Abstract
Pterygium is a common ocular disease with a high recurrence rate, characterized by hyperplasia of subconjunctival fibrovascular tissue. Autophagy, an important process to maintain cellular homeostasis, participates in the pathogenic fibrosis of different organs. However, the exact role of autophagy in pterygium pathogenesis remains unknown. Here, we found that autophagic activity was decreased in human pterygium tissues compared with adjacent normal conjunctival tissues. The in vitro model of fibrosis was successfully established using human primary conjunctival fibroblasts (ConFB) treated with transforming growth factor-β1 (TGF-β1), evidenced by increased fibrotic level and strong proliferative and invasive capabilities. The autophagic activity was suppressed during TGF-β1- or ultraviolet-induced fibrosis of ConFB. Activating autophagy dramatically retarded the fibrotic progress of ConFB, while blocking autophagy exacerbated this process. Furthermore, SQSTM1, the main cargo receptor of selective autophagy, was found to significantly promote the fibrosis of ConFB through activating the PKCι–NF-κB signaling pathway. Knockdown of SQSTM1, PKCι, or p65 in ConFB delayed TGF-β1-induced fibrosis. Overexpression of SQSTM1 drastically abrogated the inhibitory effect of rapamycin or serum starvation on TGF-β1-induced fibrosis. Collectively, our data suggested that autophagy impairment of human ConFB facilitates fibrosis via activating the SQSTM1–PKCι–NF-κB signaling cascades. This work was contributory to elucidating the mechanism of autophagy underlying pterygium occurrence.
Keywords: pterygium, autophagy, fibrosis, SQSTM1, NF-κB
Introduction
Pterygium is a common ocular disease characterized by hyperplasia of subconjunctival fibrovascular tissue (Kim et al., 2016). The existence of pterygium affects patients’ esthetic appearance considerably. When invading into the cornea, pterygium causes significant symptoms, including irritation and vision impairment due to increased corneal astigmatism (Chu et al., 2020). Pterygium can be effectively removed by surgery, but the high recurrence rate is still a major concern (Clearfield et al., 2016). Novel strategies to inhibit pterygium growth and prevent recurrence are of great significance.
Although the main risk factor for pterygium is now recognized as ultraviolet (UV) radiation (Di Girolamo et al., 2004; Bradley et al., 2010), the exact pathogenesis remains unknown. Currently, the fibrotic process is thought to play a key role in the onset of pterygium. The pterygium fibroblasts (pFB) displayed abnormal ability of proliferation and invasion as compared with normal conjunctival fibroblasts (ConFB) (Mai et al., 2019). Deposit and remodeling of extracellular matrix (ECM) were observed in pterygium tissues (Seet et al., 2012). Moreover, the pterygium tissue contained abundant alpha-smooth muscle actin (α-SMA) (Touhami et al., 2005; Lee et al., 2019), a cellular marker for myofibroblast and a sign of fibrosis (Shu and Lovicu, 2017). Particularly, for the pathogenesis of recurrent pterygium, the fibrotic process is even more pivotal (Kim et al., 2013). It has been established that excessive wound healing after pterygium excision surgery led to fibrosis and eventually resulted in recurrence (Zada et al., 2018). Therefore, fibrosis has been regarded as the key mechanism for pterygium development and may offer the clue to develop potential targeted therapies.
Macroautophagy (hereafter referred to as autophagy) is a highly evolutionarily conservative process, in which damaged organelles and protein aggregates are degraded in lysosomes and their degradation products are reused (Deretic, 2021). Autophagy has important physiological significance, as it provides new synthetic materials, maintains cellular homeostasis, and protects cells from stress and injury (Boya et al., 2016). The dysregulation of autophagic activity was involved in the onset of multiple diseases, including tumor, infection, cardiovascular disease, and neurodegenerative disease (Dikic and Elazar, 2018). Of note, recent evidences suggested that autophagy may play an important role in fibrotic diseases, such as cirrhosis, renal fibrosis, and skin fibrosis (Del Principe et al., 2013; Lodder et al., 2015; Li et al., 2016; Livingston et al., 2016; Nishida and Otsu, 2016; Nwosu et al., 2016). Therefore, it can be speculated that autophagy is also essential to the pterygium, which is closely associated with fibrosis. However, the effect of autophagy on the fibrotic process is controversial. Most investigators believe that downregulated autophagy is responsible for fibrosis (Del Principe et al., 2013; Lodder et al., 2015; Li et al., 2016; Nishida and Otsu, 2016), while some hold the opposite view that activation of autophagy promotes fibrosis (Livingston et al., 2016; Nwosu et al., 2016). Currently, the exact role of autophagy in pterygium pathogenesis remains unrevealed.
Autophagy is regulated by a series of intracellular signaling pathways, among which the mechanistic target of rapamycin (mTOR) pathway plays an important negative regulatory role (Dikic and Elazar, 2018). A previous study reported that mTORC1 signaling was highly activated in the pterygium tissue, which led to inhibition of autophagy and reduction of apoptosis (Liu et al., 2017). Two other studies reported the positive correlation between mTOR and α-SMA expression in pterygium, and activation of mTOR promoted the transdifferentiation of pFB to myofibroblasts (Zhao et al., 2017; Zhao et al., 2018). These findings provided evidence that the dysregulation of autophagy might be associated with fibrosis and eventually result in the occurrence of pterygium. Nevertheless, the exact mechanism is still far from being fully elucidated.
