Abstract
OBJECTIVE
To investiage the effect of electroacupuncture (EA) at a single acupoint of Shenmen (HT7), Baihui (GV20), Sanyinjiao (SP6) and at combined acupoints of Shenmen (HT7) and Baihui (GV20) and Sanyinjiao (SP6) on the PKA/CREB and BDNF/TrkB signaling, as well as neuroapoptosis and neurogenesis in hippocampus and elucidate the underlying mechanism of single and combined acupoints on ameliorating spatial learning and memory deficits in a rat model of primary insomnia.
METHODS
Primary insomnia was modeled by intraperitoneal injection of para-chlorophenylalanine (PCPA) once daily for 2 d. EA was applied at Shenmen (HT7), Baihui (GV20), Sanyinjiao (SP6), or Shenmen (HT7) + Baihui (GV20) + Sanyinjiao (SP6) (combined) for 30 min daily for 4 d. Spatial learning and memory function was evaluated by the Morris water maze (MWM) test. Protein expressions of hippocampal cAMP-dependent protein kinase (PKA)-Cβ, phosphorylated cAMP-responsive element-binding protein (p-CREB), brain-derived neurotrophic factor (BDNF), and tyrosine kinase receptor B (TrkB) were evaluated by Western blotting. Neuronal apoptosis in the hippocampus was detected with the transferase-mediated dUTP-X nick end labeling assay. Endogenous neurogenesis was examined with bromodeoxyuridine staining. The MWM test and hippocampal p-CREB, BDNF, and TrkB protein levels in the combined acupoints group were evaluated after the administration of a PKA-selective inhibitor (H89).
RESULTS
Spatial learning and memory were significantly impaired in rats with insomnia. The spatial learning deficits were ameliorated in the Shenmen (HT7), Baihui (GV20), Sanyinjiao (SP6), and combined groups; this improvement was significantly greater in the combined group than the single acupoint groups. The spatial memory impairment was improved in the combined, Baihui (GV20), and Shenmen (HT7) groups, but not the Sanyinjiao (SP6) group. The expressions of PKA-Cβ, p-CREB, BDNF, and TrkB were decreased in rats with insomnia. All these proteins were significantly upregulated in the combined group. PKA/p-CREB protein levels were elevated in the Baihui (GV20) and Shenmen (HT7) groups, whereas BDNF/TrkB expression was upregulated in the Sanyinjiao (SP6) group. The staining results showed significant attenuation of hippocampal cell apoptosis and increased numbers of proliferating cells in the combined group, whereas the single acupoint groups only showed decreased numbers of apoptotic cells. In the combined group, the PKA inhibitor reversed the improvement of spatial memory and upregulation of p-CREB expression caused by EA, but did not affect its activation of BDNF/TrkB signaling.
CONCLUSIONS
EA at the single acupoints Baihui (GV20), Shenmen (HT7), or Sanyinjiao (SP6) had an ameliorating effect on the spatial learning and memory deficits induced by insomnia. EA at combined acupoints exerted a synergistic effect on the improvements in spatial learning and memory impairment in rats with insomnia by upregulating the hippocampal PKA/CREB and BDNF/TrkB signaling, facilitating neurogenesis, and inhibiting neuronal apoptosis. These findings indicate that EA at combined acupoints [(Baihui (GV20), Shenmen (HT7), and Sanyinjiao (SP6)] achieves a more pronounced regulation of hippocampal neuroplasticity than EA at single acupoints, which may partly explain the underlying mechanisms by which EA at combined acupoints exerts a better ameliorative effect on the cognitive dysfunction caused by insomnia.
Keywords: sleep initiation and maintenance disorders, learning, memory, hippocampus, neuronal plasticity, acupoints combination
1. INTRODUCTION
Insomnia is the most common subjective sleep complaint in adults, affecting 30% to 50% of the general adult population.1 Insomnia is characterized by persistent difficulty in initiating and/or maintaining sleep or a lack of restorative sleep,2 and is associated with daytime cognitive functional impairments in attention, executive function, emotional regulation, learning, and memory.1,3 The cognitive impairment induced by sleep deprivation (SD) is well-established in human adults and rodents.4,5 In clinical practice, insomnia is linked to significant impairment of daytime cognitive performance; patients with insomnia reportedly have greater performance deficits6 and impaired memory capacity,7 which greatly decreases quality of life and activity efficiency and negatively affects physical and mental health.
Neural circuits are capable of structural and/or functional modification known as neuroplasticity, which is fundamental to memory and learning.8 Learning, memory, and neuroplasticity are largely dependent on the hippocampus in rodents and humans.4 During memory formation, activity-dependent synaptic plasticity is induced at appropriate synapses;9 thus, enhancement or blockade of synaptic plasticity has commensurate effects on learning or memory.9 Long-term potentiation (LTP) is a cellular form of synaptic plasticity comprising a sustained enhancement of excitatory synaptic efficacy thought to underlie the mechanism of learning and memory.10 cAMP-dependent protein kinase (PKA) is a signal transduction molecule that is critical for the expression of some forms of LTP in the hippocampus, and for the regulation of hippocampus-dependent learning and memory.9,⇓-11 Once activated, the catalytic subunit of PKA (PKA-Cβ) translocates to the nucleus, where it phosphorylates the transcription factor cAMP-responsive element-binding protein (CREB) and promotes the synthesis of proteins that contribute to structural and/or functional changes at synapses required for the maintenance of late-phase LTP and induction of long-term memory.12,13 Emerging evidence suggests that PKA/CREB signaling play crucial roles in mediating LTP and memory,14 and inhibition of PKA hinders the maintenance of LTP in an activity-dependent manner.15
Brain-derived neurotrophic factor (BDNF) is activated by a number of transcription factors (including CREB) and is another important mediator in the process of hippocampal LTP and learning and memory.16 BDNF regulates the induction and maintenance of stable LTP, and most BDNF actions on LTP are attributed to the activation of tyrosine kinase receptor B (TrkB) receptor, which has been detected in multiple areas in the hippocampus.17 In addition to the relationship of BDNF with LTP, increasing evidence suggests that the BDNF/TrkB system is involved in improving learning and memory deficits by enhancing hippocampal neurogenesis18,19 and inhibiting neuronal apoptosis.20
Sleep disorders affect the stability of the hippocampal cellular composition. In particular, the subgranular zone in the dentate gyrus (DG) of the hippocampus is one of the regions of neurogenesis in the adult brain.21 Studies have shown that SD impairs spatial memory in mice, accompanied by decreased hippocampal neurogenesis in the DG and increased apoptosis in CA1 and CA3 regions,22 and attenuates cognitive performance and promotes hippocampal neuronal apoptosis in rats and humans.23,24
In Traditional Chinese Medicine theory, insomnia is called ‘Bu Mei’.25 The Evidence-Based Guideline of Chinese Medicine for Insomnia recommends treatment at three main acupoints for insomnia: Shenmen (HT7), Baihui (GV20), and Sanyinjiao (SP6).26 In clinical practice, combined acupoints are most frequently used, as the use of combined acupoints produces synergistic reactions to enhance the clinical effect.27 We previously reported that electroacupuncture (EA) at the combined acupoints of Shenmen (HT7), Baihui (GV20), and Sanyinjiao (SP6) achieves a better effect in alleviating insomnia than EA at single acupoints.28 However, it is unclear whether EA ameliorates the learning and memory impairments caused by insomnia. Furthermore, no study has investigated whether EA at combined acupoints produces better cognitive improvements than EA at single acupoints by modulating neuroplasticity via regulating the hippocampal PKA/CREB and/or BDNF/TrkB signaling, neurogenesis, and apoptosis in rats with insomnia.
