Summary
Background
Chronic liver diseases of all etiologies exist along a spectrum with varying degrees of hepatic fibrosis. Despite accumulating evidence implying associations between liver fibrosis and cognitive functioning, there is limited research exploring the underlying neurobiological factors and the possible mediating role of inflammation on the liver-brain axis.
Methods
Using data from the UK Biobank, we examined the cross-sectional association of liver fibrosis (as measured by Fibrosis-4 score) with cognitive functioning and regional grey matter volumes (GMVs) while adjusting for numerous covariates and multiple comparisons. We further performed post-hoc preliminary analysis to investigate the mediating effect of C-reactive protein (CRP) on the association between liver fibrosis and both cognitive functioning and GMVs.
Findings
We analysed behaviour from up to 447,626 participants (N ranged from 45,055 to 447,533 per specific cognitive metric) 37 years and older. 38,244 participants (age range 44–82 years) had GMV data collected at a median 9-year follow-up. Liver fibrosis showed significant associations with cognitive performance in reasoning, working memory, visual memory, prospective memory, executive function, and processing speed. Subgroup analysis indicated larger effects sizes for symbol digital substitution but smaller effect sizes for trail making in middle-aged people than their old counterparts. Neuroimaging analyses revealed significant associations between liver fibrosis and reduced regional GMVs, primarily in the hippocampus, thalamus, ventral striatum, parahippocampal gyrus, brain stem, and cerebellum. CRP levels were significantly higher in adults with advanced liver fibrosis than those without, indicating an elevated systemic inflammation. Moreover, the serum CRP significantly mediated the effect of liver fibrosis on most cognitive measures and regional GMVs in the hippocampus and brain stem.
Interpretation
This study provides a well-powered characterization of associations between liver fibrosis, cognitive impairment, and grey matter atrophy. It also highlights the possibly mediating role of systemic inflammation on the liver-brain axis. Early surveillance and prevention of liver diseases may reduce cognitive decline and brain GMV loss.
Funding
National Science Foundation, and National Institutes of Health.
Keywords: Hepatic fibrosis, Grey matter atrophy, Fibrosis-4 score, Cognitive functioning, Systemic inflammation
Research in context.
Evidence before this study
Recent years have witnessed increasing interest in the extrahepatic manifestations of chronic liver diseases in both hepatology and neuroscience. Available evidence suggests that neurological health is affected by advanced liver dysfunction, like hepatic encephalopathy, and may also be worsened by less severe forms of chronic liver diseases including hepatic fibrosis. We searched Pubmed for articles published in English between January 2000 and October 2022, using the search terms “liver fibrosis”, “chronic liver disease”, "cognitive functioning", “inflammation”, "brain structure", and "grey matter volume" in relevant combinations. We found limited research exploring the underlying neurobiological factors and the possible mediating role of inflammation on the liver-brain axis.
Added value of this study
Using data from up to 447,626 participants from UK Biobank, this study provides a well-powered characterization of associations between liver fibrosis, cognitive impairment, and grey matter atrophy. We found that people with advanced liver fibrosis had worse cognitive functioning and grey matter atrophy in the hippocampus, thalamus, ventral striatum, parahippocampal gyrus, brain stem, and cerebellum. Serum C-reactive protein levels were significantly higher in adults with advanced liver fibrosis than those without, indicating an elevated inflammation. Moreover, we demonstrated that the association of liver fibrosis with cognitive functioning and grey matter volume was significantly mediated by serum C-reactive protein, hinting at the potential role of systemic inflammation in the liver-brain axis.
Implications of all the available evidence
Our results support the existence of the liver-brain axis and highlight the mediating effect of systemic inflammation. Since early-stage liver fibrosis is a reversible syndrome, early surveillance and prevention of liver diseases may reduce cognitive decline and brain volume loss.
Introduction
Liver disease is a leading contributor to the global burden of morbidity and the only major chronic disease with increasing mortality.1, 2, 3 Chronic liver diseases of all etiologies exist along a spectrum. Varying degrees of hepatic fibrosis occur in response to liver injury and constitute the main determinant of disease progression to end-stage cirrhosis and hepatocellular carcinoma.4,5
Recently, extrahepatic manifestations of chronic liver diseases are attracting increasing attention in both hepatology and neuroscience. Interest is particularly high in cognitive functioning and brain health owing to their shared risk factors and pathophysiology with liver diseases.6 Neurological health is affected by advanced liver dysfunction, like hepatic encephalopathy, and may also be worsened by less severe forms of chronic liver diseases. Specifically, previous works have demonstrated the association between hepatic fibrosis and cognitive impairments in executive function,7 abstract reasoning,8 attention,5 and memory.9 Moreover, recent efforts to understand the liver-brain axis identified a link between liver function and imaging markers of brain structure, indicating a possible process of brain ageing.5,6,10, 11, 12 For example, a study using data from the Framingham offspring cohort found that nonalcoholic fatty liver disease (NAFLD) was associated with smaller total cerebral volume, corresponding to a 4.2-year advance in brain ageing.6 Likewise, a recent study identified a similar relationship between liver fibrosis and lower total brain volumes, implying the impact of liver disease on neurodegeneration.5
This body of research offers important preliminary insights into the relationship between liver fibrosis, cognition, and brain health. However, several issues must be addressed to improve our understanding of the liver-brain axis. First, most prior investigations involved highly selected cohorts (such as individuals with diabetes, NAFLD, and Alzheimer's disease) or included people 60 years and older, who have increased cognitive impairments.11,13 There is limited research exploring how the severity of liver fibrosis relates to multiple cognitive functions in a large population-based cohort. Second, previous studies maintained a specific focus on general measures of neuroimaging markers like total brain volume. Nevertheless, it remains largely unknown whether liver fibrosis exerts a global effect or more specifically localised effects on the brain. In this context, focused investigations to identify which brain regions are most sensitive to liver fibrosis are required. An additional gap is whether systemic inflammation mediates the liver-brain axis, despite the abundance of research relating inflammatory cytokines to the pathogenesis of liver diseases and psychopathology.14, 15, 16 Relatedly, a preclinical study17 found that NAFLD-related chronic inflammation outside of the brain can induce an increased number of activated microglia in the brain and contribute to neurodegeneration, suggesting the mediating role of inflammatory cytokines. More recently, a study demonstrated that the association between liver fibrosis and COVID-19-related mortality can be mediated by monocyte-associated inflammatory cytokines.18 Therefore, systemic inflammation may mediate the association of liver fibrosis with cognition and brain imaging markers, yet such data is scarce. Filling these knowledge gaps could inform the development of therapeutic strategies to delay the progression and mitigate the ramifications of liver fibrosis.
To address these gaps, the primary aim of the current research was to investigate the association of liver fibrosis with cognitive functioning, brain structure, and systemic inflammation using data from the population-based UK Biobank cohort.19 Our secondary aim was to examine whether the associations between liver fibrosis, cognition, and regional grey matter volume (GMV) were mediated by inflammatory biomarkers. The current study was designed to advance our understanding of the role of systemic inflammation on the liver-brain axis.
