Summary
Background
Neurofilament light chain (NfL), a neuronal cytoskeletal protein that is released upon neuroaxonal injury, is associated with multiple sclerosis (MS) relapsing activity and has demonstrated some prognostic ability for future relapse-related disease progression, yet its value in assessing non-relapsing disease progression remains unclear.
Methods
We examined baseline and longitudinal blood NfL levels in 1421 persons with relapsing MS (RMS) and 596 persons with primary progressive MS (PPMS) from the pivotal ocrelizumab MS trials. NfL treatment-response and risk for disease worsening (including disability progression into the open-label extension period and slowly expanding lesions [SELs] on brain MRI) at baseline and following treatment with ocrelizumab were evaluated using time-to-event analysis and linear regression models.
Findings
In persons from the RMS control arms without acute disease activity and in the entire PPMS control arm, higher baseline NfL was prognostic for greater whole brain and thalamic atrophy, greater volume expansion of SELs, and clinical progression. Ocrelizumab reduced NfL levels vs. controls in persons with RMS and those with PPMS, and abrogated the prognostic value of baseline NfL on disability progression. Following effective suppression of relapse activity by ocrelizumab, NfL levels at weeks 24 and 48 were significantly associated with long-term risk for disability progression, including up to 9 years of observation in RMS and PPMS.
Interpretation
Highly elevated NfL from acute MS disease activity may mask a more subtle NfL abnormality that reflects underlying non-relapsing progressive biology. Ocrelizumab significantly reduced NfL levels, consistent with its effects on acute disease activity and disability progression. Persistently elevated NfL levels, observed in a subgroup of persons under ocrelizumab treatment, demonstrate potential clinical utility as a predictive biomarker of increased risk for clinical progression. Suppression of relapsing biology with high-efficacy immunotherapy provides a window into the relationship between NfL levels and future non-relapsing progression.
Funding
F. Hoffmann-La Roche Ltd.
Keywords: Multiple sclerosis, Ocrelizumab, NfL, Biomarker, Disease progression
Research in context.
Evidence before this study
Elevated neurofilament light chain (NfL) levels can reflect neuroaxonal injury and are abnormally increased in the cerebrospinal fluid and blood of persons with multiple sclerosis (MS) compared with healthy individuals of similar age. To date, the abnormal elevations in MS have been best linked with injury from acute (relapsing) disease biology as they (i) correlate well with the development of new focal inflammatory lesions (eg, T1-weighted gadolinium-enhancing lesions) and clinical relapses; (ii) can predict future relapse-related worsening of disability; and (iii) are decreased with therapies that effectively limit the development of new disease relapses. Current literature is mixed with regard to potential correlations of NfL with clinical progression outcomes, which typically have not distinguished progression due to relapse-biology (both clinical and subclinical) from progression that is relapse-biology independent. An important outstanding question is whether NfL measurements can be used to assess and predict injury from non-relapsing progressive disease.
Added value of this study
We postulated that suppression of relapsing biology with high-efficacy immunotherapy in patients assessed for outcomes of progression with sufficient duration of follow-up might allow definition of the relationships between NfL, non-relapsing progressive disease biology, and future progression of clinical disability. Our study utilises 3 large, well-characterised clinical datasets in persons with either relapsing MS (RMS) (OPERA I and II) or primary progressive MS (PPMS) (ORATORIO), with up to 9 years of long-term follow-up allowing the detailed assessment of baseline and longitudinal NfL levels (11,806 samples from 2194 patients) and long-term progression outcomes including magnetic resonance imaging (MRI) quantitation of slowly expanding lesions (SELs), an emerging measure of non-relapsing progressive injury, as well as assessment of insidious clinical progression independent of relapse activity (PIRA). Treatment with ocrelizumab, a B-cell-depleting anti-CD20 antibody with clinical efficacy in both relapsing and progressive MS, provides a useful tool for comparing progression outcomes between persons with different on-treatment NfL levels.
We show that beyond highly elevated NfL from acute MS disease activity, a more subtle NfL abnormality is related to insidious progressive biology. While baseline NfL levels strongly associate with MRI worsening (including whole brain and thalamic volume loss as well as SELs), the prognostic relationship between baseline NfL and confirmed disability progression is most evident when measured in persons with PPMS or in those with RMS without acute disease activity. Ocrelizumab, which significantly reduces NfL levels in both RMS and PPMS, also abrogates the prognostic relationship between baseline NfL and disease progression. Importantly, ocrelizumab treatment offers a window for assessing and predicting ongoing underlying neuroaxonal injury, as persistently elevated NfL levels measured at week 24 or 48 on treatment (when the abnormality due to acute MS disease activity is removed) are strongly associated with future disease progression in both persons with RMS and PPMS. This key observation was not reported in previous studies examining NfL on other high-efficacy therapies, possibly reflecting smaller cohort size and/or insufficient duration of follow-up to detect the association with insidious non-relapsing progressive injury. Our findings reveal that, beyond the recognised association with acute relapsing MS biology, elevated NfL levels in the absence (or during effective suppression) of acute MS disease activity can reflect injury due to non-relapsing progressive biology.
Implications of all the available evidence
A minimally invasive biomarker that could accurately assess the future risk of disease progression would be transformative for the treatment of MS and the development of novel therapies targeting progression. “Silent progression” is incompletely assessed using existing tools, including MRI, and remains an important unmet need in the field. While NfL as a putative biomarker has appeared promising for some time, the ability to dissect out what, if any, component of NfL could consistently and reliably provide information on non-relapsing progressive disease has been elusive. These important data suggest that assessment of NfL in the absence of relapsing disease activity or after abrogation of such activity, in this case by ocrelizumab, reveals the key to utilisation of the remaining abnormality in NfL to assess the risk of persons with MS for future clinical disease progression. Under these conditions, NfL demonstrates the potential to accelerate drug development by acting as an enrichment or treatment-response biomarker for progression in Phase 2 trials, thereby de-risking larger Phase 3 trials, or to ultimately allow physicians to identify persons with MS who have increased risk of progression who require earlier intervention or additional support beyond current high-efficacy therapies that target relapse biology. In addition, ongoing further work to validate NfL as a biomarker in real-world cohorts, including application of normalisation methods to address demographic and disease heterogeneity, and the successful development of NfL assays being considered for regulatory diagnostic usage will be needed to establish NfL measurements in clinical practice.
Introduction
Multiple sclerosis (MS) is an immune-mediated disorder of the central nervous system (CNS) characterised by acute focal inflammatory injury and chronic compartmentalised CNS inflammation and neurodegeneration.1 Relapsing MS (RMS) and progressive forms of MS, including primary progressive MS (PPMS), are increasingly recognised as part of the same disease continuum, and insidious non-relapsing injury contributing to disease progression is now thought to occur across the continuum, whether in presence or absence of clinically overt relapses.2, 3, 4 Unlike the injury associated with relapsing disease, the non-relapsing progressive injury, which continues to represent a major therapeutic gap, is less well understood and has been harder to measure, monitor, or predict.
Ocrelizumab, an MS disease-modifying therapy (DMT), significantly reduces relapsing disease activity and partially limits progression of disability in MS.5, 6, 7 Thus, it provides a valuable tool to interrogate biomarkers, imaging, or clinical metrics across a range of progression outcomes.5,6,8,9 Neurofilament light chain (NfL) is a marker of neuroaxonal injury in neurological diseases,10 including MS.11,12 Elevated circulating NfL levels correlate with and may be predictive of MS relapses and acute inflammatory disease activity4,13, 14, 15 typically identified as gadolinium (Gd)-enhancing lesions or new/enlarging T2 hyperintense lesions on magnetic resonance imaging (MRI), as well as measures of CNS injury including accelerated brain volume loss.12,14, 15, 16 However, it is unclear whether NfL measurements can capture elements of insidious non-relapsing progressive biology, including chronic active lesions, as partially quantified using MRI-derived measures, such as slowly expanding lesions (SELs).17 NfL levels are also impacted by age and body mass index (BMI),18,19 potentially necessitating adjustments when evaluating diverse populations and individual cases. NfL reduction is observed with several MS DMTs11,15,20, 21, 22; however, its potential utility as a treatment surrogate biomarker beyond reduction of relapses and focal MRI activity remains to be established. The prognostic value of NfL in progressive MS has been investigated23; however, these studies did not distinguish relapse-biology– related progression from progression that is relapse-biology independent, and the evidence remains conflicting.24 Identifying a biomarker with treatment surrogacy on non-relapsing disability progression in MS would accelerate clinical development, including in populations with major unmet need.