This study aimed to investigate the effect of EA at combined acupoints versus single acupoints in improving learning and memory impairments in rats with insomnia, and investigate the underlying cellular and molecular mechanisms to aid in the selection of acupoints to treat insomnia-induced cognitive dysfunction.
2. MATERIALS AND METHODS
2.1. Animals
Adult male specific pathogen free-grade Sprague-Dawley rats [(220 ± 20) g], six to seven-week-old, were supplied by Beijing Union Medical College (certificate of quality No. SCXK[Jing] 2019-0010). The rats were housed under standard laboratory conditions (12 h alternate light-dark cycle) at a controlled temperature [(25 ± 3) ℃ and humidity (45% ± 5%) with free access to standard diet and water before and throughout the experimental period. The experimental protocols were approved by the ethics committee of the Institute of Acupuncture-Moxibustion, China Academy of Chinese Medical Sciences (reference No. 20190010) and conformed to the Guidelines for Laboratory Animal Care and Use of the Chinese Ministry of Science and Technology (2006) and the National Institutes of Health Guide for Care and Use of Laboratory Animals (1996).
2.2. Experimental design
2.2.1. Experiment 1
To compare the effects of EA at combined acupoints on the amelioration of cognitive deficits and hippocampal neuroplasticity with those of EA at single acupoints in a rat model of primary insomnia, we used the random number table method to randomly divide 66 rats into six groups: normal, model, EA-Baihui (GV20), EA-Shenmen (HT7), EA-Sanyinjiao (SP6), and EA-Baihui (GV20) + Shenmen (HT7) + Sanyinjiao (SP6) (combined acupoint) (n = 11 per group).
2.2.2. Experiment 2
To assess the roles of PKA/CREB signaling in the cognitive improvement and BDNF/TrkB signaling activation resulting from EA at combined acupoints in rats with insomnia, the random number table method was used to randomly assign a further 28 rats to four groups: normal, model, combined acupoints + normal saline (combined + NS) group, and combined acupoints + H89 (PKA inhibitor, Sigma-Aldrich, St. Louis, MO, USA) (combined + H89) group (n = 7 per group).
2.3. Establishment of a rat model of insomnia
Insomnia was induced by intraperitoneal injection of PCPA (an inhibitor of 5-hydroxytryptamine biosynthesis, C8655, Sigma-Aldrich, St. Louis, MO, USA) suspended in 0.5% normal saline at a dose of 300 mg/kg between 08:00 and 09:00 am once a day for 2 d.29 After PCPA injection, the rats lost their circadian rhythm and did not sleep for the entire day, suggesting that the insomnia model was successfully created.30
2.4. Surgery and drug administration
Rats were anesthetized with 2.0% isoflurane and fixed with a stereotaxic instrument (RWD, Shenzhen, China). The stainless steel guide cannula was implanted bilaterally into the CA1 regions (AP: —3.8 mm; ML: ± 2.2 mm; DV: 2.7 mm), as previously described.31 H89 was intrahippocampally infused through the guide cannula by an injector (1 mm beyond the tip of the guide cannula) that was connected by polyethylene tubing to a 5 µL Hamilton micro-syringe. Rats in the combined + H89 group were infused with H89 at a concentration of 5 μmol/L, 1 μL/side, at a speed of 0.3 µL/min by a microinjection pump (RWD, Shenzhen, China). Rats in the combined + NS group were intrahippocampally infused with the same volume of saline at the same speed. At the end of the infusion, the injector was left in place for an additional 1 min to allow diffusion from the injector tip. H89 or saline was administered 3 h before applying EA treatment once per day for 4 d.
2.5. EA intervention
Under light anesthesia with 1.5% isoflurane, the EA groups underwent EA stimulation following the insertion of acupuncture needles (0.25 mm × 25 mm; Huatuo, Suzhou Medical Co. Ltd., Suzhou, China) at Baihui (GV20), bilateral Shenmen (HT7), bilateral Sanyinjiao (SP6), or Baihui (GV20) + bilateral Shenmen (HT7) + bilateral Sanyinjiao (SP6) at a depth of about 2-3 mm. Baihui (GV20) is located in the center of the parietal bone32 in the frontal lobe of the anterior precentral sulcus.33 The remaining points were located in accordance with an atlas of experimental animal acupuncture points.34 Shenmen (HT7) is located at the end of the transverse crease of the ulnar wrist of the forepaw.35,36 Sanyinjiao (SP6) is located 10 mm above the prominence of the lateral malleolus of the hindlimb.37 After insertion, the needle handles were connected to a HANS-200 EA device (Nanjing Jisheng Medical Technology Co., Ltd., Nanjing, China) and stimulated for 30 min at an alternating frequency of 2/15 Hz and an intensity of 1 mA. The treatment was conducted from day 1 to 4 after modeling. The model group underwent the same anesthetic procedures, but without EA treatment.
2.6. Morris water maze (MWM) test
The MWM test was used to measure spatial learning and memory ability, as previously described,38,39 and was performed on the 3rd day after PCPA injection. The rats were trained from days 3 to 5, and the testing took place on day 6. The test consisted of two parts: the orientation navigation trial and the spatial exploration trial. A circular pool (1.2 m wide, 35 cm high) filled with water containing a platform located 2.0 cm below the water surface was equally divided into four quadrants. During the orientation navigation trial, rats were randomly placed in one of the quadrants facing the wall of the pool and allowed to swim for a maximum of 90 s until they discovered the hidden platform. If the rat failed to locate the platform within 90 s, it was manually guided to the platform and allowed to rest on the platform for 20 s; this process was repeated four times a day starting from different quadrants with a 2-min interval for 3 consecutive days. After the final experiment, the hidden platform was removed and rats were placed in the pool in the same quadrant and allowed to explore for 60 s. The time spent in the quadrant previously containing the platform was measured to evaluate the level of spatial reference memory for the given task. All behavioral tracks from the trials were recorded and analyzed using video tracking software.