Methods
Study design and participants
The current study used data from the UK Biobank project, a prospective, nationwide cohort study of more than half a million adults recruited from the general population across the UK.19 Between 2006 and 2010, participants aged 37–73 years attended their closest of 22 dedicated assessment centres and gave written informed consent before completing an extensive set of assessments (the baseline visit). Post 2014, a subsample of participants was invited back for neuroimaging (the imaging visit). A flowchart illustrating the selection of samples for each analysis performed in the study is provided in Figure S1.
Assessment of liver fibrosis and inflammation
The severity of liver fibrosis was quantified using the Fibrosis-4 (Fib-4) score,20 a validated marker of liver fibrosis of multiple etiologies and a first-line tool for risk stratification in clinical practice. Fib-4 is defined based on clinical and liver function markers, including aspartate transaminase (AST), alanine aminotransferase (ALT), and platelet count (PLT), as:
| Fib-4 = Age × AST ÷ (PLT × √[ALT]) |
with age in years, aminotransferase in units/L, and PLT in 109/L. As per previous studies,5,9 participants with possible acute hepatitis (AST or ATL >250 units/L) and severe thrombocytopenia (PLT<5 × 1010/L) were excluded from further analysis to minimise their influences. Following earlier studies,5,21,22 participants with a Fib-4 score>2.67 were identified as having a high risk of advanced liver fibrosis while those with a Fib-4 score≤2.67 as having a low/indeterminate risk of liver fibrosis.
Inflammation was measured by C-reactive protein (CRP) at the baseline visit. CRP is a sensitive and stable hallmark of systemic inflammation and infection—produced principally by hepatocytes in response to changes in homeostasis due to insults.23,24 Serum CRP was measured by immunoturbidimetric high-sensitivity analysis on a Beckman Coulter AU5800.
Assessment of cognitive functioning
Participants performed a variety of cognitive tests. Overall, a total of 11 cognitive measures were included: fluid intelligence, prospective memory, reaction time, numeric memory, trail making (two measures), symbol digit substitution, and pairs matching (four measures). A detailed description of these cognitive measures can be found in Supplement. Field identifications used in this study are described in Table S1.
Cognitive measures that were assessed using response time or total error made were reversed by multiplying by −1 so that a higher value always corresponds to better performance. Positively skewed data were log-transformed.25,26
MRI data preprocessing
MRI data were acquired on a 3T Siemens Skyra scanner using a standard 32-channel head coil according to a freely available protocol (http://www.fmrib.ox.ac.uk/ukbiobank/protocol/V4_23092014.pdf). Of relevance to this study, T1 weighted MPRAGE data were acquired using the following parameters: sagittal orientation, resolution = 1 × 1 × 1 mm, TR = 2000 ms, field-of-view (FOV) = 208 × 256 × 256 matrix, duration = 5 min.
The estimates of GMVs were processed and quality controlled by the UK Biobank imaging team and made available to approved researchers as imaging-derived phenotypes. In brief, tissue-type segmentation was applied using FAST (FMRIB's Automated Segmentation Tool), and subcortical structures were modelled using FIRST (FMRIB's Integrated Registration and Segmentation Tool). The current study included 139 regional GMVs derived using parcellations from the Harvard–Oxford cortical and subcortical atlases (111 GMVs) and the Diedrichsen cerebellar atlas (28 GMVs). An extensive overview of the imaging preprocessing is available at https://biobank.ctsu.ox.ac.uk/crystal/crystal/docs/brain_mri.pdf and elsewhere. As in prior studies,27 GMVs were normalised for head size by multiplying the raw values by the head scaling factor. Further, extreme outlying data points (outside a range of four standard deviations) which may result from processing errors or severe brain irregularities, were excluded case-wise.27,28
Association analyses
We employed the linear mixed-effect model (LMM) to assess how cognitive functioning associates with liver fibrosis while accounting for relevant covariates. Separate models were constructed for each of the 11 cognitive tests. The Fib-4 score was fitted as a fixed effect and each of the cognitive tests was fitted as a response variable. As performed in other studies using UK Biobank,25,26 the data acquisition site was modelled as a random effect (assuming a random normal distribution) to account for nonindependence between distinct data centres and other potentially associated variables.29 Covariate variables, including age, self-reported sex, education level (with and without university or college degree-level qualifications), body mass index (BMI), waist-to-hip ratio, race (White vs ethnic minorities), and material deprivation (measured using the Townsend deprivation index), were used as additional predictors of no interest (Fig. 1). These covariates, which were likely to influence both liver fibrosis and cognitive functioning, were chosen on the basis of the existing literature.5,9 We conducted analyses on samples with complete data and excluded participants with missing data who responded “do not know” or “prefer not to answer” for any component of Fib-4 scores and confounding variables. We reported the unstandardised coefficient β, 95% confidence interval (CI), and two-tailed P-values for each analysis. Moreover, we also reported standardised effect sizes (Cohen's d, which was transformed from the standardised coefficient as described in30,31), as they are easier to interpret and compare with other studies, which can inform future studies.
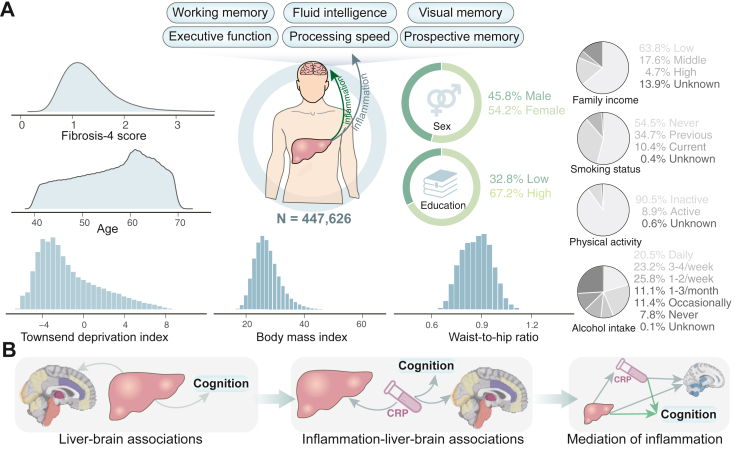
Fig. 1.
Summary of cohort characteristics at baseline and analyses performed in the current study. (A) The current study incorporated up to 447,626 participants who have complete data for liver fibrosis scores, covariate variables, and at least one cognitive test at baseline. The severity of liver fibrosis was calculated using the Fibrosis-4 (Fib-4) score. The behavioural analysis included a total of 11 cognitive functions covering domains of reasoning, working memory, visual memory, prospective memory, executive function, and processing speed. (B) We first investigate the association of liver fibrosis with cognitive functioning and brain structure, and then the association between systemic inflammation and brain health. After that, we examine whether the association between liver fibrosis and cognition and regional grey matter volumes can be mediated by differences in inflammatory biomarkers as measured by C-reactive protein.