Here, we analysed 3 large, well-controlled clinical trials in persons with RMS (pwRMS) (OPERA I, OPERA II)7 or persons with PPMS (pwPPMS) (ORATORIO)8 to unravel patterns of both baseline and on-treatment blood NfL elevations and their particular prognostic potential for non-relapsing disease progression, and we assessed the impact of ocrelizumab on levels of NfL and its potential utility as a treatment-effect surrogate biomarker.
Methods
Study population
Overall, 1421 pwRMS (OPERA I [NCT01247324] and OPERA II [NCT01412333]; randomised to ocrelizumab vs. interferon [IFN] β-1a) and 596 pwPPMS (ORATORIO [NCT01194570]; randomised to ocrelizumab vs. placebo) who consented and provided ≥1 NfL sample were included for post hoc analysis (Supplementary Fig. S1).7,8 Subgroups were defined by the presence or absence of acute or recent disease activity at baseline (RMS: Gd-enhancing lesions and/or relapse in prior 3 months; PPMS: Gd-enhancing lesions).
Ethics
The current study is a secondary analysis of anonymised patient data from clinical trials fully approved by institutional review boards and local regulatory authorities. All patients provided written informed consent.
NfL measurements
Serum NfL (sNfL) and/or plasma NfL (pNfL) were measured using the Simoa NfL Advantage V1 Kit (HD-X, Quanterix)25; sNfL and pNfL levels were highly correlated.25 Baseline sNfL levels were used to assess NfL distribution and prognostic value across trials. Treatment response for NfL and on-treatment NfL associations were assessed using available longitudinal serum samples (baseline and weeks 12, 24, 48, 72, and 96) in pwRMS (OPERA I/II) and plasma samples (baseline and weeks 12, 24, 48, 72, 96, and 120) in pwPPMS (ORATORIO).
MRI and clinical outcome measures
Definitions of MRI and clinical outcomes are provided in Supplementary Table S1. New or enlarging T2 lesions were enumerated as the total number of such lesions identified on brain MRI from baseline to the end of the controlled treatment period. Annualised percent change in whole brain volume (BV) and thalamic volume (THV) change were calculated from week 24 (rather than baseline, to account for potential rapid volume changes related to resolution of acute inflammation) to the end of the controlled treatment period. SELs were identified as concentrically and constantly expanding existing T2 lesions from baseline to the end of the controlled treatment period.17
Clinical worsening was defined as 24-week confirmed disability progression (CDP) on the Expanded Disability Status Scale (EDSS) from the time of NfL measurement to week 96 in OPERA I and II (independent replicate cohorts and combined cohort) or to week 144 in ORATORIO (controlled treatment period), or to week 432 (open-label extension [OLE]). The comparison EDSS was the baseline value when no CDP event occurred prior to NfL measurement, or a re-baselined EDSS value when a CDP event occurred prior to NfL measurement. The re-baselined EDSS value at the time of NfL measurement was also used as the comparison EDSS in a sensitivity analysis. Exploratory clinical outcomes included 24-week CDP on the Nine-Hole Peg Test (9HPT) (20% worsening), Timed 25-Foot Walk (T25FW) (20% worsening), Symbol Digit Modalities Test (SDMT) (≥4-point reduction, OPERA), progression independent of relapse activity (PIRA) on EDSS (OPERA), time to walking-aid dependence (EDSS 6; OPERA), time to wheelchair dependence (EDSS 7; ORATORIO).
Statistics
Factors affecting baseline NfL levels
A healthy donor (HD) cohort (n = 118) of similar age range (24–66 years) enabled calculation of age-adjusted NfL levels for comparison across cohorts.26 Baseline (unadjusted and adjusted) and on-treatment (adjusted) values were compared between MS populations and with those of HDs using the Kolmogorov–Smirnov (K–S) test. Relationships between baseline disease characteristics (independent variables) and NfL levels (dependent variable) were determined by (1) a basic linear regression model that included adjustments for sex (self-reported by participants), age, region (US vs. rest of world), and study for RMS (OPERA I vs. II); and (2) a multiple linear regression (MLR) model that included covariates from (1) plus body weight, years since MS symptom onset, years since last relapse (OPERA), prior MS treatment (bivariate), T1 Gd-enhancing lesion count, log-T2 lesion volume (T2LV), EDSS, 9HPT (seconds), T25FW (seconds), SDMT (OPERA), and Paced Auditory Serial Addition Test (ORATORIO). A stepwise (forward and backward) selection by minimising the Akaike information criterion was used to identify significant variables in the MLR model.
Baseline NfL levels and association with MRI outcomes
All model equations are provided in Supplementary Table S2. Relationships between baseline log-NfL levels (independent variable) and MRI outcomes were examined using the negative binomial model (for new/enlarging T2 lesions) and MLR models (for BV and THV change), adjusted for the same covariates. The number of SELs, baseline T2LV of lesions subsequently defined as SELs, change in SEL-related T1 volume, and change in T1 signal intensity within SELs during the controlled treatment period were compared between persons with high or low baseline NfL using the Wilcoxon rank-sum test.
NfL levels and association with clinical outcomes
The relationship between baseline or on-treatment NfL (bivariate independent variable using baseline median as putative threshold) and subsequent clinical progression (dependent variable) was assessed using a log-rank test (unadjusted) and Cox regression (adjusted for age, sex, region, baseline Gd + lesion count, baseline T2LV, and baseline EDSS). Relationships between baseline log-NfL levels (continuous independent variable) and subsequent clinical progression (dependent variable) were assessed using Cox regression (same adjustments as bivariate analysis). This analysis was performed within each treatment arm and in the combined treatment arms (including NfL-arm interaction variable to assess potential interaction effect).
Identification of an NfL threshold for disease progression risk in this dataset was performed by evaluating all observed unadjusted NfL values and by calculating the positive predictive value (PPV) and 1 minus negative predictive value (1-NPV) corresponding to the likelihood of 24-week CDP on EDSS ≤5 years following NfL measurement in the high and low NfL groups, respectively. The optimal NfL threshold was defined as the value at which there was greatest apparent separation between PPV and 1-NPV. To assess whether disease progression risk stratification improves with age adjustment, we used the same method to identify a threshold for age-adjusted on-treatment NfL levels.
Effect of ocrelizumab treatment on NfL levels
On-treatment NfL levels (dependent variable) in persons receiving ocrelizumab vs. IFN (OPERA I/II) or placebo (ORATORIO) were compared using a mixed model of repeated measures (MMRM) adjusting for log-baseline NfL, region, and baseline EDSS. Changes from baseline NfL levels were analysed by log-transformed concentrations and reported as geometric means and geometric mean ratios in each treatment arm.
Factors impacting on-treatment NfL levels
Associations between age-adjusted log-NfL levels measured at the end of the controlled treatment period (dependent variable)26 and MRI measures of insidious progressive injury (independent variable), including the baseline SEL-related T1 volume and BV change (week 24 to end of the controlled treatment period), were determined in each treatment arm using MLR adjusting for weight, sex, study, geographic region, and new or enlarging T2 lesions to account for potential confounding due to demographics and disease activity.
