Abstract
Alcoholic liver cirrhosis (ALC) is caused by chronic alcohol overconsumption and might be linked to dysregulated immune responses in the gut-liver axis. However, there is a lack of comprehensive research on levels and functions of innate lymphocytes including mucosal-associated invariant T (MAIT) cells, NKT cells, and NK (NK) cells in ALC patients. Thus, the aim of this study was to examine the levels and function of these cells, evaluate their clinical relevance, and explore their immunologic roles in the pathogenesis of ALC. Peripheral blood samples from ALC patients (n = 31) and healthy controls (HCs, n = 31) were collected. MAIT cells, NKT cells, NK cells, cytokines, CD69, PD-1, and lymphocyte-activation gene 3 (LAG-3) levels were measured by flow cytometry. Percentages and numbers of circulating MAIT cells, NKT cells, and NK cells were significantly reduced in ALC patients than in HCs. MAIT cell exhibited increased production of IL-17 and expression levels of CD69, PD-1, and LAG-3. NKT cells displayed decreased production of IFN-γ and IL-4. NK cells showed elevated CD69 expression. Absolute MAIT cell levels were positively correlated with lymphocyte count but negatively correlated with C-reactive protein. In addition, NKT cell levels were negatively correlated with hemoglobin levels. Furthermore, log-transformed absolute MAIT cell levels were negatively correlated with the Age, Bilirubin, INR, and Creatinine score. This study demonstrates that circulating MAIT cells, NKT cells, and NK cells are numerically deficient in ALC patients, and the degree of cytokine production and activation status also changed. Besides, some of their deficiencies are related to several clinical parameters. These findings provide important information about immune responses of ALC patients.
Keywords: Alcoholic liver cirrhosis, Mucosal-associated invariant T cells, Natural killer T cells, Natural killer cells, Prognosis
INTRODUCTION
Alcohol use disorder is a common disease worldwide, of which alcoholic liver disease (ALD) accounts for a major portion (1). ALD is a leading cause of liver-related mortality. With gradually increasing prevalence, ALD is becoming a considerable socioeconomic burden in many countries (2). Chronic alcohol overconsumption might trigger the liver to undergo several pathologic changes, ranging from hepatic steatosis and alcoholic steatohepatitis to alcoholic liver cirrhosis (ALC) and hepatocellular carcinoma (HCC) (3,4). Such development and progression of ALD are known as a result of repeated complex immune reactions. Accumulating data suggest that dysregulated immune responses in the “gut-liver axis” might contribute to the worsening of various liver diseases (4,5). Therefore, research on immune cells associated with the intestine and the liver is crucial for revealing the ALD pathophysiology.
Recently, studies on pathogenetic functions of innate-like and innate lymphocytes such as mucosal-associated invariant T (MAIT) cells, NKT cells, and NK cells are emerging (6,7). In humans, MAIT cells and NKT cells are subsets of innate-like lymphocytes that express invariant T cell receptor α-chain (TCR Vα7.2-Jα33/12/20) and invariant TCR Vα24-Jα18 chain, respectively (8,9). NK cells are a subset of innate lymphoid cells that use various surface receptors to identify changes of infected or injured host cells (10). These immune cells are reported to be abundant in the intestinal mucosa and liver. They can directly or indirectly affect other Antigen-presenting cells and T lymphocytes, eventually provoking liver damage or regeneration (11,12).
Several studies have described pathologic roles of these innate immune cells in ALD. A myriad of studies have suggested their profibrogenic role in the liver (13,14). In addition, depletion of MAIT cells can induce fibrogenesis and augment bacterial translocation due to loss of antibacterial potency in ALD patients (15,16). In murine models, NKT cells play a pathogenic role in ALD in that they can migrate from the intestine to the liver and induce hepatocyte apoptosis and fibrosis (17,18). NK cells are reported to play an ambivalent role against liver injury. Alcohol consumption inhibits anti-fibrotic function of NK cells, thereby causing more fibrosis in the liver (19,20). However, there is a lack of comprehensive research on levels and functions of these immune cells coupled with their association with clinical indicators in ALC patients. Thus, this study aimed to examine levels and functions of MAIT cells, NKT cells, and NK cells in ALC patients, evaluate their clinical relevance, and explorer their immunologic roles in the pathogenesis of ALC.
MATERIALS AND METHODS
Patients
The study cohort included 31 decompensated ALC patients (8 females and 23 males; mean age ± SD: 52.7 ± 9.7 years) hospitalized at Chonnam National University Hospital and 31 healthy controls (HCs; 8 females and 23 males; mean age ± SD: 59.8 ± 8.0 years). Patients with radiological findings consistent with cirrhosis (caudate and left lateral segmental enlargement, right and left lobe medial segmental atrophy, and regenerative nodules) on abdominal computed tomography were included in the liver cirrhosis (LC) cohort, and the presence of hepatic encephalopathy, varices, and ascites with no other cause than alcohol consumption was defined as decompensated ALC (21,22). HCs had no history of autoimmune disease, infectious disease, malignancy, chronic pulmonary, liver or renal disease, immunosuppressive therapy, or fever within 72 h prior to enrollment. To assess the severity of ALC, the Discriminant Function Index (DFI, mean score ± SD: 61.0 ± 43.1); Glasgow Alcoholic Hepatitis Score (GAHS, mean score ± SD: 8.7 ± 1.5); the Age, Bilirubin, INR, and Creatinine (ABIC, mean score ± SD: 8.3 ± 1.4) score; and the Model of End-Stage Liver Disease (MELD) score (mean score ± SD: 23.3 ± 12.8) were calculated (23,24,25,26). Clinical and laboratory characteristics of patients are summarized in Table 1.
Table 1. Clinical and laboratory characteristics of 31 patients with alcoholic liver cirrhosis.
| Parameters | Findings | |
|---|---|---|
| Sex (male/female) | 23/8 | |
| Age (yr) | 52.7 ± 9.7 | |
| Modified DFI | 61.0 ± 43.1 | |
| GAHS | 8.7 ± 1.5 | |
| ABIC score | 8.3 ± 1.4 | |
| MELD score | 23.3 ± 12.8 | |
| Laboratory variables | ||
| Leukocytes (cells/μl) | 10,506 ± 7,509 | |
| Lymphocytes (cells/μl) | 1,089 ± 635 | |
| Neutrophils (cells/μl) | 8,244 ± 6,964 | |
| Hemoglobin (g/dl) | 9.1 ± 1.8 | |
| Platelets (×103/μl) | 103 ± 63 | |
| Total bilirubin (mg/dl) | 14.4 ± 13.5 | |
| Total protein (g/dl) | 5.8 ± 1.0 | |
| Albumin (g/dl) | 2.8 ± 0.6 | |
| AST (U/l) | 136.6 ± 139.5 | |
| ALT (U/l) | 55.3 ± 86.3 | |
| Alkaline phosphatase (U/l) | 131.7 ± 57.9 | |
| Prothrombin time (INR) | 1.98 ± 0.69 | |
| LDH (U/l) | 710 ± 345 | |
| Creatinine (mg/dl) | 1.0 ± 0.6 | |
| CRP (mg/dl) | 2.9 ± 3.3 | |
Data are presented as number or the mean ± SD.
