Abstract
The mesenchymal–epithelial transition factor (MET) proto-oncogene encodes the MET receptor tyrosine kinase. MET aberrations drive tumorigenesis in several cancer types through a variety of molecular mechanisms, including MET mutations, gene amplification, rearrangement, and overexpression. Therefore, MET is a therapeutic target and the selective type Ib MET inhibitor, tepotinib, was designed to potently inhibit MET kinase activity. In vitro, tepotinib inhibits MET in a concentration-dependent manner irrespective of the mode of MET activation, and in vivo, tepotinib exhibits marked, dose-dependent antitumor activity in MET-dependent tumor models of various cancer indications. Tepotinib penetrates the blood–brain barrier and demonstrates strong antitumor activity in subcutaneous and orthotopic brain metastasis models, in-line with clinical activity observed in patients. MET amplification is an established mechanism of resistance to EGFR tyrosine kinase inhibitors (TKI), and preclinical studies show that tepotinib in combination with EGFR TKIs can overcome this resistance. Tepotinib is currently approved for the treatment of adult patients with advanced or metastatic non–small cell lung cancer harboring MET exon 14 skipping alterations. This review focuses on the pharmacology of tepotinib in preclinical cancer models harboring MET alterations and demonstrates that strong adherence to the principles of the Pharmacological Audit Trail may result in a successful discovery and development of a precision medicine.
Introduction
The mesenchymal–epithelial transition factor (MET) proto-oncogene, which is located on chromosome 7q21–31, encodes the MET receptor tyrosine kinase (1). Activation of MET, through binding of hepatocyte growth factor (HGF) to the extracellular domain, stimulates auto-phosphorylation of several tyrosine residues, triggering downstream activation of the rat sarcoma viral oncogene (RAS)/MAPK, PI3K–protein kinase B (Akt), and STAT signaling pathways (Fig. 1A; refs. 1–5). In cancer, MET pathway activation promotes cell proliferation, survival, angiogenesis, migration, and invasion (1, 6). MET is a therapeutic target in several cancers, including non–small cell lung cancer (NSCLC), hepatocellular carcinoma (HCC), and gastric cancer (1, 7). MET activation is associated with a poor prognosis and resistance to standard-of-care anticancer treatments (1, 7).
Figure 1.
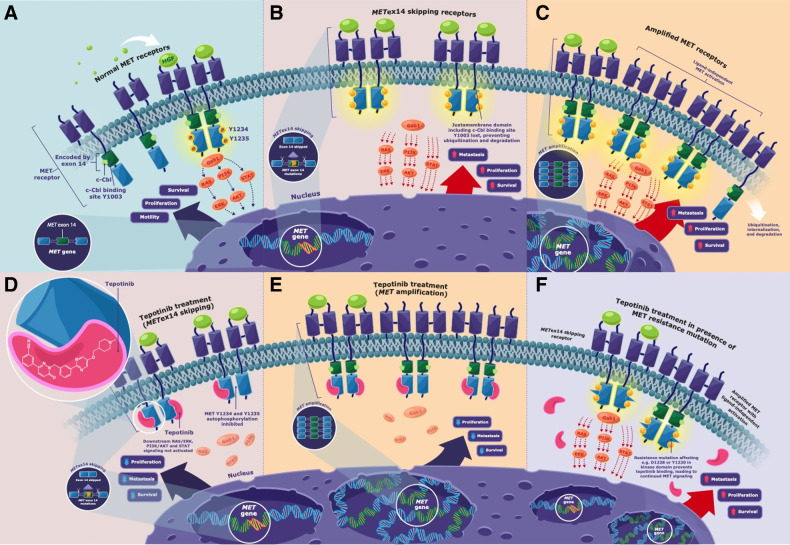
Simplified diagrammatic schema showing A, In a physiologically normal context, MET signaling is activated when the HGF ligand binds to the extracellular domain of the MET receptor that induces homodimerization and stimulates auto-phosphorylation of the tyrosine residues Y1234 and Y1235 in the cytoplasmic regions of the receptor. This leads to activation and recruitment of the adaptor/scaffold protein Gab1 and activation of downstream signaling pathways (including RAS/ERK, PI3K/AKT, and STAT), resulting in cell survival, proliferation, and motility. Under normal physiological conditions there is a balance between MET signaling and downregulation of MET (3, 4). In addition to MET signaling from the interaction between the ligand and the MET receptor, there have been published reports of MET internalization promoting additional signaling whereby the formation of early MET-containing endosomes can (i) trigger the activation of ERK leading to focal adhesions of the phosphorylated ERK that may mediate HGF-induced cell migration, or (ii) MET may be trafficked along the microtubule network and accumulate in a perinuclear compartment where it may trigger the phosphorylation of STAT3 leading to the translocation of the phosphorylated STAT3 into the nucleus, which could induce downstream signaling (3–5). Publications have also reported the downregulation of MET may occur through (i) endocytosis of MET and the formation of multivesicular bodies leading to MET undergoing lysosomal degradation, or (ii) MET degradation may occur through sequential proteolytic cleavage at the juxtamembrane site, with the cleaved intracellular MET fragment being destroyed in the proteasome, whereas the cleaved extracellular MET may generate an extracellular “decoy MET” that could capture the HGF ligand and interfere with other intact MET receptors (3, 4). B, Dysregulation of the MET pathway can occur through several mechanisms including alterations in the MET gene, such as METex14 skipping (METex14 encodes the MET receptor juxtamembrane domain, which contains negative regulatory elements such as the Y1003-binding site for c-Cbl E3 ubiquitin ligase, which under normal conditions would facilitate the ubiquitination of the MET receptor, resulting in the internalization, trafficking to late endosomes and degradation. However, in tumors harboring METex14 skipping, the receptor is truncated and the loss of this binding site for c-Cbl E3 ubiquitin ligase results in reduced ubiquitination and degradation of the receptor, through decreased lysosomal receptor degradation, leading to sustained MET signaling that can promote uncontrolled proliferation, survival, and metastasis. C, Dysregulation of the MET pathway can also occur through METamp (where the increase in the MET copy number results in increased synthesis of MET, leading to increased MET signaling and subsequent increased cell proliferation, survival, and metastasis). Frazier et al. have reported ligand-independent phosphorylation of receptor tyrosine kinases (RTK) in cancer cells with METamp, where co-localization of MET and RTKs can occur in the Golgi apparatus, and the researchers postulated that when MET is overexpressed, it may accumulate in the Golgi apparatus and this overcrowding facilitates the nonspecific interaction between MET and newly synthesized RTKs (during the RTK trafficking to the plasma membrane) leading to the premature phosphorylation of RTKs and their subsequent downstream effect (5). In tumors harboring METex14 skipping (D) or METamp (E), the MET inhibitor tepotinib binds to the kinase domain and blocks the autophosphorylation of the intracellular domain of the MET receptor, thereby impeding the activation of the downstream signaling pathways (including RAS/ERK, PI3K/AKT, and STAT), and inhibiting tumor cell proliferation, survival, and metastasis. F, Secondary MET kinase domain mutations affecting for example Y1230 and D1228 prevent the binding of tepotinib to the MET receptor, leading to continued MET signaling. AKT, protein kinase B; c-Cbl, Casitas B-lineage lymphoma; ERK, extracellular signal-regulated kinase; Gab1, Grb2-associated binder 1; HGF, hepatocyte growth factor; MET, mesenchymal–epithelial transition proto-oncogene; METamp, MET amplification; METex14, MET exon 14; PI3K, phosphoinositide 3-kinase; RAS, rat sarcoma viral oncogene; STAT, signal transducer and activator of transcription.
MET pathway dysregulations occur through alterations in the MET gene, such as MET exon 14 (METex14) skipping (Fig. 1B) or MET amplification (METamp; Fig. 1C; refs. 1, 2). METex14 encodes the juxtamembrane domain of MET, which contains various negative regulatory sites, including: the protein kinase C (PKC) phosphorylation site Ser985; Tyr1003, which is phosphorylated to activate recruitment of the E3 ubiquitin ligase Casitas B-lineage lymphoma (CBL); and a caspase cleavage site involved in apoptosis (1, 8). Because Ser985 and PKC control cellular HGF responsiveness, METex14 skipping can desensitize to PKC-induced inhibitory signals, thereby increasing MET activation (8). Similarly, METex14 skipping results in MET receptors lacking the ubiquitin-binding site Tyr1003, leading to escape of the receptor from lysosomal degradation and its recycling to the surface and, hence, sustained MET activation (1, 9). Because caspase cleavage leads to MET inactivation and generates a pro-apoptotic receptor fragment, METex14 skipping also results in loss of this negative regulatory mechanism (8). Consequently, METex14 skipping alterations cause oncogenic MET activation by expression of a truncated receptor with increased stability, and augmented and prolonged signaling capability (1, 8). METex14 skipping occurs in approximately 3%–4% of lung adenocarcinomas, in approximately 2% of squamous cell lung cancers, with a higher incidence of approximately 8%–30% in pulmonary sarcomatoid carcinoma (1, 2, 10), and may be more common in lung cancer brain metastases than primary lung tumors (11). Besides NSCLC, METex14 skipping has only rarely been noted in other solid tumors, including brain glioma (12), although a higher prevalence has been reported in secondary glioblastoma (13).
