Abstract
The present study aimed to explore the significance and molecular mechanisms of galectin-1 (LGALS1) in ovarian cancer (OC). Using the Gene Expression Omnibus database and The Cancer Genome Atlas database, the results of the present study demonstrated that LGALS1 mRNA expression was markedly increased in OC and associated with advanced tumor, lymphatic metastasis and residual lesions. In Kaplan-Meier analysis, patients who expressed LGALS1 highly had a poor prognosis. Furthermore, using The Cancer Genome Atlas database, differentially expressed genes that are potentially regulated by LGALS1 in OC were determined. Gene Ontology, Kyoto Encyclopedia of Genes and Genomes, and Gene Set Enrichment Analysis were used to build a biological network of upregulated differentially expressed genes. The results of the enrichment analysis revealed that the upregulated differentially expressed genes were primarily associated with ‘ECM-receptor interaction’, ‘cell-matrix adhesion’ and ‘focal adhesion’, which are closely associated with the metastasis of cancer cells. Subsequently, cell adhesion was selected for further analysis. The results demonstrated that LGALS1 was co-expressed with the candidate genes. Subsequently, the elevated expression levels of candidate genes were verified in OC tissues, and survival analysis indicated that high expression of candidate genes was associated with shortened overall survival of patients with OC. In the present study, OC samples were also collected to verify the high protein expression levels of LGALS1 and fibronectin 1. The results of the present study highlighted that LGALS1 may regulate cell adhesion and participate in the development of OC. Therefore, LGALS1 exhibits potential as a therapeutic target in OC.
Keywords: galectin-1, ovarian cancer, cell adhesion, extracellular matrix, metastasis
Introduction
Ovarian cancer (OC) is a common malignancy, with 200,000 cancer-related deaths in 2020, marking it the eighth most fatal female malignant tumor worldwide. In addition, OC possesses the worst prognosis and highest mortality rate among all gynecological cancers (1–3). As OC cells are extremely invasive and spread rapidly from the primary site to achieve extensive metastases, the majority of patients with OC have often progressed to an advanced stage at the preliminary diagnosis. Following the completion of initial treatment for OC, including cytoreductive surgery and platinum-based chemotherapy, patients with BRCA1/BRCA2 genetic mutations or HRD(Homologous Recombination Defect) often select PARP inhibitor maintenance therapy as a subsequent therapeutic option (4,5). Notably, this is the most well-established treatment strategy. The five-year overall survival (OS) rate of patients with OC remains at <50% (3,6,7), despite the addition of multiple molecular targeted therapeutic signaling cascades into treatment regimes. In addition, the adverse clinical outcomes of widespread metastasis and recurrence remain.
Galectin-1 (LGALS1), the first member of the galectin family with carbohydrate recognition structure, is a 14.5 KDa homodimer that encodes galectin-1 (8). When LGALS1 is secreted, it interacts with extracellular matrix (ECM) glycoproteins, such as laminin or fibronectin, to play a role in cell division, migration, adhesion, invasion, immune response and other activities that promote the metastasis of tumor cells (8–11). Results of previous studies demonstrated that LGALS1 is overexpressed in carcinoma-associated fibroblasts (CAFs), and is positively correlated with the expression of epithelial-mesenchymal transition (EMT) interstitial markers (12), supporting the invasion and metastasis of tumors (13). In clinical practice, the elevated expression of LGALS1 has been detected in lung cancer (14), liver cancer (15), colorectal cancer (16), OC (17) and other diseases, demonstrating the potential role of LGALS1 as a marker for disease monitoring and therapy. However, further research into the transcriptional network and functional mechanisms of LGALS1 in OC is required. In recent years, the rapid processing of millions of library clones has become a reliable laboratory process, due to the development of high-throughput sequencing techniques. The detection of genes that play roles in key biological processes provides novel insights into therapeutic targets and mechanisms of cancer development.
In the present study, a total of five datasets were downloaded from the Gene Expression Omnibus (GEO) database, and LGALS1 was found to be highly expressed in OC tissues. Kaplan-Meier analysis demonstrated that high expression of LGALS1 leads to a poor prognosis in patients with OC. Using The Cancer Genome Atlas (TCGA) database, differentially expressed genes (DEGs) were identified according to the median expression of LGALS1. Subsequently, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were performed using the DEGs. Gene Set Enrichment Analysis (GSEA)is used to explore genome-wide molecular mechanisms. Thus, genes involved in the biological processes of cell-matrix adhesion were selected, and their impact on the survival of patients with OC were analyzed. The results were further validated using an OC dataset. After analyzing the association between these cell adhesion molecules (CAMs) and the expression of LGALS1 using TCGA database, the expression levels of LGALS1 and fibronectin 1 (FN1) in clinical samples were determined, and the characteristics of clinical cases were further investigated. Results of the present study support the hypothesis that LGALS1, as a gene impacting OC development, modulates gene expression levels and may exhibit potential as a therapeutic target.
Materials and methods
Database resource
A total of five datasets that met the inclusion criteria were downloaded from the GEO database (http://www.ncbi.nlm.nih.gov/geo). The inclusion criteria was as follows: i) :(Available tissue samples that were derived from human epithelial OC tissues, the healthy ovarian epithelium and fallopian tube epithelium; and ii) sample quantities within each dataset were not <10. A total of 83 control samples and 271 OC samples were included in the present study (Table I). The original matrix data were standardized using the RMA algorithm from R software (version 4.0.0). Differences in LGALS1 mRNA expression levels were compared between the two groups using an unpaired and paired Student's t-test, and P<0.05 was considered to indicate a statistically significant difference.
Table I.
Summary of Gene Expression Omnibus OC microarray datasets.
| Dataset | Sample number (tumor/control) | Tumor group | Control group | Platform |
|---|---|---|---|---|
| GSE26712 | 185/10 | HGSOC | OSE | GPL96; Affymetrix Human Genome U133A Array |
| GSE10971 | 7/24 | HGSOC | FTE | GPL570; Affymetrix Human Genome U133 Plus 2.0 Array |
| GSE69428 | 10/10 | HGSOC | FTE | GPL570; Affymetrix Human Genome U133 Plus 2.0 Array |
| GSE12172 | 60/30 | SOC | SO LMP tumor | GPL570; Affymetrix Human Genome U133 Plus 2.0 Array |
| GSE30587 | 9/9 | Primary | Omental | GPL6244; Affymetrix Human Gene 1.0 ST Array |
| OC | Metastases |
HGSOC, High-grade serous ovarian cancer; OSE, ovarian surface epithelium; GPL, Gene Expression Omnibus Platform; FTE, fallopian tube epithelium; SOC, serous ovarian cancer; SO, serous ovarian; LMP, low malignant potential; OC, ovarian cancer.
