Abstract
Spinal cord injury (SCI) is a debilitating condition with significant personal, societal, and economic burden. The highest proportion of traumatic injuries occur at the cervical level, which results in severe sensorimotor and autonomic deficits. Following the initial physical damage associated with traumatic injuries, secondary pro-inflammatory, excitotoxic, and ischemic cascades are initiated further contributing to neuronal and glial cell death. Additionally, emerging evidence has begun to reveal that spinal interneurons undergo subtype specific neuroplastic circuit rearrangements in the weeks to months following SCI, contributing to or hindering functional recovery. The current therapeutic guidelines and standards of care for SCI patients include early surgery, hemodynamic regulation, and rehabilitation. Additionally, preclinical work and ongoing clinical trials have begun exploring neuroregenerative strategies utilizing endogenous neural stem/progenitor cells, stem cell transplantation, combinatorial approaches, and direct cell reprogramming. This review will focus on emerging cellular and noncellular regenerative therapies with an overview of the current available strategies, the role of interneurons in plasticity, and the exciting research avenues enhancing tissue repair following SCI.
Keywords: Spinal cord injury, Neuroplasticity, Neuroregeneration, Neural stem cells, Early surgical decompression, Rehabilitation, Spinal interneurons
Introduction
Spinal cord injury (SCI) is a devastating condition which results in impaired sensory and motor function at and below the spinal level of injury. The incidence of traumatic SCI in North America is 39 cases per million individuals [1] and lifetime medical costs amount to up to $4.6 million USD per person, which is in addition to losses incurred from lost productivity. The highest proportion of traumatic SCIs in North America are a result of car accidents, with 60% of injuries occurring at the cervical level [1].
Despite the significant personal, societal, and economic burden of SCI, to date there are no established neuroprotective or neuroregenerative therapies. Though in recent years, advances in our understanding of endogenous plasticity and regeneration of the injured spinal cord have enhanced the armamentarium of tools and approaches aimed at improving recovery after SCI. This review will explore cutting edge strategies to enhance neural regeneration after SCI, with a focus on the role of spinal interneurons in plasticity, endogenous neural stem/progenitor cells (NSPCs), stem cell transplantation, combinatorial approaches, and cell reprogramming.
Pathophysiology of SCI
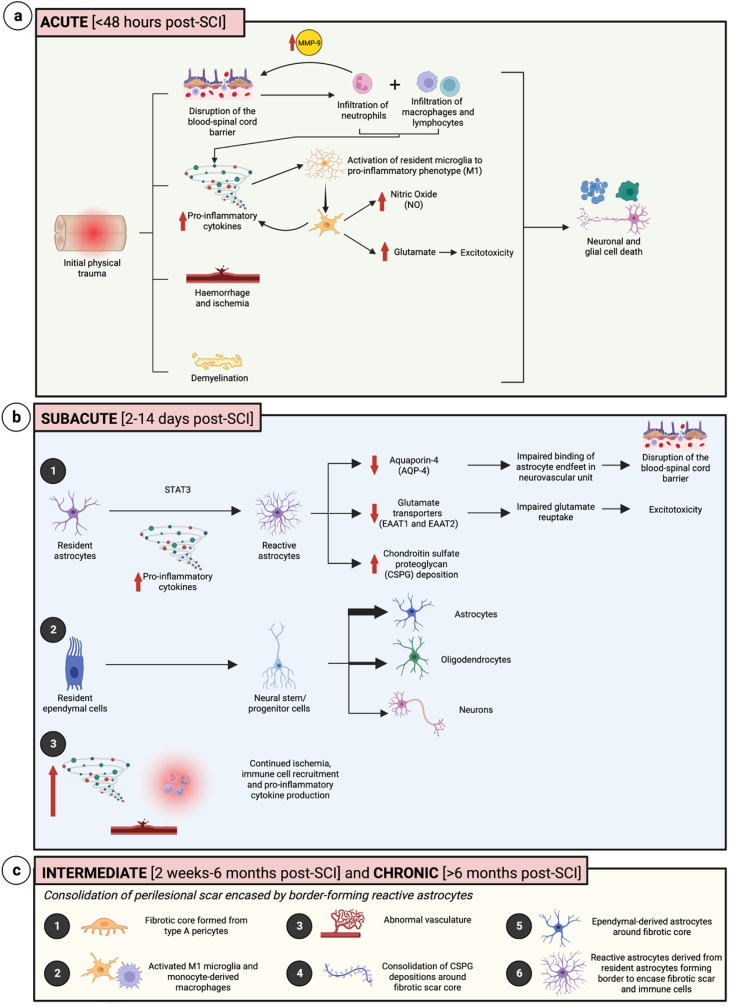
Following the initial physical damage associated with traumatic SCI, exacerbation of cell loss and inflammation persists as the result of secondary injury, preventing recovery of lost neural networks and impacting patient autonomy (Fig. 1).
Fig. 1.
Timeline of pathophysiological changes following spinal cord injury. (A) The acute phase of injury involves the initiation of inflammation, along with disruption of the blood-spinal cord barrier, hemorrhage and ischemia, demyelination, and activation of resident microglia to a pro-inflammatory phenotype (M1), resulting in nitric oxide production and glutamate excitotoxicity. (B) The subacute phase involves the STAT3 mediated activation of resident astrocytes to reactive astrocytes, further disrupting the blood-spinal cord barrier, dysregulating glutamate reuptake, and contributing to chondroitin sulfate proteoglycan (CSPG) deposition. There is also conversion of resident ependymal cells to neural stem/progenitor cells, which primarily differentiate into astrocytes, along with continued inflammation and ischemia. (C) The intermediate and chronic phases involve various changes to consolidate the type A pericyte-derived fibrotic scar core along with immune cells, CSPGs, abnormal vasculature, and ependymal-derived astrocytes, encased within a reactive astrocyte glial border. Created with BioRender.com.
Within the first 48 hours postinjury, known as the acute phase, several cellular processes occur including hemorrhage, demyelination, and necrosis, paired with disruption of the blood-spinal cord barrier (BSCB), leading to the initiation of the immune response [1,2]. This begins with the infiltration of neutrophils [3,4]. Neutrophils produce metalloproteinase 9 (MMP-9), which degrades the extracellular matrix, contributing to increased permeability of the BSCB and allowing for further infiltration of other immune cells including macrophages and lymphocytes [5]. There is a concurrent upregulation of pro-inflammatory cytokines, including tumor necrosis factor alpha (TNFα), interleukin-1 beta (IL–1β), and interleukin-6 (IL–6), which activate resident microglia biasing them towards a pro-inflammatory phenotype, often termed M1 microglia [2]. Increased activation of microglia results in the release of pro-inflammatory cytokines, as well as cytotoxic release of glutamate and nitric oxide to further impact cell survival [5,6].
The subacute phase, from 2 to 14 days postinjury [7], is characterized by increased proliferation of astrocytes paired with upregulation of glial fibrillary acidic protein (GFAP) and activation towards a reactive phenotype. These reactive astrocytes lose aquaporin-4 (AQP4) activity, further compromising the BSCB, as well as impaired glutamate re-uptake and border-forming activity around the injury site mediated by signal transducer and activator of transcription 3 (STAT3) [8,9]. Self-renewing ependymal cells, which are ciliated cells of the central canal that normally remain quiescent, become activated and migrate to the site of injury forming a portion of astrocytes in the center of the injury site [10]. There is also increased formation of chondroitin sulfate proteoglycans (CSPGs) primarily from resident astrocytes [1,8], along with continued ischemia [1].
In the intermediate and chronic phases of spinal cord injury, the perilesional scar is consolidated, with a fibrotic core formed from type A pericytes compacted by 14 days postinjury, and this prevents neural outgrowth and extensions. This fibrotic core is surrounded by activated endogenous microglia and monocyte-derived macrophages, CSPGs, abnormal vasculature, and contained by border-forming reactive astrocytes, to prevent the spread of immune cells beyond the lesion epicenter [1,8].
Although the injury microenvironment is often nonconducive to neural regeneration, modulation of the immune response has been shown to promote conversion of astrocytes and microglia to more neuroprotective phenotypes. Specific CD4+ myelin basic protein (MBP)-autoimmune T lymphocytes may participate in protective autoimmunity, helping activate microglia towards an anti-inflammatory phenotype [5,[11], [12], [13], denoted as M2 microglia. However, this classification is highly debated and may be further characterized by various spatial, environmental, and transcriptomic factors [2,[14], [15], [16]. These adaptive microglia allow for the reduction of pro-inflammatory cytokines such as TNFα, upregulation of neurotrophic factors like brain-derived neurotrophic factor (BDNF) and anti-inflammatory cytokines like interleukin-4 (IL–4) and interleukin-10 (IL–10), as well as buffering of excitatory glutamate production [2,3,11]. Following modulation of the immune response, reactive astrocytes may alter their phenotype to form bridges across the fibrotic scar to aid in guiding axonal growth [8].
Neuroplasticity after SCI
Adaptive and maladaptive plasticity of spinal cord interneurons following SCI
Neuronal circuits in the spinal cord are vital for the coordination and execution of sensory processing [17], sensorimotor control [18,19], and autonomic regulation [20]. These diverse functions are coordinated by heterogeneous interneurons assembled throughout the spinal cord. Interneuron subtypes form functionally autonomous circuits that both integrate and modulate descending supraspinal and ascending sensory commands. Together, this modular arrangement of spinal interneuron subtypes enables the dynamic and context-specific coordination of somatic and visceral outputs.
Traumatic SCI results in an abrupt silencing of descending and ascending communication between neuronal circuits caudal and rostral to the injury site. For interneuron networks caudal to the lesion, the silencing of supraspinal regulation results in a compensatory reorganization of sensory and interneuron circuits contributing to both adaptive and maladaptive neuroplastic changes. Importantly, these spinal interneuron reorganizations are not only dependent on the injury severity and postinjury period, but are also highly interneuron subtype dependent. That is, distinct spinal interneuron subtypes can display either adaptive or maladaptive changes while under the same injury and postinjury conditions (Table 1). Thus, defining and investigating spinal interneuron subtypes is crucial to understanding the mechanisms underlying spinal cord recovery and dysfunction after injury.
Table 1.