METamp is thought to be a mechanism of overexpression of the MET receptor and its constitutive, ligand-independent activation, thereby dysregulating the MET pathway and promoting tumor growth (1, 2). De novo high-level METamp occurs in approximately 1%–2% of NSCLCs and has been identified as a primary oncogenic driver (1, 14–16). METamp is also detectable in 8%–14% of tumors with METex14 skipping (17, 18). METamp manifests in up to 30% of patients with acquired resistance to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKI; refs. 19) and is reported in approximately 15% of patients with resistance to ALK, RET or ROS1 inhibitors (20, 21).
Other mechanisms of MET dysregulation include MET fusions and activating MET kinase domain mutations (22). MET fusions are rare, may be a primary oncogenic driver in NSCLC, and are also reported in other cancers, including gastric cancer and glioma (23). Activating MET kinase domain mutations primarily occur in papillary renal-cell carcinoma, including the hereditary type 1 form (24).
Molecularly targeted cancer therapeutics have transformed cancer management, with departure from a “one-size-fits-all” approach and increasing focus on precision medicine. Although the oncogenic role of MET was established >30 years ago, early attempts to develop MET inhibitors were hampered by inadequate pharmacologic potency and/or selectivity, and suboptimal target population selection for clinical testing [for further information, please see Schadt and colleagues (ref. 25) and Wu and colleagues (ref. 2) and references therein]. Recently, several selective MET-targeted small-molecule TKIs have been developed, of which three – tepotinib hydrochloride hydrate (hereafter “tepotinib”), capmatinib, and savolitinib – have been approved on the basis of clinical benefit for patients with advanced/metastatic NSCLC harboring METex14 skipping alterations (26, 27).
Considering the principles of the Pharmacological Audit Trail (PhAT; ref. 28), this review focuses on key preclinical pharmacology data generated during the discovery and development of tepotinib, as an orally available and highly selective MET TKI, and precision medicine targeting oncogenic MET alterations (10, 29). Furthermore, we summarize ongoing clinical development and discuss potential mechanisms of pre-existing and acquired tepotinib resistance.
In Vitro Pharmacology of Tepotinib
Tepotinib is a highly selective, type Ib, ATP-competitive, small-molecule MET inhibitor (Fig. 1D and E). It binds to MET in a U-shaped conformation by interacting with Y1230, D1222, and M1160 in the hinge region (2, 22, 30, 31).
Tepotinib is a potent and highly selective MET inhibitor in biochemical assays
Tepotinib inhibition of MET kinase activity was analyzed in biochemical flash-plate assays using a His6-tagged recombinant human MET kinase domain (amino acid residues 974-end), [γ33P]-labeled ATP and a biotinylated peptide substrate (biotin-poly-AlaGluLysTyr, 6:2:5:1; ref. 30). Tepotinib inhibited MET kinase activity in a concentration-dependent manner with IC50 values of 1.7 and 1.8 nmol/L, respectively, in two independent experiments (30, 32, 33).
Selective type Ib MET inhibitors reduce the risk of off-target effects and are likely to have a superior safety profile compared with multi-kinase inhibitors, which is critical for treating biomarker-selected patients with monotherapy and especially with combination treatments (2, 10). Tepotinib was therefore designed and optimized to selectively inhibit MET and avoid poly-pharmacology. Overall, tepotinib selectivity was tested against >400 kinases and kinase variants, other than MET and mutant MET versions, at clinically relevant concentrations and above (32, 33). A tepotinib concentration of 0.1 μmol/L corresponds to approximately 200% of the free steady-state maximum concentration of tepotinib of 52 nmol/L in patients with the 500 mg once daily (QD) clinical dose. At this clinically relevant concentration, MET was completely inhibited (≥99%) whereas very few of the >300 MET-unrelated kinases were weakly inhibited, with tropomyosin receptor-kinase A (TrkA) being the most strongly inhibited (35%) non-MET kinase (33). Tepotinib selectivity was also measured at supratherapeutic concentrations of 1 and 10 μmol/L, corresponding to approximately 19- and 190-fold, respectively, of the average free steady-state maximum tepotinib concentration with 500 mg QD (33). Although tepotinib completely inhibited MET (100%) at 1 μmol/L, the strongest inhibition of a MET-unrelated kinase at this concentration was observed for TrkC (91%) and TrkA (70%) in panels of 399 and 305 kinases, respectively. At 10 μmol/L, the strongest inhibition was observed for TrkB (94% in a panel of 36 kinases from which TrkB was the only Trk family member; ref. 33). The high selectivity of tepotinib was further confirmed by an independent study analyzing 243 kinase inhibitors using a chemical kinome screen (Kinobeads) in tumor cell lysates, in which tepotinib did not interact with any non-MET kinase at up to 1 μmol/L (33, 34).
As TrkA was the MET-unrelated kinase most strongly inhibited by tepotinib at 0.1 μmol/L, and TrkA (NTRK1) is a relevant target in NSCLC, an in vitro study evaluated whether tepotinib at ≥0.1 μmol/L had antitumor activity against TrkA (NTRK1)-dependent, TPM3-NTRK1–expressing, colorectal cancer KM-12 cells (33). Test inhibitors included tepotinib, crizotinib [a multi-kinase inhibitor of MET (type Ia), ALK, and ROS1], and the NTRK inhibitors larotrectinib and entrectinib (entrectinib is a pan-Trk inhibitor of TrkA, TrkB and TrkC, with activity against ROS1 and ALK; refs. 1, 35, 36). In this study, entrectinib, larotrectinib, or crizotinib markedly decreased cellular viability with IC50 values ranging from 1 to 8 nmol/L, 9 to 22 nmol/L, and 55 to 112 nmol/L (33), respectively, in line with published IC50 values for entrectinib and larotrectinib in the low nanomolar range (35). In contrast, up to 1.3 μmol/L, tepotinib did not have antitumor activity in the same TrkA (NTRK1)-dependent KM-12 cells and, even at the highest supratherapeutic concentration (5 μmol/L), only a 50% reduction of cellular viability was observed (33). These findings corroborate the high selectivity of tepotinib and support the concept that partial, incomplete inhibition of unrelated kinases is insufficient for robust antitumor activity, particularly at clinically relevant concentrations (33).
Tepotinib is a potent inhibitor of MET in tumor cells, irrespective of the mode of MET activation
On the basis of the PhAT, one of six important aspects for successful drug development is to define the potential patient population early (28). Consistent with this concept and based on the high selectivity of tepotinib, the drug-discovery phase of tepotinib development placed a strong emphasis on biomarker-driven selection of tumor models for in vitro and in vivo pharmacology studies. Another critical aspect of the PhAT is the identification of a pharmacodynamic (PD) biomarker with close functional proximity to the target, and a robust assay to measure modulation of this biomarker. As has become standard in MET inhibitor development, MET autophosphorylation on Y1234 and Y1235 was established as a suitable proximal PD biomarker, and was used to successfully define the extent of target modulation needed to achieve antitumor efficacy with tepotinib preclinically and later clinically (30, 37, 38). Use of tumor pY1234/1235MET/total MET ratio as a PD biomarker to guide tepotinib dose selection was further supported in an independent study using a MET-amplified gastric cancer xenograft model (SNU-5; ref. 39).
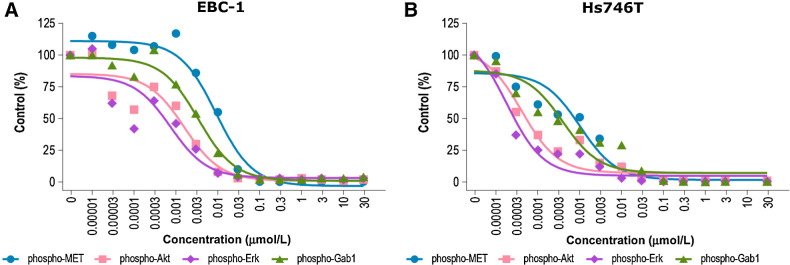
In vitro, in EBC-1 lung cancer cells harboring high-level METamp, tepotinib potently inhibited MET phosphorylation (IC50 = 1.1 nmol/L), as measured using a capture Enzyme-linked Immunosorbent Assay (30, 33). Strong MET inhibition was also observed in the gastric cancer cell lines Hs746T (harboring METex14 skipping and high-level METamp) and GTL-16 (harboring high-level METamp), with IC50 values of 2.5 and 2.9 nmol/L, respectively (33). To assess the effect of tepotinib-mediated MET inhibition on downstream signal transduction, phosphorylation of MET Y1234/1235, Grb2-associated binder 1 (Gab1) Y627, Akt S473, and ERK1/2 T202/Y204 was analyzed in EBC-1 cells using phospho-site–specific antibodies (Fig. 2A; refs. 30, 33). Inhibition of MET phosphorylation by tepotinib in the low nanomolar range was confirmed (IC50 = 9.2 nmol/L), and phosphorylation of the downstream adaptor/scaffold protein Gab1 was inhibited with an IC50 value of 3.4 nmol/L. Activation of anti-apoptotic Akt signaling and ERK phosphorylation was also efficiently inhibited in EBC-1 cells with low or sub-nanomolar IC50 values (30, 33). Similar results were observed in Hs746T cells (Fig. 2B; ref. 33). A549 cells were used to study tepotinib effects on HGF-dependent MET phosphorylation (33), because these cells do not harbor an oncogenic MET alteration and require HGF stimulation to activate MET (40). To measure the effect of tepotinib on HGF-mediated MET phosphorylation, cells were incubated in serum-free medium for 20 hours, pretreated with tepotinib in serum-free medium for 45 minutes, and stimulated with 100 ng/mL HGF for 5 minutes (33). As in the HGF-independent models, tepotinib showed potent and concentration-dependent MET kinase inhibition, with an IC50 value of 5.4 nmol/L (30, 33).