Surrogate treatment biomarker evaluation
Evaluation of candidate surrogate markers for treatment response on clinical progression was assessed by testing the effect of treatment on progression conditional on the surrogate by using Cox regression in the combined treatment arms of OPERA I and II or ORATORIO.27 A surrogacy relationship was defined as a reduction in the ocrelizumab treatment hazard ratio on clinical progression (24-week CDP during the controlled treatment period) following adjusting for surrogate markers, compared with the unadjusted treatment arm effect size. Candidate surrogate markers included on-treatment (week 48) log-NfL levels, new/enlarging T2 lesions from baseline to week 48, and BV change from baseline to week 48.
Statistical analyses were performed in R (v4.0.3) and associated packages (stats, DescTools, MASS, survival, ggplot). Results for all described analyses are provided.
Role of the funders
F. Hoffmann-La Roche Ltd. provided funding and contributed to study design, data collection, data analysis, data interpretation, and writing of the report.
Results
NfL study population
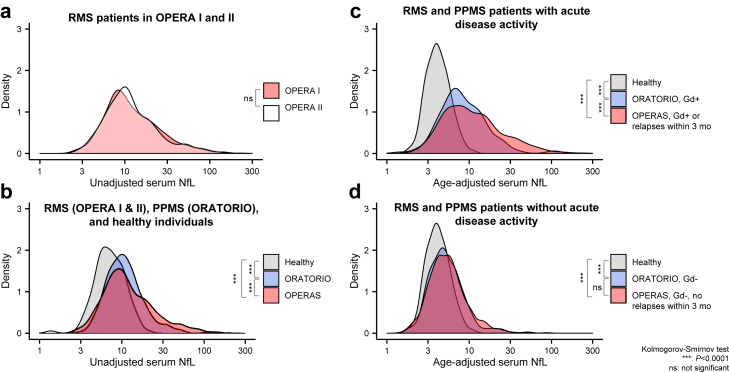
In total, 11,806 NfL samples were collected across 2194 participants (OPERA I, n = 787; OPERA II, n = 790; ORATORIO, n = 617), and longitudinal samples were available in 1835 (OPERA I, n = 672; OPERA II, n = 674; ORATORIO, n = 489) participants. Baseline demographic and clinical characteristics were comparable between the NfL study and intention-to-treat populations (Table 1, Supplementary Table S3). Baseline NfL distributions were similar in pwRMS from OPERA I and II (Fig. 1a); therefore, data were pooled (n = 1577) for subsequent analysis.
Table 1.
Baseline characteristics in the NfL study population and ITT population.
| Baseline characteristics | OPERA I and II (NfL study population)a |
OPERA I and II (ITT population) |
ORATORIO (NfL study population)a |
ORATORIO (ITT population) |
||||
|---|---|---|---|---|---|---|---|---|
| IFNβ-1a (n = 701) | OCR (n = 720) | IFNβ-1a (n = 829) | OCR (n = 827) | PBO (n = 205) | OCR (n = 391) | PBO (n = 244) | OCR (n = 488) | |
| Age, median (range), years | 38 (18–55) | 38 (18–56) | 37 (18–55) | 38 (18–56) | 46 (19–56) | 47 (20–56) | 46 (18–56) | 45 (20–56) |
| Female, n (%) | 447 (63.8) | 476 (66.1) | 552 (66.6) | 541 (65.4) | 100 (48.8) | 196 (50.1) | 124 (50.8) | 237 (48.6) |
| Male, n (%) | 254 (36.2) | 244 (33.9) | 277 (33.4) | 286 (34.5) | 105 (51.2) | 194 (49.7) | 120 (49.2) | 251 (51.4) |
| Region USA, n (%) | 175 (25.0) | 181 (25.1) | 219 (26.4) | 217 (26.2) | 31 (15.1) | 59 (15.1) | 34 (13.9) | 67 (13.7) |
| Weight, median (range), kg | 75 (42.1–163.6) | 75 (38–170) | 75 (42.1–163.6) | 75 (38–170) | 74 (45–136) | 72 (40.2–135) | 72 (45.0–136.0) | 72 (40.2–135.9) |
| Years since MS diagnosis, median (range) | 1.6 (0.1–28.5) | 1.8 (0–28.9) | 1.7 (0.1–28.5) | 1.8 (0–28.9) | 1.2 (0.1–13.8) | 1.5 (0.1–16.8) | 1.3 (0.1–23.8) | 1.6 (0.1–16.8) |
| Years since last relapse, median (range) | 0.4 (0.1–1.9) | 0.4 (0.1–2) | 0.4 (0.1–2) | 0.4 (0.1–2) | – | – | – | – |
| Patients with T1 Gd+ lesions, n (%) | 273 (39.3) | 289 (40.6) | 327 (39.8) | 333 (40.7) | 50 (24.4) | 93 (24) | 60 (24.7) | 133 (27.5) |
| No. of T1 Gd+ lesions, median (range) | 0 (0–54) | 0 (0–56) | 0 (0–54) | 0 (0–56) | 0 (0–10) | 0 (0–18) | 0 (0–50) | 0 (0–77) |
| No. of T2 lesions, median (range) | 42 (0–226) | 39 (1–209) | 42 (0–226) | 40 (1–233) | 42 (0–208) | 41 (0–226) | 43 (0–208) | 42 (0–249) |
| T2 lesion volume, median (range), mL | 6.2 (0–76.1) | 5.2 (0–96) | 6.2 (0–76.1) | 5.4 (0–96) | 6.2 (0–59.2) | 6.9 (0–89.4) | 6.2 (0–81.1) | 7.3 (0–90.3) |
| EDSS score, median (range) | 2.5 (0–6) | 2.5 (0–6) | 2.5 (0–6) | 2.5 (0–6) | 4.5 (2.5–6.5) | 4.5 (2.5–6.5) | 4.5 (2.5–6.5) | 4.5 (2.5–6.8) |
| 9HPT score, median (range) | 22.4 (11.5–149.1) | 22.4 (10–300) | 22.2 (11.3–149.1) | 22.2 (10–300) | 26.4 (13.2–120.7) | 26.6 (13.3–300) | 26.9 (11.1–120.7) | 26.8 (12.3–300) |
| T25FW score, median (range) | 5.5 (2.7–116.3) | 5.6 (2.6–149.8) | 5.5 (2.7–180) | 5.6 (2.6–149.8) | 7.3 (2.6–145) | 7.8 (3.3–180) | 7.4 (2.6–145.0) | 7.8 (3.3–180) |
| Serum NfL, median (range), pg/mL | 10.4 (2.7–339) | 10.7 (2.7–230.7) | 10.4 (2.7–339) | 10.7 (2.7–230.7) | 10.3 (3.3–102) | 10.3 (2.7–198.9) | 10.3 (2.7–198.9) | 10.3 (2.7–198.9) |
There were no appreciable differences in baseline demographics, clinical measures, and MRI characteristics between the NfL study population and ITT populations of OPERA I and II and ORATORIO. Compared with OPERA I and II, persons in ORATORIO were on average older, had greater disease burden (greater T2 lesion volume and higher EDSS), and had less disease activity (fewer patients had T1 Gd+ lesions) at baseline.
9HPT = Nine-Hole Peg Test; EDSS = Expanded Disability Status Scale; Gd = gadolinium; IFN = interferon; ITT = intention to treat; MS = multiple sclerosis; NfL = neurofilament light; OCR = ocrelizumab; PBO = placebo; T25FW = timed 25-foot walk.
Safety assessment population patients who completed baseline serum collection in OPERA and ORATORIO.
Fig. 1.
Comparison of baseline serum NfL distributions in (a) persons with RMS in OPERA I (n = 701) and II (n = 720); (b) persons with RMS pooled from OPERA I/II (n = 1421), persons with PPMS from ORATORIO (n = 596), and HDs (n = 118); (c) persons with RMS (n = 560) or PPMS (n = 141) with acute disease activity grouped by study; and (d) persons with RMS (n = 688) or PPMS (n = 464) without acute disease activity grouped by study. Statistical comparisons were conducted with the Kolmogorov–Smirnov test. HD = healthy donor; Gd = gadolinium; NfL = neurofilament light; ns = not significant; PPMS = primary progressive multiple sclerosis; RMS = relapsing multiple sclerosis. ∗∗∗P < 0.0001.