Spinal interneuron subtype plasticity following SCI.
| Interneuron subtype | Location to lesion | SCI model | Preinjury | Postinjury | References |
|---|---|---|---|---|---|
| Cervical descending propriospinal neurons | Rostral to lesion | Incomplete (hemisection) SCI | Coordination of locomotion, reaching and grasping | Form descending relay circuits around spinal cord lesion. | [18,[41], [42], [43], [44],47] |
| Excitatory thoracolumbar propriospinal neurons | Caudal to lesion | Complete thoracic SCI | ? | Activation improves hindlimb standing recovery. | [53] |
| Inhibitory thoracolumbar propriospinal neurons | Caudal to lesion | Complete thoracic SCI | ? | Inhibition improves hindlimb stepping recovery. | [53] |
| Ascending/Descending Interenlargement propriospinal neurons | Spared axons projecting through lesion | Incomplete thoracic SCI | Task-specific interlimb coordination | Inhibition improves functional locomotor recovery. | [51,[54], [55], [56], [57] |
| Spinocerebellar neurons | Caudal to lesion | Incomplete thoracic SCI | Locomotor rhythm generation via axon collateral outptus to lumbar circuits | Increased regenerative associated gene expressions. Increased lumbar axon sprouting. Unknown function in locomotor recovery. | [60,65] |
| V2a Interneurons | Caudal to lesion | Incomplete Thoracic SCI | Left-right hindlimb alternation at intermediate locomotor speed | Necessary for slow speed locomotor recovery mediated by treadmill rehabilitation and electrical epidural stimulation. Increased sensory innervation. Increased serotonin sensitivity. Neurotransmitter inhibitory phenotype switching of subset. | [58,59,69] |
| dI3 Interneurons | Caudal to lesion | Complete Thoracic SCI | Limited function in hindlimb locomotion | Necessary for spontaneous locomotor recovery. Neurotransmitter inhibitory phenotype switching of subset. | [58,68] |
| Shox2+ Interneurons | Caudal to lesion | Complete Thoracic SCI | Locomotor rhythm generation | Increased afferent evoked excitation. Increased serotonin sensitivity. Unknown function in locomotor recovery. | [64,152] |
| V3 Interneurons | Caudal to lesion | Complete Sacral (S2) SCI | Multiple roles in locomotor control: left-right extensor centre synchronization; forelimb-hindlimb coordination; motor neuron excitation. | Mediate sensory-evoked tail spasms | [55,75,[153], [154], [155]] |
Almost 25 years ago, pioneering works defined cardinal spinal cord interneuron classes by their embryonic progenitor domains of origin and unique postmitotic transcription factor expression profiles [21,22]. During early embryonic development, counteracting morphogen gradients organize dividing spinal cord cells into 11 discrete progenitor domains across the dorsoventral axis. These progenitor domains give rise to molecularly distinct postmitotic dorsal interneuron classes (dI1–dI6), ventral interneuron classes (V0–V3), and motor neurons. While cardinal interneuron classes display characteristic molecular, anatomical, and functional properties [23], they each further diversify into distinct subpopulations defined by increasingly refined molecular expressions and functional outputs [24,25]. By postnatal and adult stages, single cell sequencing experiments in rodent models have further revealed gene expression networks delineating excitatory and inhibitory spinal interneuron subtypes [26], [27], [28], [29], [30], [31], [32]. Several interneuron subtypes discovered in the adult have been linked to interneuron class origins [33]. However, further work is required to understand how embryonically defined interneuron classes translate into molecular interneuron clusters in the adult. Regardless, both the embryonic and adult molecular delineation of spinal interneurons has enabled the experimental targeting of distinct spinal circuits across uninjured and injured states.
Spinal interneuron subtype-specific circuit changes have been implicated in autonomic dysfunction [34,35], neuropathic pain [36], respiratory compensation [37], [38], [39], locomotor recovery (reviewed below), and sensory-evoked muscle spasms (reviewed below) following SCI. Investigations into the interneurons involved in locomotor control have particularly revealed key pathophysiologic principles underlying the adaptive and maladaptive spinal circuit changes postinjury. For the remainder of the current review, we will focus on post-SCI interneuron changes underlying locomotor recovery and dysfunction.
The role of propriospinal neurons connecting across spinal segments
Both human patients and animal models display varying degrees of spontaneous and therapeutically enhanced locomotor recoveries in the weeks to months following an incomplete SCI. However, full locomotor recovery is rarely obtained. These phenomena have prompted some of the key investigations into spinal circuit reorganizations following SCI.
Propriospinal neurons diffusely project through ventral and intermediate spinal white matter tracts connecting distant spinal cord segments. They often display a substantial degree of axonal sparing following incomplete SCI and have thus been extensively studied [40]. Beyond their well-established roles in bridging transected supraspinal commands to caudal spinal cord circuits, recent works have begun to reveal their heterogeneous functions in coordinating locomotor recovery following both complete and incomplete SCIs.
Descending propriospinal circuit reorganizations are essential for the recovery of supraspinal directed locomotion following incomplete SCI. Severed supraspinal axons can regain access to caudal locomotor circuits through the formation of interneuron relay circuits around an incomplete lesion [41], [42], [43], [44]. Following spinal cord injury, severed corticospinal axons sprout collaterals forming new synaptic connections onto interneuron networks rostral to the lesion. Immediately following an injury, corticospinal axon sprouting is unspecified to both local and distant projecting spinal interneurons. With subsequent spontaneous locomotor recovery, the corticospinal synaptic contacts are preferentially maintained on distant descending propriospinal neurons that project around the lesion [41]. Additionally, the descending propriospinal neurons bridging the injury site increase their synaptic innervations onto caudal motor neurons [41]. Thus, severed supraspinal axons and downstream descending propriospinal interneurons can form and maintain new circuit connections with caudal spinal networks following injury. Moreover, Courtine and colleagues (2008) demonstrated that the formation of propriospinal relay circuits was necessary for therapy-induced locomotor recovery following incomplete cervical SCI [43].
Severed corticospinal axons may also reorganize via axonal sprouting to locomotor nuclei in the medulla with postinjury rehabilitation and electrical epidural stimulation [45]. In turn, both spared [45] and transected [46] descending reticulospinal tracts are able to sprout axon collaterals to caudal and rostral spinal circuits, respectively. Thus, descending propriospinal neurons are vital for re-establishing corticospinal communication both directly through corticospinal-spinal relay circuits and indirectly through corticospinal-brainstem-spinal relay circuits.
Beyond relaying corticospinal commands to caudal locomotor circuits, descending propriospinal neurons coordinate locomotor outputs in both pre- and postinjury states [47]. They are distributed throughout the rostrocaudal spinal cord axis and are comprised of heterogeneous cardinal interneuron subtypes with distinct laminar distributions, neurotransmitter phenotypes, and projection profiles [48], [49], [50], [51], [52]. Post-SCI, descending propriospinal neurons display varied functional roles in coordinating locomotor recovery depending on their subtype identity and proximity to the spinal cord lesion.
Brommer and colleagues utilized a complete thoracic SCI model and investigated the role of descending propriospinal neurons caudal to the lesion. They showed that general activation of propriospinal neurons projecting from mid-thoracic to lumbar segments improved locomotor recovery post-SCI. However, they uncovered functional distinctions between excitatory and inhibitory subtypes for postinjury hindlimb recovery. Activation of excitatory propriospinal neurons improved the recovery of extension during standing. In contrast, inhibitory propriospinal neurons played a more significant role in the recovery of flexion during the swing phase of locomotor stepping. Furthermore, inhibition of inhibitory propriospinal neurons resulted in improved locomotor stepping post-SCI. Thus, while thoracic-lumbar propriospinal neurons caudal to a complete SCI site facilitate hindlimb recovery, distinct subtypes may be differentially targeted to preferentially facilitate the recovery of hindlimb stepping versus standing [53].
With incomplete SCI, there is often sparing of propriospinal neurons through the lesion site. This raises the important question of how propriospinal projections spared through a lesion site contribute to the recovery of locomotor coordination. Both ascending and descending interenlargement (lumbar and cervical) propriospinal neurons are necessary for task-specific interlimb coordination and locomotor stability in the uninjured state [51,54,55]. However, both long lumbar ascending [56] and cervical descending [57] propriospinal projections that are spared following incomplete thoracic SCI maladaptively inhibit locomotor recovery. In both cases, silencing of spared interenlargement propriospinal neurons resulted in improved locomotor recovery [56,57]. Thus, while long ascending and descending propriospinal neurons coordinate locomotor output in the uninjured state, their spared projections paradoxically hinder locomotor recovery in an incomplete SCI state. Together, these works suggest that a subset of functionally necessary long propriospinal neurons may form maladaptive circuit organizations post-SCI inhibiting functional locomotor recovery. Further, these works highlight the necessity of investigating spinal interneuron functions in both uninjured and injured models.
Adaptive lumbar interneuron plasticity
Lumbar sensorimotor circuits contained caudal to SCI sites undergo interneuron subtype specific alterations in molecular expression [58], [59], [60], intraspinal connectivity [59], [60], [61], [62], and sensory integration [59,63,64]. These postinjury interneuron changes often result in altered subtype specific functions. While some interneuron subtypes form circuit rearrangements that are integral for sensorimotor recovery, others undergo maladaptive alterations leading to pathophysiological phenotypes such as sensory-evoked muscle spasms.
A recent investigation demonstrated that while the relative proportions of molecularly defined spinal interneuron subtypes did not change caudal to the SCI lesion, select interneuron subtypes displayed altered gene expression patterns [60]. Expression of genes associated with axon regeneration, postsynaptic densities, and neurotransmitter receptors were distinctly elevated in Shox2+ V2d interneurons and ascending spinocerebellar neurons. Moreover, severed spinocerebellar neurons displayed significant axon collateral sprouting caudal to the injury site [60].
Recently, ventral spinocerebellar tract neurons were revealed to possess extensive axon collaterals innervating local lumbar interneurons and motor neurons in the uninjured state [65]. These spinocerebellar neurons were both sufficient and necessary for locomotor rhythm generation in the uninjured mouse spinal cord [65]. Together, these combined studies indicate spinocerebellar neurons as potential high yield therapeutic targets for locomotor recovery post-SCI. However, further work is required to determine the functional consequences of spinocerebellar circuit reorganizations following SCI.
