Abstract
BACKGROUND
Owing to a growing number of young and adolescent Hodgkin lymphoma (HL) survivors, awareness of (long-term) adverse effects of anticancer treatment increases. The risk of impaired reproductive ability is of great concern given its impact on quality of life. There is currently no review available on fertility after childhood HL treatment.
OBJECTIVE AND RATIONALE
The aim of this narrative review was to summarize existing literature on different aspects of reproductive function in male and female childhood, adolescent, and young adult HL survivors.
SEARCH METHODS
PubMed and EMBASE were searched for articles evaluating fertility in both male and female HL survivors aged <25 years at diagnosis. In females, anti-Müllerian hormone (AMH), antral follicle count, premature ovarian insufficiency (POI), acute ovarian failure, menstrual cycle, FSH, and pregnancy/live births were evaluated. In males, semen-analysis, serum FSH, inhibin B, LH, testosterone, and reports on pregnancy/live births were included. There was profound heterogeneity among studies and a lack of control groups; therefore, no meta-analyses could be performed. Results were presented descriptively and the quality of studies was not assessed individually.
OUTCOMES
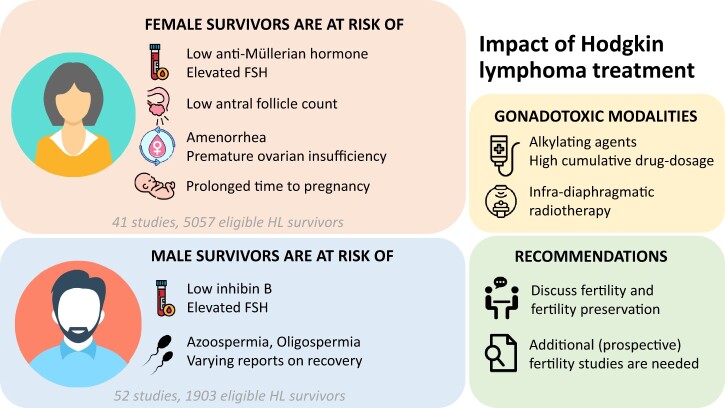
After screening, 75 articles reporting on reproductive markers in childhood or adolescent HL survivors were included. Forty-one papers reported on 5057 female HL survivors. The incidence of POI was 6–34% (median 9%; seven studies). Signs of diminished ovarian reserve or impaired ovarian function were frequently seen (low AMH 55–59%; median 57%; two studies. elevated FSH 17–100%; median 53%; seven studies). Most survivors had regular menstrual cycles. Fifty-one studies assessed fertility in 1903 male HL survivors. Post-treatment azoospermia was highly prevalent (33–100%; median 75%; 29 studies). Long-term follow-up data were limited, but reports on recovery of semen up to 12 years post-treatment exist. FSH levels were often elevated with low inhibin B (elevated FSH 0–100%; median 51.5%; 26 studies. low inhibin B 19–50%; median 45%; three studies). LH and testosterone levels were less evidently affected (elevated LH 0–57%, median 17%; 21 studies and low testosterone 0–43%; median 6%; 15 studies). In both sexes, impaired reproductive ability was associated with a higher dose of cumulative chemotherapeutic agents and pelvic radiotherapy. The presence of abnormal markers before treatment indicated that the disease itself may also negatively affect reproductive function (Females: AMH<p10 9%; one study and Males: azoospermia 0–50%; median 10%; six studies). Reports on chance to achieve pregnancy during survivorship are reassuring, although studies had their limitations and the results are difficult to evaluate. In the end, a diminished ovarian reserve does not exclude the chance of a live birth, and males with aberrant markers may still be able to conceive.
WIDER IMPLICATIONS
This review substantiates the negative effect of HL treatment on gonadal function and therefore young HL survivors should be counseled regarding their future reproductive life, and fertility preservation should be considered. The current level of evidence is insufficient and additional trials on the effects of HL and (current) treatment regimens on reproductive function are needed. In this review, we make a recommendation on reproductive markers that could be assessed and the timing of (repeated) measurements.
Keywords: Hodgkin lymphoma, childhood cancer, (future) fertility, reproductive ability, adverse effects, gonadotoxicity, azoospermia, premature ovarian insufficiency
Graphical Abstract
Graphical Abstract.
Effects of treatment at <25 years of age on reproductive markers in survivors of Hodgkin lymphoma (HL).
Introduction
Hodgkin lymphoma (HL) is a hematological malignancy, characterized by a bimodal age distribution at diagnosis with incidence peaks in young adulthood (15–35 years old) and after the age of 50 years. Over the past decades, HL treatment regimens have evolved gradually. Survival rates first improved when MOPP (mechlorethamine, oncovin (vincristine), procarbazine, and prednisolone) and ABVD (adriamycin, bleomycin, vinblastine, and dacarbazine) were introduced as a multi-agent strategies, supplemented by conventional radiotherapy. Subsequent follow-up data suggested high gonadotoxicity, and since then trials aim to replace gonadotoxic alkylating agents and reduce radiation doses and fields, without comprising effectivity rates. Current HL treatment is response- and risk adapted, and adult- and childhood HL patients are treated with distinct treatment protocols. The 5-year survival rate nowadays exceeds 90% in pediatric HL patients (Borchmann et al., 2012; Mauz-Körholz et al., 2022).
Still, administered chemotherapy and radiotherapy often cause toxicity with long lasting adverse effects, including increased risks of cardiovascular diseases and second malignancies that cause a substantially reduced life expectancy for HL survivors, when compared to the general population (van Leeuwen et al., 2000; Ng et al., 2002; Aleman et al., 2007; Myrehaug et al., 2008; Andersson et al., 2011; Swerdlow et al., 2011; Schaapveld et al., 2015; van Nimwegen et al., 2015; van Leeuwen and Ng, 2017; de Vries et al., 2021). Therapeutic effects that impede reproductive function are of great concern given their impact on future health and quality of life, particularly in young cancer survivors for whom parenthood is often among the most important future life goals (Carter et al., 2010; Canada and Schover, 2012; Benedict et al., 2016, 2018; Ellis et al., 2016; Johnson et al., 2018).
Anticancer treatment can damage the female gonads by inducing apoptosis of the primordial follicles, changes in vascularization of the ovaries, and cortical fibrosis, leading to atrophy (Meirow, 2000; Oktem and Oktay, 2007; Sonigo et al., 2019). Patients may experience (temporal) menstrual irregularities, climacteric symptoms, and hormonal disturbances but gonadal dysfunction may also lead to sub- or infertility because of ovarian failure and/or a shorter fertile lifespan because of the reduced number of primordial follicles (Spears et al., 2019).
In males, the deleterious effects of chemotherapy and radiotherapy primarily affect the germinal epithelium. Within the seminiferous epithelium of the testis, spermatogonial stem cells form the basis of spermatogenesis by a process of division and differentiation, which is initiated at the onset of puberty. Cytotoxic treatment and especially radiation may damage and deplete germ cells and differentiating spermatogonia, leading to a (temporary) cessation of spermatogenesis and diminished sperm quality (Brougham et al., 2003; Meistrich, 2013; Goossens et al., 2020). Potential recovery depends on survival of the spermatogonial stem cells, and if the entire pool is depleted, males are infertile (Goossens et al., 2020). Testosterone-producing Leydig cells are thought to be more resistant, but after high cumulative doses of anticancer drugs, Leydig cell dysfunction may also become apparent and hypoandrogenism may (although very uncommon) present as fatigue, impaired libido, premature osteoporosis, and metabolic disorders (Romerius et al., 2009; Kenney et al., 2012; Brignardello et al., 2016).
Problems with fertility do not become apparent until puberty or years thereafter. While the absence of puberty indicates infertility, the occurrence of pubertal development does not guarantee adequate gonadal function. Survivors may still have to deal with impaired reproductive ability and females may face a shortened fertile lifespan. Arguably, the ultimate proof of fertility is a live birth after pregnancy, but several markers are available to predict reproductive ability in (young) females and males as well.
In females, premature depletion of ovarian function is referred to as premature ovarian insufficiency (POI). POI is diagnosed in women with evidence of hypergonadotropic-hypogonadism in the setting of amenorrhea before the age of 40 years (Webber et al., 2016). Deterioration of ovarian function, meaning a diminished ovarian reserve, can be measured by ovarian reserve tests. The antral follicle count (AFC) and measurement of circulating anti-Müllerian hormone (AMH) are commonly used markers of ovarian reserve (Hansen et al., 2011; Anderson and Su, 2020). AFC, the number of visible antral follicles on a (transvaginal) ultrasound, reflects the size of the primordial follicle pool. AMH is produced in the granulosa cells of the ovary by the primary, secondary, pre-antral and antral follicles up to 8 mm in diameter, and these developing follicles also reflect the size of the remaining primordial follicle pool (Depmann et al., 2018).
In females, AMH levels are low or almost undetectable at birth and increase during childhood, with a peak during late puberty (Kelsey et al., 2011). In general, AMH levels rise up to the age of 25 years old and then start to decline. AFC and AMH naturally decrease during reproductive aging. Prior to disturbances in other hormones (e.g. FSH, estradiol, and inhibin B) or cycle length changes, a decrease in AMH can be seen, and AMH is predictive for timing of menopause (Broer et al., 2014).
AMH was introduced as a novel marker for reproductive lifespan in the 2010s (Rosen et al., 2012; Broer et al., 2014). Traditionally, studies referred to regularity of the menstrual cycle (with ‘regular’ defined as cycles within the range of 20–45 days) or increased serum FSH concentration (>10 IU/l) to evaluate gonadal function. However, FSH levels are strongly cycle dependent and values do not reflect ovarian reserve, nor can they individually reflect ovarian function (Broer et al., 2014).
Semen analysis is the gold standard to assess male fertility. Semen can be qualified and quantified, and lower limit reference values for concentration, motility, and morphology are published by the World Health Organization (Cooper et al., 2010). However, semen analysis can only be performed after puberty. The body of literature on (pre-pubertal) spermatogenial numbers in testicular tissue is growing (Masliukaite et al., 2016; Stukenborg et al., 2018a), but assessment of such counts in cancer patients is still in the context of (experimental) testicular biopsies and estimation of reproductive function in pre-pubertal boys depends on serum markers.
Testicular damage affects the levels of circulating hormones (Franchimont et al., 1972; Dhabhar et al., 1993; Mackie et al., 1996). Inhibin B is the active form of inhibin, a glycoprotein hormone produced by the testicular Sertoli cells. Inhibin B regulates FSH secretion, which further affects the hypothalamic–pituitary–gonadal axis. It is believed that low inhibin B reflects a decreased function of the seminiferous tubules (Robertson et al., 1988; Dhabhar et al., 1993; Jensen et al., 1997; Klingmüller and Haidl, 1997; Brugo-Olmedo et al., 2001). Generally, FSH serum levels >10 IU/l and/or inhibin B levels of <100 ng/l are considered to indicate damage to spermatogenesis in males (Lähteenmäki et al., 1999; Kelsey et al., 2017). In contrast, the measurement of serum LH and testosterone as markers for fertility seems to be of limited value, although both hormones reflect Leydig cell function and testosterone plays a vital role in pubertal development and spermatogenesis (Smith and Walker, 2014; Keskin et al., 2015).
The number of childhood and adolescent HL survivors grows, based on stable incidences combined with high survival rates. Over the recent decades, treatment protocols have rapidly evolved and awareness of the gonadotoxicity of anticancer treatment has increased. Fertility preservation programmes are initiated in effort to preserve fertility of (young) cancer patients receiving high-risk treatments (Mulder et al., 2021a,b; van der Perk et al., 2021).
Although (future) fertility after treatment for HL is discussed in an increasing number of studies, as far as we know no reviews of the literature are available. The aim of the current review was to summarize existing literature on different aspects of gonadal function and fertility in both female and male childhood, adolescent, and young adult survivors of HL. We aimed to: identify patient and treatment characteristics associated with an increased risk of impaired reproductive ability; provide personalized, risk-based information and counseling for patients on their future fertility; and identify patients that could benefit from fertility preservation at diagnosis, before start of treatment.
Methods
Search strategy
A comprehensive search was performed in the bibliographic databases PubMed and Embase.com, from inception to 2 December 2021, in collaboration with a medical librarian (L.J.S.). Search terms included controlled terms (MeSH in PubMed and Emtree in Embase) as well as free text terms. Search terms expressing ‘Hodgkin lymphoma’ were used in combination with search terms comprising ‘treatment’ and ‘fertility’. The search was performed without date or language restrictions. A search filter was used to exclude animal studies, and case reports and congress abstracts were also excluded. The full search strategies for all databases can be found in Supplementary Data File S1. Duplicate articles were excluded (by L.J.S.) using Endnote X20.0.1 (Clarivatetm), following the Amsterdam Efficient Deduplication-method and the Bramer-method (Bramer et al., 2016; Otten et al., 2019).
Study selection and data extraction
At least two reviewers (from M.C.F.P., E.v.D.-d.B., M.A.V., S.M., and K.C.E.D.) independently screened all potentially relevant titles and abstracts for eligibility using Covidence (Covidence systematic review software, 2022). Inconsistencies were discussed with a third (independent) reviewer.
Studies were included if they met the following criteria:
study design; cohort studies, case-control studies, or randomized controlled trials (RCTs); either prospective, retrospective, or cross-sectional,
study population; male and/or female HL patients/survivors OR childhood, young adult or adolescent cancer patients/survivors, aged <25 years at diagnosis, outcomes; evaluating fertility in a broad sense, reporting at least one of the following outcome measures;
Males: semen analysis, serum markers of gonadal function (i.e. elevated FSH, low inhibin B, FSH/inhibin B ratio, elevated LH, low testosterone), pregnancy, or live birth rates;
Females: markers of ovarian reserve (i.e. low AMH, low AFC), markers of ovarian failure (i.e. POI, acute ovarian failure (AOF), menstrual cycle characteristics (i.e. regular cycle, amenorrhea, oligomenorrhea), elevated serum FSH, and pregnancy or live birth rates).
Studies were excluded if they met any of the following the criteria:
wrong publication type, i.e. case reports, case series, letters, animal studies, reviews, conference abstracts, guidelines,
no full text available, or not available in English, German, French or Dutch language,
did not report separate results for HL patients or there were <10 HL patients in total cohort for which results are reported separately,
>25% of the study population was aged 25 years or older at diagnosis and there were no separate outcomes reported for age <25 years,
age at diagnosis for HL unknown,
co-treatment with GnRH analogues, and
overlapping cohort (only the largest study was included).
Data synthesis
Data on trial design and setting, study population, age at cancer diagnosis and time of study, follow-up period, and treatment characteristics were extracted by two reviewers (M.C.F.P. and K.C.E.D.) working independently, using a previously designed and piloted data extraction form (not shown).
In females, markers of ovarian reserve (AMH, AFC; pre- or post-treatment) and reports on POI were included as primary outcomes. All reports on premature ovarian failure or insufficiency before the age of 40 years, sometimes within papers referred to as non-surgical premature menopause, were defined as POI in this review. AOF, elevated serum (basal) FSH (pre- or post-treatment), menstrual cycle characteristics (i.e. regular cycle, amenorrhea, oligomenorrhea), and pregnancy or live birth rates (defined as prevalence or absolute number of pregnancies or live births, with or without ART) were included as secondary outcomes.
In males, primary outcomes comprised semen analysis pre- or post-treatment (i.e. semen volume, sperm concentration, total sperm count in ejaculate, classification azoospermia/(severe) oligospermia, normospermia, percentage progressive motility, percentage normal morphology, percentage vitality) and several serum markers of gonadal function (i.e. (basal) FSH, inhibin B, FSH/inhibin B ratio; pre- or post-treatment). Additional serum markers (i.e. (basal) LH, testosterone) and pregnancy and live birth rates were included as secondary outcomes.
In studies that did not report study characteristics for the eligible HL-subgroup specifically (i.e. aged <25 years at diagnosis), data were extracted for the entire cohort (e.g. in case of mixed cancer cohorts or studies that included older HL patients). The cutoff values used for increased or decreased markers were extracted if available. Commonly used definitions of markers of gonadal function and a list of abbreviations are included in Supplementary Table SI. If available, reports on recovery of gonadal function and sequential analysis of markers in both males and females were extracted as well.
Owing to the profound heterogeneity in included studies, it was not possible to perform additional analyses. The present review is a narrative review and no structured critical appraisal was performed.
Results
Search results
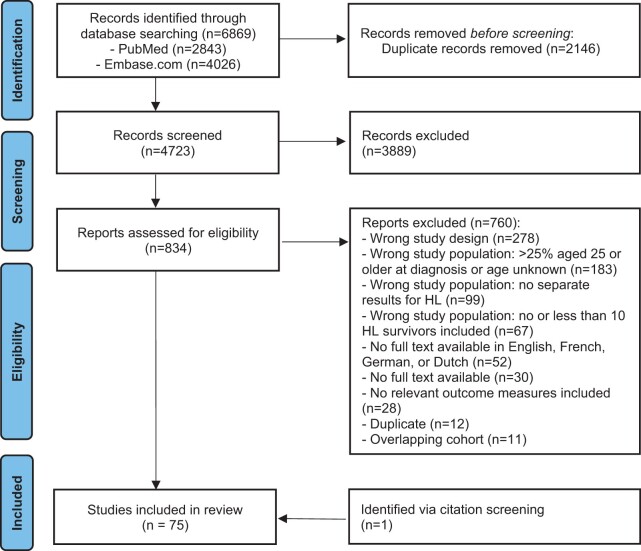
The literature search generated a total of 6869 references: 2843 in PubMed and 4026 in Embase.com. After removing duplicates of references that were selected from more than one database, 4723 references remained. Records were screened and 834 full-text articles were assessed for eligibility. Of these, 74 articles were eligible for inclusion in this review. One additional study was added via citation screening. The flow chart of the search and selection process is presented in Fig. 1.
Figure 1.
Flow chart of literature database search results and exclusion of studies. HL, Hodgkin lymphoma. Diagram adapted from Page et al. (2021).