The main analysis treated Fib-4 score as a categorical variable to investigate whether people with a high risk of advanced liver fibrosis exhibit worse cognitive performance. A secondary approach considered Fib-4 score as a continuous variable.32 Based on the covariates listed above, an additional set of covariates were added to the model in a sensitivity analysis. These additional variables included smoking status, physical activity, alcohol intake frequency (responses comprised six levels rating from never to daily), average total household income, and APOE ε4 status.33
Associations between liver fibrosis and regional GMVs, as well as between serum CRP and cognitive functioning and regional GMVs were investigated using the same analytical framework. Notably, models on regional GMVs also adjusted for the total intracranial volume. All analyses were corrected for multiple comparisons using the Benjamini-Hochberg false discovery rate (FDR) method.
Mediation analyses
A standard mediation analysis was performed using the “mediation” package in R (version 4.1.2) to investigate whether the association between two variables can be explained by the third variable (the mediator).34,35 Overall, two hypotheses were tested here (Fig. 1B). The first hypothesis examined the mediation effect of systemic inflammation on the association between liver fibrosis and cognitive functioning. Specifically, the Fib-4 score was used as an independent variable, the cognitive test as a response variable, and the CRP levels constituted the mediator. The second hypothesis examined the mediation effect of systemic inflammation on the association between liver fibrosis and regional GMVs. The independent variable and mediator were the same as in the first hypothesis, but the response variable was the regional GMVs. We controlled the same set of covariates as in the association analyses. Notably, the mediation analysis was only performed on cognitive tests or regional GMVs that were significantly correlated with both liver fibrosis and CRP levels. The statistical significance of the mediation effects was determined based on the bias-corrected bootstrap approach (5000 iterations).25
Ethics
The UK Biobank received ethical approval from the North West Multi-Centre Research Ethics Committee in the UK (REC reference 11/NW/0382), and these ethical regulations cover the work in this study. Written informed consent was obtained from all participants. The present analyses were conducted under UK Biobank application numbers 95864 and 42,009.
Role of the funding
The funder had no role in the study design, data collection, data analysis, interpretation, or writing of this manuscript.
Results
Population characteristics
After exclusion, the behavioural analysis included up to 447,626 participants who have complete data for Fib-4 score, covariate variables, and at least one cognitive test at the baseline visit (Figure S1). The median age was 58 years (IQR 50–63 years), 54.19% females, 94.67% White, and 32.77% had an education level above college. The number of participants with available data for each cognitive measure varied from a minimum of 45,055 (for the numeric memory task) to a maximum of 447,533 (for pairs matching). Of these, 9691 (2.16%) participants were considered to have a high probability of advanced liver fibrosis. People with liver fibrosis were generally older, more likely to be males, materially deprived, and physically inactive, had lower education levels and family income, and higher waist-to-hip ratio, compared with those without. They were also more likely to smoke and drink frequently. Table 1 illustrates cohort characteristics by liver fibrosis risk. After a median 9-year follow-up, a maximum sample of 38,244 participants with complete data available for at least one regional GMV were used for neuroimaging analyses (the sample size ranged between 38,080 and 38,231 per specific brain region). The median age of participants at the imaging visit was 64 years (IQR 58–70 years).
Table 1.
Characteristic of participants used for behavioural analyses.
| Total | Low risk of liver fibrosis | High risk of liver fibrosis | |
|---|---|---|---|
| Total N | 447,626 | 437,935 | 9691 |
| Age, years, median (IQR) | 58 (50, 63) | 58 (50, 63) | 64 (60, 67) |
| Female, N (%) | 242,571 (54.19%) | 239,307 (54.64%) | 3264 (33.68%) |
| White, N (%) | 423,768 (94.67%) | 414,671 (94.69%) | 9097 (93.87%) |
| BMI, kg/m2, median (IQR) | 26.72 (24.13, 29.86) | 26.72 (24.13, 29.86) | 26.62 (23.92, 29.93) |
| Waist-to-hip ratio, median (IQR) | 0.87 (0.80, 0.94) | 0.87 (0.80, 0.94) | 0.91 (0.84, 0.96) |
| Townsend deprivation index, median (IQR) | −2.17 (−3.66, 0.48) | −2.17 (−3.66, 0.47) | −2.02 (−3.59,0.77) |
| Average household income, N (%) | |||
| Low (<£51,999) | 285,641 (63.81%) | 278,734 (63.65%) | 6907 (71.27%) |
| Middle (£52,000–£100,000) | 78,878 (17.62%) | 77,874 (17.78%) | 1004 (10.36%) |
| High (>£100,000) | 21,023 (4.70%) | 20,793 (4.75%) | 230 (2.37%) |
| Unknown | 62,084 (13.87%) | 60,534 (13.82%) | 1550 (15.99%) |
| Physical activity (N/%) | |||
| Physically active | 405,271 (90.54%) | 396,597 (90.56%) | 8674 (89.51%) |
| Physically inactive | 39,874 (8.91%) | 38,935 (8.89%) | 939 (9.69%) |
| Unknown | 2481 (0.55%) | 2403 (0.55%) | 78 (0.80%) |
| Above College qualifications N (%) | 146,704 (32.77%) | 143,889 (32.86%) | 2815 (29.05%) |
| Smoking status, N (%) | |||
| Never | 244,003 (54.51%) | 239,282 (54.64%) | 4721 (48.72%) |
| Ever | 155,334 (34.70%) | 151,334 (34.56%) | 4000 (41.28%) |
| Current | 46,705 (10.43%) | 45,772 (10.45%) | 933 (9.63%) |
| Unknown | 1584 (0.35%) | 1547 (0.35%) | 37 (0.38%) |
| Alcohol intake frequency, N (%) | |||
| Daily or almost daily | 91,795 (20.51%) | 89,077 (20.34%) | 2718 (28.05%) |
| Three or four times a week | 104,033 (23.24%) | 101,987 (23.29%) | 2046 (21.11%) |
| Once or twice a week | 115,678 (25.84%) | 113,554 (25.93%) | 2124 (21.92%) |
| One to three times a month | 49,740 (11.11%) | 48,882 (11.16%) | 858 (8.85%) |
| Special occasions only | 50,931 (11.38%) | 49,916 (11.40%) | 1015 (10.47%) |
| Never | 35,074 (7.84%) | 34,159 (7.80%) | 915 (9.44%) |
| Unknown | 375 (0.08%) | 360 (0.08%) | 15 (0.15%) |
| AST, U/L, median (IQR) | 24.40 (21.00, 28.80) | 24.30 (20.90, 28.60) | 33.70 (27.30, 48.40) |
| ALT, U/L, median (IQR) | 20.13 (15.41, 27.38) | 20.10 (15.41, 27.29) | 21.60 (5.25, 33.81) |