This study aims to investigate the role of autophagy in the pathogenesis of pterygium. We found that the human pterygium tissues exhibited decreased autophagic activity compared with adjacent normal conjunctival tissues. Furthermore, autophagic activity was suppressed in human primary ConFB during the process of transforming growth factor-β1 (TGF-β1)- or UV-induced fibrosis. Elevating the autophagic activity almost blocked the fibrotic progress via degrading SQSTM1 protein (also known as p62 protein) and therefore suppressing the SQSTM1–PKCι–NF-κB signaling pathway. Our work may provide the clues to better understand the fibrogenesis in pterygium occurrence and, hence, develop the potential target for clinical therapy.
Results
The pterygium tissue exhibits decreased autophagic activity and severe fibrosis
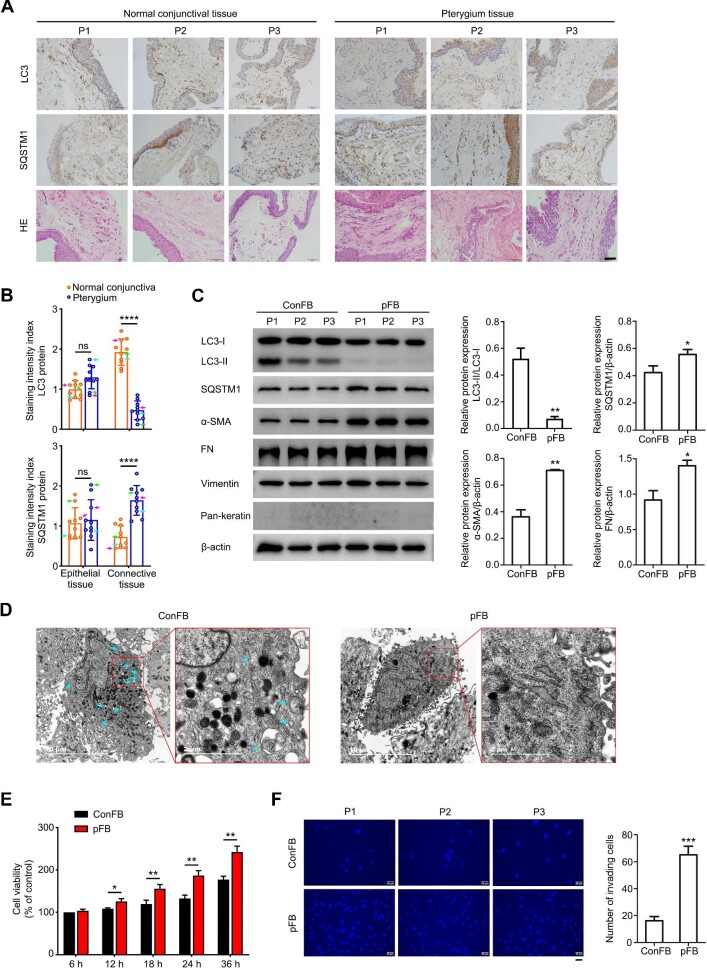
To investigate the role of autophagy in pterygium development, pterygium tissues and adjacent normal conjunctival tissues from the same individuals were collected, and the expression levels of LC3 and SQSTM1 were detected (Figure 1A and B). Remarkable hemangiectasis and abundant fibroblasts in pterygium tissues were observed after hematoxylin–eosin (HE) staining (Figure 1A). The immunohistochemistry staining results showed a significant lower level of LC3 protein in pterygium compared with the normal conjunctiva, especially in the connective tissue of pterygium, where the fibroblast proliferation and extracellular collagen deposition were predominant. In line with this, the stronger staining of SQSTM1 was detected in the connective tissue of pterygium compared with the normal conjunctiva (Figure 1B). As an important autophagic adaptor protein, SQSTM1 is thought to be degraded by the autophagy–lysosomal pathway after recruiting target organelles and proteins into the autophagosome. The decreased LC3 protein level and increased SQSTM1 protein level indicated the reduced autophagic activity in the connective tissue of pterygium. On the other hand, the protein levels of LC3 and SQSTM1 in the epithelial cells did not show a significant difference between pterygium tissues and adjacent normal conjunctival tissues (Figure 1A and B). Therefore, we focused on the effect of autophagy on the fibrotic process of pterygium in this study.
Figure 1.
Decreased autophagic activity and aggravated fibrosis in pterygium tissues and pFB. (A) Representative immunohistochemistry images of normal conjunctival tissues and pterygium tissues from three pterygium patients (P1, P2, and P3). Scale bar, 50 μm. (B) Staining intensity index of LC3 and SQSTM1 proteins for normal conjunctival tissues and pterygium tissues from 10 pterygium patients (mean ± SD; ns, not significant; ****P < 0.0001; paired Student's t-test; magenta arrows, P1; green arrows, P2; cyan arrows, P3). (C) Immunoblots showing protein levels of LC3, SQSTM1, α-SMA, FN, vimentin, and pan-keratin in primary ConFB and primary pFB isolated from tissues of three pterygium patients (left). Quantified protein levels were displayed as bar graphs (right). Data are presented as mean ± SD from three independent experiments (*P < 0.05, **P < 0.01 vs. ConFB; unpaired Student's t-test). (D) Representative electron microscopic images of ConFB and pFB. Cyan arrows point to autophagosome or autolysosome-like structures. Scale bar, 10 μm and 2 μm (magnified). (E) Viability of ConFB and pFB after cultured for the indicated time, determined by the CCK8 assay. Data are presented as mean ± SD from three independent experiments (*P < 0.05, **P < 0.01; unpaired Student's t-test). (F) Invasive ability of ConFB and pFB isolated from tissues of three pterygium patients (P1, P2, and P3), determined by the transwell assay (left). Scale bar, 50 μm. The average numbers of invading cells per visual field were displayed in a bar graph (right). Data are presented as mean ± SD from three independent experiments (***P < 0.001 vs. ConFB; unpaired Student's t-test).