NfL in MS and factors affecting baseline NfL levels
Baseline sNfL levels were elevated in pwRMS (12.1 [11.6–12.5] pg/mL) and pwPPMS (10.9 [10.4–11.3] pg/mL) vs. HD (7.2 [6.6–7.7] pg/mL; geometric mean [95% CI]), all P < 0.0001 [K–S test] (Fig. 1).26 Persons with acute disease activity showed the greatest NfL elevation vs. HD (RMS, 16.2 [15.3–17.1] pg/mL; PPMS, 14.4 [14.1–15.9] pg/mL; vs. HD 7.2 [6.6–7.7] pg/mL, all P < 0.0001 [K–S test]), while those without acute disease activity showed a more subtle albeit significant elevation (RMS, 8.9 [8.5–9.2] pg/mL; PPMS, 9.9 [9.5–10.3] pg/mL; vs. HD 7.2 [6.6–7.7] pg/mL), all P < 0.0001 [K–S test]. Upon age-adjustment (Supplementary Fig. S2),26 in persons with acute disease activity, NfL was elevated in pwRMS (12.7 [11.9–13.5] pg/mL) vs. pwPPMS (8.4 [7.6–9.4] pg/mL), P = 0.0012 [K–S test]. In persons without acute disease activity, no difference was observed in age-adjusted NfL levels between pwRMS (5.6 [5.4–5.9] pg/mL) and pwPPMS (5.3 [5.0–5.5] pg/mL); however, both groups remained elevated compared with HD (4.1 [3.9–4.4] pg/mL), all P < 0.0001 [K–S test].
MLR modelling showed that higher baseline NfL levels were independently associated with greater Gd-enhancing lesion count, higher T2LV, shorter disease duration, higher EDSS, shorter duration since last relapse (RMS), lack of prior treatment, and lower body weight in both pwRMS and pwPPMS (Supplementary Fig. S3, Supplementary Table S4). In pwPPMS, older age, higher T25FW, and higher 9HPT were also independently associated with higher NfL, in addition to the aforementioned characteristics.
Baseline NfL levels and impact on disease outcomes
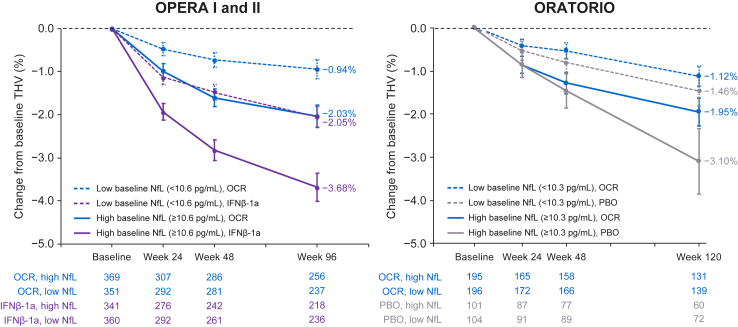
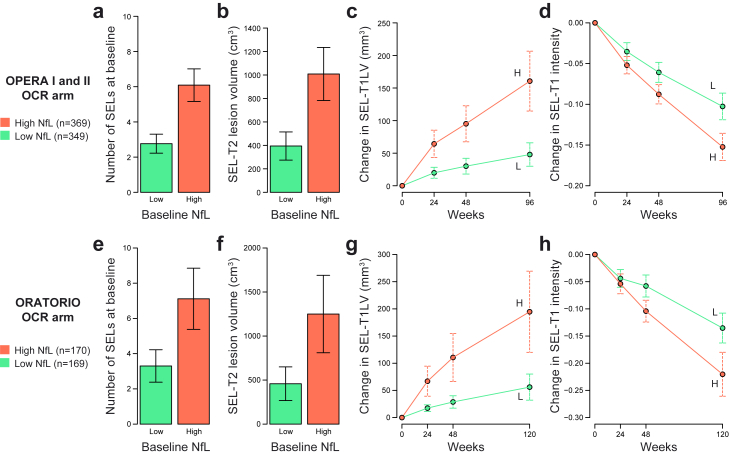
A simple median threshold (RMS, 10.6 pg/mL; PPMS, 10.3 pg/mL) was initially used to assess the relationship between high baseline sNfL and subsequent MRI and clinical outcomes. In both pwRMS and pwPPMS, high baseline NfL was prognostic for greater THV (Fig. 2) and BV reduction (Supplementary Fig. S4) during the controlled treatment period. Ocrelizumab treatment was associated with lesser BV and THV reduction in both high and low NfL groups in pwRMS and showed similar trends in pwPPMS. Moreover, high baseline NfL was associated with greater SEL count and SEL-related T2LV and was prognostic for greater SEL-related T1 volume expansion and T1 intensity reduction (Fig. 3, Supplementary Fig. S5).
Fig. 2.
Relationship between baseline serum NfL and thalamic volume reduction during the controlled treatment period in each treatment arm of OPERA I and II and ORATORIO.∗ The mean and 95% CI of percentage volume reduction at each brain MRI time point compared with baseline are shown. In both studies, a greater reduction in THV was observed at the end of the controlled treatment period in persons with high baseline NfL (above median, solid lines) compared with low baseline NfL (below median, dotted lines). Moreover, greater THV reduction was observed in persons randomised to comparator treatment (IFNβ-1a in OPERA, purple; PBO in ORATORIO, grey) vs. ocrelizumab (blue). IFN = interferon; NfL = neurofilament light; MRI = magnetic resonance imaging; OCR = ocrelizumab; PBO = placebo; THV = thalamic volume. ∗Study individuals included persons with RMS with MRI data at weeks 24 and 96 (OPERA I and II) and persons with PPMS with MRI data at weeks 24 and 120 (ORATORIO).
Fig. 3.
Relationship between baseline NfL and baseline SEL burden and SEL volume expansion during the controlled treatment period in the ocrelizumab treatment arms of OPERA I and II and ORATORIO. High or low NfL was determined by levels above or below the baseline median. In both studies, NfL elevation at baseline was associated with greater baseline SEL count (a and e), greater baseline SEL-T2LV (b and f), greater SEL-T1LV expansion (c and g), and greater T1 signal intensity reduction within SELs (d and h). The mean and 95% CI of each SEL measure is shown. SEL = slowly expanding lesion; NfL = neurofilament light; OCR = ocrelizumab; T1LV = T1 lesion volume; T2LV = T2 lesion volume; H = high; L = low.
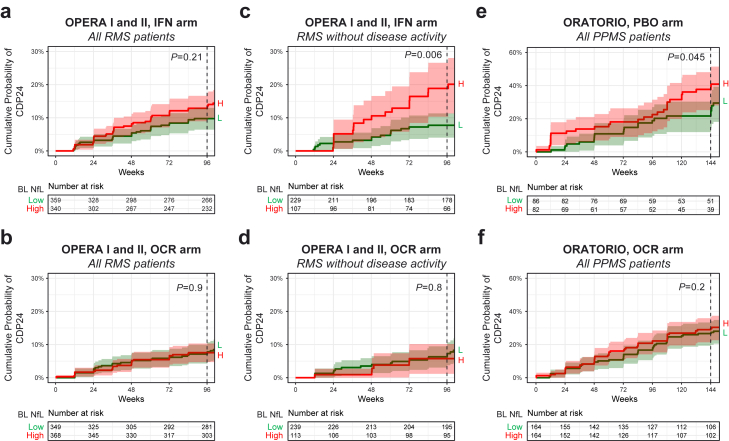
High baseline NfL was also prognostic for clinical progression (24-week CDP during the controlled treatment period) in pwPPMS receiving comparator therapy (HR = 1.8 [1.0–3.4]; P = 0.045) and in pwRMS without acute disease activity receiving comparator therapy (HR = 2.5 [1.3–5.0]; P = 0.006 [log-rank]) but not in all pwRMS regardless of disease activity (HR = 1.4 [0.8–2.2]; P = 0.21 [log-rank]) (Fig. 4). Adjustment for acute disease activity using a Cox model did not uncover an association between baseline NfL and CDP in all pwRMS receiving comparator therapy. Baseline NfL was not associated with CDP in pwRMS or pwPPMS receiving ocrelizumab.
