Abstract
Positive allometry of signalling traits has often been taken as evidence for sexual selection. However, few studies have explored interspecific differences in allometric scaling relationships among closely related species that vary in their degree of ecological similarity. Anolis lizards possess an elaborate retractable throat fan called a dewlap that is used for visual communication and differs greatly in size and colour among species. We observed that Anolis dewlaps demonstrate positive allometry: relative dewlap size increases with body size. We also observed that coexisting species are divergent in signal size allometries, while convergent species—similar in other aspects of ecology, morphology and behaviour—typically share similar dewlap allometric scaling relationships. These patterns suggest that dewlap scaling relationships may follow the same pattern as other traits in the anole radiation, where ecologically different sympatric species have evolved a suite of divergent traits.
Keywords: allometry, scaling, signal, sexual selection, dewlap, Anolis
1. Introduction
Visual signals represent some of the most elaborate biological features on Earth. From peacock tail feathers to fiddler crab claws, signalling traits have evolved to convey a range of information during visual communication [1–3]. Such traits often exhibit positive static allometry [4], whereby signal size is disproportionately larger with increasing body size [5]. As signalling traits have often been considered to influence reproductive fitness [4], positive allometric scaling of signal size has often been taken as evidence for sexual selection, although this has attracted some debate [4,6]. However, most studies have only explored allometric scaling in single species [5]; comparative studies that explore interspecific differences in signal size allometry among closely related species are rare (although see [7]).
In lizards, extendable throat fans called dewlaps have independently evolved in multiple lineages as a signal for visual communication [8,9]. The most speciose clade is Anolis lizards (anoles), in which sexual dimorphism in dewlap size is typical and male anoles usually possess relatively larger dewlaps than females [10]. However, evidence for positive allometry is mixed and comparative analyses are rare [11–14].
Anoles have independently radiated into convergent communities comprised of species specialized to use different portions of the structural environment (‘ecomorphs’; [15]) on each of the four large islands of the Greater Antilles: Cuba, Hispaniola, Jamaica and Puerto Rico [16]. Co-occurring species of different ecomorph classes rarely have dewlaps comprised of similar colours or patterns [14], which suggests a role for dewlaps as signals for species recognition ([17–19], but see [20]). However, members of the same ecomorph class, though convergent in ecology, morphology and behaviour, rarely are convergent in dewlap phenotype [14,20]. Comparative studies of dewlap size allometry, among either convergent species of the same ecomorph class or co-occurring species of different ecomorph classes, have been surprisingly overlooked.
Here, we explore three questions: (1) is there positive allometric scaling of dewlap size in Anolis lizards? (2) Do co-occurring species differ in signal size allometry? And (3) do convergent species have similar signal size allometry?
2. Methods
(a) . Species sampling and field collection
Between 6 April–3 June and 2 November–16 December 2019, we sampled male Anolis lizards (table 1) in three communities: Jamaica (Long Mountain, Kingston), the Dominican Republic (Barahona province) and the Bahamas (Andros Island). In all communities no two species are of the same ecomorph class, and members of the same ecomorph class in different communities are not closely related (with the exception of A. distichus and A. brevirostris).
Table 1.
Allometric relationships between dewlap size and body length in 10 Anolis lizard species. Slopes greater than 1 represent positive allometry where signal size (dewlap area) is disproportionately larger with increasing body size; p-values reflect a statistical difference of b to isometry (i.e. 1).
