Abstract
Marsupials are born very immature yet must be sufficiently autonomous to crawl on the mother’s belly, find a teat and attach to it to pursue their development. Sensory inputs are necessary to guide the newborn to a teat and induce attachment. The vestibular system, which perceives gravity and head movements, is one of the senses proposed to guide newborns towards the teats but there are conflicting observations about its functionality at birth (postnatal day (P) 0). To test if the vestibular system of opossum newborns is functional and can influence locomotion, we used two approaches. First, we stimulated the vestibular apparatus in in vitro preparations from opossums aged from P1 to P12 and recorded motor responses: at all ages studied, mechanical pressures applied on the vestibular organs induced spinal roots activity whereas head tilts did not induce forelimb muscle contractions. Second, using immunofluorescence, we assessed the presence of Piezo2, a protein involved in mechanotransduction in vestibular hair cells. Piezo2 labeling was scant in the utricular macula at birth, but observed in all vestibular organs at P7, its intensity increasing up to P14; it seemed to stay the same at P21. Our results indicate that neural pathways from the labyrinth to the spinal cord are already in place around birth but that the vestibular organs are too immature to influence motor activity before the end of the second postnatal week in the opossum. It may be the rule in marsupial species that the vestibular system becomes functional only after birth.
Keywords: Development, Hair cells, Marsupials, Piezo2, Sensorimotor behaviors, Vestibular labyrinth
Highlights
-
•
Question: is the vestibular system functional at birth in opossums?
-
•
Nervous pathways from the labyrinth to the spinal cord are functional at birth
-
•
Head elevations in vitro do not induce motor response even at postnatal day (P) 12
-
•
Piezo2 expression suggests that hair cells are not functional before P5 at best
-
•
Answer: no. Vestibular sensory organs and afferents are the limiting factors
1. Introduction
Marsupials give birth to extremely altricial young after a short gestational period, 4 days to 2 weeks after the beginning of gastrulation (Hughes and Hall, 1988, Smith and Keyte, 2019). The newborns are glabrous, blind and deaf; they weigh between 0.01 and 1 g and are less developed than even the most altricial placental newborn (Ferner et al., 2017, Russell, 1982). Despite their immature state, the newborns must navigate their way to a teat to which they attach to pursue their development. The mother helps the newborns mainly by removing the embryonic membranes and fluids but does not place the newborns on the teats nor does she help them get there. To reach a teat, newborn marsupials use a more or less advanced form of locomotion depending on the species (Hughes and Hall, 1988, Gemmell et al., 2002, Nelson and Gemmell, 2004), from rudimentary undulations of the body to ‘swim’ in viscous amniotic liquid on the mother’s belly, as in bandicoots or native cats, to rhythmic and alternate forelimbs extensions to crawl by grabbing the fur of the mother, as in kangaroos or opossums. In all species, the hindlimbs are less developed than the forelimbs and are immobile. The precocious locomotor behaviors follow simple rhythmic patterns that could result from the activation of spinal central pattern generators which are present in all vertebrates (Kiehn, 2006, Grillner, 2011, Grillner and El Manira, 2020). However the functionality of these networks is not well studied in marsupials (but see the work of S.M. Ho, 1997, Ho, 1998 on the tammar wallaby).
In numerous marsupial species, the newborns show a negative geotropism, i.e., they orient their head and crawl against gravity. This behavior, in conjunction with the parturition posture adopted by the mother, helps to direct the newborn towards the teats which are either covered by a skin fold (i.e., marsupium) or exposed (i.e., marsupial field). This negative geotropism suggests that the vestibular system, responsible for the sense of balance, can affect the locomotor behavior of the newborns. The vestibular perception depends on hair cells present on the vestibular sensory organs located in the inner ear (Angelaki and Cullen, 2008, Goldberg, 2012). There are five organs: the utricular and saccular maculae perceive the position of the head relative to gravity, a static component of the vestibular sense, and the ampullary crests of the three semicircular canals perceive displacement of the head, a dynamic component. The hair cells are innervated by sensory primary afferents that relay sensory inputs mainly to four brainstem nuclei - i.e., the superior (SVe), Medial (MVe), Spinal or Inferior (SpVe) and Lateral (LVe) vestibular nuclei – and to the cerebellum. The central vestibular neurons affect spinal motor activity via two major systems of projections, the vestibulospinal and the vestibulo-reticulo-spinal pathways.
The earliest behavioral and anatomical studies in different marsupial species suggested that the vestibular system is not mature enough to influence locomotor behaviors at birth (e.g., Larsell et al., 1935, Hartman, 1952 Chapter 13; Cannon et al., 1976). Other mechanisms were proposed such as the hindquarters and hindlimbs acting as a dead weight (Larsell et al., 1935, Hartman, 1952) passively orienting the head against gravity, or the action of neck receptors acting on the spinal activity in an undescribed manner (Cannon et al., 1976). Moreover, studies on the development of behavioral reflexes in marsupials showed that air righting, a reflex that has a strong vestibular components, is not expressed before at least two weeks after birth (Pellis et al., 1992, Cassidy et al., 1994).
However, the idea that the vestibular system influences precocious locomotor behaviors of newborn marsupials has also found support. Even if the vestibular labyrinth at birth is immature (Ashwell and Shulruf, 2013), all five sensory organs are generally present, the utricular macula being the most developed. A distinct macula containing hair cells and an otolithic membrane has been described in the utricle at birth in at least two opossum species (Didelphis virginia, Krause, 1991; M. domestica, Pflieger and Cabana, 1996), the tammar wallaby (Macropus eugenii; McCluskey et al., 2008) and the northern quoll (Dasyurus hallucatus; Gemmell and Nelson, 1989, Gemmell and Nelson, 1992). Furthermore, neural tracing experiments in opossums (Pflieger and Cabana, 1996) and wallabies (McCluskey et al., 2008) showed that the MVe, SpVe and LVe, as well as the cerebellum, already receive primary vestibular afferents at birth. Projections from the LVe, and to a lesser extent MVe and SpVe, already reach the cervical spinal cord at birth in the opossum (Wang et al., 1992, Pflieger and Cabana, 1996). In the newborn wallaby, vestibular projections do not reach the lower cervical cord (McCluskey et al., 2008) but, in those two species, reticulospinal pathways are developed at birth and may relay secondary vestibular inputs to the cord. Electrophysiological stimulation of the vestibular nuclei on in vitro preparations of M. domestica opossums induce movements of the forelimbs, indicating that the pathways relaying vestibular inputs to the spinal cord are functional at birth (Adadja et al., 2013). However, even if a pathway between the labyrinth, vestibular complex and spinal cord appears to be present at birth in marsupials, it is not yet known if the vestibular sensory organs and vestibular primary afferents are functional.