Meanwhile, primary fibroblasts were obtained from pterygium tissues and adjacent normal conjunctival tissues of three patients, respectively, and the autophagic level of fibroblasts was further assessed based on the conversion of LC3-I to LC3-II protein. The cultured fibroblasts were first identified by the mesenchymal cell marker, vimentin, and the epithelial cell marker, pan-keratin (Figure 1C). The LC3-II/LC3-I ratio in pFB was much lower than that in normal ConFB, confirming the decreased autophagic activity in pFB. Accordingly, the protein level of SQSTM1 was upregulated in pFB (Figure 1C). Under transmission electron microscopy, a large number of autophagosomes and autolysosomes were observed in ConFB, while few were seen in pFB (Figure 1D). The autophagic activity of ConFB and pFB was also evaluated via immunofluorescence staining of LC3 and SQSTM1 (Supplementary Figure S1). The reduced autophagic activity with fewer LC3 puncta and higher immunofluorescent intensity of SQSTM1 was observed in pFB, when compared with ConFB.
We also assessed the characteristics of pFB and ConFB as well as their potency of proliferation and invasion. α-SMA, the marker of myofibroblast, was more obviously accumulated in pFB compared with that in ConFB, indicating the enhanced myofibroblast transdifferentiation. The protein level of fibronectin (FN), an important component of ECM, was higher in pFB than in ConFB (Figure 1C). Importantly, pFB exhibited higher proliferative activity than ConFB in a time-dependent pattern, as shown by the Cell Counting Kit-8 (CCK8) assay results (Figure 1E). Moreover, the pterygium has a high invasive ability, as its head can invade the Bowman's membrane. The invasive ability of pFB was also higher than that of ConFB, which was detected by the transwell assay (Figure 1F).
Downregulation of autophagy during the process of TGF-β1- or UV-induced fibrosis
UV radiation, the major risk factor of pterygium development, can induce oxidative stress and lead to cell proliferation of conjunctival tissues (Wu et al., 2020). Oxidative stress was also associated with the fibrotic process of various organs, including the liver, lung, and kidney (Liu and Gaston Pravia, 2010). In this study, ConFB were exposed to various durations of UV radiation (Supplementary Figure S2). Increased fibrosis was observed in ConFB cultured for 24 h after exposed to UV radiation for 12 min, as evidenced by the significantly increased protein levels of α-SMA and FN. Accompany with this, the LC3-II/LC3-I ratio slightly increased after short UV exposure (6 min) and then significantly declined after prolonged UV radiation. SQSTM1 protein was accumulated after prolonged UV exposure, indicating that the autophagic activity of ConFB was inhibited under oxidative stress.
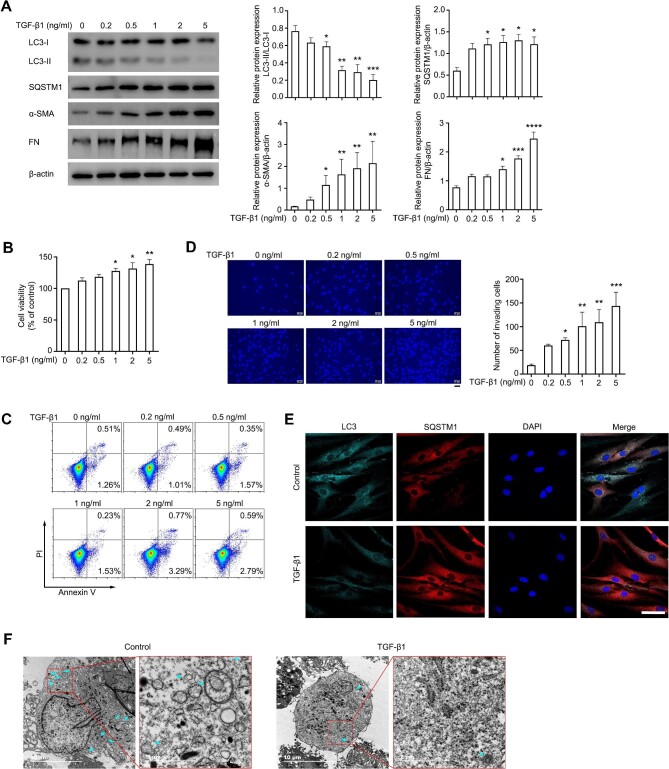
TGF-β1, a key cytokine in the fibrotic process, was found to be obviously upregulated in various fibrotic tissues (Kim et al., 2018). Thus, TGF-β1 was used as a common inducer for tissue fibrosis, including pterygium (Kim et al., 2016; Chen et al., 2019; Kim et al., 2019). We treated ConFB with various doses of TGF-β1 and investigated their fibrosis degree and autophagic level. The accumulation of α-SMA and FN in ConFB was induced by TGF-β1 treatment in a dose-dependent pattern, indicating an accelerated fibrotic process (Figure 2A). Consistently, the ConFB exhibited enhanced proliferative ability after treated with TGF-β1, evidenced by the increased viability (Figure 2B) and unchanged dead cell ratio (Figure 2C). The invasive ability of ConFB was also significantly increased after TGF-β1 treatment (Figure 2D). As expected, a significantly decreased LC3-II/LC3-I ratio and an increased SQSTM1 protein level were observed following 1 ng/ml TGF-β1 treatment for 48 h (Figure 2A). The numbers of LC3 puncta and autophagosomes/autolysosomes decreased after TGF-β1 treatment, as detected by immunofluorescence microscopy and transmission electron microscopy (Figure 2E and F). Altogether, these results indicated that the fibrotic process of ConFB was accompanied with the downregulation of autophagic activity.
