Summary
Background
Prostatic artery embolisation (PAE) is a minimally invasive treatment of symptomatic benign prostatic hyperplasia (BPH). Our aim was to compare patient's symptoms improvement after PAE and medical treatment.
Methods
A randomised, open-label, superiority trial was set in 10 French hospitals. Patients with bothersome lower urinary tract symptoms (LUTS) defined by International Prostatic Symptom Score (IPSS) > 11 and quality of life (QoL) > 3, and BPH ≥50 ml resistant to alpha-blocker monotherapy were randomly assigned (1:1) to PAE or Combined Therapy ([CT], oral dutasteride 0.5 mg/tamsulosin hydrochloride 0.4 mg per day). Randomisation was stratified by centre, IPSS and prostate volume with a minimisation procedure. The primary outcome was the 9-month IPSS change. Primary and safety analysis were done according to the intention-to-treat (ITT) principle among patients with an evaluable primary outcome. ClinicalTrials.gov Identifier: NCT02869971.
Findings
Ninety patients were randomised from September 2016 to February 2020, and 44 and 43 patients assessed for primary endpoint in PAE and CT groups, respectively. The 9-month change of IPSS was −10.0 (95% confidence interval [CI]: −11.8 to −8.3) and −5.7 (95% CI: −7.5 to −3.8) in the PAE and CT groups, respectively. This reduction was significantly greater in the PAE group than in the CT group (−4.4 [95% CI: −6.9 to −1.9], p = 0.0008). The IIEF-15 score change was 8.2 (95% CI: 2.9–13.5) and −2.8 (95% CI: −8.4 to 2.8) in the PAE and CT groups, respectively. No treatment-related AE or hospitalisation was noticed. After 9 months, 5 and 18 patients had invasive prostate re-treatment in the PAE and CT group, respectively.
Interpretation
In patients with BPH ≥50 ml and bothersome LUTS resistant to alpha-blocker monotherapy, PAE provides more urinary and sexual symptoms benefit than CT up to 24 months.
Funding
French Ministry of Health and a complementary grant from Merit Medical.
Keywords: Lower urinary tract symptoms, Benign prostatic hyperplasia, International prostatic symptom score, Erectile function, Prostatic artery embolisation, Alpha-blockers, 5- alpha-reductase inhibitors, Cost, Economics
Research in context.
Evidence before this study
The standard of care for patients having bothersome lower urinary tract symptoms due to benign prostatic hyperplasia (BPH) is watchful waiting and life-style modifications. When this initial strategy has failed and patient's quality of life is still significantly impaired, the first step of treatment is medical therapy using alpha-blockers which reduces international prostatic symptom score (IPSS) by an average of 4 points at 4 weeks. If the prostate is larger than 50 ml, and when symptoms improvement is not sufficient, the next step is addition of 5-alpha-reductase inhibitors which reduces the IPSS by 5–6 points on average at 9 months. Invasive or minimally invasive treatment are considered in case of failure of medical treatment.
To reduce the invasiveness and length of hospitalization, several minimally invasive techniques have been proposed. Prostatic Artery Embolisation (PAE) which has shown a good risk/efficacy profile, is an increasingly recognized treatment option.
We searched Pubmed for articles published between January 1, 2003 and December 31, 2015 without language restrictions using the term “bothersome lower urinary tract symptoms”, “alpha-blockers”, “5-alpha-reductase inhibitors”, “benign prostatic hyperplasia”, “BPH”, “prostatic artery embolisation”. This Search identified no previous trial comparing combined medical therapy against PAE.
Added value of this study
To our knowledge, this is the first randomized trial showing that PAE provides a clinically significant benefit over combined medical therapy. We also found that embolisation provided better outcomes on sexual function in this population.
Implications of all the available evidence
The PARTEM trial met its primary endpoint of clinically significant superior reduction of IPSS after PAE compared to combined medical treatment, in patients with BPH >50 ml who failed to improve after single alpha-blockers therapy. Therefore, PAE can be offered as an alternative to combined medical therapy when alpha-blockers treatment has failed.
Introduction
Benign Prostatic Hyperplasia (BPH) is the primary cause of male lower urinary tract symptoms (LUTS) and affects more than 50% of men over 60 years old.1 The primary goal of treatment is to reduce bothersome LUTS and prevent long-term complications such as renal function deterioration and acute urinary retention.2 Stepwise management consists in watchful waiting, behavioural and dietary modifications followed by medications, starting with an alpha-blocker.3 In case of insufficient results, 5-alpha-reductase inhibitors (5-ARI) can be combined with alpha-blockers which allows reduction of the size of the prostate as well as LUTS improvement.3,4 The CombAT study demonstrated that combination treatment is superior to either monotherapy (alpha-blockers or dutasteride).5 Both in the MTOPS and CombAT studies, combination therapy was superior to monotherapy in preventing clinical progression as defined by an international prostatic symptom score (IPSS) increase of at least 4 points, the occurrence of acute urinary retention, urinary tract infection, incontinence, or an increase in creatinine >50%.6 However, this treatment carries a significant risk of side effects that can lead to treatment discontinuation.
Prostatic artery embolisation (PAE) is a minimally invasive approach consisting in the occlusion of the prostatic arteries performed under fluoroscopic guidance by trained interventional radiologists. It can be completed in an outpatient setting and typically allows reduction of IPSS of 10–12 points at 6 months.7, 8, 9, 10, 11 A recent Cochrane review concluded that PAE and transurethral resection of the prostate may work similarly well in helping to relieve symptoms.12 To the best of our knowledge, PAE has not yet been compared to medical treatment.
The aim of the trial was to compare effect on LUTS of PAE versus medical treatment in patients with symptomatic BPH who still complained of bothersome LUTS despite >1 month treatment with alpha-blockers.
Methods
Study design
The PARTEM trial was an academic, multicentre, open-label, randomised, controlled, superiority trial conducted at 10 hospitals in France. The trial protocol (see Study Supplementary Appendix; ClinicalTrials.gov identifier: NCT02869971) was approved by an ethics committee (Comité de Protection des Personnes Ile de France VII). All participants provided written informed consent.
Participants
Patients aged between 50 and 85 years referred to the participating urology clinics with bothersome LUTS despite a treatment with alpha-blockers >1 month were considered for inclusion. Both the urologist and the interventional radiologist provided full information on the current treatment options including combined therapy (CT) and PAE.