We addressed this question in the gray short-tailed opossum (M. domestica), which is born after about 14 days of gestation. We used in vitro preparations of newborn opossums (P1-P12) to test if motor responses in the forelimbs can be induced by mechanical pressures exerted on the vestibular labyrinth and by head elevations. Moreover, we used Piezo2 immunohistochemistry to evaluate the development of vestibular hair cells. Piezo2 is one of the mechanosensitive cation channels expressed by mammalian cochlear and vestibular hair cells. We focused on it because of its specific location at the apical surface of hair cells and its involvement in the acquisition of precocious mechanosensory properties by these cells in rodents (Beurg and Fettiplace, 2017; see Discussion). Our working hypothesis was that the vestibular system, both its central and peripheral components, is sufficently functional at birth to influence motricity.
2. Material and methods
Opossums were obtained from a colony maintained at the University animal facility according to conditions optimised for Monodelphis domestica (for details see Vandeberg and Williams-Blangero, 2010; Desmarais et al., 2016). The first 24 h following birth are considered as Postnatal day 0 (P0). A total of 47 opossums aged from P1 to P21 were used in this study. The in vitro and in vivo experiments presented herein were all carried out starting a few hours after the beginning of the light cycle, at a time when opossums are less active. All the experiments followed the guidelines of the NIH and the CCAC and were approved by the University’s Animals Ethics Committee.
2.1. Electrophysiology
In vitro preparations of opossums aged from P1 to P12 were used to test if mechanical stimulations of the inner ear or movements of the head induce neural responses in the spinal cord. These preparations are adapted from those used in other studies (Lavallée and Pflieger, 2009, Adadja et al., 2013, Corriveau-Parenteau et al., 2019). All dissection procedures that follow were done under a dissecting microscope (Olympus BX61WIF). For the duration of dissections and experiments, preparations were maintained in petri dishes lined with Sylgard (Dow Corning) and filled with an oxygenated physiological solution (125 mM NaCl; 3 mM KCl; 25 mM NaHCO3; 1 mM NaH2PO4; 1 mM MgCl2 and 15 mM dextrose, pH 7.4). A peristaltic pump (Watson Marlow 120 S/DM3) was used to constantly renew the solution in the dishes from a tank oxygenated with 95% O2 / 5% CO2.
Each animal was deeply anesthetized by hypothermia and, when unresponsive to stimulation (tail or hand pinching), was eviscerated before being pinned to the floor of a petri dish and immersed in cold physiological solution (∼ 4 °C). First, the skin and skull tissues over the brain and the spinal cord were dissected before a transverse section was done in front of the diencephalon and all nervous tissues rostral to the cut were removed. The jaw, the tongue and the part of the skull rostral to the diencephalon were also cut off. The preparation was then transferred in a custom-made dish, lined with Sylgard, and filled with physiological solution at room temperature (20–24 °C). Most of the cranial nerves were sectioned close to the brainstem on both sides, leaving only the vestibulocochlear and facial nerves, as they are abutted. The facial nerve was cut peripheraly to the osseous labyrinth. To maximise the exposure of the nervous tissues to the physiological solution, the skin and muscles were removed over the head and neck as much as possible. A complete section was made at the level of the last thoracic vertebra to minimize movements of the hindquarter. Further dissection depended on the experimental paradigm (see below).
During all in vitro experiments, the responsiveness of the preparations was assessed at different times by applying pressure on the face or one forepaw to induce a reflex reaction.
Four preparations were made from P13 to P16 opossums for the ‘Head Elevation’ protocol (see below) but became unresponsive before they could be tested. They are not included in the results.
2.2. Mechanical stimulation of the labyrinth
Experiments were aimed at recording spinal activity after mechanical stimulation of the labyrinth in 10 in vitro preparations (one specimen each at ages P1, P3, P4 and P6; two specimens each at ages at P5, P7 and P9). Animals were prepared as described previously. Then, the head of each specimen was pinned down on the floor of the petri dish to restrain it. An opening was cut with microscissors in the dorsal part of the developing petrous bone on both sides to expose the lumen of the vestibule, which is the largest chamber of the labyrinth where the maculae are located. The size of the opening depended on the age of the specimen but was always sufficiently large to insert the tip of a micropipette under visual guidance. The membranous labyrinth was cut without damaging the maculae, which could be visualized either as dark patches under the microscope or by the reflection of light over the otolithic membrane. Then, the spinal cord was dissected out of the vertebral column, taking care to preserve its integrity and its connection with the brainstem (left in the skull). The resected tissues from the neck down were discarded and the ventral side of the spinal cord was turned upside-down to access the ventral roots without affecting input transmission. The spinal cord was then pinned to the bottom of the dish and the latter was transferred on a movable top plate (Scientifica) located under a microscope: (BX61WI, Olympus).
The preparations were left undisturbed for 0.5–1 h to recover from the dissection procedures. Then, a suction electrode was attached to a ventral root of one of the segments innervating the forelimbs (cervical 4–8). The electrodes were made with a micropipette puller (P-97, Sutter Instrument) from glass capillaries (external diameter: 1.2 mm; internal: 0.6 mm; A-M systems). Their tip opening was about 60–80 µm, corresponding to the size of the ventral root. The electrode was put on a microelectrode holder (MEH7W, WPI) and a ventral root was suctioned before the tip was delicately pressed on the cord to increase sealing. The electrode was then connected to a high impedance module (HZP, Grass Technologies). Automatized micromanipulators (MPC-200, Sutter Intruments) were used to move the suction electrodes.
For stimulation, a micropipette was pulled to obtain a long tip of about 100–150 µm in diameter with a blunted ending. It was attached to a micromanipulator (MX100R, Newport), inserted in the opening practiced in the labyrinth and, under visual control, manually advanced toward the floor of the membranous labyrinth, as close as possible to the utricular macula. A stimulation consisted of three round-trips of 0.05–0.2 mm in amplitude (depending of the age of the preparation) at a fast but steady pace; stimulations were repeated at least 10 times with 1 min between repetitions. In some cases, the rod was then removed before being inserted in the contralateral labyrinth, which was stimulated using the same protocol. A pedal was pressed at the start of each stimulation to produce an artefact on the recordings (‘Stim’ in Fig. 2).