| Platelet count, 109 cell/L, median (IQR) | 248.00 (213.60, 287.00) | 249.70 (215.90, 288.10) | 154.40 (126.53, 182.00) |
| Fib-4 score, median (IQR) | 1.24 (0.97, 1.57) | 1.22 (0.97, 1.54) | 3.07 (2.83, 3.60) |
| CRP, mg/L, median (IQR) | 1.30 (0.65, 2.67) | 1.30 (0.65, 2.67) | 1.34 (0.65, 2.81) |
Associations between liver fibrosis and cognitive functions
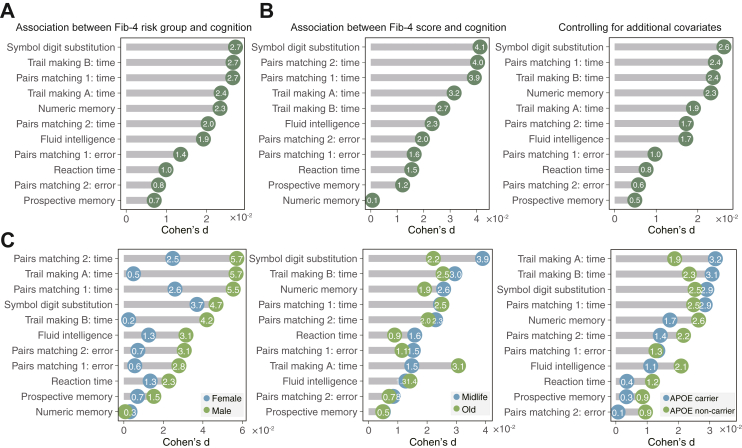
As shown in Fig. 2A and Table S2, the association analysis provided support for the association between liver fibrosis and cognitive measures after controlling for a set of covariates and multiple comparisons: fluid intelligence (N = 146,439, β = −0.124, d = −0.019, PFDR<0.001), prospective memory (N = 151,345, β = −0.075, d = −0.007, PFDR = 0.035), reaction time (N = 443,725, β = −0.007, d = −0.010, PFDR<0.001), numeric memory (N = 45,055, β = −0.101, d = −0.023, PFDR = 0.013), trail making A (N = 94,511, β = −0.028, d = −0.024, PFDR<0.001), trail making B (N = 94,509, β = −0.033, d = −0.027, PFDR<0.001), symbol digit substitution (N = 107,511, β = −0.511, d = −0.027, PFDR< 0.001), pairs matching: time (trail 1: N = 435,803, β = −0.048, d = −0.027; trail 2: N = 434,952, β = −0.028, d = −0.020; PFDR<0.001), and pairs matching: error (N = 447,533; trail 1: β = −0.053, d = −0.014, PFDR<0.001; trail 2: β = −0.091, d = −0.008, PFDR = 8.40 × 10−03). All associations were in the expected direction with participants having liver fibrosis showing inferior performance in all tested cognitive domains. Likewise, significant associations were observed in all cognitive tests other than numeric memory when Fib-4 score was treated as a continuous variable (Fig. 2B and Table S3). Compared with treating Fib-4 score as a categorical variable (using 2.67 as the cutoff), the effect sizes were larger for most cognitive measures. The strongest effect was observed for symbol digit substitution (β = −0.208, d = 0.041, PFDR< 0.001), followed by pairs matching: time (trail 2: β = −0.015, d = 0.040; PFDR<0.001). The results were nearly unchanged in a sensitivity analysis that controlled for additional covariates (including smoking status, average family income, physical activity state, alcohol intake frequency, and APOE ε4 status) (Table S4), or treating each of the cognitive measures as binary variables and analysing the associations using a generalised LMM (Figure S2). Results also remained similar when adjusting for a minimum set of covariates identified from directed acyclic graphs (DAGs) (Figure S3, Table S5).36
Fig. 2.
Associations between liver fibrosis and cognitive functioning. (A) The presence of advanced liver fibrosis showed significant associations with all cognitive tests after controlling for a set of confounders including age, sex, education level, BMI, waist-to-hip ratio, race, material deprivation, and data acquisition site (PFDR<0.05). The number of participants with available data for each cognitive measure varied from 45,055 to 447,533. (B) Significant associations were observed in most cognitive tests when treating Fib-4 score as a continuous variable or additionally controlling for smoking status, family income, alcohol intake, physical activity frequency, and APOE ε4 status. (C) Associations between liver fibrosis and cognitive tests stratified by age, sex, and APOE ε4 status.
When the analyses were stratified by subgroups, similar findings were observed, although some fell short of significance after multiple comparisons (Fig. 2C). Specifically, liver fibrosis in males showed numerously higher associations with most cognitive tests than in females. In addition, individuals aged 45–60 years had a stronger association with symbol digital substitution but a weaker association with trail making A than their older counterparts. Using 65 years as the cutoff yielded similar findings (Figure S4). Moreover, liver fibrosis was more strongly associated with worse performance on trail making among APOE ε4 carriers than noncarriers. In contrast, fluid intelligence was more strongly associated with liver fibrosis in APOE ε4 noncarriers than in carriers.
Associations between liver fibrosis and regional GMVs
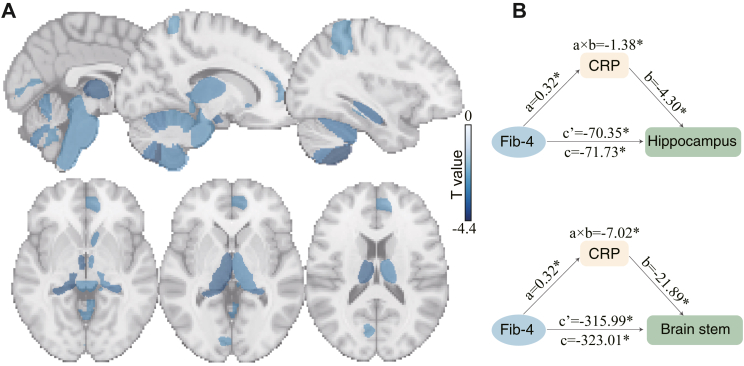
The neuroimaging analysis was performed across 139 brain regions. After adjustment for covariates and multiple comparisons, no statistically significant association was found between liver fibrosis and the total GMV. However, the regional analysis indicated strong evidence for significant associations between liver fibrosis and 24 GMVs out of 139 examined (PFDR<0.05, Figure S5, Table S6). The most significant areas primarily included the bilateral hippocampus, thalamus, ventral striatum, parahippocampal gyrus, the brain stem, and several subregions of the cerebellum (Fig. 3A). All these associations were negative, indicating an accelerated GMV loss in patients with liver fibrosis. When the total intracranial volume was not controlled, 28 brain regions showed significant associations with liver fibrosis (Figure S6).
Fig. 3.
Regional GMVs associated with liver fibrosis and the mediation effect of serum CRP on the association between liver fibrosis and GMVs. (A) The regional analysis revealed 24 brain GMVs that were significantly correlated with liver fibrosis after controlling for confounders and multiple comparisons (PFDR<0.05). The most significant brain areas primarily included the bilateral hippocampus, thalamus, ventral striatum, parahippocampal gyrus, brain stem, and cerebellum. T-statistics are visualised here. (B) The right hippocampus and brain stem emerged as the only two brain regions showing consistent associations with both liver fibrosis and serum CRP. The serum CRP significantly and partially mediated the effect of liver fibrosis on the GMV in the right hippocampus and brain stem (PFDR = 0.006). Path a: the effect of the independent variable (liver fibrosis) on the mediator (serum CRP); Path b: the effect of the mediator (serum CRP) on the outcome variable (GMV); Path ab: the indirect effect of liver fibrosis on regional GMV; Path c: the total effect of liver fibrosis on the regional GMV; Path c’: the direct effect of liver fibrosis on regional GMV controlling for serum CRP. ‘Proportion mediated’ was defined as the proportion of the total effect that is explained by a mediator (ab/c). Covariates were controlled in all paths.