| ecomorph | species | country | location | N | slope (b) | s.e. | R2 | p-value |
|---|---|---|---|---|---|---|---|---|
| trunk | A. brevirostris | Dom. Rep. | Barahona | 118 | 1.108 | 0.149 | 0.324 | 0.469 |
| trunk | A. distichus | Bahamas | Andros Island | 47 | 1.255 | 0.264 | 0.335 | 0.338 |
| trunk–crown | A. grahami | Jamaica | Long Mountain | 103 | 1.453 | 0.094 | 0.703 | <0.001 |
| trunk–crown | A. coelestinus | Dom. Rep. | Barahona | 39 | 1.486 | 0.078 | 0.907 | <0.001 |
| trunk–crown | A. smaragdinus | Bahamas | Andros Island | 32 | 1.787 | 0.249 | 0.632 | 0.004 |
| twig | A. angusticeps | Bahamas | Andros Island | 52 | 1.800 | 0.290 | 0.436 | 0.008 |
| twig | A. valencienni | Jamaica | Long Mountain | 53 | 1.337 | 0.078 | 0.853 | <0.001 |
| trunk–ground | A. cybotes | Dom. Rep. | Barahona | 73 | 1.771 | 0.106 | 0.797 | <0.001 |
| trunk–ground | A. sagrei | Bahamas | Andros Island | 89 | 2.316 | 0.083 | 0.900 | <0.001 |
| trunk–ground | A. lineatopus | Jamaica | Long Mountain | 261 | 2.426 | 0.064 | 0.849 | <0.001 |
(b) . Measurement of dewlap size
Dewlap size (total area) was calculated from digital photographs obtained in the field. Dewlaps were extended via pulling of the hyoid bone using a pair of small forceps (see electronic supplementary material, figure S1) and digital photographs were taken (Canon EOS Rebel T5 with X-Rite ColorChecker Passport for scale). We used a digital drawing tablet (Wacom Intuos Pro) to manually trace dewlaps in ImageJ [21] (all by B.K.). Body size (snout-to-vent length; SVL) was measured in the field using digital calipers (Mahr EWRi digital caliper; all by J.T.S.).
(c) . Statistical analyses
Allometry describes the size of morphological traits relative to each other, most typically to body size. Specifically, allometric slopes (b) can be described using the equation Y = aXb, where Y in our study represents dewlap size and X represents body size. Dewlap size (area) was first linearized with respect to body size (SVL) by square-root transformation and then both variables were natural log transformed [5,14]. Ordinary least squares regressions were used to determine allometric scaling relationships [5,14]. Isometry (b = 1) describes a relationship where relative trait size is constant with increasing body size. Positive allometry (b > 1) represents increasing relative trait size with body size; negative allometry (b < 1) describes the opposite. We used t-tests to investigate whether allometric slopes differed statistically from isometry. To test for divergence in static allometries (i.e. intraspecific relationships between variation in dewlap and body size) between co-occurring species in different ecomorph classes and convergence in static allometries between non-coexisting species of the same ecomorph class, we included a dewlap size × species interaction in independent multiple regression models for each community and ecomorph class. All analyses were conducted in R [22] using RStudio [23].
3. Results
(a) . Static allometry of dewlap size
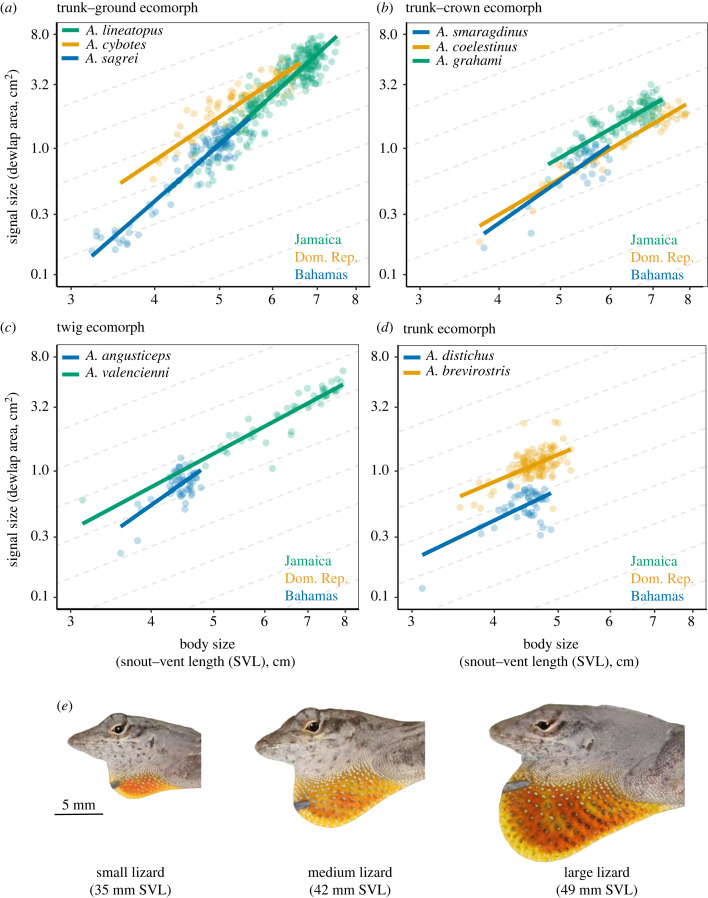
We sampled the dewlap sizes of 867 lizards. All species exhibited positive allometric scaling of dewlap size relative to body size (i.e. b > 1). Trunk–ground anoles exhibited the strongest allometry, while trunk anoles exhibited the weakest (and did not differ statistically from isometry; table 1, figure 1).
