Abstract
Background:
The purpose of this study is to describe human epidermal growth factor 2 (HER2) overexpression in head and neck squamous cell carcinoma (HNSCC) and re-evaluate its potential as a target for HER2-directed immunotherapies.
Methods:
A retrospective cohort of patients with HNSCC receiving curative treatment was identified, and HER2 expression evaluated in archival tissue by immunohistochemistry and correlated with clinicopathological characteristics. HER2 expression data were also determined for HNSCC patients in The Cancer Genome Atlas.
Results:
Nineteen percent of HNSCC and 39% of oropharyngeal HNSCC (OPSCC) were HER2 positive. HER2 expression positively correlated with nodal metastasis (p = 0.035). Patients with HER2-positive tumors had decreased overall survival (p = 0.012), including within the human papilloma virus-positive OPSCC subgroup (p = 0.007).
Conclusions:
A substantial fraction of HNSCC overexpresses HER2 protein, suggesting it may be a suitable target for antigen-directed immunotherapy. HER2 expression and its correlation with survival vary across HNSCC subsites, making it unsuitable as a prognostic marker.
Keywords: head and neck squamous cell carcinoma, human epidermal growth factor receptor 2 human papilloma virus immunotherapy oropharynx
1 |. INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) has an overall 5-year survival rate of 40%–60%1; TNM staging, pathologic grade of differentiation, and other clinical features often fail to adequately predict treatment response and survival. Several clinical studies have demonstrated that there is a prognostic role for molecular indicators of tumor progression, including p53, cyclin D1, and the epidermal growth factor receptor (EGFR) family.2–4 Overexpression of EGFR has been found in about 90% of HNSCC cases and is associated with a poor prognosis.5 However, the response to cetuximab, a monoclonal antibody targeting the EGFR receptor, does not correlate with EGFR expression levels in the primary tumor, and any response is typically not long-lasting.6 It has been suggested that one possible mechanism of EGFR resistance is overactivation of other EGFR family receptors, including human epidermal growth factor receptor 2 (HER2).7
HER2 amplification and overexpression has been linked to aggressive tumor growth and poor prognosis in a variety of cancers including breast, ovary, and lung.8–10 As such, HER2 has emerged as a common therapeutic target. In solid tumors such as breast cancers, monoclonal antibodies and kinase inhibitors such as trastuzumab and neratinib have effectively targeted the overexpressed receptor.11 These targeted therapy approaches rely on high levels of receptor overexpression.10 Although the HER2 gene is frequently amplified in breast and other cancers, resulting in receptor overexpression at high levels, HER2 overexpression in HNSCC is often independent of gene amplification, leading to a lower overall level of expression relative to other cancers.12–14 HER2 overexpression has been previously described in HNSCC; however, reports vary widely as to immunohistochemical (IHC) methods, degree and frequency of HER2 overexpression, and potential prognostic significance.15–18 Several studies have found generally low levels of HER2 overexpression in HNSCC and concluded that HER2 expression level is a weak prognostic factor, while others found high levels of overexpression and concluded that HER2 is both a useful prognostic marker and therapeutic target.15,19–21 However, the majority of these studies have focused on suitability for HER2 molecular targeted therapy (e.g., receptor-targeting tyrosine kinase inhibitors or monoclonal antibodies), which are effective against tumors with high-level (e.g., amplified) HER2 expression.
Recent advances in antigen-specific immunotherapies such as therapeutic tumor vaccines and adoptive cellular therapies often utilize tumor-antigen-specific T cells as the final common pathway of anti-tumor activity. Since T cells can identify antigen-expressing tumor cells even at expression levels far below levels found in receptor-amplified tumors, immunotherapy makes it possible to effectively target tumors with even limited HER2 overexpression.22,23 This opens new opportunities for therapeutic development in diseases such as HNSCC where HER2 is overexpressed but not amplified. The present study utilized a consistent protocol for IHC evaluation across multiple HNSCC subsites to examine the extent and distribution of HER2 overexpression in HNSCC and evaluate the impact of overexpression on HNSCC clinical outcomes.
2 |. MATERIALS AND METHODS
2.1 |. Clinical data collection
We reviewed the records of Veterans with laryngeal, oral cavity or oropharyngeal squamous cell carcinomas between January 1, 1997, and January 1, 2018, who received curative-intent treatment. Oropharyngeal sites included tonsil, base of tongue, pharyngeal wall, glossopharyngeal sulcus, and soft palate. Oral cavity sites included tongue, floor of mouth, hard palate, and buccal mucosa. Approval was obtained from Baylor College of Medicine and the Michael E. Debakey Veteran’s Administration Medical Center (MEDVAMC) Institutional Review Boards, with waiver of consent for retrospective analysis of previously collected tissue specimens. Curative intent treatment for HNSCC was defined as surgical resection, and/or the use of chemo-radiation, either as the primary treatment or as adjuvant therapy after surgery. Data collection and analysis were performed in a manner consistent with existing standards for clinical research (Declaration of Helsinki, US Federal Policy for the Protection of Human Subjects). Inclusion criteria included primary untreated HNSCC of the oral cavity, oropharynx, or larynx; tissue diagnosis at the MEDVAMC; sufficient pathologic tissue for IHC analysis; and curative treatment delivery at the MEDVAMC. Exclusion criteria included treatment at an outside institution, palliative treatment, or recurrent disease at presentation. Demographic information was recorded including age, sex, race, and ethnicity. Clinicopathologic features were collected including clinical stage according to the American Joint Commission on Cancer (Staging Manual seventh edition) staging system.
2.2 |. Tissue microarray construction
Thirty-two laryngeal, 57 oral cavity, and 31 oropharyngeal cancers were used for tissue microarray (TMA) construction. Formalin-fixed, paraffin-embedded (FFPE) biopsy tissue blocks were retrieved from the archive maintained at the MEDVAMC Department of Pathology. Prior to incorporation into a TMA block, hematoxylin and eosin (H&E)-stained sections were created from each donor tissue block to identify the most appropriate tumor areas. A 1-mm diameter tissue core was removed from selected areas of each tissue block using a Beecher TMA instrument (Sun Prairie, Wisconsin, USA) and placed into a gridded recipient paraffin block. H&E-stained sections from the TMA blocks were generated for histological evaluation. A single TMA block each was constructed for the laryngeal and oropharyngeal tumors; the oral cavity tumors spanned two TMA blocks.
2.3 |. Immunohistochemical analysis
The anti-HER2 monoclonal antibody CB11 (ab8054, Abcam Inc, Cambridge, MA), generated to a synthetic peptide sequence of predicted antigenicity near the C-terminus of the protein, was used to detect the HER2 protein in tumor sections.24 Paraffin-embedded TMA sections were cut and mounted on positively charged glass slides. These were de-paraffinized in xylene and rehydrated in serial alcohol solutions. Endogenous peroxidase activity was blocked by incubation in a hydrogen peroxide/methanol solution followed by blockade of non-specific binding sites using 1.5% normal horse serum in Tris-buffered saline (TBS). The slides were incubated for 16 h at 4°C with the anti-HER2 antibody at a dilution of 1:40.25 Following washing with TBS, binding of the primary antibody was demonstrated with a standard avidin-biotin-peroxidase complex technique.26 The TMAs were then counterstained with hematoxylin. Two sets of negative controls were made, either by substituting the primary antibody for normal serum in the staining protocol or by substituting the secondary antibody for normal serum. Breast cancer TMAs including tumors of known HER2 expression were stained and used as positive controls, for pathologist use as a grading reference.