Key inclusion criteria were an IPSS >11, a quality of life (QoL) > 3 and a prostate volume ≥50 ml as assessed by magnetic resonance or ultrasound imaging. Key exclusion criteria were severe allergy to iodine contrast agent, severe renal failure and treatment with 5-ARI in the last 6 months.
During the study, two protocol amendments affecting the trial recruitment were approved by the ethics committee. The first one was the opportunity of a revision embolisation for the second artery when there was successful embolisation of only one of the two prostatic arteries. The second one was the suppression of the urinary flow threshold as an exclusion criterion because this assessment was not part of follow-up recommendations for these patients and because this threshold has been shown to be useless regarding the trial's design and needs.
Randomisation and masking
Eligible patients were randomly assigned (1:1) to receive either PAE (experimental group) or combined therapy (control group). Randomisation was done using a centralised web-based system with a minimisation algorithm13 to obtain balanced assignment in each treatment group according to the stratification factors: treatment centre, IPSS (moderate/severe) and prostate volume (<80 g/≥80 g). A 30% random component was introduced to reduce the predictability of the allocation. The random allocation sequence was masked and was generated by the CleanWeb software (Telemedicine Technologies, http://www.tentelemed.com/la-solution-cleanweb/). Treatment group allocation could not be masked from the investigators or the participants.
Procedures
Patients in the control group (combined therapy) were given oral dutasteride 0.5 mg/tamsulosin hydrochloride 0.4 mg per day for at least 9 months. In case of poor tolerance, patient could be treated by tamsulosin only.
Patients in the experimental group (PAE) were given oral tamsulosin hydrochloride 0.4 mg per day from randomisation to 15 days after PAE. The PAE was performed by the interventional radiological teams according to a standardised technique using a single type of calibrated microspheres (Embosphere® 300–500 μm, Merit medical, Souths Jordan, USA) with proctoring of the first case in each study site by the trial principal investigator's team. This organisation was chosen to avoid the learning curve effect that can be a challenge when evaluating new interventional or surgical techniques.14 Briefly, after a femoral arterial access under local anaesthesia, the 2 prostatic arteries were catheterised super-selectively with a micro-catheter. Proximal embolisation using calibrated microparticles was performed until complete stasis followed by distal embolisation. Patient were given prophylactic antibiotic therapy during PAE (cefazoline 2 g flash injection). After arterial sheath insertion, patient received an intravenous bolus of heparin (40 UI/kg). Esomeprazole 20 mg and prednisone 20 mg once-a-day were prescribed for 5 days.
Follow-up was conducted during outpatient clinic visits scheduled at 1, 3, 9, 18 and 24 months after randomisation. IPSS/QoL and international index erectile function (IIEF) questionnaires, adverse events and BPH medication were assessed at each follow-up.
Flow max, post-voiding residue and prostate volume were assessed at the 3-, 9- and 24-month visits. Medication adherence of CT was assessed at 3 and 9 months using units' account and Girerd's patient questionnaire. After 9 months, the treatment options were left to the discretion of the patient and the referring urologist based on a patient's choice questionnaire.
Outcomes
The primary outcome was the change in IPSS 9 months after randomisation. IPSS consists in seven questions dealing with voiding (incomplete emptying, intermittency, weak stream and straining to void) and storage symptoms (frequency, urgency and nocturia): the higher the IPSS score, the worse the symptoms. The secondary outcomes at 9 and 24 months included QoL score (last question of IPSS questionnaire), flow max, IIEF-15 score (a higher score means less dysfunction), post-voiding residual volume, prostate volume, number of BPH medication, PSA value, number of patients with surgical treatment, number of embolisations in the CT group, and number of adverse events (AE). Medication adherence defined as a composite of the Girerd's questionnaire score and pills count (non-adherence was defined by either a proportion of non-taken pills ≥20% or a Girerd's questionnaire score ≥3) was measured at the 3- and 9-month outpatient visit. Patient's opinion for both groups regarding his potential choice for surgery or embolisation for the following 6 months was recorded at 9 months.15
Statistical analysis
The sample size calculation was performed using nQuery Advisor® 7.0 (Statistical Solutions, Saugus, MA, USA) using a two-sided two-sample equal-variance t-test. To detect a clinically significant 4-point difference of IPSS between PAE and CT, assuming a standard deviation of 6, and using a 0.05 two-sided type I error and a 80% power, 37 patients per group were needed.5 We arbitrarily increased this sample size by 20%, (i.e., 90 patients), in order to take into account patients with missing primary outcome and therefore maintaining the power of our analysis.
All analyses are reported according to the CONSORT statement (http://www.consort-statement.org/). The main analysis was conducted according to the intention-to-treat (ITT) principle considering patients with an evaluable primary outcome (modified ITT). We also conducted analyses in a per-protocol population (patients who underwent randomisation and had no major protocol deviations). Continuous data are presented as means (standard deviations) or as medians [interquartile ranges (IQR)]. Categorical data are summarised as number and percentages.
Treatment effect was assessed using analysis of covariance (ANCOVA) including the baseline value of the IPSS as covariate. IPSS and IIEF-15 score were also analysed using a repeated-measures, mixed-effects linear regression model that included baseline value as a covariate, with treatment group, visit and treatment group-by-visit interaction as fixed effects. Adjustment on randomisation factors was also considered in both analyses. Residual analyses were used to verify the assumptions of the models.
Planned sub-group analyses of the primary outcome were performed according to IPSS (moderate vs. severe) and prostatic volume (<80 g vs. ≥80 g).
Additional details regarding the statistical analysis are provided in the Study Supplementary Appendix. Analyses were done with SAS software, version 9.4. We deemed a two-sided p value less than 0.05 to be significant.
Economic evaluation
The choice of an optimal treatment strategy for BPH is also informed by economic data due to the high prevalence of the condition and the costs of surgery. In the USA, the incremental cost of BPH was estimated $1536 yearly per person for a population of over 12 million and about €1000 per treated patient in Europe.16, 17, 18
The within-trial cost-effectiveness analysis was undertaken from a healthcare perspective in the French setting which means that where possible actual costs of resource use have been determined and used for cost calculation, rather than national tariffs. The comparison tested the hypothesis of an incrementally cost-effective strategy; endpoints for the economic analysis were treatment success defined by an IPSS of 11 or less and the absence of serious adverse event. The 9-month ICER (Institute for Clinical and Economic Review) was calculated as the difference in costs divided by the difference in treatment success. The selection outcome for the economic evaluation was driven by the clinical relevance of treatment success at 9 months and the limited sensitivity of generic quality of life measures EQ-5D over a short period.