2.7. Western blot analysis
The hippocampus tissues were lysed with a Sonic Dismembrator (Thermo Fisher Scientific, Waltham, MA, USA) in radioimmunoprecipitation assay lysis buffer. The soluble tissue lysates were transferred into new tubes. Equivalent amounts of lysates (20 µg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to nitrocellulose, and probed by immunoblotting using standard procedures. Primary rabbit antibodies against PKA-Cβ (1∶200), p-CREB (1∶200), BDNF (1∶300), and TrkB (1∶300) were used (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Secondary antibodies conjugated to horseradish peroxidase, including anti-rabbit IgG and anti-goat IgG, were obtained from Jackson ImmunoResearch (Lancaster, PA, USA). Luminol reagents were obtained from Millipore (Billerica, MA, USA). Chemiluminescent signals within the linear range of detection were quantified using Labwork 4.6 image analysis software (Gene Company Limited, Hongkong, China). The expression ratios were normalized in accordance with β-tubulin levels.
2.8. Transferase-mediated dUTP-X nick end labeling (TUNEL)
Apoptosis was quantified by TUNEL staining as per the manufacturer’s instructions (Roche, Basilea, Switzerland) with the following modifications. The hippocampal sections were incubated with proteinase K (15 µg/mL) for 30 min and were then incubated in 3% hydrogen peroxide/methanol for 10 min in the dark at room temperature to block endogenous peroxidase activity. After being washed three times (5 min each time), the sections were incubated with the TUNEL reaction mixture for 1 h at 37 ℃. After the sections were mounted on slides and 200 µL of fluoromount-G with 4',6-diamidino-2-phenylindole (DAPI) (Southern Biotech, Birmingham, AL, USA) was placed on the slide with a coverslip, the number of TUNEL-positive neurons was counted in three hippocampal sections per animal. Representative section photographs were taken using a laser scanning confocal microscope (FV1000; Olympus Corporation, Tokyo, Japan). Digital images were processed with the ImageQuant TL 10.0 software system (GE Healthcare Life Sciences, Pittsburgh, PA, USA).
2.9. 5-Br omo-2′-Deoxyuridine (BrdU) injection and immunodetection
BrdU (Sigma-Aldrich, St. Louis, MO, USA) was diluted in warm saline (0.9% w/v NaCl in sterile H2O) by gentle vortexing to make a sterile solution of 10 mg/mL. The rats were intraperitoneally injected with BrdU solution (200 mg/kg body weight) 24 h before the last EA treatment on day 6,40 and were perfused 30 min after the last EA treatment.
The tissue sections were washed in 0.1 M phosphate buffered solution (PBS) blocked with 5% donkey serum for 30 min and incubated with BrdU primary antibody (1∶1000, Abcam, Cambridge, UK) at 4℃ overnight. After being washed with 0.1 M PBS, the sections were incubated with donkey anti-mouse IgG (Alexa Fluor 488; Thermo Fisher Scientific, Waltham, MA, USA) for 2 h in the dark at room temperature. The sections were washed and mounted on slides. Approximately 200 μL of Fluoromount-G with DAPI (Southern Biotech, Birmingham, AL, USA) was placed on the slide with a coverslip. Representative section photographs were taken using a laser scanning confocal microscope (FV1000; Olympus Corporation, Tokyo, Japan). Digital images were processed with the ImageQuant TL 10.0 software system (GE Healthcare Life Sciences, Pittsburgh, PA, USA).
2.10. Statistical analyses
Data were analyzed with SPSS version 22.0 (IBM Corp., Armonk, NY, USA). All values are presented as mean ± standard deviation ($\bar{x}±s$). One-way analysis of variance followed by the least significant difference test was used for multiple comparisons between groups. P < 0.05 was considered statistically significant.
3. RESULTS
3.1. EA at combined acupoints exerted a synergistic effect in ameliorating cognitive impairment induced by insomnia
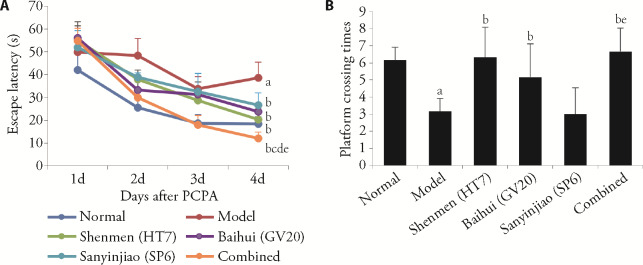
The spatial learning and memory ability of the rats were measured by the MWM test on day 6 after the establishment of insomnia. All rats learned to find the hidden platform, and the latency time required to locate the platform was reduced by varying degrees in each group in the orientation navigation trial (P < 0.05; Figure 1A). However, the model group took significantly longer to find the platform than the EA-Shenmen (HT7), EA-Baihui (GV20), EA-Sanyinjiao (SP6), and EA-combined acupoints groups (P < 0.05; Figure 1A). The results suggested that the spatial learning capacity was significantly deteriorated in rats with insomnia compared with normal rats; however, EA treatment significantly reduced the time required by rats with insomnia to locate the platform on day 6. Furthermore, the EA-combined acupoints group spent much less time locating the platform than the EA-Shenmen (HT7), EA-Baihui (GV20), and EA-Sanyinjiao (SP6) groups (P < 0.05).
Figure 1. Evaluation of the effects of EA on spatial learning and memory dysfunction of rats with insomnia using the MWM test.
A: escape latency time of the six groups in the orientation navigation trial on days 1-4. B: platform crossing times of the six groups in the space exploration trial on day 4. Normal: control group, Model: injected with PCPA, Shenmen (HT7): model mice with EA at Shenmen (HT7), Baihui (GV20): model mice with EA at Baihui (GV20), Sanyinjiao (SP6): model mice with EA at Sanyinjiao (SP6), Combined: model mice with EA at Shenmen (HT7), Baihui (GV20) and Sanyinjiao (SP6). PCPA: para-chlorophenylalanine; EA: electroacupuncture; MWM: Morris water maze. aP < 0.05, compared with the normal group; bP < 0.05, compared with the model group; cP < 0.05, compared with the Shenmen (HT7) group; dP < 0.05, compared with the Baihui (GV20) group; eP < 0.05, compared with the Sanyinjiao (SP6) group (n = 11). Data are presented as mean ± standard deviation. Statistics by one-way repeated measures analysis of variance followed by the least significant difference test.
When the hidden platform was removed in the space exploration trial, the rats with insomnia passed through the original position of the platform much fewer times than the normal rats (P < 0.05; Figure 1B), suggesting that the rats with insomnia had impaired spatial memory. However, after EA treatment, the EA groups except EA-Sanyinjiao (SP6) crossed the original position of the platform significantly more times than the model group (P < 0.05). These findings suggested that the rats with insomnia had cognitive impairment, and that this insomnia-induced cognitive impairment was ameliorated by EA at the single acupoints Shenmen (HT7), Baihui (GV20), or Sanyinjiao (SP6), but was more effective when performed at the combined acupoints of Shenmen (HT7), Baihui (GV20), and Sanyinjiao (SP6).