Female HL survivors
Overall, there were 41 studies included that reported on fertility related outcomes in 5057 female childhood or adolescent HL survivors (Supplementary Table SII). Seventeen studies were cross-sectional, 10 were executed prospectively, and 14 were executed retrospectively. Most studies originated from the USA (n = 17), the UK (n = 5), the Netherlands (n = 5), and Italy (n = 4). The remaining 10 trials were initiated from Canada, Portugal, France, Germany, Poland, Slovenia, Sweden, Copenhagen, Tunisia, and Turkey. Studied populations often only comprised patients with HL, but in 11 papers the cohort included multiple childhood cancer diagnoses (mixed childhood cancer: MCC); in those papers, the results on gonadal markers were extracted for the subgroup of HL patients aged below 25 years. Patients were treated between 1940 and 2018. Administered chemotherapy-drugs as well as radiotherapy (dose and site) varied widely, and the included (abbreviated) HL-treatment protocols are listed in Supplementary Table SI. Seven studies mentioned that (some of) the included females had oophoropexy before radiotherapy. Median duration of follow-up or time until execution of a (cross-sectional) study ranged from a few years post-treatment up to 48 years. Results were compared to a control or reference group (healthy females or siblings) in 10 studies.
Anti-Müllerian hormone
Six of the included studies reported on AMH. Low AMH serum levels in childhood and adolescent HL survivors were reported in three articles (91 patients) (van Beek et al., 2007b; Charpentier et al., 2014; van Dorp et al., 2014). Of these, one article evaluated pre-treatment AMH serum levels in females with median age 6.6 years (range 0–17.4) and reported on nine (28%) females with ‘low’ AMH values, defined as <p10 percentile of healthy controls (van Dorp et al., 2014). The other two studies evaluated AMH serum levels after a follow-up period of median ±11 years (range 1.4–25.1) after treatment. The studies compared AMH serum levels in female HL survivors aged 23–25 years old (range 18.2–40.4) to AMH values in healthy controls and found 55–59% of the study population to have low AMH (<14 pmol/l = 6.2 ng/ml or <95% CI of healthy controls), especially in those survivors treated with chemotherapy protocols including procarbazine (van Beek et al., 2007b). In both studies, none of the patients had received pelvic radiotherapy. Three additional papers (89 patients) reported a significant decrease in AMH before and after treatment for HL (Krawczuk-Rybak et al., 2013a; Berjeb et al., 2020; Policiano et al., 2020). A summary of results is presented in Table I and Supplementary Fig. S1.
Table I.
Anti-Müllerian hormone and antral follicle count in childhood or adolescent females diagnosed with Hodgkin lymphoma.
| Study | N patients | Age at diagnosis | Age at time of study | Follow-up | Treatment | Outcome |
|---|---|---|---|---|---|---|
| (years) | (years) | (years) | ||||
| Pre-treatment | ||||||
| van Dorp et al., 2014 | 32 | 6.6 (0–17.4) | NA | NA | NA | Pre-treatment, n = 9/32 (28%) patients with low AMH (i.e. <p10 percentile of healthy controls) |
| Post-treatment | ||||||
| Charpentier et al., 2014 | 29 | 11.9 (1.8–17.3) | 23.3 (18.2–34.2) | 11.5 (1.4–25.1)* | Chemo: n.s. | n = 16/29 (55%) females with low AMH (i.e. <14 pmol/l) |
| No pelvic RT | ||||||
| van Beek et al., 2007b | 30 | 14.0 (5.0–17.2) | 25 (19.2–40.4) | 11.6 (5.7–24.5) | Chemo: ABVD, EBVD, MOPP | n = 10/17 (59%) patients with low AMH (i.e. <95% CI of healthy controls) |
| No pelvic RT |
|
|||||
| Decrease in AMH or AFC pre-/post -treatment | ||||||
| Berjeb et al., 2020 | 32 | 21.6 ± 4.4 | n.s. | (1.3–1.5) | Chemo: BEACOPP, DHAOX, ABVD | Significant decrease in AMH before and after chemotherapy. 2.30 ± 1.96 and 1.54±1.96 ng/ml; P = 0.002 |
| No pelvic RT | In n = 7/32 (22%) women, AMH values were higher after chemotherapy, when compared to AMH values pre-treatment | |||||
| Krawczuk-Rybak et al., 2013a | 13 | 14 ± 3.45 | 20.1 ± 3.5 | (6–10) | Chemo: MVPP + BDOPA | Significant decrease in AMH before and after treatment 2.96 ng/ml ± 2.05 and 1.26 ng/ml ± 0.84, P = 0.001 |
| Pelvic RT: n = 8/13 (62%), 15 Gy | ||||||
| Policiano et al., 2020 | 44 | 22.6 (IQR 18.8–26.4) | n.s. | 2.8 | Chemo: ABVD | Decrease in AMH before and after treatment 2.2 ng/ml (IQR 1.4–4.4 ng/ml) and 1.3 ng/ml (IQR 0.9–2.5 ng/ml), P not given |
| No pelvic RT | Decrease in AFC before and after treatment 19 (IQR 14–23), and 10 (IQR 7–15), P not given | |||||
| Patients with AFC <6 or AMH <0.84 ng/ml at baseline were excluded | ||||||
Follow-up period defined as years off treatment.
ABVD, Adriamycin (Doxorubicin), Bleomycin, Vinblastine, Dacarbazine; AMH, anti-Müllerian hormone; AFC, antral follicle count; BDOPA, Bleomycin, Dacarbazine, Vincristine (Oncovin), Adriamycin, Prednisone; BEACOPP, Bleomycin, Etoposide, Adiamycin (Doxorubicin), Cyclophosphamide, Vincristine (Oncovin), Procarbazine, Prednisone; Chemo, chemotherapy; DHAOX, Dexamethasone, high-dose Cytarabine, and Oxaliplatin; EBVD, Epirubicin, Bleomycin, Vinblastine, Dacarbazine; Gy, Gray; IQR, interquartile range; MOPP, Mechlorethamine, Vincristine (Oncovin), Procarbazine, Prednisone; MVPP, Mechlorethamine, Vinblastine, Procarbazine, Prednisone; NA, not applicable; n.s., not specified; N, number; RT, radiotherapy.
Age at diagnosis and time of study reported in median years (range) or mean ± SD.
The number of patients that received pelvic radiotherapy as (part of their) cancer treatment is reported. If mentioned in the article (pelvic) radiation dosage is specified.
Antral follicle count
Only the paper by Policiano et al. (2020) reported AFC in HL survivors, and they mentioned a decrease in AFC post-treatment in comparison to values pre-treatment after a follow-up of ±2.8 years (44 patients, pre-treatment AFC 19 (interquartile range (IQR) 14–23), and post-treatment 10 (IQR 7–15), P value not given). Patients with an AFC below 6 or serum AMH below 0.84 ng/ml at baseline were excluded from this study.
Premature ovarian insufficiency and ovarian failure
The number of female survivors with POI was reported in seven of the included studies (1516 patients) (Table II and Supplementary Fig. S1) (Green et al., 1981; Sklar et al., 2006; Zaletel et al., 2010; van der Kaaij et al., 2012b; Swerdlow et al., 2014; Levine et al., 2018; Felicetti et al., 2020). The percentage of female HL survivors with signs of POI ranged from 6% to 34% (median 9%) and was especially high in patients receiving radiotherapy and BEAM (i.e. high-dose chemotherapy before autologous hemopoietic stem cell transplantation (HSCT)) (Sklar et al., 2006; Swerdlow et al., 2014). Long-term follow-up data were lacking. Only in one study the median age of included HL survivors at time of the study exceeded 40 years, while in two other studies the oldest patients at time of follow-up assessment were 24 and 29 years old.
Table II.
Premature ovarian insufficiency and (acute) ovarian failure in childhood or adolescent females diagnosed with Hodgkin lymphoma.
| Study | N patients | Age at diagnosis (years) | Age at time of study (years) | Follow-up (years) | Treatment | Outcome |
|---|---|---|---|---|---|---|
| Premature ovarian insufficiency (POI) | ||||||
| Felicetti et al., 2020 | 33 | <18 | 24.7 (21.5–28.3) | n.s. |
|
n = 2/33 (6%) patients with POI, defined as amenorrhea and consistently low 17beta-estradiol levels and elevated gonadotropin levels |
| Green et al., 1981 | 7 | 14.6 (11.2–16.8 | 18.5 (15.4–24.2) | 2.6 (1.3–5.6) |
|
n = 2/7 (29%) patients with gonadotropin patterns consistent with ovarian failure (not defined) |
| Levine et al., 2018 | 348 | 6 (0–20) | 35 (18–58) | n.s. |
|
n = 56/348 (16%) patients with non-surgical premature menopause, defined as sustained menses cessation occurring for 6 months beginning 5 years after the cancer diagnosis but before age 40 years that was not due to pregnancy, surgery, or medications |
| Sklar et al., 2006 | 404 | 7 (0–20) | 9 (18–50) | n.s. |
|
n = 38/404 (9%) with NSPM, defined as not experienced a spontaneous menses for at least 6 months. Other causes (e.g. pregnancy; use of agents such as injectable progesterone and gonadotropin-releasing hormone analogs) had been excluded.
|
| Swerdlow et al., 2014 | 508 | (0–25) | n.s. | 17.8 (0.3–48.4) |
|
n = 173/508 (34%) with menopause <age 40 years.
|
| van der Kaaij et al., 2012b | 192 | (15–24) | 49 (25–76) | 15 (5–45) |
|
|
| Zaletel et al., 2010 | 24 | 13 (3–16) | 21 (13–34) | 10 (4–27) |
|
|
| Ovarian failure (undefined) and acute ovarian failure (AOF) | ||||||
| Chemaitilly et al., 2006 | 553 | (0–20) | n.s. | n.s. |
|
n = 66/553 (12%) survivors who self-reported never menstruating or reported that they had ceased having spontaneous menses within 5 years after cancer diagnosis (in article referred to as: AOF, acute ovarian failure) |
| Hudson et al., 1993 | 36 | 14.6 (4.2–20) | n.s. | 4 (1–9.7) |
|
n = 6/36 (17%) with ovarian failure (not defined)
|
| Mackie et al., 1996 | 32 | 13.0 (9.0–15.2) | n.s. | 4.3 (1.9–11.5)* |
|
n = 10/32 (31%) with ‘symptomatic’ ovarian failure (not defined) |
| Ortin et al., 1990 | 86 | 13 (12–15) | n.s. | 9 (up to 26) |
|
n = 11/86 (13%) with ovarian failure (not defined) |
Follow-up period defined as years off treatment.
Reported number only available within total cohort (i.e. not specified for HL diagnosis or age-subgroup).
ABV, Adriamycin (Doxorubicin), Bleomycin, Vinblastine; ABVD, Adriamycin (Doxorubicin), Bleomycin, Vinblastine, Dacarbazine; AOF, acute ovarian failure; BEACOPP, Bleomycin, Etoposide, Adiamycin (Doxorubicin), Cyclophosphamide, Vincristine (Oncovin), Procarbazine, Prednisone; BEAM, BCNU (bis-chloroethylnitrosourea), Etoposide, Cytarabine, Melphalan; Chemo, chemotherapy; CHlVPP, Chlorambucil, Vinblastine, Procarbazine, Prednisone; COP-ABVD; Cyclophosphamide, Vincristine (Oncovin), Procarbazine, Adriamycin (Doxorubicin), Bleomycin, Vinblastine, Dacarbazine; COPP, Cyclophosphamide, Vincristine (Oncovin), Procarbazine, Prednisone; CVPP, Cyclophosphamide, Vinblastine, Procarbazine, Prednisone; EBVP, Epirubicin, Bleomycin, Vinblastine, Prednisone; Gy, gray; HL, Hodgkin lymphoma; LOPP, Chlorambucil, Vincristine (Oncovin), Procarbazine, Prednisone; MOPP, Mechlorethamine, Vincristine (Oncovin), Procarbazine, Prednisone; MVPP, Mechlorethamine, Vinblastine, Procarbazine, Prednisone; NA, not applicable; n.s., not specified; N, number; OPPA, Vincristine (Oncovin), Prednisone, Procarbazine, Adriamycin (Doxorubicin); PAVe, Procarbazine, Alkeran, Velban; POI, premature ovarian insufficiency; RT, radiotherapy; VBM, Velban, Bleomycin, Methotrexate.
Age at diagnosis and time of study reported in median years (range) or mean ± SD.
The number of patients that received pelvic radiotherapy as (part of their) cancer treatment is reported. If mentioned, (pelvic) radiation dosage is included. If oophoropexy is mentioned in the paper, number of patients is specified.
Three additional studies (154 patients) reported on ovarian failure, without presenting an applied definition or classification (Ortin et al., 1990; Hudson et al., 1993; Mackie et al., 1996). It could not be assumed that the term ‘ovarian failure’ was exclusively used to refer to POI and, therefore, results were presented separately. Nevertheless, reported incidences were comparable to reports on the incidence of POI (12–31% ovarian failure and 4–34% POI, respectively). All patients with ovarian failure in the study by Mackie et al. (1996) had received pelvic irradiation.
One study reported on AOF (553 patients) and stated that 12% of the included females reported never menstruating or ceased having spontaneous menses within 5 years after cancer diagnosis (Chemaitilly et al., 2006). The occurrence of AOF was associated with age at cancer diagnosis >12 years old. Nevertheless, this study was a cross-sectional study, and potential cycle recovery was not evaluated. Gonadotrophin patterns were not assessed either.
Menstrual cycle
Information on menstrual cycle of HL survivors was provided in 18 included studies (387 patients), see Supplementary Table SIII and Supplementary Fig. S1. Most studies reported that the majority of HL survivors developed or maintained normal and regular menstrual cycles (median percentage of HL survivors with regular cycle 100%, range 79–100%, nine studies; 168 patients) (Horning et al., 1981; Whitehead et al., 1982; Andrieu and Ochoa-Molina, 1983; Perrone et al., 1989; Hudson et al., 1993; Gözdasoglu et al., 1995; Madsen et al., 1995; Donaldson et al., 2007; Zaletel et al., 2010). Hudson et al. (1993) mentioned that three patients developed amenorrhea during therapy but resumed spontaneous menses 2–4 years after completion of therapy. Other articles reported on rates ranging from 0% to 71% (median 4%) of patients experiencing amenorrhea post-treatment (Wilimas et al., 1980; Green et al., 1981; Koziner et al., 1986; Green and Hall, 1988; Madsen et al., 1995; Mackie et al., 1996; Brusamolino et al., 2000; Zaletel et al., 2010).
FSH
Eight studies (155 patients) included elevated FSH serum levels in female HL survivors at follow-up as an outcome measurement (Green et al., 1981; Perrone et al., 1989; Mackie et al., 1996; Papadakis et al., 1999; van Beek et al., 2007b; Zaletel et al., 2010; Krawczuk-Rybak et al., 2013a; Salih et al., 2015). The percentage of patients with elevated FSH after a median follow-up of ±6 years (range 0–30) varied from 17% to 100%, median 53% (values above cutoff value 8–30 IU/l, or high in comparison to healthy controls or survivors of other types of childhood cancer). Elevated FSH values were observed more frequently in patients treated with procarbazine/MOPP and those treated with abdominal/pelvic radiotherapy (Green et al., 1981; Papadakis et al., 1999; van Beek et al., 2007b; Zaletel et al., 2010). Results on serial sampling were available in 14 patients from one study (Papadakis et al., 1999). All patients were treated with procarbazine and cyclophosphamide, and none had received pelvic radiotherapy. Seven patients had elevated FSH values at time of first evaluation, in three patients the values remained abnormal up to 18 years post-diagnosis and in the other four patients FSH concentrations normalized over time (at 2, 3, 4, and 9 years post-diagnosis). Serum FSH levels of the remaining seven patients remained normal throughout the entire assessment period. Results are summarized in Supplementary Table SIV and Supplementary Fig. S1.
Pregnancy/live birth
There were 19 studies that reported at least one pregnancy or live birth in 1262 out of the 2388 (53%) included female HL survivors (Supplementary Table SV). Pivetta et al. (2011) reported a significantly lower ratio of observed (O) to expected (E) number of liveborn children for 110 married/cohabitant female HL survivors, in comparison to general population, ratio 0.53; 95% CI 0.42–0.64. However, the ratio was not significantly different when all surviving women of the studied population were included (i.e. married, never married and unknown marital status, n = 271, ratio 0.88; 95% CI 0.71–1.08) (Pivetta et al., 2011). Brämswig et al. (2015) stated that the proportion of women HL survivors having children was comparable to the German general population, except for the 66 HL survivors aged 40–44 years old, in whom the proportion of mothers was significantly lower (61% of 66 HL survivors versus 78% of 2 847 000 women, P = 0.001) (Brämswig et al., 2015): the study observed no statistically significant difference in parenthood among females aged 45–49 years old. Two additional studies specifically mentioned they only included female HL survivors who attempted to become pregnant. In the study by van der Kaaij et al. (2012a), 176 out of the 218 (81%) females gave birth to at least one child after treatment for HL. Horning et al. (1981) reported 28 pregnancies in 20 of the 26 (77%) included women who attempted to become pregnant, with a median time to pregnancy of 42 months (3–100 months). Only three studies mentioned whether females used ART to become pregnant. The number of pregnancies achieved via IUI or IVF were n = 4/176, n = 2/17 and n = 1/10 (Papadakis et al., 1999; van Beek et al., 2007b; van der Kaaij et al., 2012a, respectively). One paper assessed parenthood according to several treatment- and diagnosis related factors and stated that pelvic radiation appeared to have a substantial effect on the hazard ratio (HR) for parenthood (HR 0.66 95% CI 0.48–0.90, P = 0.006) (Brämswig et al., 2015).