Monoclonal Abs (mAbs) and flow cytometry
The following mAbs and reagents were used in this study: Allophycocyanin (APC)-conjugated anti-CD3, APC-conjugated anti-IL-4, APC-conjugated anti-TCRVα24-Jα18, APC-Cy7-conjugated anti-CD3, FITC-conjugated anti-CD3, FITC-conjugated anti-CD45, FITC-conjugated anti-CD56, FITC-conjugated anti-TCRγδ, FITC-conjugated anti-IFN-γ, PE-conjugated anti-TCRVα24-Jα18, PE-conjugated anti-CD45, PE-conjugated anti-CD56, PE-conjugated anti-CD69, PE-conjugated anti-IL-17A, PE-conjugated anti-lymphocyte-activation gene 3 (anti-LAG3), PE-Cy5-conjugated anti-CD161, PE-Cy7-conjugated anti-TNF-α, PerCP-conjugated anti-CD3, PerCP-conjugated anti-CD45, FITC-conjugated mouse IgG isotype, PE-conjugated mouse IgG isotype and PE-Cy7-conjugated mouse IgG isotype control (all from Becton Dickinson, San Diego, CA, USA); PE-conjugated anti-programmed death-1 (anti-PD-1; eBioscience, San Diego, CA, USA) and APC-conjugated anti-TCR Vα7.2 (BioLegend, San Diego, CA, USA). Cells were stained with combinations of appropriate mAbs for 20 min at 4°C. Stained cells were analyzed on a Navios flow cytometer using Kaluza software (version 1.5a; Beckman Coulter, Brea, CA, USA).
Isolation of PBMCs and the identification of MAIT, NK, and NKT cells
Peripheral venous blood samples were collected into heparin-containing tubes, and PBMCs were isolated by density-gradient centrifugation using Ficoll-Paque Plus solution (Amersham Biosciences, Uppsala, Sweden). MAIT, NK, and NKT cells were identified phenotypically as CD3+TCRγδ−Vα7.2+CD161high cells, CD3−CD56+ cells, and CD3+TCRVα24-Jα18+ cells, respectively, by flow cytometry as previously described (27,28,29).
Functional MAIT cell assay
IFN-γ, IL-17, and TNF-α expression levels in MAIT cells were detected by intracellular cytokine flow cytometry as previously described (11,28). Briefly, freshly isolated PBMCs (1×106/well) were incubated in 1 ml complete media, consisting of RPMI 1640, 2 mM L-glutamine, 100 units/ml of penicillin, and 100 μg/ml of streptomycin, and supplemented with 10% FBS (Welgene, Gyeongsan, Korea) for one hour in the presence of PMA (100 ng/mL; Sigma, St Louis, MO, USA) and ionomycin (IM) (1 μM; Sigma). For intracellular cytokine staining, 1 μl of brefeldin A for 1 ml of cell culture (GolgiPlug; BD Biosciences, San Diego, CA, USA) was added. The final concentration of brefeldin A was 10 μg/ml. After incubation for an additional 4 h, cells were stained with APC-Cy7-conjugated anti-CD3, PE-Cy5-conjugated anti-CD161, and APC-conjugated anti-TCR Vα7.2 mAbs for 20 min at 4°C, fixed in 4% paraformaldehyde for 15 min at room temperature, and permeabilized with Perm/Wash solution (BD Biosciences) for 10 min. Cells were then stained with FITC-conjugated anti-IFN-γ, PE-conjugated anti-IL-17A and PE-Cy7-conjugated anti-TNF-α mAbs for 30 min at 4°C and analyzed by flow cytometry.
Functional NKT cell assay
Freshly isolated PBMCs (1×106/well) were incubated in 1 ml complete media, consisting of RPMI 1640, 2 mM L-glutamine, 100 units/ml of penicillin, and 100 μg/ml of streptomycin, and supplemented with 10% FBS for 2 h in the presence of α-GalCer (100 ng/ml; Alexis Biochemicals, San Diego, CA, USA) or 0.1% DMSO as control. For intracellular cytokine staining, 1 μl of brefeldin A for 1 ml of cell culture was added. After incubation for an additional 4 h, cells were stained with FITC-conjugated anti-CD3 and APC-conjugated anti-TCRVα24-Jα18 mAbs for 20 min at 4°C, fixed in 4% paraformaldehyde for 15 min at room temperature, and permeabilized with Perm/Wash solution for 10 min. Cells were then stained with PE-conjugated anti-IFN-γ and PE-conjugated anti-IL-4 mAbs for 30 min at 4°C and analyzed by flow cytometry.
Functional NK cell assay
Freshly isolated PBMCs (1×106/well) were incubated in 1 ml complete media, consisting of RPMI 1640, 2 mM L-glutamine, 100 units/ml of penicillin, 100 μg/ml of streptomycin, and supplemented with 10% FBS for 24 h in the presence of IL-12 (50 ng/ml; Miltenyi Biotec, Bergisch Gladbach, Germany) and IL-18 (50 ng/ml; Medical and Biological Laboratories, Woburn, MA, USA). For intracellular cytokine staining, 1 μl of brefeldin A for 1 ml of cell culture was added. After incubation for an additional 4 h, cells were stained with APC-Cy7-conjugated anti-CD3 and PE-conjugated anti-CD56 mAbs for 20 min at 4°C, fixed in 4% paraformaldehyde for 15 min at room temperature, and permeabilized with Perm/Wash solution for 10 min. Cells were then stained with FITC-conjugated anti-IFN-γ for 30 min at 4°C and analyzed by flow cytometry.
Statistical analysis
All comparisons of percentages and absolute numbers of MAIT, NK, and NKT cells were performed by analysis of covariance after adjusting for age and sex using Bonferroni correction for multiple comparisons. The Mann-Whiney U test was used to compare expression levels of CD69, PD-1, LAG3, and cytokines in MAIT, NK, or NKT cells from ALC patients versus HCs. Linear regression analysis was used to test associations between cell levels and clinical or laboratory parameters. The p-values less than 0.05 were considered statistically significant. All statistical analysis and graphic works were performed using SPSS version 26.0 software (IBM Corp., Armonk, NY, USA) and GraphPad Prism version 5.03 software (GraphPad Software, San Diego, CA, USA), respectively.