The gene expression information and the corresponding clinical data of 354 OC samples were downloaded from the University of California Santa Cruz database (https://xenabrowser.net/datapages/). Transcripts per million (TPM) were used to normalize the RNA-seq data. The specimen belonged to the primary tumor, and the fresh tissue was preserved at −80°C. LGALS1 expression is presented as the mean ± standard deviation and analyzed in combination with the clinical characteristics. Unpaired Student's t-test was used to compare the differences of two groups.
Acquisition of DEGs
According to the median expression of LGALS1 in TCGA database, LGALS1 expression was divided into a high expression group (> median) and low expression group (< median). The R bioconductor package DESeq2 (18) was utilized to search for DEGs. The following criteria were applied to find DEGs: A false discovery rate <0.05, |Log2 FC| >1.8 and P<0.05. A heatmap was subsequently created for the DEGs in each sample.
Annotation of the biological functions of DEGs
The clusterProfiler and Goplot packages in the R software were used to analyze and visualize GO terms and KEGG pathway enrichment results of DEGs. P<0.05 and q<0.05 were considered to indicate a statistically significant difference.
GSEA
GSEA (version 4.2.2) software was used to perform GSEA analysis on all genes of TCGA OC dataset. The following conditions were set: i) The grouping method was the same as for screening DEGs; ii) 1,000 genomic permutations were performed per analysis; iii) P<0.05 was considered to indicate a statistically significant difference; and iv) FDR(False Discovery Rates) <25%, and normalized enrichment score (NES) >1.0 demonstrated that the enrichment to gene set was significant.
Kaplan-Meier analysis
Kaplan-Meier Plotter database (www.kmplot.com/ovar) was used to analyze the effects of LGALS1 mRNA and CAMs [FN1, integrin α 11 (ITGA11), gremlin 1 (GREM1), collagen type I α 1 (COL1A1), collagen type 3 α 1 (COL3A1) and periostin (POSTN)] on the prognosis of patients with OC. This database contains 1,793 OC samples, and 15 datasets were involved in the present analysis [GSE14764 (n=80), GSE15622 (n=35), GSE18520 (n=63), GSE19829 (n=28), GSE23554 (n=28), GSE26193 (n=107), GSE26712 (n=195), GSE27651 (n=49), GSE30161 (n=58), GSE3149 (n=116), GSE51373 (n=28), GSE63885 (n=101), GSE65986 (n=55), GSE9891 (n=285) and TCGA (n=565)]. Data on the histological type of serous OC were included in the present analysis. To determine whether there was a difference in OS between the two groups, and to create a Kaplan-Meier survival curve, patients with serous OC were separated into groups with high and low expression of the target genes, based on the optimal cut-off value. Subsequently, subgroup analysis was further conducted, including the following: i) Stage; ii) histological grade; iii) TP53 mutation; iv) surgical treatment; and v) different chemotherapy regimens. A P-value of log-rank <0.05 was used to establish whether the target genes were a protective factor [HR(Hazard Ratio)<1] or a risk factor (HR>1).
Identification of the expression of CAMs
Using r-GGStatsplot package in R software, the association between LGALS1 and the expression of CAMs in TCGA database was calculated. The correlation between expression levels was investigated using Pearson's correlation analysis, and significance was assessed using a Student's t-test. The mRNA expression levels of CAMs in OC tissue were verified using the dataset GSE66957 from platform GPL15048, containing 12 ovarian samples from healthy controls (HC) and 57 OC samples.
Clinical samples
Tissues of patients who received surgical treatment in the Department of Gynecology, The Second Hospital of Jilin University from July 2020 to December 2020, were collected. Samples were obtained from 43 patients with OC and 29 patients with benign gynecological diseases that required surgical removal of the ovaries. The ovaries of HC were confirmed to be healthy by an independent pathologist, and the relevant clinical characteristics of patients with OC were recorded. The surgical treatment of OC was comprehensive staging laparotomy and cytoreductive surgery. Patients who had received neoadjuvant radiotherapy, chemotherapy and other specific therapies prior to surgery were excluded. An independent pathologist confirmed that the tissue was epithelial OC. The age of onset of OC ranged from 34 to 79 years old, with a median age of 55 years. A total of 21 patients were younger than 55 years old, and 22 patients were older than 55 years old. The Ethics Committee of The Second Hospital of Jilin University approved tissue collection (ethics approval no. 2020069). All patients provided written informed consent prior to inclusion in the study.
Immunohistochemistry
Immunohistochemistry was used to examine the expression levels of LGALS1 and FN1 in the tissues of 43 patients with OC and 29 HC. The resected OC and benign ovarian epithelial tumor tissues were fixed with formalin, and 4-µm-thick tissue sections were cut and heated in EDTA repair solution (PH, 9.0) for antigen repair. Tissues were incubated with primary antibodies against LGALS1 (1:300; cat. no. 11858-1-AP; ProteinTech Group, Inc.) and FN (1:200; cat. no. WL00712a; WANLEIBIO) overnight at 4°C. Following primary incubation, tissues were incubated with the conjugate secondary antibody (1:500; cat. no. 115-035-003; Jackson ImmunoResearch Laboratories, Inc.) for 50 min at room temperature. Tissues were washed with PBS three times for 5 min each time. Intensity was scored as follows: Colorless, 0; light yellow, 1; yellowish brown, 2; and brown, 3. The percentage of the total cell population that was positive within the visual field was scored as follows: <10%, 0 scores; 11–24%, 1 score; 25–49%, 2 scores; and >50%, 3 scores. When two scores were multiplied, a result ≤2 was considered to indicate a negative expression, and a result >2 was considered to indicate a positive expression.
Statistical analysis
Statistical analysis was performed using R Software 4.0.0 (R Development Core Team) and GraphPad Prism 9.0 (GraphPad Software). All experiments were repeated three times. The count data were represented by the number of cases (percentage), and the other data were shown as the mean ± standard deviation. Two groups were compared with the paired and unpaired Student's t-test. A paired T test was used to compare the difference in LGALS1 expression between the two groups (Data sets: GSE69428 and GSE30587). Unpaired T test was used to compare the difference of LGALS1 expression (Data sets: GSE26712, GSE10971, GSE12171), and to examine the relationship between LGALS1 expression and clinicopathological characteristics in OC. The differences in CAM mRNA expression between OC and HC were also analyzed using an unpaired t-test. Kaplan-Meier curve was used to evaluate the effects of LGALS1 and CAMs on OS of OC patients. The significance of the survival differences between the groups was assessed using a log-rank test. Immunohistochemical difference between the two groups was Chi-square test. Pearson's correlation was used to analyze the correlation between LGALS1 and CAMs expression. P<0.05 was considered to be statistically significant.