A trained clinical pathologist (WY) scored the stained TMA slides in a blinded fashion without knowledge of the clinical data. Each section was scored according to the conventional FDA-approved HER2 staining scoring system (0: negative, 1+: faint membrane staining >10% of cells, 2+: moderate complete membrane staining >10% cells, 3+: strong, complete membrane staining >10% of cells). In addition, each section was scored for staining intensity (0: background, minimal; 1+: weak; 2+: moderate; 3+: strong) and the proportion of cell staining (0: <1%; 1+: 1%–25%; 2+: >25%–50%; 3+: >50%–75%; 4+: >75%). An H-score was then calculated for each section by multiplying the intensity value by the proportion score as a percent. H-scores for duplicate sections were averaged together. Representative images with different scores are shown in Figure 1. Since there is no standard threshold for HER2 positivity in HNSCC, we considered an H-score of ≥50 as HER2 positive, based on the distribution of our data and recent published literature.15,27 For reference, at a H-score ≥100, 6.7% of tumors were positive for HER2; while a cutoff ≥ 50 (e.g., 25% of tumor staining at moderate intensity) yielded 19% HER2 positivity. For the conventional scoring system, a score of 2+ or greater was considered HER2 positive.
FIGURE 1.
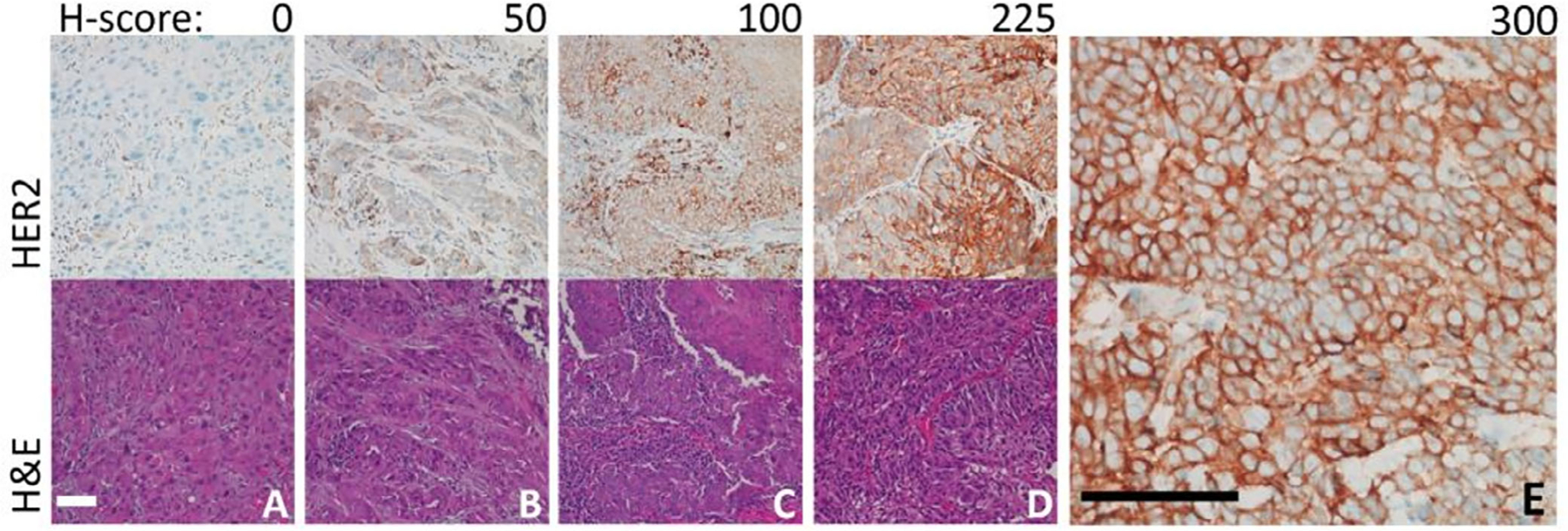
Representative immunohistochemical staining of human epidermal growth factor receptor 2 positive cells in head and neck squamous cell carcinoma. (A) Tumors with complete absence or nonspecific staining were scored as a 0. (B) Tumors with an H-score of 50 demonstrated at least weak staining in up to 50% of the cells present. (C) Tumors with an H-score of 100 demonstrated moderate staining in up to 50% of the cells present. (D) Tumors with H-scores of 225 demonstrated strong staining in up to 75% of the cells present. (E) The maximum H-score possible is 300, with strong staining in over 75% of cells. This image was taken at higher magnification (40X objective) to demonstrate membrane staining. All scale bars represent 100 μm [Color figure can be viewed at wileyonlinelibrary.com]
2.4 |. TCGA dataset
RNAseq data from The Cancer Genome Atlas (TCGA) projects, previously downloaded from the TCGA Data Coordinating Center, harmonized with a common calling/mapping algorithm and publicly available from the University of California Santa Cruz TOIL RNAseq recompute project, were used to collect fragments per kilobase of exon model per million reads mapped (FPKM) gene expression values of HER2 for all 301 oral cavity, 79 oropharyngeal, and 137 laryngeal SCC patient samples.28 Clinical data and human papilloma virus (HPV) status for the same set of samples from TCGA were downloaded from cbioportal.org. HER2 protein expression values were downloaded from The Cancer Proteome Atlas (tcpaportal.org).29,30 For all statistical tests, the upper tertile of expression was compared to the lower tertile. A proportion test was used to determine correlation between HPV status and HER2 gene expression, and HER2 protein expression, for each of the subsites. For survival analysis, comparisons between groups were made using log-rank statistics.
2.5 |. Study endpoints and statistical analysis
Endpoints of interest included time to recurrence or death, reported in months. In the absence of a pathological report documenting recurrent disease, imaging was used as a surrogate measure of local, regional, or distant recurrence. Disease-free survival (DFS) was defined as time from date of primary diagnosis to date of diagnosis of loco-regional and/or distant recurrence (whichever was diagnosed first). Overall survival (OS) was defined as time from date of primary diagnosis to date of death or last recorded follow-up in hospital records. Fisher’s exact test was used to report likelihood of association between categorical variables. Actuarial survival rates were generated using the Kaplan-Meier method, and log-rank statistics were used to make comparisons between groups. For all computations, p-values were considered statistically significant if below a threshold of 0.05. Statistical calculations were performed with SPSS (version 1.0.0.1347, IBM) and Rstudio (Version 1.2.1335). GraphPad Prism (Version 8.4.1) was used to generate figures.