Figure 2.
Autophagic activity is inhibited during the process of TGF-β1-induced fibrosis in ConFB. (A–D) Primary ConFB were stimulated with different concentrations of TGF-β1 for 48 h. (A) Immunoblots showing protein levels of LC3, SQSTM1, α-SMA, and FN (left). Quantified protein levels were displayed as bar graphs (right). (B) Viability determined by the CCK8 assay. (C) Apoptosis determined by the Annexin V–PI assay. (D) Invasive ability determined by the transwell assay (left). Scale bar, 50 μm. The average numbers of invading cells per visual field were displayed in a bar graph (right). (E and F) Primary ConFB were stimulated with 1 ng/ml TGF-β1 for 48 h. (E) Confocal images of LC3 (cyan), SQSTM1 (red), and DAPI (blue). Scale bar, 50 μm. (F) Representative electron microscopic images. Cyan arrows point to autophagosome or autolysosome-like structures. Scale bar, 10 μm and 2 μm (magnified). All quantitative data are presented as mean ± SD from three independent experiments (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs. control group; one-way ANOVA followed by Bonferroni post hoc test).
Activating autophagy dramatically retards TGF-β1-induced fibrosis
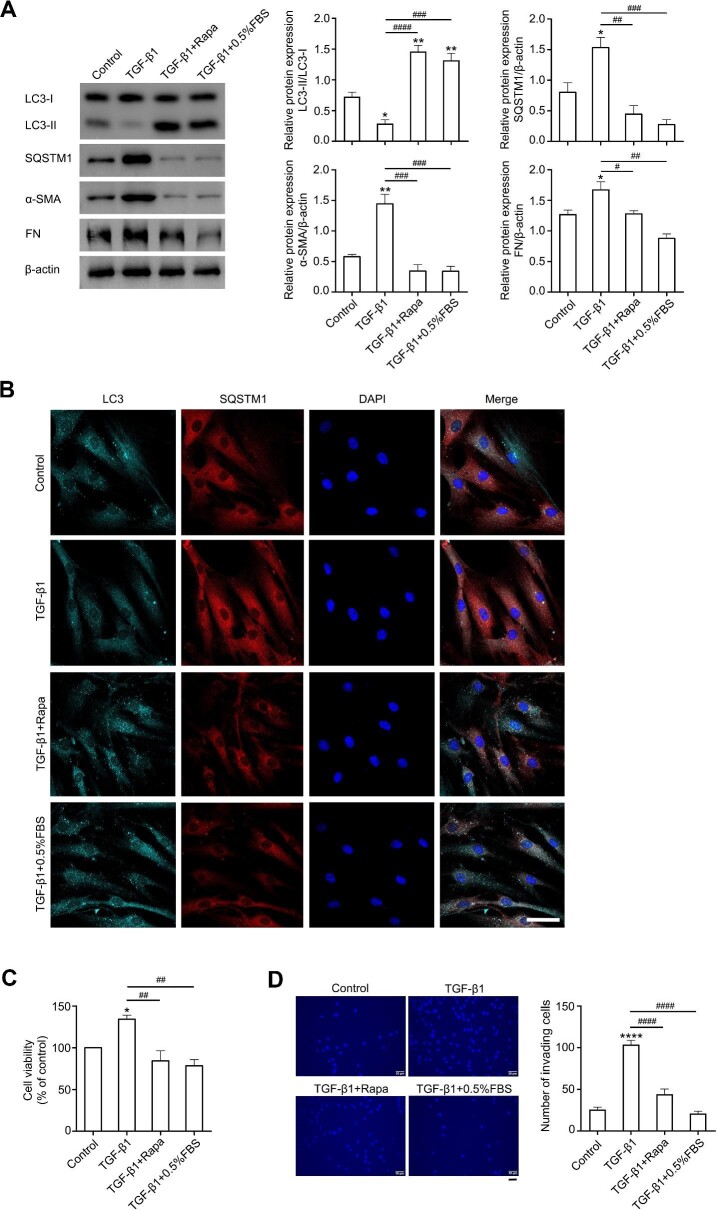
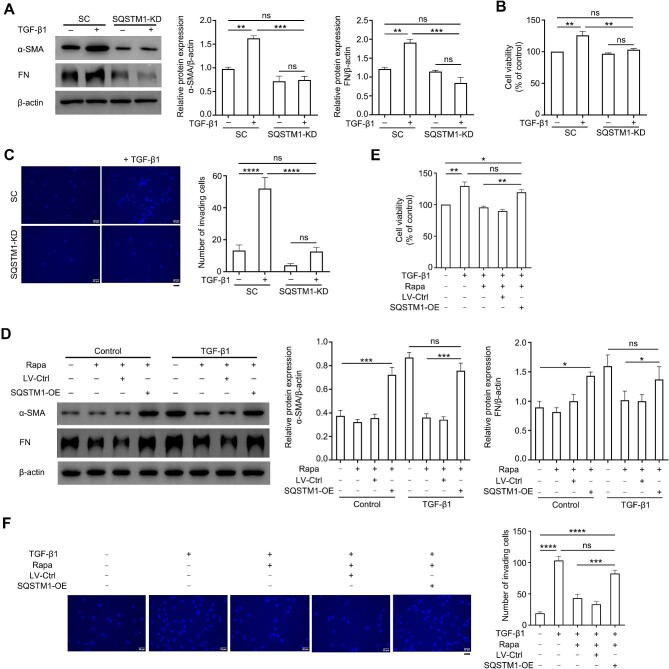
Because of the controversial role of autophagy in the fibrotic process of different tissues, the exact effect of autophagy on pterygium development was investigated via elevating the autophagic flux in ConFB and pFB by rapamycin or serum starvation [0.5% fetal bovine serum (FBS)]. In ConFB, both rapamycin and serum starvation treatments induced a high level