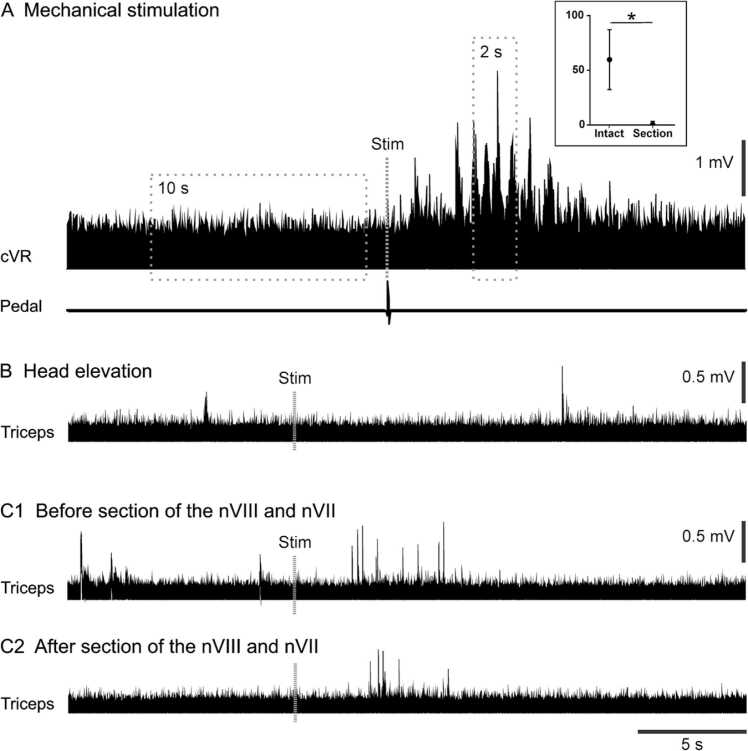
Fig. 2.
Motor responses after vestibular stimulation recorded in in vitro preparations. (A) Electroneurogram of a cervical ventral root (cVR) activity of a preparation from a P5 opossum before and after mechanical stimulation of the sensory vestibular organs (see text for details). The start of the stimulation (‘Stim’, vertical dashed line) is indicated by an artefact generated by a pedal. The dashed squares delineate approximately the areas used to estimate the respective amplitudes of the baseline (‘10 s′) and the response (‘2 s′). Graph in inset: four specimens were tested before (Intact) and after vestibulochlear and facial nerves sectioning (Section), the mean amplitude of the responses was 59.8 ± 13.7% over the baseline before the section and 1.0 ± 1.2% after (* p = 0.0286, Mann-Withney non parametric t-test). (B,C) Electromyograms of triceps brachii muscles activity before and after head elevation of preparations at P11 (B) and P12 (C1,C2). The start of the elevation is indicated by a vertical dashed line (‘Stim’). Head elevations did not induced motor response in most of the trials (B). However, in a few cases, bursts of activity could be recorded following stimulation (C1), but similar bursts were observed after complete section of the vestibulochlear (nVIII) and facial (nVII) nerves (C2).
2.3. Head elevation
Experiments were aimed at recording spinal activity after pitch rotation of the head in 10 in vitro preparations of opossums (one specimen each at ages P4, P6 and P8; two specimens each at ages P9 and P11; three specimens at P12). As these movements induce small displacements of the brain and the spinal cord which can dislodge the suction electrode when put on the ventral roots, we did not record ventral root responses, but the activity of the triceps brachii, a forelimb extensor muscle used when newborn opossums locomote (Adadja et al., 2013, Desmarais et al., 2016). For that purpose, the spinal cord was left in the carcass but the skin and fascia of the forelimbs were removed to expose the muscles. The preparation was then pinned to the petri dish to minimize movements from the neck down to the thoracic segments, with the forelimbs slightly abducted. The skull was left free to move vertically.
The petri dish was then transferred to the electrophysiology set-up and a suction electrode such as described previously for ventral root recordings was tightly positioned on a triceps muscle belly. After 0.5–1 h of recovery time, a microspatula attached to a micromanipulator was inserted under the skull, longitudinally along the specimen axis. To stimulate the preparation, the spatula was elevated from the floor of the petri dish until the base of the skull formed an angle of 45–60° relative to the floor, and then lowered back to the initial position. Stimulations were done under visual guidance and lasted around 3 s from the beginning of the elevation to the return to the horizontal position. Specimens were stimulated at least 5 times, consecutively, with 5 min between each stimulation. As before, a pedal was pressed at the start of stimulations.
During stimulation, care was taken to minimize neck and body movements but it was not possible to remove all stresses on the neck. To ensure that such stresses did not induce motor activity, the C1–3 spinal roots (both ventral and dorsal) were sectioned before stimulation in 6 specimens.
2.4. Recording and analysis
Signals from the electrodes and pedal were amplified and filtered (bandwidth, 3 Hz to 3 kHz; CP511 Amplifier, Grass Technologies), digitized (Digidata 1322 A interface, Clampex 9.2 software; Molecular Devices) and stored on a hard disk. The recordings were then rectified and analyzed with the software Clampfit 10.6 (Molecular Devices). For quantification, areas under the curve were measured at specific times before and after stimulation using the command “Means” of the software. A burst of activity was considered a response to simulation when a) it followed the artefact indicating the start of stimulation, b) its amplitude reached at least twice the average baseline as computed over the 10 s preceding the stimulation and c) it lasted at least 2 s
Statistical analyses were performed using Prism 5 (Graphpad, USA). Data are presented as averages ± SEM in the text and the figures.
2.5. Piezo2 immunohistochemistry
27 specimens aged from P0 to P21 were studied (eight specimens at P0, three at P5, nine at P7, five at P14 and two at P21). They were deeply anesthetized by hypothermia and decapitated. The heads were immersed in 4% PFA (J61899; ThermoFisher) for 1 week, then in sucrose 15% and 30% for 24–48 h at 4°C. They were then embedded in Optimal Cutting Temperature Compound (OCT; Tissue Tek Sakura), rapidly frozen on dry ice, sectioned transversally at 20 µm with a cryostat (CM3050S, Leica) and put on Superfrost Plus slides (Fisherbrand). They were stored at 4 °C until processing.
Immunohistochemistry was carried out at room temperature unless noted otherwise. The sections were washed with TBST (0.05 M Tris buffer, 1.5% NaCL, 0.3% Triton X-100, pH 7.4) for 20 min then incubated at 4 °C for 24 h with primary anti-Piezo2 polyclonal IgG rabbit antibodies (1:500, Sigma-Aldrich HPA040616) and 5% NGS dissolved in TBST. After rinsing with TBST, the sections were incubated with secondary goat anti-rabbit IgG HandL antibodies coupled with Alexa Fluor-488 (1:400 in TBST, Abcam ab150077) for 2 h. The sections were washed three times with TBST before the slides were coverslipped using Fluoromount G (SouthernBiotech) as mounting medium.