Figure 2.
Inhibition of MET phosphorylation and downstream signaling molecules with tepotinib in EBC-1 lung cancer cells (A; ref. 30) and Hs746T gastric cells (B; ref. 33). A, Adapted with permission from Bladt F, et al. Clin Cancer Res 2013;19 (11):2941–51. AKT, protein kinase B; ERK, extracellular signal-regulated kinase; Gab1, Grb2-associated binder 1; MET, mesenchymal–epithelial transition factor.
Tepotinib has a fast cellular uptake and long retention in tumor cells, as demonstrated by washout experiments (30). A549 cells were treated for 45 minutes with tepotinib and then incubated in tepotinib-free medium for 14 hours, before stimulation with 100 ng/mL HGF (30). The 45-minute incubation with tepotinib was sufficient to completely inhibit HGF-induced MET phosphorylation 14 hours after withdrawal of tepotinib, with an IC50 value of 5.3 nmol/L (30, 33), demonstrating rapid uptake and cellular retention of tepotinib, with long-lasting MET inhibition. Persistence of tepotinib inhibition under washout conditions may be explained by the long residence time of tepotinib and its retention in the lysosomal compartment to provide a local drug reservoir (41). Furthermore, 10% (v/v) serum only moderately affected tepotinib inhibition of HGF-induced MET phosphorylation in A549 cells (average IC50 values of 21 and 23 nmol/L with murine and human serum, respectively; ref. 30), suggesting that tepotinib activity will be largely maintained under whole-blood conditions in vivo.
Tepotinib selectively inhibits proliferation of MET-dependent tumor cells in 2D and 3D cell culture
MET promotes tumor cell proliferation and survival, particularly in cells with METamp (30). To investigate the effect of tepotinib on tumor cell viability in vitro, MKN-45 (METamp) and SNU-16 gastric cancer cells (non-METamp) were incubated with increasing concentrations of tepotinib, and cellular metabolic activity was assessed (30). Whereas incubation with tepotinib for 48 hours considerably inhibited MKN-45 cell viability (IC50 = 6 nmol/L), SNU-16 cells were less sensitive (IC50 = 3 μmol/L), thus demonstrating a selective inhibitory effect of tepotinib on METamp cells (30, 33). In another study, tepotinib had the highest MET inhibitory activity among 1,449 FDA-approved agents in METamp SNU-620 gastric cancer cells (42). Further studies showed concentration-dependent inhibition of METamp SNU-620 and MKN-45 cell growth by tepotinib, with average IC50 values of 9 and 7 nmol/L, respectively (42). In vitro wound-healing tests evaluating the effect of tepotinib, with or without HGF, on closure of a gap induced by scratching a layer of NCI-H441 lung cancer cells demonstrated that tepotinib inhibits cancer cell migration at clinically relevant concentrations (30).
Anchorage-independent growth is a hallmark of anoikis resistance and the path to invasive, metastatic tumor growth. To mimic the ability of cancer cells to grow in an anchorage-independent three-dimensional manner, murine NIH-3T3 cells co-transfected with human HGF and MET were cultured in a three-dimensional soft agar matrix (33). Treatment of established colonies with tepotinib for 5 days caused dose-dependent growth inhibition (IC50 = 1.8 nmol/L; ref. 33).
In Vivo Pharmacology of Tepotinib
PD activity of tepotinib
According to the PhAT, establishing the pharmacokinetic (PK) profile of a drug and understanding the PK/PD relationship in preclinical in vivo models is essential (28, 30, 32, 43). The tepotinib PK/PD relationship was first investigated in a single-administration study, using the Hs746T gastric cancer model (harboring METex14 skipping and METamp) with constitutive MET phosphorylation (30, 33). Consistent with the high volume of distribution of tepotinib of >8 L/kg in mice, tepotinib concentrations were greater in the tumor than plasma at all tested doses (30). At oral doses ≥10 mg/kg, tumor drug concentrations were within the active pharmacological range identified in vitro (i.e., one-digit nanomolar IC50 values) at all time points tested (3–96 hours; ref. 30). The lowest tested tepotinib dose (3 mg/kg) induced transient MET phosphorylation inhibition, which peaked (90%) at 6 hours and decreased (to 30%) at 24 hours (30). In contrast, the other doses tested (10, 30, and 100 mg/kg) resulted in >90% inhibition of MET phosphorylation for ≥72 hours, in line with the long cellular retention of tepotinib in vitro (30).
Tepotinib has strong antitumor activity in MET-dependent tumor models
In vivo, the antitumor efficacy of tepotinib was tested in mice bearing human tumor xenografts, representing various mechanisms of MET activation across multiple indications, including NSCLC and gastric cancer (30, 33, 37, 44). The dose-dependent inhibition of MET phosphorylation by tepotinib generally translated into dose-dependent antitumor activity in MET-dependent subcutaneous xenograft models, thus demonstrating preclinical proof of concept (30, 37). In mice bearing HGF-independent subcutaneous EBC-1 tumors with METamp, tepotinib (free base) 15 mg/kg QD led to tumor growth inhibition, with complete regressions in 7/10 animals, whereas 25 mg/kg QD led to complete regression in 10/10 mice (30, 33). In mice bearing HGF-independent Hs746T xenografts with METex14 skipping/METamp, effective tumor growth inhibition and regression, respectively, were observed at 3 and 6 mg/kg QD (30, 33).
Dose-dependent tepotinib antitumor activity was also observed in vivo in tumors with MET fusions (33). In one study, tepotinib induced strong tumor growth inhibition in subcutaneously implanted TPR-MET–transformed NIH-3T3 tumor cells at 12.5 mg/kg QD, and induced complete tumor regression in 4 out of 8 cases at 25 mg/kg QD (33, 43). Anecdotal evidence that patients with cancers with MET fusions may benefit from MET inhibitors comes from a case report of a woman with NSCLC and brain metastases harboring an HLA–DRB1–MET fusion. Following recurrence/progression on previous treatments, the patient received tepotinib and showed complete response in the brain, lung, and liver, which was sustained for almost 9 months (45).
In addition, tepotinib has demonstrated antitumor activity in preclinical models of liposarcoma (46), HCC (37, 47), head and neck squamous cell carcinoma (also showing a radiosensitization effect of tepotinib; ref. 48), bladder cancer (49), and neuroblastoma (50).
Tepotinib Is a Brain-Penetrating Molecule with Intracranial Antitumor Activity
The ability of tepotinib to cross the blood–brain barrier (BBB) was assessed in Wistar rats (44). After intravenous infusion of tepotinib for 24 hours, the average brain-to-plasma ratio was 2.87 at steady state (44). Because of its considerably higher brain tissue protein-binding versus plasma protein-binding, the partition coefficient of unbound drug in plasma and brain (Kp u,u) was 0.25, which would allow for intracranial target inhibition (44). This suggests that tepotinib is not freely diffusible but exhibits brain penetration (44). Brain penetration of tepotinib compares favorably with available data for capmatinib (brain-to-plasma ratio of 0.09 in rats) and crizotinib (cerebrospinal fluid-to-plasma ratio of 0.0006–0.003 in patients with brain metastases; refs. 51, 52).
In 20 subcutaneously implanted lung cancer brain metastasis patient-derived xenograft models, tepotinib at a suboptimal oral dose of 30 mg/kg QD (mimicking BBB-restricted drug exposure in the brain compartment) caused regression in 2/20 models (LU5349, −12%; LU5406, −88%; ref. 44). Molecular profiling revealed that only these two responding models had high-level METamp, confirming the selective efficacy of tepotinib in tumors with oncogenic MET alterations (44). The identified tepotinib-sensitive METamp NSCLC brain metastasis models were used to further evaluate the antitumor efficacy of tepotinib in a subcutaneous and orthotopic (intracranial) setting at a clinically relevant dose (125 mg/kg QD orally; ref. 44). In the subcutaneous setting, tepotinib treatment induced strong tumor shrinkage with complete regressions in 5/5 mice in both models (33, 44). In the orthotopic setting, tepotinib resulted in intracranial tumor shrinkage, as monitored by magnetic resonance imaging, with median tumor volume changes of −84% for LU5349 and −63% for LU5406 (44).