RESULTS
Reduced numbers of circulating MAIT cells, NKT cells, and NK cells in ALC patients
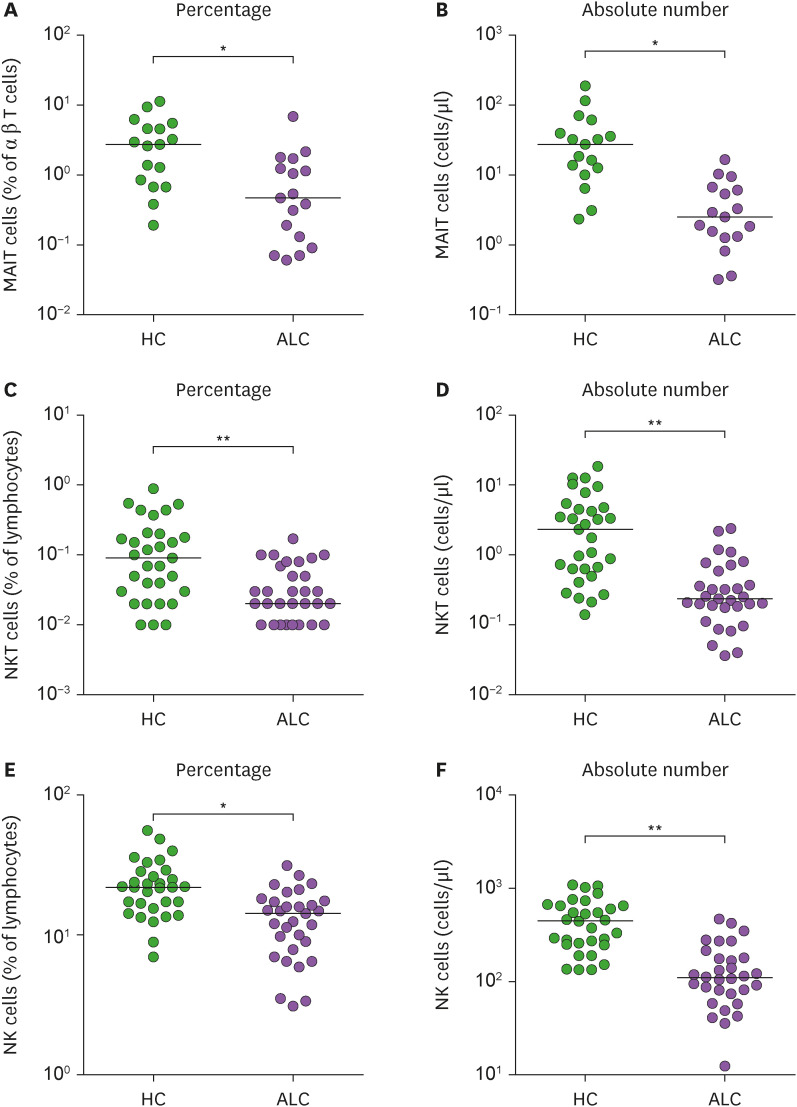
Percentages and absolute numbers of MAIT cells, NKT cells, and NK cells in peripheral blood samples were determined by flow cytometry. All comparisons of percentages and absolute numbers of these cells were performed by analysis of covariance (ANCOVA) after adjusting for age and sex using the Bonferroni correction for multiple comparisons as described in the Materials and Methods. MAIT cells were defined as CD3+TCRγδ− T-cells expressing TCRVα7.2 and CD161high. NKT cells were defined as CD3+TCRVα24-Jα18+ cells, and NK cells were defined as CD3−CD56+ cells (Supplementary Fig. 1). Percentage of circulating MAIT cells, NKT cells, and NK cells were significantly lower in ALC patients than in HCs (for MAIT cells, median 0.47% vs. 2.73%, p<0.005; for NKT cells, median 0.02% vs. 0.09%, p<0.0001; for NK cells, median 14.32% vs. 21.90%, p<0.005; Fig. 1A, C, and E). Absolute numbers of circulating MAIT cells, NKT cells, and NK cells were calculated by multiplying each cell fraction by total lymphocyte numbers (per μl of peripheral blood). ALC patients had significantly lower absolute numbers of MAIT cells, NKT cells, and NK cells than HCs (for MAIT cells, median 2.51 vs. 27.44 cells/μl, p<0.005; for NKT cells, median 0.24 vs. 2.33 cells/μl, p<0.0001; for NK cells, median 109.4 vs. 450.3 cells/μl, p<0.0001; Fig. 1B, D, and F).
Figure 1. Decreased innate immune cells in the peripheral blood of patients with alcoholic liver cirrhosis. Freshly isolated PBMCs from HCs and patients with alcoholic liver cirrhosis were stained with APC-Cy7-conjugated anti-CD3, FITC-conjugate anti-CD3, FITC-conjugate anti-CD56, FITC-conjugated anti-TCRγδ, APC-conjugated anti-TCRVα7.2, PE-conjugated anti-CD3, PE-conjugated anti-TCRVα24-Jα18, PE-Cy5-conjugated anti-CD161, PerCP-conjugated anti-CD45 mAbs and then analyzed by flow cytometry. (A) Percentages of MAIT cells were calculated using a αβ T cell gate. (B) Absolute MAIT cell numbers (per μl of blood). (C) Percentages of NKT cells were calculated using a CD45/SSC gate. (D) Absolute NKT cell numbers (per μl of blood). (E) Percentages of NK cells were calculated using a CD45/SSC gate. (F) Absolute NK cell numbers (per μl of blood). Data in (A and B) were obtained from 17 HCs and 17 patients. Data in (C, D, E, and F) were obtained from 31 HCs and 31 patients.
Symbols represent individual subjects and horizontal lines show median values. *p<0.005, **p<0.0001 by ANCOVA.
Altered cytokine production by MAIT cells and NKT cells from ALC patients
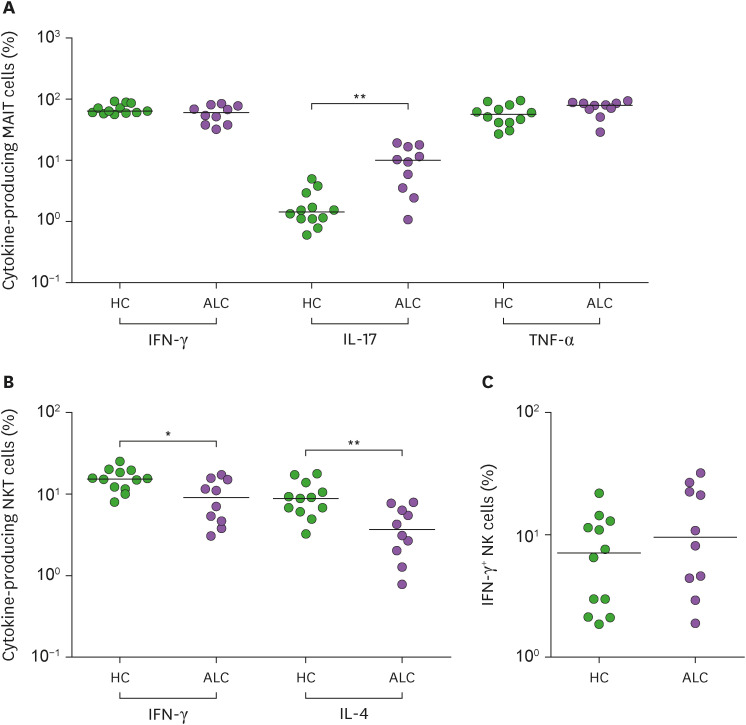
We next examined cytokine-releasing profiles of MAIT cells, NKT cells, and NK cells from ALC patients. IFN-γ, IL-17 and TNF-α-producing MAIT cells, IFN-γ and IL-4 producing NKT cells, and IFN-γ-producing NK cells were obtained from 10 patients and then compared with those from 12 HCs. The percentage of IL-17+ MAIT cells was significantly higher in ALC patients than in HCs (median 10.0 vs. 1.4%, p<0.005; Fig. 2A). Besides, NKT cells from ALC patients showed decreased IFN-γ and IL-4 expression than those from HCs (for IFN-γ, median 9.0% vs. 15.2%, p<0.05; for IL-4, median 3.6% vs. 8.9%, p<0.005; Fig. 2B). However, the expression of IFN-γ from NK cells showed a declining tendency in ALC patients without showing statistical significance (Fig. 2C).