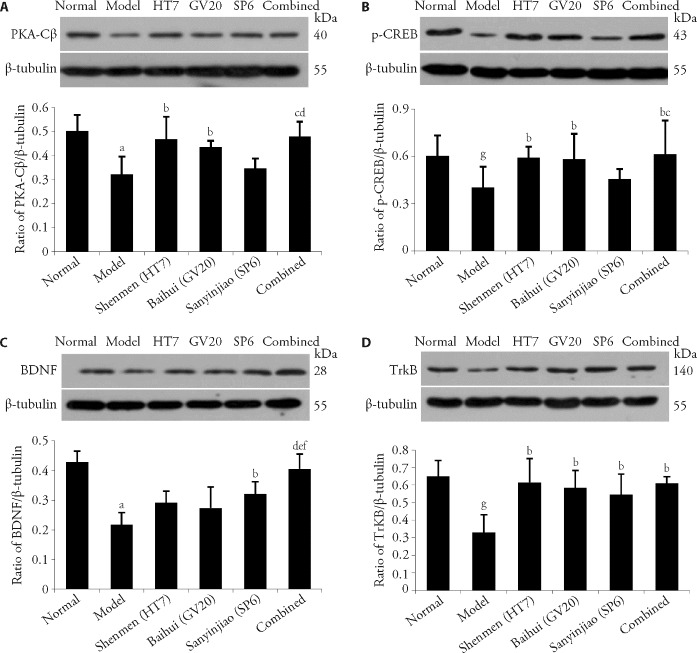
3.2. EA at combined acupoints enhanced the hippocampal expression of PKA/CREB and BDNF/TrkB signaling
Western blot analysis showed that the protein expression levels of PKA-Cβ and p-CREB were decreased in the model group compared with the normal group (P < 0.01 and P < 0.05, respectively). Compared with the model group, the expression levels of PKA-Cβ and p-CREB were upregulated in the EA-Shenmen (HT7) group (both P < 0.05, respectively), EA-Baihui (GV20) group (both P < 0.05, respectively), and EA-combined acupoints group (P < 0.01 and P < 0.05, respectively).
Compared with the normal group, the expression levels of BDNF and TrkB were reduced in the model group (P < 0.01 and P < 0.05, respectively), and were higher in the EA-combined acupoints group (P < 0.01 and P < 0.05, respectively) and EA-Sanyinjiao (SP6) group (both P < 0.05, respectively). Compared with the model group, the expression level of TrkB was increased in the EA-Shenmen (HT7) and EA-Baihui (GV20) groups (both P < 0.05, respectively) (Figure 2).
Figure 2. Effects of EA stimulation on the expressions of PKA-Cβ, p-CREB, BDNF, and TrkB.
A: PKA-Cβ; B: p-CREB; C: BDNF; D: TrkB. PKA-Cβ: catalytic subunits Cβ of cyclic AMP dependent protein kinase; Normal: control group, Model: injected with PCPA, Shenmen (HT7): model mice with EA at Shenmen (HT7), Baihui (GV20): model mice with EA at Baihui (GV20), Sanyinjiao (SP6): model mice with EA at Sanyinjiao (SP6), Combined: model mice with EA at Shenmen (HT7), Baihui (GV20) and Sanyinjiao (SP6). p-CREB: phosphorylated cAMP-responsive element-binding protein; PCPA: para-chlorophenylalanine; BDNF: brain-derived neurotrophic factor; TrkB: tyrosine kinase receptor B. aP < 0.01, compared with the normal group; bP < 0.05, compared with the model group; cP < 0.05, compared with the Sanyinjiao (SP6) group; dP < 0.01, compared with the model group; eP < 0.05, compared with the Baihui (GV20) group; fP < 0.05, compared with the Shenmen (HT7) group; gP < 0.05, compared with the normal group (n = 6). Data are presented as mean ± standard deviation. Statistics by one-way repeated measures analysis of variance followed by the least significant difference test.
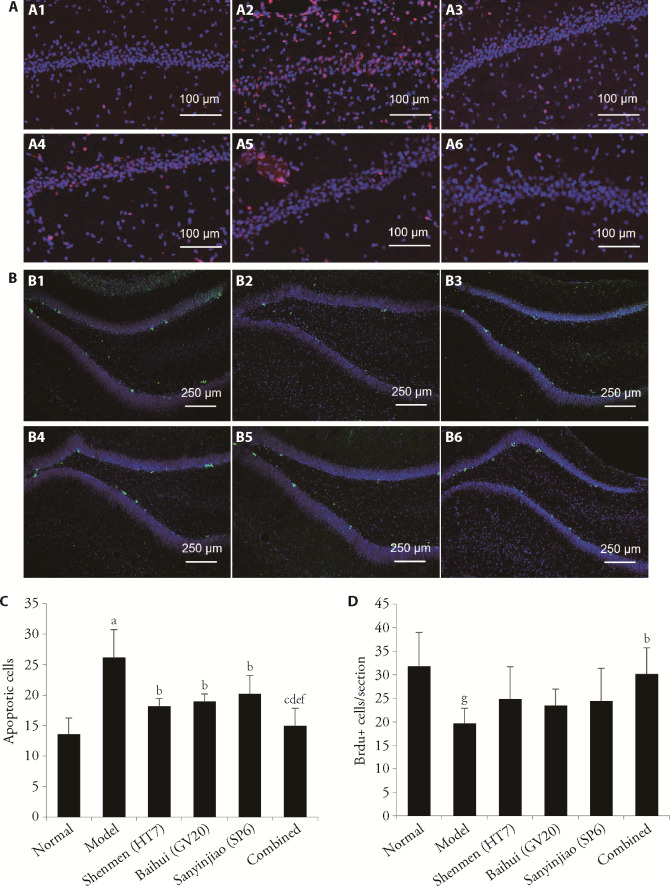
3.3. EA at combined acupoints increased the attenuation of neuronal apoptosis in the hippocampal CA1 region
TUNEL staining was performed to assess the neuronal apoptosis in the hippocampal regions. An increased number of apoptotic cells in the hippocampal CA1 region were detected in the model group compared with the normal group (P < 0.01). The EA-Shenmen (HT7), EA-Baihui (GV20), EA-Sanyinjiao (SP6), and EA-combined acupoints groups had decreased numbers of apoptotic cells in the hippocampal CA1 region compared with the model group (P < 0.05, P < 0.05, P < 0.05, and P < 0.01, respectively) (Figure 3A, 3C). Furthermore, the number of apoptotic cells was lower in the EA-combined acupoints group than the EA-Shenmen (HT7), EA-Baihui (GV20), and EA-Sanyinjiao (SP6) groups (all P < 0.05, respectively).