of autophagy, with elevated LC3-II/LC3-I ratio, increased LC3 puncta, and decreased SQSTM1 protein level (Figure 3A and B). Meanwhile, the expression levels of α-SMA and FN in TGF-β1 + rapamycin and TGF-β1 + 0.5% FBS treatment groups were lower than those in the TGF-β1 treatment alone group and similar to those in the control group, suggesting the inhibition of TGF-β1-induced fibrosis by autophagy activation (Figure 3A). TGF-β1-induced proliferation and invasion of ConFB were also significantly inhibited after autophagy activation (Figure 3C and D). Similarly, in pFB, rapamycin or serum starvation led to a high level of autophagy and decreased levels of α-SMA and FN (Supplementary Figure S3A). The pFB displayed weakened proliferative and invasive abilities when autophagy was reinforced (Supplementary Figure S3B–D). Taken together, these data indicated that elevating autophagic activity dramatically retarded the fibrotic progress of ConFB.
Figure 3.
Activating autophagy alleviates TGF-β1-induced fibrosis in primary ConFB. Primary ConFB were stimulated with 1 ng/ml TGF-β1 for 48 h, with co-treatment of 2 μM rapamycin or serum starvation (0.5% FBS) for 18 h. (A) Immunoblots showing protein levels of LC3, SQSTM1, α-SMA, and FN (left). Quantified protein levels were displayed as bar graphs (right). (B) Confocal images of LC3 (cyan), SQSTM1 (red), and DAPI (blue). Scale bar, 50 μm. (C) Viability determined by the CCK8 assay. (D) Invasive ability determined by the transwell assay (left). Scale bar, 50 μm. The average numbers of invading cells per visual field were displayed in a bar graph (right). All