Kathe and colleagues recently employed single cell sequencing experiments to uncover the interneuron subtypes mediating locomotor recovery induced by electrical epidural stimulation with rehabilitation training in an incomplete SCI mouse model. Locomotor recovery evoked by electrical epidural stimulation and rehabilitation results in an overall reduction in spinal neuronal activity in both human patients [59] and animal models [59,66]. This is likely owed to spinal circuit rearrangements favoring the recruitment of functionally relevant interneuron subtypes. Indeed, Hoxa10+ V2a interneurons displayed significantly increased recruitments with post-SCI therapeutic targeting [59]. Furthermore, their genetic ablation abolished therapy-induced locomotor recovery [59]. Thus, Hoxa10+ V2a interneurons are necessary for locomotor recovery induced by electrical epidural stimulation and rehabilitation. Interestingly, V2a interneurons are not required for low-speed walking in uninjured mice [59,67]. Furthermore, without post-SCI therapy, genes associated with regeneration were not expressed in V2a interneurons as they were in Shox2+ V2d and spinocerebellar neurons [60]. These results suggest that V2a interneurons adopt distinct post-SCI locomotor functions dependent on targeted electrical stimulation and task-specific rehabilitation.
In the absence of intensive postinjury therapies, dI3 interneurons are necessary for spontaneous locomotor recovery in the mouse [68]. Similar to V2a interneurons, dI3 interneurons exhibit a limited functional role for slow speed locomotion in the uninjured state [68]. Thus, these results again highlight the functional adaptations of spinal interneuron subtypes underlying spontaneous (dI3) and therapy mediated (V2a) locomotor recovery post-SCI. Though, it is likely that other spinal interneuron subtypes are also involved in locomotor recovery post-SCI and further studies are required.
Understanding how distinct spinal interneuron subtypes rearrange into postinjury circuits enabling or hindering locomotor recovery is critical for the development of novel therapeutic strategies. Neuronal recruitment is determined by synaptic inputs combined with intrinsic electrophysiological properties. Intracellular patch-clamp recordings revealed that while spinal interneuron subtypes maintain stable electrophysiological properties [64,69], they display increased serotonin sensitivities [64,69] and enhanced afferent-evoked excitatory drives [63,64].
Achieving optimal spinal afferent reorganization is key to enhancing locomotor recovery and preventing sensory-evoked muscle spasms post-SCI. Targeted and task-specific excitation of proprioceptive afferents is vital for adaptive spinal interneuron rearrangements and locomotor recovery. Electrical epidural stimulation over the dorsal spinal cord must cooperatively interact with muscle spindle feedback during locomotion to enhance recovery [70,71]. Similarly, locomotor recovery associated with rehabilitation training requires intact proprioceptive afferents [72]. In incomplete SCI, silencing proprioceptive afferents also results in reduced descending circuit rearrangements around the spinal lesion [72]. Thus, task-specific sensory afferent activation is likely a key mechanism reorganizing spinal interneuron subtypes into circuit assemblies necessary for locomotor recovery.
Maladaptive lumbar interneuron plasticity
In addition to adaptive lumbar spinal circuit rearrangements, distinct maladaptive spinal interneuron changes counteract functional sensorimotor recovery. Bertels and colleagues recently discovered that lumbar excitatory spinal interneuron subtypes undergo neurotransmitter phenotype switching to inhibitory interneurons post-SCI. Experimentally suppressing inhibitory phenotype switching significantly enhanced locomotor recovery [58]. Endogenous phenotype switching may therefore contribute to the inhibition of spinal circuits necessary for locomotor recovery. Interestingly, only select excitatory interneuron subtypes exhibited neurotransmitter switching. dI3, dI5, and V2a interneurons displayed significant inhibitory phenotype switching while V3 interneurons did not [58].
Sensorimotor dysfunction caudal to SCI sites can also manifest as hyper excitation of sensory reflexes, resulting in uncontrolled muscle spasms. In addition to synaptic inhibition dysfunction [73] and motor neuron serotonin receptor dysregulation [74], excitatory interneuron circuit rearrangements are involved in both initiating and maintaining sensory-evoked tail spasms in the chronic SCI mouse model [63]. Interestingly, optogenetic stimulation of excitatory V3 interneurons produces characteristic sensory-evoked muscle spasm output patterns in the chronic SCI mouse model [75]. Additionally, optogenetic silencing of V3 interneurons resulted in diminished spasms. Thus, excitatory V3 interneurons likely play a key role in mediating sensory evoked muscle spasms post-SCI.
Taken together, these recent investigations have revealed several important principles of interneuron circuit plasticity following SCI. First, spinal interneuron reorganizations are subtype specific and may be adaptive or maladaptive for a given functional output. Second, spinal interneuron subtypes may adopt new functional roles post-SCI that were not evident in the uninjured spinal cord. The interneuron circuits involved in specific functional recoveries post-SCI may not necessarily be inferred from studying uninjured models alone. Third, task-specific therapeutic targeting can significantly enhance adaptive spinal interneuron reorganizations and functional improvements post-SCI. These findings underscore the importance of interneuron subtype diversity in the reorganization of spinal cord circuits postinjury. Going forward, it will be essential to further identify the key adaptive and maladaptive interneuron subtype-specific circuit changes to properly target and effectively treat SCI.
Promoting regeneration after SCI
Endogenous stem cells
Following SCI there is extensive neuronal as well as glial cell death, and thus cellular replacement strategies are critical for recovering lost neural networks. In the healthy spinal cord, most of the progenitor population of proliferating cells comes from oligodendrocyte progenitor cells (OPCs), which have unipotent potential to differentiate and replace oligodendrocytes [76,77]. Compared to the brain, which has well established neurogenic regions such as the hippocampal dentate gyrus and the sub ventricular zone [2,76], the spinal cord is often characterized as a non-neurogenic region, with limited NSPC potential [77].
Most of the NSPC potential in the spinal cord comes from radial and tanycyte ependymal cells within the central canal, with the dorsal pole of the ependymal zone thought to contain the majority of NSPCs [78]. During SCI, normally self-renewing ependymal cells have been shown to demonstrate NPSC multipotent potential, with downregulation of ependymal marker FoxJ1, and upregulation of stem/progenitor cell markers such as nestin, CD113, and prominin [76,[79], [80], [81]. Activating transcription factor 3 (ATF3) is a novel nuclear marker expressed by migrating NSPCs from the central canal to the surrounding parenchyma [78].
These ependymal-derived NSPCs have been shown to be able to differentiate in vitro into neurons, oligodendrocytes, and astrocytes [79]. For in vivo SCI models, however, the differentiation is primarily glial-biased, with a fraction forming Olig2+ oligodendrocytes and the majority forming astrocytes positive for SOX9 and vimentin [76,79]. These astrocytes are functionally distinct from resident border-forming reactive astrocytes, typically lacking increased GFAP expression and production of CSPGs, and contributing to the center of the scar following injury [77,79,81]. Sabelström et al. demonstrated that impairing NSPC proliferation through inducible knockout of Ras resulted in secondary expansion of the lesion site [81].
Work by Yamamato et al. [82] demonstrated that there may be a small population of nonependymal-derived NSPCs in the periventricular area, along with medial and lateral regions of the cord, however, most studies to date demonstrate NSPC potential to be restricted to ependymal cells, confirmed through genetic cell fate mapping [79].
Ohori et al. [83] demonstrated that genetic manipulation through retroviral administration of transcription factors Neurogenin2 (NGN2) and Mash1 can bias endogenous NSPC differentiation towards neuron or oligodendrocyte lineages, respectively. Furthermore, use of fibroblast growth factor 2 (FGF2) and epidermal growth factor (EGF) can promote expression of immature neuronal markers including HuC/D and TuJ1 [83]. Further in vitro studies confirm the role of EGF and basic fibroblast growth factor (bFGF) in proliferation and differentiation of endogenous NSPCs into neurons, astrocytes, and oligodendrocytes [84].
A clinical trial (NCT04054414) testing the safety of the drug Sovateltide (PMZ-1620), an endothelin B (ETB) receptor agonist, showed enhanced endogenous progenitor cell activity, improved neurogenesis and angiogenesis, and reduced apoptosis [85]. Strategies to activate these endogenous NSPCs to promote neuron-specific differentiation would provide an important noninvasive regeneration strategy following SCI.
Modification of IPSC-derived NPCs prior to transplantation in the injured spinal cord
Induced pluripotent stem cells (IPSCs), which can be made from easily accessible autologous somatic cells, have strong translational potential for transplantation after SCI [86]. Ideal transplantation candidates are tripotent neural progenitor cells (NPCs) that can be developed from IPSCs via dual SMAD inhibition [87].
Several studies have examined biasing human IPSC-derived NPCs prior to transplantation in the injured spinal cord to enhance motor recovery as well as to improve the integration of transplanted cells with spared endogenous circuitry. Upon transplantation, NPCs form a higher proportion of astrocytes due to the inflammatory SCI environment [88]. Biasing these cells prior to transplantation aims to maintain tripotency while also enhancing the generation of neurons and/or oligodendrocytes.
Biasing NPCs to an oligodendrocytic fate prior to transplantation in the injured cervical spinal cord demonstrated an earlier and significant restoration of motor function 7 weeks postinjury with improved myelination as compared to the transplantation of NPCs alone [89]. Oligodendrocyte biasing of NPCs involved small molecule treatments using a caudalizing agent, a ventralizing agent and platelet-derived growth factor.
The transplantation of GDNF-expressing NPCs promoted a more neuronal fate following transplantation into the injured cervical spinal cord [90]. The expression of GDNF counteracted the elevated Notch-1 signaling found in the SCI microenvironment, which promoted a pro-astrocytic fate in transplanted NPCs. Neuronal biasing of GDNF-expressing NPCs promoted the sparing of endogenous tissue, improved the electrical integration of the transplanted cells and enhanced motor recovery.
Biasing NPCs prior to transplantation combines the improved survival of NPCs in the inflammatory injury environment with an enriched capacity for electrical integration (neuronal biasing) and/or improved myelination (oligodendrocytic biasing), both of which can lead to improved motor recovery and sparing of endogenous tissue. Several new protocols exist to develop specific neuronal populations through the small molecule treatment of NPCs, such as glutamatergic V2a interneurons [91], motor neurons [92] and dorsal sensory interneurons [93].
Biasing NPCs towards a specific neuronal fate, such as an interneuron or motor neuron, prior to transplantation is a promising therapeutic strategy. The immature progenitors more readily survive in the injured spinal cord upon transplantation and would be biased to produce an enriched population of specific neurons involved in motor function that will mature within the injured spinal cord to form connections with spared endogenous circuitry.