Figure 2. Change of cytokine expression in innate immune cells of patients with alcoholic liver cirrhosis. (A) Freshly isolated PBMCs (1×106/well) were incubated for 1 hr in the presence of PMA (100 ng/ml) and IM (1 μM). IFN-γ, IL-17 and TNF-α expressions levels in MAIT cell population were determined by intracellular flow cytometry after stimulation with PMA and IM. (B) Freshly isolated PBMCs (1×106/well) were incubated for 2 hr in the presence of α-GalCer (100 ng/ml). IFN-γ, and IL-4 expression levels in the NKT cell population were determined by intracellular flow cytometry after stimulation with α-GalCer. (C) Freshly isolated PBMCs (1×106/well) were incubated for 24 hr in the presence of IL-12 (50 ng/ml) and IL-18 (50 ng/ml). IFN-γ expression levels in the NK cell population were determined by intracellular flow cytometry after stimulation with IL-12 and IL-18. Data were obtained from 12 HCs and 10 patients.
Symbols represent individual subjects and horizontal lines indicate median values. *p<0.05, **p<0.005 by the Mann-Whitney U test.
Activities of MAIT cells, NKT cells, and NK cells in ALC patients
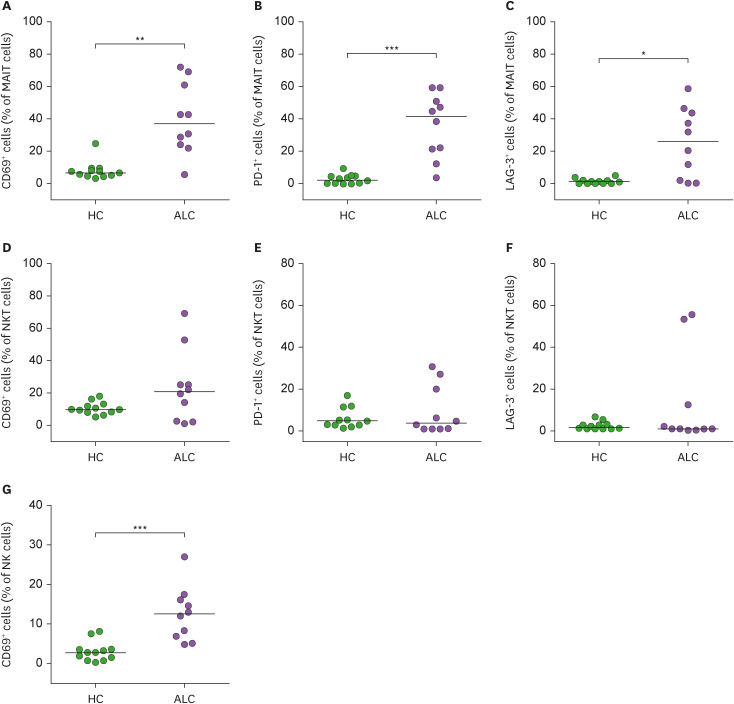
CD69 is considered an early activation marker, whereas PD-1 and LAG-3 are relatively late activation markers (30,31,32). To examine activities of MAIT cells, NKT cells, and NK cells, expression levels of these activation markers were compared between 10 ALC patients and 12 HCs using flow cytometry. Percentages of CD69+ MAIT cells and CD69+ NK cells were found to be significantly higher in ALC patients than in HCs (for MAIT cells, median 36.81% vs. 6.9%, p<0.005; for NK cells, median 12.5% vs. 2.7%, p<0.0005; Fig. 3A and G). Moreover, both PD-1+ and LAG-3+ MAIT cells were significantly elevated in ALC patients than in HCs (for PD-1, median 41.6 vs. 2.4, p<0.0005; for LAG-3, median 26.2 vs. 1.1, p<0.01; Fig. 3B and C). However, expression levels of CD69, PD-1, and LAG-3 molecules in NKT cells were comparable between ALC patients and HCs (Fig. 3D, E, and F).
Figure 3. Expression levels of CD69, PD-1, and LAG-3 in circulating innnate immune cells of patients with alcoholic liver cirrhosis. Percentages of CD69-expressing cells (A, D, G), PD-1-expressing cells (B, E), and LAG-3-expressing cells (C, F) among MAIT, NKT, or NK cell population are shown. Data were obtained from 12 HCs and 10 patients.
Symbols represent individual subjects and horizontal lines indicate median values. *p<0.01, **p<0.005, ***p<0.0005 by the Mann-Whitney U test.
Relationship between innate immune cells and clinical parameters in ALC patients
To evaluate clinical relevance of MAIT, NKT, and NK cells in ALC, we first determined relationships between laboratory parameters and percentages of MAIT, NKT, and NK cells in the peripheral blood using linear regression analysis. Since their distributions were distorted, absolute percentages of MAIT, NKT, and NK cells were log-transformed for the analysis. Results revealed that MAIT cell percentages were significantly correlated with lymphocyte count and C-reactive protein (CRP) level (p=0.03, and p=0.01, respectively). NKT cell percentages were found to be correlated with hemoglobin level (p=0.012). However, no significant correlations were observed between NK cell percentages and clinical parameters such as sex, age, leukocyte count, lymphocyte count, neutrophil count, hemoglobin level, platelet count, total bilirubin level, total protein level, albumin level, aspartate aminotransferase level, alanine aminotransferase level, alkaline phosphatase level, prothrombin time, lactate dehydrogenase level, creatinine level, or CRP level (Table 2). Next, we calculated several validated and clinically used scores in ALC and compared these values with log-transformed absolute number of peripheral MAIT, NKT, and NK cells. Among the clinical scores including DFI, GAHS, ABIC, and MELD scores, ABIC showed a negative correlation with the log-transformed absolute number of MAIT cells (p=0.044). However, these clinical scores showed no correlations with log-transformed absolute number of NKT or NK cells (Table 3).
Table 2. Regression coefficients for log-transformed percentages of MAIT, NKT, and NK cells with respect to laboratory findings in patients with alcoholic liver cirrhosis.