Male HL survivors
Included studies
A total of 52 studies, reporting on 1903 males diagnosed with HL before the age of 25 years, were included in this review. An overview of included studies is provided in Supplementary Table SVI. Studies were cross-sectional (n = 26) or had a retrospective (n = 20) or prospective (n = 6) design, there were no RCTs included in this review. Trials originated from 18 different countries, including the USA (n = 13), the UK (n = 7), Italy (n = 6), the Netherlands (n = 5), Germany (n = 5), France (n = 3), Sweden (n = 2), and others (Brazil, Uganda, India, Israel, Iran, Turkey, Slovenia, Czech Republic, Denmark, Poland, and Switzerland—all n = 1). Of the 52 studies, 32 included only HL patients, 1 non-Hodgkin lymphoma (NHL) and HL patients, 16 MCC patients, 2 included childhood hematological malignancy patients, and 1 included a mixed cancer cohort with patients of all ages. In all, 12 studies used a control group or reference cohort in their analyses. Patients were treated with varying HL treatment protocols, between 1940 and 2017.
Semen analysis
A large number of the studies reported on sperm quality pre-treatment (12 studies, 324 patients) and/or post-treatment (30 studies, 809 patients). Most studies reported on (quantitative) sperm quality by defining sperm concentration in HL patients as ‘normospermic’, ‘oligospermic’, or ‘azoospermic’ (i.e. sperm concentrations of >15 million/ml, 5–15 million/ml, and 0 sperm/ml, respectively). Some articles reported on qualitative sperm deterioration by mentioning asthenezospermia (abnormal motility) and theratospermia (abnormal morphology). Results are summarized in Table III and Supplementary Fig. S2.
Table III.
Semen analysis in childhood or adolescent males diagnosed with Hodgkin lymphoma.
| Study | N patients | Age at diagnosis (years) | Age at time of study (years) | Follow-up (years) | Treatment | Outcome |
|---|---|---|---|---|---|---|
| Pre-treatment | ||||||
| Adam et al., 2020 | 24 | 17.2 ± 1.7 | NA | NA | NA |
|
| Bahadur et al., 2002 | 36 | 16.44 | NA | NA | NA |
|
| DiNofia et al., 2017 | 54 | 16.9 ± 2.6 | NA | NA | NA | n = 8/54 (15%) azoospermia |
| Ginsberg et al., 2008 | 13 | 16.1 ± 0.5 | NA | NA | NA | n = 0/13 (0%) azoospermia. n = 5/13 (39%) oligospermia |
| Hagenäs et al., 2010 | 19 | 16.4 (12.7–17.9) | NA | NA | NA |
|
| Heikens et al., 1996 | 19 | 11.0 (5–15) | NA | NA | NA | Sperm count 3.4 ± 5.5 |
| Keene et al., 2012 | 38 | 16.2 ± 1.2 | NA | NA | NA |
|
| Krawczuk-Rybak et al., 2012 | 10 | 15–18 | NA | NA | NA |
|
| Laddaga et al., 2022 | 11 | (17–24) | NA | NA | NA | n = 8/11 (73%) with dyspermia (6× oligospermia, 5× azoospermia, 1× theratospermia). |
| Menon et al., 2009 | 30 | 17.8 ± 0.1 (13–20) | NA | NA | NA |
|
| Paoli et al., 2016 | 50 | 15.8 ± 1.1 | NA | NA | NA |
|
| van Casteren et al., 2008 | 20 | 16.3 (SEM 0.3) | NA | NA | NA |
|
| Post-treatment | ||||||
| Anselmo et al. 1990 | 20 | 20 (16–24) | n.s. | (0.5–1) |
|
|
| Aubier et al., 1989 | 10 | 10 (8–15) | n.s. | 9 (1–20) |
|
n = 7/10 (70%) azoospermia |
| Ben Arush et al., 2000 | 12 | 13.7 (2.1–16.4) | 22 (14.8–29.3) | 9.8 (4–19) |
|
|
| Bordallo et al., 2004 | 21 | 10 (6–19) | 18 (17–23) | (3–11) |
|
|
| Brämswig et al., 1990 | 75 | 12.44 ± 2.1 | 17.24 ± 2.19 | 4.3 ± 1.9 |
|
n = 4/4 (100%) azoospermia in patients with elevated FSH |
| da Cunha et al., 1984 | 12 | 20 (15–24) | n.s. | 4 (1.2–10.6) |
|
|
| Dhabhar et al., 1993 | 26 | 12 (4–15) | 17 (15–23) | 6.1 (2.3–11) |
|
n = 18/18 (100%) azoospermia |
| Donaldson et al., 2007 | 75 | 13.3 (3.6–21) | n.s. | 9.6 (1.7–15.0) |
|
n = 1/1 (100%) azoospermia |
| Gözdasoglu et al., 1995 | 10 | n.s. | 18 (11–29) | (5–24) |
|
n = 4/4 (100%) azoospermia in patients with elevated FSH |
| Green and Hall, 1988 | 48 | 14.9 (5.1–19.9) | (18.1–42.3) | n.s. |
|
n = 8/8 (100%) azoospermia |
| Heikens et al., 1996 | 19 | 11.0 (5–15) | 19 (16–27) | 14 (13–20) |
|
|
| Hobbie et al., 2005 | 11 | 13.2 (6–19) | 21 (18–31) | 6.5 (1.5–21) |
|
|
| Jaffe et al., 1988 | 13 | 11 (6–15) | 22 (20–27) | 12 (5–15) |
|
|
| Koziner et al., 1986 | 11 | 21 (9–29) | n.s. | 3 (0.8–2.4) |
|
n = 8/11 (73%) azoospermia |
| Kruseová et al., 2021 | 81 | 13.7 (0.1–19.1) | 23.6 (14.9–40.3) | 11.6 (5.1–32) | n = 60/81 (74%) with spermatogenesis damage (i.e. azoospermia, oligozoospermia or asthenozoospermia) | |
| Laddaga et al., 2022 | 11 | (17–24) | n.s. | 8.6 (4.4–14) |
|
n = 4/11 (36%) with dyspermia (i.e. n = 3 oligospermia, n = 4 azoospermia, n = 1 theratospermia) |
| Mackie et al., 1996 | 46 | 12.2 (8.2–15.3) | n.s. | 6 (2.5–11.1)* |
|
n = 7/7 (100%) azoospermia |
| Müller et al., 1996 | 13 | 14 (3–17) | 21 (19–34) | 8 (1–18) |
|
n = 6/6 (100%) azoospermia. |
| Ortin et al., 1990 | 20 | 13 (12–15) | n.s. | 9 (up to 26) |
|
|
| Papadakis et al., 1999 | 36 | 13.0 (2.4–22.6) | 22.3 (15.1–32.5) | 6.8 (2.0–19.3)* |
|
n = 2/2 (100%) azoospermia |
| Perrone et al., 1989 | 7 | 9.0 (2.4–12.0) | 10.8 (3.0–18.0) | 1.2 (0.2–6.5) |
|
n = 3/3 (100%) azoospermia |
| Rafsanjani et al., 2007 | 33 | 9 (5–15) | 19 (17–29) | 7 (2–20)* |
|
|
| Relander et al., 2000 | 11 | 13 (6–16) | n.s. | 11.4 (6.3–22.2) |
|
|
| Romerius et al., 2010 | 19 | 10 (0.1–17) | 29 (20–46) | >4* |
|
n = 10/19 (53%) azoospermia |
| Shafford et al., 1993 | 40 | n.s. | >16 | >6* |
|
|
| van Beek et al., 2007a | 56 | 11.4 (3.7–15.9) | 27.0 (17.7–42.6) | 15.5 (5.6–30.2) |
|
|
| van Casteren et al., 2008 | 20 | 16.3 (SEM 0.3) | n.s. | 3.4 (0.8–14.0) |
|
n = 2/4 (50%) azoospermia. |
| van den Berg et al., 2004 | 33 | 11.8 (3.8–17.2) | n.s. | 11.3 (0.5–24) |
|
|
| Whitehead et al., 1982 | 15 | 11.2 (4.8–14.8) | n.s. | 3.3 (0,7–8)* |
|
n = 6/6 (100%) azoospermia |
| Zaletel et al., 2010 | 40 | 13 (3–16) | 21 (13–34) | 10 (4–27) |
|
n = 6/6 (100%) azoospermia in males with primary hypogonadism (defined as basal serum FSH and/or LH level above the normal upper limit and exaggerated response after the administration of LH-RH. Gonadotropin releasing hormone) |
Follow-up period defined as years off treatment.
Reported number only available within total cohort (i.e. not specified for HL diagnosis or age-subgroup).
ABV, Adriamycin (Doxorubicin), Bleomycin, Vinblastine; ABVD, Adriamycin (Doxorubicin), Bleomycin, Vinblastine, Dacarbazine; CCNU, Chlorambucil, Etoposide; Chemo, chemotherapy; CHlVPP, Chlorambucil, Vinblastine, Procarbazine, Prednisone; C-MOPP, Cyclophosphamide, Nitrogen mustard, Vincristine (Oncovin), Procarbazine, Prednisone; COPP, Cyclophosphamide, Vincristine (Oncovin), Procarbazine, Prednisone; CPP, Cetuximab, Paclitaxel, Cisplatin; EBVD, Epirubicin, Bleomycin, Vinblastine, Dacarbazine; Gy, gray; LOPP, Chlorambucil, Vincristine (Oncovin), Procarbazine, Prednisone; MDP, Adriamycin (Doxorubicin), Procarbazine, Prednisone, Vincristine (Oncovin), Cyclophosphamide; MOPP, Mechlorethamine, Vincristine (Oncovin), Procarbazine, Prednisone; MVPP, Mechlorethamine, Vinblastine, Procarbazine, Prednisone; NA, not applicable; n.s., not specified; N, number; OPPA, Vincristine (Oncovin), Prednisone, Procarbazine, Adriamycin (Doxorubicin); PAVE, Prednisolone, Adriamycin (Doxorubicin), Vinblastine, Etoposide; PAVe, Procarbazine, Alkeran, Velban; RT, radiotherapy; VAMP, Vincristine (Oncovin), Adriamycin (Doxorubicin), Methotrexate, Prednisone; VBM, Velban, Bleomycin, Methotrexate; VELBE, Vinblastine.
Age at diagnosis and time of study reported in median years (range) or mean ± SD.
The number of patients that received pelvic radiotherapy as (part of their) cancer treatment is reported. If mentioned, (pelvic) radiation dosage is included.
Reference values for semen analysis are summarized in Supplementary Table SI.
Most studies detected a high incidence of abnormal semen (pre-treatment azoospermia range 0–50%; median 10%; six studies, pre-treatment oligospermia range 39–68%; median 55%; three studies, post-treatment azoospermia range 33–100%; median 75%; 29 studies, post-treatment oligospermia range 0–33%; median 17.5%; 14 studies). Treatment with high doses of alkylating agents, such as procarbazine and cyclophosphamide, appeared to be a risk factor for both decreased sperm counts and quality, and negative effects on semen appeared to be even stronger when alkylating chemotherapy was combined with testicular irradiation (Green and Hall, 1988; Dhabhar et al., 1993; Shafford et al., 1993; Heikens et al., 1996; van den Berg et al., 2004; Hobbie et al., 2005; van Beek et al., 2007a; Romerius et al., 2010). Some studies specifically evaluated semen quality in patients with elevated FSH, and most of these patients were azoospermic (Brämswig et al., 1990; Gözdasoglu et al., 1995; Zaletel et al., 2010). One study compared pre-treatment semen storage in cancer patients and reported, in addition to abnormal semen characteristics, a trend toward fewer straws (and thus lower sperm volume produced) among patients with HL (P = 0.051) compared to other patients with an indication for sperm cryopreservation (i.e. sarcoma, testicular cancer, NHL, other malignant hemopathies or other/non-oncological indications) (Adam et al., 2020).
Most studies evaluated semen quality in HL survivors at a single time point. Three studies evaluated the semen quality both pre- and post-cancer treatment (Heikens et al., 1996; van Casteren et al., 2008; Laddaga et al., 2022). The study by Laddaga et al. (2022) reported that 8 out of 11 males (73%) had sperm abnormalities at time of diagnosis (i.e. oligospermia, azoospermia, and/or theratospermia), four of these males (50%) had abnormal sperm counts at 54 months (range 26–137 months) after treatment. Three of these patients had undergone allogeneic or autologous HSCT which, according to the study results, was associated with severe fertility impairment in terms of sperm motility (74% motility at diagnosis versus 22% motility after HSCT in the entire study cohort with both young and older HL patients, P = 0.025) (Laddaga et al., 2022). The other two studies reported abnormal (mean) semen analysis characteristics pre-treatment (Heikens et al: mean sperm count 3.4 ± 5.5 million, van Casteren et al: mean sperm concentration 12.0 (SEM 5.1) million/ml with low volume and motility), and 50–63% of the HL survivors had azoospermia post-treatment (Heikens et al., 1996; van Casteren et al., 2008).
In total, four papers mentioned results of sequential semen analysis for years after treatment (da Cunha et al., 1984; Anselmo et al. 1990; Ortin et al. 1990; Heikens et al., 1996). Three studies reported on recovery of spermatogenesis in males receiving six cycles of MOPP (Anselmo et al. 1990; Ortin et al. 1990; Heikens et al., 1996). Heikens et al. observed no recovery of spermatogenesis up to 20 years after treatment in 19 patients (two received pelvic irradiation), while 3 out of 9 (33%) azoospermatic patients aged <25 years old at diagnosis had late recovery in the paper by Anselmo et al. (at 30, 57, 108 months post-treatment, respectively) (Anselmo et al. 1990; Heikens et al., 1996). In a study by Ortin et al. (1990) two azoospermic boys (one received supra-diaphragmatic radiation) had late recovery (both 12 years after treatment, and they both successfully fathered a child). The semen analysis of all other 10 boys with azoospermia (four received pelvic radiation) showed no recovery during follow-up (up to 11 years after treatment). In the paper by da Cunha et al. (1984) recovery of semen to normozoospermic counts (at 3 and 9 years post-treatment) was only observed in two patients with initial oligospermia post-treatment who had received no more than three MOPP courses. Two patients treated with six courses ABVD had a relatively faster normalization of oligospermia, at 18 and 19 months post-treatment, respectively (Anselmo et al. 1990).
FSH
A total of 28 included studies (738 patients) reported on (elevated) serum FSH levels in male HL patients or survivors (Table IV and Supplementary Fig. S2). One assessed pre-treatment serum FSH levels in 10 boys and reported a significantly higher mean FSH concentration in patients diagnosed with HL, when compared to healthy controls (6.3 ± 3.6 mIU/ml versus 4.6 ± 2.2 mIU/ml, respectively, P = 0.05) (Krawczuk-Rybak et al., 2012).
Table IV.
Serum FSH and inhibin B in childhood or adolescent males diagnosed with Hodgkin lymphoma.
| Study | N patients | Age at diagnosis (years) | Age at time of study (years) | Follow-up (years) | Treatment | Outcome |
|---|---|---|---|---|---|---|
| Pre-treatment | ||||||
| Krawczuk-Rybak et al., 2012 | 10 | 15–18 | NA | NA | NA |
|
| Post-treatment | ||||||
| Aubier et al., 1989 | 10 | 10 (8–15) | n.s. | 9 (1–20) |
|
n = 4/4 (100%) with elevated FSH (i.e. >5 mcl/ml) |
| Ben Arush et al., 2000 | 12 | 13.7 (2.1–16.4) | 22 (14.8–29.3) | 9.8 (4.0–19.0) | n = 8/12 (67%) with elevated FSH (i.e. >14 mU/ml) | |
| Bordallo et al., 2004 | 21 | 10 (6–19) | 18 (17–23) | (3–11) |
|
|
| Brämswig et al., 1990 | 75 | 12.4 ± 2.1 | 17.2 ± 2.2 | 4.3 ± 1.9 |
|
n = 26/65 (40%) with elevated FSH (i.e. >2 SD of controls) |
| Brignardello et al., 2016 | 40 | <18 | n.s. | 14.01 |
|
n = 20/40 (50%) with elevated FSH (i.e. >10 IU/l) and low Inhibin B (<100 pg/ml) |
| Dhabhar et al., 1993 | 26 | 12 (4–15) | 17 (15–23) | 6.1 (2.3–11) |
|
n = 14/23 (61%) with elevated FSH (i.e. >500 ng/ml) |
| Felicetti et al., 2020 | 55 | <18 | 24.6 (21.8–29.4) | n.s. |
|
n = 25/55 (45%) with elevated FSH (i.e. >10 IU/l) and low Inhibin B (i.e. <100 pg/ml) |
| Gerres et al., 1998 | 46 | 14.9 ± 1.5 | n.s. | 11.7 ± 1.2 |
|
n = 7/45 (16%) with elevated FSH (i.e. >2 SD of controls) |
| Gözdasoglu et al., 1995 | 10 | n.s. | 18 (11–29) | (5–24) |
|
n = 4/10 (40%) with elevated FSH (i.e. >2 SD of controls) |
| Green et al., 1981 | 17 | 12.1 (5.4–16.8) | 16.3 (8.3–24.4) | 3.4 (0.5–8.2) |
|
n = 11/17 (65%) with elevated FSH (i.e. >16 mU/ml) |
| Hassel et al., 1991 | 25 | 13.6 ± 1.2 | 16.2 ± 1.2 | 2.4 (0.5–3.8) |
|
n = 0/25 (0%) with elevated FSH (i.e. >10 IU/l) |
| Heikens et al., 1996 | 19 | 11.0 (5–15) | 19 (16–27) | 14 (13–20) |
|
n = 15/19 (79%) with elevated FSH (i.e. >10 IU/l) |
| Hobbie et al., 2005 | 11 | 13.2 (6–19) | 21 (18–31) | 6.5 (1.5–21) |
|
n = 5/11 (45%) with elevated FSH (i.e. >8 mIU/ml) |
| Mackie et al., 1996 | 46 | 12.2 (8.2–15.3) | n.s. | 6 (2.5–11.1)* |
|
n = 41/46 (89%) with elevated FSH (i.e. >10 IU/l) |
| Müller et al., 1996 | 13 | 14 (3–17) | 21 (19–34) | 8 (1–18) |
|
n = 4/6 (66%) with elevated FSH (i.e. >10 IU/l) |
| Ortin et al., 1990 | 20 | 13 (12–15) | n.s. | 9 (up to 26) |
|
n = 7/10 (70%) with elevated FSH (i.e. >18 mIU/ml) |
| Papadakis et al., 1999 | 36 | 13.0 (2.4–22.6) | 22.3 (15.1–32.5) | 6.8 (2.0–19.3)* |
|
|
| Perrone et al., 1989 | 7 | 9.0 (2.4–12.0) | 10.8 (3.0–18.0) | 1.2 (0.2–6.5) |
|
n = 2/7 (29%) with elevated FSH (i.e. >2 SD of controls) |
| Rafsanjani et al., 2007 | 33 | 9 (5–15) | 19 (17–29) | 7 (2–20)* |
|
n = 6/33 (18%) with elevated FSH (>15 mIU/ml) |
| Schellong, 1998 | 31 | 13 | >15 | >4 |
|
n = 0/31 (0%) with elevated FSH (cutoff n.s.), in patients who received only 2 cycles OEPA |
| Servitzoglou et al., 2015 | 50 | 10.8 (2.1–17.3) | 21.1 (17.0–30.4) | 9.3 (2.0–22.4)* |
|
|
| Shafford et al., 1993 | 40 | n.s. | >16 | >6* |
|
|
| Sherins et al., 1978 | 15 | (3–16) | n.s. | >2 |
|
n = 8/15 (53%) with elevated FSH (i.e. >10 IU/l)
|
| van Beek et al., 2007a | 56 | 11.4 (3.7–15.9) | 27.0 (17.7–42.6) | 15.5 (5.6–30.2) |
|
|
| van den Berg et al., 2004 | 33 | 11.8 (3.8–17.2) | n.s. | 11.3 (0.5–24) |
|
n = 14/33 (42%) with elevated FSH (i.e. >10 IU/l).
|
| Whitehead et al., 1982 | 15 | 11.2 (4.8–14.8) | n.s. | 3.3 (0,7–8)* |
|
n = 10/18* (56%) with elevated FSH (cutoff n.s., in text, within figures depicting basal and peak FSH concentrations, normal range of values are shown, the following cutoff values appears to be used: prepuberal and early puberty ±4 mU/ml, late pubertal ±7 mU.ml).
|
| Zaletel et al., 2010 | 40 | 13 (3–16) | 21 (13–34) | 10 (4–27) |
|
|
Follow-up period defined as years off treatment.