Total costs were estimated from the date of recruitment until the earliest of death, withdrawal from study and 9 months. Treatment costs per patient were determined by multiplying observed medical consumption with costs per unit. Unit costs are detailed in Supplementary Appendix Table S1. Measures of within-trial use of hospital resources were based on routine hospital data via patient-level information and costing systems, and entries in case report forms; drug utilization was extracted from the case report form. Procedural (direct) costs for PAE included interventional radiology suite staffing and interventional radiology supplies. Radiology supplies included catheters, wires, and contrast. We used current French 2021 values for all resources (€). Costs of co-morbidities were excluded. Neither costs nor outcomes were discounted.
Quantitative data were presented using means (standard deviation). Standard deviations, 95% confidence intervals and the uncertainty surrounding the ICER on the cost effectiveness plane were calculated by 3000 bootstrap replications. All health-economic analyses were done with R, version 3.4.3 (R Foundation for Statistical Computing).
Role of the funding source
The sponsor and the funding source were not involved in study design; in collection, analysis, and interpretation of data; in writing the manuscript; or in the decision to submit the manuscript for publication. The trial was conducted under the guidance of an independent data and safety monitoring board convened by the sponsor. The corresponding author had full access to all the study data and had final responsibility for the decision to submit for publication.
Results
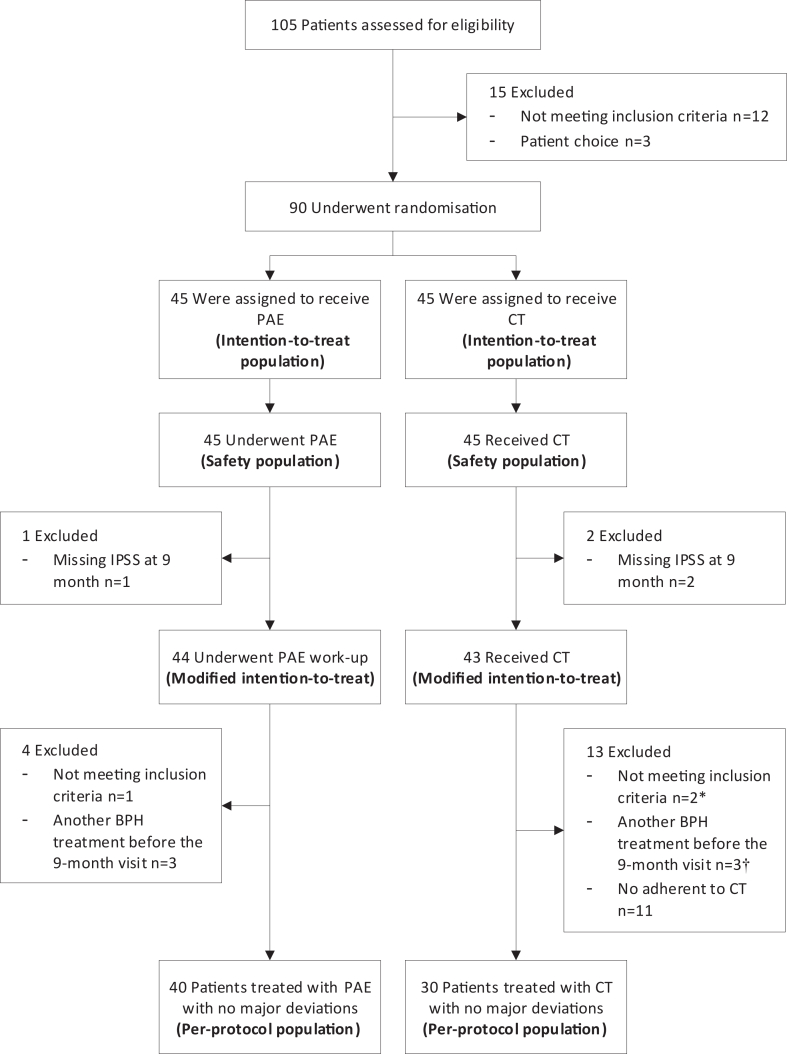
Participants were recruited between September 19, 2016 and February 17, 2020. Forty-five patients were randomised in each group; 44 and 43 patients could be assessed for primary endpoint in PAE and CT groups respectively (Fig. 1). Participant's baseline characteristics are detailed in Table 1.
Fig. 1.
Study flow-chart. ∗ One patient was also non adherent to the combined therapy (Tamsulosin and Dutasteride in a combined pill). † Two patients were also non adherent to the combined therapy (Tamsulosin and Dutasteride in a combined pill). PAE denotes prostatic artery embolisation, CT combined therapy, IPSS International Prostatic Symptom Score and BPH benign prostatic hyperplasia.
Table 1.
Clinical and biological characteristics of patients randomised to the prostatic artery embolisation or combined therapy groupsa.
| Clinical characteristics | n | Prostatic artery embolisation group (n = 45) | n | Combined therapy group (n = 45) |
|---|---|---|---|---|
| Age (years) | 45 | 67.3 ± 6.1 | 45 | 65.2 ± 5.5 |
| BMI (kg/m2) | 45 | 25.9 ± 3.5 | 45 | 26.8 ± 3.3 |
| Prostate volume (ml) | 45 | 92.0 ± 35.3 | 45 | 95.2 ± 38.5 |
| Prostate volume ≥80 (ml)–no. (%) | 45 | 24 (53.3) | 45 | 26 (57.8) |
| Median PSA (ng/ml) (IQR) | 43 | 4.2 (2.2–8.5) | 44 | 5.4 (3.1–10.2) |
| Flow max (ml/sec) | 44 | 9.3 ± 4.6 | 45 | 8.6 ± 4.2 |
| Median post-voiding residual (ml) (IQR) | 43 | 75.0 (27.0–132.0) | 43 | 81.0 (20.0–145.0) |
| IPSS | 45 | 18.9 ± 5.3 | 45 | 19.4 ± 6.1 |
| QoL score | 45 | 4.7 ± 0.9 | 45 | 4.9 ± 0.8 |
| Median IIEF-15 score (IQR) | 43 | 42.0 (19.0–61.0) | 41 | 52.0 (22.0–63.0) |
SD, Denotes standard deviation, BMI, Body mass index, PSA, Prostate specific antigen, IQR, Interquartile range, IPSS, International prostatic symptom score, QoL, Quality of life, IIEF, International index of erectile function.