Figure 3. Effects of EA stimulation on the neuronal apoptosis and neurogenesis in the hippocampus (immunofluorescence, × 200, × 100).
A: representative images of TUNEL staining (red) and DAPI (blue) in the hippocampal CA1 region to identify apoptotic cells (A1-A6, × 200). A1: normal group; A2: model group; A3: EA-Shenmen (HT7) group; A4: EA-Baihui (GV20) group; A5: EA-Sanyinjiao (SP6) group; A6: EA-combined group. Scale bar: 100 µm. B: representative images of immunofluorescent staining for BrdU (green) and DAPI (blue) in the hippocampal DG regions (B1-B6 is × 100). B1: normal group; B2: model group; B3: EA-Shenmen (HT7) group; B4: EA-Baihui (GV20) group; B5: EA-Sanyinjiao (SP6) group; B6: EA-combined group. Scale bar: 250 µm; C: quantification of TUNEL staining in the sections; D: quantification of BrdU-positive cells in the sections. EA: electroacupuncture; TUNEL: transferase-mediated dUTP-X nick end labeling; DAPI: fluoromount-G with 4',6-diamidino-2-phenylindole; DG: dentate gyrus; BrdU: 5-Bromo-2′-Deoxyuridine. aP < 0.01, compared with the normal group; bP < 0.05, compared with the model group; cP < 0.05, compared with the Shenmen (HT7) group; dP < 0.05, compared with the Baihui (GV20) group; eP < 0.05, compared with the Sanyinjiao (SP6) group;fP < 0.01, compared with the model group; gP < 0.05, compared with the normal group (n = 5). Data are presented as mean ± standard deviation. Statistics by one-way repeated measures analysis of variance followed by the least significant difference test.
3.4. EA at combined acupoints facilitated neurogenesis in the DG region
Compared with the normal group, the mean number of BrdU-positive cells (green, reflecting cell proliferation) was significantly reduced on day 6 after PCPA injection in the model group (Figure 3B). The mean number of BrdU-positive cells in the hippocampal DG region was increased in the EA-combined acupoints group (P < 0.05). The EA-Shenmen (HT7), EA-Baihui (GV20), and EA-Sanyinjiao (SP6) groups had similar numbers of BrdU-positive cells compared with the model group (Figure 3B, 3D).
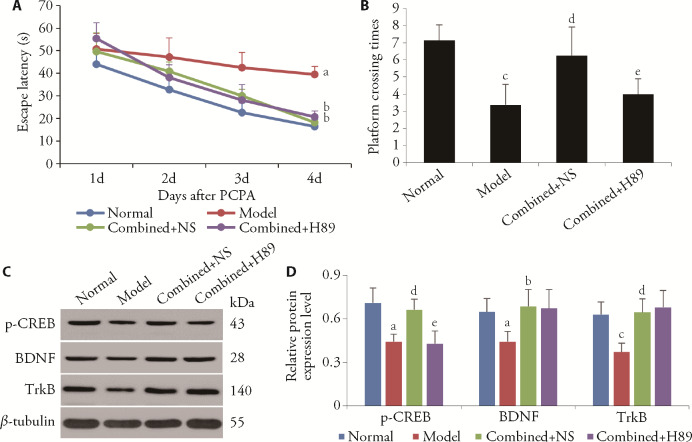
3.5. Administration of a PKA inhibitor reversed the ameliorative effect of EA at combined acupoints on the spatial memory impairment of rats with insomnia
After the application of saline, rats treated with EA at the combined acupoints showed obvious improvements in spatial learning and memory on the 4th day of EA treatment, indicated by the reduced latency in locating the platform (P < 0.05) and the increased number of times the position of the platform was crossed compared with the model group (P < 0.01) (Figure 4). The administration of the PKA inhibitor H89 abolished the effect of EA at the combined acupoints on platform crossing times (P < 0.05) (Figure 4B), but did not significantly affect the effect of EA treatment at the combined acupoints in improving the escape latency (P > 0.05) (Figure 5A). Furthermore, compared with the combined + NS group, the p-CREB expression was decreased in the combined + H89 group (P < 0.05), whereas the BDNF and TrkB protein levels were not significantly altered (Figure 4C, 4D). These results indicated that PKA/CREB signaling may be necessary for EA at the combined acupoints to improve the spatial memory impairment induced by insomnia, but not for its activation of hippocampal BDNF/TrkB signaling.
Figure 4. Effects of EA at the combined acupoints on spatial memory and learning function, and on hippocampal p-CREB, BDNF, and TrkB expression in the presence of a PKA inhibitor.
A: escape latency of the four groups in the orientation navigation trial on days 1-4. B: platform crossing times of the four groups in the space exploration trial on day 4. C: representative Western blots of p-CREB, BDNF, TrkB, and the corresponding β-tubulin band. D: densitometric analysis of p-CREB, BDNF, and TrkB protein levels in the normal, model, combined + NS, and combined+H89 groups. Normal: control group; Model: injected with PCPA; combined + NS: model mice with EA at Shenmen (HT7), Baihui (GV20) and Sanyinjiao (SP6) intrahippocampally infused with NS (1 μL/side); combined + H89: model mice with EA at Shenmen (HT7), Baihui (GV20) and Sanyinjiao (SP6) intrahippocampally infused with H89 (5 μmol/L, 1 μL/side). p-CREB: phosphorylated cAMP-responsive element-binding protein; BDNF: brain-derived neurotrophic factor; TrkB: tyrosine kinase receptor B. H89: PKA inhibitor. aP < 0.05, compared with the normal group; bP < 0.05, compared with the model group; cP < 0.01 compared with the normal group; dP < 0.01 compared with the model group; eP < 0.05 compared with the combined + NS group (n = 7). Data are presented as mean ± standard deviation. Statistics by one-way repeated measures analysis of variance followed by the least significant difference test.
4. DISCUSSION
This is one special study to investigate the underlying mechanisms of the improvements in spatial learning and memory after EA at single acupoints versus combined acupoints in a rat model of primary insomnia based on the regulation of hippocampal neuroplasticity by modulating PKA/CREB and BDNF/TrkB signaling, neurogenesis, and neuronal apoptosis. Our study had several important findings. First, EA at the combined acupoints of Baihui (GV20), Shenmen (HT7), and Sanyinjiao (SP6) exerted a more significant effect than EA at the three single acupoints in improving spatial learning and memory impairments in rats with insomnia. Second, EA at the combined acupoints activated both hippocampal PKA/CREB and BDNF/TrkB signaling, whereas EA at the single acupoints only activated either one of these pathways. Third, EA at the combined acupoints not only inhibited neuronal apoptosis more significantly than EA at the single acupoints, but also promoted hippocampal neurogenesis, which was not achieved by EA at the single acupoints.