Associations of CRP with liver fibrosis, cognitive functions, and regional GMVs
The serum CRP was significantly and positively correlated with liver fibrosis, indicating elevated inflammation in patients with advanced hepatic fibrosis (N = 441,768, d = 0.014, P < 0.001). With regards to its association with cognitive functions, eight measures reached statistical significance (PFDR<0.05), but statistical significance was not reached for trail making A, symbol digit substitution, and pairs matching 2: error (Table S7). The effect size for significant associations ranged from d = 0.007 (pairs matching 2: time) to d = 0.056 (numeric memory).
The neuroimaging analyses also provided strong evidence for associations between serum CRP and 15 regional GMVs after adjustment for covariates and multiple comparisons (PFDR<0.05, Table S8). These brain areas primarily included the middle frontal gyrus, posterior supramarginal gyrus, anterior cingulate, anterior fusiform cortex, angular gyrus, pallidum, hippocampus, frontal pole, inferior temporal gyrus, and the brain stem (Figure S7). Notably, the right hippocampus and brain stem emerged as the only two brain regions showing consistent associations with both liver fibrosis and serum CRP.
Mediation effects of CRP on the association between liver fibrosis and cognitive functions and brain health
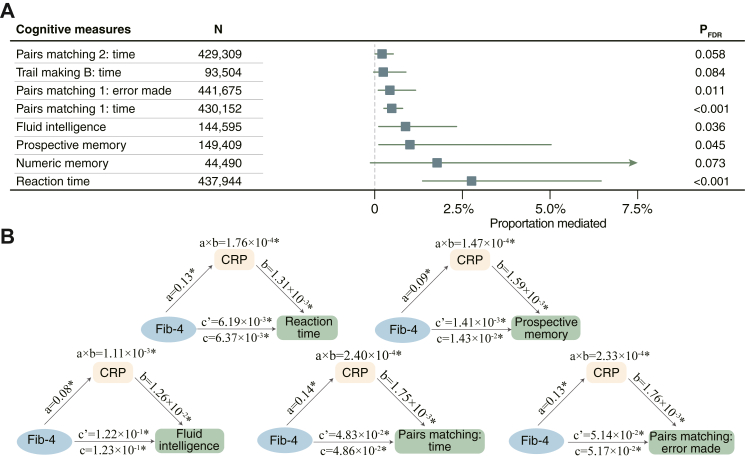
The mediation analyses revealed a partial and significant effect of serum CRP levels on the association between liver fibrosis and cognitive functions (Fig. 4A), including reaction time (bootstrapping test, PFDR<0.001, proportion mediated = 2.76%, [95% CI: 1.37%–6.46%]), prospective memory (PFDR = 0.045, 1.00%, [0.12%–5.03%]), fluid intelligence (PFDR = 0.036, 0.88%, [0.12%–2.33%]), pairs matching 1: completion time (PFDR<0.001, 0.49%, [0.26%–0.80%]), and pairs matching 1: error made (PFDR = 0.011, 0.43%, [0.10%–1.16%]), but nonsignificant for the other measures (Fig. 4B). The CRP levels also significantly and partially mediated the effect of liver fibrosis on the GMV in the right hippocampus (PFDR = 0.006, proportion mediated = 1.92%, [0.41%–5.97%]) and brain stem (PFDR = 0.006, 2.18%, [0.52%–7.94%], Fig. 3B). Mediation results adjusting for a minimum set of covariates identified from DAGs were provided in Figures S8 and S9.
Fig. 4.
Mediation effect of serum CRP on the association between liver fibrosis and cognitive functioning. (A) The mediation analysis was performed on eight cognitive tests that were significantly associated with both liver fibrosis and serum CRP levels. (B) The serum CRP significantly and partially mediated the effect of liver fibrosis on reaction time (PFDR<0.001, N = 437,944), prospective memory (PFDR = 0.045, N = 149,409), fluid intelligence (PFDR = 0.036, N = 144,595), pairs matching 1: completion time (PFDR<0.001, N = 430,152), and pairs matching 1: error made (PFDR = 0.011, N = 441,675).
Discussion
Using data from UK Biobank, the current study provides a well-powered characterization of the association between liver fibrosis, cognitive functioning, brain structure, and inflammation. We found that people with advanced liver fibrosis had worse cognitive functioning and grey matter atrophy in the hippocampus, thalamus, ventral striatum, parahippocampal gyrus, brain stem, and cerebellum. Moreover, we demonstrated that the association of liver fibrosis with cognitive functioning and GMVs was partially and significantly mediated by serum CRP, hinting at the potential role of systemic inflammation in the liver-brain axis.
The observation that liver fibrosis was associated with inferior cognitive performance is consistent with a growing body of literature highlighting the critical role of liver fibrosis in cognitive functioning.5,7, 8, 9 However, most prior studies were performed in smaller samples and included relatively older or highly selected populations. Also supporting our findings are studies that found a possible link between liver fibrosis or the presence of NAFLD and increased risk of incident dementia, a severe form of cognitive impairment.32,37, 38, 39, 40 Our current study replicates these findings and extends them in several aspects. The most prominent strength is a much larger cohort of population-scale adults and the inclusion of a broad range of cognitive functions covering reasoning, working memory, visual memory, executive function, and processing speed. The large sample size provides greater statistical power to detect subtle but significant liver fibrosis effects that may be undetectable in smaller studies. It also increases the confidence that these results may extrapolate to the general populations and allows for examining how the liver–cognition associations vary by subgroups.41 Specifically, the subgroup analyses revealed 1) larger effect sizes in males for most cognitive tests than females; 2) larger effects sizes for symbol digital substitution but smaller effect sizes for trail making in middle-aged people than their old counterparts; 3) stronger association for trail making but weaker association for fluid intelligence in APOE ε4 carriers than noncarriers. This result can be partially attributed to the low sensitivity of Fib-4 scores in these people21,42 but may also imply a high vulnerability of those individuals to liver fibrosis. In this regard, our findings argue for increased attention to those individuals with liver fibrosis.
Chronic liver diseases may contribute to cognitive decline across the whole spectrum.9 As such, early surveillance and prevention of liver diseases before the onset of overt clinical manifestations could improve public health. Further, since approximately 15% of people with subclinical liver fibrosis are asymptomatic and have normal liver enzyme levels,43 the assessment of liver fibrosis could become part of routine health screening delivered to older people within primary health care and general practice settings. Fib-4 scores are non-invasive and easily calculated from routine laboratory indices without extra costs,44 suggesting their feasibility as a screening tool in a community setting to identify high-risk individuals with liver fibrosis.