Combinatorial approaches employing scaffolds and stem cells
The injury microenvironment with scar formation, reactive astrocyte border formation, inflammatory cell infiltration, and neurotoxic compounds, is often not conducive to cellular regeneration and survival. When transplanting NSCs, the disrupted extracellular matrix (ECM) and perineuronal networks (PNNs) needed for cell grafting are not available, thus NSCs are not retained at the injury site. Research is examining the use of scaffolds transplanted along with NSCs to provide a temporary ECM for newly generated neurons and glial cells, until the PNNs are regenerated. Key advances in preclinical and clinical studies are highlighted in this section.
The INSPIRE trials (NCT02138110; NCT03762655) are examining the safety and efficacy of a bioresorbable polymer of the Neuro-Spinal Scaffold, consisting of poly(lactic-co-glycolic acid)-b-poly(L-lysine) (PLGA-PLL) in traumatic acute thoracic SCI. Preclinical work transplanting the PLGA with NSCs in rat [94] and monkey [95] models demonstrated preliminary safety and improvements in locomotion, as well as reduced scar formation. Pilot data [96], along with 6-month [97] and 24-month [98] follow-ups from the INSPIRE clinical trials, demonstrate improvements in American Spinal Cord Injury (ASIA) impairment scores (AIS), with no serious side effects [99].
A set of clinical trials (NCT02688049, NCT02510365, NCT02352077) are examining the use of a collagen-based scaffold (NeuroRegen) in combination with either umbilical cord mesemchymal stem cells (hUCB-MSCs) or autologous bone marrow mononuclear cells (BMMCs) in chronic and acute SCI [99]. In vitro studies with linearly-ordered collagen scaffolds (LOCs) demonstrate its potential for guiding neurite outgrowth [100]. No adverse side effects were observed and there were sensory and AIS motor improvements [101,102] with hUCB-MSC transplantation, but no motor improvements with BMMCs [103]. Preclinical in vitro and rat models have assessed the NeuroRegen scaffold bound with epidermal growth factor receptor (EGFR) antibody to allow for binding of NSCs, as well as reduced EGFR signalling to promote neuronal differentiation [104].
Preclinical animal and in vitro models are examining additional biomaterial scaffolds. Self-assembling peptides, such as K2 (QL)6K2 (QL6), form a 3D nanomatrix upon injection into the injury cavity. QL6 is a multidomain protein, which when transplanted with NPCs, helped increase neuronal differentiation, reduced cystic cavitation's and inflammation, and improved functional recovery [105], [106], [107], [108].
In addition to helping cell grafting, biomaterials can also be used to provide localized growth factors for improved cell survival and recovery at the injury site. A fast-gelling, noncell adhering hydrogel polymer of hyaluronin (HA) and methylcellulose (MC) [HAMC] has been tested to be nonimmunogenic, localized to the injury epicenter, and biocompatible [105,109]. Modification of HAMC with platelet-derived growth factor-A (rPDGF-A) to target NSPC differentiation to oligodendrocytes allowed for improved locomotion and reduced lesion size compared to NSPCs alone [110]. HAMC can be further modified by encapsulating growth factors in poly(lactic-co-glycolic acid) (PLGA) nanoparticles for extended, slow release, to provide continual support to neurons [111].
Cell reprogramming strategies
Astrocytes are important support glial cells present in the CNS. After injury, there is upregulation of astrocyte production from both ependymal-derived NSPCs and resident astrocytes that form components of the glial scar [8,77,79,81]. Recent work has examined strategies to directly reprogram these somatic terminally differentiated astrocytes directly into neurons through direct cell reprogramming or transdifferentiation.
Direct cell reprogramming can be conducted using a number of different techniques. Small molecule or pharmacological reprogramming has been used by Ma et al. [112] employing agents such as forskolin with support from bFGF to form functional glutamatergic and GABAergic neurons capable of action potentials. The most common method, however, is the overexpression of select transcription factors via lentiviral vectors [113,114]. The first report of transdifferentiation of astrocytes to neurons was conducted by Heins et al., using retroviral expression of Paired box protein Pax6 (Pax6) [115,116]. A few research groups have examined the impact of ectopic expression of Sry-related HMG-box 2 (Sox2) [2,114,[117], [118], [119]. Su et al. found that Sox2 expression using a human GFAP promotor targeted-lentiviral delivery resulted in the formation of doublecortin positive (DCX+) neuroblasts, confirmed to be of astrocyte origin using genetic lineage tracing. Further modification with the histone deacetylase inhibitor valproic acid (VPA) helped these neuroblasts differentiate into GABAergic interneurons that were able to form functional connections with spinal motor neurons, with survival for up to 30 weeks postinjury. Mature neuron formation, observed 8 weeks postinjury, was confirmed with expression of MAP2 and NeuN [117]. Wang et al. [118] confirmed the use of Sox2 expression to form neuroblasts and later VGUT2+ excitatory glutamatergic interneurons. The p53-p21 pathway was identified to be involved in controlling Sox2 mediated transdifferentiation, with downregulation of either p53 or p21 increasing the number of neuroblasts and neurons obtained. Furthermore, supplementing with BDNF and noggin (NOG) improves maturation to glutamatergic neurons. Yang et al. [119] combined Sox2 expression with running wheel rehabilitation and found improved motor recovery postinjury.
Ectopic expression of other transcription factors has proved successful in the transdifferentiation of astrocytes to neurons using lentiviral or adenoviral vectors. Zarei-Kheirabadi et al. demonstrated more efficient reprogramming with zinc finger protein 521 (Zfp521) in comparison to Sox2, with motor neuron-evoked potentials observed and improvements in motor function [120,121]. Neurogenin2 (Ngn2) converted astrocytes specifically into glutamatergic and GABAergic neurons with induced neurons able to respond to inputs from the dorsal root ganglion [122]. Neurod1 transformed astrocytes to functional glutamatergic neurons with 95% efficiency that integrated into spinal networks, with distal-free homolog2 (Dlx2) administration biasing differentiation to GABAergic neurons [123]. Ghazale et al. mutated proneural gene Asc11 by mutating 6 serine prospho-acceptor sites (Asc11SA6), which was found to enhance reprogramming and downregulate astrocytic markers such as Sox9 and GFAP [124].
Overexpression of transcription factors often does not have sufficient reprogramming efficiency, and there remain concerns with regards to the integration of viral vectors into host cell DNA and clinical translation [113,125]. CRISPR/Cas9 can be used to modulate transcription factors [113,125]. Zhou et al. used CRISPRa to activate expression of Ngn2 and Islet-1 (Isl1) in vitro and in vivo. ROCK inhibitor Y-27632 administration allowed for improved motor neuronal maturation and branching to the sciatic nerves [120,125]. dCas9 is a mutant nuclease deactivated form of Cas9 that can be used to either augment or decrease gene expression without disruption of the DNA strands [113]. Russo et al. found that early dCas9-mediated activation of neuronal mitochondrial proteins aids to enhance reprogramming [126].
Additional therapies for spinal cord injury
In addition to the cellular-based strategies highlighted earlier in this review, several additional management approaches and noncellular therapies have emerged for the treatment of SCI. These include early surgical decompression, hemodynamic management, targeted rehabilitation, along with small molecule and drug therapies that may further target the secondary injury of SCI, creating an environment conducive for neural growth and repair (Table 2).
Table 2.
Summary of regenerative strategies for spinal cord injury.
| Cellular strategies | |
|---|---|
| Endogenous stem cells |
|
| IPSC-derived NPCs |
|
| Direct cell reprogramming |
|
| Scaffolds and stem cells |
|
| Noncellular strategies | |
| Early surgery |
|
| Hemodynamic regulation |
|
| Targeted rehabilitation and functional electrical stimulation |
|
| Riluzole |
|
| Anti-Nogo-A Antibody |
|
| Anti-RGMa Antibody |
|
Early surgical decompression
With the initiation of secondary injury cascades occurring within the first 24 hours after the physical insult associated with SCI, providing early treatment options is key to enhancing preservation of tissue and cell survival. Early surgical decompression aims to reduce compression on the cord to improve blood flow [127]. The Surgical Timing in Acute Spinal Cord Injury Study (STASCIS) found that early decompressive surgery (<24 hours) resulted in 19.8% of patients observing an increase in AIS scores by 2 grades, with minimal differences in complications between early and late decompression surgery groups [128,129]. Clinical Practice guidelines released in 2017 by AO Spine and the American Association of Neurological Surgeons/Congress of Neurological Surgeons (AANS/ CNS), based on a systematic review of the literature, suggest early decompressive surgery within 24 hours for acute SCI as a treatment option with weak evidence [129]. Further evidence has emerged supporting the role of early surgery in improving outcomes after SCI [130]. Earlier time frames for decompressive surgery have also been considered (eg, 8–12 hours postinjury), however, the evidence base is lacking [127].
Hemodynamic management
Hypoxic-ischemic injury is a major component of the secondary injury post-SCI. Injury to blood vessels impairs spinal cord perfusion, which is further compounded by hypotension, vasoconstriction and free radical formation. Maintaining spinal cord perfusion is critical for mitigating the deleterious effects of ischemia on the cord. As such, current guidelines recommend a MAP of 85 and 90 mm Hg be maintained for 7 days post-SCI [131]. Spinal cord perfusion pressure (SCPP) has also emerged as a potentially more accurate predictor of outcomes as compared to MAP. It is recommended to maintain SCPP above 50 mm Hg [132] in the acute injury period. Typically, maintenance of these parameters requires the use of vasopressors in the acute injury period, which may present additional complications. As such, further research into the hemodynamic management of SCI is critical for developing evidence-based guidelines that best enhance recovery.
Targeted rehabilitation and electrical stimulation
Rehabilitation has become standard of care in the management of SCI with the aim of improving patient independence and activities of daily living. Targeted rehabilitation strategies offer goal-oriented motor therapies with the potential to further improve recovery [133]. Recently, preclinical studies have demonstrated that rehabilitation can augment the integration of grafted stem cells into the lessoned spinal cord and improve functional recovery [134].