3 |. RESULTS
3.1 |. Patient and tumor characteristics
We analyzed data from 120 patients with SCC of the larynx, oral cavity, or oropharynx. The majority of HNSCC patients in this Veteran population were white (72.5%) and male (97.5%); the average age was 62 years old (Table 1). Smoking status was known for 108 patients; patients were stratified based on a threshold of 10 pack years of exposure. Only 11 (10.2%) of our patients had less than 10 pack years of exposure. The most common primary site was oral cavity (47.5%), followed by larynx (26.7%) and oropharynx (25.8%); 49.2% of patients presented with T1–T2 tumors and 50.8% with T3–T4 tumors; 53 (44.2%) patients presented with nodal metastasis.
TABLE 1.
Association of human epidermal growth factor 2 (HER2) protein expression with patient demographics and clinicopathologic features
| No. of patients (%) by HER2+ status |
p-values |
|||||
|---|---|---|---|---|---|---|
| H-score |
Conventional score (0–3+) |
|||||
| No. of patients (%) (n = 120) |
Positive (n = 23) |
Negative (n = 97) |
Positive (n = 13) |
Negative (n = 107) |
H-score, Conventional score |
|
| Primary tumor site | 0.004, <0.001 | |||||
| Larynx | 32 (26.7) | 6 (26.1) | 26 (27.0) | 2 (15.4) | 30 (28.0) | |
| Oral cavity | 57 (47.5) | 5 (21.7) | 52 (54.2) | 0 (0.0) | 57 (53.3) | |
| Oropharynx | 31 (25.8) | 12 (52.2) | 19 (19.8) | 11 (84.6) | 20 (18.7) | |
| Sex | ||||||
| Male | 117 (97.5) | 23 (100.0) | 94 (96.9) | 13 (100.0) | 104 (97.2) | 1, 1 |
| Female | 3 (2.5) | 0 (0.0) | 3 (3.1) | 0 (0.0) | 3 (2.8) | |
| Race | ||||||
| White | 86 (71.7) | 19 (82.6) | 67 (69.1) | 11 (84.6) | 75 (70.1) | 0.418, 0.653 |
| Black | 31 (25.8) | 4 (17.4) | 27 (27.8) | 2 (15.4) | 29 (27.1) | |
| Hispanic | 3 (2.5) | 0 (0.0) | 3 (3.1) | 0 (0.0) | 3 (2.8) | |
| pT classification | 0.819, 0.391 | |||||
| pT1–pT2 | 59 (49.2) | 12 (52.2) | 47 (48.4) | 8 (61.5) | 51 (47.7) | |
| pT3–pT4 | 61 (50.8) | 11 (47.8) | 50 (51.6) | 5 (38.5) | 56 (52.3) | |
| TNM stage | 0.207, 0.545 | |||||
| Stage I | 16 (13.3) | 1 (4.3) | 15 (15.6) | 0 (0.0) | 16 (15.0) | |
| Stage II | 12 (10.0) | 2 (8.7) | 10 (10.4) | 1 (7.7) | 11 (10.3) | |
| Stage III | 27 (22.5) | 3 (13.1) | 24 (25.0) | 3 (23.1) | 24 (22.4) | |
| Stage IV | 65 (54.2) | 17 (73.9) | 48 (50.0) | 9 (69.2) | 56 (52.3) | |
| N classification | 0.035, 0.002 | |||||
| N0 | 67 (55.8) | 8 (34.8) | 59 (60.8) | 2 (15.4) | 65 (60.7) | |
| N+ | 53 (44.2) | 15 (65.2) | 38 (39.2) | 11 (84.6) | 42 (39.3) | |
| Histological grading | 0.648, 0.274 | |||||
| GX | 2 (1.7) | 1 (4.3) | 1 (1.0) | 1 (7.7) | 1 (0.9) | |
| G1 | 10 (8.3) | 2 (8.7) | 8 (8.3) | 0 (0.0) | 10 (9.3) | |
| G2 | 44 (36.7) | 8 (34.8) | 36 (37.5) | 4 (30.8) | 40 (37.4) | |
| G3 | 64 (53.3) | 12 (52.2) | 52 (54.2) | 8 (61.5) | 56 (52.4) | |
| Tobacco usage (n = 108) | ||||||
| <10 pack years | 11 (10.2) | 4 (20.0) | 7 (8.0) | 3 (27.3) | 8 (8.2) | 0.119, 0.385 |
| ≥10 pack years | 97 (89.8) | 16 (80.0) | 81 (92.0) | 8 (72.7) | 89 (91.8) | |
| p16 statusa (n = 31) | ||||||
| Negative | 14 (45.2) | 4 (33.3) | 10 (52.6) | 3 (27.3) | 11 (55.0) | 0.461, 0.258 |
| Positive | 17 (54.8) | 8 (66.7) | 9 (47.4) | 8 (72.7) | 9 (45.0) | |
p16 status is only available for the 31 oropharyngeal tumors.
3.2 |. Clinicopathologic features and HER2 expression
Twenty-three tumors (19.2%) were HER2 positive, defined as H-score ≥50 (Table 1). Twelve (52.2%) of these 23 were oropharyngeal tumors, while six (26.1%) were laryngeal and five (21.7%) were from the oral cavity. The majority of the HER2+ tumors were stage IV (73.9%); only one (4.3%) was stage I, two (8.7%) were stage II, and three (13.1%) were stage III. Fifteen (65.2%) of the 23 had positive lymph nodes (N+). Over half (52.2%) of the tumors had a histological grading of G3 (poorly differentiated), while 34.8% were G2 (moderately differentiated) and only 8.7% were G1 (well differentiated). Using the conventional HER2 scoring system, 13 tumors were positive (10.8%); the majority of these tumors were stage IV (69.2%), poorly differentiated (61.5%), and with positive nodal disease (84.6%). HER2 positivity, both by H-score and conventional scoring, was correlated with primary tumor site (p = 0.004, <0.001) and nodal status (p = 0.035 and 0.002).
3.3 |. HER2 expression and clinical outcomes, TMA
From the total set of 120 patient tumors, survival data were available for 119, but four were excluded from the oropharyngeal TMA since they did not receive curative intent treatment. For this mixed HNSCC population, site, T-classification, nodal status, and smoking status did not impact overall (OS) or DFS (Figure 2(A)–(F), Table 2). HER2 overexpression by H-score was associated with decreased OS (p = 0.012) and DFS, although this difference was not statistically significant for DFS (p = 0.066) (Figure 2(G), (H)). Median survival was 11 months for HER2+ patients and 49 months for HER2− patients. HER2 positivity by conventional scoring, however, was not associated with a significant difference in OS or DFS. The results of univariate analysis for all clinicopathologic variables are detailed in Table 2.
FIGURE 2.