Figure 1.
(a–d) Allometric scaling patterns of dewlap size in Anolis lizard ecomorphs. Positive allometry occurs when slopes are steeper than isometric dashed lines (i.e. b = 2, as dewlap area data presented here are not linearized). (e) Typical dewlap size progression with body size in Anolis sagrei.
(b) . Comparative analyses of dewlap size allometry
In all communities, co-occurring species exhibited different allometries of dewlap size (table 2). Between communities, species of the same ecomorph class typically exhibited similar allometries (table 2). Trunk–ground anoles were an exception: the allometric slope of A. cybotes was lower than that of both A. sagrei (F1,158 = 60.8, p < 0.001) and A. lineatopus (F1,158 = 29.9, p < 0.001). The removal of small-bodied outliers in both A. distichus and A. valencienni did not change overall results (see electronic supplementary material).
Table 2.
Differences in allometric scaling of dewlap size in (a) co-occurring species of different ecomorph classes and (b) convergent but allopatric species of the same ecomorph class.
| sum sq. | d.f. | F | p | |
|---|---|---|---|---|
| (a) ecomorph differences within a community | ||||
| Jamaica × ecomorph | 1.37 | 2,411 | 67.05 | <0.001*** |
| Bahamas × ecomorph | 0.24 | 3,212 | 7.15 | <0.001*** |
| Dom. Rep. × ecomorph | 0.14 | 2,224 | 7.07 | 0.001** |
| (b) ecomorph similarities between communities | ||||
| trunk × species | <0.01 | 1,161 | 0.28 | 0.595 |
| trunk–ground × species | 0.25 | 2,411 | 11.12 | <0.001*** |
| (A. cybotes excluded) | 0.01 | 1,343 | 0.90 | 0.344 |
| trunk–crown × species | 0.02 | 2,168 | 1.26 | 0.285 |
| twig × species | 0.03 | 1,106 | 2.64 | 0.107 |
4. Discussion
All Anolis lizards in our study exhibited positive allometry in dewlap size (table 1 and figure 1). Specifically, we observed that dewlap allometry is very similar for allopatric species of the same ecomorph class (figure 1 and table 2b), but that co-occurring species—those of different ecomorph classes—vary significantly in dewlap size allometry (table 2a).
As positive signal allometry is often taken as evidence for sexual selection [4], interspecific differences in dewlap allometry may result from divergent sexual selection pressures. A comparative analysis of Anolis social behaviour found high variation among different ecomorphs in dewlap display rates and spatially structured social landscapes (e.g. interaction frequency of adult males overlapping in space; [24]). For example, male trunk–ground anoles spatially overlap with six times as many males as twig anoles but encounter a similar number of females [24]. These differences may lead to variable sexual selection pressures, driving evolutionary differences in dewlap allometry. Conversely, species of the same ecomorph class are typically very similar in social [24] and dewlap attributes (table 2, [20]). In the same vein, convergence within ecomorphs could result from similar sexual selection pressures. For example, trunk–ground anoles typically exhibit the highest rates of dewlap display behaviours associated with courtship and competitive social conflict [24–26], suggesting strong sexual selection may occur. Concomitantly, trunk–ground species in our study displayed the steepest allometric slopes of dewlap size (table 1).
