE) Hum Mol Genet. 2019;28:133–142. doi: 10.1093/hmg/ddy334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bhoopalan V., Kaur A., Hein N., Maclachlan K., Ferreira R., Man S., et al. Ribosomal biogenesis inhibition facilitates megakaryocyte/platelet biased haematopoiesis. Res Pract Thromb Haemost. 2022;6 [Google Scholar]
- 102.Barrachina M., Pernes G., Becker I., Freire D., Groeneveld D., Luyendyk J., et al. Thrombopoiesis has a unique lipidomic profile enriched in polyunsaturated fatty acids that facilitates megakaryocyte maturation and platelet production. Res Pract Thromb Haemost. 2022;6 [Google Scholar]
- 103.Boiron O., Lanza F., Strassel C., Knapp Y. In-depth analysis of the effects of unsteady flows on platelet release from suspensions of proplatelet-bearing megakaryocytes. Res Pract Thromb Haemost. 2022;6 [Google Scholar]
- 104.Tarassova N., Zhao X., Poole A. Investigating the health and function of murine platelets generated using a new ex-vivo lung system. Res Pract Thromb Haemost. 2022;6 [Google Scholar]