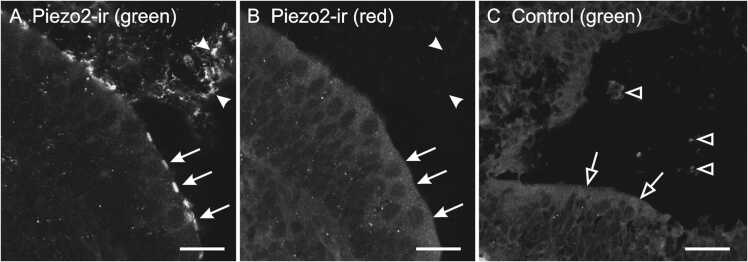
Control sections were routinely performed for all specimens either by omitting the primary antibodies during the first incubation or, in fewer cases, by pre-absorption of the primary antibodies with the antigen. All sections were then exposed to the secondary antibodies and none showed fluorescent labeling. An example of control section is given in Fig. 1: Fig. 1A shows Piezo2-labeling in a section of the utricular macula (filled arrows) and otoconial membrane (filled arrowheads) of a P7 opossum whereas in a serial section processed without the primary antibody (Fig. 1C), the macula (empty arrows) and the otoconial membrane (empty arrowheads) are devoid of labeling. Fig. 1B shows the same section as in Fig. 1A but photographed using a fluorescent filter not specific for Alexa Fluor-488 (see below), as evidenced by the absence of labeling (filled arrows and arrowheads in Fig. 1B).
Fig. 1.
(A,B) Section of the utricule of a P7 opossum processed with the primary antibodies against Piezo2 and observed with a filter specific for the fluorophore (Alexa Fluor-488; green, A) or not specific (red, B). The arrows and arrowheads point respectively at the surface of the macula and the otoconial membrane, labeling is observable only in A. (C) Adjacent section to that shown in A but processed without the primary antibody. No labeling is observed at the surface of the macula (empty arrows) when exposed to the right fluorescence (green). Empty arrowheads point unlabeled portions of the otoconial membrane showing slight intrinsic fluorescence. All microphotographs were taken with the same exposure time. Scale bars, 20 µm for A and B, 40 µm for C.
2.6. Microscopy
Sections were observed under a fluorescence microscope (either Olympus BX61WI or Leica ASLMD) mounted with a digital camera (either Retiga 2000R Qimaging or Evolution QEi Monochrome), controlled by an image acquisition software (Image Pro Plus 7.0 Media Cybernetics). Two fluorescent filters were routinely used: a FITC (green) filter specific for Alexa-488 (peak of excitation: 450–490 nm, emission: 520 nm) and TRDA (red) filter (excitation: 510–560 nm, emission: 590 nm) to better discriminate labeling from autofluorescence of the tissues. Some photographs of Piezo2-labeled sections were taken and edited using a confocal microscope (Zeiss LSM 800) equiped with Airyscan. The figures were designed using Corel Draw (version X6; Corel Corporation). The color images were converted to gray scale with Image Pro Plus, and contrast adjustment was performed on the whole photograph when needed.
3. Results
3.1. Mechanical stimulation of the labyrinth
To evaluate if stimulation of the vestibular sensory organs can induce motor responses in the spinal cord in newborn opossums, pressures were applied with a glass rod on the membranous labyrinth of in vitro preparations of brainstem and spinal cord of opossums aged P1 to P9 while the spinal ventral roots activity was recorded (i.e., electroneurograms). The pressure was primarily aimed at the utricular macula but, due to the small size of the labyrinth, could not be precisely applied, often moving larger portions of the membranous labyrinth. Except for the facial and vestibulocochlear nerves, spinal roots and cranial nerves were sectioned to eliminate undesirable stimulation of sensory nerves or receptors.
Such mechanical stimulations of the labyrinth induced motor responses in the ventral roots at all ages tested. Overall, motor responses (Fig. 2 A) were observed in 69% of trials (99/143) distributed as follows: 50% (5/10) at P1, 40% (8/20) at P3, 100% (10/10) at P4, 73% (24/33) at P5, 100% (5/5) at P6, 52% (14/27) at P7, and 75% (33/44) at P9. Following at least ten stimulations, the vestibulocochlear and facial nerves were sectioned in all specimens but two (a P1 and a P7), and they were stimulated as before. No response was recorded after these sections, confirming that the previous responses were due to the stimulation of the labyrinth.
As the stimulations were done manually, it was difficult to assess with precision the beginning and end of the responses. Moreover, some preparations showed spontaneous bursting activity which may have overlapped with the responses. These caveats in mind, we still measured the size of the responses by comparing the mean amplitude (i.e., area under the trace) of the 2 s of consecutive strongest activity (‘2 s′ square in Fig. 2 A) with a baseline measured before stimulation (‘10 s′ square in Fig. 2 A). For all six specimens tested, the responses showed an increase in amplitude of 62.8 ± 46.3% from the baseline. For the four specimens in which the vestibulocochlear and facial nerves were sectioned, the amplitude of the responses was on average increased by 59.8 ± 13.7% before the section and 1.0 ± 1.2% after (p = 0.0286, Mann-Withney non parametric t-test).
When the six specimens with the nerves intact are considered, a statistically significant decrease in the amplitude of responses is observed between those aged P1–4 (74.6 ± 36.5%) and those aged P7–9 (22.4 ± 21.3%)(p < 0.0001, Mann-Withney non parametric t-test).
3.2. Head elevations
We tested if displacements of the head can induce motor responses on in vitro preparations of brainstem and spinal cord with the forelimbs attached. These preparations were subjected to head elevations while triceps activity was recorded to detect muscle responses (i.e., electromyograms, EMGs).
A total of 50 trials were made in 10 preparations of specimens aged from P4 to P12. In 96,0% of trials, the stimulation induced no recordable EMG response that could be distinguished from spontaneous activity (Fig. 2B). In two trials from two different specimens aged P9 and P12, short bursts of activity were observed following the head elevation (Fig. 2C1), but similar bursting was also observed in the P12 specimen after further sectioning of the vestibulocochlear nerves (Fig. 2C2). The presence or absence of the cervical roots in the preparations did not influence these results.