Reported number only available within total cohort (i.e. not specified for HL diagnosis or age-subgroup).
ABV, Adriamycin (Doxorubicin), Bleomycin, Vinblastine; ABVD, Adriamycin (Doxorubicin), Bleomycin, Vinblastine, Dacarbazine; ABVP, Adriamycin (Doxorubicin), Bleomycin, Vinblastine, Prednisone; BOPP, 1,3-bis(2-chloroethyI)-l-nitrosourea, Vincristine (Oncovin), Procarbazine, Prednisone; CCNU, Chlorambucil, Etoposide; Chemo, chemotherapy; CHlVPP, Chlorambucil, Vinblastine, Procarbazine, Prednisone; C-MOPP, Cyclophosphamide, Nitrogen mustard, Vincristine (Oncovin), Procarbazine, Prednisone; COMP, CCNU (Chlorambucil, Etoposide), Vincristine (Oncovin), Amethopterine, Procarbazine; COPP, Cyclophosphamide, Vincristine (Oncovin), Procarbazine, Prednisone; CPP, Cetuximab, Paclitaxel, Cisplatin; CVPP, Cyclophosphamide, Vinblastine, Procarbazine, Prednisone; EBVD, Epirubicin, Bleomycin, Vinblastine, Dacarbazine; Gy, gray; LOPP, Chlorambucil, Vincristine (Oncovin), Procarbazine, Prednisone; MDP, Adriamycin (Doxorubicin), Procarbazine, Prednisone, Vincristine (Oncovin), Cyclophosphamide; MOPP, Mechlorethamine, Vincristine (Oncovin), Procarbazine, Prednisone; MVPP, Mechlorethamine, Vinblastine, Procarbazine, Prednisone; NA, not applicable; n.s., not specified; N, number; OEPA, Vincristine (Oncovin), Etoposide, Prednisone, Adriamycin (Doxorubicin); OPA, Vincristine (Oncovin) Prednisone, Adriamycin (Doxorubicin); OPPA, Vincristine (Oncovin), Prednisone, Procarbazine, Adriamycin (Doxorubicin); PAVE, Prednisolone, Adriamycin (Doxorubicin), Vinblastine, Etoposide; PAVe, Procarbazine, Alkeran, Velban; RT, radiotherapy; VBM, Velban, Bleomycin, Methotrexate; VELBE, Vinblastine.
Age at diagnosis and time of study reported in median years (range) or mean ± SD.
The number of patients that received pelvic radiotherapy as (part of their) cancer treatment is reported. If mentioned, (pelvic) radiation dosage is included.
Among the eligible studies, the reported percentage of patients with elevated FSH ranged from 0% to 100% (median 51.5%). Nine studies used the generally accepted cutoff value of 10 IU/L to determine high FSH serum level, indicating damage to spermatogenesis (Sherins et al., 1978; Hassel et al., 1991; Heikens et al., 1996; Mackie et al., 1996; Müller et al., 1996; van den Berg et al., 2004; Servitzoglou et al., 2015; Brignardello et al., 2016; Felicetti et al., 2020). The other studies used higher cutoff values (i.e. >14, >15, >16, or >18 IU/l), lower cutoff values (i.e. >5, >6, or >8 IU/l), >2 SD of controls or did not mention the definition of elevated FSH.
In the study that reported no HL survivors with elevated FSH concentrations (0%), patients had received only two cycles of chemotherapy (OEPA: Vincristine (Oncovin), Etoposide, Prednisone, Adriamycin (Doxorubicin)) (Schellong, 1998). FSH was especially higher in boys who received multiple courses of procarbazine-containing chemotherapy regimens, boys who were late-pubertal or young adults in comparison to younger boys, and boys who had HL at a more advanced stage and received more intensive treatment protocols (Sherins et al., 1978; Green et al., 1981; Whitehead et al., 1982; Brämswig et al., 1990; Gerres et al., 1998; van den Berg et al., 2004; van Beek et al., 2007a).
Two studies mentioned there was no recovery of elevated serum FSH over time (up to 8.2 and 17 years follow-up, respectively) (Papadakis et al., 1999; Shafford et al. 1993). Two other studies observed normalization of FSH during follow-up in a few subjects (in 1 out 4 survivors and 2 out of 20 survivors, respectively) (Whitehead et al., 1982; Servitzoglou et al., 2015).
Inhibin B
Five papers (182 patients) reported on inhibin B serum levels in HL patients (Table IV and Supplementary Fig. S2). Krawczuk-Rybak et al. (2012) detected significantly lower mean inhibin B concentration in newly diagnosed HL patients when compared to healthy controls (100.4 ± 67.5 ng/L versus 153.6 ± 71.4 ng/L, respectively, P = 0.03). The percentage of patients with low inhibin B (serum concentration <100 pg/ml was considered low) after treatment ranged from 19% to 50% (median 45%). Van Beek et al. observed that median FSH and LH values were significantly higher, and inhibin B levels significantly lower in patients treated with MOPP, when compared to patients that did not receive MOPP (P = 0.01). However, only inhibin B (not FSH or LH) serum levels showed an independent correlation with sperm concentration (r = 0.86; P < 0.001), suggesting that the measurement of inhibin B serum levels might be favored over other serum markers as being representative of male gonadal function (van Beek et al., 2007a). Only one of the included studies mentioned the inhibin B/FSH ratio. Within this study, the median inhibin B/FSH ratio was lower in HL survivors when compared to controls (31 (3.8–267.9) in HL survivors versus 142.1 (47.6–767.3) in healthy controls, P = 0.0002, respectively) (Bordallo et al., 2004).
LH
One study reported on LH levels pre-treatment, and mean serum LH values were significantly higher in HL patients when compared to healthy controls (5.9 ± 4.0 mIU/ml in 10 HL patients versus 3.6 ± 1.8 mIU/ml in 14 controls, respectively, P = 0.05) (Krawczuk-Rybak et al., 2012).
A total of 21 studies (528 patients) assessed the percentage of HL survivors with elevated LH serum concentrations (Supplementary Table SVII and Supplementary Fig. S2). Applied cutoff values to determine elevated concentrations varied widely (i.e. >3 up to >30 IU/l, >2 SD of controls, or not specified). Overall, the reported percentage of patients with elevated LH levels ranged from 0% to 59% (median 17%). The study by Servitzoglou et al. (2015) mentioned that a significantly higher proportion of patients with elevated LH levels had received abdominal radiotherapy, when compared to patients exhibiting normal LH levels (n = 6/7 (85%) versus n = 17/42 (40%), P = 0.03, respectively). Another study found that median LH levels were significantly higher in patients treated with MOPP when compared to patients receiving ABVD or EBVD (Epirubicin, Bleomycin, Vinblastine, Dacarbazine) (5.9 U/l (range 1.68–15.0, 40 patients) versus 2.5 U/l (range 1.2–9.0, 16 patients), P = 0.01) (van Beek et al., 2007a). Interestingly, a study by Ben Arush and colleagues mentioned that four HL survivors (33%) had low LH serum concentrations (<5 IU/L) post-treatment, while none of the 12 patients had elevated serum concentrations after treatment (Ben Arush et al., 2000).
A single study mentioned the results of sequential LH serum sampling during follow-up. LH concentrations normalized in two patients with initial high LH levels, and in eight other patients LH levels were initially within the normal reference limits but subsequently became elevated over time (Shafford et al., 1993).
Testosterone
Most studies reported that serum testosterone concentrations in HL survivors were within normal range. One paper studied pre-treatment serum testosterone levels and reported no statistically significant difference between newly diagnosed HL patients and healthy boys (466.0 ± 67.5 ng/dl versus 466.8 ± 242.2 ng/dl, P not given) (Krawczuk-Rybak et al., 2012). The percentage of patients with low testosterone values after treatment ranged from 0% to 43% (median 9%); 15 studies, 339 patients. Most studies used cutoff values close to 9–12 IU/l, but some studies applied much lower thresholds (e.g. <3 ng/dl = ±0.1 IU/l). Two studies stated that HL survivors sometimes had (relatively) high testosterone levels (Ben Arush et al.: n = 4/12 (33%) with testosterone levels >35 nmol/l post-treatment, Gerres et al.: mean testosterone levels were higher in 45 HL survivors when compared to 37 controls, P not given) (Gerres et al., 1998; Ben Arush et al., 2000). Data on testosterone levels are presented in Supplementary Table SVII and Supplementary Fig. S2.
Pregnancy/live birth
In total, 13 of the included studies reported that 374 out of the 755 (50%) male childhood or adolescent HL survivors fathered a (biological) child. The study by Zaletel et al. (2010) specifically evaluated the number of males with signs of germinal epithelium damage (i.e. elevated FSH concentrations) having children (n = 5/24, 21%). Reulen et al. (2009) found no significant variation in odds ratio for live births by type of cancer, exposure to chemotherapy, or gonadal irradiation (odds for live birth 1.2 (95% CI: 0.9–1.8) in females, 0.9 (95% CI: 0.5–1.6) in males).
In studies that reported results of pre-treatment semen analysis, semen samples were retrieved for cryobanking in an effort to preserve fertility. Laddaga et al (2022) mentioned that none of the 11 males used their stored sperm after a median follow-up of 8.6 years (range 4.4–14 years, males were aged 17–24 years at diagnosis). None of these males achieved pregnancy, but it is unknown whether they even attempted pregnancy. None of the other included studies reported on the use of stored semen.
The results are summarized in Supplementary Table SVIII.
Discussion
In this review, markers of gonadal function and fertility in female and male childhood and adolescent HL survivors were evaluated. Aberrant concentrations of reproductive hormone serum markers, risk of (premature) ovarian insufficiency in females, and abnormal semen analyses in males mark the risk of gonadotoxicity after exposure to (high) doses of chemotherapy and radiotherapy. An overview of the results is presented in Table V.
Table V.
Summary of evidence on markers of reproductive ability in female and male childhood or adolescent Hodgkin lymphoma survivors.
| Reported outcome | N studies | N HL survivors | Results |
|---|---|---|---|
| Females | 41 | 5057 | |
| Serum anti-Müllerian hormone (AMH) | 6 | 180 |
Pre-treatment
|
Post-treatment
| |||
| Antral follicle count (AFC) | 1 | 44 |
Post-treatment
|
| Premature ovarian insufficiency (POI)* | 7 | 1516 |
Post-treatment
|
| ‘Ovarian failure’ (not defined) | 3 | 154 |
Post-treatment
|
| Acute ovarian failure (AOF) | 1 | 553 |
Post-treatment
|
| Cycle (ir)regularity or amenorrhea | 18 | 387 |
Post-treatment
|
| Serum follicle stimulating hormone (FSH) | 8 | 155 |
Post-treatment
|
| Pregnancy or live birth | 20 | 2681 |
Post-treatment
|
| Males | 52 | 1903 | |
| Semen-analysis | 39 | 1118 |
Pre-treatment
|
Post-treatment
| |||
| Serum follicle stimulating hormone (FSH) | 28 | 738 |
Pre-treatment
|
Post-treatment
| |||
| Serum Inhibin B | 5 | 182 |
Pre-treatment
|
Post-treatment
| |||
| Serum luteinizing hormone (LH) | 24 | 604 |
Pre-treatment
|
Post-treatment
| |||
| Serum testosterone | 21 | 498 |
Pre-treatment
|
Post-treatment
| |||
| Pregnancy or live birth | 13 | 755 |
Post-treatment
|
AFC, antral follicle count; AOF, acute ovarian failure; AMH, anti-Müllerian hormone; HL, Hodgkin lymphoma; MOPP, Mechlorethamine, Vincristine (Oncovin), Procarbazine, Prednisone; N, number; POI, premature ovarian insufficiency.
All reports on (premature) ovarian failure before the age of 40 years, sometimes within papers referred to as non-surgical premature menopause (NSPM), were defined as POI in this review.
Female HL survivors
Results show that female HL survivors are at increased risk of POI (6–34% of HL survivors had POI, median 9%; seven studies; 1516 patients) in comparison to healthy controls but owing to the few studies available, personalized treatments, and heterogeneity in the applied definition of POI, it is difficult to express the risk in odds or relative risk for individual patients. The studies evaluated young cancer survivors and the follow-up period was often relatively short, meaning that POI could still occur in the coming years and the stated incidence of POI could be underestimated.
The diagnosis of POI is definite. Recommendations on initial assessment and management of females with POI are established in the international ESHRE guidelines (Webber et al., 2016). Hormone replacement treatment can provide relief of vasomotor symptoms, such as hot flushes or night sweats, but there is currently no proven treatment to restore ovarian function in women with POI and the chance of having a biological child is minimal. Therefore, the timely recognition of patients ‘at risk’ of comprised reproductive potential at a young age is crucial, as this will give these women the possibility to expedite their plans to become pregnant or opt for fertility preservation. However, reproductive staging in cancer survivors is complicated as adverse effects of treatment may be transitory (Harlow et al., 2012).
AOF with temporary cessation of menses is normal during chemotherapy. Only one of the included articles specifically reported on AOF, finding that 12% of the survivors had ceased spontaneous menses within 5 years of diagnosis, but long-term follow-up data were lacking (Chemaitilly et al., 2006). In most other papers, the majority of HL survivors self-reported regular menstrual cycles during follow-up (median follow-up ±5 years after treatment; 79–100% with regular cycle; median 100%; nine studies; 168 patients; 0–71% with amenorrhea; median 4%; 13 studies; 240 patients). The resumption of menstruation after therapy may be reassuring, but it remains uncertain for how long the cycle will be ovulatory. A regular cycle after cancer-treatment at a young age does not guarantee future fertility and these women may still be at increased risk of POI.
Increased serum FSH levels in female HL survivors were frequently reported (17–100% with elevated FSH; median 53%; seven studies; 132 patients), which may be useful to help recognize the preliminary ‘phase’ of POI, also known as incipient ovarian failure. However, serum FSH levels rise relatively late and, when compared to FSH, assessment of AMH appears to be a better predictor of the remaining ovarian reserve after anticancer treatments (van Beek et al., 2007b; Bedoschi et al., 2016). Usually, an immense initial decline in AMH is seen directly after chemotherapy, which indicates damage to the granulosa cells of the developing follicles (Anderson and Su, 2020; Anderson et al., 2022). Subsequent recovery of AMH levels varies greatly, and measurement of AMH concentration over time may help to determine treatment gonadotoxicity, predict ovarian reserve and diagnose (permanent) ovarian insufficiency (Krawczuk-Rybak et al., 2013b; Oktem et al., 2018; Anderson et al., 2022). In a study by Berjeb et al. (2020) a significant drop in AMH was observed at 6 months post-chemotherapy, and in three out of five HL patients a subsequent recovery of AMH was detected but in the other two patients AMH serum levels became even lower in the year thereafter. All patients were treated with BEACOPP (Bleomycin, Etoposide, Adiamycin (Doxorubicin), Cyclophosphamide, Vincristine (Oncovin), Procarbazine, Prednisone), but detailed information on treatment received as well as baseline characteristics (including age) were not provided. In another study that followed AMH levels up to 24 months post-treatment in HL and NHL patients (up to 35 years old), varying recovery patterns were observed as well (Decanter et al., 2021). In general, patients receiving ABVD (n = 67) had an earlier and more pronounced increase of AMH levels when compared to patients receiving higher doses of alkylating agents (n = 55). In this study, patients receiving ABVD showed pre-treatment AMH levels after ±6 months, while none of the patients treated with the previously mentioned regimens had reached pre-treatment AMH levels during follow-up (Decanter et al., 2021).