Rehabilitation strategies combined with electrical stimulation paradigms have further shown promise in promoting recovery after SCI. Functional electrical stimulation provides electrical currents to nerves and muscles to enhance activity. Epidural spinal cord stimulation applies rhythmic electrical currents to the spinal cord to activate locomotor circuits along with circuits underlying pain and cardiorespiratory systems [135]. Exoskeletons are additional devices that serve as motorized orthoses that can facilitate locomotion through either hand/mouth-controlled devices or through detection of micro-movements.
Emerging noncellular regenerative strategies
Several small molecule and pharmacological therapies have been assessed in preclinical and clinical trials for SCI, which are highlighted in this section.
Riluzole
Riluzole is a benzothiazole anticonvulsant neuroprotective drug that has been shown to block sodium channels, thereby helping to reduce glutamate-associated excitotoxicity [99,136,137]. Satkunendrarah et al. demonstrated that following administration of riluzole in a rodent cervical hemisection model, there was improved preservation of glutamatergic synapses and motor neurons caudal to the injury, with improvements in locomotion and respiratory activity [138]. Multiple clinical trials have assessed the use of riluzole as a therapeutic following SCI. A phase I trial (NCT00876889) testing the safety and pharmacokinetics of riluzole in acute traumatic SCI found variable levels of peak plasma concentration with no serious effects from riluzole, although some patients did observe mild-moderate increases in liver enzymes [136,[139], [140], [141]. Greater improvements in mean motor scores were also seen in the riluzole-treated group [141]. A phase IIB/III clinical trial (NCT01597518), termed the Riluzole in Spinal Cord Injury Study (RISCIS), aimed to examine improvements in the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) motor scores, although it was terminated due to challenges with enrollment [99,136,142]. A further study aims to examine improvements in spasticity following administration of riluzole in chronic SCI, although the status of this trial is unknown (NCT02859792).
Anti-Nogo-A antibody
Nogo-A is a myelin protein that contains an extracellular domain involved in growth-cone collapse [99,143,144]. This is mediated in part through the activation of the Rho-ROCK pathway upon binding to the Nogo receptor [99,145]. Preclinical work by Freund et al. demonstrated improvements in axonal sprouting and dexterity following cervical-level lesions in a macaque model [99,146,147]. The first human pilot intrathecal administration of anti-Nogo-A antibody (ATI355) found some improvements in motor scores and overall good tolerability of the antibody in acute SCI [99,148]. The RESET (NCT03989440) and Nogo Inhibition in Spinal Cord Injury (NISCI; NCT03935321) clinical trials are currently testing administration of anti-Nogo antibodies in chronic and acute SCI, respectively [99]. Administration of antibodies for Nogo may allow for improved axonal growth and inhibition of the Rho-ROCK pathway, thus enhancing neural regeneration post-SCI.
Anti-RGMa antibody
Repulsive guidance molecule A (RGMa) binds to the Neogenin receptor, activating the RhoA-Rho kinase pathway, and resulting in the inhibition of axon growth [99,149,150]. RGMa is expressed by oligodendrocytes as well as neurons and is upregulated at the lesion epicenter and in myelinated regions following SCI [149,150]. Antibodies targeting RGMa have been shown to help improve locomotion, neuron survival, and axon regeneration in vitro and in rodent SCI models [99,149,150], along with ameliorate dexterity in rhesus monkeys [151]. Elezanumab (ABT-555) is a human anti-RGMa monoclonal antibody being tested for safety, efficacy, and upper extremity motor improvements for acute SCI in the ELASCI trial (NCT04295538), along with intravenous expanded access being approved (NCT04278235) [99].
Conclusions
This review summarizes the key advances in the field of neural regeneration and neuroplasticity. Regenerative medicine shows promise to help enhance tissue regeneration following SCI, with various cellular and noncellular strategies being tested in the preclinical phase and through clinical trials. There will be ongoing work as researchers further optimize these strategies and establish standardized personalized treatment options. Furthermore, assessing combinatorial regenerative approaches with current standard of care guidelines will be important to target various aspects of secondary injury in order to improve sensorimotor functional recovery and enhance patient quality of life following SCI.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
MGF would like to acknowledge support from the Robert Campeau Family Foundation/Dr. C.H. Tator Chair in Brain and Spinal Cord Research at UHN.
Footnotes
FDA device/drug status: Not applicable.
Author disclosures: NP: Nothing to disclose. DDG: Nothing to disclose. LDH: Nothing to disclose. MA: Nothing to disclose. MGF: Nothing to disclose.
References
- 1.Ahuja CS, Wilson JR, Nori S, et al. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3:1–21. doi: 10.1038/nrdp.2017.18. [DOI] [PubMed] [Google Scholar]
- 2.Rodríguez-Barrera R, Rivas-González M, García-Sánchez J, Mojica-Torres D, Ibarra A. Neurogenesis after spinal cord injury: State of the Art. Cells. 2021;10:1499. doi: 10.3390/cells10061499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hellenbrand DJ, Quinn CM, Piper ZJ, Morehouse CN, Fixel JA, Hanna AS. Inflammation after spinal cord injury: A review of the critical timeline of signaling cues and cellular infiltration. J Neuroinflamm. 2021;18:284. doi: 10.1186/s12974-021-02337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tzekou A, Fehlings MG. Treatment of spinal cord injury with intravenous immunoglobulin g: preliminary evidence and future perspectives. J Clin Immunol. 2014;34:132–138. doi: 10.1007/s10875-014-0021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammopharmacol. 2007;15:252–259. doi: 10.1007/s10787-007-0013-x. [DOI] [PubMed] [Google Scholar]
- 7.Ahuja CS, Nori S, Tetreault L, et al. Traumatic spinal cord injury—repair and regeneration. Neurosurgery. 2017;80:S9. doi: 10.1093/neuros/nyw080. [DOI] [PubMed] [Google Scholar]
- 8.Chio JCT, Punjani N, Hejrati N, Zavvarian M-M, Hong J, Fehlings MG. Extracellular matrix and oxidative stress following traumatic spinal cord injury: Physiological and pathophysiological roles and opportunities for therapeutic intervention. Antioxid Redox Signal. 2022;37:184–207. doi: 10.1089/ars.2021.0120. [DOI] [PubMed] [Google Scholar]
- 9.Sofroniew MV. Astrocyte reactivity: Subtypes, states, and functions in CNS innate immunity. Trends Immunol. 2020;41:758–770. doi: 10.1016/j.it.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gazdic M, Volarevic V, Harrell CR, et al. Stem cells therapy for spinal cord injury. Int J Mole Sci. 2018;19:1039. doi: 10.3390/ijms19041039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crutcher KA, Gendelman HE, Kipnis J, et al. Debate: “Is Increasing Neuroinflammation Beneficial for Neural Repair? Jrnl Neuroimmune Pharm. 2006;1:195–211. doi: 10.1007/s11481-006-9021-7. [DOI] [PubMed] [Google Scholar]
- 12.Moalem G, Leibowitz–Amit R, Yoles E, Mor F, Cohen IR, Schwartz M. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- 13.Yoles E, Hauben E, Palgi O, et al. Protective autoimmunity is a physiological response to CNS trauma. J Neurosci. 2001;21:3740–3748. doi: 10.1523/JNEUROSCI.21-11-03740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jurga AM, Paleczna M, Kuter KZ. Overview of general and discriminating markers of differential microglia phenotypes. Front Cell Neurosci. 2020;14:198. doi: 10.3389/fncel.2020.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paolicelli RC, Sierra A, Stevens B, et al. Microglia states and nomenclature: A field at its crossroads. Neuron. 2022;110:3458–3483. doi: 10.1016/j.neuron.2022.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koch SC, Acton D, Goulding M. Spinal circuits for touch, pain, and itch. Annu Rev Physiol. 2018;80:189–217. doi: 10.1146/annurev-physiol-022516-034303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alstermark B, Isa T. Circuits for skilled reaching and grasping. Annu Rev Neurosci. 2012;35:559–578. doi: 10.1146/annurev-neuro-062111-150527. [DOI] [PubMed] [Google Scholar]
- 19.Kiehn O. Decoding the organization of spinal circuits that control locomotion. Nat Rev Neurosci. 2016;17:224–238. doi: 10.1038/nrn.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karnup S. Spinal interneurons of the lower urinary tract circuits. Auton Neurosci. 2021;235 doi: 10.1016/j.autneu.2021.102861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 22.Lu DC, Niu T, Alaynick WA. Molecular and cellular development of spinal cord locomotor circuitry. Front Mol Neurosci. 2015;8:25. doi: 10.3389/fnmol.2015.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci. 2009;10:507–518. doi: 10.1038/nrn2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gosgnach S, Bikoff JB, Dougherty KJ, El Manira A, Lanuza GM, Zhang Y. Delineating the diversity of spinal interneurons in locomotor circuits. J Neurosci. 2017;37:10835–10841. doi: 10.1523/JNEUROSCI.1829-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deska-Gauthier D, Zhang Y. The functional diversity of spinal interneurons and locomotor control. Curr Opin Physiol. 2019;8:99–108. doi: 10.1016/j.cophys.2019.01.005. [DOI] [Google Scholar]