Given that brain metastases are common in METex14 skipping NSCLC and are associated with a poor prognosis and reduced quality of life (44), there is considerable interest in the use of tepotinib as a CNS-penetrating drug in these patients. Consistent with the preclinical data, intracranial antitumor activity of tepotinib was observed in patients with NSCLC with intracranial metastasis (53–55). Two case reports of patients with METex14 skipping NSCLC and brain (leptomeningeal) metastases showed marked intracranial tumor responses to tepotinib, with one patient showing disappearance of multiple intracranial metastases within 2 weeks of treatment (across both cases, the cerebrospinal fluid penetration rate of tepotinib ranged from 1.2% to 1.8%; refs. 53, 54). In a third report, in a patient with METex14 skipping NSCLC and multiple brain lesions, who had received prior crizotinib, chemotherapy, and immunotherapy, pronounced intracranial response to tepotinib was observed, with all brain lesions too small to measure by day 23 (56). Complete response to adjuvant tepotinib has also been reported in a patient with glioblastoma multiforme with METamp (57). Furthermore, in patients with METex14 skipping NSCLC in the VISION study, tepotinib demonstrated an intracranial disease control rate of 88.4% [95% confidence interval (CI), 74.9–96.1] in patients with target or non-target brain lesions (n = 43) and an intracranial objective response rate (ORR) of 66.7% (95% CI, 38.4–88.2) in patients with target brain lesions (n = 15), according to Response Assessment in Neuro-Oncology Brain Metastases criteria (58). No significant neurotoxicity has been reported with tepotinib in the clinical setting (55, 59).
Tepotinib Can Overcome MET-Mediated Resistance to Targeted Cancer Therapies
In metastatic NSCLC, activating EGFR mutations are a common oncogenic driver and positive predictive marker for EGFR-TKIs (19). However, METamp is a mechanism of resistance occurring in up to 30% of patients with NSCLC treated with various EGFR-TKIs (1, 2, 19). Under EGFR blockade, METamp provides a bypass resistance mechanism, allowing EGFR-independent activation of ErbB3 and the downstream PI3K/AKT pathway (1). Thus, MET inhibition may help overcome METamp-driven resistance (19).
Preclinical in vivo studies demonstrated that tepotinib can overcome EGFR-TKI resistance in NSCLC xenografts harboring METamp (60). In mice bearing patient-derived LU0858 tumors (harboring the EGFR-activating L858R mutation and METamp), gefitinib and afatinib had no inhibitory effect on tumor growth whereas tepotinib alone delayed tumor growth significantly, and tepotinib combined with EGFR-TKIs caused complete tumor regression (60). In mice bearing DFCI081 xenografts (harboring EGFR Del19 and METamp), tepotinib, alone or combined with EGFR-TKIs (rociletinib, erlotinib, or afatinib), induced complete tumor regression (60). In mice bearing HCC827-GR-T790M xenografts (harboring endogenous EGFR Del19, exogenous EGFR T790M, and METamp), monotherapy with tepotinib or rociletinib only moderately affected tumor growth, whereas afatinib and erlotinib had no effect. Tepotinib in combination with afatinib or erlotinib also moderately inhibited tumor growth but tepotinib combined with the third-generation EGFR-TKI rociletinib induced complete regression (60). Interestingly, preclinical evidence suggests that some EGFR-mutant, METamp NSCLCs may develop exclusive dependence on MET signaling, and so may be amenable to MET inhibitor monotherapy (61). Although clinical data indicate limited benefit of tepotinib monotherapy in this setting (62), characterization of responders may help identify a subset who could be candidates for a monotherapy strategy.
A recent study in preclinical breast cancer models provided evidence that a combination of pan-HER inhibitors with MET inhibitors (including tepotinib) may help overcome HER2 inhibitor resistance among patients with cooperating pan-HER and MET dysregulation (63). A maximal inhibitory effect on HCC1954 breast cancer xenografts was achieved with a combination of HER and MET receptor antagonists (63).
Tepotinib activity has also been evaluated in triple-negative breast cancer (TNBC) cell lines (64). In TNBC, EGFR expression and downstream pathway activation are common; however, anti-EGFR treatments have not been clinically effective (64). As MET is overexpressed in breast cancer (20%–30%), and METamp and MET overexpressions are associated with anti-EGFR resistance in NSCLC, it was hypothesized that MET contributes to anti-EGFR resistance in TNBC (64). In TNBC cell lines, the combination of an EGFR inhibitor (gefitinib or cetuximab) plus tepotinib demonstrated a synergistic anti-proliferative effect, thus targeting that both EGFR and MET simultaneously may provide an effective therapeutic strategy in TNBC (64).
Rationale for Tepotinib Clinical Dose Selection
Establishing a dose that is pharmacodynamically active and safe is a mainstay in drug development, especially for targeted treatments that are less prone to toxicities. It is therefore essential to develop a thorough understanding of the PK/PD relationship on the basis of in vivo preclinical models, to optimize assessment and selection of the clinical dose and schedule. Moreover, the characteristics of in vivo models and their predictive value in humans should be established and carefully considered.
For tepotinib, antitumor activity was demonstrated in several studies of xenograft models harboring METamp, METex14 skipping, and MET fusions, as described above (30, 33, 37, 44, 60). Although these models provided preclinical proof of principle for tepotinib, they are limited by their independence from human HGF and their consequent high sensitivity to MET inhibition, which could bias clinical dose selection. To address this, dedicated single- and repeated-dose PK/PD studies were executed in the HGF/MET autocrine KP-4 model, which showed comparably lower tepotinib sensitivity (moderate tumor shrinkage), meaning that higher tepotinib doses were required to achieve maximal response than in very sensitive tumors with oncogenic alterations (43). Thus, the KP-4 model allowed a more conservative estimation of the tepotinib dose–efficacy relationship (43).
Results from these experiments and subsequent PK/PD modeling demonstrated that near-complete (>95%) phospho-MET inhibition for ≥24 hours was required to achieve tumor regression in the KP-4 model (43). After accounting for inter-species differences in plasma protein binding (2.9% in mice and 1.6% in humans), it was estimated that tepotinib concentrations of 390–823 ng/mL in humans were required to attain 90%–95% maximum tumor inhibition. Subsequently, population PK and MET phosphorylation results derived from paired tumor biopsies acquired in the first-in-human (FIH) trial were integrated into the translational model and indicated that ≥95% target inhibition is achieved in >90% of patients with the standard dose of 500 mg (43), and in >80% of patients with a reduced dose of 250 mg (59). On the basis of this model, a biologically active dose of 500 mg QD was defined as the recommended phase II dose (RP2D) for subsequent studies (32).
Clinical Development Path toward Regulatory Approval in NSCLC
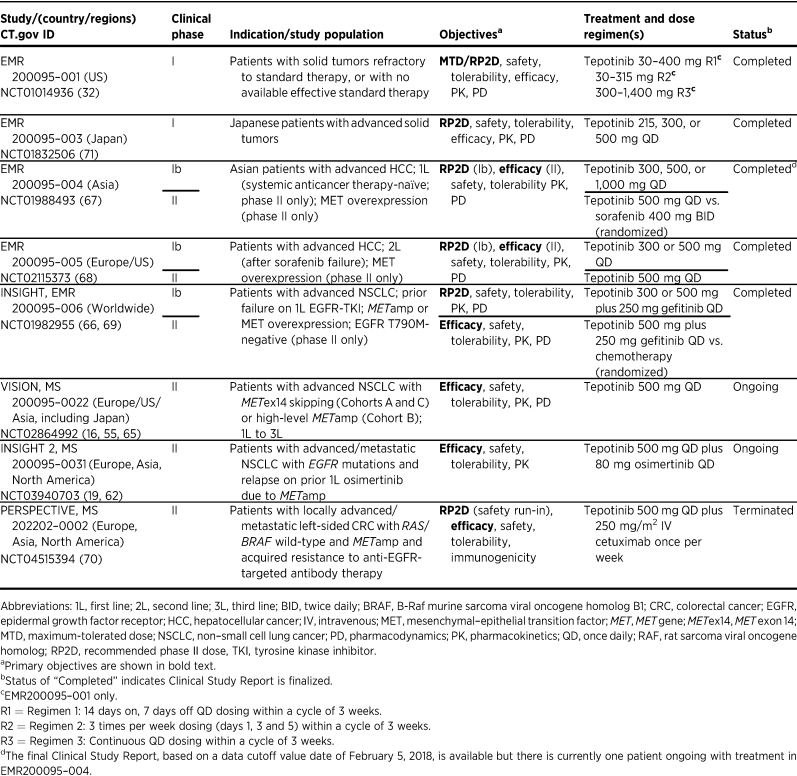
As of December 2022, the tepotinib clinical development program includes 17 phase I and II clinical studies (including clinical pharmacology studies in healthy participants) sponsored by the healthcare business of Merck KGaA, Darmstadt, Germany (see Table 1 for a summary of trials in patients with cancer; refs. 16, 19, 32, 55, 62, 65–71). Of these, 15 studies have been completed or terminated and two phase II studies (VISION and INSIGHT 2) are ongoing. Several investigator-sponsored studies are also underway in patients with cancers with MET alterations, including gastric cancer (NCT05439993) and brain tumors (NCT05120960).
Table 1.
Summary table of completed and ongoing sponsor-initiated phase I and II clinical trials with tepotinib in patients with cancer.
The FIH trial was an open-label, non-randomized, dose-escalation phase I trial in patients with advanced solid tumors conducted to determine the maximum tolerated dose (MTD) and RP2D of tepotinib (NCT01014936; ref. 32). Overall, tepotinib was well tolerated up to the highest dose administered (1,400 mg QD) and demonstrated signs of activity, particularly in tumors with high levels of MET expression or METamp, consistent with the previously described preclinical pharmacology data (32, 72). Although no MTD was identified, an RP2D of 500 mg QD was defined using the translational modeling approach, using preclinical PK/PD data, tumor growth data, and clinical PK/PD data from this trial (32, 43).