| Variable | MAIT cells | NKT cells | NK cells | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | p-value | β | SE | p-value | β | SE | p-value | |
| Sex (male) | −0.110 | 0.362 | 0.765 | 0.095 | 0.160 | 0.557 | −0.159 | 0.102 | 0.130 |
| Age (yr) | −0.011 | 0.019 | 0.688 | 0.002 | 0.007 | 0.756 | 0.002 | 0.005 | 0.690 |
| Leukocytes (cells/μl) | −0.001 | 0.001 | 0.288 | −0.001 | 0.001 | 0.726 | 0.001 | 0.001 | 0.147 |
| Lymphocytes (cells/μl) | 0.001 | 0.001 | 0.030* | −0.001 | 0.001 | 0.972 | −0.001 | 0.001 | 0.985 |
| Neutrophils (cells/μl) | −0.001 | 0.001 | 0.515 | −0.001 | 0.001 | 0.665 | 0.001 | 0.001 | 0.126 |
| Hemoglobin (g/dl) | −0.055 | 0.076 | 0.482 | −0.092 | 0.035 | 0.012* | −0.004 | 0.026 | 0.867 |
| Platelets (cells/μl) | 0.001 | 0.003 | 0.845 | 0.001 | 0.001 | 0.285 | −0.001 | 0.001 | 0.206 |
| Total bilirubin (mg/dl) | −0.009 | 0.009 | 0.334 | 0.004 | 0.005 | 0.405 | −0.004 | 0.003 | 0.311 |
| Total protein (g/dl) | 0.035 | 0.181 | 0.850 | −0.023 | 0.070 | 0.749 | −0.043 | 0.047 | 0.363 |
| Albumin (g/dl) | 0.010 | 0.214 | 0.965 | −0.012 | 0.105 | 0.911 | 0.002 | 0.071 | 0.973 |
| AST (U/l) | 0.001 | 0.001 | 0.694 | −0.001 | 0.001 | 0.962 | 0.001 | 0.001 | 0.930 |
| ALT (U/l) | 0.001 | 0.004 | 0.893 | 0.001 | 0.001 | 0.400 | 0.001 | 0.001 | 0.777 |
| Alkaline phosphatase (U/l) | 0.002 | 0.002 | 0.486 | 0.001 | 0.001 | 0.969 | 0.001 | 0.001 | 0.794 |
| Prothrombin time (INR) | −0.099 | 0.138 | 0.482 | 0.017 | 0.077 | 0.828 | −0.020 | 0.051 | 0.704 |
| LDH (U/l) | 0.001 | 0.001 | 0.483 | 0.001 | 0.001 | 0.716 | 0.001 | 0.001 | 0.110 |
| Creatinine (mg/dl) | −0.070 | 0.164 | 0.676 | −0.087 | 0.084 | 0.308 | −0.063 | 0.056 | 0.270 |
| CRP (mg/dl) | −0.179 | 0.061 | 0.010* | 0.001 | 0.021 | 0.957 | −0.007 | 0.014 | 0.605 |
*p<0.05.
Table 3. Regression coefficients for log-transformed absolute numbers of MAIT, NKT, and NK cells with respect to clinical findings in patients with alcoholic liver cirrhosis.
| Variable | MAIT cells | NKT cells | NK cells | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | p-value | β | SE | p-value | β | SE | p-value | |
| Modified DFI | −0.004 | 0.002 | 0.057 | 0.001 | 0.002 | 0.882 | −0.001 | 0.001 | 0.295 |
| GAHS | −0.103 | 0.074 | 0.184 | 0.018 | 0.057 | 0.748 | −0.008 | 0.041 | 0.845 |
| ABIC | −0.126 | 0.058 | 0.044* | −0.011 | 0.049 | 0.824 | −0.055 | 0.034 | 0.122 |
| MELD | −0.010 | 0.008 | 0.247 | 0.003 | 0.007 | 0.622 | −0.003 | 0.005 | 0.530 |
DISCUSSION
To the best of our knowledge, this is a comprehensive study that examines levels and functions of MAIT cells, NKT cells, and NK cells in ALC patients and assesses their clinical relevance. We found that ALC patients exhibited lower percentages and absolute numbers of circulating MAIT cells, NKT cells, and NK cells than HCs. Compared with HCs, MAIT cells in ALC patients exhibited increased IL-17 production and expression level of CD69, PD-1, and LAG-3. NKT cells displayed a decrease in the production capability of IFN-γ and IL-4 without any difference in the expression of activation markers. NK cells showed only increased expression of CD69. Besides, circulating MAIT cell levels were positively correlated with lymphocyte count, but inversely correlated with CRP level. Circulating NKT cell levels showed an inverse correlation with hemoglobin levels. However, NK cell levels in peripheral blood did not show any correlation with clinical parameters. Of note, log-transformed absolute numbers of MAIT cells showed a negative correlation with the ABIC score, suggesting that these cells might play a role as a disease severity indicator. Overall, these findings imply that these altered levels and function of MAIT cells, NKT cells, and NK cells might reflect the altered immunologic milieu of ALC.
Our study revealed that percentages and absolute numbers of innate and innate-like lymphocytes in peripheral blood of ALC patients were decreased than those of HCs. In line with our research, several previous studies have shown that the frequency of MAIT cells appears to be decreased in various liver diseases (13,33). In particular, the number of MAIT cells is decreased in chronically alcohol-fed groups and further decreased in the presence of alcoholic hepatitis (34). Mechanisms involved in such decrease remain uncertain. Several hypotheses such as cell apoptosis or their migration to liver tissue have been proposed (13,35). Further research studies are needed to clarify the mechanisms involved in such disease. However, the frequency of NKT cells varied depending on the target disease and study design. Circulating NKT cells are usually decreased in the viral hepatitis B and hepatitis C patients than in HCs (36,37). However, a few studies have shown no difference in peripheral NKT cell levels between HCV patients and HCs (38). Besides, whether circulating NKT cells in non-alcoholic fatty liver disease increase or decrease remains unclear due to conflicting results among studies (39). Such contradictory results might be due to the use of different markers when defining NKT cells depending on studies or the lack of a clear distinction about the condition of a liver disease (40,41). Therefore, further research is warranted to examine the number of circulating NKT cells consistently and comprehensively depending on each cause of cirrhosis. Lastly, the number of circulating NK cells was decreased in ALC patients of the present study, similar to previous studies on viral hepatitis and HCC patients (42,43). Although the leukocyte count was comparable, total lymphocyte counts were significantly lower in ALC patients than in HCs (median 1,041 vs. 2,209 cells/μl, p<0.0001). Thus, these findings suggest that the reduction in innate and innate-like cells may due to the reduced total lymphocyte count.
These reduced innate and innate-like cells in ALC patients displayed different cytokine production profiles and activation status, respectively. Circulating MAIT cells of ALC patients showed increased production of IL-17. However, there was no significant difference in IFN-γ or TNF-α expression between HCs and ALC patients. Besides, MAIT cells of ALC patients showed increased expression of CD69 (an early activation marker) and PD-1/LAG-3 (late activation/anergy markers). Activation of MAIT cells and elevation of IL-17 production have been reported in previous studies (13,44). It is known that MAIT cells can secrete pro-inflammatory cytokines in Th1 or Th17 patterns upon TCR mediation and that IL-17 secreted by MAIT cells plays a role in the pathogenesis of various diseases as well as regulation of liver inflammation (11,45,46,47). Likewise, our findings support that MAIT cells are likely to play a role in the pathogenesis of ALC. In the present study, IL-4 and IFN-γ secreted by NKT cells in the peripheral blood of ALC patients were decreased compared to those of HCs, although there was no difference in the expression of activation markers. Previous studies of NKT cells in ALD have mostly been carried out using hepatic tissue of murine models. Results have revealed that chronic alcohol consumption can cause NKT cells to be accumulated and activated in the liver, leading to neutrophil infiltration (17,48). NKT cells are known to secrete cytokines such as IL-4 and IFN-γ (17,49). Whether they are involved in liver protection or damage in ALD remains unclear. Overall, the role of NKT in peripheral blood of ALC patients is still unclear. Several reasons could possibly explain these controversies. First, most studies were conducted using murine models. Second, experimental conditions that influence the NKT cell activation and polarization might differ among studies. Lastly, our data revealed an elevated level of CD69+ NK cells in patients with ALC without showing statistical difference in the expression of IFN-γ between ALC patients and HCs. Despite no statistical significance, IFN-γ level from NK cells tended to decrease in ALC patients. This finding was also observed in several previous studies, suggesting that alcohol consumption could downregulate IFN-γ expression from NK cells and inhibit NK cells’ protective effect against liver steatosis and hepatic fibrosis (19,49).