- 26.Baek M, Menon V, Jessell TM, Hantman AW, Dasen JS. Molecular logic of spinocerebellar tract neuron diversity and connectivity. Cell Rep. 2019;27:2620–2635. doi: 10.1016/j.celrep.2019.04.113. .e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Häring M, Zeisel A, Hochgerner H, et al. Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types. Nat Neurosci. 2018;21:869–880. doi: 10.1038/s41593-018-0141-1. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi M, Hinckley CA, Driscoll SP, et al. Graded arrays of spinal and Supraspinal V2a interneuron subtypes underlie forelimb and hindlimb motor control. Neuron. 2018;97:869–884. doi: 10.1016/j.neuron.2018.01.023. .e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osseward PJ, Amin ND, Moore JD, et al. Conserved genetic signatures parcellate cardinal spinal neuron classes into local and projection subsets. Science. 2021;372:385–393. doi: 10.1126/science.abe0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenberg AB, Roco CM, Muscat RA, et al. Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science. 2018;360:176–182. doi: 10.1126/science.aam8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sathyamurthy A, Johnson KR, Matson KJE, et al. Massively parallel single nucleus transcriptional profiling defines spinal cord neurons and their activity during behavior. Cell Rep. 2018;22:2216–2225. doi: 10.1016/j.celrep.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeisel A, Hochgerner H, Lönnerberg P, et al. Molecular architecture of the mouse nervous system. Cell. 2018;174:999–1014. doi: 10.1016/j.cell.2018.06.021. .e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russ DE, Cross RBP, Li L, et al. A harmonized atlas of mouse spinal cord cell types and their spatial organization. Nat Commun. 2021;12:5722. doi: 10.1038/s41467-021-25125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noble BT, Brennan FH, Wang Y, et al. Thoracic VGluT2+ Spinal interneurons regulate structural and functional plasticity of sympathetic networks after high-level spinal cord injury. J Neurosci. 2022;42:3659–3675. doi: 10.1523/JNEUROSCI.2134-21.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueno M, Ueno-Nakamura Y, Niehaus J, Popovich PG, Yoshida Y. Silencing spinal interneurons inhibits immune suppressive autonomic reflexes caused by spinal cord injury. Nat Neurosci. 2016;19:784–787. doi: 10.1038/nn.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kramer JLK, Minhas NK, Jutzeler CR, Erskine ELKS, Liu LJW, Ramer MS. Neuropathic pain following traumatic spinal cord injury: Models, measurement, and mechanisms. J Neurosci Res. 2017;95:1295–1306. doi: 10.1002/jnr.23881. [DOI] [PubMed] [Google Scholar]
- 37.Cregg JM, Chu KA, Hager LE, et al. A latent propriospinal network can restore diaphragm function after high cervical spinal cord injury. Cell Rep. 2017;21:654–665. doi: 10.1016/j.celrep.2017.09.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satkunendrarajah K, Karadimas SK, Laliberte AM, Montandon G, Fehlings MG. Cervical excitatory neurons sustain breathing after spinal cord injury. Nature. 2018;562:419–422. doi: 10.1038/s41586-018-0595-z. [DOI] [PubMed] [Google Scholar]
- 39.Zholudeva LV, Karliner JS, Dougherty KJ, Lane MA. Anatomical recruitment of spinal V2a interneurons into phrenic motor circuitry after high cervical spinal cord injury. J Neurotrauma. 2017;34:3058–3065. doi: 10.1089/neu.2017.5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flynn JR, Graham BA, Galea MP, Callister RJ. The role of propriospinal interneurons in recovery from spinal cord injury. Neuropharmacology. 2011;60:809–822. doi: 10.1016/j.neuropharm.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 41.Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- 42.Chen B, Li Y, Yu B, et al. Reactivation of dormant relay pathways in injured spinal cord by KCC2 manipulations. Cell. 2018;174:1599. doi: 10.1016/j.cell.2018.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Courtine G, Song B, Roy RR, et al. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cowley KC, MacNeil BJ, Chopek JW, Sutherland S, Schmidt BJ. Neurochemical excitation of thoracic propriospinal neurons improves hindlimb stepping in adult rats with spinal cord lesions. Exp Neurol. 2015;264:174–187. doi: 10.1016/j.expneurol.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 45.Asboth L, Friedli L, Beauparlant J, et al. Cortico-reticulo-spinal circuit reorganization enables functional recovery after severe spinal cord contusion. Nat Neurosci. 2018;21:576–588. doi: 10.1038/s41593-018-0093-5. [DOI] [PubMed] [Google Scholar]
- 46.May Z, Fenrich KK, Dahlby J, Batty NJ, Torres-Espín A, Fouad K. Following spinal cord injury transected reticulospinal tract axons develop new collateral inputs to spinal interneurons in parallel with locomotor recovery. Neural Plast. 2017;2017 doi: 10.1155/2017/1932875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laliberte AM, Goltash S, Lalonde NR, Bui TV. Propriospinal neurons: Essential elements of locomotor control in the intact and possibly the injured spinal cord. Front Cell Neurosci. 2019;13:512. doi: 10.3389/fncel.2019.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flynn JR, Conn VL, Boyle KA, et al. Anatomical and molecular properties of long descending propriospinal neurons in mice. Front Neuroanat. 2017;11:5. doi: 10.3389/fnana.2017.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ni Y, Nawabi H, Liu X, et al. Characterization of long descending premotor propriospinal neurons in the spinal cord. J Neurosci. 2014;34:9404–9417. doi: 10.1523/JNEUROSCI.1771-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pivetta C, Esposito MS, Sigrist M, Arber S. Motor-circuit communication matrix from spinal cord to brainstem neurons revealed by developmental origin. Cell. 2014;156:537–548. doi: 10.1016/j.cell.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 51.Ruder L, Takeoka A, Arber S. Long-distance descending spinal neurons ensure quadrupedal locomotor stability. Neuron. 2016;92:1063–1078. doi: 10.1016/j.neuron.2016.10.032. [DOI] [PubMed] [Google Scholar]
- 52.Saywell SA, Ford TW, Meehan CF, Todd AJ, Kirkwood PA. Electrophysiological and morphological characterization of propriospinal interneurons in the thoracic spinal cord. J Neurophysiol. 2011;105:806–826. doi: 10.1152/jn.00738.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brommer B, He M, Zhang Z, et al. Improving hindlimb locomotor function by Non-invasive AAV-mediated manipulations of propriospinal neurons in mice with complete spinal cord injury. Nat Commun. 2021;12:781. doi: 10.1038/s41467-021-20980-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pocratsky AM, Shepard CT, Morehouse JR, et al. Long ascending propriospinal neurons provide flexible, context-specific control of interlimb coordination. Elife. 2020;9:e53565. doi: 10.7554/eLife.53565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang H, Shevtsova NA, Deska-Gauthier D, et al. The role of V3 neurons in speed-dependent interlimb coordination during locomotion in mice. Elife. 2022;11:e73424. doi: 10.7554/eLife.73424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shepard CT, Pocratsky AM, Brown BL, et al. Silencing long ascending propriospinal neurons after spinal cord injury improves hindlimb stepping in the adult rat. Elife. 2021;10:e70058. doi: 10.7554/eLife.70058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shepard CT, Brown BL, Rijswijck MAV, et al. Silencing long-descending inter-enlargement propriospinal neurons improves hindlimb stepping after contusive spinal cord. injuries. 2022 doi: 10.1101/2022.08.30.505848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bertels H, Vicente-Ortiz G, El Kanbi K, Takeoka A. Neurotransmitter phenotype switching by spinal excitatory interneurons regulates locomotor recovery after spinal cord injury. Nat Neurosci. 2022;25:617–629. doi: 10.1038/s41593-022-01067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kathe C, Skinnider MA, Hutson TH, et al. The neurons that restore walking after paralysis. Nature. 2022;611:540–547. doi: 10.1038/s41586-022-05385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matson KJE, Russ DE, Kathe C, et al. Single cell atlas of spinal cord injury in mice reveals a pro-regenerative signature in spinocerebellar neurons. Nat Commun. 2022;13:5628. doi: 10.1038/s41467-022-33184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rank MM, Flynn JR, Battistuzzo CR, Galea MP, Callister R, Callister RJ. Functional changes in deep dorsal horn interneurons following spinal cord injury are enhanced with different durations of exercise training. J Physiol. 2015;593:331–345. doi: 10.1113/jphysiol.2014.282640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Skup M, Gajewska-Wozniak O, Grygielewicz P, Mankovskaya T, Czarkowska-Bauch J. Different effects of spinalization and locomotor training of spinal animals on cholinergic innervation of the soleus and tibialis anterior motoneurons. Eur J Neurosci. 2012;36:2679–2688. doi: 10.1111/j.1460-9568.2012.08182.x. [DOI] [PubMed] [Google Scholar]
- 63.Bellardita C, Caggiano V, Leiras R, et al. Spatiotemporal correlation of spinal network dynamics underlying spasms in chronic spinalized mice. ELife n.d. 2023;6:e23011. doi: 10.7554/eLife.23011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garcia-Ramirez DL, Ha NTB, Bibu S, Stachowski NJ, Dougherty KJ. Spinal cord injury alters spinal Shox2 interneurons by enhancing excitatory synaptic input and serotonergic modulation while maintaining intrinsic properties in mouse. J Neurosci. 2021;41:5833–5848. doi: 10.1523/JNEUROSCI.1576-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chalif JI, Martínez-Silva M de L, Pagiazitis JG, Murray AJ, Mentis GZ. Control of mammalian locomotion by ventral spinocerebellar tract neurons. Cell. 2022;185:328–344. doi: 10.1016/j.cell.2021.12.014. .e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ichiyama RM, Courtine G, Gerasimenko YP, et al. Step training reinforces specific spinal locomotor circuitry in adult spinal rats. J Neurosci. 2008;28:7370–7375. doi: 10.1523/JNEUROSCI.1881-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crone SA, Zhong G, Harris-Warrick R, In Sharma K. Mice lacking V2a interneurons, gait depends on speed of locomotion. J Neurosci. 2009;29:7098–7109. doi: 10.1523/JNEUROSCI.1206-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bui TV, Stifani N, Akay T, Brownstone RM. Spinal microcircuits comprising dI3 interneurons are necessary for motor functional recovery following spinal cord transection. ELife. 2016;5:e21715. doi: 10.7554/eLife.21715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Husch A, Van Patten GN, Hong DN, Scaperotti MM, Cramer N, Harris-Warrick RM. Spinal cord injury induces serotonin supersensitivity without increasing intrinsic excitability of mouse V2a interneurons. J Neurosci. 2012;32:13145–13154. doi: 10.1523/JNEUROSCI.2995-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moraud EM, Capogrosso M, Formento E, et al. Mechanisms underlying the neuromodulation of spinal circuits for correcting gait and balance deficits after spinal cord injury. Neuron. 2016;89:814–828. doi: 10.1016/j.neuron.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 71.Formento E, Minassian K, Wagner F, et al. Electrical spinal cord stimulation must preserve proprioception to enable locomotion in humans with spinal cord injury. Nat Neurosci. 2018;21:1728–1741. doi: 10.1038/s41593-018-0262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takeoka A, Vollenweider I, Courtine G, Arber S. Muscle spindle feedback directs locomotor recovery and circuit reorganization after spinal cord injury. Cell. 2014;159:1626–1639. doi: 10.1016/j.cell.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 73.Boulenguez P, Liabeuf S, Bos R, et al. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat Med. 2010;16:302–307. doi: 10.1038/nm.2107. [DOI] [PubMed] [Google Scholar]