The neuroimaging analyses further support the existence of the liver-brain axis by showing significantly negative associations between Fib-4 score and regional but not total brain GMV, implying a region-specific effect of liver fibrosis on brain health.45 The most extensively affected brain areas primarily include the hippocampus, thalamus, ventral striatum, parahippocampal gyrus, brain stem, and cerebellum. As brain atrophy is an established signature of neurodegeneration,46 these results may suggest a disproportionate vulnerability of these brain regions to liver fibrosis. Nevertheless, it should be noted that the brain imaging indices were collected a median 9 years after the baseline assessment. The status of liver fibrosis may have changed over time, although the liver-brain associations were consistent after controlling for the years between the baseline and imaging visits (Figures S6 and S8). Some people who were healthy at baseline may progress to have liver fibrosis, which may underestimate the liver-brain associations. However, given that it may take years for disease-related changes to be evident in brain structure,47 this long time interval can allow us to observe the associations between liver fibrosis and lower GMVs.5 Supporting evidence for the direct association of liver fibrosis with regional brain structure in the literature is sparse, but these results accord with emerging evidence relating chronic liver diseases to Alzheimer's disease (AD) pathology and progression. Specifically, the liver is the origin of brain Aβ deposits and plays an integral role in clearing Aβ from circulating blood.48 Consequently, hepatic dysfunction may lead to insufficient peripheral Aβ clearance, worsening brain Aβ deposition.49,50 This hypothesis concords with our observation that brain regions, including the hippocampus, thalamus, and striatum, often amongst the first regions to show structural atrophy in ageing and early AD,51 exhibit the most significant associations with liver fibrosis. Moreover, it is reported that neurotoxic metabolites produced in the injured liver may cross the blood–brain barrier and affect the activity of specific brain regions, particularly those with high metabolic demands and those crucial for memory and learning, like the hippocampus, cerebellum, and prefrontal cortex.52 Consistently, an animal study observed that NAFLD could decrease metabolic brain activity in the prefrontal cortex, thalamus, hippocampus, and amygdala, dopamine in the prefrontal and cerebellum, and noradrenaline in the striatum.53
Our study provides further evidence of the potential mechanism through which liver fibrosis may exert its effect on cognitive impairment and brain atrophy. Specifically, intrahepatic fat accumulation can damage hepatocytes, stimulating the excess secretion of pro-inflammatory cytokines.54 The elevated peripheral inflammatory cytokines can cross the blood–brain barrier, activate microglia, release various inflammatory mediators into the brain, and induce elevated expression of neuroinflammation and silent lesions in the brain.55 Neuroinflammation may decrease the expression of brain-derived neurotrophic factors in the hippocampus and striatum, resulting in cognitive impairment via disrupted neurogenesis.56 In addition, liver fibrosis may trigger gut microbiota and urea cycle functional impairment and promote ammonia accumulation. In turn, these can exacerbate the effect of liver diseases on cognition and the brain via synergizing with systemic inflammation. Moreover, inflammation can contribute to cognitive impairments by interacting with other non-inflammatory processes, including endothelial dysfunction, insulin resistance, and oxidative stress.57 Overall, these findings, together with ours, open up mechanistic prospects for preventing cognitive decline and brain atrophy in patients with hepatic fibrosis.
The following limitations need to be noted. First, the current research relies on a cross-section design. The observed mediation effects are strictly a measure of association58 and cannot disentangle the causality of the interplay between liver fibrosis, inflammation, and brain health. Given the potential flaws related to mediation analysis,59 these results should be considered preliminary. In this regard, combining brain-wide imaging-genetics associations with co-localisation analysis60 could potentially identify shared causal variants between liver fibrosis and cognitive functioning, thus enabling unique insights into the liver-brain axis. Second, the Fib-4 score is less accurate than a liver biopsy and has a low sensitivity in assessing hepatic fibrosis, which may result in misclassification and underestimating the observed associations.42 Future studies can leverage the combination of proteomic analyses and elastography to quantify the severity of liver fibrosis. Moreover, although cognitive decline is considered a syndrome limited to the brain, the mechanisms responsible for cognitive impairments may share with aberrations in other organs. Evidence from a recent study indicates interdependencies of the heart–brain–liver axis.61 As such, a multi-organ approach mapping the relationship between imaging measures of the liver (like proton density fat fraction, liver iron concentration, and iron-corrected T1) and other organs, especially the brain, using state-of-the-art MRI phenotypes also merits further study. Third, the serum CRP was the only inflammatory biomarker studied in this research, and its mediation effects were relatively low, albeit statistically significant. This result can be attributed to the fact that CRP reflects the general inflammatory levels and is not sensitive enough to capture more specific liver-related inflammatory pathways.62 As such, other inflammatory biomarkers like interleukin1 (IL-1), IL-6, and tumor necrosis factor-α, which are known to be present in specific brain areas, especially the hippocampus, may better explain the liver-brain axis.16 Fourth, despite adjustment for a list of potential covariates, unmeasured or residual confounding resulting from imperfections in the measurement of some variables (especially those based on self-report) is inevitable.61 Moreover, the complete case analysis can also introduce bias since data may not be missing at random, thereby underscoring the need to replicate the findings. Our sensitivity analysis using imputed data for covariate variables yields largely unaltered results (Tables S9–S11). Finally, participants in UK Biobank are more likely to have healthier behaviour, a higher socioeconomic status, and be predominantly of European ancestry.63 Therefore, summary statistics like the prevalence of liver fibrosis may not represent the general population, although the effect sizes of liver-brain associations were generally consistent with other studies.
Conclusions
In summary, the current study, using one of the largest population-scale cohorts currently available, provides evidence of negative associations of liver fibrosis with cognitive functioning and GMV, informing our understanding of the mediation role of inflammation in the liver-brain axis. Considering that early-stage liver fibrosis is a reversible syndrome, the burden of cognitive impairment and brain atrophy attributable to liver diseases may be delayed and prevented by interventions. Nevertheless, further investigations should consider whether public strategies aiming at improving chronic liver diseases, especially those related to liver fibrosis, can contribute to reducing cognitive decline and brain atrophy.
Contributors
R.J., and D.S. conceived and designed the experiment. R.J. performed the analyses with support from D.S., J.W., B.X., Q.L., and W.D. R.J. and J.W. drafted the manuscript with comments from M.R., R.X.R., J.S., S.Q., and Q.M. B.X. and V.D.C. contributed to interpreting the results. R.J., Q.L., W.D., and D.S. accessed and verified the underlying data reported in the manuscript. All authors read and approved the final manuscript.
Data sharing statement
All data used in this study are publicly accessible from UK Biobank via their standard data access procedure at https://www.ukbiobank.ac.uk/. Researchers can apply for access to the UK Biobank data via the Access Management System (AMS) (https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access).
Declaration of interests
The authors declare no competing interests.
Acknowledgments
This work is supported in part by the National Science Foundation (2112455), National Institutes of Health (RF1AG063153, R01MH117107), National Key Research and Development Program (2021YFE0202500), and an internal Yale University fund. We are grateful to all participants who donated their time to this project and the UK Biobank team for collecting, processing, and disseminating data used in this study.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104679.
Contributor Information
Rongtao Jiang, Email: rongtao.jiang@yale.edu.
Bin Xu, Email: xubin1016@ccmu.edu.cn.
Dustin Scheinost, Email: dustin.scheinost@yale.edu.