Fig. 4.
Relationship between baseline NfL and disability progression during the controlled treatment period in each treatment arm of OPERA I and II (a) and ORATORIO (e). High or low NfL was determined by levels above or below the baseline median. NfL elevation at baseline was prognostic for CDP24 only in persons with RMS without acute disease activity receiving IFNβ-1a (c) and in persons with PPMS receiving placebo therapy (e). Baseline NfL was not predictive of CDP24 in persons receiving ocrelizumab treatment (b, d, and f). P values are shown for log-rank tests comparing high vs. low NfL groups. IFN = interferon; NfL = neurofilament light; OCR = ocrelizumab; PBO = placebo; CDP24 = 24-week confirmed disability progression; PPMS = primary progressive multiple sclerosis; RMS = relapsing multiple sclerosis; BL = baseline; H = high; L = low.
Analysis of baseline log-NfL as a continuous variable confirms its prognostic relationship with MRI outcomes (BV and THV change, new/enlarging T2 lesions) in both populations and with clinical progression (24-week CDP) in pwRMS without disease activity and pwPPMS receiving comparator treatments (Supplementary Fig. S6, Supplementary Table S5). Moreover, baseline NfL level was associated with exploratory clinical outcomes PIRA (RMS), EDSS 6 (RMS), and 9HPT progression (RMS and PPMS) only in pwRMS without disease activity and in pwPPMS receiving comparator treatment (Supplementary Table S5).
Interactions between high baseline NfL and treatment arm were detected for some outcomes including new/enlarging T2 lesions (RMS; P < 0.01 [negative binomial]), thalamic volume change (PPMS; P < 0.001 [linear model]), EDSS and PIRA (RMS without disease activity; all P < 0.05 [CoxPH]), time to walking aid (RMS without disease activity; P = 0.055 [CoxPH]), and 9HPT (PPMS; P < 0.05 [CoxPH]) (Supplementary Fig. S7, Supplementary Table S5). Such interactions were not detected for brain volume change, annualized relapse rate (RMS only), T25FW, SDMT (RMS only), or time to wheelchair (PPMS only).
Effect of ocrelizumab treatment on NfL levels
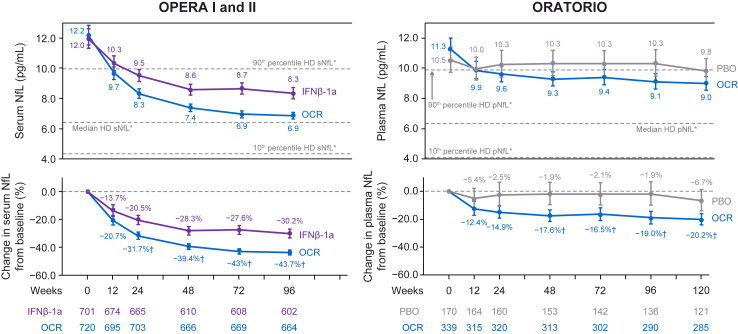
Significant reductions in NfL levels from baseline were observed at the earliest measured time point (3 months) following ocrelizumab initiation (geometric mean ratio [95% CI]; pwRMS, 0.79 [0.76–0.83]; pwPPMS, 0.88 [0.83–0.93]) and were more pronounced by the end of controlled treatment (pwRMS [96 weeks], 0.56 [0.55–0.58]; pwPPMS [120 weeks], 0.80 [0.76–0.84]) (all P < 0.0001 [MMRM]; Fig. 5). Moreover, a greater reduction in NfL was observed with ocrelizumab vs. comparator treatments as early as week 24 in pwRMS (−31.7% vs. −20.5%, P < 0.0001 [MMRM]) and week 48 in PPMS (−17.6% vs. −1.9%, P < 0.01 [MMRM]). Strikingly, age-adjusted NfL levels in pwRMS receiving ocrelizumab were reduced to levels observed in HDs by week 96, while NfL reductions in pwPPMS were significant but less pronounced (Supplementary Fig. S8).
Fig. 5.
Blood NfL levels (GM and 95% CI, top)∗ and relative change from baseline (% reduction in GM and 95% CI, bottom) during the controlled treatment in OPERA I and II (left) and ORATORIO (right). NfL levels were significantly reduced following ocrelizumab treatment in persons with RMS and persons with PPMS, both vs. comparator treatment and vs. baseline levels. GM = geometric mean; HD = healthy donor; IFN = interferon; NfL = neurofilament light; OCR = ocrelizumab; PBO = placebo; pNfL = plasma neurofilament light; sNfL = serum neurofilament light. ∗NfL levels from the HD cohort were adjusted to median ages in OPERA (38 years) and ORATORIO (47 years) to determine median, 10th percentile, and 90th percentile levels. †Significant reduction in NfL following ocrelizumab treatment vs. comparator arms; plots show GMs of NfL and 95% CIs.
Relationship between on-treatment NfL levels and clinical progression
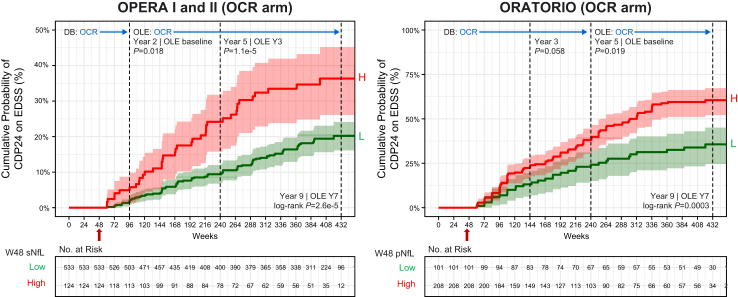
We used ocrelizumab’s ability to robustly suppress acute disease activity as an opportunity to assess the utility of NfL for monitoring risk for relapse-independent clinical progression following treatment initiation. We observed a significant association between high NfL at week 48 (sNfL >10.6 pg/mL in RMS [optimised threshold approximately equalled baseline median], pNfL >7.5 pg/mL in PPMS [optimised threshold was lower than baseline median]) and risk for future 24-week CDP in both pwRMS and pwPPMS receiving ocrelizumab (Fig. 6, Supplementary Fig. S9, Supplementary Table S6), including within the replicate cohorts OPERA I and OPERA II (Supplementary Fig. S10), and in a sensitivity analysis using EDSS re-baselined at time of NfL measurement (Supplementary Fig. S11). The association with risk for CDP was observed at the end of controlled treatment period (HR [95% CI]; RMS, 3.3 [1.2–9.6]; PPMS, 1.7 [1.2–2.5]; all P < 0.05 [CoxPH]) and up to week 432 in the OLE (≤9 years following ocrelizumab initiation) in both populations (RMS, 2.2 [1.5–3.3]; PPMS, 2.2 [1.4–3.3]; all P < 0.001 [CoxPH]). Using these optimised thresholds, 18.9% of pwRMS and 67.3% of pwPPMS receiving ocrelizumab had high NfL at week 48, reflecting risk for future non-relapsing progression.
Fig. 6.