- 74.Murray KC, Nakae A, Stephens MJ, et al. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat Med. 2010;16:694–700. doi: 10.1038/nm.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin S, Li Y, Lucas-Osma AM, et al. Locomotor-related V3 interneurons initiate and coordinate muscles spasms after spinal cord injury. J Neurophysiol. 2019;121:1352–1367. doi: 10.1152/jn.00776.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barnabé-Heider F, Göritz C, Sabelström H, et al. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell. 2010;7:470–482. doi: 10.1016/j.stem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 77.Stenudd M, Sabelström H, Frisén J. Role of endogenous neural stem cells in spinal cord injury and repair. JAMA Neurology. 2015;72:235–237. doi: 10.1001/jamaneurol.2014.2927. [DOI] [PubMed] [Google Scholar]
- 78.Hachem LD, Mothe AJ, Tator CH. Unlocking the paradoxical endogenous stem cell response after spinal cord injury. Stem Cells. 2020;38:187–194. doi: 10.1002/stem.3107. [DOI] [PubMed] [Google Scholar]
- 79.Meletis K, Barnabé-Heider F, Carlén M, et al. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008;6:e182. doi: 10.1371/journal.pbio.0060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mothe AJ, Tator CH. Proliferation, migration, and differentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord injury in the adult rat. Neuroscience. 2005;131:177–187. doi: 10.1016/j.neuroscience.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 81.Sabelström H, Stenudd M, Réu P, et al. Resident neural stem cells restrict tissue damage and neuronal loss after spinal cord injury in mice. Science. 2013;342:637–640. doi: 10.1126/science.1242576. [DOI] [PubMed] [Google Scholar]
- 82.Yamamoto S, Yamamoto N, Kitamura T, Nakamura K, Nakafuku M. Proliferation of parenchymal neural progenitors in response to injury in the adult rat spinal cord. Experimental Neurology. 2001;172:115–127. doi: 10.1006/exnr.2001.7798. [DOI] [PubMed] [Google Scholar]
- 83.Ohori Y, Yamamoto S, Nagao M, et al. Growth factor treatment and genetic manipulation stimulate neurogenesis and oligodendrogenesis by endogenous neural progenitors in the injured adult spinal cord. J Neurosci. 2006;26:11948–11960. doi: 10.1523/JNEUROSCI.3127-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weiss S, Dunne C, Hewson J, et al. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16:7599–7609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Briyal S, Ranjan AK, Hornick MG, Puppala AK, Luu T, Gulati A. Anti-apoptotic activity of ETB receptor agonist, IRL-1620, protects neural cells in rats with cerebral ischemia. Sci Rep. 2019;9:10439. doi: 10.1038/s41598-019-46203-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khazaei M, Ahuja CS, Fehlings MG. Induced pluripotent stem cells for traumatic spinal cord injury. Front Cell Dev Biol. 2017;4:152. doi: 10.3389/fcell.2016.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McIntyre WB, Pieczonka K, Khazaei M, Fehlings MG. Regenerative replacement of neural cells for treatment of spinal cord injury. Expert Opin Biol Ther. 2021;21:1411–1427. doi: 10.1080/14712598.2021.1914582. [DOI] [PubMed] [Google Scholar]
- 89.Nagoshi N, Khazaei M, Ahlfors J-E, et al. Human spinal oligodendrogenic neural progenitor cells promote functional recovery after spinal cord injury by axonal remyelination and tissue sparing. Stem Cells Transl Med. 2018;7:806–818. doi: 10.1002/sctm.17-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khazaei M, Ahuja CS, Nakashima H, et al. GDNF rescues the fate of neural progenitor grafts by attenuating Notch signals in the injured spinal cord in rodents. Sci Transl Med. 2020;12:eaau3538. doi: 10.1126/scitranslmed.aau3538. [DOI] [PubMed] [Google Scholar]
- 91.Butts JC, Iyer N, White N, Thompson R, Sakiyama-Elbert S, McDevitt TC. V2a interneuron differentiation from mouse and human pluripotent stem cells. Nat Protoc. 2019;14:3033–3058. doi: 10.1038/s41596-019-0203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thiry L, Hamel R, Pluchino S, Durcan T, Stifani S. Characterization of human iPSC-derived spinal motor neurons by single-cell RNA sequencing. Neuroscience. 2020;450:57–70. doi: 10.1016/j.neuroscience.2020.04.041. [DOI] [PubMed] [Google Scholar]
- 93.Gupta S, Sivalingam D, Hain S, et al. Deriving dorsal spinal sensory interneurons from human pluripotent stem cells. Stem Cell Reports. 2018;10:390–405. doi: 10.1016/j.stemcr.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Teng YD, Lavik EB, Qu X, et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci U S A. 2002;99:3024–3029. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pritchard CD, Slotkin JR, Yu D, et al. Establishing a model spinal cord injury in the African green monkey for the preclinical evaluation of biodegradable polymer scaffolds seeded with human neural stem cells. J Neurosci Methods. 2010;188:258–269. doi: 10.1016/j.jneumeth.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Theodore N, Hlubek R, Danielson J, et al. First human implantation of a bioresorbable polymer scaffold for acute traumatic spinal cord injury: A clinical pilot study for safety and feasibility. Neurosurgery. 2016;79:E305. doi: 10.1227/NEU.0000000000001283. [DOI] [PubMed] [Google Scholar]
- 97.Kim KD, Lee KS, Coric D, et al. A study of probable benefit of a bioresorbable polymer scaffold for safety and neurological recovery in patients with complete thoracic spinal cord injury: 6-month results from the INSPIRE study. J Neurosurg Spine. 2021;34:1–10. doi: 10.3171/2020.8.SPINE191507. [DOI] [PubMed] [Google Scholar]
- 98.Kim KD, Lee KS, Coric D, Harrop JS, Theodore N, Toselli RM. Acute implantation of a bioresorbable polymer scaffold in patients with complete thoracic spinal cord injury: 24-month follow-up from the INSPIRE study. Neurosurgery. 2022;90:668–675. doi: 10.1227/neu.0000000000001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Senthilnathan V, Punjani N, Nagoshi N, Ahuja CS, Fehlings MG. In: Neural repair and regeneration after spinal cord injury and spine trauma. Fehlings MG, Kwon BK, Vaccaro AR, Oner FC, editors. Academic Press (Cambridge, Massachusetts); 2022. Chapter 26 - Clinical trials: Noncellular regenerative approaches; pp. 473–500. [DOI] [Google Scholar]
- 100.Lin H, Chen B, Wang B, Zhao Y, Sun W, Dai J. Novel nerve guidance material prepared from bovine aponeurosis. J Biomed Materi Res A. 2006;79:591–598. doi: 10.1002/jbm.a.30862. [DOI] [PubMed] [Google Scholar]
- 101.Xiao Z, Tang F, Zhao Y, et al. Significant improvement of acute complete spinal cord injury patients diagnosed by a combined criteria implanted with neuroregen scaffolds and mesenchymal stem cells. Cell Transplant. 2018;27:907–915. doi: 10.1177/0963689718766279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhao Y, Tang F, Xiao Z, et al. Clinical study of NeuroRegen scaffold combined with human mesenchymal stem cells for the repair of chronic complete spinal cord injury. Cell Transplant. 2017;26:891–900. doi: 10.3727/096368917X695038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen W, Zhang Y, Yang S, et al. NeuroRegen scaffolds combined with autologous bone marrow mononuclear cells for the repair of acute complete spinal cord injury: A 3-year clinical study. Cell Transplant. 2020;29 doi: 10.1177/0963689720950637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu B, Zhao Y, Xiao Z, et al. A dual functional scaffold tethered with EGFR antibody promotes neural stem cell retention and neuronal differentiation for spinal cord injury repair. Adv Healthc Mater. 2017;6 doi: 10.1002/adhm.201601279. [DOI] [PubMed] [Google Scholar]
- 105.Ahuja CS, Mothe A, Khazaei M, et al. The leading edge: Emerging neuroprotective and neuroregenerative cell-based therapies for spinal cord injury. Stem Cells Transl Med. 2020;9:1509–1530. doi: 10.1002/sctm.19-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iwasaki M, Wilcox JT, Nishimura Y, et al. Synergistic effects of self-assembling peptide and neural stem/progenitor cells to promote tissue repair and forelimb functional recovery in cervical spinal cord injury. Biomaterials. 2014;35:2617–2629. doi: 10.1016/j.biomaterials.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 107.Zweckberger K, Liu Y, Wang J, Forgione N, Fehlings MG. Synergetic use of neural precursor cells and self-assembling peptides in experimental cervical spinal cord injury. J Vis Exp. 2015 doi: 10.3791/52105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zweckberger K, Ahuja CS, Liu Y, Wang J, Fehlings MG. Self-assembling peptides optimize the post-traumatic milieu and synergistically enhance the effects of neural stem cell therapy after cervical spinal cord injury. Acta Biomaterialia. 2016;42:77–89. doi: 10.1016/j.actbio.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 109.Gupta D, Tator CH, Shoichet MS. Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord. Biomaterials. 2006;27:2370–2379. doi: 10.1016/j.biomaterials.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 110.Mothe AJ, Tam RY, Zahir T, Tator CH, Shoichet MS. Repair of the injured spinal cord by transplantation of neural stem cells in a hyaluronan-based hydrogel. Biomaterials. 2013;34:3775–3783. doi: 10.1016/j.biomaterials.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 111.Ho MT, Teal CJ, Shoichet MS. A hyaluronan/methylcellulose-based hydrogel for local cell and biomolecule delivery to the central nervous system. Brain Res Bull. 2019;148:46–54. doi: 10.1016/j.brainresbull.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 112.Ma Y, Xie H, Du X, et al. In vivo chemical reprogramming of astrocytes into neurons. Cell Discov. 2021;7:1–13. doi: 10.1038/s41421-021-00243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Grath A, Dai G. Direct cell reprogramming for tissue engineering and regenerative medicine. J Biol Engineer. 2019;13:14. doi: 10.1186/s13036-019-0144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tai W, Xu X-M, Zhang C-L. Regeneration through in vivo cell fate reprogramming for neural repair. Front Cell Neurosci. 2020;14:107. doi: 10.3389/fncel.2020.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bajohr J, Faiz M. In: Cell biology and translational medicine, Volume 6: Stem Cells: Their Heterogeneity, Niche and Regenerative Potential. Turksen K, editor. Springer International Publishing; Cham: 2020. Direct lineage reprogramming in the CNS; pp. 31–48. editor. [DOI] [Google Scholar]