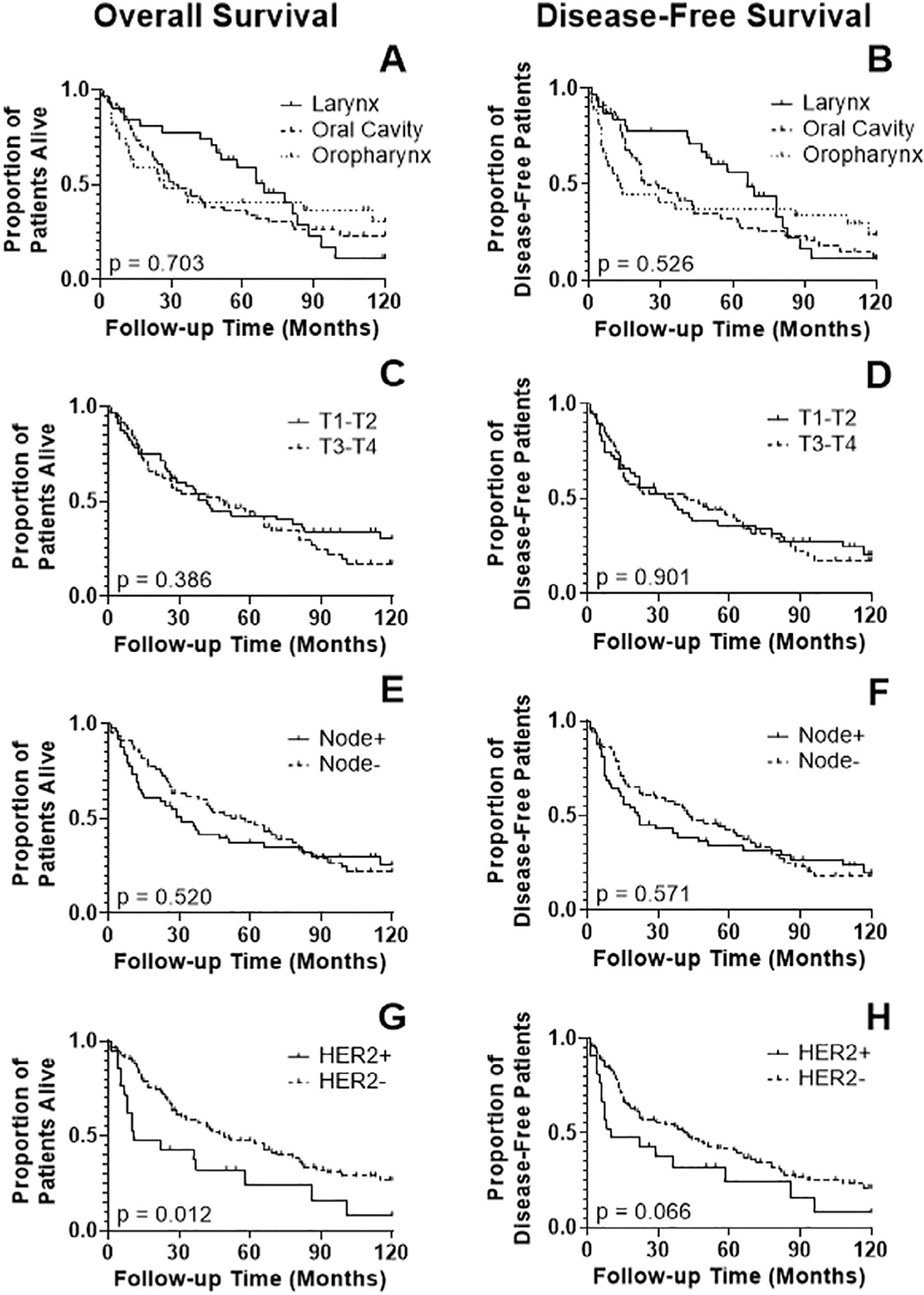
Kaplan-Meier analysis of overall survival (OS), disease-free survival (DFS) according to clinicopathologic parameters for 115 patients with primary head and neck squamous cell carcinoma: (A,B) Primary tumor site (larynx, oral cavity, oropharynx); (C,D) T classification; (E,F) lymph node status; (G,H) human epidermal growth factor receptor 2 (HER2) expression. The OS correlated significantly with HER2 expression (p = 0.012); DFS showed a similar trend with HER2 expression but it was not significant (p = 0.066)
TABLE 2.
Univariate analysis of prognostic factors in head and neck squamous cell carcinoma
| Univariate analysis |
|||
|---|---|---|---|
| Variable | Variable category | Hazard ratio (95% CI) | p-value |
| OS | |||
| Primary tumor site | Larynx | – | – |
| Oral cavity | 0.797 (0.479–1.327) | 0.097 | |
| Oropharynx | 1.004 (0.535–1.884) | 0.989 | |
| T classification | pT1–pT2 | – | – |
| pT3–pT4 | 0.825 (0.532–1.278) | 0.386 | |
| N classification | N0 | – | – |
| N+ | 1.115 (0.737–1.810) | 0.520 | |
| Smoking | <10 pack years | – | – |
| ≥10 pack years | 0.651 (0.322–1.317) | 0.632 | |
| H-score | HER2− | – | – |
| HER2+ | 1.942 (0.994–3.794) | 0.012 | |
| Conventional score | HER2− | – | – |
| HER2+ | 1.290 (0.599–2.779) | 0.467 | |
| DFS | |||
| Primary tumor site | Larynx | – | – |
| Oral cavity | 0.720 (0.430–1.183) | 0.199 | |
| Oropharynx | 0.912 (0.497–1.673) | 0.754 | |
| T classification | pT1–pT2 | – | – |
| pT3–pT4 | 0.974 (9.632–1.490) | 0.901 | |
| N classification | N0 | – | – |
| N+ | 1.131 (0.732–1.747) | 0.571 | |
| Smoking | <10 pack years | – | |
| ≥10 pack years | 0.583 (0.278–1.141) | 0.192 | |
| H-score | HER2− | – | – |
| HER2+ | 1.629 (0.871–3.047) | 0.066 | |
| Conventional score | HER2− | – | |
| HER2+ | 1.485 (0.659–3.348) | 0.253 | |
3.4 |. HER2 expression and survival, TCGA
In the TCGA cohort, HER2 mRNA expression was associated with a significant improvement in OS for oropharyngeal squamous cell carcinoma (OPSCC) (p = 0.020) (Figure 3(A)); however, there was no effect of HER2 protein expression (p = 0.327) (Figure 3(C)) on survival. For SCC of the larynx, HER2 mRNA and protein expression were both associated with an improvement in OS (p = 0.002 and p = 0.004, respectively) (Figure 3(B),(D)). For oral cavity SCC, there was no difference in OS according to HER2 mRNA or protein expression (data not shown). HER2 gene expression (ERBB2) by RNA seq did not correlate strongly with HER2 protein expression (Figure 3(E)). Of note, protein expression data were only available for a fraction of the total tumors in each of the subsites: 49% of OP, 68% of the oral cavity, and 68% of the larynx.
FIGURE 3.