Sexual selection is not the only factor affecting dewlaps, however; rather, dewlaps are multi-purpose signals, communicating information not only to male rivals [27] and potential mates [28,29], but also congeneric competitors [17] or even predators [30,31]. Therefore, while dewlap traits may have direct reproductive fitness consequences through their function in courtship interactions, as well as many indirect fitness consequences, the relative importance of these biotic interactions could be expected to differ among ecomorphs classes that inhabit different parts of the structural environmental and differ in behaviour and social structures [24]. The variation in dewlap allometry that we observed could have evolved due to this variation in function: species with social systems where signals function primarily for courtship often have different optimal allometric slopes than those that function as threat displays [32]. The patterns of dewlap size allometry that we observed—both the divergence in sympatry and convergence within ecomorphs—are likely the result of a complex trade-off between the multiple functions of the dewlap (e.g. courtship and antagonism) which may vary among ecomorphs.
While dewlap phenotypes likely evolve primarily due to the dual roles of signalling behaviours that have both direct (i.e. courtship) and indirect (e.g. mediation of agonistic interactions among competing males) fitness effects, the context of communication between the signaller and the receiver may also play a major role [33]. In anoles, it is likely that dewlap size is a proxy for overall body size (as the two are highly correlated; table 1). However, variation in the strength of this correlation—as well as the allometric slope—exists among ecomorphs. One possible explanation is due to variation in signalling distance among ecomorphs. Anolis visual systems are complex, possessing fovea that facilitate binocular visual acuity [34–36], and are very similar among species [37,38], suggesting little interspecific variation in visual acuity as it pertains to distance [39]. As small differences in absolute size are more difficult to discern with increasing distance [40], species that display from further distances would be expected to have allometric slopes that are both steeper and more highly correlated. In this way, increased allometry would exaggerate the signal's message while increased correlation would increase the signal's accuracy. Trunk–ground anoles have the largest dewlaps of all ecomorphs [41] and also display from greater distances and in environments with the fewest visual obstructions [24,26]; our results suggest that these species also exhibit the strongest positive allometry in dewlap size and dewlaps most accurately represent body size (table 1). The strength of allometry in dewlap size may therefore be a function of the distance at which different species communicate, with allometric slopes being strongest in those that display from the greatest distance.
Allometric scaling patterns may also be affected by the viability costs associated with dewlap size, which may vary among microhabitats and so among ecomorphs. However, comparative data on viability among Anolis ecomorphs are lacking but would be exceptionally valuable in clarifying these dynamics.
A recent study of dewlap size allometry in Caribbean anoles reported surprising evidence for hypo-allometry, i.e. dewlap size was proportionately smaller with increasing body size [14]. This result was especially surprising as it was counter to all other studies (e.g. [11–13]), including our results here. The paper introduced a new method based on measurements taken from videos of lizards displaying in nature [14]. Though an innovative and potentially important method, when we examined the underlying data associated with the paper, we found that the dewlap size measurements are vastly larger than our measurements of individuals of the same species (see electronic supplementary material). These video-derived size estimates were not validated by additional measurement methods (e.g. the methods used in this article) and so their accuracy remains unclear. Further studies using such video-based measurements of anole dewlaps would benefit from detailed validation of the accuracy of trait size estimates before such studies can challenge the results consistently discovered by other studies of anole dewlap allometry.
Here, we observed that all Anolis species exhibit positive allometry in dewlap size: larger individuals had proportionately larger dewlaps for their body size than small individuals, although the strength of this allometry varied among species. As our study represents one of the first comparative analyses of signal size allometry in lizard dewlaps, it remains unclear if these patterns are specific only to anoles or are consistent across in the multiple independent lineages that have evolved dewlap structures [8,9].