3.3. Piezo2 labeling
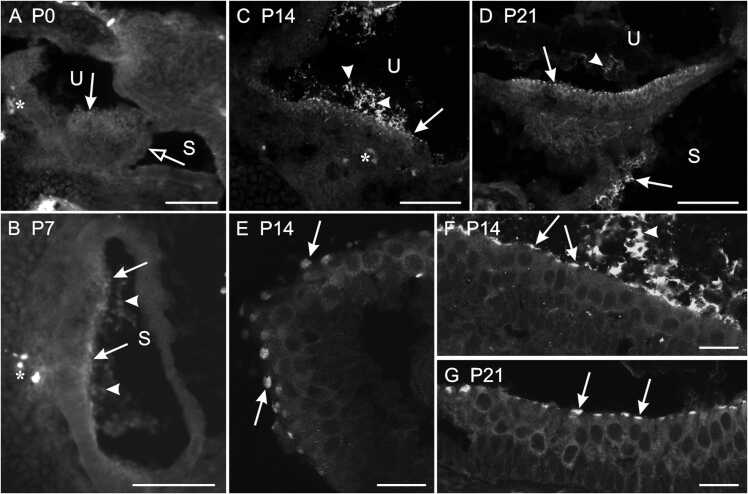
The presence of Piezo2 in the vestibular sensory organs was studied by immunohistochemistry on sections of the head. At birth, Piezo2 labeling was observed as faint patches of fluorescence sparsely disseminated throughout the surface of the utricular macula (filled arrows in Fig. 3 A) and was absent in the saccular macula (empty arrowhead in Fig. 3 A). The latter started to show faint labeling at P5. At P7, Piezo2 labeling took the form of disk-shaped patches of fluorescence homogenously distributed over the two maculae (arrows in Fig. 3B). They were more numerous and intense than what was observed in the utricule at P0 and in the saccule at P5. At P14 (Fig. 3 C) and P21 (Fig. 3D), the labeling was similar to that observed at P7, except that the number of patches seemed to have increased. At all ages, the labeling was restricted to the apical surface of the macula and had diameters corresponding to those of the epithelial cells (arrows in Fig. 3 F,G; see also Fig. 1 A). The otoconial membrane over the maculae also showed Piezo2 labeling (filled arrowheads in Fig. 1 A, 3B-D,F) which increased in intensity from P0 to P14, but seemed stable thereafter. The ampullary crests of the semicircular canals showed patterns of labeling similar to that observed for the saccular maculae, starting at P5 with faint labeling and showing well delineated patches at P14 (arrows in Fig. 3E). As for the maculae, the labeling in the ampulary crests was restricted to the apical surface of the epithelium. Contrary to what was observed for the otoconial membranes, the cupula covering the ampulary crests showed no Piezo2-labeling.
Fig. 3.
Transverse sections of the inner ear showing Piezo2 immunoreactivity in opossums at different ages. The filled arrows point to examples of Piezo2 labeling at the surface of the maculae and ampullary crest whereas the filled arrowheads point labeling in the otoconial membrane. (A) At P0, Piezo2 labelling is observed in the utricule (U) but not in the saccule (S; the empty arrow points the macula). (B) Saccule of a P7 opossum. (C) Utricule of a P14 opossum. (D) Utricule and saccule of a P21 opossum. (E) Ampullary crest of a P14 opossum. (F,G) Magnifications of the utricular maculae shown respectively in (C) and (D). Dorsal is up in all microphotographs. Asterix in A-C indicate some blood vessels showing intrinsic fluorescence. Scale bars, 100 µm for A-D; 20 µm for E-G.
To our knowledge, the presence of Piezo2 in the otoconial membranes of the macular organs have not been reported in other vertebrate species. The present observation suggests that Piezo2 is secreted in these membranes during their synthesis in the opossum, but further studies are needed to understand what purpose it may serve.
4. Discussion
This study was aimed at evaluating if the vestibular system can influence the precocious motor behaviors of newborn opossums. Electrophysiological experiments on in vitro preparations suggest that movements of the head do not evoke motor responses before P12 even if neural fibers are able to convey inputs from the labyrinth to vestibulo- or reticulospinal neurons around birth. These observations support the idea that the neural substrate of the vestibular system is functional at birth but not the sensory organs. This is supported by the low level of Piezo2 labeling of hair cells at birth that indicates that their maturation is not advanced at this age.
The small sizes of the newborn heads prevented us from using fine stimulation of the vestibular sensory organs in situ. Thus, to see if neural signals can be transmitted from the labyrinth to the spinal cord, we used an invasive paradigm consisting of pressures mechanically applied on the maculae to evoke non-physiological discharges of either the ciliated cells, the primary afferents innervating them, or both. As most cranial nerves and spinal roots were cut close to either the brainstem or the spinal cord, the only sensory fibers that may have been non-specifically stimulated would be those associated to the facial and cochlear nerves. The facial nerve runs outside the vestibule, from which it is separated by a layer of periotic cartilage, and is mainly composed of motor axons with a lesser component of sensory fibers that innervate facial subcutaneous tissues. To stimulate the cochlear nerve or the organ of Corti, the rod used would have to go through the vestibular sensory organs. The decrease in the amplitude of the motor responses between P1–4 and P7–9 (from 74.6% to 22.4%, respectively) may reflect that the stimulations affected other sensory structures other than the maculae in the specimens with a smaller labyrinth, where the cochlear components (organ of Corti and cochlear nerve fibers) are closer to the vestibular ones. Thus, while we cannot totaly exclude non-specific stimulation of cochlear components, the most plausible explanation to the spinal root activity after labyrinthic stimulation is that they originate primarily from vestibular organs or vestibular primary afferents. Our observations thus suggest that, already at P1, the primary afferents could mediate neural inputs to the vestibular nuclei which, in turn, influence the spinal cord either via vestibulospinal or vestibulo-reticulospinal projections. However, the vestibular responses were obtained in conditions that are not physiologically relevant.
To evaluate the functionality of the system in more physiological conditions, we tested if head elevation in vitro could induce motor responses. Previous studies in different marsupial species indicated that the most mature vestibular organs at birth are the maculae, especially that of the utricle (Gemmell and Nelson, 1989, Krause, 1991, Pflieger and Cabana, 1996, McCluskey et al., 2008, Ashwell and Shulruf, 2013). The utricular macula is mainly stimulated by displacements from the horizontal plane (Day and Fitzpatrick, 2005, Barrett et al., 2012, Khan and Chang, 2013) and this is why we used elevation and lowering of the head (pitch rotation) to stimulate the in vitro preparations. However, we were unable to induce significant motor responses with such stimulations in all specimens aged from P0 to P12. Muscle activity was recorded in all specimens by pinching the hands or tail after the series of head elevation, precluding a lack of viability of the preparations. This suggests either that the displacements were inadequate to induce responses or that the maculae were not sufficiently developed to respond to head movement even at P12.