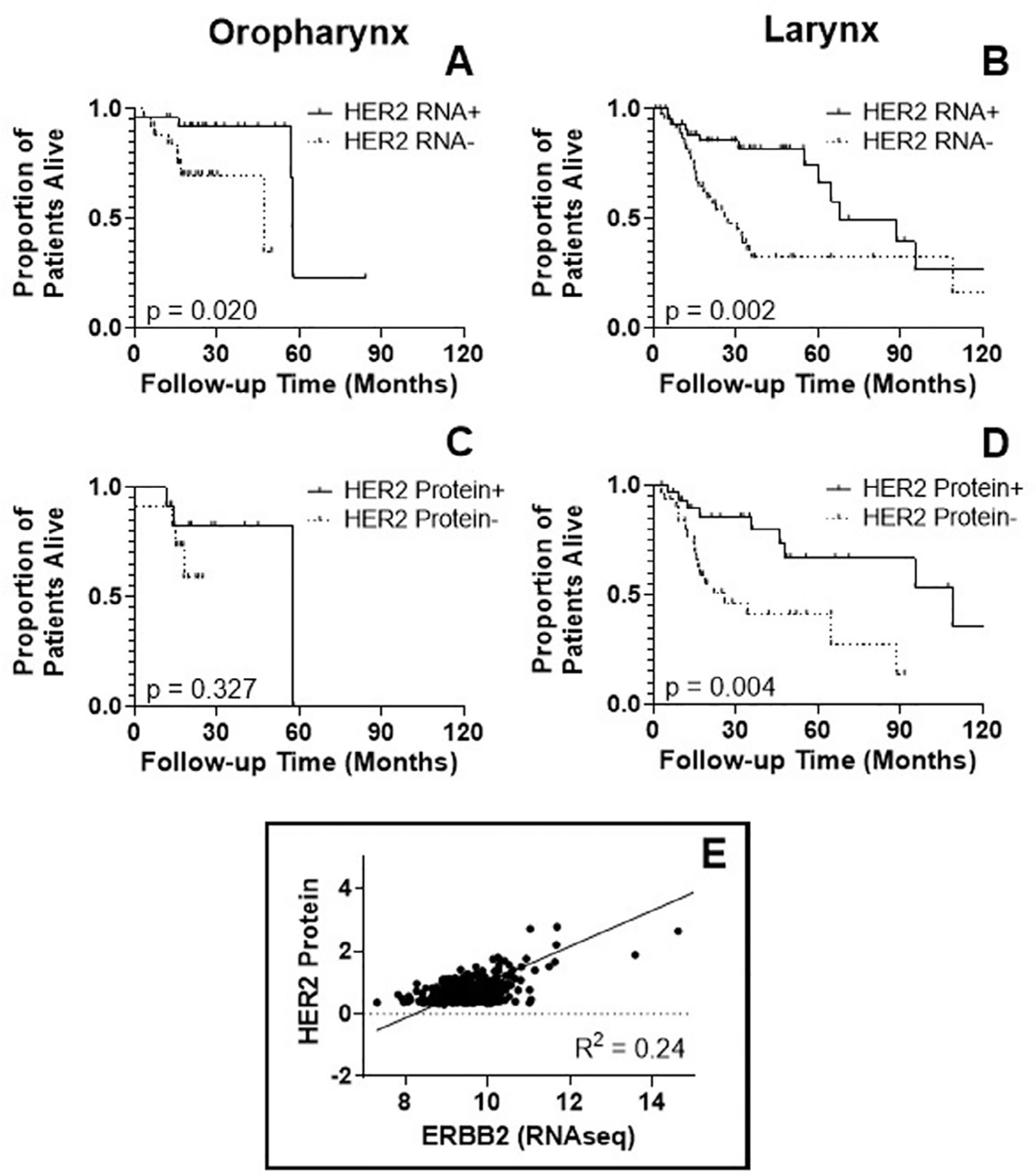
Kaplan-Meier survival curves of TCGA database patients with oropharyngeal or laryngeal SCC, according to: (A,B) human epidermal growth factor receptor 2 (HER2) RNA expression and (C,D) HER2 protein expression. Overall survival (OS) is significantly improved in the upper tertian of HER2 RNA expression (“HER2 RNA+”) (p < 0.001). There is no difference in OS based on HER2 protein expression. For laryngeal SCC, there is better OS for the upper tertian of both HER2 RNA expression and protein expression (p = 0.327 and 0.004). (E) Based on a linear regression, HER2 RNA expression (ERBB2 RNA seq) and HER2 protein expression are only loosely correlated in head and neck squamous cell carcinoma from all three sites, with an R squared of 0.24
3.5 |. HER2 expression and HPV status
HPV infection plays an important role in the etiology of OPSCC and has been shown to impact treatment response and survival.2,31,32 Over half (54.8%) of the OPSCC patients in our TMA series overexpressed p16 by IHC (a surrogate marker of HPV-positivity) (Table 1). As expected, OPSCC patients who overexpressed p16 demonstrated improved survival (OS p < 0.001, DFS p < 0.001) (Figure 4(A),(B)). Patients with HER2-positive tumors had significantly decreased OS (p = 0.037) (Figure 4(C)), but no difference in DFS (p = 0.162) (Figure 4(D)). There was no correlation between HPV status and HER2 positivity (Fisher’s exact test, p = 0.461) (Table 1). However, within the HPV+ OPSCC subgroup, HER2 status did correlate with decreased OS and DFS (p = 0.007 and 0.018, respectively) (Figure 4(E),(F)).
FIGURE 4.