Plus–minus values are means ± SD.
Four patients in the PAE group underwent only unilateral embolisation, despite a second attempt in 2 patients. Unilateral embolisation was done when it was impossible to catheterize selectively the prostatic artery; this could be related to excessive angulation, calcification or stenosis.
Baseline characteristics of patients in the modified intention-to-treat population and the per-protocol analysis are presented in Supplementary Appendix Tables S2 and S3, respectively.
At 9 months, the change in IPSS was −10.0 (95% confidence interval [CI]: −11.8 to −8.3) and −5.7 (95% CI: −7.5 to −3.8) in the PAE and CT groups, respectively (Table 2). This reduction was significantly greater in the PAE group than in the CT group (−4.4 [95% CI: −6.9 to −1.9], p = 0.0008). Similar results were observed in the per-protocol analysis (−4.2 [95% CI: −7.0 to −1.5], p = 0.0034; Supplementary Appendix Table S4). Individual IPSS changes between randomisation and 9 months are presented in Fig. 2 (panel a).
Table 2.
IPSS, quality of life, IIEF, prostatic volume, PSA, flow max, post-voiding residual measurements at randomisation and after 9 months follow-up in the prostatic artery embolisation and combined therapy groups (modified intention-to-treat population).
| Prostatic artery embolisation group (n = 44) |
Combined therapy group (n = 43) |
Mean baseline-adjusted difference (95% CI) between the 2 groups at 9 months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Randomisation (mean ± SD) | 9 months (mean ± SD) | Mean baseline-adjusted difference (95% CI) | n | Randomisation (mean ± SD) | 9 months (mean ± SD) | Mean baseline-adjusted difference (95% CI) | ||
| IPSS | 44 | 19.0 ± 5.3 | 9.0 ± 5.3 | −10.0 (−11.8 to −8.3) | 43 | 19.2 ± 6.0 | 13.2 ± 7.1 | −5.7 (−7.5 to −3.8) | −4.4 (−6.9 to −1.9) |
| QOL | 44 | 4.8 ± 0.8 | 2.0 ± 1.4 | −2.8 (−3.3 to −2.4) | 43 | 4.9 ± 0.8 | 3.7 ± 1.8 | −1.1 (−1.6 to −0.6) | −1.7 (−2.4 to −1.1) |
| IIEF-15 | 38 | 39.1 ± 21.9 | 48.6 ± 21.0 | 8.2 (2.9–13.5) | 34 | 44.9 ± 22.3 | 41.0 ± 22.0 | −2.8 (−8.4 to 2.8) | 11.0 (3.3–18.7) |
| Prostate volume (ml) | 42 | 92.8 ± 36.3 | 69.9 ± 25.7 | −23.8 (−29.6 to −18.0) | 40 | 95.8 ± 39.7 | 74.5 ± 33.7 | −21.1 (−27.0 to −15.1) | −2.7 (−10.9 to 5.5) |
| PSA (ng/ml) | 40 | 5.9 ± 4.1 | 3.8 ± 2.6 | −2.6 (−3.2 to −1.9) | 39 | 8.0 ± 7.0 | 4.4 ± 4.8 | −3.2 (−3.9 to −2.5) | 0.6 (−0.3 to 1.6) |
| Flow max (ml/sec) | 36 | 9.5 ± 3.9 | 11.7 ± 3.8 | 2.4 (1.0–3.8) | 34 | 9.0 ± 4.5 | 11.3 ± 6.3 | 2.1 (0.7–3.5) | 0.3 (−1.7 to 2.3) |
| PVR volume (ml) | 34 | 105.9 ± 103.9 | 79.0 ± 96.2 | −23.4 (−54.0 to 7.2) | 31 | 91.3 ± 89.9 | 67.1 ± 91.3 | −29.4 (−61.6 to 2.7) | 6.1 (−38.5 to 50.6) |
SD denotes standard deviation, CI confidence interval, IPSS international prostatic symptom score, QoL quality of life, IIEF international index of erectile function, PSA prostate specific antigen, PVR post-voiding residual.
Fig. 2.
Between-subject variability: between-subject variability in IPSS∗ (Panel a) and IIEF-15 (Panel b) score changes from baseline to 9 months in the prostatic artery embolisation and the combined therapy group (modified intention-to-treat population). ∗ The dashed line corresponds to the clinically relevant improvement of IPSS. IPSS, Denotes international prostatic symptom score and IIEF, International index of erectile function. Symptoms are improved when IPSS is decreased and when IIEF score is increased.
The reduction in storage and voiding IPSS sub-scores was also greater in the PAE than in the CT group (mean between-group difference: −2.0 [95% CI: −3.1 to −1.0] and −2.4 [95% CI: −4.1 to −0.6], respectively; Supplementary Appendix Table S5). IPSS modification from randomisation to 1, 3 and 9 months is shown on Supplementary Appendix Figure S1 (Panel a). Neither prostate size (<80 g vs. ≥80 g) nor the severity of urinary symptoms (moderate vs. severe) before randomisation had any effect on the IPSS change.
QoL change was greater in the PAE than in the CT group (mean between-group difference: −1.7 [95% CI: −2.4 to −1.1]).
At 9 months, the change of IIEF-15 score was 8.2 (95% CI: 2.9–13.5) and −2.8 (95% CI: −8.4 to 2.8) in the PAE and CT groups, respectively (Table 2). Individual IIEF-15 changes between randomisation and 9 months are presented in Fig. 2 (panel b). The same effect was observed for the 5 IIEF-15 sub-scores, i.e., erectile and orgasmic function, sexual desire, intercourse and overall satisfaction (Supplementary Appendix Table S5). Finally, the change of ejaculation scale was 0.67 ± 1.74 and −0.34 ± 1.73 in the PAE and CT groups, respectively.