quantitative data are presented as mean ± SD from three independent experiments (*P < 0.05, **P < 0.01, ****P < 0.0001 vs. control group; #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001 vs. TGF-β1 treatment group; one-way ANOVA followed by Bonferroni post hoc test). Rapa, rapamycin.
Inhibition of autophagy exacerbates TGF-β1-induced fibrosis
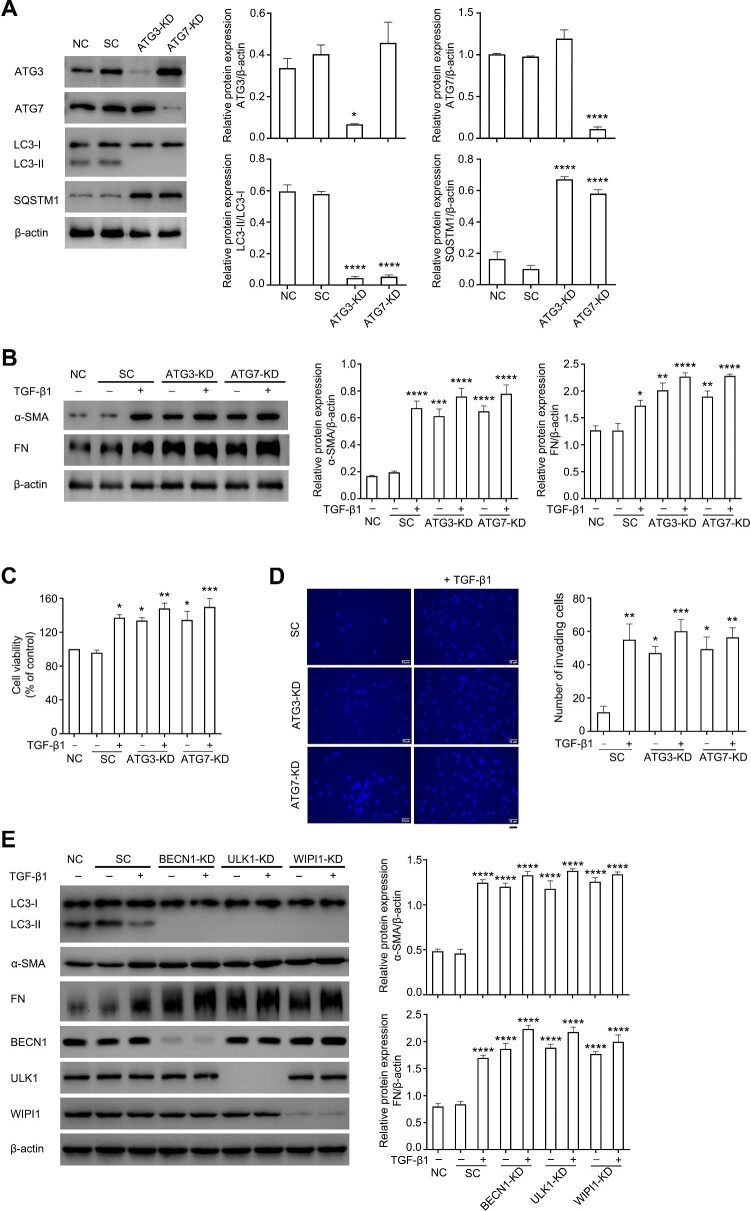
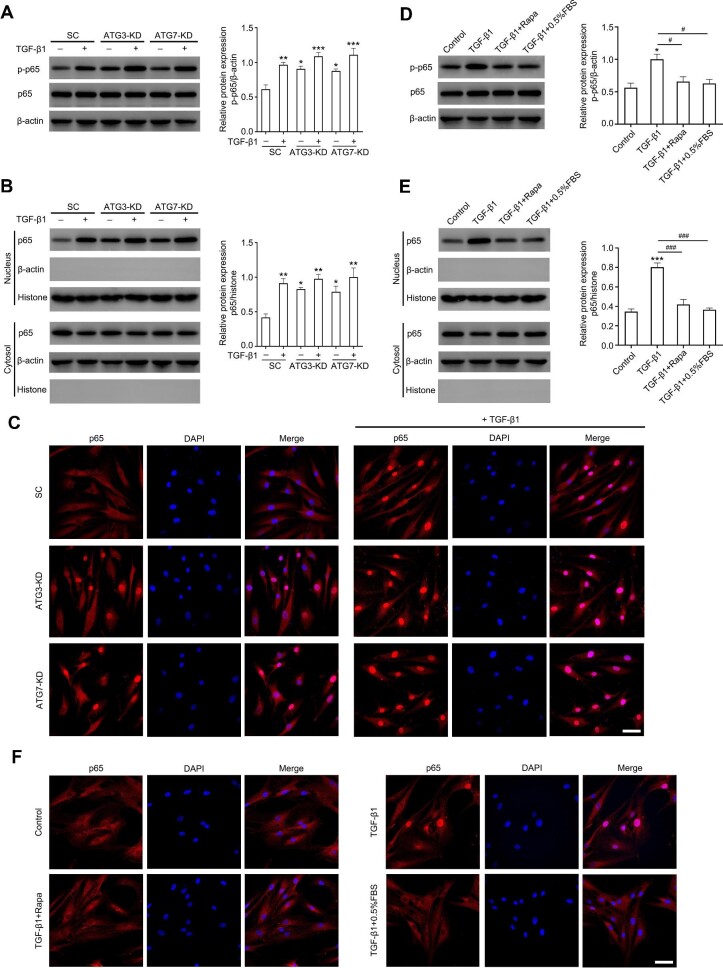
To explore whether blocking autophagy aggravates the fibrotic process, autophagy-related gene 3 (ATG3) or ATG7 was knocked down in ConFB using a lentiviral-shRNA system. As shown in Figure 4A, knockdown of ATG3 or ATG7 almost abrogated the autophagy flux in ConFB. The α-SMA and FN expression levels were increased after ATG3 or ATG7 knockdown, compared with scramble control, and were further elevated with additional treatment of 1 ng/ml TGF-β1 (Figure 4B). Knockdown of ATG3 or ATG7 also promoted cell proliferative rate and invasive potential of ConFB, both in the presence and absence of TGF-β1 (Figure 4C and D). To further rule out the effect of non-canonical autophagy, BECN1, ULK1, and WIPI1 were knocked down, respectively. These proteins are essential for canonical autophagy, but can be bypassed during the process of non-canonical autophagy (Codogno et al., 2011). Knocking down BECN1, ULK1, or WIPI1 led to the blockage of autophagic flux and obvious increase of α-SMA and FN protein expression, regardless of the presence of TGF-β1 (Figure 4E), indicating that canonical autophagy, rather than non-canonical autophagy, was involved in the fibrotic process of ConFB.
Figure 4.