Acknowledgements
We thank Terry Ord for extremely helpful discussions of Anolis dewlaps and for insightful feedback on this manuscript. Research was conducted under permits from the Jamaican NEPA (no. 18/27), the Bahamas BEST (SRBS-018-2019-JS), the Dominican Republic Ministerio de Medio Ambiente, Washington University IACUC no. 20180101. Sarin Tiatragul, Sean Giery and Alejandra Belisario provided invaluable assistance in the field.
Ethics
All research was conducted in accordance with Washington University IACUC no. 20180101.
Data accessibility
Raw data included as electronic supplementary material.
All data associated with this study are included as electronic supplementary material [42].
Authors' contributions
J.T.S.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing—original draft and writing—review and editing; A.P.: investigation, writing—original draft and writing—review and editing; B.K.: data curation, formal analysis, investigation and writing—review and editing; K.W.: data curation, investigation and writing—review and editing; J.J.S.: data curation, investigation and writing—review and editing; J.B.L.: formal analysis, funding acquisition, resources, supervision and writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.West-Eberhard MJ. 2014. Darwin's forgotten idea: the social essence of sexual selection. Neurosci. Biobehav. Rev. 46, 501-508. ( 10.1016/j.neubiorev.2014.06.015) [DOI] [PubMed] [Google Scholar]
- 2.Detto T, Backwell PR, Hemmi JM, Zeil J. 2006. Visually mediated species and neighbour recognition in fiddler crabs (Uca mjoebergi and Uca capricornis). Proc. R. Soc. B 273, 1661-1666. ( 10.1098/rspb.2006.3503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith JM, Harper D. 2003. Animal signals. Oxford, UK: Oxford University Press. [Google Scholar]
- 4.Eberhard WG, Rodríguez RL, Huber BA, Speck B, Miller H, Buzatto BA, Machado G. 2018. Sexual selection and static allometry: the importance of function. Q. Rev. Biol. 93, 207-250. ( 10.1086/699410) [DOI] [Google Scholar]
- 5.Bonduriansky R. 2007. Sexual selection and allometry: a critical reappraisal of the evidence and ideas. Evolution 61, 838-849. ( 10.1111/j.1558-5646.2007.00081.x) [DOI] [PubMed] [Google Scholar]
- 6.Gould SJ. 1974. The origin and function of 'bizarre' structures: antler size and skull size in the 'Irish elk,’ Megaloceros giganteus. Evolution 28, 191-220. [DOI] [PubMed] [Google Scholar]
- 7.Tonini JFR, Provete DB, Maciel NM, Morais AR, Goutte S, Toledo LF, Pyron RA. 2020. Allometric escape from acoustic constraints is rare for frog calls. Ecol. Evol. 10, 3686-3695. ( 10.1002/ece3.6155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ord TJ, Klomp DA, Garcia-Porta J, Hagman M. 2015. Repeated evolution of exaggerated dewlaps and other throat morphology in lizards. J. Evol. Biol. 28, 1948-1964. ( 10.1111/jeb.12709) [DOI] [PubMed] [Google Scholar]
- 9.Hagman M, Ord TJ. 2016. Many paths to a common destination: morphological differentiation of a functionally convergent visual signal. Am. Nat. 188, 306-318. ( 10.1086/687560) [DOI] [PubMed] [Google Scholar]
- 10.Butler MA, Schoener TW, Losos JB. 2000. The relationship between sexual size dimorphism and habitat use in Greater Antillean Anolis lizards. Evolution 54, 259-272. [PubMed] [Google Scholar]
- 11.Echelle AF, Echelle AA, Fitch HS. 1978. Inter- and intraspecific allometry in a display organ: the dewlap of Anolis (Iguanidae) species. Copeia 1978, 245-250. ( 10.2307/1443558) [DOI] [Google Scholar]