Change in IIEF-15 score from randomisation to 1, 3 and 9 months is shown on Supplementary Appendix Figure S1 (Panel b). A comparable improvement at 9 months was observed in the 2 groups for prostate volume, PSA value, flow max and post-voiding residual volume (Table 2). Similar results were observed for secondary outcomes in the per-protocol analysis (Supplementary Appendix Tables S4 and S6).
At 9 months, among the 43 patients of the CT group, 10 (23.3%) were considered non-adherent to CT and 3 patients received other BPH medications (alpha-blockers: n = 1; alpha-blockers + herbal remedies: n = 1, phosphodiesterase type 5 inhibitors: n = 1). Among the 44 patients of the PAE group, 3 patients received BPH medication (alpha-blockers: n = 1; herbal remedies: n = 1, phosphodiesterase type 5 inhibitors: n = 1).
At 9 months, a total of 31 patients in the PAE group and 21 patients in the CT group presented at least one AE (Table 3). No hospitalization or prolongation of hospitalization induced by an AE related to the treatment was observed.
Table 3.
Number of adverse events at 9 months among patients randomised to prostatic artery embolisation or combined therapy groups (ITT population).
| Reaction/event | Prostatic artery embolisation group (M = 51)a | Combined therapy group (M = 42)b |
|---|---|---|
| Abdominopelvic pain | 2 | 2 |
| Acute Urinary Retention | 0 | 1 |
| Ankle oedema | 0 | 1 |
| Arrhythmia | 1 | 3 |
| Arterial dissection/perforation | 3 | 0 |
| Balanitis | 3 | 0 |
| BPH increase | 1 | 0 |
| Decreased libido | 0 | 5 |
| Difficulty with ejaculation | 0 | 1 |
| Difficulty with sleeping | 0 | 2 |
| Dizziness | 0 | 1 |
| Dyspnoea | 0 | 1 |
| Dysuria | 3 | 0 |
| Excessive radiation dose | 1 | 0 |
| Fatigue | 0 | 1 |
| Foot pain | 1 | 0 |
| Heartburn | 0 | 1 |
| Hematoma at Femoral access | 2 | 0 |
| Hematospermia | 0 | 1 |
| Hydrocele | 1 | 0 |
| Impotence | 0 | 3 |
| Knee inflammation | 0 | 1 |
| Myalgia | 1 | 0 |
| Night sweating | 0 | 1 |
| Obstructive renal insufficiency | 1 | 0 |
| Orthostatic hypotension | 0 | 2 |
| Pain and swelling in the testicles | 0 | 1 |
| Pain following urinary catheterisation | 1 | 0 |
| Pain/burning during miction | 1 | 4 |
| Polyuria/nocturia | 4 | 1 |
| Post-embolisation syndrome | 17 | 0 |
| Prostatitis | 1 | 1 |
| Renal tumour ablation | 1 | 0 |
| Rhinitis | 1 | 0 |
| Sexual dysfunction | 0 | 1 |
| Skin melanoma | 1 | 0 |
| Skin rash/itching | 0 | 2 |
| Suspicion of acute coronary ST syndrome | 0 | 1 |
| Thoracic pain | 1 | 0 |
| Tinnitus | 0 | 1 |
| Total hip replacement | 1 | 0 |
| Transient ischemic attack | 1 | 0 |
| Urinary tract infection | 1 | 2 |
| Weight gain | 0 | 1 |
M denotes the number of adverse events. Post-embolisation syndrome consists in mild pelvic pain and fever with spontaneous resolution in 3–5 days.
A total of 31 patients in the prostatic artery embolisation group presented at least one adverse event.
A total of 21 patients in the combined therapy group presented at least one adverse event.
At 9 months, when asked which treatment they would consider in the following 6 months, 4 (9.1%) patients considered surgery in the PAE group compared to 9 (20.9%) in the CT group and 4 (9.1%) considered PAE in the PAE group compared to 18 (41.9%) in the CT group.
Eighty patients (42 in the PAE group and 38 patients in the CT group) completed the 24 months follow-up, of whom 23 received invasive treatment of their BPH between 9 and 24 months after randomization. In the PAE group, 5 patients underwent prostatic surgery (mainly Holmium Laser Prostate Surgery) after a mean delay from randomisation of 18 months. All these 5 patients were taking alpha-blockers at 24 months. Of the remaining 37 patients, 1 was taking combined therapy, 8 were taking alpha-blockers and 2 patients were taking herbal remedies at 24 months. In the CT group, 14 patients underwent PAE (4 were taking alpha-blockers at 24 months), and 4 patients underwent prostatic surgeries (mainly Holmium Laser Prostate Surgery) at an average of 14 months after their randomization. Of the remaining 20 patients, 4 were taking combined therapy, 2 were taking combined therapy plus herbal remedies, 1 was taking alpha-blockers and 1 was taking alpha-blockers plus herbal remedies at 24 months. Both reduction of IPSS and the sexual function as assessed by IIEF-15 global and its subdomains remained stable for the PAE group (Supplementary Appendix Tables S7 and S8).
The average duration of the intervention was 134 min (min = 36 min, max = 260 min). The initial cost of the intervention was €4013 (386), half of which was the hospital stay. The total 9-month costs were €5252 (3089) in the PAE group (including readmissions) and €1311 (1297) in the CT group (p < 0.01) (Supplementary Appendix Table S9). The point estimate of the cost effectiveness ratio was €29,356 (95% CI: −136,741 to 178,656) per % of patients with improvement in urinary symptoms (4 points off the IPSS), the uncertainly on the joint distribution of costs and outcomes is presented on Supplementary Appendix Figure S2 and shows a 94% probability that the PAE is both more effective and more expensive than the medical treatment. In the deterministic analysis, the cost of PAE was found to be most sensitive to the type of hospital admission: switching from inpatient to outpatient reduced the initial cost by 10% (Supplementary Appendix Figure S3).