Our results demonstrated that in contrast to normal rats, the rats with insomnia showed increased escape latency and decreased platform crossing times in the MWM test, indicating spatial learning and memory deficits and cognitive impairment. EA at the combined acupoints of Baihui (GV20), Shenmen (HT7), and Sanyinjiao (SP6) significantly reversed these deficits; EA at the single acupoints of Baihui (GV20), Shenmen (HT7), or Sanyinjiao (SP6) also significantly decreased the escape latency, but to a significantly lesser degree than EA at the combined acupoints. EA at the single acupoint of Sanyinjiao (SP6) did not lead to a significant change in the platform crossing times compared with the model rats. Overall, the behavioral results showed that EA at Baihui (GV20), Shenmen (HT7), or Sanyinjiao (SP6) alone has a potentially positive impact on the learning and memory dysfunction induced by insomnia, and EA at the combined acupoints produced a more significant synergistic effect.
In Traditional Chinese Medicine theory, Baihui (GV20) belongs to the Du meridian (the governing vessel) and is located on the highest place of the head where the Yang meridians meet.41 Acupuncture at Baihui (GV20) clears the mind, lifts the spirits, tonifies Yang, strengthens the ascending function of the spleen, eliminates interior wind, and promotes resuscitation.42 Thus, Baihui (GV20) is used in the treatment of neurological and psychiatric diseases such as stroke,43 Alzheimer's disease,44 and insomnia.26 Sanyinjiao (SP6) belongs to the spleen meridian, which has the functions of tonifying Yin and blood, and coordinating and reinforcing qi and blood owing to its intersection with the liver and kidney meridians.45 Sanyinjiao (SP6) is frequently used in the treatment of insomnia and other anxiety-related emotions.42 Shenmen (HT7) is the original acupoint of the heart meridian, and nourishes the heart and tranquilizes the mind.45 The combination of Baihui (GV20), Sanyinjiao (SP6), and Shenmen (HT7) exerts the effects of harmonizing Yin and Yang, calming the heart, and soothing the nerves.45
At the molecular level, both PKA/CREB and BDNF/TrkB signaling play crucial roles in hippocampal-dependent cognitive function, and the two signaling pathways regulate neural plasticity, learning, and memory separately or in combination.46 Several animal experiments have indicated that SD selectively impairs PKA/CREB-dependent forms of synaptic plasticity,47 decreases the protein levels of BDNF and TrkB in the hippocampus,48,49 and suppresses the expression of hippocampal CREB and BDNF;50 all of these effects result in learning and memory impairments. Consistent with these reports, our results revealed that both hippocampal PKA/CREB and BDNF/TrkB signaling were significantly inhibited in the rats with insomnia with cognitive impairment. However, EA at the single acupoints of Shenmen (HT7) or Baihui (GV20) enhanced PKA/CREB signaling, EA at Sanyinjiao (SP6) facilitated BDNF/TrkB signaling, and EA at the combined acupoints synergistically activated both PKA/CREB and BDNF/TrkB signaling. These findings are in agreement with previous reports that have shown that EA at Baihui (GV20) and Dazhui (GV14) ameliorates learning and memory dysfunction by activating hippocampal PKA/CREB signaling and its downstream apoptosis-related proteins in rats with cerebral hypoperfusion,51 and that acupuncture improves cognition and synaptic plasticity in rats with traumatic brain injury by enhancing BDNF/TrkB signaling in a CREB-independent manner.52 Furthermore, when the phosphorylation of CREB was inhibited by the PKA inhibitor H89, we found that EA at the combined acupoints still enhanced the expression of the BDNF/TrkB system, but failed to ameliorate the spatial memory dysfunction. This finding not only indicated that EA at the combined acupoints separately facilitated PKA/CREB and BDNF/TrkB signaling, but also demonstrated that the activation of PKA/CREB signaling was essential for EA at the combined acupoints to improve spatial memory. Taken together, our results suggested that EA at different single acupoints or at the combined acupoints activated PKA/CREB signaling, BDNF/TRKB signaling, or both, and was accompanied by the improvement of cognitive function.
The hippocampus plays a critical role in learning and memory processes,53 and adult neurogenesis and neuronal apoptosis affect the hippocampal cellular composition and cognitive functions. Newly generated neurons in the hippocampal DG contribute to maintaining or improving learning and memory functions,54,55 whereas increased apoptosis of hippocampal neurons results in learning and memory dysfunction.56 In particular, hippocampal CA1 pyramidal neurons are more vulnerable than other hippocampal neurons,57 and SD induces LTP deficits and neuronal apoptosis in the hippocampal CA1 region.23,58 However, inhibition of neuronal apoptosis in the hippocampal CA1 area alleviates learning and memory deficits in mice.59 Therefore, in the present study, we examined the effects of EA on neurogenesis in the DG and neuronal apoptosis in the hippocampus CA1 region. Our results revealed that rats with insomnia showed significantly increased apoptosis of CA1 hippocampal neurons and decreased neurogenesis in the DG. Furthermore, both EA at the single acupoints of Baihui (GV20), Shenmen (HT7), or Sanyinjiao (SP6) and EA at the combined acupoints significantly inhibited the neuronal apoptosis, whereas only EA at the combined acupoints increased neurogenesis. This finding is in agreement with previous studies that have shown that EA ameliorates learning and memory impairment by inhibiting the apoptosis of hippocampal CA1 neurons51,60 and facilitating neurogenesis in the DG.61 EA at the combined acupoints of Baihui (GV20), Shenmen (HT7), and Sanyinjiao (SP6) simultaneously enhanced the neurogenesis and reduced neuronal apoptosis, which may partly explain the possible underlying cellular mechanisms by which EA at the combined acupoints exerted better cognitive improvement than EA at the single acupoints.
In conclusion, our findings suggest that EA at the combined acupoints of Baihui (GV20), Shenmen (HT7), and Sanyinjiao (SP6) achieved enhanced synergistic effects compared with EA at these single acupoints in ameliorating the spatial learning and memory impairment induced by insomnia. The underlying mechanisms of the difference in the ameliorating effect may be that EA at the combined acupoints exerted more comprehensive regulation of cognition-related neuroplasticity by activating both PKA/CREB and BDNF/TrkB signaling, and by promoting neurogenesis as well as attenuating neuronal apoptosis in the hippocampus to enhance the hippocampal LTP and memory formation. Future research is warranted to further elucidate the precise neurobiological regulatory mechanisms by which EA ameliorates the cognitive impairment induced by insomnia, thus contributing to the development of acupuncture in clinical settings.
5. ACKNOWLEDGMENTS
We thank Liwen Bianji (Edanz) (https://www.liwenbianji.cn) for editing the language of a draft of this manuscript.