Results
High expression of LGALS1 in OC is associated with prognosis
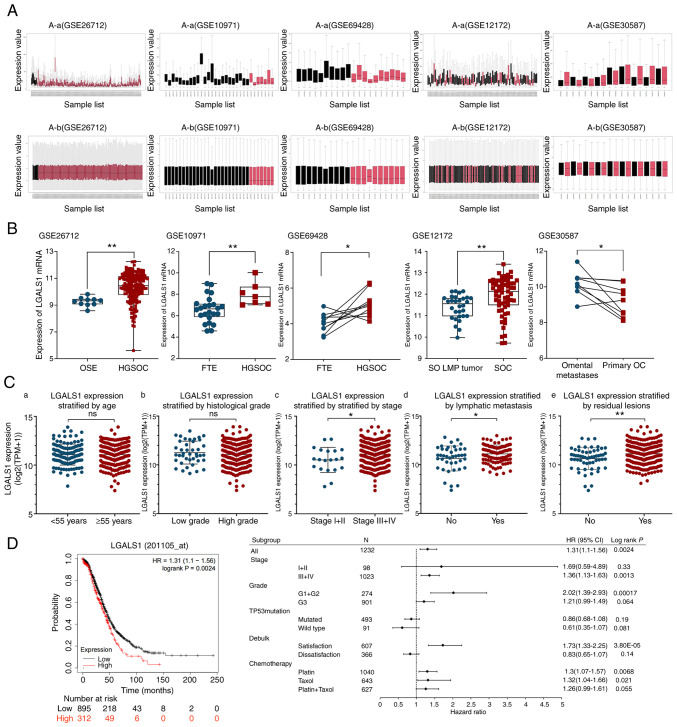
To understand the expression of LGALS1 mRNA in OC, the GEO database was used for analysis. Each dataset was normalized (Fig. 1A). Results of previous studies demonstrated that serous OC arises from the tubal epithelium and is secondary to the ovary; whereas epithelial OC was initially considered to originate from the epithelium on the ovary surface (19,20). Therefore, healthy tubal tissue was included in the control group. Compared with healthy ovarian epithelial tissue, healthy fallopian tube epithelium and serous ovarian low malignant potential tumor, results of the present study demonstrated that LGALS1 mRNA was highly expressed in OC (Fig. 1B). Further analysis demonstrated that LGALS1 mRNA expression in omental metastasis lesions was higher than in primary OC.
Figure 1.
Expression of LGALS1 in OC and its relationship with prognosis of patients. (A) GEO dataset standardization processing: (A-a) Before data processing and (A-b) after data processing. (B) Differential expression of LGALS1 mRNA in the tumor and control groups, and primary tumor and tumor metastasis groups was examined using the relevant GEO database datasets. Statistical analysis was performed using an unpaired t-test (GSE26712, GSE10971 and GSE12172) or paired t-test (GSE69428 and GSE30587). (C) Relationship between LGALS1 expression and different clinicopathological features. Association between the LGALS1 expression and (C-a) the age of patients, (C-b) histological grade, (C-c) the stage of OC, (C-d) lymphatic metastasis and (C-e) residual lesions. An unpaired t-test was used to compare the two groups. (D) Survival curves and subgroup analysis of overall survival in patients with OC, stratified by high and low expression of LGALS1 based on the best cutoff value. *P<0.05, **P<0.01. GEO, Gene Expression Omnibus; HGSOC, high-grade serous ovarian cancer; OSE, ovarian surface epithelium; FTE, fallopian tube epithelium; SO, serous ovarian; LMP, low malignant potential; SOC, serous ovarian cancer; HR, hazard ratio; LGALS1, galectin-1; OC, ovarian cancer; ns, not significant; TPM, transcripts per million.
Among 354 patients with OC in the TCGA database, the median age of diagnosis was 59, 88.4% patients had high-grade histological type, 94.3% patients had advanced stage (stage III–IV) at the first diagnosis, 69.9% of the patients with lymph node dissection had lymphatic metastasis, 81.6% OC patients could not reach R0 resection at the first operation (Table II). LGALS1 mRNA expression was markedly higher in the advanced stage (stage III–IV), lymphatic metastasis and residual lesion groups. However, high expression of LGALS1 was not significantly associated with other clinical features (Fig. 1C). The association between LGALS1 expression and patient survival was investigated using Kaplan-Meier analysis. LGALS1 mRNA expression was negatively correlated with the OS of patients with serous OC. Moreover, results of the subgroup analysis demonstrated that Grade 1 and 2, stages III and IV, and treatment with platinum or Taxol exhibited statistical significance (Fig. 1D)
Table II.
The Cancer Genome Atlas data of clinicopathological features of patients with ovarian cancer (n=354).
| Characteristics | No. (%) |
|---|---|
| Age, years (median, 59 years) | |
| <55 | 123 (34.7) |
| ≥55 | 231 (65.3) |
| Histological grade | |
| Low grade | 41 (11.6) |
| High grade | 311 (88.4) |
| Stage | |
| I+II | 20 (5.7) |
| III+IV | 331 (94.3) |
| Lymphatic metastasis | |
| Yes | 95 (69.9) |
| No | 41 (30.1) |
| Residual lesions | |
| Yes | 257 (81.6) |
| No | 58 (18.4) |
The total number of patients for some categories is <354, due to missing information in the database.