Low AMH levels are especially found in survivors who were treated with alkylating agents/procarbazine containing regimens, but the effect of radiotherapy on (the recovery of) AMH is less frequently described (van Beek et al., 2007b; Brougham et al., 2012; Decanter et al., 2021). Within the papers included in this review, only a few patients were treated with radiotherapy and no additional analyses were performed. In studies on childhood cancer patient with varying diagnoses, lower AMH values were typically observed in patients receiving abdominal radiotherapy or total body irradiation (Lie Fong et al., 2009; Gracia et al., 2012; Miyoshi et al., 2013; Elchuri et al., 2016; van den Berg et al., 2018; George et al., 2019).
Interpretation of the results is complicated by the known decrease in AMH with age, inter-individual variation, the potential confounding effect of hormonal contraceptive use, and additional variations caused by sample instability in storage, as well as variations between assays and kits (Krawczuk-Rybak et al., 2013b; Yates et al., 2019). A recent systematic review evaluated AMH as a biomarker of ovarian reserve and POI in children and women with a cancer diagnosis (Anderson et al., 2022): 11 of the included papers evaluated AMH in patients with different types of lymphoma and although not all of these studies were eligible for this review, their results were comparable to ours, as all studies reported significant declines in AMH concentrations during or after anticancer treatment (≤3 months post-treatment). Large prospective studies including pre-treatment and long-term follow-up AMH measurements are lacking, whereas the change in AMH in the individual girl or young woman may be helpful to estimate gonadotoxicity of the treatment given. This is valuable information for counseling not only female HL patients but also for HL survivors, since there may still be an opportunity to harvest oocytes in survivorship.
Male HL survivors
In this review, high incidences of abnormal semen analyses (i.e. azoospermia, oligospermia, asthenezospermia and theratospermia) were observed in male HL survivors (post-treatment: azoospermia 33–100%; median 75%; 29 studies; 332 patients. oligospermia 0–33%; median 17.5%; 14 studies; 223 patients). Adverse effects with spermatogenesis damage were specifically present in patients treated with alkylating agents or pelvic radiotherapy, which is line with previous literature reports (Skinner et al., 2017). If spermatogenesis is impaired, FSH blood levels rise and, as expected, many studies found that males with increased FSH concentration had abnormal sperm counts (Franchimont et al., 1972; Brämswig et al., 1990; Gözdasoglu et al., 1995; Rafsanjani et al., 2007; Zaletel et al., 2010). However, a normal serum FSH does not exclude germinal epithelium damage, and azoospermia was also reported in boys with normal endocrine markers (de Kretser et al., 1974; Green and Hall, 1988; Brämswig et al., 1990; Kader and Rostom, 1991; Chan et al., 2001; Bordallo et al., 2004; Hobbie et al. 2005). It is thought that the detection of serum inhibin B, in the presence of FSH (inhibin B/FSH ratio), is the best indirect marker of male infertility (Bordallo et al., 2004; van Casteren et al., 2008). Unfortunately, inhibin B serum concentrations were not often included as an outcome measurement in the studies included in this review. Only four studies reported on inhibin B serum concentrations after treatment, and one specifically evaluated the inhibin B/FSH ratio (van Beek et al., 2007a; Bordallo et al., 2004; Brignardello et al., 2016; Felicetti et al., 2020). In general, inhibin B levels appeared to be lower in the HL group when compared to healthy controls and a significant association was observed with sperm quality (Bordallo et al., 2004).
The value of LH and testosterone as serum markers of reproductive ability remains debatable (Krawczuk et al., 2012; Keskin et al., 2015). Primarily, the rapidly dividing stem cells and Sertoli cells are affected by irradiation and cytostatic agents, and often Leydig cells appear to be spared. Most male childhood cancer survivors undergo normal pubertal development with normal testosterone concentrations, and androgen replacement therapy in cancer survivors is rare (Stukenborg et al., 2018b). Within this review, LH and testosterone levels were indeed less evidently affected when compared to FSH levels (median percentage of patients with elevated LH 17%, low testosterone 6%, elevated FSH 51.5%). Still, boys with decreased testosterone values during puberty may be at risk of delayed sexual maturation, although such cases are almost exclusively described in boys with testicular cancer or those receiving extremely high doses of pelvic radiation (or non-malignant disorders) (Sklar et al., 1990; Amano et al., 2013; Goossens et al., 2020).
Studies presented varying results on recovery of reproductive ability in male HL survivors during follow-up (da Cunha et al., 1984; Green and Hall, 1988; Anselmo et al., 1990; Ortin et al., 1990; Heikens et al. 1996; Bordallo et al., 2004). In most patients, serum markers remained abnormal, although only a limited number of patients underwent sequential sampling for a long period of time. Recovery of semen quality is usually reported within the first months after treatment but can also occur years after treatment completion. For example, late recovery was established in survivors with (initially) azoospermia at 114 months post-treatment or even 12 years after treatment (da Cunha et al., 1984; Ortin et al, 1990). Although recovery of sperm appears to be correlated with the cumulative total dose of chemotherapy and pelvic radiotherapy, it is very difficult to predict whether spermatogenesis will normalize after treatment, and full recovery does not appear to occur often (Sherins et al., 1978; Bonadonna, 1982; da Cunha et al., 1984; Naysmith et al., 1998). For some male cancer survivors, testicular sperm extraction followed by ICSI can help in achieving biologic fatherhood but, generally, the therapeutic options are limited and prognosis is poor in case of severely damaged gonads (Meseguer et al., 2003; Hsiao et al., 2011). Therefore, it is highly recommended to offer pre-treatment sperm banking to all boys who are able to produce semen physically, but this would also be based on emotional and cognitive status (Wallace, 2011; Loren et al., 2013). In previous studies including young boys diagnosed with HL, the youngest patient with an ejaculate containing motile spermatozoa was 12.2 years old, and the smallest testicular volumes were 6–7 ml (Bahadur et al., 2002; Hagenäs et al., 2010; Daudin et al., 2015; Adam et al., 2020). For young, pre-pubertal boys at high risk of infertility, a testicular tissue biopsy for cryopreservation could be considered (Goossens et al., 2020; Mulder et al., 2021a).
Treatment-related factors
In both sexes, adverse effects were specifically present in treatment protocols using alkylating chemotherapy agents, a higher cumulative dose of chemotherapy, a large number of treatment cycles, and/or infra-diaphragmic radiotherapy (Whitehead et al., 1982; da Cunha et al., 1984; Anselmo et al., 1990; Brämswig et al., 1990; Bokemeyer et al., 1994; Ben Arush et al., 2000; van Beek et al., 2007a; 2007b; Aubier et al., 1989; van der Kaaij et al., 2012b; Brignardello et al., 2016). Patients also appeared to be at increased risk of disturbed spermatogenesis after HSCT, probably linked to the use of radiotherapy and high doses of alkylating treatment in the myeloablative treatment pre-HSCT (Brignardello et al., 2016; Laddaga et al., 2022). To ensure the highest survival rates with the least adverse effects, treatment protocols for childhood HL are constantly being evaluated and adapted. Over the past decades, many chemotherapy regimens have been used for HL treatment, all with (slightly) different working mechanisms, and differing gonadal toxicity as well. The ABVD regimen was shown to be as effective as the classical MOPP regimen, but less damaging for gonadal function (Bonadonna et al., 1984, 1989; Viviani et al., 1985; Canellos et al., 1992). Some of the included studies compared ABVD to the MOPP regimen and confirmed this statement: ABVD resulted in less azoospermia and there was no statistically significant decrease in AMH serum levels after treatment with ABVD, while the MOPP regimen was associated with a significantly lower AMH post-treatment (Anselmo et al., 1990; Dhabhar et al., 1993; Berjeb et al., 2020). Another study used ∼50% less cyclophosphamide and procarbazine in their C-MOPP/ABV treatment protocol but still found severe damage to germinal epithelium (Bordallo et al., 2004). These results suggest that even a small dosage of alkylating agents may be gonadotoxic in males. Efforts should be made to avoid or minimize the potential risk of drug-induced gonadal failure by avoiding the use of alkylating agents if possible.
Recently, robust evidence for the beneficial effects of replacing procarbazine with dacarbazine on gonadotoxicity was provided by the (randomized) Euronet-PHL-C1 study (Mauz-Körholz et al., 2022): the paper was published after completion of our literature search, therefore these findings were not included in the results section of this review. Male patients receiving COPP (Cyclophosphamide, Vincristine (Oncovin), Procarbazine, Prednisone) treatment (with procarbazine) more often had azoospermia, significantly higher FSH serum levels, and lower inhibin B concentrations when compared to patients receiving COPDAC (with dacarbazine). The 5-year event-free survival rates of both treatment arms were within the same range (around 90%). The Euronet-PHL-C1 study also revealed that radiotherapy could safely be omitted in patients who responded adequately to treatment, which further contributes to the reduction of treatment-related gonadotoxicity.
The current Euronet-PHL-C2 study is evaluating whether radiotherapy can be reduced even more, by intensifying chemotherapy with higher doses of cyclophosphamide (Clinical trials; NCT02684708). The effects of the C2 treatment protocol on reproductive potential are being studied in detail within the ongoing fertility add-on study. Moreover, several other newly introduced drugs are considered to be non-gonadotoxic, such as Brentuximab, Nivolumab and Pembrolizumab, but studies still need to confirm their safety and effectivity.
Effect of HL
In addition to the adverse effects of treatment for HL on fertility, there might also be a negative effect of the disease itself. In a study that compared pre-treatment semen analyses of males with different cancer types, semen parameters were particularly affected in HL patients and testicular tumors, in comparison to patients with other types of cancer and controls (Caponecchia et al., 2016). Within this review, we noticed that abnormalities in semen analysis were often present before anticancer treatment was administered (pre-treatment analyses: 0–50% azoospermia; median 7.5%; six studies; 109 patients, 39–68% oligospermia; median 54.5%; three studies; 43 patients). Based on studies in adult HL patients, the higher metabolic and inflammatory state in HL patients appears to affect semen quality negatively (Botchan et al., 1997; Hallak et al., 2000; Agarwal and Allamaneni, 2005; Trottmann et al., 2007; van der Kaaij et al., 2009). Pre-treatment azoospermia is mostly seen in patients with B-symptoms (weight loss, fever and drenching night sweats), which might in part be related to the well-known adverse effect of elevated scrotal temperature on spermatogenesis (Carlsen et al., 2003; van der Kaaij et al., 2009; Caponecchia et al., 2016). A study in pediatric HL patients revealed that not only sperm counts but also serum inhibin B concentrations were significantly lower in patients with B-symptoms, when compared to patients presenting without B-symptoms (P = 0.05 and P < 0.05, respectively) (van Beek et al., 2007a).
One of the studies included in this review, demonstrated that not all patients with abnormal semen at time of diagnosis had abnormal semen quality after treatment (n = 4/8, 50%) (Laddaga et al., 2022). Hypothetically, disease activity may temporarily affect spermatogenesis, but it remains highly complex to analyze a potential association of HL itself with semen quality after treatment, because of the confounding effects of treatment.
Moreover, the study by van Dorp et al. reported decreased AMH levels in their pre-treatment analyses in girls with newly diagnosed lymphoma, which may also be explained by the high cytokine expression associated with HL (van Dorp et al., 2014; Paradisi et al., 2016). One of the included studies assessed the presence of B-symptoms as a risk factor of having POI after treatment and observed no statistically significant correlation (van der Kaaij et al., 2012b). Additional exploration of the hypothesized pathophysiological pathways would be appropriate, as a compromised reproductive function before the start of chemotherapy would affect the chance of successful fertility preservation.
Age at diagnosis
Pennisi et al. (1975) suggested that chemotherapy-induced gonadal damage is related to the age of the cancer patient at treatment, with young age being a relative protecting factor. In their small study, they treated 23 boys and 11 girls with nephrosis with cyclophosphamide, and almost exclusively found abnormal spermatogenesis and increased FSH levels in post-pubertal boys. It was thought that the epithelium of the seminiferous tubules is relatively dormant before puberty and thereby less sensitive to cytotoxic agents (Sherins et al., 1978; Bayle-Weisgerber et al., 1984). Likewise, Brämswig et al. (1990) found a correlation between a lower quality of semen and being post-pubertal at treatment. Another study reported a significantly lower probability of developing POI for NHL and HL survivors below the age of 25 years, compared with those over 25 years (P < 0.03) (Bokemeyer et al., 1994). Nevertheless, multiple additional studies did not find such a correlation of age at treatment with chemotherapy-induced gonadotoxicity (Shafford et al., 1993; Mackie et al., 1996; Ben Arush et al., 2000; Hobbie et al., 2005; van Beek et al., 2007b; Aubier et al., 1989; Romerius et al., 2010; Zaletel et al., 2010; van der Kaaij et al., 2012b; Brignardello et al., 2016). In most studies the number of patients is limited, which affects the reliability of sensitivity analyses, and the results may well be affected by other patient- or treatment-related characteristics.
Chance to conceive
Based on the included reports on parenthood, having children as a HL survivor is certainly not impossible. Yet, it remains very complex to evaluate data on fertility-related outcomes and to define the chance to achieve pregnancy in HL survivors. There are many factors that affect fertility (e.g. age, BMI, alcohol and drug use, menstrual cycle, additional medical history, fertility potential of the partner), but these are often poorly documented in papers presenting pregnancy outcomes in cancer survivors. In most of the included studies, results were restricted to the number of patients obtaining a pregnancy or live birth, while it would be appropriate to also mention the percentage of females wishing to have (more) children but failing to succeed. Nonetheless, pregnancy cases were documented in male survivors with ‘abnormal’ fertility markers, such as high FSH values or (severe) oligospermia, which demonstrates that the presence of aberrant markers of fertility does not explicitly exclude the chance to conceive (Chapman et al., 1979; Waxman et al., 1982; Kreuser et al., 1987; van den Berg et al., 2004; Bordallo et al., 2004; van Casteren et al., 2008). Similarly, females with low AMH may still have a chance to become pregnant, as long as they have an ovulatory cycle, as a low oocyte quantity does not define the quality of the follicles (di Paola et al., 2013). Nevertheless, reproductive treatment may help males and females with a history of cancer treatment to extend their reproductive lifespan and chance of pregnancy. Therefore, personalized fertility counseling is strongly recommended.
Strengths and limitations
This review is the first complete overview of the literature on pre-existing and treatment-related gonadotoxicity in both male and female childhood HL survivors.
The present review is a narrative review. Owing to the lack of standardized evaluation of reproductive potential among HL survivors, the available evidence on fertility after HL treatment is heterogenous and it is difficult to compare or combine results. Only a few eligible studies included control or reference groups. Therefore, the results presented remain descriptive and meta-analyses could not be performed, while stratified analyses would have been favored to analyze the effect of treatment- or diagnosis-related factors (such as age at diagnosis or treatment protocol applied) on reproductive potential. Quality of studies was not assessed systematically within this review, as the overall level of evidence was poor and a time-consuming appraisal of each study would not have provided useful information. If future studies on fertility after cancer treatment are performed in a more structured way, a systematic review with critical appraisal could be conducted, in order to achieve a higher level of evidence. Nonetheless, the current review gives an overview of best available evidence of a clinically relevant topic, useful for both HL survivors and the clinicians treating them in terms of potential late effects.
Still, several comments on quality of included studies should be made. Most studies were performed in small groups of childhood HL survivors, or in cohorts with multiple types of childhood cancer survivors with only a few HL survivors included. Studies did not always include a control group, or the comparison groups were small or based on another group of cancer patients. Many studies on menstrual cycle and pregnancy outcomes used questionnaires to collect results, which increases risk of bias and imprecision. The included trials were published between 1978 and 2021, and the older studies in females may not have assessed AMH as a reproductive marker, as it can be considered a relatively new approach. Moreover, poor methodology for semen analysis due to minimal standardization at the time of older studies may have affected the results. In 2015, a checklist for author and reviewers was published to help ensure that an appropriate and robust methodology of semen analysis is carried out, which may contribute to more reliable results on semen quality in the future (Björndahl et al., 2016). Nevertheless, for male cancer survivors, mainly azoospermia is of clinical relevance and we expect less uncertainty in semen analysis reporting a zero sperm count.
In general, the young age of cancer patients may complicate the evaluation of some markers of reproductive function. Young boys may be physically or cognitively unable to produce semen and AFC measurement in girls may be disregarded owing to the invasive measurement via transvaginal ultrasound.
Many studies were cross-sectional, meaning that they only performed one measurement at a single time point. Gonadotoxic effects may become clearer after time passes by, and to evaluate potential recovery multiple assessments are crucial to provide valid results. Studies assessing POI require a minimal follow-up of the entire study population after the age of 40 years old, but a long follow-up period is highly recommended for all studies in female or male HL survivors.
Over the past years, treatment for HL has improved and some of applied treatment protocols are not commonly used anymore. Since the severe gonadotoxicity of alkylating agents was pointed out, treatment regimens have been adjusted, and the dosage of alkylating drugs and radiation are reduced, or replaced if possible (Mauz-Körholz et al., 2010, 2022). The results of the studies included in this review may be mainly valuable for survivors who have been treated with these particular regimens and less relevant for patients who are currently undergoing treatment.
Implications for further research and daily practice
The effects of HL and (current) treatment regimens on reproductive ability should be studied more intensely. We recommend focusing on longitudinal prospective studies with repeated measurements (for example before and during treatment, up to yearly during the first few years after treatment, with subsequent longer time-intervals during follow-up) and a combination of reproductive markers (such as semen analysis, FSH and inhibin B in males, and AMH, AFC and FSH in females). We highly encourage future researchers to further analyze the effect of specific treatment- and diagnosis-related factors on direct and indirect adverse effects as well as (failure of) recovery over time. We suggest analyzing the commonly assessed parameters such as abdominal radiotherapy (yes/no), CED score (cyclophosphamide equivalent dose, using the cutoff >6000 mg/m2 in girls and >4000 mg/m2 in boys in line with the PANCARE guidelines (Mulder et al., 2021a,b)), and age at diagnosis (pre-/post-pubertal), but trials should, above all, aim to study the effects of more unknown agents (e.g. dacarbazine) and newly introduced drugs that are thought to be non-gonadotoxic (e.g. Brentuximab, Nivolumab and Pembrolizumab).