In widely used rodents such as rats and mice, which compare to the opossum in size and are born altricial, the first vestibulo- and reticulospinal fibers grow in the spinal cord before embryonic day (E)17 and these projection systems become functional prenatally (Lakke, 1997, Auclair et al., 1999, Kasumacic et al., 2010, Kasumacic et al., 2015). The acquisition of physiological properties in vestibular hair cells starts around E16 in mice (Géléoc and Holt, 2003) and around birth in rats (Beurg and Fettiplace, 2017) but the maturation of these properties continues late postnatally in both species (see Burns and Stone, 2017 for a review on vestibular hair cells development). The primary afferents reach the vestibular nuclei around E15 (Morris et al., 1988). Transmission of vestibular inputs to the central nuclei has been recorded in P0 rat pups subjected to horizontal rotations of very high angular acceleration (Lannou et al., 1979). However, the synaptic contacts between vestibular neurons and vestibular afferents are poorly developed at birth and their physiological maturation continues postnataly (Lannou et al., 1979). This is well exemplified by a series of in situ hybridization experiments to measure the number of c-Fos-labeled central vestibular neurons in order to evaluate the sensitivity of central vestibular neurons to stimulation of the utricle (Lai et al., 2004, Lai et al., 2006) or the horizontal semicircular canals (Lai et al., 2010). C-Fos is a marker of neuronal activity expressed when neurons are sufficiently stimulated to discharge (Kovács, 1998, Kovács, 2008, Dai et al., 2005, Piché et al., 2007). In the developing rats, c-Fos labelling was observed in the vestibular nuclei at P4 after horizontal canals stimulation (Lai et al., 2010) and P7 after otolithic stimulation (Lai et al., 2004, Lai et al., 2006) but, in all cases, the number of labeled neurons did not reach values close to that observed in adults before P14. These and other studies in rats (e.g., Freeman et al., 1999) suggest that central vestibular neurons do not receive enough inputs to influence motor behaviors in response to vestibular stimulation before P4–7. In terms of days post-conception, P4 rats compare to P9–13 opossums (https://www.translatingtime.org). Thus, it is possible that the lack of motor responses after head elevations in vitro may be partly explained by the immaturity of synaptic contacts between vestibular primary afferents and central vestibular neurons.
We also evaluated the development of hair cells using Piezo2 expression as a proxy of the functional maturity of the vestibular organs. As said before, Piezo2 is a mechanically activated ion channel expressed at the apical surface of hair cells (Wu et al., 2017). This channel is thought to respond to deformation of the surface by allowing influx of cations (mainly Ca2+) in the cell (Beurg and Fettiplace, 2017, Wu et al., 2017). This current was dubbed ‘anomalous’ or ‘reverse-polarity’ because it differs from the ‘normal-polarity’ currents activated by the deflection of the stereocilia towards the largest cilia that occurs when mature hair cells are stimulated in situ (Beurg et al., 2016, Beurg and Fettiplace, 2017). In mice, the anomalous current is maximal at E16 and P0, respectively for vestibular and cochlear hair cells, and is nearly abolished at P0 and P4, ages at which the normal currents are strong (Beurg et al., 2016). We did not try to target the channels underlying the normal currents in hair cells because they are not identified with certainty even in the mature hair cells (for review see Beurg and Fettiplace, 2017; McPherson, 2018).
In opossums, Piezo2-labeling took the shape of disk-shaped patches of fluorescence on the apical membrane of the sensory epithelium resembling what was previously observed in mice (Wu et al., 2017). At P0, the labeling was sparse in the utricular macula and absent in the other vestibular organs. Stronger labeling was observed at P5 in the utricular macula and at P7 in the saccular macula and the ampullary crests, but it is at P14 that the intensity of the labeling appeared to stabilize in all sensory organs. Considering the developmental latency of about 4 days for the disappearance of the anomalous currents observed in mice (Beurg et al., 2016), this suggests that a majority of hair cells are not mature enough to perceive vestibular stimuli before P9–12 in opossums and that their maturation extends at minimum into the third postnatal week. This observation should be confirmed by electrophysiological recordings of hair cells at these ages, but it could explain why we did not record motor responses after head elevations in vitro.
Contrary to our working hypothesis, the present study reveals that the peripheral components, either the hair cells, the primary afferents, or both, are too immature for the vestibular system to influence motor behaviors in newborn opossums. Ashwell and Shulruf (2013) compared the morphological development of the vestibular apparatus in different marsupial and eutherian species, including the opossum. They found that all marsupial species are born at similar stages of vestibular development (stages j-k of Ashwell and Shulruf, 2013), with the exception of the more immature dasyurids (stage d), whereas mouse and rat newborns are slightly more mature (stage l). To our knowledge, the functional development of the vestibular system is not studied in other marsupial species, which limits inferences from our results. However, these observations suggest that the vestibular system is not involved in motor control at birth in all marsupials.
Funding source
This work was supported by a Grant from the Natural Sciences and Engineering Research Council of Canada to J-FP (NSERC, RGPIN-2016-06518). FL received student Fellowships from Fonds de Recherche du Québec - Nature et Technologies (FRQNT) and NSERC to work on this project for his M.Sc.
CRediT authorship contribution statement
Frédéric Lanthier: Investigation, Methodology, Writing – original draft. Jessica Laforge: Investigation, Methodology, Writing – original draft. Jean-François Pflieger: Methodology, Supervision, Writing – review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no competing interests..
Acknowledgements
We are grateful to Daniel Kierzkowski and Hana Bertrand-Rakusova (Département de sciences biologiques, Université de Montréal) for their help with the acquisition of confocal photographs at the Institut de recherche en biologie végétale. We thank Thérèse Cabana for her comments on this manuscript.
Author contributions
They performed experiments, analyzed data, and wrote the paper. JFP designed research, analyzed data, and wrote the paper.