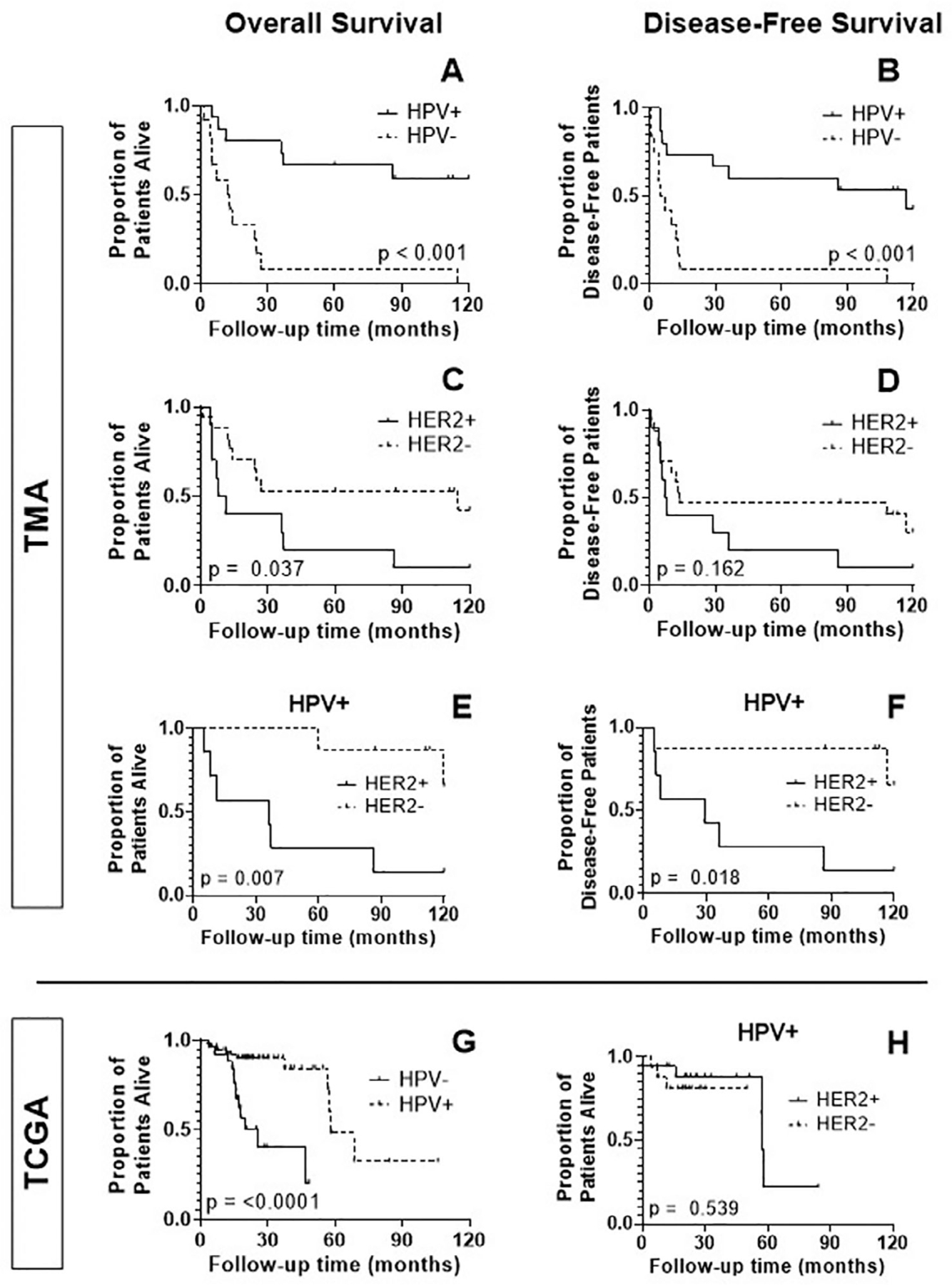
Kaplan-Meier analysis of overall survival (OS), disease-free survival (DFS) in the TMA and TCGA subsets of OPSCC according to HPV status and human epidermal growth factor receptor 2 (HER2) expression. (A,B) HER2 expression. There is a significant improvement in OS and DFS in HPV positive patients (p < 0.001 and 0.001). (C,D) Conversely, HER2 positivity corresponds to a significant decrease in OS (p = 0.037). There is a non-significant trend toward improved DFS in patients with HER2 overexpression (p = 0.162). (E, F) Within the HPV+ subset of patients, those who were HER2+ had significantly worse OS and DFS (p = 0.007, 0.018). (G, H) In the TCGA group of OPSCC, HPV+ patients experienced improved OS (p < 0.0001); there was no effect of HER2 positivity on OS within the HPV+ subset (p = 0.539)
In the TCGA cohort, 67.1% of OPSCC tumors were HPV+, and this subset of patients also demonstrated better OS (p < 0.0001) (Figure 4(G)). This group’s HPV positivity significantly correlated with increased HER2 RNA expression (two-sample proportion test, p = 0.003) but not with HER2 protein expression (Fisher’s test, p = 0.411). HER2 status did not correlate with survival in the HPV-positive subgroup (Figure 4(H), p = 0.539).
For tumors in the larynx or oral cavity, there was no effect of HPV status on survival (p = 0.910, data not shown) and it did not correlate with HER2 gene expression (two-sample proportion test, p = 0.520 and 0.630, respectively).
4 |. DISCUSSION
The American Society of Clinical Oncology (ASCO) publishes guidelines for assessing HER2 status in breast carcinomas via IHC and FISH, and specific protocols have also been established for gastric cancer; however, no standardized evaluation methods have been developed for most other solid tumors, including HNSCC.33,34 Instead, IHC/FISH guidelines for breast carcinomas are typically applied to HNSCC and other solid tumors, despite their different etiology, biology, and clinical features. Most notably, HER2 is amplified frequently in breast cancer, but rarely if ever in HNSCC. Reported HER2 overexpression in HNSCC ranges from 0% to 88%.15,16,35,36 A careful review of many studies reveals variations in detection techniques (including the use of different antibodies that recognize different epitopes of the protein) and interpretation methods, which contribute to varying reports of HER2 overexpression in HNSCC. To date, no threshold for HER2 positivity has been definitively established for HNSCC. Most studies apply the same 0–3+ scoring system that is used in the FDA-approved HercepTest for breast cancer, but application of this system to HNSCC is problematic since it was developed for evaluation of HER2-amplified tumors. The HercepTest scoring system focuses on membrane staining as the standard criterion for positivity; however, depending on the anti-HER2 antibody used, there may a significant component of cytoplasmic staining. The importance of cytoplasmic staining in HNSCC is controversial and its interpretation has not yet been delineated. Some assert that cytoplasmic staining may be a technical artifact while others argue that it may represent incomplete receptor degradation.37,38 Since many immunologic methods of targeting HER2-expressing tumors, including therapeutic tumor vaccines and receptor-engineered T cells, would recognize both cytoplasmic and membrane-expressed HER2, it makes sense to consider total HER2 expression levels when assessing suitability for these methods.
For the current study, we utilized a HER2 H-score threshold of 50, taking into account both the intensity of staining and proportion of cells staining for characterization of HER2 expression. Using this threshold, the overall HER2 positivity rate was roughly 20% across all three subsites, similar to several previously published studies.15,27
According to the conventional HER2 scoring system, however, positivity was lowered to 10%. In examining the differences in positivity between the two scoring systems based on tumor subsite, the laryngeal and oral cavity tumors were disproportionately affected. All but one of the oropharyngeal tumors were considered HER2+ by both scoring systems, yet none of the five oral cavity tumors positive by H-score retained positive status by conventional scoring.
Regardless, by both scoring systems, there was a significant correlation between HER2 overexpression and positive lymph node status in HNSCC. Because lymph node invasion is a sign of tumor aggressiveness, it is possible that overexpression of HER2 plays a role in regional metastatic spread.39,40 While none of the typical clinicopathologic variables (primary tumor site, T classification, and lymph node status) influenced OS or DFS, HER2 positivity by H-score was associated with decreased OS. There was no survival difference observed for HER2 positive tumors by conventional scoring. Furthermore, there was no significant difference in survival by smoking status, possibly because there were very few non-smokers in our patient cohort.41
Analysis of data available on HNSCC patients in the TCGA database revealed a survival benefit in oropharyngeal and laryngeal SCC for patients with increased HER2 RNA expression; for OPSCC; however, the same benefit was not observed based on HER2 protein expression. This discrepancy may be related to a diminished sample size: while RNA expression data were available for all 79 OPSCC patients, HER2 protein expression information was only present for 38 (48%). Nevertheless, since high HER2 RNA expression in this dataset significantly correlated with HPV positivity (two-sample proportion test, p = 0.003), the effect on survival here may largely be a factor of HPV status. For laryngeal SCC, on the other hand, greater expression of HER2 protein continued to demonstrate a survival benefit, and HPV status not only had no effect on OS but also had no relationship to HER2 gene expression. For oral cavity SCC, none of the 3 parameters had any significant effect on OS.