Our data revealed relationships between innate-like lymphocytes and several clinical parameters. In the present study, MAIT cell levels were positively correlated with lymphocyte count but negatively correlated with CRP. Such results were also observed in other infectious and inflammatory diseases (50,51). A recent study on ALD patients has also shown a decrease in the number and function of MAIT cells after exposure to gut microbiota and microbial products (16). Collectively, the reduction of MAIT cell frequency might be a consequence of contact with microbial products or microbiota released by gut leakage in an inflammatory condition of ALC patients. Besides, NKT cell levels were negatively correlated with hemoglobin levels. This could be explained to some extent by recent studies implying that NKT cells might secrete inflammatory cytokines in an infectious condition to induce anemia or that NKT cells might be engaged in hepcidin production, which could affect iron homeostasis (52,53). However, NK cells showed no significant correlation with any clinical or laboratory parameters in the present study.
Since there is no known single parameter to predict the severity or prognosis of ALC, several prognostic models have been developed and used (54). Direct biomarkers associated with byproducts in the process of ALD’s immune response or metabolism have recently been emphasized (55). Our novel findings revealed that the ABIC score, one of several prognostic markers, showed a negative correlation with log transformed absolute MAIT cell numbers. It implies that a decrease in the number of MAIT cells in ALC could be used as a predictor for estimating disease mortality.
A limitation of our study is that we only studied patients with ALC, so there are no data available on patients with other causes of LC, including hepatitis B virus (HBV)-LC. However, some relevant findings have been reported. According to a recent paper on MAIT cell behavior in decompensated LC, the frequency of MAIT cells was reduced in LC patients compared to HC, and there was no significant difference between hepatitis-induced LC and ALC. Functionally, however, PD-1 expression was significantly higher in LC patients, and MAIT cells in hepatitis-induced LC patients had higher PD-1 activity compared to ALC (56). Regarding NK cells, studies have shown that the frequency of TNF-related apoptosis-inducing ligand (TRAIL)+CD56bright NK cells of HBV patients was higher in liver tissue than in peripheral blood, and the expression of CD69 was also higher, which was associated with liver damage (57,58). For the invariant NKT (iNKT) cells, the frequency of peripheral iNKT cells was significantly reduced in HBV-LC patients, but the expression levels of CD25, IL-4, IL-13, and IFN-γ were increased (59). Taken together, the frequency of innate and innate-like lymphocytes seems to be decreased in HBV-LC, which is in line with our results. However, further studies are needed to determine the aspects of the function of these cells according to the cause of LC.
In conclusion, we comprehensively analyzed levels and functions of circulating MAIT cells, NKT cells, and NK cells in ALC patients. Frequencies of these innate lymphocytes were diminished, and the degree of cytokine production and activation status also changed according to each cell. Some of their deficiencies were related to several clinical parameters. Especially, MAIT cell reduction was associated with a prognostic model that could be used to estimate mortality. These findings provide important information about the innate immune response of ALC patients.
ACKNOWLEDGEMENTS
This study was supported by grants (2019R1A2C1003238, 2021R1A2C2013961, 2019R1F1A1058244, 2019R1I1A1A01040762) from the National Research Foundation of Korea funded by the Korean Government. It was also supported by grants (BCRI20063, BCRI20024) funded by Chonnam National University Hospital Biomedical Research Institute.
Abbreviations
- ABIC
Age, Bilirubin, INR, and Creatinine
- ALC
alcoholic liver cirrhosis
- ALD
alcoholic liver disease
- ALT
alanine aminotransferase
- ANCOVA
analysis of covariance
- APC
allophycocyanin
- AST
aspartate aminotransferase
- CRP
C-reactive protein
- DFI
Discriminant Function Index
- GAHS
Glasgow Alcoholic Hepatitis Score
- HC
healthy control
- HCC
hepatocellular carcinoma
- IM
ionomycin
- iNKT
invariant NK T
- LAG-3
lymphocyte-activation gene 3
- LC
liver cirrhosis
- LDH
lactate dehydrogenase
- mAb
monoclonal Ab
- MAIT
mucosal-associated invariant T
- MELD
Model of End-Stage Liver Disease
- TRAIL
TNF-related apoptosis-inducing ligand
- β
regression coefficient
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Park YW.
- Data curation: Yoon JH.
- Formal analysis: Park KJ, Jin HM, Cho YN, Yoon JH, Kee SJ, Park YW.
- Funding acquisition: Park YW.
- Investigation: Park KJ, Jin HM, Cho YN, Kee SJ.
- Methodology: Kee SJ.
- Writing - original draft: Park KJ, Jin HM, Kim HS.
- Writing - review & editing: Yoon JH, Kim HS, Park YW.
SUPPLEMENTARY MATERIAL
Gating strategy to define circulating MAIT cells, NKT cells, and NK cells by flow cytometry analysis. Freshly isolated PBMCs from patients with alcoholic liver cirrhosis were stained with APC-Cy7-conjugated anti-CD3, FITC-conjugate anti-CD3, FITC-conjugate anti-CD56, FITC-conjugated anti-TCRγδ, APC-conjugated anti-TCRVα7.2, PE-conjugated anti-CD3, PE-conjugated anti-TCRVα24-Jα18, PE-Cy5-conjugated anti-CD161, PerCP-conjugated anti-CD45 mAbs and then analyzed by flow cytometry. Representative dot plots for circulating MAIT, NKT, and NK cells.