References
- Adadja T., Cabana T., Pflieger J.F. Cephalic sensory influence on forelimb movement in newborn opossums. Monodelphis Domest. Neurosci. 2013;228:259–270. doi: 10.1016/j.neuroscience.2012.10.029. [DOI] [PubMed] [Google Scholar]
- Angelaki D.E., Cullen K.E. Vestibular system: the many facets of a multimodal sense. Annu Rev. Neurosci. 2008;31:125–150. doi: 10.1146/annurev.neuro.31.060407.125555. [DOI] [PubMed] [Google Scholar]
- Auclair F., Marchand R., Glover J.C. Regional patterning of reticulospinal and vestibulospinal neurons in the hindbrain of mouse and rat embryos. J. Comp. Neurol. 1999;411:288–300. doi: 10.1002/(sici)1096-9861(19990823)411:2%3C288::aid-cne9%3E3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Ashwell K.W., Shulruf B. Vestibular development in marsupials and monotremes. J. Anat. 2013;224:447–458. doi: 10.1111/joa.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett K.E., Barman S.M., Boitano S., Brooks H.L. Chapter 10. Hearing & equilibrium. Ganong’s review of medical physiology. 24th ed.., McGraw-Hill,; New York: 2012. p. 768. [Google Scholar]
- Beurg M., Fettiplace R. Piezo2 as the anomalous mechanotranducer channel in auditory hair cell. J. Physiol. 2017;595:7039–7048. doi: 10.1113/jp274996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M., Goldring A.C., Ricci A.J., Fettiplace R. Development and localization of reverse-polarity mechanotransducer channels in cochlear hair cells. PNAS. 2016;113 doi: 10.1073/pnas.1601067113. 6767-6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J.C., Stone J.S. Development and regeneration of vestibular hair cells in mammals. Semin Cell Dev. Biol. 2017;65:96–105. doi: 10.1016/j.semcdb.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J.R., Bakker H.R., Bradshaw S.D., McDonald I.R. Gravity as the sole navigational aid to the newborn quokka. Nature. 1976;259:42. doi: 10.1038/259042a0. [DOI] [PubMed] [Google Scholar]
- Cassidy G., Boudrias D., Pflieger J.F., Cabana T. The development of sensorimotor reflexes in the Brazilian opossum Monodelphis domestica. Brain Behav. Evol. 1994;43:244–253. doi: 10.1159/000113638. [DOI] [PubMed] [Google Scholar]
- Corriveau-Parenteau E., Beauvais A., Angers A., Pflieger J.F. Influence of temperature on motor behaviors in newborn opossums (Monodelphis domestica): An in vitro study. eNeuro. 2019;6:1–18. doi: 10.1523/eneuro.0347-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X., Noga B.R., Douglas J.R., Jordan L.M. Localization of spinal neurons activated during locomotion using the c-fos immunohistochemical method. J. Neurophysiol. 2005;93:3442–3452. doi: 10.1152/jn.00578.2004. [DOI] [PubMed] [Google Scholar]
- Day B.L., Fitzpatrick R.C. The vestibular system. Curr. Biol. 2005;15:R583–R586. doi: 10.1016/j.cub.2005.07.053. [DOI] [PubMed] [Google Scholar]
- Desmarais M.J., Beauregard F., Cabana T., Pflieger J.F. Facial mechanosensory influence on forelimb movement in wewborn opossums, Monodelphis domestica. PLoS One. 2016;11:1–15. doi: 10.1371/journal.pone.0148352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferner K., Schultz J.A., Zeller U. Comparative anatomy of neonates of the three major mammalian groups (monotremes, marsupials, placentals) and implications for the ancestral mammalian neonate morphotype. J. Anat. 2017;231:798–822. doi: 10.1111/joa.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman S., Plotnik M., Elidan J., Sohmer H. Development of short latency vestibular evoked potentials in the neonatal rat. Hear Res. 1999;137:51–58. doi: 10.1016/s0378-5955(99)00137-9. [DOI] [PubMed] [Google Scholar]
- Géléoc G.S.G., Holt J.R. Developmental acquisition of sensory transduction in hair cells of the mouse inner ear. Nat. Neurosci. 2003;6:1019–1020. doi: 10.1038/nn1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemmell R.T., Nelson J. Vestibular system of the newborn marsupial cat Dasyurus hallucatus. Anat. Rec. 1989;225:203–208. doi: 10.1002/ar.1092250305. [DOI] [PubMed] [Google Scholar]
- Gemmell R.T., Nelson J. Development of the vestibular and auditory system of the northern native cat, Dasyurus hallucatus. Anat. Rec. 1992;234:136–143. doi: 10.1002/ar.1092340115. [DOI] [PubMed] [Google Scholar]
- Gemmell R.T., Veitch C., Nelson J. Birth in marsupials. Comp. Biochem Physiol. B Biochem Mol. Biol. 2002;131:621–630. doi: 10.1016/S1096-4959(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Goldberg J.M. Oxford University Press,; New York: 2012. The vestibular system: a sixth sense. [DOI] [Google Scholar]
- Grillner, S. (2011). Control of Locomotion in Bipeds, Tetrapods, and Fish. In Comprehensive Physiology, R. Terjung (Ed.). https://doi.org/10.1002/cphy.cp010226.