REFERENCES
- 1. Brownlow JA, Miller KE, Gehrman PR. . Insomnia and cognitive performance. Sleep Med Clin 2020; 15: 71-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. . Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med 2008; 4: 487-504. [PMC free article] [PubMed] [Google Scholar]
- 3. Tarokh L, Saletin JM, Carskadon MA. . Sleep in adolescence: physiology, cognition and mental health. Neurosci Biobehav Rev 2016; 70: 182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abel T, Havekes R, Saletin JM, Walker MP. . Sleep, plasticity and memory from molecules to whole-brain networks. Curr Biol 2013; 23: R774-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim SM, Zhang S, Park J, et al. REM sleep deprivation impairs learning and memory by decreasing brain O-GlcNAc cycling in mouse. Neurotherapeutics 2021; 18: 2504-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Edinger JD, Means MK, Carney CE, Krystal AD. . Psychomotor performance deficits and their relation to prior nights' sleep among individuals with primary insomnia. Sleep 2008; 31: 599-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fortier-Brochu E, Morin CM. . Cognitive impairment in individuals with insomnia: clinical significance and correlates. Sleep 2014; 37: 1787-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cassilhas RC, Tufik S, de Mello MT. . Physical exercise, neuroplasticity, spatial learning and memory. Cell Mol Life Sci 2016; 73: 975-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martin SJ, Grimwood PD, Morris RG. . Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci 2000; 23: 649-711. [DOI] [PubMed] [Google Scholar]
- 10. Duffy SN, Craddock KJ, Abel T, Nguyen PV. . Environmental enrichment modifies the PKA-dependence of hippocampal LTP and improves hippocampus-dependent memory. Learn Mem 2001; 8: 26-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kandel ER. . The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol Brain 2012; 5: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bailey CH, Kande ER, Harris KM. . Structural components of synaptic plasticity and memory consolidation. Cold Spring Harb Perspect Biol 2015; 7: a021758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dunning J, During MJ. . Molecular mechanisms of learning and memory. Expert Rev Mol Med 2003; 5: 1-11. [DOI] [PubMed] [Google Scholar]
- 14. Nguyen PV, Woo NH. . Regulation of hippocampal synaptic plasticity by cyclic AMP-dependent protein kinases. Prog Neurobiol 2003; 71: 401-37. [DOI] [PubMed] [Google Scholar]
- 15. Blitzer RD, Wong T, Nouranifar R, Iyengar R, Landau EM. . Postsynaptic cAMP pathway gates early LTP in hippocampal CA1 region. Neuron 1995; 15: 1403-14. [DOI] [PubMed] [Google Scholar]
- 16. Lu Y, Christian K, Lu B. . BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem 2008; 89: 312-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Drake C, Milner T, Patterson S. . Ultrastructural localization of full-length trkB immunoreactivity in rat hippocampus suggests multiple roles in modulating activity-dependent synaptic plasticity. J Neurosci 1999; 19: 8009-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Han H, Wu LM, Han MX, Yang WM, Wang YX, Fang ZH. . Diabetes impairs spatial learning and memory and hippocampal neurogenesis via BDNF in rats with transient global ischemia . Brain Res Bull 2016; 124; 269-77. [DOI] [PubMed] [Google Scholar]
- 19. Stagni F, Giacomini A, Sandra G, et al. A flavonoid agonist of the TrkB receptor for BDNF improves hippocampal neurogenesis and hippocampus-dependent memory in the Ts65Dn mouse model of DS. Exp Neurol 2017; 298: 79-96. [DOI] [PubMed] [Google Scholar]
- 20. Che H, Zhang LY, Ding L, et al. EPA-enriched ethanolamine plasmalogen and EPA-enriched phosphatidylethanolamine enhance BDNF/TrkB/CREB signaling and inhibit neuronal apoptosis in vitro and in vivo. Food Funct 2020; 11: 1729-39. [DOI] [PubMed] [Google Scholar]
- 21. Nam MH, Ahn KS, Choi SH. . Acupuncture stimulation induces neurogenesis in adult brain. Int Rev Neurobiol 2013; 111: 67-90. [DOI] [PubMed] [Google Scholar]
- 22. Soto-Rodriguez S, Lopez-Armas G, Luquin S, et al. Rapid eye movement sleep deprivation produces long-term detrimental effects in spatial memory and modifies the cellular composition of the subgranular zone. Front Cell Neurosci 2016; 10: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xie G, Huang X, Li H, Wang P, Huang P. . Caffeine-related effects on cognitive performance: roles of apoptosis in rat hippocampus following sleep deprivation. Biochem Biophys Res Commun 2021; 534: 632-8. [DOI] [PubMed] [Google Scholar]
- 24. Joo EY, Kim H, Suh S, Hong SB. . Hippocampal substructural vulnerability to sleep disturbance and cognitive impairment in patients with chronic primary insomnia: magnetic resonance imaging morphometry. Sleep 2014; 37: 1189-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu D, Hang HY, Liu M, et al. Literature study on distribution features of TCM syndromes and syndrome elements of peptic ulcer. Chin J Basic Med Tradit Chin Med 2012; 18: 722-4. [Google Scholar]
- 26. Shergis JL, Ni XJ, Jackson ML, et al. A systematic review of acupuncture for sleep quality in people with insomnia. Complement Ther Med 2016; 26: 11-20. [DOI] [PubMed] [Google Scholar]
- 27. Wang H, Liang FX. . Thoughts and prospects of research on acupoints compatibility. Zhong Guo Zhen Jiu 2012; 32: 359-62. [PubMed] [Google Scholar]
- 28. Qiao LN, Gao QL, Tan LH, et al. Electroacupuncture of multiple acupoints is significantly superior to that of single acupoint in improving sleep by regulating serum sleep-promoting and awakening-promoting factors in insomnia rats. Zhen Ci Yan Jiu 2018; 43: 651-6. [DOI] [PubMed] [Google Scholar]
- 29. Hong J, Chen J, Kan J, Liu M, Yang D. . Effects of acupuncture treatment in reducing sleep disorder and gut microbiota alterations in PCPA-Induced insomnia mice. Evid Based Complement Alternat Med 2020; 2020: 3626120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Du CH, Yan Y, Shen CX, Cui XF, Pei XP, Qin XM. . Comparative pharmacokinetics of six major compounds in normal and insomnia rats after oral administration of Ziziphi Spinosae Semen aqueous extract. J Pharm Anal 2020; 10: 385-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hosseini-Sharifabad A, Ghahremani MH, Sabzevari O, et al. Effects of protein kinase A and G inhibitors on hippocampal cholinergic markers expressions in rolipram- and sildenafil-induced spatial memory improvement. Pharmacol Biochem Behav 2012; 101: 311-9. [DOI] [PubMed] [Google Scholar]