Discussion
This randomised trial shows that the urinary function as assessed by both IPSS and QoL was significantly more improved at 9 months after PAE than after combined therapy, in both modified ITT and per-protocol analyses. This reduction in the PAE group was of a magnitude that is clinically significant to patients.2
The improvement of IPSS and QoL after PAE in the modified ITT population was in line with previously published results of randomised controlled trial comparing PAE to Trans-Urethral Resection of the Prostate or to sham intervention, or in large prospective non-randomised cohort studies.7, 8, 9, 10,19 This improvement was observed in both the storage and the voiding symptoms as previously observed.20,21 In the CT group, the 5.7 points reduction at 9 months in IPSS was also consistent with previously published results, as for example, in the COMBAT trial, where the adjusted mean reduction in IPSS from baseline to 9 months was 5.4.5 The improvement in IPSS could not be judged over a period of more than 9 months, because our design assumed that patients in the CT group would not consent to the study if waiting more time for PAE. The improvement was indirectly documented over the longer term at 24 months by the higher rate of invasive treatment in the CT group (18/38, 47%) than in the PAE group (2/42.12%).
Men suffering from bothersome LUTS have frequently co-existing sexual impairment. As described in the ICS- BPH international study conducted on men aged ≥45 years, >70% of men of any age group found that the effect of LUTS on their sex lives was a problem, and 45% reported that their sex lives were spoiled by LUTS.22 We observed the same trend with an average decrease of 2.8 on IIEF-15 in the CT group, in line with findings of the meta-analysis of Corona in 2017 who reported that overall, 5-ARI determined an increased risk of hypoactive sexual desire [odds ratio [OR] = 1.54 (95% CI: 1.29; 1.82); p < 0.0001] and erectile dysfunction [OR = 1.47 (95% CI: 1.29; 1.68); p < 0.0001].23 This side effect of 5-ARI on sexuality could be responsible for the 25.6% non-adherent patients in the CT group of our trial. Indeed, side effects are reported to be the main reason for treatment discontinuation.24,25 A large United Kingdom cohort recently reported an even lower adherence rates with only 45.6% of patients still adherent to BPH combination therapy at one year.26 A better IPSS improvement would have probably been observed in the CT group with a better medication adherence. The latter may have been achieved by including in the trial only patients with non-active sexual life, but the trial population would have been far apart from the “real life” population.
In contrast, the significant increase of IIEF-15 after PAE at 9 months in our trial, illustrated by an improvement in all sexuality sub-domains, including ejaculation, is an important result in the patient's perspective. Previous reports have also shown an increase in all components of IIEF-15 after PAE although the mechanism isn't clear.27 The absence of retrograde ejaculation after PAE likely contributes to sexual quality of life improvement.12
In the 2 years follow up findings, we report that the sexual function remained stable at years in both groups but it is difficult to draw any conclusion as the treatment was left open and that ultimately a large number of patients initially treated medically underwent PAE between the 9 and the 24 months follow-up.
Regarding functional outcomes, we observed that the improvement in flow max was minor and not different between CT and PAE group. This finding is well recognised and is a limitation in the efficacy of PAE when compared to surgery. Nonetheless, patients are usually more focused on other symptoms such as polyuria or nocturia.
The 62.8% of patients envisioning intervention (surgery or PAE) 6 months after completion of the 9 months outcome in CT arm reflects the overall limited effect of CT as well as its deleterious sexual side effects. In the PAE group only three patients were under medical treatment for BPH at 9 months which is less than the 13.8% prevalence of pharmacologic treatment after surgery reported by Lukacs in 2013.28
We did not observe any treatment-related serious adverse event requiring surgical treatment or re-hospitalisation in both groups confirming the favourable safety profile of PAE, as previously described. The minor side effects of CT comprising sexual function deterioration were also in line with the literature as well as the nearly 50% incidence of the self-contained post-embolisation syndrome after PAE.7,10,19,21,23 Non-severe local complications of PAE such as groin hematoma or reversible balanitis have also been previously described.7,8,10 Although technically demanding, PAE was safely performed in all participating centres, thanks to initial proctoring of operators. It is important to note that 4 patients could not undergo bilateral embolisation despite a second attempt in 2 of them. The initial technical failure of PAE can be related to patient's anatomy, especially severe atheromatous lesions, and to operator's experience. Nevertheless, despite these suboptimal technical result in 4 patients, a significant improvement in symptoms was observed after PAE in the mITT population.
Regarding stability of results of PAE, we found maintained IPSS reduction as well as a need for secondary surgery of 5 patients which is less than what was reported at 2 years in the trial of Abt et al. (21%).8 This could be related to the smaller prostate volume (25–80 ml) in this study compared to our trial (>90 ml). The clinical failure rate of PAE over the 2-year follow-up period is a recognized limitation of EPA. Indeed, in most case series, approximately 20% of patients may show no clinical response despite a good technical result. The stability of PAE results over a longer period of time is still unclear, but our trial was not designed to provide longer-term results.
The economic evaluation found that PAE was more costly and efficacious than CT with an incremental cost effectiveness ratio of per treatment success at 9 months. Our study is, to our knowledge, the first to report both outcomes and costs and duration of efficacy as part of the analysis for PAE.
The external validity of cost calculations is often questioned because of differences in prices and practices. In the case of PAE however the prices of Embosphere microspheres are remarkably similar between Europe and the USA and a study of PAE in a US hospital found both procedural and hospital costs amazingly close to our results, of $1667 and $1678 respectively with PAE patients discharged after 3 h of observation and no overnight stay.29 Another cost study in Switzerland reported much higher costs for PAE with a total of €8185 ± 1630 per patient. The difference with US and French results was explained by the higher cost of medical supplies alone (€2590 ± 628) and the inpatient stay (€3837 ± 1179).30
Our trial has several limitations. First of all, it is an open-label trial because it was obviously not possible to blind the patient and the investigators to treatment. Second, our trial did not compare PAE to other medical treatment such as muscarinic receptor agonist or phosphodiesterase-5 inhibitors.3 Third, embolisation was performed in centres having various level of experience. Anyway, we did not observe side effects and higher technical expertise could only improve the already favourable results of PAE compared to CT. Fourth, the duration of the combined treatment was 9 months, a period perhaps too short to observe its full effect. Full efficacy in the CT group could have been hampered by an insufficient duration of treatment and poor adherence to medication. The choice of this duration was voluntary and was intended to avoid too many cross-overs of patients from the CT group to embolisation or surgery. This limit can however be balanced by the fact that, at 24 months, the results of the PAE group remained better than those of the CT group. Fifth, we did not consider multiple comparison problem: readers should therefore pay attention to effect size since some “significant” p-values may be due to multiple testing. Finally, given both the subjective nature of IPSS, our primary endpoint, and the open design of our trial, the risk of detection bias (i.e., a systematic difference between groups in how the IPSS was rated by patients) cannot be eliminated.