- Grillner S., El Manira A. Current Principles of Motor Control, with Special Reference to Vertebrate Locomotion. Physiol. Rev. 2020;100:271–320. doi: 10.1152/physrev.00015.2019. [DOI] [PubMed] [Google Scholar]
- Hartman C.G. Univ. Texas Press,; 1952. Possums; p. 174. [Google Scholar]
- Ho S.M. Rhythmic motor activity and interlimb co-ordination in the developing pouch young of a wallaby (Macropus eugenii) J. Physiol. 1997;501(3):623–636. doi: 10.1111/j.1469-7793.1997.623bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S.M. Strychnine- and bicuculline-induced changes in the firing pattern of motoneurones during in vitro fictive locomotion reveal a possible N-methyl-D-aspartic acid (NMDA)-mediated suppression of motor discharge in wallaby (Macropus eugenii) pouch young. Somat. Mot. Res. 1998;15:325–332. doi: 10.1080/08990229870736. [DOI] [PubMed] [Google Scholar]
- Hughes R.L., Hall L.S. In: Tyndale-Biscoe H., Janssens P.A., editors. vol. 2. Springer-Verlag; New York: 1988. Structural adaptations of the newborn marsupial; pp. 8–27. (The developing marsupial). [Google Scholar]
- Khan S., Chang R. Anatomy of the vestibular system: a review. NeuroRehabilitation. 2013;32:437–443. doi: 10.3233/NRE-130866. [DOI] [PubMed] [Google Scholar]
- Kasumacic N., Glover J.C., Perreault M.C. Segmental patterns of vestibular- mediated synaptic inputs to axial and limb motoneurons in the neonatal mouse assessed by optical recording. J. Physiol. 2010;588:4905–4925. doi: 10.1113/jphysiol.2010.195644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasumacic N., Lambert F.M., Coulon P., Bras H., Vinay L., Perreault M.C., Glover J.C. Segmental organization of vestibulospinal inputs to spinal interneurons mediating crossed activation of thoracolumbar motoneurons in the neonatal mouse. J. Neurosci. 2015;35(21):8158–8169. doi: 10.1523/jneurosci.5188-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev. Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- Kovács K.J. c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem. Int. 1998;33:287–297. doi: 10.1016/S0197-0186(98)00023-0. [DOI] [PubMed] [Google Scholar]
- Kovács K.J. Measurement of Immediate-Early Gene Activation- c-fos and Beyond. JNE. J. Neuroendocr. 2008;20:665–672. doi: 10.1111/j.1365-2826.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- Krause W.J. The vestibular apparatus of the opossum (Didelphis virginiana) prior to and immediately after birth. Acta Anat. 1991;142:57–59. doi: 10.1159/000147160. [DOI] [PubMed] [Google Scholar]
- Lakke E.A.J.F. The projections to the spinal cord of the rat during development, a time-table of descent. Adv. Anat. Embryol. Cell Biol. 1997;135:1–143. doi: 10.1007/978-3-642-60601-4. [DOI] [PubMed] [Google Scholar]
- Lai S.K., Lai C.H., Yung K.K.L., Shum D.K.Y., Chan Y.S. Maturation of otolith-related brainstem neurons in the detection of vertical linear acceleration in rats. Eur. J. Neurosci. 2006;23:2431–2446. doi: 10.1111/j.1460-9568.2006.04762.x. [DOI] [PubMed] [Google Scholar]
- Lai C.H., Tse Y.C., Shum D.K.Y., Yung K.K.L., Chan Y.S. Fos expression in otolith-related brainstem neurons of postnatal rats following off-vertical axis rotation. J. Comp. Neurol. 2004;470:282–296. doi: 10.1002/cne.11048. [DOI] [PubMed] [Google Scholar]
- Lai C.H., Yiu C.N., Lai S.K., Ng K.P., Yung K.K., Shum D.K., Chan Y.S. Maturation of canal-related brainstem neurons in the detection of horizontal angular acceleration in rats. J. Comp. Neurol. 2010;518:1742–1763. doi: 10.1002/cne.22300. [DOI] [PubMed] [Google Scholar]
- Lannou J., Precht W., Cazin L. The postnatal development of functional properties of central vestibular neurons in the rat. Brain Res. 1979;175:219–232. doi: 10.1016/0006-8993(79)91002-3. [DOI] [PubMed] [Google Scholar]
- Larsell O., McCrady E., Zimmermann A. Morphological and functional development of the membranous labyrinth in the opossum. J. Comp. Neurol. 1935;63:95–118. doi: 10.1002/cne.900630107. [DOI] [Google Scholar]
- Lavallée A., Pflieger J.F. Developmental expression of spontaneous activity in the spinal cord of postnatal opossums, Monodelphis domestica: an anatomical study. Brain Res. 2009;1282:1–9. doi: 10.1016/j.brainres.2009.05.068. [DOI] [PubMed] [Google Scholar]
- McCluskey S.U., Marotte L.R., Ashwell K.W.S. Development of the vestibular apparatus and central vestibular connections in a wallaby (Macropus eugenii) Brain Behav. Evol. 2008;71:271–286. doi: 10.1159/000127047. [DOI] [PubMed] [Google Scholar]
- McPherson D.R. Sensory Hair Cells: An Introduction to Structure and Physiology. Integr. Comp. Biol. 2018;58:282–300. doi: 10.1093/icb/icy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R.J., Beech J.N., Heizmann C.W. Two distinct phases and mechanisms of axonal growth shown by primary vestibular fibres in the brain, demonstrated by parvalbumin immunohistochemistry. Neuroscience. 1988;27:571–596. doi: 10.1016/0306-4522(88)90290-4. [DOI] [PubMed] [Google Scholar]
- Nelson J., Gemmell R. Implications of marsupial births for an understanding behavioural development. Int J. Comp. Psychol. 2004;17:53–70. doi: 10.46867/ijcp.2004.17.01.03. [DOI] [Google Scholar]
- Pellis S.M., Pellis V.C., Nelson J.E. The development of righting reflexes in the pouch young of the marsupial Dasyurus hallucatus. Dev. Psychobiol. 1992;25:105–125. doi: 10.1002/dev.420250204. [DOI] [PubMed] [Google Scholar]
- Pflieger J.F., Cabana T. The vestibular primary afferents and the vestibulospinal projections in the developing and adult opossum, Monodelphis domestica. Anat. Embryol. 1996;194:75–88. doi: 10.1007/bf00196317. [DOI] [PubMed] [Google Scholar]
- Piché M., Chabot N., Bronchti G., Miceli D., Lepore F., Guillemot J.P. Auditory responses in the visual cortex of neonatally enucleated rats. Neuroscience. 2007;145:1144–1156. doi: 10.1016/j.neuroscience.2006.12.050. [DOI] [PubMed] [Google Scholar]
- Russell E.M. Patterns of parental care and parental investment in marsupials. Biol. 1982;57:423–486. doi: 10.1111/j.1469-185x.1982.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Smith K.K., Keyte A.L. Adaptations of the marsupial newborn: Birth as an extreme environment. Anat. Rec. 2019;0:1–15. doi: 10.1002/ar.24049. [DOI] [PubMed] [Google Scholar]
- Vandeberg J.L., Williams-Blangero S. The Laboratory Opossum. The UFAW Handbook on the Care and Management of Laboratory and Other Research. Animals. 2010;8:246–261. [Google Scholar]
- Wang X.M., Xu X.M., Qin Y.Q., Martin G.F. The origins of supraspinal projections to the cervical and lumbar spinal cord at different stages of development in the gray short-tailed Brazilian opossum, Monodelphis domestica. Dev. Brain Res. 1992;68:203–216. doi: 10.1016/0165-3806(92)90062-2. [DOI] [PubMed] [Google Scholar]
- Wu Z., Grillet N., Zhao B., Cunningham C., Harkins-Perry S., Coste B., Ranade S., Zebarjadi N., et al. Mechanosensory hair cells express two molecularly distinct mechanotransduction channels. Nat. Neurosci. 2017;20:24–33. doi: 10.1038/nn.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]