Appendix A. Supplementary data
References
- 1.Ramakrishnan A., Velmurugan G., Somasundaram A., et al. Prevalence of abnormal liver tests and liver fibrosis among rural adults in low and middle-income country: a cross-sectional study. eClinicalMedicine. 2022;51 doi: 10.1016/j.eclinm.2022.101553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gines P., Graupera I., Lammert F., et al. Screening for liver fibrosis in the general population: a call for action. Lancet Gastroenterol Hepatol. 2016;1(3):256–260. doi: 10.1016/S2468-1253(16)30081-4. [DOI] [PubMed] [Google Scholar]
- 3.Taylor R.S., Taylor R.J., Bayliss S., et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta -analysis. Gastroenterology. 2020;158(6):1611–1625. doi: 10.1053/j.gastro.2020.01.043. [DOI] [PubMed] [Google Scholar]
- 4.Angulo P., Kleiner D.E., Dam-Larsen S., et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389–397. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parikh N.S., Kamel H., Zhang C., et al. Association of liver fibrosis with cognitive test performance and brain imaging parameters in the UK Biobank study. Alzheimers Dement. 2022;19(4):1518–1528. doi: 10.1002/alz.12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinstein G., Zelber-Sagi S., Preis S.R., et al. Association of nonalcoholic fatty liver disease with lower brain volume in healthy middle-aged adults in the Framingham study. JAMA Neurol. 2018;75(1):97–104. doi: 10.1001/jamaneurol.2017.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuriakose L., Moulton C., Rokakis A., Rahim M., Ismail K., Heneghan M. The association between liver fibrosis and cognitive impairment in type 2 diabetes: a cross-sectional sub-study of the south London diabetes cohort. J Hepatol. 2020;73:S144. [Google Scholar]
- 8.Weinstein G., Davis-Plourde K., Himali J.J., Zelber-Sagi S., Beiser A.S., Seshadri S. Non-alcoholic fatty liver disease, liver fibrosis score and cognitive function in middle-aged adults: the Framingham Study. Liver Int. 2019;39(9):1713–1721. doi: 10.1111/liv.14161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parikh N.S., Kumar S., Rosenblatt R., et al. Association between liver fibrosis and cognition in a nationally representative sample of older adults. Eur J Neurol. 2020;27(10):1895–1903. doi: 10.1111/ene.14384. [DOI] [PubMed] [Google Scholar]
- 10.Nho K., Kueider-Paisley A., Ahmad S., et al. Association of altered liver enzymes with alzheimer disease diagnosis, cognition, neuroimaging measures, and cerebrospinal fluid biomarkers. JAMA Netw Open. 2019;2(7) doi: 10.1001/jamanetworkopen.2019.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basu E., Mehta M., Zhang C., et al. Association of chronic liver disease with cognition and brain volumes in two randomized controlled trial populations. J Neurol Sci. 2022;434 doi: 10.1016/j.jns.2021.120117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng Z., Duggan M.R., Dark H.E., et al. Association of liver disease with brain volume loss, cognitive decline, and plasma neurodegenerative disease biomarkers. Neurobiol Aging. 2022;120:34–42. doi: 10.1016/j.neurobiolaging.2022.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang R., Scheinost D., Zuo N., et al. A neuroimaging signature of cognitive aging from whole-brain functional connectivity. Adv Sci. 2022;9(24) doi: 10.1002/advs.202201621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furman D., Campisi J., Verdin E., et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kronsten V.T., Tranah T.H., Pariante C., Shawcross D.L. Gut-derived systemic inflammation as a driver of depression in chronic liver disease. J Hepatol. 2022;76(3):665–680. doi: 10.1016/j.jhep.2021.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Seki E., Schwabe R.F. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology. 2015;61(3):1066–1079. doi: 10.1002/hep.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim D.G., Krenz A., Toussaint L.E., et al. Non-alcoholic fatty liver disease induces signs of Alzheimer's disease (AD) in wild-type mice and accelerates pathological signs of AD in an AD model. J Neuroinflammation. 2016;13:1. doi: 10.1186/s12974-015-0467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y.J., Regan J., Fajnzylber J., et al. Liver fibrosis index FIB-4 is associated with mortality in COVID-19. Hepatol Commun. 2021;5(3):434–445. doi: 10.1002/hep4.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bycroft C., Freeman C., Petkova D., et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterling R.K., Lissen E., Clumeck N., et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 21.McPherson S., Hardy T., Dufour J.-F., et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol. 2017;112(5):740. doi: 10.1038/ajg.2016.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao G., Zhu S., Xiao X., Yan L., Yang J., Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology. 2017;66(5):1486–1501. doi: 10.1002/hep.29302. [DOI] [PubMed] [Google Scholar]
- 23.Pepys M.B., Hirschfield G.M. C-reactive protein: a critical update. J Clin Invest. 2003;111(12):1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sproston N.R., Ashworth J.J. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9 doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang R., Westwater M.L., Noble S., et al. Associations between grip strength, brain structure, and mental health in> 40,000 participants from the UK Biobank. BMC Med. 2022;20(1):1–14. doi: 10.1186/s12916-022-02490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Firth J., Firth J.A., Stubbs B., et al. Association between muscular strength and cognition in people with major depression or bipolar disorder and healthy controls. JAMA Psychiatr. 2018;75(7):740–746. doi: 10.1001/jamapsychiatry.2018.0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daviet R., Aydogan G., Jagannathan K., et al. Associations between alcohol consumption and gray and white matter volumes in the UK Biobank. Nat Commun. 2022;13(1):1–11. doi: 10.1038/s41467-022-28735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox S.R., Lyall D.M., Ritchie S.J., et al. Associations between vascular risk factors and brain MRI indices in UK Biobank. Eur Heart J. 2019;40(28):2290–2300. doi: 10.1093/eurheartj/ehz100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marshall A.T., Betts S., Kan E.C., McConnell R., Lanphear B.P., Sowell E.R. Association of lead-exposure risk and family income with childhood brain outcomes. Nat Med. 2020;26(1):91–97. doi: 10.1038/s41591-019-0713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grant R.L. Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ. 2014:348. doi: 10.1136/bmj.f7450. [DOI] [PubMed] [Google Scholar]