Identification and functional annotation of DEGs
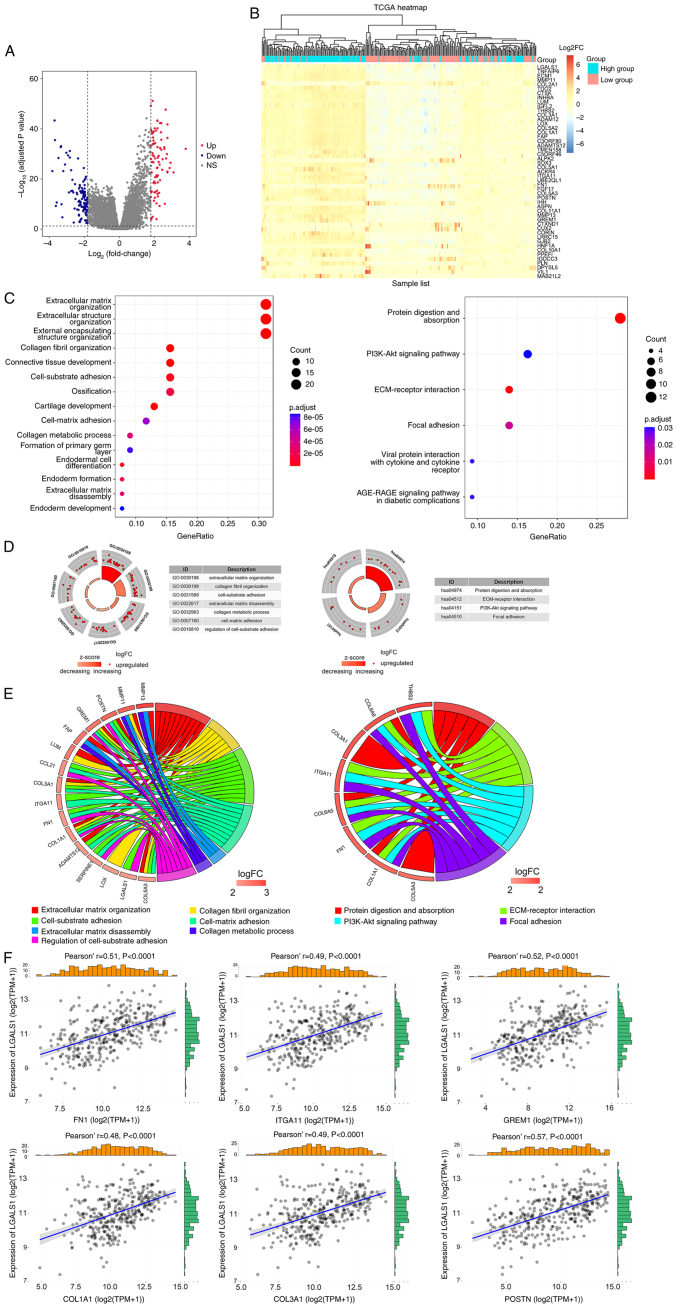
A total of 208 DEGs were obtained using the filtering criteria. In total, 83 genes exhibited significant upregulation and 125 genes exhibited significant downregulation. A volcano map was used to demonstrate DEGs associated with LGALS1 expression (Fig. 2A). The significant DEGs were merged to create a heatmap, according to the levels of expression (Fig. 2B).
Figure 2.
Identification and function annotation of DEGs. (A) Volcano map of DEGs between high and low LGALS1 groups, using abslog2(FC)>1.8 and FDR <0.05/adjusted P-value <0.05 as the threshold. Red dots represent upregulated genes, blue dots represent downregulated genes and gray dots represent genes that were not significantly differentially expressed. (B) Hierarchical clustering of DEGs in high and low LGALS1 groups. Each column represents a sample and each row represents a gene. The expression of each sample is represented as a log2FC value (scale of −6 to 6). The color gradient from blue to red represents downregulation to upregulation of gene expression. The top 50 DEGs are displayed ordered according to the adjusted P-value (smallest to largest). (C) Top 15 representative GO biological processes of DEGs, and the six representative KEGG pathways of DEGs. (D) Enrichment results. The outer circle is the term, the middle circle is the difference and the inner circle is the z-score (indicating whether the term is upregulated or suppressed). The term name corresponds to the GO ID. (E) Distribution of DEGs for biological processes is shown as a chord map. The DEGs are depicted on the left side of the map, and the FC values are represented by the color scale. Colored lines show the connection between genes and biological functions. (F) Correlation between LGALS1 and CAM genes in OC examined using TCGA. CAM, cell adhesion molecule; DEGs, differentially expressed genes; ECM, extracellular matrix; abs, absolute value FC, fold change; FDR, false discovery rate; GO, Gene Ontology; KEGG, Kyoto Encyclopaedia of Genes and Genomes; LGALS1, galectin-1; OC, ovarian cancer; TCGA, The Cancer Genome Atlas; FN1, fibronectin 1; ITGA11, integrin α 11; GREM1, gremlin 1; COL1A1, collagen type I α 1; COL3A1, collagen type III α 1; POSTN, periostin; NS, not significant; TPM, transcripts per million.
As LGALS1 may act as an oncogene in OC, clusterProfiler and Goplot were used for GO and KEGG annotation and visualization, using upregulated DEGs. Enrichment analysis of biological processes demonstrated that DEGs were involved in ECM organization, collagen fibril organization, cell-substrate adhesion, cell-matrix adhesion, collagen metabolic processes and ECM disassembly. Moreover, analysis of KEGG pathways demonstrated that DEGs were involved in protein digestion and absorption, ECM-receptor interaction, the PI3K-Akt signaling pathway and focal adhesion (Fig. 2C-E). CAM genes involved in cell matrix adhesion were selected to analyze the association with LGALS1 expression. Results of the present study demonstrated that LGALS1 was positively co-expressed with FN1, ITGA11, GREM1, COL1A1, COL3A1 and POSTN (Fig. 2F).
LGALS1 activation pathway in OC
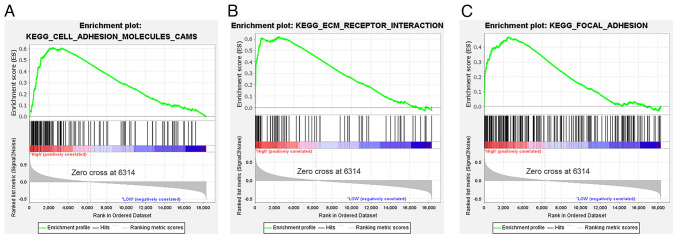
GSEA of TCGA genes demonstrated that the significantly enriched gene sets were mainly concentrated in the LGALS1 high expression group. These gene sets included CAMs, ECM-receptor interaction and focal adhesion gene sets (Fig. 3A-C).
Figure 3.
GSEA based on The Cancer Genome Atlas data. (A) ‘Cell adhesion molecules CAMs’. (B) ‘ECM-receptor interaction’. (C) ‘Focal adhesion’. CAMs, cell adhesion molecules; ECM, extracellular matrix; ES, enrichment score; GSEA, Gene Set Enrichment Analysis; KEGG, Kyoto Encyclopedia of Genes and Genomes.