- 116.Heins N, Malatesta P, Cecconi F, et al. Glial cells generate neurons: the role of the transcription factor Pax6. Nat Neurosci. 2002;5:308–315. doi: 10.1038/nn828. [DOI] [PubMed] [Google Scholar]
- 117.Su Z, Niu W, Liu M-L, Zou Y, Zhang C-L. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat Commun. 2014;5:3338. doi: 10.1038/ncomms4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang L-L, Su Z, Tai W, Zou Y, Xu X-M, Zhang C-L. The p53 pathway controls SOX2-mediated reprogramming in the mouse adult spinal cord. Cell Rep. 2016;17:891–903. doi: 10.1016/j.celrep.2016.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang T, Xing L, Yu W, Cai Y, Cui S, Chen G. Astrocytic reprogramming combined with rehabilitation strategy improves recovery from spinal cord injury. The FASEB Journal. 2020;34:15504–15515. doi: 10.1096/fj.202001657RR. [DOI] [PubMed] [Google Scholar]
- 120.Yang R, Pan J, Wang Y, et al. Application and prospects of somatic cell reprogramming technology for spinal cord injury treatment. Front Cell Neurosci. 2022;16 doi: 10.3389/fncel.2022.1005399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zarei-Kheirabadi M, Hesaraki M, Kiani S, Baharvand H. In vivo conversion of rat astrocytes into neuronal cells through neural stem cells in injured spinal cord with a single zinc-finger transcription factor. Stem Cell Res Ther. 2019;10:380. doi: 10.1186/s13287-019-1448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu F, Zhang Y, Chen F, et al. Neurog2 directly converts astrocytes into functional neurons in midbrain and spinal cord. Cell Death Dis. 2021;12:225. doi: 10.1038/s41419-021-03498-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Puls B, Ding Y, Zhang F, et al. Regeneration of functional neurons after spinal cord injury via in situ NeuroD1-Mediated Astrocyte-to-Neuron conversion. Front Cell Dev Biol. 2020;8 doi: 10.3389/fcell.2020.591883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ghazale H, Park E, Vasan L, et al. Ascl1 phospho-site mutations enhance neuronal conversion of adult cortical astrocytes in vivo. Front Neurosci. 2022;16 doi: 10.3389/fnins.2022.917071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhou M, Tao X, Sui M, et al. Reprogramming astrocytes to motor neurons by activation of endogenous Ngn2 and Isl1. Stem Cell Reports. 2021;16:1777–1791. doi: 10.1016/j.stemcr.2021.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Russo GL, Sonsalla G, Natarajan P, et al. CRISPR-mediated induction of neuron-enriched mitochondrial proteins boosts direct glia-to-neuron conversion. Cell Stem Cell. 2021;28:524–534. doi: 10.1016/j.stem.2020.10.015. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ahuja CS, Badhiwala JH, Fehlings MG. “Time is spine”: the importance of early intervention for traumatic spinal cord injury. Spinal Cord. 2020;58:1037–1039. doi: 10.1038/s41393-020-0477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fehlings MG, Vaccaro A, Wilson JR, et al. Early versus delayed decompression for traumatic cervical spinal cord injury: Results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS) PLOS ONE. 2012;7:e32037. doi: 10.1371/journal.pone.0032037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fehlings MG, Tetreault LA, Wilson JR, et al. A clinical practice guideline for the management of patients with acute spinal cord injury and central cord syndrome: recommendations on the timing (≤24 Hours Versus >24 Hours) of decompressive surgery. Global Spine J. 2017;7:195S–202S. doi: 10.1177/2192568217706367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Badhiwala JH, Wilson JR, Witiw CD, et al. The influence of timing of surgical decompression for acute spinal cord injury: a pooled analysis of individual patient data. Lancet Neurol. 2021;20:117–126. doi: 10.1016/S1474-4422(20)30406-3. [DOI] [PubMed] [Google Scholar]
- 131.Ryken TC, Hurlbert RJ, Hadley MN, et al. The acute cardiopulmonary management of patients with cervical spinal cord injuries. Neurosurgery. 2013;72(Suppl 2):84–92. doi: 10.1227/NEU.0b013e318276ee16. [DOI] [PubMed] [Google Scholar]
- 132.Squair JW, Bélanger LM, Tsang A, et al. Spinal cord perfusion pressure predicts neurologic recovery in acute spinal cord injury. Neurology. 2017;89:1660–1667. doi: 10.1212/WNL.0000000000004519. [DOI] [PubMed] [Google Scholar]
- 133.Lorusso M, Tagliamonte NL, Tramontano M, et al. Technology-assisted balance assessment and rehabilitation in individuals with spinal cord injury: A systematic review. NeuroRehabilitation. 2022;51:213–230. doi: 10.3233/NRE-220060. [DOI] [PubMed] [Google Scholar]
- 134.Lu P, Freria CM, Graham L, et al. Rehabilitation combined with neural progenitor cell grafts enables functional recovery in chronic spinal cord injury. JCI Insight. 2022;7 doi: 10.1172/jci.insight.158000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hachem LD, Ahuja CS, Fehlings MG. Assessment and management of acute spinal cord injury: From point of injury to rehabilitation. J Spinal Cord Med. 2017;40:665–675. doi: 10.1080/10790268.2017.1329076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nagoshi N, Nakashima H, Fehlings MG. Riluzole as a neuroprotective drug for spinal cord injury: From bench to bedside. Molecules. 2015;20:7775–7789. doi: 10.3390/molecules20057775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wilson JR, Fehlings MG. Riluzole for acute traumatic spinal cord injury: A promising neuroprotective treatment strategy. World Neurosurg. 2014;81:825–829. doi: 10.1016/j.wneu.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 138.Satkunendrarajah K, Nassiri F, Karadimas SK, Lip A, Yao G, Fehlings MG. Riluzole promotes motor and respiratory recovery associated with enhanced neuronal survival and function following high cervical spinal hemisection. Exp Neurol. 2016;276:59–71. doi: 10.1016/j.expneurol.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 139.Chow DSL, Teng Y, Toups EG, et al. Pharmacology of Riluzole in acute spinal cord injury. J Neurosurg Spine. 2012;17:129–140. doi: 10.3171/2012.5.AOSPINE12112. [DOI] [PubMed] [Google Scholar]
- 140.Fehlings MG, Wilson JR, Frankowski RF, et al. Riluzole for the treatment of acute traumatic spinal cord injury: rationale for and design of the NACTN Phase I clinical trial. J Neurosurg Spine. 2012;17:151–156. doi: 10.3171/2012.4.AOSPINE1259. [DOI] [PubMed] [Google Scholar]
- 141.Grossman RG, Fehlings MG, Frankowski RF, et al. A prospective, multicenter, phase I matched-comparison group trial of safety, pharmacokinetics, and preliminary efficacy of riluzole in patients with traumatic spinal cord injury. J Neurotrauma. 2014;31:239–255. doi: 10.1089/neu.2013.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fehlings MG, Nakashima H, Nagoshi N, Chow DSL, Grossman RG, Kopjar B. Rationale, design and critical end points for the Riluzole in Acute Spinal Cord Injury Study (RISCIS): A randomized, double-blinded, placebo-controlled parallel multi-center trial. Spinal Cord. 2016;54:8–15. doi: 10.1038/sc.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen MS, Huber AB, van der Haar ME, et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- 144.Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- 145.Liu J, Gao H, Wang X. The role of the Rho/ROCK signaling pathway in inhibiting axonal regeneration in the central nervous system. Neural Regen Res. 2015;10:1892. doi: 10.4103/1673-5374.170325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Freund P, Schmidlin E, Wannier T, et al. Nogo-A–specific antibody treatment enhances sprouting and functional recovery after cervical lesion in adult primates. Nat Med. 2006;12:790–792. doi: 10.1038/nm1436. [DOI] [PubMed] [Google Scholar]
- 147.Freund P, Wannier T, Schmidlin E, et al. Anti-Nogo-A antibody treatment enhances sprouting of corticospinal axons rostral to a unilateral cervical spinal cord lesion in adult macaque monkey. J Comp Neurol. 2007;502:644–659. doi: 10.1002/cne.21321. [DOI] [PubMed] [Google Scholar]
- 148.Kucher K, Johns D, Maier D, et al. First-in-man intrathecal application of neurite growth-promoting Anti-Nogo-A antibodies in acute spinal cord injury. Neurorehabil Neural Repair. 2018;32:578–589. doi: 10.1177/1545968318776371. [DOI] [PubMed] [Google Scholar]
- 149.Hata K, Fujitani M, Yasuda Y, et al. RGMa inhibition promotes axonal growth and recovery after spinal cord injury. J Cell Biol. 2006;173:47–58. doi: 10.1083/jcb.200508143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mothe AJ, Tassew NG, Shabanzadeh AP, et al. RGMa inhibition with human monoclonal antibodies promotes regeneration, plasticity and repair, and attenuates neuropathic pain after spinal cord injury. Sci Rep. 2017;7:10529. doi: 10.1038/s41598-017-10987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nakagawa H, Ninomiya T, Yamashita T, Takada M. Treatment with the neutralizing antibody against repulsive guidance molecule-a promotes recovery from impaired manual dexterity in a primate model of spinal cord injury. Cerebral Cortex. 2019;29:561–572. doi: 10.1093/cercor/bhx338. [DOI] [PubMed] [Google Scholar]
- 152.Dougherty KJ, Zagoraiou L, Satoh D, et al. Locomotor rhythm generation linked to the output of spinal shox2 excitatory interneurons. Neuron. 2013;80:920–933. doi: 10.1016/j.neuron.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 153.Chopek JW, Nascimento F, Beato M, Brownstone RM, Zhang Y. Sub-populations of Spinal V3 Interneurons Form Focal Modules of Layered Pre-motor Microcircuits. Cell Rep. 2018;25:146–156. doi: 10.1016/j.celrep.2018.08.095. .e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Danner SM, Zhang H, Shevtsova NA, et al. Spinal V3 Interneurons and left-right coordination in mammalian locomotion. Front Cell Neurosci. 2019;13:516. doi: 10.3389/fncel.2019.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang Y, Narayan S, Geiman E, et al. V3 spinal neurons establish a robust and balanced locomotor rhythm during walking. Neuron. 2008;60:84–96. doi: 10.1016/j.neuron.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]