- 12.Driessens T, Huyghe K, Vanhooydonck B, Van Damme R. 2015. Messages conveyed by assorted facets of the dewlap, in both sexes of Anolis sagrei. Behav. Ecol. Sociobiol. 69, 1251-1264. ( 10.1007/s00265-015-1938-5) [DOI] [Google Scholar]
- 13.Petelo M, Swierk L. 2017. Trait allometries generate super-honesty in Anolis dewlaps and may underlie sexual dimorphism. Integr. Zool. 12, 97-111. ( 10.1111/1749-4877.12238) [DOI] [PubMed] [Google Scholar]
- 14.Summers TC, Ord TJ. 2022. Signal detection shapes ornament allometry in functionally convergent Caribbean Anolis and Southeast Asian Draco lizards. J. Evol. Biol. 35, 1508-1523. ( 10.1111/jeb.14102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Losos JB. 2009. Lizards in an evolutionary tree: ecology and adaptive radiation of anoles. Berkeley, CA: University of California Press. [Google Scholar]
- 16.Losos JB. 1992. The evolution of convergent structure in Caribbean Anolis communities. Syst. Biol. 41, 403-420. ( 10.1093/sysbio/41.4.403) [DOI] [Google Scholar]
- 17.Rand AS, Williams EE. 1970. An estimation of redundancy and information content of anole dewlaps. Am. Nat. 104, 99-103. ( 10.1086/282643) [DOI] [Google Scholar]
- 18.Losos JB. 1985. An experimental demonstration of the species-recognition role of Anolis dewlap color. Copeia 1985, 905-910. ( 10.2307/1445240) [DOI] [Google Scholar]
- 19.Stroud JT, Losos JB. 2020. Bridging the process-pattern divide to understand the origins and early stages of adaptive radiation: a review of approaches with insights from studies of Anolis lizards. J. Hered. 111, 33-42. ( 10.1093/jhered/esz055) [DOI] [PubMed] [Google Scholar]
- 20.Nicholson KE, Harmon LJ, Losos JB. 2007. Evolution of Anolis lizard dewlap diversity. PLoS ONE 2, e274. ( 10.1371/journal.pone.0000274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671-675. ( 10.1038/nmeth.2089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.R Core Team. 2021. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 23.RStudio Team. 2021. RStudio: integrated development environment for R. Boston, MA: RStudio, PBC. [Google Scholar]
- 24.Johnson MA, Revell LJ, Losos JB. 2010. Behavioral convergence and adaptive radiation: effects of habitat use on territorial behavior in Anolis lizards. Evolution 64, 1151-1159. ( 10.1111/j.1558-5646.2009.00881.x) [DOI] [PubMed] [Google Scholar]
- 25.Lailvaux SP, Irschick DJ. 2007. The evolution of performance-based male fighting ability in Caribbean Anolis lizards. Am. Nat. 170, 573-586. ( 10.1086/521234) [DOI] [PubMed] [Google Scholar]
- 26.Stroud JT, Colom M, Ferrer P, Palermo N, Vargas V, Cavallini M, Lopez J, Jones I. 2019. Behavioral shifts with urbanization may facilitate biological invasion of a widespread lizard. Urban Ecosyst. 22, 425-434. ( 10.1007/s11252-019-0831-9) [DOI] [Google Scholar]
- 27.Jenssen TA, Orrell KS, Lovern MB. 2000. Sexual dimorphisms in aggressive signal structure and use by a polygynous lizard, Anolis carolinensis. Copeia 2000, 140-149. ( 10.1643/0045-8511(2000)2000[0140:SDIASS]2.0.CO;2) [DOI] [Google Scholar]
- 28.Sigmund WR. 1983. Female preference for Anolis carolinensis males as a function of dewlap color and background coloration. J. Herpetol. 17, 137-143. ( 10.2307/1563454) [DOI] [Google Scholar]