Validation of CAMs
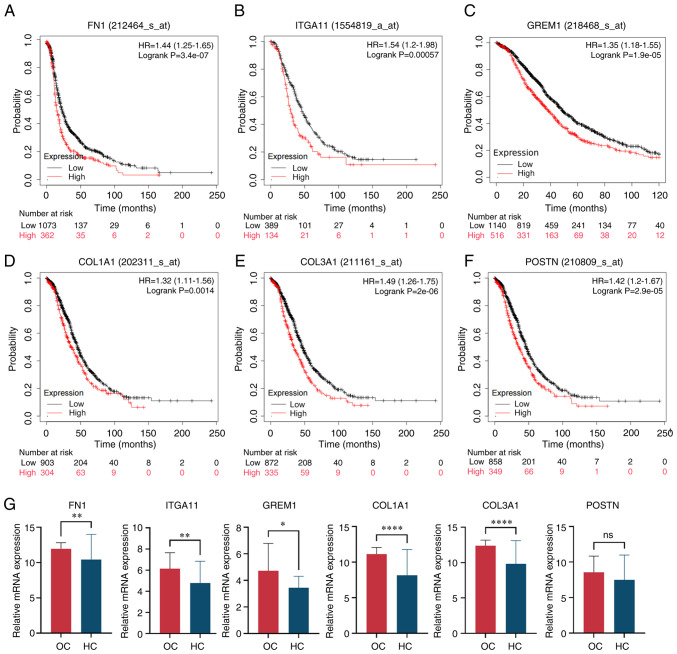
Kaplan-Meier Plotter was used to analyze how the mRNA expression of CAMs affected the OS of OC patients (Fig. 4A-F). Results of the present study demonstrated that the expression levels of FN1, ITGA11, GREM1, COL1A1, COL3A1 and POSTN were significantly correlated with poor OS rates. Gene expression levels of CAMs in HC and OC were compared using the dataset GSE66957 (Fig. 4G). The results demonstrated that the expression levels of the aforementioned CAM genes were markedly increased in OC, except for POSTN.
Figure 4.
Validation of CAMs. Expression of CAMs, including (A) FN1, (B) ITGA11, (C) GREM1, (D) COL1A1, (E) COL3A1 and (F) POSTN, and overall survival rate of patients with OC. (G) CAM mRNA was highly expressed in OC samples compared with HC samples, and unpaired t-test was used for comparison between the two groups. Data are presented as the mean ± standard deviation. *P<0.05, **P<0.01, ****P<0.0001. ns, not significant; CAM, cell adhesion molecule; OC, ovarian cancer; HC, healthy controls; FN1, fibronectin 1; ITGA11, integrin α11; GREM1, gremlin 1; COL1A1, collagen type I α 1; COL3A1, collagen type III α 1; POSTN, periostin; TPM, transcripts per million.
Clinical sample validation
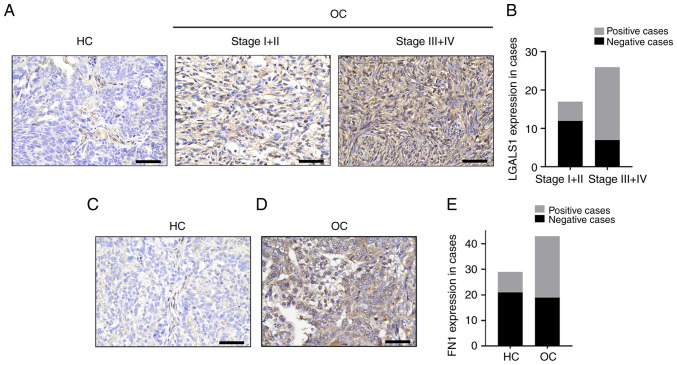
Compared with HC, the protein expression levels of LGALS1 and FN1 were significantly upregulated in OC. The positive rate of LGALS1 expression in FIGO (Federation Internationale Of Gynecologie And Obstetrigue) III/IV epithelial OC (73.08%) was significantly higher than that in FIGOI/II epithelial OC (29.41%; Fig. 5A and B). In addition, the expression rate of LGALS1 in patients with high-grade OC (67.86%) was significantly higher than that in patients with low-grade OC (33.33%; Table III). Notably, there was no significant difference in LGALS1 expression between patients of different ages and patients with lymph node metastases. When comparing the clinical samples obtained during the present study with data obtained from TCGA, the trend of LGALS1 expression in staging was consistent. The expression rate of FN1 protein in HC was 27.59%, and that in OC tissue was 62.79%. Moreover, when compared with HC, the expression of FN1 protein in patients with OC was significantly upregulated (Fig. 5C-E).
Figure 5.
LGALS1 and FN1 protein expression in epithelial OC, determined using immunohistochemical staining. (A) LGALS1 expression levels in HC and OC. Magnification, ×400. Scale bar, 50 µm. (B) LGALS1 expression levels were quantified according to immunostaining scores. (C) FN1 protein levels in HC. Magnification, ×400. Scale bar, 50 µm. (D) FN1 protein levels in OC. Magnification, ×400. Scale bar, 50 µm. (E) FN1 expression levels were quantified according to immunostaining scores in HC and OC. FN1, fibronectin 1; LGALS1, galectin-1; OC, ovarian cancer; HC, healthy controls.
Table III.
Protein expression of LGALS1 and pathological features in patients with ovarian cancer.
| LGALS1 expression | ||||
|---|---|---|---|---|
|
|
||||
| Characteristics | No. (%) | Positive cases (%) | Negative cases (%) | P-value |
| Age, years | ||||
| <55 | 21 (48.84) | 10 (47.62) | 11 (52.38) | 0.290 |
| ≥55 | 22 (51.16) | 14 (63.64) | 8 (36.36) | |
| Grade | ||||
| Low grade | 15 (34.88) | 5 (33.33) | 10 (66.67) | 0.030a |
| High grade | 28 (65.12) | 19 (67.86) | 9 (32.14) | |
| Stage | ||||
| I+II | 17 (39.53) | 5 (29.41) | 12 (70.59) | 0.005b |
| III+IV | 26 (60.47) | 19 (73.08) | 7 (26.92) | |
| Lymphatic metastasis | ||||
| Yes | 17 (39.53) | 7 (41.18) | 10 (58.82) | 0.118 |
| No | 26 (60.47) | 17 (65.38) | 9 (34.62) | |
P<0.05,
P<0.01. χ2 test was used. LGALS1, galectin-1.