The present study highlights some of the difficulties associated with developing a clear understanding of the association between HER2 expression and HNSCC survival. In the smaller TMA dataset, HER2 positivity was 19% and was generally associated with decreased OS, consistent with findings in earlier studies.15,42 Since over half of the HER2 positive HNSCC tumors were oropharyngeal tumors, the poorer survival of patients with HER2 overexpressing tumors is all the more striking, given the well-known improved outcomes in HPV positive oropharynx cancer. In the larger TCGA dataset for OPSCC, the correlation between HER2 and HPV is strong with respect to HER2 RNA expression; however, there was no correlation with HER2 protein expression, which may explain the lack of association between HER2 expression and worse survival in this cohort. Another potential explanation for this discrepancy is the substantially higher proportion of heavy smokers in our TMA cohort, which we and others have found to have markedly worse survival of non-smokers with HPV-associated OPSCC.43 And finally, the different methods used to measure HER2 protein expression in TMA and TCGA tumors (IHC versus reverse-phase protein array, respectively) may also contribute to the difference in findings.
Taken together, our results suggest that: (1) HER2 overexpression, detected using conventional IHC, is found in a significant fraction of HNSCC tumors and (2) associations between HER2 overexpression and survival are inconsistent and vary among subsites. For OPCSCC, the association between HER2 status and survival is impacted by HPV status but not other clinical parameters (site, T-classification, N-classification). A more definitive analysis of HER2 impact on clinical outcomes could be performed by performing IHC on specimens from a site-defined clinical trial cohort with known HPV/p16 status and fairly homogenous treatment. The recently completed RTOG1016 and De-EscalateHPV clinical trial cohorts would be ideal for addressing this persistent question using large OPSCC cohorts and high-quality clinical data.44,45
In conclusion, we found HER2 to be overexpressed in roughly one-fifth of HNSCC tumors across multiple HNSCC sites, suggesting that HER2 may be an appropriate target for antigen-specific immunotherapy approaches, such as tumor vaccination, T cell receptor engineered T cells, and in some cases (overexpression at the cell membrane), chimeric antigen receptor T cells.22,23 As has been the case in other single-institution cohort studies, associations of HER2 overexpression on clinical outcomes are inconsistent across tumor sites and study populations, and its impact on survival may be partially confounded by HPV status. While HER2 is unlikely to be a useful prognostic marker or direct drug target in HNSCC, our findings and others suggest that it may be a suitable target for clinical trials of antigen-specific immunotherapy directed against HER2-expressing tumors.
ACKNOWLEDGMENTS
This work was supported by CPRIT RP160283-Baylor College of Medicine Comprehensive Cancer Training Program, NCI-CA125123 P30 Cancer Center Support Grant, and NIH/NCI 5T32CA174647–05 Ruth L. Kirschstein Institutional National Research Service Award. This work was also supported in part by an unrestricted research grant from Tessa Therapeutics. This material is also the result of work supported with resources and the use of facilities at the Michael E. DeBakey Veterans Affairs Medical Center. VCS is supported by a Career Development Award from the Veterans Administration Clinical Science Research and Development division (1IK2CX001953).
Footnotes
Section Editor: James Rocco
CONFLICT OF INTEREST
Contents do not represent the views of the US Department of Veterans Affairs or the US Government. The authors report no conflicts of interest for the existing work.
DATA AVAILABILITY STATEMENT
Data availability statement. TCGA data is publicly available for use and re-analysis through tcpaportal.org. Institutional data from patient specimens as well as clinical data can be provided upon reasonable inquiry/request in an aggregated, de-identified fashion following approval from the appropriate hospital and institutional administrative bodies in a manner compliant with all institutional, local, state and federal regulations pertaining to transfer of patient data and materials.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63(1):11–30. 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Ang KK, Berkey BA, Tu X, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res 2002;62(24):7350–7356. [PubMed] [Google Scholar]
- 3.Sartor M, Steingrimsdottir H, Elamin F, et al. Role of P16/MTS1, cyclin D1 and RB in primary oral cancer and oral cancer cell lines. Br J Cancer 1999;80(1–2):79–86. 10.1038/sj.bjc.6690505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyle JO, Hakim J, Koch W, et al. The incidence of P53 mutations increases with progression of head and neck cancer. Cancer Res 1993;53(19):4477–4480. [PubMed] [Google Scholar]
- 5.Grandis JR, Tweardy DJ. Elevated levels of transforming growth factor α and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res 1993;53(15):3579–3584. [PubMed] [Google Scholar]
- 6.Vermorken JB, Trigo J, Hitt R, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based. J Clin Oncol 2007;25(16): 2171–2177. 10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 7.Yonesaka K, Zejnullahu K, Okamoto I, et al. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med 2011;3(99): 99ra86–99ra86. 10.1126/scitranslmed.3002442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berchuck A, Kamel A, Whitaker R, et al. Overexpression of HER-2/Neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer Res 1990;50(13):4087–4091. [PubMed] [Google Scholar]
- 9.Slamon D, Clark G, Wong S, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/Neu oncogene. Science 1987;235(4785):177–182. 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 10.Iqbal N, Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol Biol Int 2014;2014:852748 10.1155/2014/852748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rimawi MF, Schiff R, Osborne CK. Targeting HER2 for the treatment of breast cancer. Annu Rev Med 2015;66(1):111–128. 10.1146/annurev-med-042513-015127. [DOI] [PubMed] [Google Scholar]
- 12.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/Neu proto-oncogene in human breast and ovarian cancer. Science 1989;244(4905):707–712. 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 13.Ménard S, Pupa SM, Campiglio M, Tagliabue E. Biologic and therapeutic role of HER2 in cancer. Oncogene Nature Publishing Group. 2003;22(42):6570–6578. 10.1038/sj.onc.1206779. [DOI] [PubMed] [Google Scholar]
- 14.Pollock NI, Grandis JR. HER2 as a therapeutic target in head and neck squamous cell carcinoma. Clin Cancer Res 2015;21 (3):526–533. 10.1038/jid.2014.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavalot A, Martone T, Rogero N, et al. Prognostic impact of HER-2/Neu expression on squamous head and neck carcinomas. Head Neck 2007;29:655–664. 10.1002/HED. [DOI] [PubMed] [Google Scholar]
- 16.Sardari Y, Pardis S, Tadbir AA, et al. HER2/Neu expression in head and neck squamous cell carcinoma patients is not significantly elevated. Asian Pacific J Cancer Prev 2012;13(6):2891–2896. 10.7314/APJCP.2012.13.6.2891. [DOI] [PubMed] [Google Scholar]