Blocking autophagy aggravates TGF-β1-induced fibrosis in primary ConFB. (A) Knockdown of ATG3 or ATG7 using lentiviral-shRNA resulted in autophagy blockage and accumulation of SQSTM1, confirmed by immunoblots (left). Quantified protein levels were displayed as bar graphs (right). Data are presented as mean ± SD from three independent experiments (*P < 0.05, ****P < 0.0001 vs. normal control group; one-way ANOVA followed by Bonferroni post hoc test). (B–D) Primary ConFB before and after ATG3 or ATG7 knockdown were incubated in the presence or absence of 1 ng/ml TGF-β1 for 48 h. (B) Immunoblots showing protein levels of α-SMA and FN (left). Quantified protein levels were displayed as bar graphs (right). (C) Viability determined by the CCK8 assay. (D) Invasive ability determined by the transwell assay (left). Scale bar, 50 μm. The average numbers of invading cells per visual field were displayed in a bar graph (right). Data are presented as mean ± SD from three independent experiments (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs. normal control group (B and C) or scramble control group (D); one-way ANOVA followed by Bonferroni post hoc test). (E) Immunoblots showing protein levels of LC3, α-SMA, FN, BECN1, ULK1, and WIPI1 in primary ConFB before and after knockdown of BECN1, ULK1, or WIPI1 incubated in the presence or absence of 1 ng/ml TGF-β1 for 48 h (left). Quantified protein levels were displayed as bar graphs (right). Data are presented as mean ± SD from three independent experiments (****P < 0.0001 vs. normal control group; one-way ANOVA followed by Bonferroni post hoc test). SC, scramble control; KD, knockdown.
In addition to RNA interference, chemical autophagy inhibitors, 3-MA and chloroquine (CQ), were also applied to suppress the autophagic activity of ConFB. As a lysosomotropic autophagy inhibitor, CQ blocked the fusion of autophagosome to lysosome and subsequently caused accumulation of LC3-II (Supplementary Figure S4A). As expected, both 3-MA and CQ further aggravated TGF-β1-induced fibrosis of ConFB compared with TGF-β1 treatment alone. The expression levels of α-SMA and FN, as well as the ability of proliferation and invasion, were significantly enhanced when autophagy was inhibited (Supplementary Figure S4A–C).
Autophagy alleviates TGF-β1-induced fibrosis depending on SQSTM1 protein
SQSTM1 is mainly defined as a cargo receptor for selective autophagy to recruit the damaged organelles and protein aggregates into the autophagosome (Katsuragi et al., 2015). Besides this role, SQSTM1 also functions as a central signaling hub for the activation of the NF-κB and NRF2 signaling pathways, linking SQSTM1 to inflammation, oxidative defense system, nutrient sensing, and tumor initiation and progress, respectively (Fan et al., 2018; Sanchez-Martin and Komatsu, 2018; Sanchez-Martin et al., 2019). In this study, we found the negative regulation of SQSTM1 protein by the autophagic activity in the fibrotic process (Figures 3A, B and 4A). Based on these findings, it could be speculated that autophagy may influence pterygium development and the fibrotic process via regulating SQSTM1 protein.
To further determine the role of SQSTM1 in TGF-β1-induced fibrosis of primary ConFB, the expression of SQSTM1 was silenced in ConFB using a lentiviral-shRNA system (Supplementary Figure S5A). Knockdown of SQSTM1 fully abrogated the signatures of fibrosis induced by TGF-β1, resulting in reduced accumulation of α-SMA and FN as well as reduced cellular proliferative and invasive abilities (Figure 5A–C). These signatures in the SQSTM1-silenced ConFB remained unchanged regardless of TGF-β1 treatment, suggesting that TGF-β1-induced fibrosis was dependent on SQSTM1. In parallel with this, overexpression of SQSTM1 protein in ConFB accelerated the accumulation of α-SMA and FN and elevated the proliferative and invasive potential of ConFB (Supplementary Figure S5B–D). More importantly, overexpression of SQSTM1 protein could drastically abrogate the inhibitory effect of rapamycin and serum starvation on TGF-β1-induced fibrosis, as evidenced by the increased α-SMA and FN expression levels, as well as elevated cellular proliferative and invasive abilities (Figure 5D–F; Supplementary Figure S5E–G). Therefore, these data indicated that the regulatory effect of autophagy on the fibrotic process of ConFB was largely dependent on the SQSTM1 protein level.
Figure 5.
Autophagy alleviates TGF-β1-induced fibrosis depending on SQSTM1 protein. (A–C) Primary ConFB with or without SQSTM1 knockdown were incubated in the presence or absence of 1 ng/ml TGF-β1 for 48 h. (A) Immunoblots showing protein levels of α-SMA and FN (left). Quantified protein levels were displayed as bar graphs (right). (B) Viability determined by the CCK8 assay. (C) Invasive ability determined by the transwell assay (left). Scale bar, 50 μm. The average numbers of invading cells per visual field were displayed in a bar graph (right). (D–F) Primary ConFB with or without SQSTM1 overexpression were incubated in the presence or absence of 1 ng/ml TGF-β1 for 48 h and 2 μM rapamycin for 18 h. (D) Immunoblots showing protein levels of α-SMA and FN (left). Quantified protein levels were displayed as bar graphs (right). (E) Viability determined by the CCK8 assay. (F) Invasive ability determined by the transwell assay (left). Scale bar, 50 μm. The average numbers of invading cells per visual field were displayed in a bar graph (right). All quantitative data are presented as mean ± SD from three independent experiments (ns, not significant; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; one-way ANOVA followed by Bonferroni post hoc test). SC, scramble control; KD, knockdown; OE, overexpression; Rapa, rapamycin.