References
- 1.Nutt DJ, Rehm J. Doing it by numbers: a simple approach to reducing the harms of alcohol. J Psychopharmacol. 2014;28:3–7. doi: 10.1177/0269881113512038. [DOI] [PubMed] [Google Scholar]
- 2.Xiao J, Wang F, Wong NK, He J, Zhang R, Sun R, Xu Y, Liu Y, Li W, Koike K, et al. Global liver disease burdens and research trends: analysis from a Chinese perspective. J Hepatol. 2019;71:212–221. doi: 10.1016/j.jhep.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH. ACG clinical guideline: alcoholic liver disease. Am J Gastroenterol. 2018;113:175–194. doi: 10.1038/ajg.2017.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orman ES, Odena G, Bataller R. Alcoholic liver disease: pathogenesis, management, and novel targets for therapy. J Gastroenterol Hepatol. 2013;28(Suppl 1):77–84. doi: 10.1111/jgh.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. 2010;16:1321–1329. doi: 10.3748/wjg.v16.i11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Kaer L, Postoak JL, Wang C, Yang G, Wu L. Innate, innate-like and adaptive lymphocytes in the pathogenesis of MS and EAE. Cell Mol Immunol. 2019;16:531–539. doi: 10.1038/s41423-019-0221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett MS, Round JL, Leung DT. Innate-like lymphocytes in intestinal infections. Curr Opin Infect Dis. 2015;28:457–463. doi: 10.1097/QCO.0000000000000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 9.Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13:101–117. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- 10.Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, et al. Innate lymphoid cells: 10 years on. Cell. 2018;174:1054–1066. doi: 10.1016/j.cell.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 11.Dusseaux M, Martin E, Serriari N, Péguillet I, Premel V, Louis D, Milder M, Le Bourhis L, Soudais C, Treiner E, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117:1250–1259. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- 12.Mehrfeld C, Zenner S, Kornek M, Lukacs-Kornek V. The contribution of non-professional antigen-presenting cells to immunity and tolerance in the liver. Front Immunol. 2018;9:635. doi: 10.3389/fimmu.2018.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hegde P, Weiss E, Paradis V, Wan J, Mabire M, Sukriti S, Rautou PE, Albuquerque M, Picq O, Gupta AC, et al. Mucosal-associated invariant T cells are a profibrogenic immune cell population in the liver. Nat Commun. 2018;9:2146. doi: 10.1038/s41467-018-04450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao B, Radaeva S. Natural killer and natural killer T cells in liver fibrosis. Biochim Biophys Acta. 2013;1832:1061–1069. doi: 10.1016/j.bbadis.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurioka A, Walker LJ, Klenerman P, Willberg CB. MAIT cells: new guardians of the liver. Clin Transl Immunology. 2016;5:e98. doi: 10.1038/cti.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riva A, Patel V, Kurioka A, Jeffery HC, Wright G, Tarff S, Shawcross D, Ryan JM, Evans A, Azarian S, et al. Mucosa-associated invariant T cells link intestinal immunity with antibacterial immune defects in alcoholic liver disease. Gut. 2018;67:918–930. doi: 10.1136/gutjnl-2017-314458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathews S, Feng D, Maricic I, Ju C, Kumar V, Gao B. Invariant natural killer T cells contribute to chronic-plus-binge ethanol-mediated liver injury by promoting hepatic neutrophil infiltration. Cell Mol Immunol. 2016;13:206–216. doi: 10.1038/cmi.2015.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee KC, Chen P, Maricic I, Inamine T, Hu J, Gong S, Sun JC, Dasgupta S, Lin HC, Lin YT, et al. Intestinal iNKT cells migrate to liver and contribute to hepatocyte apoptosis during alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2019;316:G585–G597. doi: 10.1152/ajpgi.00269.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeong WI, Park O, Gao B. Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology. 2008;134:248–258. doi: 10.1053/j.gastro.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sehgal R, Kaur S, Shasthry SM, Agrawal T, Dwivedi V, Seth D, Ramakrishna G, Sarin SK, Trehanpati N. Natural killer cells contribute to pathogenesis of severe alcoholic hepatitis by inducing lysis of endothelial progenitor cells. Alcohol Clin Exp Res. 2020;44:78–86. doi: 10.1111/acer.14242. [DOI] [PubMed] [Google Scholar]
- 21.Jepsen P, Ott P, Andersen PK, Sørensen HT, Vilstrup H. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology. 2010;51:1675–1682. doi: 10.1002/hep.23500. [DOI] [PubMed] [Google Scholar]
- 22.Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749–1761. doi: 10.1016/S0140-6736(14)60121-5. [DOI] [PubMed] [Google Scholar]
- 23.Maddrey WC, Boitnott JK, Bedine MS, Weber FL, Jr, Mezey E, White RI., Jr Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193–199. [PubMed] [Google Scholar]
- 24.Forrest EH, Evans CD, Stewart S, Phillips M, Oo YH, McAvoy NC, Fisher NC, Singhal S, Brind A, Haydon G, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut. 2005;54:1174–1179. doi: 10.1136/gut.2004.050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dominguez M, Rincón D, Abraldes JG, Miquel R, Colmenero J, Bellot P, García-Pagán JC, Fernández R, Moreno M, Bañares R, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol. 2008;103:2747–2756. doi: 10.1111/j.1572-0241.2008.02104.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, Edwards E, Therneau TM. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin E, Treiner E, Duban L, Guerri L, Laude H, Toly C, Premel V, Devys A, Moura IC, Tilloy F, et al. Stepwise development of MAIT cells in mouse and human. PLoS Biol. 2009;7:e54. doi: 10.1371/journal.pbio.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho YN, Kee SJ, Kim TJ, Jin HM, Kim MJ, Jung HJ, Park KJ, Lee SJ, Lee SS, Kwon YS, et al. Mucosal-associated invariant T cell deficiency in systemic lupus erythematosus. J Immunol. 2014;193:3891–3901. doi: 10.4049/jimmunol.1302701. [DOI] [PubMed] [Google Scholar]
- 29.Lee SJ, Cho YN, Kim TJ, Park SC, Park DJ, Jin HM, Lee SS, Kee SJ, Kim N, Yoo DH, et al. Natural killer T cell deficiency in active adult-onset Still’s Disease: correlation of deficiency of natural killer T cells with dysfunction of natural killer cells. Arthritis Rheum. 2012;64:2868–2877. doi: 10.1002/art.34514. [DOI] [PubMed] [Google Scholar]
- 30.Simms PE, Ellis TM. Utility of flow cytometric detection of CD69 expression as a rapid method for determining poly- and oligoclonal lymphocyte activation. Clin Diagn Lab Immunol. 1996;3:301–304. doi: 10.1128/cdli.3.3.301-304.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown KE, Freeman GJ, Wherry EJ, Sharpe AH. Role of PD-1 in regulating acute infections. Curr Opin Immunol. 2010;22:397–401. doi: 10.1016/j.coi.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldberg MV, Drake CG. LAG-3 in cancer immunotherapy. Curr Top Microbiol Immunol. 2011;344:269–278. doi: 10.1007/82_2010_114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gebru YA, Choi MR, Raja G, Gupta H, Sharma SP, Choi YR, Kim HS, Yoon SJ, Kim DJ, Suk KT. Pathophysiological roles of mucosal-associated invariant T cells in the context of gut microbiota-liver axis. Microorganisms. 2021;9:296. doi: 10.3390/microorganisms9020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li W, Lin EL, Liangpunsakul S, Lan J, Chalasani S, Rane S, Puri P, Kamath PS, Sanyal AJ, Shah VH, et al. Alcohol abstinence does not fully reverse abnormalities of mucosal-associated invariant T cells in the blood of patients with alcoholic hepatitis. Clin Transl Gastroenterol. 2019;10:e00052. doi: 10.14309/ctg.