Relationship between week 48 (unadjusted) blood NfL and risk for subsequent 24-week CDP in persons with RMS and those with PPMS receiving ocrelizumab treatment. High or low NfL was determined using sNfL above or below 10.6 pg/mL in OPERA I and II, and pNfL above or below 7.5 pg/mL in ORATORIO. The comparison EDSS was the baseline value when no CDP event occurred prior to NfL measurement or a re-baselined EDSS value when a CDP event occurred prior to NfL measurement. Differences between high vs. low NfL groups on risk for CDP was assessed using a log-rank test. IFN = interferon; NfL = neurofilament light; OCR = ocrelizumab; PBO = placebo; pNfL = plasma neurofilament light; sNfL = serum neurofilament light; DB = double-blind period; OLE = open-label extension period; H = high; L = low; CDP = confirmed disability progression.
In pwRMS, high NfL as early as week 24 was also associated with risk for CDP in both treatment arms during the controlled treatment period and in the ocrelizumab arm for the OLE (Supplementary Fig. S12, Supplementary Table S6). Ocrelizumab treatment was still associated with reduced CDP vs. comparator treatment in the low NfL group (HR = 0.52 [0.28–0.97]; P = 0.039 [log-rank]) and showed a trend for reduced CDP in the high NfL group (HR = 0.65 [0.34–1.21]; P = 0.16 [log-rank]), the latter being limited by sample size (27.4% of ocrelizumab arm, 38.2% of IFN arm at week 24).
Age-adjusted on-treatment NfL levels at week 48 showed similar associations with future 24-week CDP up to week 432 in pwRMS (optimised threshold sNfL >7.0 pg/mL) and pwPPMS (optimised threshold pNfL >4.8 pg/mL) (HR [95% CI]; RMS, 1.8 [1.1–2.9]; PPMS, 1.8 [1.2–2.5]; all P < 0.05 [CoxPH]) (Supplementary Figs. S13 and S14, Supplementary Table S7). Using these adjusted thresholds, 14.9% of pwRMS and 50.6% of pwPPMS receiving ocrelizumab had high NfL at week 48.
Factors impacting on-treatment NfL levels
Further investigation into factors driving elevated on-treatment NfL levels identified greater total BV reduction and higher SEL-related T1 lesion volume as associated with higher age-adjusted NfL levels at the end of the controlled treatment period in pwRMS and pwPPMS treated with ocrelizumab and with greater new/enlarging T2 lesions in the comparator arms (all P ≤ 0.05 [linear model]; Supplementary Fig. S15, Supplementary Table S8). Additional factors associated with higher on-treatment NfL levels included lower body weight, male sex, and region (US) in both populations (all P < 0.05 [linear model]).
NfL as a potential surrogate treatment biomarker
Adjusting for log-NfL (week 48) as a candidate surrogate marker reduced the ocrelizumab treatment hazard ratio on clinical progression (CDP) during the controlled treatment period in pwRMS (unadjusted HR for OCR vs. comparator 0.65 [0.44–0.94], P = 0.023; adjusted for NfL, 0.72 [0.49–1.1], P = 0.098; explaining 22% of treatment effect [all, CoxPH]) and in pwPPMS (unadjusted, 0.71 [0.51–0.99], P = 0.045; adjusted for NfL, 0.73 [0.52–1.02], P = 0.064; explaining 6% of treatment effect [all, CoxPH]). Compared with NfL, adjusting for new/enlarging T2 lesions and percent BV change (baseline to week 48) showed lesser impact on treatment effect (explaining 12% and 13%, respectively) in pwRMS (Table 2). In pwPPMS, adjusting for new/enlarging T2 lesions showed a greater impact on treatment effect (unadjusted, 0.76 [0.57–1.00], P = 0.049; adjusted for T2 lesions, 0.84 [0.63–1.12], P = 0.23; explaining 33% of treatment effect [all, CoxPH]).
Table 2.
Evaluation of new/enlarging T2 lesions, brain volume change, and blood NfL at week 48 as potential surrogate markers for CDP24 during controlled treatment period (week 96 in OPERA, week 144 in ORATORIO).
| Study | Sample size (biomarker- available population) | Candidate surrogate marker | Unadjusted CoxPH model CDP24 ∼ treatment arm |
Adjusted CoxPH model CDP24 ∼ treatment arm + surrogate marker |
Treatment effect explained by surrogate | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR for OCR vs. comparator arm (95% CI) | P value | HR for OCR vs. comparator arm (95% CI) | OCR arm P value | 1 SD in surrogate marker | HR per 1 SD in surrogate marker (95% CI) | Candidate surrogate P value | ||||
| OPERA I and II | 1656 | None (ITT population) | 0.60 (0.43–0.84) | 0.003 | – | – | – | – | – | – |
| 1404 | New/enlarging T2 lesions from BL to W48 | 0.62 (0.44–0.88) | 0.008 | 0.67 (0.47–0.95) | 0.026 | 5.6 | 1.15 (1.04–1.27) | 0.0065 | 13% | |
| 1261 | % Brain volume change from BL to W48 | 0.63 (0.44–0.91) | 0.013 | 0.68 (0.47–0.98) | 0.039 | 0.75 | 0.77 (0.66–0.91) | 0.0014 | 12% | |
| 1270 | log10 (W48 sNfL) | 0.65 (0.44–0.94) | 0.023 | 0.72 (0.49–1.1) | 0.098 | 0.21 | 1.39 (1.17–1.64) | 0.00012 | 22% | |
| ORATORIO (n = 463) | 732 | None (ITT population) | 0.75 (0.58–0.98) | 0.04 | – | – | – | – | – | – |
| 662 | New/enlarging T2 lesions from BL to W48 | 0.76 (0.57–1.00) | 0.049 | 0.84 (0.63–1.12) | 0.23 | 7.0 | 1.17 (1.05–1.3) | 0.004 | 33% | |
| 612 | % Brain volume change from BL to W48 | 0.68 (0.51–0.91) | 0.009 | 0.67 (0.5–0.89) | 0.006 | 0.74 | 0.81 (0.71–0.91) | 0.0007 | −4.3% | |
| 463 | CDP24 ∼ arm + log10 (W48 pNfL) | 0.71 (0.51–0.99) | 0.045 | 0.73 (0.52–1.02) | 0.064 | 0.20 | 1.23 (1.04–1.45) | 0.013 | 6.4% | |
In persons with RMS, week 48 NfL shows elements of a surrogate biomarker, as addition of NfL to a Cox PH model that evaluates the impact of ocrelizumab treatment on CDP24 during the controlled treatment period reduces the treatment effect size compared with a model without NfL. Analysis is limited to the ITT study population with available week 48 data for candidate surrogate markers.
HR = hazard ratio; OCR = ocrelizumab; CDP24 = 24-week confirmed disability progression; NfL = neurofilament light; ITT = intention to treat.
Discussion
The potential utility of NfL measurements to assess or predict MS disease progression independent of relapsing disease activity has been unclear.15,23,24,28 While the bulk of abnormal NfL elevation in MS reflects injury from acute relapsing activity,11,14,19,21 we used 3 large, well-characterised MS clinical trials of ocrelizumab to identify a more subtle and previously less appreciated component of NfL abnormality that appears to reflect ongoing progressive biology, with utility for prognosticating relapse-independent disease progression. We observed a modest baseline NfL elevation in both pwRMS without apparent acute disease activity and in pwPPMS that was prognostic for CDP (EDSS, PIRA, EDSS 6, and 9HPT in pwRMS without disease activity and EDSS and 9HPT in pwPPMS). These baseline NfL elevations were also prognostic for greater BV or THV reduction and greater SEL volume expansion in both populations. Together, these findings suggest that such NfL elevation might be explained by injury processes that occur beyond acute “relapsing” disease activity (ie, damage from CNS-compartmentalised chronic active lesions, as well as potentially subpial microscopic inflammatory lesions, and/or neuroaxonal degeneration mediated through non-inflammatory mechanisms).