- 29.Crews D. 1975. Effects of different components of male courtship behaviour on environmentally induced ovarian recrudescence and mating preferences in the lizard, Anolis carolinensis. Anim. Behav. 23, 349-356. ( 10.1016/0003-3472(75)90083-4) [DOI] [Google Scholar]
- 30.Leal M, Rodríguez-Robles JA. 1997. Signalling displays during predator–prey interactions in a Puerto Rican anole, Anolis cristatellus. Anim. Behav. 54, 1147-1154. ( 10.1006/anbe.1997.0572) [DOI] [PubMed] [Google Scholar]
- 31.Leal M. 1999. Honest signalling during prey–predator interactions in the lizard Anolis cristatellus. Anim. Behav. 58, 521-526. ( 10.1006/anbe.1999.1181) [DOI] [PubMed] [Google Scholar]
- 32.Rodríguez RL, Eberhard WG. 2019. Why the static allometry of sexually-selected traits is so variable: the importance of function. Integr. Comp. Biol. 59, 1290-1302. ( 10.1093/icb/icz039) [DOI] [PubMed] [Google Scholar]
- 33.Fleishman LJ, Perez-Martinez CA, Leal M. 2022. Can sensory drive explain the evolution of visual signal diversity in terrestrial species? A test with Anolis lizards. Am. Nat. 200, 236-249. ( 10.1086/720267) [DOI] [PubMed] [Google Scholar]
- 34.Rasys AM, Pau SH, Irwin KE, Luo S, Kim HQ, Wahle MA, Trainor PA, Menke DB, Lauderdale JD. 2021. Ocular elongation and retraction in foveated reptiles. Dev. Dyn. 250, 1584-1599. ( 10.1002/dvdy.348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sannan NS, Shan X, Gregory-Evans K, Kusumi K, Gregory-Evans CY. 2018. Anolis carolinensis as a model to understand the molecular and cellular basis of foveal development. Exp. Eye Res. 173, 138-147. ( 10.1016/j.exer.2018.05.012) [DOI] [PubMed] [Google Scholar]
- 36.Fleishman LJ. 1992. The influence of the sensory system and the environment on motion patterns in the visual displays of anoline lizards and other vertebrates. Am. Nat. 139, S36-S61. ( 10.1086/285304) [DOI] [Google Scholar]
- 37.Persons MH, Fleishman LJ, Frye MA, Stimphil ME. 1999. Sensory response patterns and the evolution of visual signal design in anoline lizards. J. Comp. Physiol. A 184, 585-607. ( 10.1007/s003590050358) [DOI] [Google Scholar]
- 38.Loew ER, Fleishman LJ, Foster RG, Provencio I. 2002. Visual pigments and oil droplets in diurnal lizards: a comparative study of Caribbean anoles. J. Exp. Biol. 205, 927-938. ( 10.1242/jeb.205.7.927) [DOI] [PubMed] [Google Scholar]
- 39.Fleishman LJ, Yeo AI, Perez CW. 2017. Visual acuity and signal color pattern in an Anolis lizard. J. Exp. Biol. 220, 2154-2158. [DOI] [PubMed] [Google Scholar]
- 40.Fleishman LJ, Prebish MG, Leal M. 2020. The effects of limited visual acuity and context on the appearance of Anolis lizard dewlaps. J. Herpetol. 54, 355-360. ( 10.1670/19-108) [DOI] [Google Scholar]
- 41.Losos JB, Chu L. 1998. Examination of factors potentially affecting dewlap size in Caribbean anoles. Copeia 1998, 430-438. ( 10.2307/1447437) [DOI] [Google Scholar]
- 42.Stroud JT, Petherick A, Krasnoff B, Walker K, Suh JJ, Losos JB. 2023. Signal size allometry in Anolis lizard dewlaps. Figshare. ( 10.6084/m9.figshare.c.6708279) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Stroud JT, Petherick A, Krasnoff B, Walker K, Suh JJ, Losos JB. 2023. Signal size allometry in Anolis lizard dewlaps. Figshare. ( 10.6084/m9.figshare.c.6708279) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Raw data included as electronic supplementary material.
All data associated with this study are included as electronic supplementary material [42].