With these data, clinical guideline for fertility preservation in future HL patients can be updated, and patients at risk of impaired gonadal function or a reduced fertility lifespan can be better informed on the methods available to preserve fertility and timing of parenthood (Mulder et al., 2021a,b). Until then, (future) fertility and the possibility of fertility preservation (e.g. storage of semen or harvesting testicular tissue for boys, and oocyte or ovarian tissue cryopreservation in girls) should always be considered when treating (young) cancer patients (Wallace, 2011; Loren et al., 2013; Goossens et al., 2020; Mulder et al., 2021a,b; van der Perk et al., 2021).
Conclusion
HL treatment, primarily when high doses of chemotherapy, alkylating agents and/or pelvic radiotherapy are administered, can negatively affect reproductive ability in both female and male survivors. Problems with fertility can seriously affect psychological wellbeing and quality of life, and patients at risk of impaired gonadal function or a shortened reproductive lifespan should be well informed on the methods available to preserve fertility and the timing of parenthood.
Treatment regimens for HL rapidly evolve to ensure the highest survival rates with the least adverse effects.
Studies on fertility after HL treatment are heterogeneous owing to the lack of structured evaluation of markers of reproductive potential. The overall quality of evidence is poor and additional studies on markers of reproductive function in cancer survivors are needed. We recommend the use of repeated measurements and a combination of reproductive markers next to clinical outcomes to continuously study the effects of HL and (current) treatment regimens on reproductive capacity.
Supplementary Material
Acknowledgements
The authors gratefully thank Elvira van Dalen, epidemiologist from the Princess Máxima Center, for useful discussions on the conceptualization and execution of this review.
Contributor Information
Katja C E Drechsel, Pediatric Oncology, Cancer Center Amsterdam, Emma Children’s Hospital, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Princess Máxima Center for Pediatric Oncology, Utrecht, The Netherlands; Cancer Center Amsterdam, Amsterdam UMC, Location VUmc, VU Amsterdam, Amsterdam, The Netherlands.
Maxime C F Pilon, Pediatric Oncology, Cancer Center Amsterdam, Emma Children’s Hospital, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Princess Máxima Center for Pediatric Oncology, Utrecht, The Netherlands.
Francis Stoutjesdijk, Pediatric Oncology, Cancer Center Amsterdam, Emma Children’s Hospital, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Princess Máxima Center for Pediatric Oncology, Utrecht, The Netherlands.
Salena Meivis, Pediatric Oncology, Cancer Center Amsterdam, Emma Children’s Hospital, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Princess Máxima Center for Pediatric Oncology, Utrecht, The Netherlands.
Linda J Schoonmade, Medical Library, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
William Hamish B Wallace, Department of Haematology/Oncology, Royal Hospital for Sick Children, Edinburgh, UK.
Eline van Dulmen-den Broeder, Pediatric Oncology, Cancer Center Amsterdam, Emma Children’s Hospital, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Auke Beishuizen, Princess Máxima Center for Pediatric Oncology, Utrecht, The Netherlands; Department of Haematology/Oncology, Erasmus MC—Sophia Children’s Hospital, Rotterdam, The Netherlands.
Gertjan J L Kaspers, Pediatric Oncology, Cancer Center Amsterdam, Emma Children’s Hospital, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Princess Máxima Center for Pediatric Oncology, Utrecht, The Netherlands.
Simone L Broer, Department of Reproductive Medicine & Gynecology, University Medical Center Utrecht, Utrecht, The Netherlands.
Margreet A Veening, Pediatric Oncology, Cancer Center Amsterdam, Emma Children’s Hospital, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Princess Máxima Center for Pediatric Oncology, Utrecht, The Netherlands.
Supplementary data
Supplementary data are available at Human Reproduction Update online.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary Materials.
Authors’ roles
K.C.E.D., M.C.F.P., F.S., S.M., E.v.D.-d.B., M.A.V., and S.L.B. participated in the design of the paper. L.J.S. contributed to the search. K.C.E.D., M.C.F.P., S.M., E.v.D.-d.B., and M.A.V. screened for eligible articles and K.C.E.D. and M.C.F.P. extracted data. K.C.E.D. analyzed the data and drafted the first version of the manuscript. All authors were involved in the data interpretation, contributed to the manuscript writing and approved the final version.
Funding
The authors are involved in the ongoing fertility add-on study, nested within the EuroNet-PHL-C2 study (Clinicaltrials NCT02684708; EudraCT number 2012-004053-88). The fertility add-on study was funded by the Dutch charity foundation KiKa (stichting Kinderen Kankervrij, project 257) that funds research on all forms of childhood cancer. This research received no specific funding.
Conflict of interest
The authors are involved in the ongoing fertility add-on study, nested within the EuroNet-PHL-C2 study (Clinicaltrials NCT02684708; EudraCT number 2012-004053-88). This study evaluates gonadal function and fertility in children treated according to the EuroNet-PHL-C2 protocol.
References
- Adam C, Deffert C, Leyvraz C, Primi MP, Simon JP, Beck Popovic M, Bénard J, Bouthors T, Girardin C, Streuli I. et al. Use and effectiveness of sperm cryopreservation for adolescents and young adults: a 37-year bicentric experience. J Adolesc Young Adult Oncol 2020;10:78–84. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Allamaneni SS.. Disruption of spermatogenesis by the cancer disease process. J Natl Cancer Inst Monogr 2005;34:9–12. [DOI] [PubMed] [Google Scholar]
- Aleman BMP, van den Belt-Dusebout AW, De Bruin ML, van 't Veer MB, Baaijens MHA, de Boer JP, Hart AAM, Klokman WJ, Kuenen MA, Ouwens GM. et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood 2007;109:1878–1886. [DOI] [PubMed] [Google Scholar]
- Amano T, Imao T, Takemae K.. Male infertility and androgen replacement therapy for subjects with bilateral testicular tumors. Reprod Med Biol 2013;13:103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA, Cameron D, Clatot F, Demeestere I, Lambertini M, Nelson SM, Peccatori F.. Anti-Müllerian hormone as a marker of ovarian reserve and premature ovarian insufficiency in children and women with cancer: a systematic review. Hum Reprod Update 2022;28:417–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA, Su HI.. The clinical value and interpretation of anti-Müllerian hormone in women with cancer. Front Endocrinol 2020;11:574263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson A, Enblad G, Gustavsson A, Erlanson M, Hagberg H, Molin D, Tavelin B, Melin B.. Long term risk of infections in Hodgkin lymphoma long-term survivors. Br J Haematol 2011;154:661–663. [DOI] [PubMed] [Google Scholar]
- Andrieu JM, Ochoa-Molina ME.. Menstrual cycle, pregnancies and offspring before and after MOPP therapy for Hodgkin’s disease. Cancer 1983;52:435–438. [DOI] [PubMed] [Google Scholar]
- Anselmo AP, Cartoni C, Bellantuono P, Maurizi-Enrici R, Aboulkair N, Ermini M.. Risk of infertility in patients with Hodgkin’s disease treated with ABVD vs MOPP vs ABVD/MOPP. Haematologica 1990;75:155–158. [PubMed] [Google Scholar]
- Aubier F, Flamant F, Brauner R, Caillaud JM, Chaussain JM, Lemerle J, Gonadal M, After F.. Male gonadal function after chemotherapy for solid tumors in childhood. J Clin Oncol 1989;7:304–309. [DOI] [PubMed] [Google Scholar]
- Bahadur G, Ling KL, Hart R, Ralph D, Wafa R, Ashraf A, Jaman N, Mahmud S, Oyede AW.. Semen quality and cryopreservation in adolescent cancer patients. Hum Reprod 2002;17:3157–3161. [DOI] [PubMed] [Google Scholar]
- Bayle-Weisgerber C, Lemercier N, Teillet F.. Hodgkin’s disease in children. Results of therapy in a mixed group of 178 clinical and pathologically staged patients over 13 years. Cancer 1984;54:215–222. [DOI] [PubMed] [Google Scholar]
- Bedoschi G, Navarro PA, Oktay K.. Chemotherapy-induced damage to ovary: mechanisms and clinical impact. Future Oncol 2016;12:2333–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Arush MW, Solt I, Lightman A, Linn S, Kuten A.. Male gonadal function in survivors of childhood Hodgkin and non-Hodgkin lymphoma. Pediatr Hematol Oncol 2000;17:239–245. [DOI] [PubMed] [Google Scholar]
- Benedict C, Shuk E, Ford JS.. Fertility issues in adolescent and young adult cancer survivors. J Adolesc Young Adult Oncol 2016;5:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict C, Thom B, Friedman DN, Pottenger E, Raghunathan N, Kelvin JF.. Fertility information needs and concerns post-treatment contribute to lowered quality of life among young adult female cancer survivors. Support Care Cancer 2018;26:2209–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berjeb KK, Debbabi L, Braham M, Zemni Z, Chtourou S, Hannachi H, Hamdoun M, Ayadi M, Kacem K, Zhioua F. et al. Evaluation of ovarian reserve before and after chemotherapy. J Gynecol Obstet Hum Reprod 2020;50:102035. [DOI] [PubMed] [Google Scholar]
- Björndahl L, Barratt CLR, Mortimer D, Jouannet P.. “How to count sperm properly”: checklist for acceptability of studies based on human semen analysis. Hum Reprod 2016;31:227–232. [DOI] [PubMed] [Google Scholar]
- Bokemeyer C, Schmoll HJ, van Rhee J, Kuczyk M, Schuppert F, Poliwoda H.. Long-term gonadal toxicity after therapy for Hodgkin’s and non-Hodgkin’s lymphoma. Ann Hematol 1994;68:105–110. [DOI] [PubMed] [Google Scholar]
- Bonadonna G. Chemotherapy strategies to improve the control of Hodgkin’s disease: the Richard and Hinda Rosenthal Foundation Award Lecture. Cancer Res 1982;42:4309–4320. [PubMed] [Google Scholar]
- Bonadonna G, Santoro A, Viviani S.. Gonadal damage in Hodgkin’s disease from cancer chemotherapeutic regimens. Arch Toxicol 1984;55:140–145. [DOI] [PubMed] [Google Scholar]
- Bonadonna G, Valagussa P, Santoro A, Viviani S, Bonfante V, Banfi A.. Hodgkin’s disease: the Milan Cancer Institute experience with MOPP and ABVD. Recent Results Cancer Res 1989;117:169–174. [DOI] [PubMed] [Google Scholar]
- Borchmann P, Eichenauer DA, Engert A.. State of the art in the treatment of Hodgkin lymphoma. Nat Rev Clin Oncol 2012;9:450–459. [DOI] [PubMed] [Google Scholar]
- Bordallo MA, Guimarães MM, Pessoa CH, Carriço MK, Dimetz T, Gazolla HM, Dobbin J, Castilho IA.. Decreased serum inhibin B/FSH ratio as a marker of Sertoli cell function in male survivors after chemotherapy in childhood and adolescence. J Pediatr Endocrinol Metab 2004;17:879–887. [DOI] [PubMed] [Google Scholar]
- Botchan A, Hauser R, Gamzu R, Yogev L, Lessing JB, Paz G, Yavetz H.. Sperm quality in Hodgkin’s disease versus non-Hodgkin’s lymphoma. Hum Reprod 1997;12:73–76. [DOI] [PubMed] [Google Scholar]
- Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T.. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc 2016;104:240–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brämswig JH, Heimes U, Heiermann E, Schlegel W, Nieschlag E, Schellong G.. The effects of different cumulative doses of chemotherapy on testicular function. Results in 75 patients treated for Hodgkin’s disease during childhood or adolescence. Cancer 1990;65:1298–1302. [DOI] [PubMed] [Google Scholar]
- Brämswig JH, Riepenhausen M, Schellong G.. Parenthood in adult female survivors treated for Hodgkin's lymphoma during childhood and adolescence: a prospective, longitudinal study. Lancet Oncol 2015;16:667–675. [DOI] [PubMed] [Google Scholar]
- Brignardello E, Felicetti F, Castiglione A, Nervo A, Biasin E, Ciccone G, Fagioli F, Corrias A.. Gonadal status in long-term male survivors of childhood cancer. J Cancer Res Clin Oncol 2016;142:1127–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broer SL, Broekmans FJM, Laven JSE, Fauser BCJM.. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update 2014;20:688–701. [DOI] [PubMed] [Google Scholar]
- Brougham MFH, Crofton PM, Johnson EJ, Evans N, Anderson RA, Wallace WHB.. Anti-Müllerian hormone is a marker of gonadotoxicity in pre- and postpubertal girls treated for cancer: a prospective study. J Clin Endocrinol Metab 2012;97:2059–2067. [DOI] [PubMed] [Google Scholar]
- Brougham MFH, Kelnar CJH, Sharpe RM, Wallace WHB.. Male fertility following childhood cancer: current concepts and future therapies. Asian J Androl 2003;5:325–337. [PubMed] [Google Scholar]
- Brugo-Olmedo S, de Vincentiis S, Calamera JC, Urrutia F, Nodar F, Acosta AA.. Serum inhibin B may be a reliable marker of the presence of testicular spermatozoa in patients with nonobstructive azoospermia. Fertil Steril 2001;76:1124–1129. [DOI] [PubMed] [Google Scholar]
- Brusamolino E, Lunghi F, Orlandi E, Astori C, Passamonti F, Baraté C, Pagnucco G, Baio A, Franchini P, Lazzarino M. et al. Treatment of early-stage Hodgkin’s disease with four cycles of ABVD followed by adjuvant radio-therapy: analysis of efficacy and long-term toxicity. Haematologica 2000;85:1032–1039. [PubMed] [Google Scholar]
- Canada AL, Schover LR.. The psychosocial impact of interrupted childbearing in long-term female cancer survivors. Psycho-Oncology 2012;21:134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canellos GP, Anderson JR, Propert KJ, Nissen N, Cooper MR, Henderson ES, Green MR, Gottlieb A, Peterson BA.. Chemotherapy of advanced Hodgkin’s disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med 1992;327:1478–1484. [DOI] [PubMed] [Google Scholar]
- Caponecchia L, Cimino G, Sacchetto R, Fiori C, Sebastianelli A, Salacone P, Marcucci I, Tomassini S, Rago R.. Do malignant diseases affect semen quality? Sperm parameters of men with cancers. Andrologia 2016;48:333–340. [DOI] [PubMed] [Google Scholar]
- Carlsen E, Andersson A-M, Petersen JH, Skakkebaek NE.. History of febrile illness and variation in semen quality. Hum Reprod 2003;18:2089–2092. [DOI] [PubMed] [Google Scholar]
- Carter J, Chi DS, Brown CL, Abu-Rustum NR, Sonoda Y, Aghajanian C, Levine DA, Baser RE, Raviv L, Barakat RR.. Cancer-related infertility in survivorship. Int J Gynecol Cancer 2010;20:2–8. [DOI] [PubMed] [Google Scholar]
- Chan PTK, Palermo GD, Veeck LL, Rosenwaks Z, Schlegel PN.. Testicular sperm extraction combined with intracytoplasmic sperm injection in the treatment of men with persistent azoospermia postchemotherapy. Cancer 2001;92:1632–1637. [DOI] [PubMed] [Google Scholar]
- Chapman RM, , SutcliffeSB, , ReesLH, , EdwardsCR, , Malpas JS.. Cyclical combination chemotherapy and gonadal function. Retrospective study in males. Lancet. 1979;1:285–289. [DOI] [PubMed] [Google Scholar]
- Charpentier AM, Chong AL, Gingras-Hill G, Ahmed S, Cigsar C, Gupta AA, Greenblatt E, Hodgson DC.. Anti-Müllerian hormone screening to assess ovarian reserve among female survivors of childhood cancer. J Cancer Surviv 2014;8:548–554. [DOI] [PubMed] [Google Scholar]
- Chemaitilly W, Mertens AC, Mitby P, Whitton J, Stovall M, Yasui Y, Robison LL, Sklar CA.. Acute ovarian failure in the childhood cancer survivor study. J Clin Endocrinol Metab 2006;91:1723–1728. [DOI] [PubMed] [Google Scholar]
- Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HWG, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT. et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010;16:231–245. [DOI] [PubMed] [Google Scholar]
- Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. 2022. Available at www.covidence.org.