Ocrelizumab’s ability to effectively suppress detectable acute inflammatory disease activity offered an important opportunity to understand the clinical utility of NfL for monitoring risk for relapse-independent clinical progression. A striking relationship was observed between on-treatment NfL levels, measured as early as week 24 in RMS and week 48 in PPMS, and subsequent risk for CDP ≤9 years following ocrelizumab initiation. Age-adjusted on-treatment NfL levels showed similar associations with CDP, suggesting that this relationship is not driven by an age-related process. On-treatment NfL levels were also associated with BV reduction and SEL volume, supporting the finding that a component of NfL reflects injury from underlying progressive biology.29 While other studies examining NfL levels on another high-efficacy therapy, natalizumab, found inconsistent associations with disease progression,24,28 these studies utilised smaller cohorts and/or progression outcomes with limited longitudinal follow-up. Our finding that on-treatment NfL is prognostic for longer-term disease progression is evident in RMS (replicate cohorts) and PPMS patients receiving ocrelizumab treatment and, with further investigation, may extend to persons receiving other high-efficacy DMTs.
Ocrelizumab treatment induced significant and sustained reductions in NfL levels and removed the prognostic value of baseline NfL on clinical progression observed in pwRMS and pwPPMS. A reduction in NfL was observed at the earliest time point tested of 12 weeks, was sustained throughout the treatment period, and was superior to reductions in comparator groups. Although other DMTs also lower NfL levels,14,15,19,20 the robust treatment effect and ultimate correction into the HD range for pwRMS suggest that ocrelizumab has meaningful efficacy in reducing neuroaxonal injury. An interaction between NfL and treatment arm was observed in some cases, suggesting that patients with high NfL may be at particularly greater risk for progression when receiving placebo or low-efficacy treatment, in this case IFNβ-1a. Ocrelizumab treatment was associated with reduced risk for disease progression after adjusting for baseline NfL, was associated with less BV and THV reduction regardless of baseline NfL levels, and showed lower risk for disease progression regardless of on-treatment NfL levels. These data suggest that ocrelizumab treatment has benefit in persons with MS even when NfL levels are not obviously high and that treatment should not be withheld simply on the basis of NfL levels.
In the large clinical datasets examined here, which are relatively homogeneous in disease status and demographics (including age, 18–55 years at enrolment), simple threshold cut-offs for elevated NfL levels were sufficient to identify a population at greater risk for disease progression. Thresholds for NfL measured via the Simoa assay (sNfL >10.6 pg/mL and pNfL >7.5 pg/mL in RMS and PPMS, respectively) were determined using a classical biomarker analysis assessing positive and negative predictive values for the full range of NfL values measured. These thresholds were identified following suppression of acute disease activity with ocrelizumab and reflect the need to assess insidious neuroaxonal injury in the absence of confounding acute disease activity. While these thresholds support risk stratification at the group level, NfL measurements in the current format are not yet ready to provide patient-level prognostic information. Validation of these thresholds, identified through retrospective analysis, in independent clinical trial datasets could help to accelerate drug development while, in real-world settings, normalisation for demographic factors (such as through the use of percentiles or Z-scores) and accounting for a broader range of comorbidities may be needed. Ultimately, achieving a biomarker with reliable and broadly applicable clinical utility, whether at the population or individual patient level, will require replication and/or prospective testing of proposed stratification criteria in multiple large datasets, a clinically validated in vitro diagnostic assay, and a large normative database to adjust for demographic features, including broader age-range, BMI, and comorbidities not routinely captured in clinical trials.11,19,24
Our findings support NfL as a potential surrogate endpoint useful for accelerated development of MS therapies. NfL levels at week 48 exhibited elements of a treatment surrogate biomarker in both pwRMS and pwPPMS. Using NfL levels, as opposed to BV change or new T2 lesions, accounted for the greatest reduction in ocrelizumab treatment hazard ratio on clinical progression in pwRMS, suggesting that NfL may be a better proxy for treatment effect than MRI measures in shorter clinical studies. In pwPPMS, adjusting for new/enlarging T2 lesions observed by week 48 showed a greater impact on ocrelizumab treatment effect. However, in progressive MS studies with active comparator arms, it may nonetheless be worthwhile to evaluate the impact on NfL levels. Validation in independent MS clinical trials would further support NfL as a promising surrogate endpoint for treatments that effectively target progression.
Conclusions
Using large data sets from phase 3 ocrelizumab trials in both pwRMS and pwPPMS, we provide evidence to support NfL as a potentially clinically actionable biomarker. Elevated NfL levels in the absence, or effective suppression, of acute disease activity reflect increased risk for non-relapsing disability progression. The finding that this NfL abnormality is prognostic for SEL volume expansion and disability progression further supports the notion that a more subtle NfL component partially captures chronic smouldering injury related to such non-relapsing progressive biology. Ocrelizumab induces a rapid and sustained reduction in NfL levels and obviates the prognostic value of baseline NfL levels, consistent with its demonstrated impact on acute disease activity and long-term disability. On-treatment NfL levels show potential utility for risk stratification and exhibit elements of a surrogate biomarker endpoint that, if validated, may help accelerate development of novel treatments that more effectively impact non-relapsing progressive MS.
Contributors
All authors certify that they have participated in the conceptualisation of the study and in the writing, editing, reviewing, and final approval of the manuscript. Additionally, AB-O, G-AT, CH, XJ, and AH developed the study methodology and performed formal data analysis and validation. Data were verified by AH, CH, G-AT, XJ, FM, LG, and AS. CB, UB, SF, LG, FM, AS, and HK performed study investigations. All authors read and approved the final version of the manuscript. All authors had full access to all study data and had final responsibility for the decision to submit for publication.
Data sharing statement
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here: https://vivli.org/members/ourmembers/. For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/innovation/process/clinical-trials/data-sharing/).
Declaration of interests
AB-O has received consulting fees from Gossamer, Janssen/Actelion, Atara Biotherapeutics, Biogen, BMS/Celgene/Receptos, F. Hoffmann-La Roche Ltd., Genentech, Inc., MAPI, MedImmune, Merck/EMD Serono, Novartis, Sanofi Genzyme, and GSK; has performed contracted research for Genentech, Inc., Novartis, and Biogen; and receives a salary from the University of Pennsylvania Perelman School of Medicine. G-AT, UB, LG, FM, AS, and HK are employees and shareholders of F. Hoffmann-La Roche Ltd. CH, SF, RH, XJ, and AH are employees of Genentech, Inc., and shareholders of F. Hoffmann-La Roche Ltd. CB has received consulting fees as a contractor for F. Hoffmann-La Roche Ltd. AHC has, in the past year, received fees or honoraria for consulting from Biogen, Celgene, EMD Serono/Merck, F. Hoffmann-La Roche Ltd., Genentech, Inc., Greenwich Biosciences, Janssen Pharmaceuticals, and Novartis. SLH serves on the SAB of Accure, Annexon, and Alector and on the BOD of Neurona, has consulted for NGM Bio, and has received travel reimbursement and writing assistance from F. Hoffmann-La Roche Ltd. and Novartis for CD20-related meetings and presentations. LK’s institution (University Hospital Basel) has received funds in the past 3 years that were exclusively used for research support at the Department, for steering committee and advisory board participation, consultancy services, and participation in educational activities from the following organisations: Actelion, AurigaVision AG, Bayer AG, BMS, Celgene, df-mp Molnia & Pohlman, Eli Lilly, EMD Serono, Genentech, GSK, Janssen LLC, Janssen Pharmaceuticals, Japan Tobacco Inc., Merck, MH Consulting, Merck Healthcare KGaA, Minoryx Therapeutics S.L., Novartis, Novartis Biociências S.A., Österreichische Gesellschaft für Neurologie, Roche, Sanofi, Santhera Pharmaceuticals, Senda Biosciences Inc., Shionogi BV, TG Therapeutics, and Wellmera AG; has served a leadership or fiduciary role for Foundation Clinical Neuroimmunology and Neuroscience Basel, MAGNIMS Steering Committee, European Charcot Foundation, and Neurostatus-UHB AG; the Research of the MS Center in Basel has been supported by grants from Novartis, Innosuisse, and Roche. JK received speaker fees, research support, and travel support and/or served on advisory boards for ECTRIMS, Swiss MS Society, Swiss National Research Foundation (grant no. 320030_189140/1), University of Basel, Bayer, Biogen, Celgene, Genzyme, Merck, Novartis, Roche, Sanofi, and Teva. DL is Chief Medical Officer of GeNeuro and has received travel reimbursement and personal compensation for consulting and speaking from Quanterix, Orion, Novartis, Roche, and Sanofi; has received consulting fees for GeNeuro SA as CMO, Orion, Novartis, Roche, and Sanofi; has received travel support from GeNeuro SA and Sanofi; and holds stock in GeNeuro SA.