Discussion
OC is a highly metastatic disease with a poor prognosis, and the underlying molecular mechanisms remain to be fully elucidated. Using the GEO database, results of the present study demonstrated that LGALS1 mRNA was highly expressed in OC tissues, compared with the healthy ovaries, healthy oviduct tissues and serous ovarian low malignant potential tumor. Moreover, increased expression levels of LGALS1 were associated with lymph node metastases and residual tumor lesions. Results of previous studies demonstrated that serum detection of LGALS1 exhibits potential for the diagnosis of OC (21,22), and that LGALS1 levels reduce following tumor excision and chemotherapy (21). Results of the present study also demonstrated that elevated LGALS1 expression was negatively associated with a poor prognosis, particularly in patients with advanced OC, stage III and IV, Grade 1 and 2, satisfactory reduction surgery, and treatment with platinum or paclitaxel. Moreover, similar findings have been observed in thyroid cancer, breast cancer and pancreatic cancer (21,23,24). The development of inhibitors that target LGALS1 exhibit potential as future anti-cancer therapies (25,26). Collectively, results of the present study demonstrated that LGALS1 may exhibit potential as a target for the treatment of OC.
Enrichment analysis of upregulated DEGs was carried out to further understand the oncogenic mechanisms of LGALS1 in OC. Findings of the GO annotation revealed that ECM genes, which encode collagen fibers, fibronectin and metalloproteinases, were a large proportion of the upregulated DEGs. These genes were mostly engaged in cell-matrix adhesion, ECM-receptor interaction, ECM degradation and collagen fiber decomposition. According to results of the KEGG analysis, DEGs were involved in protein digestion and absorption, ECM-receptor interaction, the PI3K-Akt signaling pathway and focal adhesion. All of these are involved in ECM alteration, and are closely associated with the proliferation and metastasis of tumor cells. The uncontrolled adhesion interaction alters the molecular characteristics of local ECM components, such as their morphology and hardness, and promotes cancer progression (27). Results of previous studies demonstrated that OC originates in the epithelium, and moves to the abdominal cavity through single cell or multicellular aggregation. These cells are attached to peritoneal mesothelial cells that are planted in the basement membrane, and to degraded ECM components for diffusion (27,28). Further investigations into the mechanisms underlying OC metastasis are required.
The use of GSEA in the present study demonstrated the significant enrichment of genes involved in focal adhesion, ECM-receptor interaction and CAMs. These results further indicated that LGALS1 may impact the mechanisms involved in cell adhesion.
As a molecular glue, LGALS1 heterotypic recognition glycoprotein provides diversity in ECM junctions and intercellular tightness, and improves the physical force for the directed invasion of tumor cells (11,29). To further understand the role of LGALS1 in cell matrix adhesion events, a total of six CAMs of the aforementioned pathway were selected for subsequent analysis. As a structural scaffold, FN1 regulates cell adhesion, growth and migration, and plays a vital role in embryonic development and wound healing (30). FN1 is both a mesenchymal marker and a promoter of EMT (31), and its abnormal expression is associated with a poor prognosis in patients with cancer (32,33) and platinum resistance (34). Moreover, members of the integrin family regulate cell-cell interactions and cell-cell adhesion. As a specific collagen receptor, ITGA11 initiates the recombination and alteration of the stiffness of the collagen matrix (35), which significantly facilitates the migration and invasion of cancer (36,37). COL1A1 and COL3A1 belong to the collagen family, and are the main components of the ECM. Levental et al (38) demonstrated that type I collagen cross-linking is associated with ECM stiffness, which stimulates focal adhesion and PI3K signal transduction, to enhance breast cancer cell growth and invasion. Gao et al (39) reported that the silencing of COL1A1 expression inhibited the EMT process and cell motility, and reduced tumor aggressiveness. COL3A1 is also an important protein in the development and progression of bladder, glioma, head and neck cancer (39–41). Following knockdown of COL3A1 using small interfering RNA, cell growth was prolonged, migration ability was weakened, and colony formation was inhibited (39). Moreover, GREM1 is a highly conserved glycoprotein that promotes intracellular infiltration and exosmosis of MDA-MB-231 cells in zebrafish, through activation of CAFs. Results of a previous study demonstrated that GREM1 expression was markedly increased at the infiltrated edge (42), highlighting the function of the protein in cancer metastasis. In addition, POSTN is expressed in pan-carcinomas, and is associated with metastasis, recurrence and poor prognosis (43). Results of a previous study demonstrated that POSTN from CAFs of OC acts as an integrin avb3 receptor, to activate the downstream PI3K-Akt signaling cascade (44). This initiates EMT and increases the malignancy of cancer. Results of this previous study demonstrated that candidate genes were positively correlated with LGALS1 expression, and exerted adverse effects on the clinical outcomes of patients with OC (44). Results of the present study verified the high expression of CAM mRNA in OC tissues, except for POSTN. These results further demonstrated that LGALS1 is involved in cell adhesion.
Collectively, results of the present study revealed that the LGALS1 protein was highly expressed in OC, and that the expression of LGALS1 was significantly associated with increasing pathological grade and clinical stage. These results indicated that LGALS1 may promote the development of OC. Notably, results of a previous study demonstrated that the addition of recombinant LGALS1 promoted the adhesion of OC cell lines to FN in a dose-dependent manner, whereas free-floating cells exhibited no response to FN under the same conditions. These results highlighted that FN altered cell spatial localization and promoted cell-ECM association under the action of LGALS1; however, this was cell state-dependent (45). Results of the present study demonstrated that FN1 protein expression was markedly upregulated in OC, compared with HC. Thus, we hypothesized that two proteins may interact to accelerate the progression of OC; however, further investigations are required.
In conclusion, abnormalities in cell adhesion cause the ECM to decompose and recombine, increasing the activity and aggressiveness of tumor cells. Notably, these are crucial processes for OC cell shedding and implantation. Results of the present study demonstrated that high expression levels of LGALS1 were associated with a poor prognosis in patients with OC. Moreover, results of the present study identified six candidate genes that may regulate cell adhesion pathways to participate in OC progression. However, further studies into the specific mechanisms are required. Transcriptional changes regulated by LGALS1 further the understanding of signal networks, and may lead to the development of novel targeted therapies for OC.
Acknowledgements
Not applicable.