Tepotinib underwent further evaluation in patients with tumors with MET alterations, including NSCLC with METex14 skipping or high-level METamp (16, 65), EGFR-mutant NSCLC with MET-driven resistance to EGFR inhibitors (66), and MET-overexpressing advanced HCC (67, 68). VISION (NCT02864992) is a phase II, multicenter, multi-cohort trial evaluating tepotinib in patients with NSCLC and METex14 skipping (Cohorts A and C) or high-level METamp (Cohort B; refs. 16, 55, 65). Results from Cohorts A and C demonstrated that tepotinib has robust and durable clinical activity in patients with METex14 skipping NSCLC (55, 58, 65). In this precision medicine trial, METex14 skipping was prospectively tested using circulating free DNA (cfDNA) from liquid biopsy (LBx) samples or with an RNA-based approach using fresh or archival tissue biopsy (TBx) samples. Independently assessed ORR was consistent between the LBx (49%; 95% CI, 41–57) and TBx groups (51%; 95% CI, 44–59), underscoring the value of non-invasive LBx testing (73). LBx also enabled collection of longitudinal on-treatment biomarker data, which showed high concordance between the molecular cfDNA response and clinical response based on RECIST (74). In line with another important aspect of the PhAT, baseline and post-progression cfDNA analysis from this trial will be used to understand pre-existing and acquired tepotinib resistance mechanisms, and guide new treatment and combination approaches to reverse resistance (74). VISION data enabled successful regulatory approval of tepotinib for treatment of adults with advanced/metastatic METex14 skipping NSCLC in several countries. In addition, the trial included a cohort of patients with high-level METamp, EGFR/ALK wild-type NSCLC without METex14 skipping, in which tepotinib demonstrated clinically meaningful activity and induced durable responses (16).
The potential for tepotinib to overcome EGFR TKI resistance was evaluated in the phase Ib/II INSIGHT trial (NCT01982955; refs. 66, 69). The randomized phase II part compared tepotinib plus gefitinib versus chemotherapy in patients with EGFR-mutant (T790M−negative) NSCLC harboring MET overexpression or METamp, who had failed prior EGFR-TKI therapy. Although no significant difference was seen in the overall population, the tepotinib and gefitinib combination greatly improved outcomes versus chemotherapy in the subgroup with METamp, with a median progression-free survival (mPFS) of 16.6 months (90% CI, 8.3–22.1) versus 4.2 months (90% CI, 1.4–7.0) and median overall survival (mOS) of 37.3 months (90% CI, 21.1–52.1) versus 13.1 months (90% CI, 3.3–22.6), respectively, in the final analysis (69). ORR was 66.7% (90% CI, 39.1–87.7) for tepotinib and gefitinib versus 42.9% for chemotherapy (90% CI, 12.9–77.5). These noteworthy results provided a rationale for the ongoing pivotal phase II study in NSCLC (NCT03940703, INSIGHT 2), evaluating the combination of tepotinib with osimertinib in patients with advanced EGFR-mutant METamp NSCLC with acquired resistance to first-line osimertinib (19). Preliminary results indicated promising activity of tepotinib plus osimertinib, with an ORR of 54.5% in patients with METamp by central fluorescence in situ hybridization (FISH) testing and ≥9 months’ follow-up (62). Clinical activity of tepotinib plus an EGFR-TKI in NSCLC with EGFR-TKI resistance due to METamp has also been documented in patients receiving this combination outside clinical trials via compassionate use requests (75).
Finally, tepotinib has demonstrated promising activity in patients with HCC with MET overexpression who were either systemic treatment-naïve (NCT01988493; ref. 67) or previously treated with sorafenib (NCT02115373; ref. 68).
The clinical safety of tepotinib and MET inhibitors in patients with METex14 skipping NSCLC and the recommendations for management of adverse events (AE) have previously been reported in detail (59, 76). Briefly, in patients with METex14 skipping NSCLC (N = 255), tepotinib had a manageable safety profile with a low frequency of treatment discontinuation due to AEs, the most frequent all-cause AEs were mostly mild/moderate and included (overall/grade ≥3): edema [composite term: 69.8%/9.4%, with peripheral edema (60.0%/7.8%) being the most common edema], nausea (26.7%/0.8%), diarrhea (26.3%/0.4%), blood creatinine increase (25.9%/0.4%), hypoalbuminemia (composite term, 23.9%/5.5%), pleural effusions (13.3%/5.1%), vomiting (12.9%/1.2%), and alanine transaminase (ALT) and/or aspartate transaminase (AST) increase (composite term, 12.2%/3.1%; ref. 59). Edema as the most frequent tepotinib AE is a common class effect AE with MET inhibitors and has been reported with capmatinib (all cause peripheral edema: 59.8%), crizotinib (treatment-related edema composite event: 50.7%), and savolitinib (treatment-related peripheral edema: 54.0%; refs. 59, 76, 77). Noteworthy, the reversible increase in blood creatinine levels observed in patients treated with MET TKIs, including tepotinib and capmatinib, is potentially related to competitive inhibition of renal transporters for the secretion of creatinine in the renal tubules (76–78). Tepotinib and its major circulating human metabolite inhibit the elimination of creatinine through inhibition of the organic cation transporter 2 (OCT2) or the multidrug and toxin extrusion (MATE) transporters that could provide an explanation for the observed blood creatinine increase in patients (79, 80). Similar to the inhibition of OCT2 and MATE by tepotinib, other MET TKIs inhibit renal transporters (e.g., capmatinib inhibits MATE1 and MATE2K, crizotinib inhibits OCT1 and OCT2, and savolitinib inhibits MATE1 and MATE2K) and the increase in blood creatinine is potentially a class effect of MET TKIs (76–81). Most AEs can be managed through monitoring, supportive measures, and/or dose reduction/treatment interruption (59, 76).
Potential Mechanisms of Resistance to Tepotinib
With MET inhibitors now clinically available (26, 27), understanding mechanisms of resistance to tepotinib and other MET inhibitors is of utmost importance to offer alternative treatments to patients who develop resistance. Potential MET TKI resistance mechanisms are heterogeneous (e.g., MET mutations, bypass signaling, mutations in downstream effectors, or histological transformation), and may differ based on the class of MET TKI (22, 82). Besides secondary MET kinase domain mutations (e.g., affecting D1228 or Y1230; Fig. 1F; ref. 31), activation of alternative signaling pathways may result in MET inhibitor resistance, which could be addressed by combination approaches (16, 82). In one in vitro study, tepotinib inhibited MET autophosphorylation in 5/8 tested NIH-3T3 cell lines stably expressing mutated MET variants (83). Tepotinib remained active in cells expressing M1268T, H1112Y, H1112L, V1110I, and V1238I mutants, but not in cells expressing Y1248H, L1213V, and V1206L mutants. The tepotinib resistance of the L1213V variant was further confirmed in an in vivo xenograft study. It should be noted that this publication adopted an older numbering system for MET amino acids in which the number of each position was increased by 18; hence, Y1248H corresponds to the well-described Y1230H mutation (83). Another study in METex14 skipping Ba/F3 cells demonstrated that Y1230C/D/S/H/N or D1228A/E/G/H/N/Y kinase domain mutations confer resistance to type 1b MET inhibitors, including tepotinib, capmatinib, and savolitinib (84).
Clinical evidence for these variants as potential resistance mutations was provided by biomarker analyses from VISION. In Cohort A (METex14 skipping), seven patients had MET codon D1228 or Y1230 mutations at the time of progression (74). More recently, initial biomarker results from Cohort B (high-level METamp) showed emergence of MET kinase domain mutations in 2/9 patients (22.2%) with available end-of-treatment biomarker profiles (D1228H/N/Y, Y1230C/H, and D1231N in one patient, and D1213N, D1228N/H, and Y1230H in the other; ref. 16).
RAS mutations may activate the MAPK pathway and could thereby impair the downstream effect of MET inhibition (85). Preclinical data suggest that MET inhibitors combined with MEK inhibitors (i.e., targeting both MET and MAPK) may overcome resistance to MET inhibitors due to RAS mutations (85). In EBC-1 cells, tepotinib combined with an inhibitor of Src homology 2 domain-containing phosphatase 2 (SHP2), a cytoplasmic tyrosine phosphatase promoting MAPK pathway activation, delayed emergence of tepotinib resistance, and synergistic inhibition of cell proliferation was observed with tepotinib plus a SHP2 inhibitor in EBC-1, Hs746T, NCI-H1993, and MKN-45 cells in vitro (86). Another in vitro study suggests that MET inhibitor–induced autophagy may mediate resistance to MET inhibitors, including tepotinib, specifically in gastric cancer models (87). In this study, a combination of MET (tepotinib or PHA665752) and autophagy inhibition (3-MA) in gastric cancer cells significantly decreased cell viability (87).
Thus, a MET inhibitor in combination with another targeted therapy to block alternative signaling pathways may be a strategy for further investigation to overcome MET inhibitor resistance (82, 85–87). Ongoing biomarker studies evaluating pre-treatment and post-progression biopsies will provide further insights into predictive biomarker-directed treatments in patients with MET inhibitor resistance.