- da Cunha MF, Meistrich ML, Fuller LM, Cundiff JH, Hagemeister FB, Velasquez WS, McLaughlin P, Riggs SA, Cabanillas FF, Salvador PG.. Recovery of spermatogenesis after treatment for Hodgkin’s disease: limiting dose of MOPP chemotherapy. J Clin Oncol 1984;2:571–577. [DOI] [PubMed] [Google Scholar]
- Daudin M, Rives N, Walschaerts M, Drouineaud V, Szerman E, Koscinski I, Eustache F, Saïas-Magnan J, Papaxanthos-Roche A, Cabry-Goubet R. et al. Sperm cryopreservation in adolescents and young adults with cancer: results of the French national sperm banking network (CECOS). Fertil Steril 2015;103:478–486.e1. [DOI] [PubMed] [Google Scholar]
- de Kretser DM, Burger HG, Hudson B.. The relationship between germinal cells and serum FSH levels in males with infertility. J Clin Endocrinol Metab 1974;38:787–793. [DOI] [PubMed] [Google Scholar]
- de Vries S, Schaapveld M, Janus CPM, Daniëls LA, Petersen EJ, van der Maazen RWM, Zijlstra JM, Beijert M, Nijziel MR, Verschueren KMS. et al. Long-term cause-specific mortality in Hodgkin lymphoma patients. J Natl Cancer Inst 2021;113:760–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decanter C, Delepine J, Behal H, Manier S, Bruno B, Barbatti M, Robin C, Labreuche J, Morschhauser F, Pigny P.. Longitudinal study of AMH variations in 122 Adolescents and Young Adults (AYA) and non-AYA lymphoma patients to evaluate the chemo-induced ovarian toxicity to further personalise fertility preservation counselling. Hum Reprod 2021;36:2743–2752. [DOI] [PubMed] [Google Scholar]
- Depmann M, Eijkemans MJC, Broer S, Tehrani FR, Solaymani-Dodaran M, Azizi F, Lambalk CB, Randolph JF, Harlow SD, Freeman EW. et al. Does AMH relate to timing of menopause? Results of an individual patient data meta-analysis. J Clin Endocrinol Metab 2018;10:3593–3600. [DOI] [PubMed] [Google Scholar]
- Dhabhar BN, Malhotra H, Joseph R, Garde S, Bhasin S, Sheth A, Advani SH.. Gonadal function in prepubertal boys following treatment for Hodgkin’s disease. Am J Pediatr Hematol Oncol 1993;15:306–310. [PubMed] [Google Scholar]
- di Paola R, Costantini C, Tecchio C, Salvagno GL, Montemezzi R, Perandini A, Pizzolo G, Zaffagnini S, Franchi M.. Anti-Müllerian hormone and antral follicle count reveal a late impairment of ovarian reserve in patients undergoing low-gonadotoxic regimens for hematological malignancies. Oncologist 2013;18:1307–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNofia AM, , WangX, , YannekisG, , OgleS, , HobbieWL, , CarlsonCA, , Ginsberg JP.. Analysis of semen parameters in a young cohort of cancer patients. Pediatr Blood Cancer 2017;64:381–386. [DOI] [PubMed] [Google Scholar]
- Donaldson SS, Link MP, Weinstein HJ, Rai SN, Brain S, Billett AL, Hurwitz CA, Krasin M, Kun LE, Marcus KC. et al. Final results of a prospective clinical trial with VAMP and low-dose involved-field radiation for children with low-risk Hodgkin’s disease. J Clin Oncol 2007;25:332–337. [DOI] [PubMed] [Google Scholar]
- Elchuri SV, Patterson BC, Brown M, Bedient C, Record E, Wasilewski-Masker K, Mertens AC, Meacham LR.. Low anti-Mullerian hormone in pediatric cancer survivors in the early years after gonadotoxic therapy. J Pediatr Adolesc Gynecol 2016;29:393–399. [DOI] [PubMed] [Google Scholar]
- Ellis SJ, Wakefield CE, McLoone JK, Robertson EG, Cohn RJ.. Fertility concerns among child and adolescent cancer survivors and their parents: a qualitative analysis. J Psychosoc Oncol 2016;34:347–362. [DOI] [PubMed] [Google Scholar]
- Felicetti F, Castiglione A, Biasin E, Fortunati N, Dionisi-Vici M, Matarazzo P, Ciccone G, Fagioli F, Brignardello E.. Effects of treatments on gonadal function in long-term survivors of pediatric hematologic malignancies: a cohort study. Pediatr Blood Cancer 2020;67:e28709. [DOI] [PubMed] [Google Scholar]
- Franchimont P, Millet D, Vendrely E, Letawe J, Legros JJ, Netter A.. Relationship between spermatogenesis and serum gonadotropin levels in azoospermia and oligospermia. J Clin Endocrinol Metab 1972;34:1003–1008. [DOI] [PubMed] [Google Scholar]
- George SA, Williamson Lewis R, Schirmer DA, Effinger KE, Spencer JB, Mertens AC, Meacham LR.. Early detection of ovarian dysfunction by anti-Mullerian hormone in adolescent and young adult-aged survivors of childhood cancer. J Adolesc Young Adult Oncol 2019;8:18–25. [DOI] [PubMed] [Google Scholar]
- Gerres L, Brämswig JH, Schlegel W, Jürgens H, Schellong G.. The effects of etoposide on testicular function in boys treated for Hodgkin’s disease. Cancer 1998;83:2217–2222. [DOI] [PubMed] [Google Scholar]
- Ginsberg JP, , OgleSK, , TuchmanLK, , CarlsonCA, , ReillyMM, , HobbieWL, , RourkeM, , ZhaoH, , Meadows AT.. Sperm banking for adolescent and young adult cancer patients: sperm quality, patient, and parent perspectives. Pediatr Blood Cancer 2008;50:594–598. [DOI] [PubMed] [Google Scholar]
- Goossens E, Jahnukainen K, Mitchell RT, van Pelt A, Pennings G, Rives N, Poels J, Wyns C, Lane S, Rodriguez-Wallberg KA. et al. Fertility preservation in boys: recent developments and new insights. Hum Reprod Open 2020;2020:hoaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gözdasoglu S, Cavdar AO, Babacan E, Mengübas K, Yavuz G, Unal E, Pamir A, Ocal G, Haluk Gökçora I.. Late effects of chemoradiotherapy in pediatric Hodgkin’s disease. J Chemother 1995;7:463–466. [DOI] [PubMed] [Google Scholar]
- Gracia CR, Sammel MD, Freeman E, Prewitt M, Carlson C, Ray A, Vance A, Ginsberg JP.. Impact of cancer therapies on ovarian reserve. Fertil Steril 2012;97:134–140.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Brecher ML, Lindsay AN, Yakar D, Voorhess ML, MacGillivray MH, Freeman AI.. Gonadal function in pediatric patients following treatment for Hodgkin disease. Med Pediatr Oncol 1981;9:235–244. [DOI] [PubMed] [Google Scholar]
- Green DM, Hall B.. Pregnancy outcome following treatment during childhood or adolescence for Hodgkin’s disease. Pediatr Hematol Oncol 1988;5:269–277. [DOI] [PubMed] [Google Scholar]
- Hagenäs I, Jørgensen N, Rechnitzer C, Sommer P, Holm M, Schmiegelow K, Daugaard G, Jacobsen N, Juul A.. Clinical and biochemical correlates of successful semen collection for cryopreservation from 12-18-year-old patients: a single-center study of 86 adolescents. Hum Reprod 2010;25:2031–2038. [DOI] [PubMed] [Google Scholar]
- Hallak J, Mahran AM, Agarwal A.. Characteristics of cryopreserved semen from men with lymphoma. J Assist Reprod Genet 2000;17:591–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ; STRAW + 10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab 2012;97:1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KR, Hodnett GM, Knowlton N, Craig LB.. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril 2011;95:170–175. [DOI] [PubMed] [Google Scholar]
- Hassel JU, Bramswig JH, Schlegel W, Schellong G.. Testicular function following OPA/COMP-chemotherapy in pubertal boys treated for Hodgkin’s disease. Results in 25 patients of the West-German therapy study DAL-HD-85 not receiving procarbazine. Klin Padiatr 1991;203:268–272. [DOI] [PubMed] [Google Scholar]
- Heikens J, Behrendt H, Adriaanse R, Berghout A.. Irreversible gonadal damage in male survivors of pediatric Hodgkin’s disease. Cancer 1996;78:2020–2024. [DOI] [PubMed] [Google Scholar]
- Hobbie WL, Ginsberg JP, Ogle SK, Carlson CA, Meadows AT.. Fertility in males treated for Hodgkins disease with COPP/ABV hybrid. Pediatr Blood Cancer 2005;44:193–196. [DOI] [PubMed] [Google Scholar]
- Horning SJ, Hoppe RT, Kaplan HS, Rosenberg SA.. Female reproductive potential after treatment for Hodgkin’s disease. N Engl J Med 1981;304:1377–1382. [DOI] [PubMed] [Google Scholar]
- Hsiao W, Stahl PJ, Osterberg EC, Nejat E, Palermo GD, Rosenwaks Z, Schlegel PN.. Successful treatment of postchemotherapy azoospermia with microsurgical testicular sperm extraction: the Weill Cornell experience. J Clin Oncol 2011;29:1607–1611. [DOI] [PubMed] [Google Scholar]
- Hudson MM, Greenwald C, Thompson E, Wilimas J, Marina N, Fairclough D, Kauffman W, Bozeman P, Mackert PW, Abromowitch M.. Efficacy and toxicity of multiagent chemotherapy and low-dose involved-field radiotherapy in children and adolescents with Hodgkin’s disease. J Clin Oncol 1993;11:100–108. [DOI] [PubMed] [Google Scholar]
- Jaffe N, , SullivanMP, , RiedH, , BorenH, , MarshallR, , MeistrichM, , MaorM, , Da Cunha M.. Male reproductive function in long-term survivors of childhood cancer. Med Pediatr Oncol 1988;16:241–247. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Andersson A-M, Hjollund NHI, Scheike T, Kolstad H, Giwercman A, Henriksen TB, Ernst E, Bonde JP, Olsen J. et al. Inhibin B as a serum marker of spermatogenesis: Correlation to differences in sperm concentration and follicle-stimulating hormone levels. A study of 349 Danish men. J Clin Endocrinol Metab 1997;82:4059–4063. [DOI] [PubMed] [Google Scholar]
- Johnson AC, Mays D, Rehberg K, Shad A, Tercyak KP.. Knowledge and beliefs about oncofertility and associations with quality of life among adolescent and young adult survivors of pediatric cancer. J Adolesc Young Adult Oncol 2018;7:424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kader HA, Rostom AY.. Follicle stimulating hormone levels as a predictor of recovery of spermatogenesis following cancer therapy. Clin Oncol (R Coll Radiol) 1991;3:37–40. [DOI] [PubMed] [Google Scholar]
- Keene DJB, , SajjadY, , MakinG, , Cervellione RM.. Sperm banking in the United Kingdom is feasible in patients 13 years old or older with cancer. J Urol 2012;188:594–597. [DOI] [PubMed] [Google Scholar]
- Kelsey TW, McConville L, Edgar AB, Ungurianu AI, Mitchell RT, Anderson RA, Wallace WHB.. Follicle stimulating hormone is an accurate predictor of azoospermia in childhood cancer survivors. PLoS One 2017;12:e0181377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WHB.. A validated model of serum anti-Müllerian hormone from conception to menopause. PLoS One 2011;6:e22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney LB, Cohen LE, Shnorhavorian M, Metzger ML, Lockart B, Hijiya N, Duffey-Lind E, Constine L, Green D, Meacham L.. Male reproductive health after childhood, adolescent, and young adult cancers: a report from the Children’s Oncology Group. J Clin Oncol 2012;30:3408–3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keskin MZ, Budak S, Zeyrek T, Çelik O, Mertoglu O, Yoldas M, Ilbey YÖ.. The relationship between serum hormone levels (follicle-stimulating hormone, luteinizing hormone, total testosterone) and semen parameters. Arch Ital Urol Androl 2015;87:194–197. [DOI] [PubMed] [Google Scholar]
- Klingmüller D, Haidl G.. Inhibin B in men with normal and disturbed spermatogenesis. Hum Reprod 1997;12:2376–2378. [DOI] [PubMed] [Google Scholar]
- Koziner B, , MyersJ, , CirrincioneC, , RedmanJ, , CunninghamI, , CaravelliJ, , NisceLZ, , MccormickB, , StrausDJ, , Mertelsmann R. et al. Treatment of stages I and II hodgkin's disease with three different therapeutic modalities. The American Journal of Medicine 1986;80:1067–1078. [DOI] [PubMed] [Google Scholar]
- Krawczuk-Rybak M, Płonowski M, Solarz E, Sega-Pondel D, Kazanowska B, Zelazowska-Rutkowska B, Wysocka J.. Assessment of gonadal function in boys and adolescents at the diagnosis of neoplastic disease. J Pediatr Endocrinol Metab 2012;25:453–458. [DOI] [PubMed] [Google Scholar]
- Krawczuk-Rybak M, Leszczynska E, Poznanska M, Zelazowska-Rutkowska B, Wysocka J.. The progressive reduction in the ovarian reserve in young women after anticancer treatment. Horm Metab Res 2013a;45:813–819. [DOI] [PubMed] [Google Scholar]
- Krawczuk-Rybak M, Leszczynska E, Poznanska M, Zelazowska-Rutkowska B, Wysocka J.. Anti-Müllerian hormone as a sensitive marker of ovarian function in young cancer survivors. Int J Endocrinol 2013b;2013:125080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuser ED, , XirosN, , HetzelWD, , Heimpel H.. Reproductive and endocrine gonadal capacity in patients treated with COPP chemotherapy for Hodgkin's disease. J Cancer Res Clin Oncol 1987;113:260–266. [DOI] [PubMed] [Google Scholar]
- Kruseová J, ČerníkováJ, , Zámečníková M, Hřivnová L, Koloušková S, Čepelová M, Kabíčková E, Čapek V, Lukš A, Eckschlager T. Semen analysis and treatment risk factors in long-term survivors of childhood cancer. Andrologia 2021;53(1):e13853. doi:10.1111/and.13853 [DOI] [PubMed] [Google Scholar]
- Lacher MJ, Toner K.. Pregnancies and menstrual function before and after combined radiation (RT) and chemotherapy (TVPP) for Hodgkin’s disease. Cancer Invest 1986;4:93–100. [DOI] [PubMed] [Google Scholar]
- Laddaga FE, Masciopinto P, Nardelli C, Vacca MP, Masciandaro P, Arcuti E, Cicinelli E, Specchia G, Musto P, Gaudio F.. In male Hodgkin lymphoma patients, impaired fertility may be improved by non-gonadotoxic therapy. Br J Haematol 2022;196:110–115. [DOI] [PubMed] [Google Scholar]
- Lähteenmäki PM, Toppari J, Ruokonen A, Laitinen P, Salmi TT.. Low serum inhibin B concentrations in male survivors of childhood malignancy. Eur J Cancer 1999;35:612–619. [DOI] [PubMed] [Google Scholar]
- Levine JM, Whitton JA, Ginsberg JP, Green DM, Leisenring WM, Stovall M, Robison LL, Armstrong GT, Sklar CA.. Nonsurgical premature menopause and reproductive implications in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer 2018;124:1044–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie Fong S, Laven JSE, Hakvoort-Cammel FGAJ, Schipper I, Visser JA, Themmen APN, de Jong FH, van den Heuvel-Eibrink MM.. Assessment of ovarian reserve in adult childhood cancer survivors using anti-Mullerian hormone. Hum Reprod 2009;24:982–990. [DOI] [PubMed] [Google Scholar]
- Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, Quinn G, Wallace WH, Oktay K.. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2500–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie EJ, Radford M, Shalet SM.. Gonadal function following chemotherapy for childhood Hodgkin’s disease. Med Pediatr Oncol 1996;27:74–78. [DOI] [PubMed] [Google Scholar]
- Madsen BL, Giudice L, Donaldson SS.. Radiation-induced premature menopause: a misconception. Int J Radiat Oncol Biol Phys 1995;32:1461–1464. [DOI] [PubMed] [Google Scholar]
- Mauz-Körholz C, Hasenclever D, Dörffel W, Ruschke K, Pelz T, Voigt A, Stiefel M, Winkler M, Vilser C, Dieckmann K. et al. Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin’s lymphoma: the GPOH-HD-2002 study. J Clin Oncol 2010;28:3680–3686. [DOI] [PubMed] [Google Scholar]
- Mauz-Körholz C, Landman-Parker J, Balwierz W, Ammann RA, Anderson RA, Attarbaschi A, Bartelt JM, Beishuizen A, Boudjemaa S, Cepelova M. et al. Response-adapted omission of radiotherapy and comparison of consolidation chemotherapy in children and adolescents with intermediate-stage and advanced-stage classical Hodgkin lymphoma (EuroNet-PHL-C1): a titration study with an open-label, embedded, multinational, non-inferiority, randomised controlled trial. Lancet Oncol 2022;23:125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliukaite I, Hagen JM, Jahnukainen K, Stukenborg JB, Repping S, van der Veen F, van Wely M, van Pelt AM.. Establishing reference values for age-related spermatogonial quantity in prepubertal human testes: a systematic review and meta-analysis. Fertil Steril 2016;106:1652–1657.e2. [DOI] [PubMed] [Google Scholar]
- Meirow D. Reproduction post-chemotherapy in young cancer patients. Mol Cell Endocrinol 2000;169:123–131. [DOI] [PubMed] [Google Scholar]
- Meistrich ML. Effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertil Steril 2013;100:1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon S, , RivesN, , Mousset-SiméonN, , SibertL, , VannierJP, , MazurierS, , MasséL, , DuchesneV, , Macé B.. Fertility preservation in adolescent males: experience over 22 years at Rouen University Hospital. Hum Reprod 2009;24:37–44. [DOI] [PubMed] [Google Scholar]
- Meseguer M, Garrido N, Remohí J, Pellicer A, Simón C, Martínez-Jabaloyas JM, Gil-Salom M.. Testicular sperm extraction (TESE) and ICSI in patients with permanent azoospermia after chemotherapy. Hum Reprod 2003;18:1281–1285. [DOI] [PubMed] [Google Scholar]
- Miyoshi Y, Ohta H, Namba N, Tachibana M, Miyamura T, Miyashita E, Hashii Y, Oue T, Isobe A, Tsutsui T. et al. Low serum concentrations of anti-Mullerian hormone are common in 53 female childhood cancer survivors. Horm Res Paediatr 2013;79:17–21. [DOI] [PubMed] [Google Scholar]