Funding Statement
The present research was supported by the Project of Jilin Provincial Department of Science and Technology (grant no. 20210101442JC).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XL, HW and ZJ conceptualized and designed the study. XL and HW wrote the manuscript. XL, HW, AJ, YC and LY carried out the experiments and analyzed the data. ZJ received funding, ensured that work-related difficulties were adequately investigated and resolved, and approved the final version of the article. XL and HW confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All tissue collection was approved by the Ethics Committee of the Second Hospital of Jilin University (ethics approval no. 2020069; Changchun, China). All patients provided written informed consent prior to study inclusion.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2017;41:3–14. doi: 10.1016/j.bpobgyn.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.Arend RC, Jackson-Fisher A, Jacobs IA, Chou J, Monk BJ. Ovarian cancer: New strategies and emerging targets for the treatment of patients with advanced disease. Cancer Biol Ther. 2021;22:89–105. doi: 10.1080/15384047.2020.1868937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falzone L, Scandurra G, Lombardo V, Gattuso G, Lavoro A, Distefano AB, Scibilia G, Scollo P. A multidisciplinary approach remains the best strategy to improve and strengthen the management of ovarian cancer (Review) Int J Oncol. 2021;59:53. doi: 10.3892/ijo.2021.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baldwin LA, Huang B, Miller RW, Tucker T, Goodrich ST, Podzielinski I, DeSimone CP, Ueland FR, van Nagell JR, Seamon LG. Ten-year relative survival for epithelial ovarian cancer. Obstet Gynecol. 2012;120:612–618. doi: 10.1097/AOG.0b013e318264f794. [DOI] [PubMed] [Google Scholar]
- 7.Yeung TL, Leung CS, Yip KP, Yeung CLA, Wong STC, Mok SC. Cellular and molecular processes in ovarian cancer metastasis. A review in the theme: Cell and molecular processes in cancer metastasis. Am J Physiol Cell Physiol. 2015;309:C444–C456. doi: 10.1152/ajpcell.00188.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camby I, Le Mercier M, Lefranc F, Kiss R. Galectin-1: A small protein with major functions. Glycobiology. 2006;16:137R–157R. doi: 10.1093/glycob/cwl025. [DOI] [PubMed] [Google Scholar]
- 9.Janiszewska M, Primi MC, Izard T. Cell adhesion in cancer: Beyond the migration of single cells. J Biol Chem. 2020;295:2495–2505. doi: 10.1074/jbc.REV119.007759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang RY, Rabinovich GA, Liu FT. Galectins: Structure, function and therapeutic potential. Exp Rev Mol Med. 2008;10:e17. doi: 10.1017/S1462399408000719. [DOI] [PubMed] [Google Scholar]
- 11.Perillo NL, Marcus ME, Baum LG. Galectins: Versatile modulators of cell adhesion, cell proliferation, and cell death. J Mol Med (Berl) 1998;76:402–412. doi: 10.1007/s001090050232. [DOI] [PubMed] [Google Scholar]
- 12.Ham IH, Lee D, Hur H. Role of cancer-associated fibroblast in gastric cancer progression and resistance to treatments. J Oncol. 2019;2019:6270784. doi: 10.1155/2019/6270784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chong Y, Tang D, Gao J, Jiang X, Xu C, Xiong Q, Huang Y, Wang J, Zhou H, Shi Y, Wang D. Galectin-1 induces invasion and the epithelial-mesenchymal transition in human gastric cancer cells via non-canonical activation of the hedgehog signaling pathway. Oncotarget. 2016;7:83611–83626. doi: 10.18632/oncotarget.13201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu YL, Hung JY, Chiang SY, Jian SF, Wu CY, Lin YS, Tsai YM, Chou SH, Tsai MJ, Kuo PL. Lung cancer-derived galectin-1 contributes to cancer associated fibroblast-mediated cancer progression and immune suppression through TDO2/kynurenine axis. Oncotarget. 2016;7:27584–27598. doi: 10.18632/oncotarget.8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang PF, Li KS, Shen YH, Gao PT, Dong ZR, Cai JB, Zhang C, Huang XY, Tian MX, Hu ZQ, et al. Galectin-1 induces hepatocellular carcinoma EMT and sorafenib resistance by activating FAK/PI3K/AKT signaling. Cell Death Dis. 2016;7:e2201. doi: 10.1038/cddis.2015.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Y, Liu M, Li Z, Wu XB, Wang Y, Wang Y, Nie M, Huang F, Ju J, Ma C, et al. LYAR promotes colorectal cancer cell mobility by activating galectin-1 expression. Oncotarget. 2015;6:32890–32901. doi: 10.18632/oncotarget.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masoodi M, Shah ZA, Beigh AH, Ahmad SZ, Mir AW, Yasin B, Rasool R, Masoodi KZ, Bhat GM. Galectin-1 as a predictive biomarker in ovarian cancer. J Ovarian Res. 2021;14:123. doi: 10.1186/s13048-021-00874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson MD, McCarthy DJ, Smyth GK. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Z, Artibani M, Alsaadi A, Wietek N, Morotti M, Shi T, Zhong Z, Gonzalez LS, El-Sahhar S, Carrami EM, et al. The repertoire of Serous Ovarian cancer non-genetic heterogeneity revealed by single-cell sequencing of normal fallopian tube epithelial cells. Cancer Cell. 2020;37:226–242. e227. doi: 10.1016/j.ccell.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Perets R, Wyant GA, Muto KW, Bijron JG, Poole BB, Chin KT, Chen JY, Ohman AW, Stepule CD, Kwak S, et al. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer Cell. 2013;24:751–765. doi: 10.1016/j.ccr.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arcolia V, Journe F, Wattier A, Leteurtre E, Renaud F, Gabius HJ, Remmelink M, Decaestecker C, Rodriguez A, Boutry S, et al. Galectin-1 is a diagnostic marker involved in thyroid cancer progression. Int J Oncol. 2017;51:760–770. doi: 10.3892/ijo.2017.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chetry M, Song Y, Pan C, Li R, Zhang J, Zhu X. Effects of Galectin-1 on biological behavior in cervical cancer. J Cancer. 2020;11:1584–1595. doi: 10.7150/jca.38538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van de Vijver MJ, He YD, Veer LJ, Dai H, Hart AAM, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, et al. A gene-expression signature as a predictor of survival in breast cancer. New Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 24.Lankadasari MB, Aparna JS, Mohammed S, James S, Aoki K, Binu VS, Nair S, Harikumar KB. Targeting S1PR1/STAT3 loop abrogates desmoplasia and chemosensitizes pancreatic cancer to gemcitabine. Theranostics. 