Conclusions
The discovery and development of tepotinib followed a stepwise approach, including the early identification of a robust PD biomarker and development of a deep understanding of the PK/PD relationship with respect to target/pathway modulation, which supported successful clinical dose and schedule selection. Alongside stringent use of biomarker-selected and predictive preclinical models to demonstrate preclinical proof of concept, these studies undergirded a successful clinical development program that enabled approval of tepotinib for advanced/metastatic METex14 skipping NSCLC. In this respect, the development of tepotinib is fully consistent with the subsequently published PhAT, which describes key questions to be addressed during discovery and development of a molecularly targeted anticancer drug (28). Importantly, the high selectivity and excellent physicochemical profile of tepotinib is a prerequisite for a precision medicine approach.
LBx is a powerful diagnostic tool for precision oncology that has recently been integrated into routine practice and is highlighted as an emerging technology in the PhAT, both to identify appropriate patients for biomarker-driven therapy, and to enable exploratory analysis of resistance mechanisms and alternative response measures (28). Implementation of prospective central testing of METex14 skipping in LBx samples in VISION significantly accelerated recruitment and generated important data to understand tepotinib resistance (88). Careful analysis of these data will guide rational combination approaches to overcome MET inhibitor resistance in the future.
By selectively targeting MET, the precision medicine tepotinib has shown durable activity in patients with hard-to-treat aggressive NSCLC tumors harboring specific oncogenic MET alterations (16, 65). The approval of tepotinib in Japan in 2020 was the first regulatory approval globally for an oral MET inhibitor for treatment of advanced NSCLC harboring METex14 skipping alterations, and was followed by approvals in multiple other countries/regions (55). Tepotinib is also recommended in clinical guidelines for eligible patients with METex14 skipping metastatic NSCLC (89–92), and as a treatment option for patients with high-level METamp metastatic NSCLC (91).
Acknowledgments
All authors participated fully in developing and reviewing the article for publication. All authors had full access to all the data in the article and had final responsibility for the decision to submit for publication. This work was funded by the healthcare business of Merck KGaA, Darmstadt, Germany. Medical writing and editorial assistance were provided by Syneos Health, UK, and funded by the healthcare business of Merck KGaA, Darmstadt, Germany.
Authors' Disclosures
J. Albers reports employment with the healthcare business of Merck KGaA, Darmstadt, Germany, other support from the healthcare business of Merck KGaA, Darmstadt, Germany during the conduct of the study; as well as other support from the healthcare business of Merck KGaA, Darmstadt, Germany outside the submitted work. O. Schadt reports employment with the healthcare business of Merck KGaA, Darmstadt, Germany, a patent for WO2009006959A1 issued and with royalties paid, a patent for WO2009007074A1 issued and with royalties paid, and a patent for WO2010078897A1 issued and with royalties paid. G. Walter-Bausch reports employment with the healthcare business of Merck KGaA, Darmstadt, Germany. C. Stroh reports employment with the healthcare business of Merck KGaA, Darmstadt, Germany. A. Johne reports employment with the healthcare business of Merck KGaA, Darmstadt, Germany, other support from the healthcare business of Merck KGaA, Darmstadt, Germany and other support from the healthcare business of Merck KGaA, Darmstadt, Germany during the conduct of the study. N. Karachaliou reports employment with the healthcare business of Merck KGaA, Darmstadt, Germany, other support from the healthcare business of Merck KGaA, Darmstadt, Germany outside the submitted work. A. Blaukat reports other support from the healthcare business of Merck KGaA, Darmstadt, Germany outside the submitted work; as well as reports a patent for Tepotinib issued. A. Blaukat is a former employee of the healthcare business of Merck KGaA, Darmstadt, Germany. M. Friese-Hamim reports employment with the healthcare business of Merck KGaA, Darmstadt, Germany. A. Clark reports employment with EMD Serono, Billerica, MA.
References
- 1. Drilon A, Cappuzzo F, Ou SI, Camidge DR. Targeting MET in lung cancer: will expectations finally be MET? J Thorac Oncol 2017;12:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu YL, Soo RA, Locatelli G, Stammberger U, Scagliotti G, Park K. Does c-Met remain a rational target for therapy in patients with EGFR TKI-resistant non–small cell lung cancer? Cancer Treat Rev 2017;61:70–81. [DOI] [PubMed] [Google Scholar]
- 3. Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol 2010;11:834–48. [DOI] [PubMed] [Google Scholar]
- 4. Lefebvre J, Ancot F, Leroy C, Muharram G, Lemière A, Tulasne D. Met degradation: more than one stone to shoot a receptor down. FASAB J 2012;26:1387–99. [DOI] [PubMed] [Google Scholar]
- 5. Frazier NM, Brand T, Gordan JD, Grandis J, Jura N. Overexpression-mediated activation of MET in the Golgi promotes HER3/ERBB3 phosphorylation. Oncogene 2019;38:1936–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang H, Rao B, Lou J, Li J, Liu Z, Li A, et al. The function of the HGF/c-Met axis in hepatocellular carcinoma. Front Cell Dev Biol 2020;8:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Safaie Qamsari E, Safaei Ghaderi S, Zarei B, Dorostkar R, Bagheri S, Jadidi-Niaragh F, et al. The c-Met receptor: implication for targeted therapies in colorectal cancer. Tumour Biol 2017;39:1010428317699118. [DOI] [PubMed] [Google Scholar]
- 8. Cortot AB, Kherrouche Z, Descarpentries C, Wislez M, Baldacci S, Furlan A, et al. Exon 14 deleted MET receptor as a new biomarker and target in cancers. J Natl Cancer Inst 2017;109. [DOI] [PubMed] [Google Scholar]
- 9. Thien CB, Langdon WY. c-Cbl and Cbl-b ubiquitin ligases: substrate diversity and the negative regulation of signalling responses. Biochem J 2005;391:153–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reungwetwattana T, Liang Y, Zhu V, Ou SI. The race to target MET exon 14 skipping alterations in non–small cell lung cancer: the why, the how, the who, the unknown, and the inevitable. Lung Cancer 2017;103:27–37. [DOI] [PubMed] [Google Scholar]
- 11. Chai RC, Liu X, Pang B, Liu YQ, Li JJ, Li YF, et al. Recurrent PTPRZ1-MET fusion and a high occurrence rate of MET exon 14 skipping in brain metastases. Cancer Sci 2022;113:796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frampton GM, Ali SM, Rosenzweig M, Chmielecki J, Lu X, Bauer TM, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov 2015;5:850–9. [DOI] [PubMed] [Google Scholar]
- 13. Hu H, Mu Q, Bao Z, Chen Y, Liu Y, Chen J, et al. Mutational landscape of secondary glioblastoma guides MET-targeted trial in brain tumor. Cell 2018;175:1665–78.e18. [DOI] [PubMed] [Google Scholar]
- 14. Noonan SA, Berry L, Lu X, Gao D, Barón AE, Chesnut P, et al. Identifying the appropriate FISH criteria for defining MET copy number-driven lung adenocarcinoma through oncogene overlap analysis. J Thorac Oncol 2016;11:1293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caparica R, Yen CT, Coudry R, Ou SI, Varella-Garcia M, Camidge DR, et al. Responses to crizotinib can occur in high-level MET-amplified non–small cell lung cancer independent of MET exon 14 alterations. J Thorac Oncol 2017;12:141–4. [DOI] [PubMed] [Google Scholar]
- 16. Le X, Paz-Ares L, Meerbeeck JV, Ramirez SV, Galvez CC, Baz DV, et al. Clinical response to tepotinib according to circulating tumor (ct) DNA biomarkers in patients with advanced NSCLC with high-level MET amplification (METamp) detected by liquid biopsy (LBx). J Clin Oncol 2022;40:9121. [Google Scholar]