- 31.Jiang R., Noble S., Sui J., et al. Associations of physical frailty with health outcomes and brain structure in 483 033 middle-aged and older adults: a population-based study from the UK Biobank. Lancet Digit Health. 2023;5(6):e350–e359. doi: 10.1016/S2589-7500(23)00043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parikh N.S., Kamel H., Zhang C., et al. Association between liver fibrosis and incident dementia in the UK Biobank study. Eur J Neurol. 2022;29(9):2622–2630. doi: 10.1111/ene.15437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lumsden A.L., Mulugeta A., Zhou A., Hypponen E. Apolipoprotein E (APOE) genotype-associated disease risks: a phenome-wide, registry-based, case-control study utilising the UK Biobank. eBioMedicine. 2020;59 doi: 10.1016/j.ebiom.2020.102954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baron R.M., Kenny D.A. The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 35.Cheng W., Rolls E., Gong W., et al. Sleep duration, brain structure, and psychiatric and cognitive problems in children. Mol Psychiatr. 2021;26(8):3992–4003. doi: 10.1038/s41380-020-0663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shrier I., Platt R.W. Reducing bias through directed acyclic graphs. BMC Med Res Methodol. 2008;8 doi: 10.1186/1471-2288-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shang Y., Widman L., Hagstrom H. Nonalcoholic fatty liver disease and risk of dementia: a population-based cohort study. Neurology. 2022;99(6):e574–e582. doi: 10.1212/WNL.0000000000200853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao T., van Kleef L., Ikram M.K., De Knegt R., Ikram M.A. Association of nonalcoholic fatty liver disease and fibrosis with incident dementia and cognition: the Rotterdam study. Neurology. 2022;99(6):e565–e573. doi: 10.1212/WNL.0000000000200770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim G.A., Oh C.H., Kim J.W., et al. Association between non-alcoholic fatty liver disease and the risk of dementia: a nationwide cohort study. Liver Int. 2022;42(5):1027–1036. doi: 10.1111/liv.15244. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y., Li Y., Liu K., et al. Nonalcoholic fatty liver disease, serum cytokines, and dementia among rural-dwelling older adults in China: a population-based study. Eur J Neurol. 2022;29(9):2612–2621. doi: 10.1111/ene.15416. [DOI] [PubMed] [Google Scholar]
- 41.Marek S., Tervo-Clemmens B., Calabro F.J., et al. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022;603(7902):654–660. doi: 10.1038/s41586-022-04492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hagstrom H., Talback M., Andreasson A., Walldius G., Hammar N. Ability of noninvasive scoring systems to identify individuals in the population at risk for severe liver disease. Gastroenterology. 2020;158(1):200–214. doi: 10.1053/j.gastro.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Harris R., Harman D.J., Card T.R., Aithal G.P., Guha I.N. Prevalence of clinically significant liver disease within the general population, as defined by non-invasive markers of liver fibrosis: a systematic review. Lancet Gastroenterol Hepatol. 2017;2(4):288–297. doi: 10.1016/S2468-1253(16)30205-9. [DOI] [PubMed] [Google Scholar]
- 44.McPherson S., Stewart S.F., Henderson E., Burt A.D., Day C.P. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59(9):1265–1269. doi: 10.1136/gut.2010.216077. [DOI] [PubMed] [Google Scholar]
- 45.Seo S.W., Gottesman R.F., Clark J.M., et al. Nonalcoholic fatty liver disease is associated with cognitive function in adults. Neurology. 2016;86(12):1136–1142. doi: 10.1212/Wnl.0000000000002498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frisoni G.B., Fox N.C., Jack C.R., Scheltens P., Thompson P.M. The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol. 2010;6(2):67–77. doi: 10.1038/nrneurol.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang R., Calhoun V.D., Noble S., et al. A functional connectome signature of blood pressure in >30,000 participants from the UK Biobank. Cardiovasc Res. 2023;119(6):1427–1440. doi: 10.1093/cvr/cvac116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sehgal N., Gupta A., Valli R.K., et al. Withania somnifera reverses Alzheimer's disease pathology by enhancing low-density lipoprotein receptor-related protein in liver. Proc Natl Acad Sci U S A. 2012;109(9):3510–3515. doi: 10.1073/pnas.1112209109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weinstein G., O'Donnell A., Davis-Plourde K., et al. Non-Alcoholic fatty liver disease, liver fibrosis, and regional amyloid-beta and Tau pathology in middle-aged adults: the Framingham study. J Alzheimers Dis. 2022;86(3):1371–1383. doi: 10.3233/JAD-215409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bassendine M.F., Taylor-Robinson S.D., Fertleman M., Khan M., Neely D. Is Alzheimer's disease a liver disease of the brain? J Alzheim Dis. 2020;75(1):1–14. doi: 10.3233/Jad-190848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fjell A.M., Walhovd K.B. Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci. 2010;21(3):187–222. doi: 10.1515/revneuro.2010.21.3.187. [DOI] [PubMed] [Google Scholar]
- 52.Colognesi M., Gabbia D., De Martin S. Depression and cognitive impairment-extrahepatic manifestations of NAFLD and NASH. Biomedicines. 2020;(7):8. doi: 10.3390/biomedicines8070229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Higarza S.G., Arboleya S., Gueimonde M., Gomez-Lazaro E., Arias J.L., Arias N. Neurobehavioral dysfunction in non-alcoholic steatohepatitis is associated with hyperammonemia, gut dysbiosis, and metabolic and functional brain regional deficits. PLoS One. 2019;14(9) doi: 10.1371/journal.pone.0223019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2(11):901–910. doi: 10.1016/S2213-8587(14)70032-4. [DOI] [PubMed] [Google Scholar]
- 55.Kang S., Kim E., Cho H., Kim D.J., Kim H.C., Jung S.J. Associations between non-alcoholic fatty liver disease and cognitive impairment and the effect modification of inflammation. Sci Rep. 2022;12(1) doi: 10.1038/s41598-022-16788-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dhanda S., Gupta S., Halder A., Sunkaria A., Sandhir R. Systemic inflammation without gliosis mediates cognitive deficits through impaired BDNF expression in bile duct ligation model of hepatic encephalopathy. Brain Behav Immun. 2018;70:214–232. doi: 10.1016/j.bbi.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 57.Gehrke N., Schattenberg J.M. Metabolic inflammation—a role for hepatic inflammatory pathways as drivers of comorbidities in nonalcoholic fatty liver disease? Gastroenterology. 2020;158(7):1929–1947. doi: 10.1053/j.gastro.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 58.Lees B., Mewton L., Jacobus J., et al. Association of prenatal alcohol exposure with psychological, behavioral, and neurodevelopmental outcomes in children from the adolescent brain cognitive development study. Am J Psychiatr. 2020;177(11):1060–1072. doi: 10.1176/appi.ajp.2020.20010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao X., Lynch J.G., Jr., Chen Q. Reconsidering baron and Kenny: myths and truths about mediation analysis. J Consum Res. 2010;37(2):197–206. [Google Scholar]
- 60.Mohammadi-Nejad A.R., Allen R.J., Kraven L.M., et al. Mapping brain endophenotypes associated with idiopathic pulmonary fibrosis genetic risk. eBioMedicine. 2022;86 doi: 10.1016/j.ebiom.2022.104356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McCracken C., Raisi-Estabragh Z., Veldsman M., et al. Multi-organ imaging demonstrates the heart-brain-liver axis in UK Biobank participants. Nat Commun. 2022;13(1):7839. doi: 10.1038/s41467-022-35321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Milton D.C., Ward J., Ward E., et al. The association between C-reactive protein, mood disorder, and cognitive function in UK Biobank. Eur Psychiatr. 2021;64(1) doi: 10.1192/j.eurpsy.2021.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Batty G.D., Gale C.R., Kivimaki M., Deary I.J., Bell S. Comparison of risk factor associations in UK Biobank against representative, general population based studies with conventional response rates: prospective cohort study and individual participant meta-analysis. BMJ. 2020;368:m131. doi: 10.1136/bmj.m131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.