- Mulder RL, Font-Gonzalez A, Green DM, Loeffen EAH, Hudson MM, Loonen J, Yu R, Ginsberg JP, Mitchell RT, Byrne J. et al. Fertility preservation for male patients with childhood, adolescent, and young adult cancer: recommendations from the PanCareLIFE Consortium and the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 2021a;22:e57–e67. [DOI] [PubMed] [Google Scholar]
- Mulder RL, Font-Gonzalez A, Hudson MM, van Santen HM, Loeffen EAH, Burns KC, Quinn GP, van Dulmen-den Broeder E, Byrne J, Haupt R. et al. Fertility preservation for female patients with childhood, adolescent, and young adult cancer: recommendations from the PanCareLIFE Consortium and the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 2021b;22:e45–e56. [DOI] [PubMed] [Google Scholar]
- Müller HL, , Klinkhammer-SchalkeM, , Seelbach-GöbelB, , HartmannAA, , Kuhl J.. Gonadal function of young adults after therapy of malignancies during childhood or adolescence. Eur J Pediatr 1996;155:763–769. [DOI] [PubMed] [Google Scholar]
- Myrehaug S, Pintilie M, Tsang R, Mackenzie R, Crump M, Chen Z, Sun A, Hodgson DC.. Cardiac morbidity following modern treatment for Hodgkin lymphoma: supra-additive cardiotoxicity of doxorubicin and radiation therapy. Leuk Lymphoma 2008;49:1486–1493. [DOI] [PubMed] [Google Scholar]
- Naysmith TE, Blake DA, Harvey VJ, Johnson NP.. Do men undergoing sterilizing cancer treatments have a fertile future? Hum Reprod 1998;13:3250–3255. [DOI] [PubMed] [Google Scholar]
- Ng AK, Patricia Bernardo M V, Weller E, Backstrand K, Silver B, Marcus KC, Tarbell NJ, Stevenson MA, Friedberg JW, Mauch PM.. Second malignancy after Hodgkin disease treated with radiation therapy with or without chemotherapy: Long-term risks and risk factors. Blood 2002;100:1989–1996. [DOI] [PubMed] [Google Scholar]
- Oktem O, Kim SS, Selek U, Schatmann G, Urman B.. Ovarian and uterine functions in female survivors of childhood cancers. Oncologist 2018;23:214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktem O, Oktay K.. Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer 2007;110:2222–2229. [DOI] [PubMed] [Google Scholar]
- Ortin TT, Shostak CA, Donaldson SS.. Gonadal status and reproductive function following treatment for Hodgkin's disease in childhood: the Stanford experience. Int J Radiat Oncol Biol Phys 1990;19:873–880. [DOI] [PubMed] [Google Scholar]
- Otten R, de Vries R, Schoonmade L. Amsterdam Efficient Deduplication (AED) method (Version 1). Zenodo. 2019.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoli D, Rizzo F, Fiore G, Pallotti F, Pulsoni A, Annechini G, Lombardo F, Lenzi A, Gandini L. Spermatogenesis in Hodgkin's lymphoma patients: a retrospective study of semen quality before and after different chemotherapy regimens. Hum Reprod. 2016;31(2):263–272. [DOI] [PubMed] [Google Scholar]
- Papadakis V, Vlachopapadopoulou E, van Syckle K, Ganshaw L, Kalmanti M, Tan C, Sklar C.. Gonadal function in young patients successfully treated for Hodgkin disease. Med Pediatr Oncol 1999;32:366–372. [DOI] [PubMed] [Google Scholar]
- Paradisi R, Vicenti R, Macciocca M, Seracchioli R, Rossi S, Fabbri R.. High cytokine expression and reduced ovarian reserve in patients with Hodgkin lymphoma or non-Hodgkin lymphoma. Fertil Steril 2016;106:1176–1182. [DOI] [PubMed] [Google Scholar]
- Pennisi AJ, Grushkin CM, Lieberman E.. Gonadal function in children with nephrosis treated with cyclophosphamide. Am J Dis Child 1975;129:315–318. [DOI] [PubMed] [Google Scholar]
- Perrone L, Sinisi AA, di Tullio MT, Casale F, Indolfi P, Manzo T, Coppola G, Bellastella A, Faggiano M.. Endocrine function in subjects treated for childhood Hodgkins’ disease. J Pediatr Endocrinol Metab 1989;3:175–179. [Google Scholar]
- Pivetta E, Maule MM, Pisani P, Zugna D, Haupt R, Jankovic M, Aricò M, Casale F, Clerico A, Cordero di Montezemolo L. et al. ; Italian Association of Pediatric Hematology and Oncology (AIEOP) Group. Marriage and parenthood among childhood cancer survivors: a report from the Italian AIEOP Off-Therapy Registry. Haematologica 2011;96:744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Policiano C, Subirá J, Aguilar A, Monzó S, Iniesta I, Rubio Rubio JM.. Impact of ABVD chemotherapy on ovarian reserve after fertility preservation in reproductive-aged women with Hodgkin lymphoma. J Assist Reprod Genet 2020;37:1755–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafsanjani KA, , FaranoushM, , HedayatiaslAA, , Vossough P.. Gonadal function and fertility in male survivors treated for Hodgkin's disease in Iran. Saudi Med J. 2007;28(11):1690–1693. [PubMed] [Google Scholar]
- Relander T, , Cavallin-StåhlE, , GarwiczS, , OlssonAM, , Willén M.. Gonadal and sexual function in men treated for childhood cancer. Med Pediatr Oncol 2000;35:52–63. [DOI] [PubMed] [Google Scholar]
- Reulen RC, Zeegers MP, Wallace WHB, Frobisher C, Taylor AJ, Lancashire ER, Winter DL, Hawkins MM; British Childhood Cancer Survivor Study. Pregnancy outcomes among adult survivors of childhood cancer in the british childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev 2009;18:2239–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson DM, Tsonis CG, McLachlan RI, Handelsman DJ, Leask R, Baird DT, McNeilly AS, Hayward S, Healy DL, Findlay JK.. Comparison of inhibin immunological and in vitro biological activities in human serum. J Clin Endocrinol Metab 1988;67:438–443. [DOI] [PubMed] [Google Scholar]
- Romerius P, Ståhl O, Moëll C, Relander T, Cavallin-Ståhl E, Wiebe T, Giwercman YL, Giwercman A.. Hypogonadism risk in men treated for childhood cancer. J Clin Endocrinol Metab 2009;94:4180–4186. [DOI] [PubMed] [Google Scholar]
- Romerius P, Ståhl O, Moëll C, Relander T, Cavallin-Ståhl E, Wiebe T, Giwercman YL, Giwercman A, Moe C, Wiebe T. et al. High risk of azoospermia in men treated for childhood cancer. Int J Androl 2010;34:69–76. [DOI] [PubMed] [Google Scholar]
- Rosen MP, Johnstone E, McCulloch CE, Schuh-Huerta SM, Sternfeld B, Reijo-Pera RA, Cedars MI.. A characterization of the relationship of ovarian reserve markers with age. Fertil Steril 2012;97:238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salih SM, Elsarrag SZ, Prange E, Contreras K, Osman RG, Eikoff JC, Puccetti D.. Evidence to incorporate inclusive reproductive health measures in guidelines for childhood and adolescent cancer survivors. J Pediatr Adolesc Gynecol 2015;28:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaapveld M, Aleman BMP, van Eggermond AM, Janus CPM, Krol ADG, van der Maazen RWM, Roesink J, Raemaekers JMM, de Boer JP, Zijlstra JM. et al. Second cancer risk up to 40 years after treatment for Hodgkin’s lymphoma. N Engl J Med 2015;373:2499–2511. [DOI] [PubMed] [Google Scholar]
- Schellong G. Pediatric Hodgkin’s disease: Treatment in the late 1990s. Annals of Oncology 1998;9:s115–s119. [DOI] [PubMed] [Google Scholar]
- Servitzoglou M, , De VathaireF, , OberlinO, , PatteC, , Thomas-Teinturier C.. Dose–Effect Relationship of Alkylating Agents on Testicular Function in Male Survivors of Childhood Lymphoma. Pediatric Hematology and Oncology 2015;32:613–623. [DOI] [PubMed] [Google Scholar]
- Shafford EA, Kingston JE, Malpas JS, Plowman PN, Pritchard J, Savage MO, Eden OB.. Testicular function following the treatment of Hodgkin’s disease in childhood. Br J Cancer 1993;68:1199–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherins RJ, Olweny CLM, Ziegler JL.. Gynecomastia and gonadal dysfunction in adolescent boys treated with combination chemotherapy for Hodgkin’s disease. N Engl J Med 1978;299:12–16. [DOI] [PubMed] [Google Scholar]
- Skinner R, Mulder RL, Kremer LC, Hudson MM, Constine LS, Bardi E, Boekhout A, Borgmann-Staudt A, Brown MC, Cohn R. et al. Recommendations for gonadotoxicity surveillance in male childhood, adolescent, and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCareSurFup Consortium. Lancet Oncol 2017;18:e75–e90. [DOI] [PubMed] [Google Scholar]
- Sklar CA, Robison LL, Nesbit ME, Sather HN, Meadows AT, Ortega JA, Kim TH, Hammond GD.. Effects of radiation on testicular function in long-term survivors of childhood acute lymphoblastic leukemia: a report from the Children Cancer Study Group. J Clin Oncol 1990;8:1981–1987. [DOI] [PubMed] [Google Scholar]
- Sklar CA, Mertens AC, Mitby P, Whitton J, Stovall M, Kasper C, Mulder J, Green D, Nicholson HS, Yasui Y. et al. Premature menopause in survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst 2006;98:890–896. [DOI] [PubMed] [Google Scholar]
- Smith LB, Walker WH.. The regulation of spermatogenesis by androgens. Semin Cell Dev Biol 2014;30:2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonigo C, Beau I, Binart N, Grynberg M.. The impact of chemotherapy on the ovaries: molecular aspects and the prevention of ovarian damage. Int J Mol Sci 2019;20:5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears N, Lopes F, Stefansdottir A, Rossi V, de Felici M, Anderson RA, Klinger FG.. Ovarian damage from chemotherapy and current approaches to its protection. Hum Reprod Update 2019;25:673–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stukenborg JB, Alves-Lopes JP, Kurek M, Albalushi H, Reda A, Keros V, Töhönen V, Bjarnason R, Romerius P, Sundin M. et al. Spermatogonial quantity in human prepubertal testicular tissue collected for fertility preservation prior to potentially sterilizing therapy. Hum Reprod 2018a;33:1677–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stukenborg JB, Jahnukainen K, Hutka M, Mitchell RT.. Cancer treatment in childhood and testicular function: the importance of the somatic environment. Endocr Connect 2018b;7:R69–R87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow AJ, Cooke R, Bates A, Cunningham D, Falk SJ, Gilson D, Hancock BW, Harris SJ, Horwich A, Hoskin PJ. et al. Risk of premature menopause after treatment for Hodgkin’s lymphoma. J Natl Cancer Inst 2014;106:dju207. [DOI] [PubMed] [Google Scholar]
- Swerdlow AJ, Higgins CD, Smith P, Cunningham D, Hancock BW, Horwich A, Hoskin PJ, Lister TA, Radford JA, Rohatiner AZS. et al. Second cancer risk after chemotherapy for Hodgkin’s lymphoma: a collaborative British cohort study. J Clin Oncol 2011;29:4096–4104. [DOI] [PubMed] [Google Scholar]
- Trottmann M, Becker AJ, Stadler T, Straub J, Soljanik I, Schlenker B, Stief CG.. Semen quality in men with malignant diseases before and after therapy and the role of cryopreservation. Eur Urol 2007;52:355–367. [DOI] [PubMed] [Google Scholar]
- van Beek RD, Smit M, van den Heuvel-Eibrink MM, de Jong FH, Hakvoort-Cammel FG, van den Bos C, van den Berg H, Weber RF, Pieters R, de Muinck Keizer-Schrama SM.. Inhibin B is superior to FSH as a serum marker for spermatogenesis in men treated for Hodgkin’s lymphoma with chemotherapy during childhood. Hum Reprod 2007a;22:3215–3222. [DOI] [PubMed] [Google Scholar]
- van Beek RD, van den Heuvel-Eibrink MM, Laven JS, de Jong FH, Themmen AP, Hakvoort-Cammel FG, van den Bos C, van den Berg H, Pieters R, de Muinck Keizer-Schrama SM.. Anti-Mullerian hormone is a sensitive serum marker for gonadal function in women treated for Hodgkin’s lymphoma during childhood. J Clin Endocrinol Metab 2007b;92:3869–3874. [DOI] [PubMed] [Google Scholar]
- van Casteren NJ, Dohle GR, Romijn JC, de Muinck Keizer-Schrama SMPF, Weber RFA, van den Heuvel-Eibrink MM.. Semen cryopreservation in pubertal boys before gonadotoxic treatment and the role of endocrinologic evaluation in predicting sperm yield. Fertil Steril 2008;90:1119–1125. [DOI] [PubMed] [Google Scholar]
- van der Perk MEM, van der Kooi ALF, van de Wetering MD, IJgosse IM, van Dulmen-den Broeder E, Broer SL, Klijn AJ, Versluys AB, Arends B, Oude Ophuis RJA. et al. Oncofertility care for newly diagnosed girls with cancer in a national pediatric oncology setting, the first full year experience from the Princess Máxima Center, the PEARL study. PLoS One 2021;16:e0246344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg H, van den Berg H, Furstner F, van den Bos C, Behrendt H.. Decreasing the number of MOPP courses reduces gonadal damage in survivors of childhood Hodgkin disease. Pediatr Blood Cancer 2004;42:210–215. [DOI] [PubMed] [Google Scholar]
- van den Berg MH, Overbeek A, Lambalk CB, Kaspers GJL, Bresters D, van den Heuvel-Eibrink MM, Kremer LC, Loonen JJ, van der Pal HJ, Ronckers CM. et al. ; DCOG LATER-VEVO study group. Long-term effects of childhood cancer treatment on hormonal and ultrasound markers of ovarian reserve. Hum Reprod 2018;33:1474–1488. [DOI] [PubMed] [Google Scholar]
- van der Kaaij MA, Heutte N, Meijnders P, Abeilard-Lemoisson E, Spina M, Moser LC, Allgeier A, Meulemans B, Dubois B, Simons AH. et al. Parenthood in survivors of Hodgkin lymphoma: an EORTC-GELA general population case-control study. J Clin Oncol 2012a;30:3854–3863. [DOI] [PubMed] [Google Scholar]
- van der Kaaij MAE, Heutte N, Meijnders P, Abeilard-Lemoisson E, Spina M, Moser EC, Allgeier A, Meulemans B, Simons AHM, Lugtenburg PJ. et al. Premature ovarian failure and fertility in long-term survivors of hodgkin’s lymphoma: a European Organisation for Research and Treatment of Cancer Lymphoma Group and Groupe d’Étude des Lymphomes de l’Adulte Cohort Study. J Clin Oncol 2012b;30:291–299. [DOI] [PubMed] [Google Scholar]
- van der Kaaij MAE, Heutte N, van Echten-Arends J, Raemaekers JMM, Carde P, Noordijk EM, Fermé C, Thomas J, Eghbali H, Brice P. et al. Sperm quality before treatment in patients with early stage Hodgkin’s lymphoma enrolled in EORTC-GELA Lymphoma Group trials. Haematologica 2009;94:1691–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dorp W, van den Heuvel-Eibrink MM, de Vries AC, Pluijm SM, Visser JA, Pieters R, Laven JS.. Decreased serum anti-Müllerian hormone levels in girls with newly diagnosed cancer. Hum Reprod 2014;29:337–342. [DOI] [PubMed] [Google Scholar]
- van Leeuwen FE, Klokman WJ, Veer MB, Hagenbeek A, Krol AD, Vetter UA, Schaapveld M, van Heerde P, Burgers JM, Somers R. et al. Long-term risk of second malignancy in survivors of Hodgkin’s disease treated during adolescence or young adulthood. J Clin Oncol 2000;18:487–497. [DOI] [PubMed] [Google Scholar]
- van Leeuwen FE, Ng AK.. Late sequelae in Hodgkin lymphoma survivors. Hematol Oncol 2017;35:60–66. [DOI] [PubMed] [Google Scholar]
- van Nimwegen FA, Schaapveld M, Janus CPM, Krol ADG, Petersen EJ, Raemaekers JMM, Kok WEM, Aleman BMP, van Leeuwen FE.. Cardiovascular disease after hodgkin lymphoma treatment 40-year disease risk. JAMA Intern Med 2015;175:1007–1017. [DOI] [PubMed] [Google Scholar]
- Viviani S, Santoro A, Ragni G, Bonfante V, Bestetti O, Bonadonna G.. Gonadal toxicity after combination chemotherapy for Hodgkin’s disease. Comparative results of MOPP vs ABVD. Eur J Cancer Clin Oncol 1985;21:601–605. [DOI] [PubMed] [Google Scholar]
- Wallace WH. Oncofertility and preservation of reproductive capacity in children and young adults. Cancer 2011;117:2301–2310. [DOI] [PubMed] [Google Scholar]
- Waxman JH, , TerryYA, , WrigleyPF, , MalpasJS, , ReesLH, , BesserGM, , Lister TA.. Gonadal function in Hodgkin's disease: long-term follow-up of chemotherapy. Br Med J (Clin Res Ed) 1982;285:1612–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber L, Davies M, Anderson R, Bartlett J, Braat D, Cartwright B, Cifkova R, de Muinck Keizer-Schrama S, Hogervorst E, Janse F. et al. ; European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI. ESHRE Guideline: Management of women with premature ovarian insufficiency. Hum Reprod 2016;31:926–937. [DOI] [PubMed] [Google Scholar]
- Whitehead E, Shalet SM, Jones PH, Beardwell CG, Deakin DP.. Gonadal function after combination chemotherapy for Hodgkin’s disease in childhood. Arch Dis Child 1982;57:287–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilimas J, Thompson E, Smith KL.. Long-term results of treatment of children and adolescents with Hodgkin’s disease. Cancer 1980;46:2123–2125. [DOI] [PubMed] [Google Scholar]
- Yates AP, Jopling HM, Burgoyne NJ, Hayden K, Chaloner CM, Tetlow L.. Paediatric reference intervals for plasma anti-Müllerian hormone: comparison of data from the Roche Elecsys assay and the Beckman Coulter Access assay using the same cohort of samples. Ann Clin Biochem 2019;56:536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaletel L, Bratanic N, Jereb B.. Gonadal function in patients treated for Hodgkin’s disease in childhood. Radiol Oncol 2010;44:187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary Materials.