- 17. Lee JK, Madison R, Classon A, Gjoerup O, Rosenzweig M, Frampton GM, et al. Characterization of non–small cell lung cancers with MET exon 14 skipping alterations detected in tissue or liquid: clinicogenomics and real-world treatment patterns. JCO Precis Oncol 2021;5:PO.21.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Le X, Hong L, Hensel C, Chen R, Kemp H, Coleman N, et al. Landscape and clonal dominance of co-occurring genomic alterations in non–small cell lung cancer harboring MET exon 14 skipping. JCO Precis Oncol 2021;5:PO.21.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smit EF, Dooms C, Raskin J, Nadal E, Tho LM, Le X, et al. INSIGHT 2: a phase II study of tepotinib plus osimertinib in MET-amplified NSCLC and first-line osimertinib resistance. Future Oncol 2022;18:1039–54. [DOI] [PubMed] [Google Scholar]
- 20. Dagogo-Jack I, Yoda S, Lennerz JK, Langenbucher A, Lin JJ, Rooney MM, et al. MET alterations are a recurring and actionable resistance mechanism in ALK-positive lung cancer. Clin Cancer Res 2020;26:2535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coleman N, Hong L, Zhang J, Heymach J, Hong D, Le X. Beyond epidermal growth factor receptor: MET amplification as a general resistance driver to targeted therapy in oncogene-driven non–small cell lung cancer. ESMO Open 2021;6:100319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Recondo G, Che J, Jänne PA, Awad MM. Targeting MET dysregulation in cancer. Cancer Discov 2020;10:922–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guo R, Luo J, Chang J, Rekhtman N, Arcila M, Drilon A. MET-dependent solid tumours—molecular diagnosis and targeted therapy. Nat Rev Clin Oncol 2020;17:569–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang Y, Ricketts CJ, Vocke CD, Killian JK, Padilla-Nash HM, Lang M, et al. Characterization of genetically defined sporadic and hereditary type 1 papillary renal cell carcinoma cell lines. Genes Chromosomes Cancer 2021;60:434–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schadt O, Dai G. The preclinical journal of MET inhibitors. In: Rudolph J, Bronsin JJ, editors. 2022Medicinal Chemistry Reviews. Volume 57. ACS Publications; 2022. p. 267–92. [Google Scholar]
- 26. Mathieu LN, Larkins E, Akinboro O, Roy P, Amatya AK, Fiero MH, et al. FDA approval summary: capmatinib and tepotinib for the treatment of metastatic NSCLC harboring MET exon 14 skipping mutations or alterations. Clin Cancer Res 2022;28:249–54. [DOI] [PubMed] [Google Scholar]
- 27. Markham A. Savolitinib: first approval. Drugs 2021;81:1665–70. [DOI] [PubMed] [Google Scholar]
- 28. Banerji U, Workman P. Critical parameters in targeted drug development: the pharmacological audit trail. Semin Oncol 2016;43:436–45. [DOI] [PubMed] [Google Scholar]
- 29. Zhong L, Li Y, Xiong L, Wang W, Wu M, Yuan T, et al. Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct Target Ther 2021;6:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bladt F, Faden B, Friese-Hamim M, Knuehl C, Wilm C, Fittschen C, et al. EMD 1214063 and EMD 1204831 constitute a new class of potent and highly selective c-Met inhibitors. Clin Cancer Res 2013;19:2941–51. [DOI] [PubMed] [Google Scholar]
- 31. Collie GW, Koh CM, O'Neill DJ, Stubbs CJ, Khurana P, Eddershaw A, et al. Structural and molecular insight into resistance mechanisms of first-generation cMET inhibitors. ACS Med Chem Lett 2019;10:1322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Falchook GS, Kurzrock R, Amin HM, Xiong W, Fu S, Piha-Paul SA, et al. First-in-man phase I trial of the selective MET inhibitor tepotinib in patients with advanced solid tumors. Clin Cancer Res 2020;26:1237–46. [DOI] [PubMed] [Google Scholar]
- 33. The healthcare business of Merck KGaA, Darmstadt, Germany. Data on file. [Google Scholar]
- 34. Klaeger S, Heinzlmeir S, Wilhelm M, Polzer H, Vick B, Koenig PA, et al. The target landscape of clinical kinase drugs. Science 2017;358:eaan4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol 2018;15:731–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hong L, Zhang J, Heymach JV, Le X. Current and future treatment options for MET exon 14 skipping alterations in non–small cell lung cancer. Ther Adv Med Oncol 2021;13:1758835921992976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bladt F, Friese-Hamim M, Ihling C, Wilm C, Blaukat A. The c-Met inhibitor MSC2156119J effectively inhibits tumor growth in liver cancer models. Cancers (Basel) 2014;6:1736–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dorsch D, Schadt O, Stieber F, Meyring M, Grädler U, Bladt F, et al. Identification and optimization of pyridazinones as potent and selective c-Met kinase inhibitors. Bioorg Med Chem Lett 2015;25:1597–602. [DOI] [PubMed] [Google Scholar]
- 39. Srivastava AK, Hollingshead MG, Govindharajulu JP, Covey JM, Liston D, Simpson MA, et al. Molecular pharmacodynamics-guided scheduling of biologically effective doses: a drug development paradigm applied to MET tyrosine kinase inhibitors. Mol Cancer Ther 2018;17:698–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lutterbach B, Zeng Q, Davis LJ, Hatch H, Hang G, Kohl NE, et al. Lung cancer cell lines harboring MET gene amplification are dependent on Met for growth and survival. Cancer Res 2007;67:2081–8. [DOI] [PubMed] [Google Scholar]
- 41. Berges N, Klug JH, Eicher A, Loehr J, Schwarz D, Bomke J, et al. Differences in sustained cellular effects of MET inhibitors are driven by prolonged target engagement and lysosomal retention. Mol Pharmacol 2022;103:77–88. [DOI] [PubMed] [Google Scholar]
- 42. Sohn SH, Sul HJ, Kim B, Kim BJ, Kim HS, Zang DY. Tepotinib inhibits the epithelial-mesenchymal transition and tumor growth of gastric cancers by increasing GSK3β, E-cadherin, and mucin 5AC and 6 levels. Int J Mol Sci 2020;21:6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xiong W, Friese-Hamim M, Johne A, Stroh C, Klevesath M, Falchook GS, et al. Translational pharmacokinetic-pharmacodynamic modeling of preclinical and clinical data of the oral MET inhibitor tepotinib to determine the recommended phase II dose. CPT Pharmacometrics Syst Pharmacol 2021;10:428–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Friese-Hamim M, Clark A, Perrin D, Crowley L, Reusch C, Bogatyrova O, et al. Brain penetration and efficacy of tepotinib in orthotopic patient-derived xenograft models of MET-driven non–small cell lung cancer brain metastases. Lung Cancer 2022;163:77–86. [DOI] [PubMed] [Google Scholar]
- 45. Blanc-Durand F, Alameddine R, Iafrate AJ, Tran-Thanh D, Lo YC, Blais N, et al. Tepotinib efficacy in a patient with non–small cell lung cancer with brain metastasis harboring an HLA-DRB1-MET gene fusion. Oncologist 2020;25:916–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bill KL, Garnett J, Ma X, May CD, Bolshakov S, Lazar AJ, et al. The hepatocyte growth factor receptor as a potential therapeutic target for dedifferentiated liposarcoma. Lab Invest 2015;95:951–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhan N, Michael AA, Wu K, Zeng G, Bell A, Tao J, et al. The effect of selective c-MET inhibitor on hepatocellular carcinoma in the MET-active, β-catenin-mutated mouse model. Gene Expr 2018;18:135–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nisa L, Francica P, Giger R, Medo M, Elicin O, Friese-Hamim M, et al. Targeting the MET receptor tyrosine kinase as a strategy for radiosensitization in locoregionally advanced head and neck squamous cell carcinoma. Mol Cancer Ther 2020;19:614–26. [DOI] [PubMed] [Google Scholar]
- 49. Lee YH, Apolo AB, Agarwal PK, Bottaro DP. Characterization of HGF/Met signaling in cell lines derived from urothelial carcinoma of the bladder. Cancers (Basel) 2014;6:2313–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Scorsone K, Zhang L, Woodfield SE, Hicks J, Zage PE. The novel kinase inhibitor EMD1214063 is effective against neuroblastoma. Invest New Drugs 2014;32:815–24. [DOI] [PubMed] [Google Scholar]
- 51. US Food and Drug Administration. Tabrecta. (capmatinib). U.S. Prescribing Information. Revised 8/2022.