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Huang B, Jiang X, Chen W, Zhang J, Wei Y, Chen Y, Lian M, Bian Z, Miao Q, et al. Mucosal-associated invariant T cells improve nonalcoholic fatty liver disease through regulating macrophage polarization. Front Immunol. 2018;9:1994. doi: 10.3389/fimmu.2018.01994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lucas M, Gadola S, Meier U, Young NT, Harcourt G, Karadimitris A, Coumi N, Brown D, Dusheiko G, Cerundolo V, et al. Frequency and phenotype of circulating Vα24/Vβ11 double-positive natural killer T cells during hepatitis C virus infection. J Virol. 2003;77:2251–2257. doi: 10.1128/JVI.77.3.2251-2257.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang X, Zhang M, Lai Q, Huang X, Li Y, Sun J, Abbott WG, Ma S, Hou J. Restored circulating invariant NKT cells are associated with viral control in patients with chronic hepatitis B. PLoS One. 2011;6:e28871. doi: 10.1371/journal.pone.0028871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Vliet HJ, Molling JW, von Blomberg BM, Kölgen W, Stam AG, de Gruijl TD, Mulder CJ, Janssen HL, Nishi N, van den Eertwegh AJ, et al. Circulating Vα24+Vβ11+ NKT cell numbers and dendritic cell CD1d expression in hepatitis C virus infected patients. Clin Immunol. 2005;114:183–189. doi: 10.1016/j.clim.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Van Herck MA, Weyler J, Kwanten WJ, Dirinck EL, De Winter BY, Francque SM, Vonghia L. The differential roles of T cells in non-alcoholic fatty liver disease and obesity. Front Immunol. 2019;10:82. doi: 10.3389/fimmu.2019.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu CF, Yu CH, Li YM, Xu L, Du J, Shen Z. Association of the frequency of peripheral natural killer T cells with nonalcoholic fatty liver disease. World J Gastroenterol. 2007;13:4504–4508. doi: 10.3748/wjg.v13.i33.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adler M, Taylor S, Okebugwu K, Yee H, Fielding C, Fielding G, Poles M. Intrahepatic natural killer T cell populations are increased in human hepatic steatosis. World J Gastroenterol. 2011;17:1725–1731. doi: 10.3748/wjg.v17.i13.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai L, Zhang Z, Zhou L, Wang H, Fu J, Zhang S, Shi M, Zhang H, Yang Y, Wu H, et al. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol. 2008;129:428–437. doi: 10.1016/j.clim.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 43.Amadei B, Urbani S, Cazaly A, Fisicaro P, Zerbini A, Ahmed P, Missale G, Ferrari C, Khakoo SI. Activation of natural killer cells during acute infection with hepatitis C virus. Gastroenterology. 2010;138:1536–1545. doi: 10.1053/j.gastro.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Fan Y, He W, Han Y, Bao H, Yang R, Wang B, Kong D, Wang H. Persistent deficiency of mucosa-associated invariant T (MAIT) cells during alcohol-related liver disease. Cell Biosci. 2021;11:148. doi: 10.1186/s13578-021-00664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haga K, Chiba A, Shibuya T, Osada T, Ishikawa D, Kodani T, Nomura O, Watanabe S, Miyake S. MAIT cells are activated and accumulated in the inflamed mucosa of ulcerative colitis. J Gastroenterol Hepatol. 2016;31:965–972. doi: 10.1111/jgh.13242. [DOI] [PubMed] [Google Scholar]
- 46.Heymann F, Tacke F. Immunology in the liver--from homeostasis to disease. Nat Rev Gastroenterol Hepatol. 2016;13:88–110. doi: 10.1038/nrgastro.2015.200. [DOI] [PubMed] [Google Scholar]
- 47.Dias J, Boulouis C, Sobkowiak MJ, Lal KG, Emgård J, Buggert M, Parrot T, Gorin JB, Leeansyah E, Sandberg JK. Factors influencing functional heterogeneity in human mucosa-associated invariant T cells. Front Immunol. 2018;9:1602. doi: 10.3389/fimmu.2018.01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cui K, Yan G, Xu C, Chen Y, Wang J, Zhou R, Bai L, Lian Z, Wei H, Sun R, et al. Invariant NKT cells promote alcohol-induced steatohepatitis through interleukin-1β in mice. J Hepatol. 2015;62:1311–1318. doi: 10.1016/j.jhep.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 49.Cui K, Yan G, Zheng X, Bai L, Wei H, Sun R, Tian Z. Suppression of natural killer cell activity by regulatory nkt10 cells aggravates alcoholic hepatosteatosis. Front Immunol. 2017;8:1414. doi: 10.3389/fimmu.2017.01414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwon YS, Cho YN, Kim MJ, Jin HM, Jung HJ, Kang JH, Park KJ, Kim TJ, Kee HJ, Kim N, et al. Mucosal-associated invariant T cells are numerically and functionally deficient in patients with mycobacterial infection and reflect disease activity. Tuberculosis (Edinb) 2015;95:267–274. doi: 10.1016/j.tube.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 51.Cho YN, Jeong HS, Park KJ, Kim HS, Kim EH, Jin HM, Jung HJ, Ju JK, Choi SE, Kang JH, et al. Altered distribution and enhanced osteoclastogenesis of mucosal-associated invariant T cells in gouty arthritis. Rheumatology (Oxford) 2020;59:2124–2134. doi: 10.1093/rheumatology/keaa020. [DOI] [PubMed] [Google Scholar]
- 52.Cnops J, De Trez C, Stijlemans B, Keirsse J, Kauffmann F, Barkhuizen M, Keeton R, Boon L, Brombacher F, Magez S. NK-, NKT- and CD8-derived IFNγ drives myeloid cell activation and erythrophagocytosis, resulting in trypanosomosis-associated acute anemia. PLoS Pathog. 2015;11:e1004964. doi: 10.1371/journal.ppat.1004964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang H, Zuzarte-Luis V, Fragoso G, Calvé A, Hoang TA, Oliero M, Chabot-Roy G, Mullins-Dansereau V, Lesage S, Santos MM. Acute invariant NKT cell activation triggers an immune response that drives prominent changes in iron homeostasis. Sci Rep. 2020;10:21026. doi: 10.1038/s41598-020-78037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rahimi E, Pan JJ. Prognostic models for alcoholic hepatitis. Biomark Res. 2015;3:20. doi: 10.1186/s40364-015-0046-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gala KS, Vatsalya V. Emerging noninvasive biomarkers, and medical management strategies for alcoholic hepatitis: present understanding and scope. Cells. 2020;9:524. doi: 10.3390/cells9030524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Niehaus CE, Strunz B, Cornillet M, Falk CS, Schnieders A, Maasoumy B, Hardtke S, Manns MP, Kraft AR, Björkström NK, et al. MAIT cells are enriched and highly functional in ascites of patients with decompensated liver cirrhosis. Hepatology. 2020;72:1378–1393. doi: 10.1002/hep.31153. [DOI] [PubMed] [Google Scholar]
- 57.Dunn C, Brunetto M, Reynolds G, Christophides T, Kennedy PT, Lampertico P, Das A, Lopes AR, Borrow P, Williams K, et al. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J Exp Med. 2007;204:667–680. doi: 10.1084/jem.20061287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang Y, Qin S, Wei X, Liu X, Guan J, Zhu H, Chang G, Chen Y, Lu H, Qian J, et al. Highly activated TRAIL+ CD56bright NK cells are associated with the liver damage in HBV-LC patients. Immunol Lett. 2021;232:9–19. doi: 10.1016/j.imlet.2020.12.008. [DOI] [PubMed] [Google Scholar]
- 59.Wei X, Qian J, Yao W, Chen L, Guan H, Chen Y, Xie Y, Lu H, Zhang Z, Shi L, et al. Hyperactivated peripheral invariant natural killer T cells correlate with the progression of HBV-relative liver cirrhosis. Scand J Immunol. 2019;90:e12775. doi: 10.1111/sji.12775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gating strategy to define circulating MAIT cells, NKT cells, and NK cells by flow cytometry analysis. Freshly isolated PBMCs from patients with alcoholic liver cirrhosis were stained with APC-Cy7-conjugated anti-CD3, FITC-conjugate anti-CD3, FITC-conjugate anti-CD56, FITC-conjugated anti-TCRγδ, APC-conjugated anti-TCRVα7.2, PE-conjugated anti-CD3, PE-conjugated anti-TCRVα24-Jα18, PE-Cy5-conjugated anti-CD161, PerCP-conjugated anti-CD45 mAbs and then analyzed by flow cytometry. Representative dot plots for circulating MAIT, NKT, and NK cells.