- 17.Pardis S, Sardari Y, Ashraf MJ, et al. Evaluation of tissue expression and salivary levels of HER2/Neu in patients with head and neck squamous cell carcinoma. Iran J Otorhinolaryngol 2012;24(69):161–170. 10.22038/ijorl.2012.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato-Kuwabara Y, Neves JI, Fregnani JHTG, Sallum RA, Soares FA. Evaluation of gene amplification and protein expression of HER-2/Neu in esophageal squamous cell carcinoma using fluorescence in situ hybridization (FISH) and immunohistochemistry. BMC Cancer 2009;9:6. 10.1186/1471-2407-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanken H, Gaudin R, Gröbe A, et al. Her2 expression and gene amplification is rarely detectable in patients with oral squamous cell carcinomas. J Oral Pathol Med 2014;43(4):304–308. 10.1111/jop.12173. [DOI] [PubMed] [Google Scholar]
- 20.Ali MAS, Gunduz M, Gunduz E, et al. Expression and mutation analysis of Her2 in head and neck squamous cell carcinoma. Cancer Invest 2010;28(5):495–500. 10.3109/07357900903476778. [DOI] [PubMed] [Google Scholar]
- 21.Xia W, Lau Y-K, Zhang H-Z, et al. Strong correlation of patients between with overexpression cell overall squamous carcinoma. Clin Cancer Res 1997;3 (January):3–9. [PubMed] [Google Scholar]
- 22.Shaw AR, Porter CE, Watanabe N, et al. Adenovirotherapy delivering cytokine and checkpoint inhibitor augments CAR T cells against metastatic head and neck cancer. Mol Ther 2017; 25(11):2440–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porter CE, Rosewell Shaw A, Jung Y, et al. Oncolytic adenovirus armed with BiTE, cytokine, and checkpoint inhibitor enables CAR T cells to control the growth of heterogeneous tumors. Mol Ther 2020;28(5):1251–1262. 10.1016/j.ymthe.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corbett IP, Henry JA, Angus B, et al. NCL-CB11, a new monoclonal antibody recognizing the internal domain of the C- Erb B-2 oncogene protein effective for use on formalin-fixed, paraffin-embedded tissue. J Pathol 1990;161(1):15–25. [DOI] [PubMed] [Google Scholar]
- 25.Gilbertson RJ, Pearson ADJ, Perry RH, Jaros E, Kelly PJ. Prognostic significance of the C-ErbB-2 oncogene product in childhood medulloblastoma. Br J Cancer 1995;71(3):473–477. 10.1038/bjc.1995.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 1981;29(4):577–580. 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 27.Beckhardt RN, Kiyokawa N, Xi L, Liu T. Her-2/Neu oncogene characterization in head and neck squamous cell carcinma. Head Neck 1995;121:1265–1270. [DOI] [PubMed] [Google Scholar]
- 28.Vivian J, Rao AA, Nothaft FA, et al. Toil enables reproducible, open source, big biomedical data analyses. Nat Biotechnol 2017;35(4):314–316. 10.1038/nbt.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Lu Y, Akbani R, et al. TCPA: a resource for cancer functional proteomics data. Nat Methods 2013;10(11):1046–1047. 10.1038/nmeth.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Akbani R, Zhao W, et al. Explore, visualize, and analyze functional cancer proteomic data using the cancer proteome atlas. Cancer Res 2017;77(21):e51–e54. 10.1158/0008-5472.CAN-17-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dahlstrom KR, Calzada G, Hanby JD, et al. An evolution in demographics, treatment, and outcomes of oropharyngeal cancer at a major cancer center: a staging system in need of repair. Cancer 2013;119(1):81–89. 10.1002/cncr.27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandulache VC, Hamblin J, Lai S, et al. Oropharyngeal squamous cell carcinoma in the veteran population: association with traditional carcinogen exposure and poor clinical outcomes. Head Neck 2015;37(9):1246–1253. 10.1002/hed.23740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolff AC, Elizabeth Hale Hammond M, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice guideline focused update. J Clin Oncol 2018;36(20):2105–2122. 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 34.Rüschoff J, Hanna W, Bilous M, et al. HER2 testing in gastric cancer: a practical approach. Mod Pathol 2012;25(5):637–650. 10.1038/modpathol.2011.198. [DOI] [PubMed] [Google Scholar]
- 35.Angiero F, Del Sordo R, Dessy E, et al. Comparative analysis of C-ErbB-2 (HER-2/Neu) in squamous cell carcinoma of the tongue: does over-expression exist? And what is its correlation with traditional diagnostic parameters? J Oral Pathol Med 2008; 37(3):145–150. 10.1111/j.1600-0714.2007.00603.x. [DOI] [PubMed] [Google Scholar]
- 36.Wei Q, Sheng L, Shui Y, et al. EGFR, HER2, and HER3 expression in laryngeal primary tumors and corresponding metastases. Ann Surg Oncol 2008;15(4):1193–1201. 10.1245/s10434-007-9771-3. [DOI] [PubMed] [Google Scholar]
- 37.Ibrahim S, Vasstrand E, Liavaag P, Johnannessen A, Lillehaug J. Expression of C-ErbB Proto-oncogene family members in squamous cell carcinoma of the head and neck. Anticancer Res 1997;17:4539–4546. [PubMed] [Google Scholar]
- 38.Field J, Spandidos D, Yiagnisis M, Gosney J, Papadimitriou K. C-ErbB-2 expression in squamous cell carcinoma of the head and neck. Anticancer Res 1992;12:613–619. [PubMed] [Google Scholar]
- 39.Warren EA, Liu H-C, Porter CE, et al. Abstract 574: overexpression of HER2 in head and neck cancer represents a potential target for T cell immunotherapy. Cancer research Vol 79. Philadelphia, PA: American Association for Cancer Research (AACR); 2019:574–574. 10.1158/1538-7445.am2019-574. [DOI] [Google Scholar]
- 40.Wang G, Zhang W, Jiang W, Luan L. Overexpression of Her-2 associated with the progression of esophageal cancer patients. Hepatogastroenterology 2013;60(128):1972–1978. 10.5754/hge13409. [DOI] [PubMed] [Google Scholar]
- 41.Sharp L, McDevitt J, Carsin AE, Brown C, Comber H. Smoking at diagnosis is an independent prognostic factor for cancer-specific survival in head and neck cancer: findings from a large, population-based study. Cancer Epidemiol Biomarkers Prev 2014;23(11):2579–2590. 10.1158/1055-9965.EPI-14-0311. [DOI] [PubMed] [Google Scholar]
- 42.Xia W, Lau Y-K, Zhang H-Z, et al. Strong correlation between C-ErbB-2 overexpression and overall survival of patients with oral squamous cell carcinoma; 1997; Vol. 3. [PubMed] [Google Scholar]
- 43.Kemnade JO, Elhalawani H, Castro P, et al. CD8 infiltration is associated with disease control and tobacco exposure in intermediate-risk oropharyngeal cancer. Sci Rep 2020;10(1):1–10. 10.1038/s41598-019-57111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gillison ML, Trotti AM, Harris J, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet 2019;393(10166):40–50. 10.1016/S0140-6736(18)32779-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehanna H, Robinson M, Hartley A, et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet 2019;393 (10166):51–60. 10.1016/S0140-6736(18)32752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data availability statement. TCGA data is publicly available for use and re-analysis through tcpaportal.org. Institutional data from patient specimens as well as clinical data can be provided upon reasonable inquiry/request in an aggregated, de-identified fashion following approval from the appropriate hospital and institutional administrative bodies in a manner compliant with all institutional, local, state and federal regulations pertaining to transfer of patient data and materials.