- 52. Angeli E, Bousquet G. Brain metastasis treatment: the place of tyrosine kinase inhibitors and how to facilitate their diffusion across the blood–brain barrier. Pharmaceutics 2021;13:1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ninomaru T, Okada H, Fujishima M, Irie K, Fukushima S, Hata A. Lazarus response to tepotinib for leptomeningeal metastases in a patient with MET exon 14 skipping mutation-positive lung adenocarcinoma: case report. JTO Clin Res Rep 2021;2:100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tanaka H, Taima K, Makiguchi T, Nakagawa J, Niioka T, Tasaka S. Activity and bioavailability of tepotinib for leptomeningeal metastasis of NSCLC with MET exon 14 skipping mutation. Cancer Commun (Lond) 2021;41:83–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Le X, Sakai H, Felip E, Veillon R, Garassino MC, Raskin J, et al. Tepotinib efficacy and safety in patients with MET exon 14 skipping NSCLC: outcomes in patient subgroups from the VISION study with relevance for clinical practice. Clin Cancer Res 2022;28:1117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Takamori S, Matsubara T, Fujishita T, Ito K, Toyozawa R, Seto T, et al. Dramatic intracranial response to tepotinib in a patient with lung adenocarcinoma harboring MET exon 14 skipping mutation. Thorac Cancer 2021;12:978–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pham L, Gann C, Schumacher KM, Vlassak S, Swanson T, Highsmith K, et al. INNV-21. Complete response to adjuvant tepotinib in a patient with newly diagnosed disseminated glioblastoma (GBM) harboring MET amplification. Neuro-oncol 2021;23:vi109. [DOI] [PubMed] [Google Scholar]
- 58. Thomas M, Garassino MC, Felip E, Sakai H, Le X, Veillon R, et al. Tepotinib in patients with MET exon 14 skipping NSCLC: primary analysis of the confirmatory VISION Cohort C [abstract]. In: Proceedings of the IASLC 2022 World Conference on Lung Cancer; 2022 Aug 6–9; Vienna, Austria: WCLC; 2022. Abstract nr OA03.05. [Google Scholar]
- 59. Veillon R, Sakai H, Le X, Felip E, Cortot AB, Smit EF, et al. Safety of tepotinib in patients with MET Exon 14 skipping NSCLC and recommendations for management. Clin Lung Cancer 2022;23:320–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Friese-Hamim M, Bladt F, Locatelli G, Stammberger U, Blaukat A. The selective c-Met inhibitor tepotinib can overcome epidermal growth factor receptor inhibitor resistance mediated by aberrant c-Met activation in NSCLC models. Am J Cancer Res 2017;7:962–72. [PMC free article] [PubMed] [Google Scholar]
- 61. Eser P, Paranal RM, Son J, Ivanova E, Kuang Y, Haikala HM, et al. Oncogenic switch and single-agent MET inhibitor sensitivity in a subset of EGFR-mutant lung cancer. Sci Transl Med 2021;13:eabb3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mazieres J, Kim TM, Lim BK, Wislez M, Dooms C, Finocchiaro G, et al. LBA52 - Tepotinib + osimertinib for EGFRm NSCLC with MET amplification (METamp) after progression on first-line (1L) osimertinib: initial results from the INSIGHT 2 study. Ann Oncol 2022:33;S808–S69. [Google Scholar]
- 63. MacNeil IA, Khan SA, Sen A, Soltani SM, Burns DJ, Sullivan BF, et al. Functional signaling test identifies HER2 negative breast cancer patients who may benefit from c-Met and pan-HER combination therapy. Cell Commun Signal 2022;20:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sohn J, Liu S, Parinyanitikul N, Lee J, Hortobagyi GN, Mills GB, et al. cMET activation and EGFR-directed therapy resistance in triple-negative breast cancer. J Cancer 2014;5:745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Paik PK, Felip E, Veillon R, Sakai H, Cortot AB, Garassino MC, et al. Tepotinib in non–small cell lung cancer with MET exon 14 skipping mutations. N Engl J Med 2020;383:931–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wu YL, Cheng Y, Zhou J, Lu S, Zhang Y, Zhao J, et al. Tepotinib plus gefitinib in patients with EGFR-mutant non–small cell lung cancer with MET overexpression or MET amplification and acquired resistance to previous EGFR inhibitor (INSIGHT study): an open-label, phase 1b/2, multicentre, randomised trial. Lancet Respir Med 2020;8:1132–43. [DOI] [PubMed] [Google Scholar]
- 67. Ryoo BY, Cheng AL, Ren Z, Kim TY, Pan H, Rau KM, et al. Randomised phase 1b/2 trial of tepotinib vs sorafenib in Asian patients with advanced hepatocellular carcinoma with MET overexpression. Br J Cancer 2021;125:200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Decaens T, Barone C, Assenat E, Wermke M, Fasolo A, Merle P, et al. Phase 1b/2 trial of tepotinib in sorafenib pretreated advanced hepatocellular carcinoma with MET overexpression. Br J Cancer 2021;125:190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Liam CK, Ahmad AR, Hsia TC, Zhou J, Kim DW, Soo RA, et al. Randomized trial of tepotinib plus gefitinib versus chemotherapy in EGFR-mutant NSCLC with EGFR inhibitor resistance due to MET amplification: INSIGHT final analysis. Clin Cancer Res 2023;29:1879–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bekaii-Saab TS, Cutsem EV, Cubillo A, Petorin-Lesens C, Rodriguez-Salas N, Raghav KPS, et al. PERSPECTIVE: tepotinib + cetuximab in patients (pts) with RAS/BRAF wild-type left-sided metastatic colorectal cancer (mCRC) and acquired resistance to anti-EGFR antibody therapy due to MET amplification (METamp). J Clin Oncol 2021;39:TPS3616. [Google Scholar]
- 71. Shitara K, Yamazaki K, Tsushima T, Naito T, Matsubara N, Watanabe M, et al. Phase I trial of the MET inhibitor tepotinib in Japanese patients with solid tumors. Jpn J Clin Oncol 2020;50:859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Falchook GS, Kurzrock R, Amin HM, Fu S, Piha-Paul SA, Janku F, et al. Efficacy, safety, biomarkers, and phase II dose modeling in a phase I trial of the oral selective c-Met inhibitor tepotinib (MSC2156119J). J Clin Oncol 2015;33:2591.26195716 [Google Scholar]
- 73. Felip E, Garassino MC, Sakai H, Le X, Veillon R, Smit E, et al. P45.03 Tepotinib in Patients with METexon 14 (METex14) skipping NSCLC as identified by liquid (LBx) or tissue (TBx) biopsy. J Thorac Oncol 2021;16:S1085. [Google Scholar]
- 74. Paik PK, Veillon R, Felip E, Cortot A, Sakai H, Mazieres J, et al. METex14 ctDNA dynamics & resistance mechanisms detected in liquid biopsy (LBx) from patients (pts) with METex14 skipping NSCLC treated with tepotinib. J Clin Oncol 2021;39:9012. [Google Scholar]
- 75. Le X, Eisert A, Himpe U, De Bondt C, Mazieres J, Petrini I, et al. EP08.02-162 Tepotinib with an EGFR-tyrosine kinase inhibitor (TKI) in patients with EGFR-mutant MET-amplified NSCLC: a case series. J Thoracic Oncol 2022:17;S483–S4.
- 76. Cortot A, Le X, Smit E, Viteri S, Kato T, Sakai H, et al. Safety of MET tyrosine kinase inhibitors in patients with MET exon 14 skipping non–small cell lung cancer: a clinical review. Clin Lung Cancer 2022;23:195–207. [DOI] [PubMed] [Google Scholar]
- 77. Wolf J, Seto T, Han J-Y, Reguart N, Garon EB, Groen HJM, et al. Capmatinib in MET exon 14–mutated or MET-amplified non–small cell lung cancer. N Engl J Med 2020;383:944–57. [DOI] [PubMed] [Google Scholar]
- 78. Mohan A, Herrmann SM. Capmatinib-induced pseudo-acute kidney injury: a case report. Am J Kidney Dis 2022;79:120–4. [DOI] [PubMed] [Google Scholar]
- 79. Xiong W, Hietala SF, Nyberg J, Papasouliotis O, Johne A, Berghoff K, et al. Exposure–response analyses for the MET inhibitor tepotinib, including patients in the pivotal VISION trial: support for dosage recommendations. Cancer Chemother Pharmacol 2022;90:53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wijtvliet V, Roosens L, De Bondt C, Janssens A, Abramowicz D. Pseudo-acute kidney injury secondary to tepotinib. Clinical Kidney J 2023:16;760–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Grande E, Giovannini M, Marriere E, Pultar P, Quinlan M, Chen X, et al. Effect of capmatinib on the pharmacokinetics of digoxin and rosuvastatin administered as a 2-drug cocktail in patients with MET-dysregulated advanced solid tumours: a phase I, multicentre, open-label, single-sequence drug-drug interaction study. Br J Clin Pharmacol 2021;87:2867–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Recondo G, Bahcall M, Spurr LF, Che J, Ricciuti B, Leonardi GC, et al. Molecular mechanisms of acquired resistance to MET tyrosine kinase inhibitors in patients with MET exon 14-mutant NSCLC. Clin Cancer Res 2020;26:2615–25. [DOI] [PubMed] [Google Scholar]
- 83. Medová M, Pochon B, Streit B, Blank-Liss W, Francica P, Stroka D, et al. The novel ATP-competitive inhibitor of the MET hepatocyte growth factor receptor EMD1214063 displays inhibitory activity against selected MET-mutated variants. Mol Cancer Ther 2013;12:2415–24. [DOI] [PubMed] [Google Scholar]
- 84. Fujino T, Kobayashi Y, Suda K, Koga T, Nishino M, Ohara S, et al. Sensitivity and resistance of MET Exon 14 mutations in lung cancer to eight MET tyrosine kinase inhibitors in vitro. J Thorac Oncol 2019;14:1753–65. [DOI] [PubMed] [Google Scholar]
- 85. Leiser D, Medová M, Mikami K, Nisa L, Stroka D, Blaukat A, et al. KRAS and HRAS mutations confer resistance to MET targeting in preclinical models of MET-expressing tumor cells. Mol Oncol 2015;9:1434–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pudelko L, Jaehrling F, Reusch C, Vitri S, Stroh C, Linde N, et al. SHP2 inhibition influences therapeutic response to tepotinib in tumors with MET alterations. iScience 2020;23:101832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Humbert M, Medová M, Aebersold DM, Blaukat A, Bladt F, Fey MF, et al. Protective autophagy is involved in resistance towards MET inhibitors in human gastric adenocarcinoma cells. Biochem Biophys Res Commun 2013;431:264–9. [DOI] [PubMed] [Google Scholar]
- 88. Le X, Kowalski D, Cho BC, Conte P, Felip E, Garassino MC, et al. Abstract 3385: liquid biopsy to detect MET exon 14 skipping (METex14) and MET amplification in patients with advanced NSCLC: biomarker analysis from VISION study. Cancer Res 2020;80:3385. [Google Scholar]
- 89. Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic non–small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv192–237. [DOI] [PubMed] [Google Scholar]
- 90. Hanna NH, Robinson AG, Temin S, Baker S Jr, Brahmer JR, Ellis PM, et al. Therapy for stage IV non–small cell lung cancer with driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol 2021;39:1040–91. [DOI] [PubMed] [Google Scholar]
- 91. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small Cell Lung Cancer V4.2022. © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed September 7, 2022. To view the most recent and complete version of the guideline, go online to https://www.nccn.org/home. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- 92. ESMO. ESMO. Clinical Practice Living Guidelines—metastatic non–small cell lung cancer. [cited 7 Sep 2022]. Available from: https://www.esmo.org/guidelines/guidelines-by-topic/lung-and-chest-tumours/clinical-practice-living-guidelines-metastatic-non-small-cell-lung-cancer.