2018;8:3824–3840. doi: 10.7150/thno.25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paz H, Joo EJ, Chou CH, Fei F, Mayo KH, Abdel-Azim H, Ghazarian H, Groffen J, Heisterkamp N. Treatment of B-cell precursor acute lymphoblastic leukemia with the Galectin-1 inhibitor PTX008. J Exp Clin Cancer Res. 2018;37:67. doi: 10.1186/s13046-018-0721-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orozco CA, Martinez-Bosch N, Guerrero PE, Vinaixa J, Dalotto-Moreno T, Iglesias M, Moreno M, Djurec M, Poirier F, Gabius HJ, et al. Targeting galectin-1 inhibits pancreatic cancer progression by modulating tumor-stroma crosstalk. Proc Natl Acad Sci USA. 2018;115:E3769–E3778. doi: 10.1073/pnas.1722434115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ricciardelli C, Lokman NA, Ween MP, Oehler MK. WOMEN IN CANCER THEMATIC REVIEW: Ovarian cancer-peritoneal cell interactions promote extracellular matrix processing. Endocr Relat Cancer. 2016;23:T155–T168. doi: 10.1530/ERC-16-0320. [DOI] [PubMed] [Google Scholar]
- 28.Kicman A, Niczyporuk M, Kulesza M, Motyka J, Lawicki S. Utility of matrix metalloproteinases in the diagnosis, monitoring and prognosis of ovarian cancer patients. Cancer Manag Res. 2022;14:3359–3382. doi: 10.2147/CMAR.S385658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Storti P, Marchica V, Giuliani N. Role of galectins in multiple myeloma. Int J Mol Sci. 2017;18:2740. doi: 10.3390/ijms18122740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li B, Shen W, Peng H, Li Y, Chen F, Zheng L, Xu J, Jia L. Fibronectin 1 promotes melanoma proliferation and metastasis by inhibiting apoptosis and regulating EMT. Onco Targets Ther. 2019;12:3207–3221. doi: 10.2147/OTT.S195703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park J, Schwarzbauer JE. Mammary epithelial cell interactions with fibronectin stimulate epithelial-mesenchymal transition. Oncogene. 2014;33:1649–1657. doi: 10.1038/onc.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu WJ, Wang Q, Zhang W, Li L. Identification and prognostic value of differentially expressed proteins of patients with platinum resistance epithelial ovarian cancer in serum. Zhonghua Fu Chan Ke Za Zhi. 2016;51:515–523. doi: 10.3760/cma.j.issn.0529-567X.2016.07.007. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 33.Yoshihara M, Kajiyama H, Yokoi A, Sugiyama M, Koya Y, Yamakita Y, Liu W, Nakamura K, Moriyama Y, Yasui H, et al. Ovarian cancer-associated mesothelial cells induce acquired platinum-resistance in peritoneal metastasis via the FN1/Akt signaling pathway. Int J Cancer. 2020;146:2268–2280. doi: 10.1002/ijc.32854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yokoi A, Matsumoto T, Oguri Y, Hasegawa Y, Tochimoto M, Nakagawa M, Saegusa M. Upregulation of fibronectin following loss of p53 function is a poor prognostic factor in ovarian carcinoma with a unique immunophenotype. Cell Commun Signal. 2020;18:103. doi: 10.1186/s12964-020-00580-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navab R, Strumpf D, To C, Pasko E, Kim KS, Park CJ, Hai J, Liu J, Jonkman J, Barczyk M, et al. Integrin α11β1 regulates cancer stromal stiffness and promotes tumorigenicity and metastasis in non-small cell lung cancer. Oncogene. 2016;35:1899–1908. doi: 10.1038/onc.2015.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ando T, Kage H, Matsumoto Y, Zokumasu K, Yotsumoto T, Maemura K, Amano Y, Watanabe K, Nakajima J, Nagase T, et al. Integrin alpha 11 in non-small cell lung cancer is associated with tumor progression and postoperative recurrence. Cancer Sci. 2020;111:200–208. doi: 10.1111/cas.14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Primac I, Maquoi E, Blacher S, Heljasvaara R, Van Deun J, Smeland HYH, Canale A, Louis T, Stuhr L, Sounni NE, et al. Stromal integrin alpha 11 regulates PDGFR beta signaling and promotes breast cancer progression. J Clin Invest. 2019;129:4509–4528. doi: 10.1172/JCI125890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao YF, Zhu T, Chen J, Liu L, Ouyang R. Knockdown of collagen-1(III) inhibits glioma cell proliferation and migration and is regulated by miR128-3p. Oncol Lett. 2018;16:1917–1923. doi: 10.3892/ol.2018.8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen Y, Li X, Wang D, Zhang L, Li X, Su L, Fan X, Yang X. COL3A1: Potential prognostic predictor for head and neck cancer based on immune-microenvironment alternative splicing. Cancer Med. 2022;12:4882–4894. doi: 10.1002/cam4.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan L, Shu B, Chen L, Qian K, Wang Y, Qian G, Zhu Y, Cao X, Xie C, Xiao Y, Wang X. Overexpression of COL3A1 confers a poor prognosis in human bladder cancer identified by co-expression analysis. Oncotarget. 2017;8:70508–70520. doi: 10.18632/oncotarget.19733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pelli A, Vayrynen JP, Klintrup K, Makela J, Makinen MJ, Tuomisto A, Karttunen TJ. Gremlin1 expression associates with serrated pathway and favourable prognosis in colorectal cancer. Histopathology. 2016;69:831–838. doi: 10.1111/his.13006. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez-Gonzalez L, Alonso J. Periostin: A matricellular protein with multiple functions in cancer development and progression. Front Oncol. 2018;8:225. doi: 10.3389/fonc.2018.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yue H, Li W, Chen R, Wang J, Lu X, Li J. Stromal POSTN induced by TGF-β1 facilitates the migration and invasion of ovarian cancer. Gynecol Oncol. 2021;160:530–538. doi: 10.1016/j.ygyno.2020.11.026. [DOI] [PubMed] [Google Scholar]
- 45.van den Brule F, Califice S, Garnier F, Fernandez PL, Berchuck A, Castronovo V. Galectin-1 accumulation in the ovary carcinoma peritumoral stroma is induced by ovary carcinoma cells and affects both cancer cell proliferation and adhesion to laminin-1 and fibronectin. Lab Invest. 2003;83:377–386. doi: 10.1097/01.LAB.0000059949.01480.40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.