This trial demonstrates that, at 9 months and up to 24 months, PAE provides better urinary and sexual symptoms score benefit than combined therapy in patients with bothersome LUTS related to BPH ≥50 ml not responsive to a one-month trial of alpha blockers.
Contributors
MS, NT, AD, GR, NBD, ADLT, HK, VV, VKS, IDZ, HP, CD, and GC participated in the design of the study. MS, NT, AD, AR, GP, RCD, GR, FP, GK, VV, TM, HVK, ADLT, HK, RM, JFH, SD, JF, NBD, and PARTEM investigators participated in patient data collection. All authors analysed and interpreted the data. GC was the methodologist of the study and HP was the study biostatistician responsible for the statistical analyses. All authors participated in writing of the report, agreed on the content of the manuscript, reviewed drafts, and approved the final version.
Data sharing statement
Individual participant data that underlie results in publication could be shared. Individual participant data detailed in the protocol of a planned meta-analysis could be shared. Data sharing must be accepted by the sponsor and the principal investigator based on scientific project and scientific involvement of the principal investigator team. The founder could be involved in the decision. Teams wishing obtain IPD must meet the sponsor and principal investigator's team to present scientific (and commercial) purpose, individual participant data needed, format of data transmission, and timeframe. Technical feasibility and financial support will be discussed before mandatory contracting.
Declaration of interests
NT, AD, CD, AR, GP, RCD, GR, FP, GK, VV, TM, HVK, ADLT, HK, RM, JFH, SD, JF, NBD, VKS, IDZ, HP, and GC declare no conflict of interest. MS reports consulting fees from Merit medical.
Acknowledgements
The trial was sponsored by Assistance Publique–Hôpitaux de Paris and funded by a grant from the French Ministry of Health (Programme Hospitalier de Recherche Clinique) and supplemented with a research grant from Merit Medical.
The authors are deeply indebted to all patients who accepted to participate in the trial, and to the physicians who took care of the patients at the participating institutions. The authors would like to thank Juliette Djadi-Prat, Yvann Frigout, Coraline Hautem and Asma Ksouri for their help in managing the PARTEM trial.
PARTEM study group:
Olivier Pellerin1,2, Brigitte Sabatier25,26, Charles Dariane3, Benjamin Gabay5, Paul Cezar Moldovan7, Olivier Rouvière7, Jean Champagnac7, Samuel Lagabrielle9, Nicolas Grenier10, Romain Boissier11, Éric Lechevallier11, Jalal-Jean Izaaryene12, Farouk Tradi12, Raphaele Arrouasse16, Julien Defontaines16, Xavier Joseph16, Philippe Le Corvoisier16, Emilie Sbidian16, Cécile Champy16, Mélanie Chiaradia17, Armand Chevrot20, Cyrille Blion20, Jean Goupil21, Julie Bulsei28, Alexandra Vappereau28.
Footnotes
For the French translation of the abstract see Supplementary Materials section.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2023.100672.
Contributor Information
Marc Sapoval, Email: marc.sapoval2@aphp.fr.
PARTEM study group:
Olivier Pellerin, Brigitte Sabatier, Charles Dariane, Benjamin Gabay, Paul Cezar Moldovan, Olivier Rouvière, Jean Champagnac, Samuel Lagabrielle, Nicolas Grenier, Romain Boissier, Éric Lechevallier, Jalal-Jean Izaaryene, Farouk Tradi, Raphaele Arrouasse, Julien Defontaines, Xavier Joseph, Philippe Le Corvoisier, Emilie Sbidian, Cécile Champy, Mélanie Chiaradia, Armand Chevrot, Cyrille Blion, Jean Goupil, Julie Bulsei, and Alexandra Vappereau
Appendix A. Supplementary data
References
- 1.Eckhardt M.D., Van Venrooij G.E., Van Melick H.H., Boon T.A. Prevalence and bothersomeness of lower urinary tract symptoms in benign prostatic hyperplasia and their impact on well-being. J Urol. 2001;166:563–568. [PubMed] [Google Scholar]
- 2.Barry M.J. Evaluation of symptoms and quality of life in men with benign prostatic hyperplasia. Urology. 2001;58:25–32. doi: 10.1016/s0090-4295(01)01300-0. [DOI] [PubMed] [Google Scholar]
- 3.Cornu J.N., Gacci M., Hashim H., et al. European Association of Urology; 2023. EAU guidelines on non neurogenic LUTS including benign prostatic obstruction.https://uroweb.org/guideline [Google Scholar]
- 4.Roehrborn C.G., Boyle P., Nickel J.C., Hoefner K., Andriole G. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology. 2002;60:434–441. doi: 10.1016/s0090-4295(02)01905-2. [DOI] [PubMed] [Google Scholar]
- 5.Roehrborn C.G., Siami P., Barkin J., et al. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol. 2010;57:123–131. doi: 10.1016/j.eururo.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 6.McConnell J.D., Roehrborn C.G., Bautista O.M., et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387–2398. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 7.Insausti I., de Ocáriz A.S., Galbete A., et al. Randomized comparison of prostatic artery embolization versus transurethral resection of the prostate for treatment of benign prostatic hyperplasia. J Vasc Interv Radiol. 2020;31:882–890. doi: 10.1016/j.jvir.2019.12.810. [DOI] [PubMed] [Google Scholar]
- 8.Abt D., Müllhaupt G., Hechelhammer L., et al. European Urology; 2021. Prostatic artery embolisation versus transurethral resection of the prostate for benign prostatic hyperplasia: 2-yr outcomes of a randomised, open-label, single-centre trial. [DOI] [PubMed] [Google Scholar]
- 9.Pisco J.M., Bilhim T., Costa N.V., et al. Randomised clinical trial of prostatic artery embolisation versus a sham procedure for benign prostatic hyperplasia. Eur Urol. 2020;77:354–362. doi: 10.1016/j.eururo.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Gao Y., Huang Y., Zhang R., et al. Benign prostatic hyperplasia: prostatic arterial embolization versus transurethral resection of the prostate—a prospective, randomized, and controlled clinical trial. Radiology. 2014;270:920–928. doi: 10.1148/radiol.13122803. [DOI] [PubMed] [Google Scholar]