Acknowledgements
The authors thank Richard Karpowicz, PhD, CMPP, of Health Interactions, Inc., Hamilton, NJ, USA, for providing medical writing support/editorial support, which was funded by F. Hoffmann-La Roche Ltd., Basel, Switzerland, in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). The authors also thank Damian Fiore, PharmD, Shweta Kotwal, MS/MD, Nipa Shah, and Teresa Davancaze for helpful discussion and/or operational and technical support.
Funding: This study is sponsored by F. Hoffmann-La Roche Ltd., Basel, Switzerland.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104662.
Appendix A. Supplementary data
References
- 1.Reich D.S., Lucchinetti C.F., Calabresi P.A. Multiple sclerosis. N Engl J Med. 2018;378(2):169–180. doi: 10.1056/NEJMra1401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kantarci O.H. Phases and phenotypes of multiple sclerosis. Continuum (Minneap Minn) 2019;25(3):636–654. doi: 10.1212/CON.0000000000000737. [DOI] [PubMed] [Google Scholar]
- 3.Kappos L., Wolinsky J.S., Giovannoni G., et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77(9):1132–1140. doi: 10.1001/jamaneurol.2020.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cree B.A.C., Hollenbach J.A., et al. University of California, San Francisco MS-EPIC Team Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol. 2019;85(5):653–666. doi: 10.1002/ana.25463. Epub 2019 Mar 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genentech Ocrevus (Ocrelizumab) [Full prescribing information] https://www.gene.com/download/pdf/ocrevus_prescribing.pdf
- 6.European Medicines Agency Ocrevus [Summary of product characteristics] https://www.ema.europa.eu/en/documents/product-information/ocrevus-epar-product-information_en.pdf
- 7.Hauser S.L., Bar-Or A., Comi G., et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221–234. doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- 8.Montalban X., Hauser S.L., Kappos L., et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209–220. doi: 10.1056/NEJMoa1606468. [DOI] [PubMed] [Google Scholar]
- 9.Wolinsky J.S., Arnold D.L., Brochet B., et al. Long-term follow-up from the ORATORIO trial of ocrelizumab for primary progressive multiple sclerosis: a post-hoc analysis from the ongoing open-label extension of the randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2020;19(12):998–1009. doi: 10.1016/s1474-4422(20)30342-2. Epub 2020 Oct 29. [DOI] [PubMed] [Google Scholar]
- 10.Olsson B., Portelius E., Cullen N.C., et al. Association of cerebrospinal fluid neurofilament light protein levels with cognition in patients with dementia, motor neuron disease, and movement disorders. JAMA Neurol. 2019;76(3):318–325. doi: 10.1001/jamaneurol.2018.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhle J., Kropshofer H., Haering D.A., et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology. 2019;92(10):e1007–e1015. doi: 10.1212/WNL.0000000000007032. Epub 2019 Feb 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhle J., Barro C., Disanto G., et al. Serum neurofilament light chain in early relapsing remitting MS is increased and correlates with CSF levels and with MRI measures of disease severity. Mult Scler. 2016;22(12):1550–1559. doi: 10.1177/1352458515623365. Epub 2016 Jan 11. [DOI] [PubMed] [Google Scholar]
- 13.Lizak N., Malpas C.B., Sharmin S., et al. Association of sustained immunotherapy with disability outcomes in patients with active secondary progressive multiple sclerosis. JAMA Neurol. 2020;77(11):1398–1407. doi: 10.1001/jamaneurol.2020.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Disanto G., Barro C., Benkert P., et al. Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81(6):857–870. doi: 10.1002/ana.24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziemssen T., Arnold D.L., Alvarez E., et al. Prognostic value of serum neurofilament light chain for disease activity and worsening in patients with relapsing multiple sclerosis: results from the phase 3 ASCLEPIOS I and II trials. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.852563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhle J., Nourbakhsh B., Grant D., et al. Serum neurofilament is associated with progression of brain atrophy and disability in early MS. Neurology. 2017;88(9):826–831. doi: 10.1212/WNL.0000000000003653. Epub 2017 Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliott C., Wolinsky J.S., Hauser S.L., et al. Slowly expanding/evolving lesions as a magnetic resonance imaging marker of chronic active multiple sclerosis lesions. Mult Scler. 2019;25(14):1915–1925. doi: 10.1177/1352458518814117. Epub 2018 Dec 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khalil M., Pirpamer L., Hofer E., et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun. 2020;11(1):812. doi: 10.1038/s41467-020-14612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benkert P., Meier S., Schaedelin S., et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol. 2022;21(3):246–257. doi: 10.1016/S1474-4422(22)00009-6. [DOI] [PubMed] [Google Scholar]
- 20.Piehl F., Kockum I., Khademi M., et al. Plasma neurofilament light chain levels in patients with MS switching from injectable therapies to fingolimod. Mult Scler. 2018;24(8):1046–1054. doi: 10.1177/1352458517715132. Epub 2017 Jun 19. [DOI] [PubMed] [Google Scholar]
- 21.Novakova L., Zetterberg H., Sundström P., et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology. 2017;89(22):2230–2237. doi: 10.1212/WNL.0000000000004683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delcoigne B., Manouchehrinia A., Barro C., et al. Blood neurofilament light levels segregate treatment effects in multiple sclerosis. Neurology. 2020;94(11):e1201–e1212. doi: 10.1212/WNL.0000000000009097. Epub 2020 Feb 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leppert D., Kropshofer H., Häring D.A.A., et al. Blood neurofilament light in progressive multiple sclerosis: post hoc analysis of 2 randomized controlled trials. Neurology. 2022;98(21):e2120–e2131. doi: 10.1212/WNL.0000000000200258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gafson A.R., Jiang X., Shen C., et al. Serum neurofilament light and multiple sclerosis progression independent of acute inflammation. JAMA Netw Open. 2022;5(2) doi: 10.1001/jamanetworkopen.2021.47588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hendricks R., Baker D., Brumm J., et al. Establishment of neurofilament light chain Simoa assay in cerebrospinal fluid and blood. Bioanalysis. 2019;11(15):1405–1418. doi: 10.4155/bio-2019-0163. Epub 2019 Aug 12. [DOI] [PubMed] [Google Scholar]
- 26.Harp C., Thanei G.A., Jia X., et al. Development of an age-adjusted model for blood neurofilament light chain. Ann Clin Transl Neurol. 2022;9(4):444–453. doi: 10.1002/acn3.51524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prentice R.L. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 1989;8(4):431–440. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]
- 28.Bridel C., Leurs C.E., van Lierop Z.Y.G.J., et al. Serum neurofilament light association with progression in natalizumab-treated patients with relapsing-remitting multiple sclerosis. Neurology. 2021;97(19):e1898–e1905. doi: 10.1212/WNL.0000000000012752. [DOI] [PubMed] [Google Scholar]
- 29.Maggi P., Kuhle J., Schädelin S., et al. Chronic white matter inflammation and serum neurofilament levels in multiple sclerosis. Neurology. 2021;97(6):e543–e553. doi: 10.1212/WNL.0000000000012326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.