- 32. Wang HL, Liu FL, Li RQ, et al. Electroacupuncture improves learning and memory functions in a rat cerebral ischemia/reperfusion injury model through PI3K/Akt signaling pathway activation. Neural Regen Res 2021; 16: 1011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xu H, Zhang YM, Sun H, Chen SH, Si YK. . Electroacupuncture at GV20 and ST36 exerts neuroprotective effects via the EPO-Mediated JAK2/STAT3 pathway in cerebral ischemic rats. Evid Based Complement Alternat Med 2017; 2017: 6027421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hua XB, Li CR, Zhou HL. . The development of the rat acupuncture points map. Shi Yan Dong Wu Yu Dong Wu Shi Yan 1991; 1: 1. [Google Scholar]
- 35. Chang SC, Ryu Y, FanY, et al. Involvement of the cuneate nucleus in the acupuncture inhibition of drug-seeking behaviors. Front Neurosci 2019; 13: 928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park HJ, Chae Y, Jang J, Shim I, Lee H, Lim S. . The effect of acupuncture on anxiety and neuropeptide Y expression in the basolateral amygdala of maternally separated rats. Neurosci Lett 2005; 377: 179-84. [DOI] [PubMed] [Google Scholar]
- 37. Chen Y, Zhao Y, Luo DN, Zheng H, Li Y, Zhou SY. . Electroacupuncture regulates disorders of gut-brain interaction by decreasing corticotropin-releasing factor in a rat model of IBS. Gastroenterol Res Pract 2019; 2019: 1759842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wen T, Zhang XF, Liang SX, et al. Electroacupuncture ameliorates cognitive impairment and spontaneous low-frequency brain activity in rats with ischemic stroke. J Stroke Cerebrovasc Dis 2018; 27: 2596-605. [DOI] [PubMed] [Google Scholar]
- 39. Wang RM, Dong Y, Lu YJ, Zhang WL, Brann DW, Zhang QG. . Photobiomodulation for global cerebral ischemia: targeting mitochondrial dynamics and functions. Mol Neurobiol 2019; 56: 1852-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Munoz-Velasco I, Ortiz R, Echeverria OM, Escobar ML, Vazquez-Nin GH. . Characterization of the pre-meiotic S phase through incorporation of BrdU during spermatogenesis in the rat. J Histochem Cytochem 2013; 61: 680-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shen EY, Chen FJ, Chen YY, Lin MF. . Locating the acupoint Baihui (GV20) beneath the cerebral cortex with MRI reconstructed 3D neuroimages. Evid Based Complement Alternat Med 2011; 2011: 362494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cheong YC, Dix S, Hung Yu Ng E, Ledger WL, Farquhar C. . Acupuncture and assisted reproductive technology. Cochrane Database Syst Rev 2013; 7: CD006920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang WW, Xie CL, Lu L, Zheng GQ. . A systematic review and Meta-analysis of Baihui (GV20)-based scalp acupuncture in experimental ischemic stroke. Sci Rep 2014; 4: 3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Park S, Lee JH, Yang EJ. . Effects of acupuncture on Alzheimer's disease in animal-based research. Evid Based Complement Alternat Med 2017; 2017: 6512520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu P, Cheng C, Song XJ, et al. Acupoint combination effect of Shenmen (HT 7) and Sanyinjiao (SP 6) in treating insomnia: study protocol for a randomized controlled trial. Trials 2020; 21: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sakamoto K, Karelina K, Obrietan K. . CREB: a multifaceted regulator of neuronal plasticity and protection. J Neurochem 2011; 116: 1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Havekes R, Abel T. . The tired hippocampus: the molecular impact of sleep deprivation on hippocampal function. Curr Opin Neurobiol 2017; 44: 13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Looti Bashiyan M, Nasehi M, Vaseghi S, Khalifeh S. . Investigating the effect of crocin on memory deficits induced by total sleep deprivation (TSD) with respect to the BDNF, TrkB and ERK levels in the hippocampus of male Wistar rats. J Psychopharmacol 2021; 35: 744-54. [DOI] [PubMed] [Google Scholar]
- 49. Mahboubi S, Nasehi M, Imani A, et al. Benefit effect of REM-sleep deprivation on memory impairment induced by intensive exercise in male wistar rats: with respect to hippocampal BDNF and TrkB. Nat Sci Sleep 2019; 11: 179-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Guzman-Marin R, Ying Z, Untsova N, et al. Suppression of hippocampal plasticity-related gene expression by sleep deprivation in rats. J Physiol 2006; 575: 807-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Han XH, Zhao XX, Lu M, et al. Electroacupuncture ameliorates learning and memory via activation of the CREB signaling pathway in the hippocampus to attenuate apoptosis after cerebral hypoperfusion. Evid Based Complement Alternat Med 2013; 2013: 156489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li XH, Chen C, Yang XP, et al. Acupuncture improved neurological recovery after traumatic brain injury by activating BDNF/TrkB Pathway. Evid Based Complement Alternat Med 2017; 2017: 8460145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Santin LJ, Rubio S, Begega A, Miranda R, Arias JL. Spatial learning and the hippocampus. . Rev Neurol 2000; 31: 455-62. [PubMed] [Google Scholar]
- 54. van Praag H, Shubert T, Zhao C, Gage FH. . Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci 2005; 25: 8680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cao L, Jiao XY, Zuzga D, et al. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet 2004; 36: 827-35. [DOI] [PubMed] [Google Scholar]
- 56. Jia Z, Geng L, Xie G, Chu Q, Zhang W. . Sevoflurane impairs acquisition learning and memory function in transgenic mice model of Alzheimer's disease by induction of hippocampal neuron apoptosis. Int J Clin Exp Med 2015; 8: 15490-7. [PMC free article] [PubMed] [Google Scholar]
- 57. Mori K, Yoshioka M, Suda N, et al. An incomplete cerebral ischemia produced a delayed dysfunction in the rat hippocampal system. Brain Res 1998; 795: 221-6. [DOI] [PubMed] [Google Scholar]
- 58. McDermott CM, LaHoste GL, Chen C, Musto A, Bazan NG, Magee JC. . Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J Neurosci 2003; 23: 9687-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ye S, Xie DJ, Zhou P, et al. Huangpu Tongqiao formula ameliorates the hippocampus apoptosis in diabetic cognitive dysfunction mice by activating CREB/BDNF/TrkB signaling pathway. Evid Based Complement Alternat Med 2021; 2021: 5514175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Feng SF, Wang Q, Wang HN, et al. Electroacupuncture pretreatment ameliorates hypergravity-induced impairment of learning and memory and apoptosis of hippocampal neurons in rats. Neurosci Lett 2010; 478: 150-5. [DOI] [PubMed] [Google Scholar]
- 61. Li XY, Guo F, Zhang QM, et al. Electroacupuncture decreases cognitive impairment and promotes neurogenesis in the APP/PS 1 transgenic mice. BMC Complement Altern Med 2014; 14: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]