- 11.Frandon J., Belaouni A., Pellerin O., et al. Efficacy and safety of prostate artery embolization for patients with lower urinary tract symptoms and indwelling urinary catheter: a retrospective multicenter study. Diagn Interv Imaging. 2022;103:601–606. doi: 10.1016/j.diii.2022.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Jung J., McCutcheon K.A., Borofsky M., et al. Prostatic arterial embolization for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database Syst Rev. 2022;12 doi: 10.1002/14651858.CD012867.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pocock S.J., Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]
- 14.Cook J.A., McCulloch P., Blazeby J.M., Beard D.J., Marinac-Dabic D., Sedrakyan A. IDEAL framework for surgical innovation 3: randomised controlled trials in the assessment stage and evaluations in the long term study stage. BMJ. 2013;346 doi: 10.1136/bmj.f2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girerd X., Hanon O., Anagnostopoulos K., Ciupek C., Mourad J.J., Consoli S. Assessment of antihypertensive compliance using a self-administered questionnaire: development and use in a hypertension clinic. Presse Med. 2001;30:1044–1048. [PubMed] [Google Scholar]
- 16.Vuichoud C., Loughlin K.R. Benign prostatic hyperplasia: epidemiology, economics and evaluation. Can J Urol. 2015;22(Suppl 1):1–6. [PubMed] [Google Scholar]
- 17.van Exel N.J.A., Koopmanschap M.A., McDonnell J., Chapple C.R., Berges R., Rutten F.F.H. Medical consumption and costs during a one-year follow-up of patients with LUTS suggestive of BPH in six european countries: report of the TRIUMPH study. Eur Urol. 2006;49:92–102. doi: 10.1016/j.eururo.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Erman A., Masucci L., Krahn M.D., Elterman D.S. Pharmacotherapy vs surgery as initial therapy for patients with moderate-to-severe benign prostate hyperplasia: a cost-effectiveness analysis. BJU Int. 2018;122:879–888. doi: 10.1111/bju.14520. [DOI] [PubMed] [Google Scholar]
- 19.Hacking N., Vigneswaran G., Maclean D., et al. Technical and imaging outcomes from the UK registry of prostate artery embolization (UK-ROPE) study: focusing on predictors of clinical success. Cardiovasc Intervent Radiol. 2019;42:666–676. doi: 10.1007/s00270-018-02156-8. [DOI] [PubMed] [Google Scholar]
- 20.Lin Y.T., Pereira H., Pellerin O., Dean C., Thiounn N., Sapoval M. Four-year impact of voiding and storage symptoms in patients with benign prostatic hyperplasia treated with prostatic artery embolization. J Vasc Interv Radiol. 2020;31:1460. doi: 10.1016/j.jvir.2019.08.034. [DOI] [PubMed] [Google Scholar]
- 21.Vigneswaran G., Maclean D., Hadi M., et al. Prostatic artery embolization (PAE) and transurethral resection of the prostate (TURP) have a differential impact on lower urinary tract symptoms (LUTS): retrospective analysis of the multicentre UK-ROPE (UK register of prostate embolization) study. Cardiovasc Intervent Radiol. 2021;44:1095–1102. doi: 10.1007/s00270-021-02821-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frankel S.J., Donovan J.L., Peters T.I., et al. Sexual dysfunction in men with lower urinary tract symptoms. J Clin Epidemiol. 1998;51:677–685. doi: 10.1016/s0895-4356(98)00044-4. [DOI] [PubMed] [Google Scholar]
- 23.Corona G., Tirabassi G., Santi D., et al. Sexual dysfunction in subjects treated with inhibitors of 5α-reductase for benign prostatic hyperplasia: a comprehensive review and meta-analysis. Andrology. 2017;5:671–678. doi: 10.1111/andr.12353. [DOI] [PubMed] [Google Scholar]
- 24.Eisen C., Lulic Z., Palacios-Moreno J.M., et al. Persistence and adherence to dutasteride/tamsulosin fixed-dose versus free-combination alpha blocker/5ARI therapy in patients with benign prostate hyperplasia in Germany. Int J Clin Pharmacol Ther. 2020;58:37. doi: 10.5414/CP203549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahn S.T., Lee D.H., Jeong H.G., et al. Treatment persistence with a fixed-dose combination of tadalafil (5 mg) and tamsulosin (0.4 mg) and reasons for early discontinuation in patients with benign prostatic hyperplasia and erectile dysfunction. Investig Clin Urol. 2020;61:81–87. doi: 10.4111/icu.2020.61.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayele H.T., Reynier P., Azoulay L., et al. Trends in the pharmacological treatment of benign prostatic hyperplasia in the UK from 1998 to 2016: a population-based cohort study. World J Urol. 2021;39:2019–2028. doi: 10.1007/s00345-020-03429-z. [DOI] [PubMed] [Google Scholar]
- 27.Marzano L., Thiounn N., Pereira H., et al. Prostatic artery embolization allows to maintain full sexual activity in patients suffering from bothersome lower urinary tracts symptoms related to benign prostatic hyperplasia. Cardiovasc Intervent Radiol. 2020;43:1202–1207. doi: 10.1007/s00270-020-02520-7. [DOI] [PubMed] [Google Scholar]
- 28.Lukacs B., Cornu J.N., Aout M., et al. Management of lower urinary tract symptoms related to benign prostatic hyperplasia in real-life practice in France: a comprehensive population study. Eur Urol. 2013;64:493–501. doi: 10.1016/j.eururo.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 29.Bagla S., Smirniotopoulos J., Orlando J., Piechowiak R. Cost analysis of prostate artery embolization (PAE) and transurethral resection of the prostate (TURP) in the treatment of benign prostatic hyperplasia. Cardiovasc Intervent Radiol. 2017;40:1694–1697. doi: 10.1007/s00270-017-1700-7. [DOI] [PubMed] [Google Scholar]
- 30.Müllhaupt G., Hechelhammer L., Engeler D.S., et al. In-hospital cost analysis of prostatic artery embolization compared with transurethral resection of the prostate: post hoc analysis of a randomized controlled trial. BJU Int. 2019;123:1055–1060. doi: 10.1111/bju.14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.