SQSTM1 promotes TGF-β1-induced fibrosis by activating the NF-κB signaling pathway
SQSTM1 is a multi-functional scaffold protein that directly interacts with different key signaling proteins through well-defined structural elements: SQSTM1 can excite the IκB–NF-κB pathway through binding to atypical protein kinase C (aPKC) by its TB domain; SQSTM1 also activates the NRF2-dependent anti-oxidative response by sequestering KEAP1 through its KIR domain (Sanchez-Martin and Komatsu, 2018). Recent studies have extended the function of NRF2 that acute activation of NRF2 causes an anti-oxidative effect, and prolonged activation of NRF2 is important to tumor initiation and progress (Ni et al., 2014; Jiang et al., 2015). Because of the hyperproliferative characteristic of the pterygium tissue, we further investigated whether SQSTM1 promotes TGF-β1-induced fibrosis through activating the NF-κB and NRF2 signaling pathways.
In TGF-β1-treated ConFB, the increased phosphorylation level of p65 was detected, while the phosphorylation level of NRF2 had no obvious change (Figure 6A). p65, the subunit of NF-κB protein, is activated by phosphorylation and migrates into the nucleus to promote the transcription of target genes, including some pro-survival genes (Oeckinghaus et al., 2011). The nuclear translocation of p65 was assessed in the cytosolic and nuclear extracts of ConFB by western blot analysis. Indeed, the level of p65 in the nucleus was elevated after TGF-β1 treatment (Figure 6B). Immunofluorescence staining results also confirmed the increased nuclear translocation of p65 after TGF-β1 treatment (Figure 6C). In line with these findings, the pterygium tissue showed a higher staining intensity of phosphorylated p65 (p-p65) than the normal conjunctival tissue, as shown by immunohistochemistry staining (Figure 6D and E).
Figure 6.
TGF-β1 promotes fibrosis of primary ConFB through activating the SQSTM1–NF-κB signaling pathway. (A–C) Primary ConFB with or without SQSTM1 knockdown were incubated in the presence or absence of 1 ng/ml TGF-β1 for 48 h. (A) Immunoblots showing total and phosphorylated levels of p65 and NRF2 (left). Quantified protein levels were displayed as bar graphs (right). (B) Immunoblots showing protein levels of p65 in the nuclear and cytosolic extracts (left). Quantified protein levels in the nucleus were displayed as a bar graph (right). (C) Confocal images showing nuclear translocation of p65 (red). The nucleus was stained with DAPI (blue). Scale bar, 50 μm. Data are presented as mean ± SD from three independent experiments (ns, not significant; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; one-way ANOVA followed by Bonferroni post hoc test). (D) Representative immunohistochemistry images for p-p65 staining in normal conjunctival tissues and pterygium tissues from three pterygium patients (P1, P2, and P3). Scale bar, 50 μm. (E) Staining intensity index of p-p65 protein for normal conjunctival tissues and pterygium tissues from 10 pterygium patients (mean ± SD; ns, not significant; ****P < 0.0001; paired Student's t-test; magenta arrows, P1; green arrows, P2; cyan arrows, P3). (F–H) Primary ConFB with or without p65 knockdown were incubated in the presence or absence of 1 ng/ml TGF-β1 for 48 h. (F) Immunoblots showing protein levels of α-SMA and FN (left). Quantified protein levels were displayed as bar graphs (right). (G) Viability determined by the CCK8 assay. (H) Invasive ability determined by the transwell assay (left). Scale bar, 50 μm. The average numbers of invading cells per visual field were displayed in a bar graph (right). Data are presented as mean ± SD from three independent experiments (ns, not significant; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; one-way ANOVA followed by Bonferroni post hoc test). SC, scramble control; KD, knockdown.
To determine the role of NF-κB and NRF2 in the process of TGF-β1-induced fibrosis, the expression of p65 or NRF2 was knocked down in ConFB (Supplementary Figure S6A and B). Interestingly, knockdown of p65 almost completely abrogated the signatures of TGF-β1-induced fibrosis, resulting in reduced accumulation of α-SMA and FN as well as decreased cellular proliferative and invasive abilities (Figure 6F–H). Meanwhile, knockdown of NRF2 slightly, but not significantly, impaired the signatures of TGF-β1-induced fibrosis (Supplementary Figure S6C–E). Thus, these results indicated that TGF-β1 promoted the fibrosis of ConFB mainly through activating the NF-κB signaling pathway, but not the NRF2 signaling pathway.
The p65 homodimer was thought to induce FN expression via directly binding to the promoter of the FN gene (Lee et al., 2002; Stanisavljevic et al., 2011). In our work, the p65 E39I mutant lacking transcriptional activity was transduced into p65-silenced ConFB and failed to elevate the α-SMA and FN expression levels or cellular proliferative and invasive abilities (Supplementary Figure S7). Thus, TGF-β1-induced fibrosis should rely on the transcriptional activity of p65.
Moreover, the negative correlation between autophagy and NF-κB activation was also confirmed. Knockdown of ATG3 or ATG7, leading to SQSTM1 protein accumulation as previously described (Figure 4A), induced the phosphorylation and nuclear translocation of p65 (Figure 7A–C). Activation of autophagy via rapamycin or serum starvation and knockdown of SQSTM1 significantly eliminated the phosphorylation and nuclear translocation of p65 induced by TGF-β1 treatment (Figures 6A–C and 7D–F), while overexpression of SQSTM1 upregulated the phosphorylation level of p65 (Supplementary Figure S8).
Figure 7.
Downregulation of autophagy activates the NF-κB signaling pathway in TGF-β1-induced fibrosis. (A–C) Primary ConFB before and after ATG3 or ATG7 knockdown were incubated in the presence or absence of 1 ng/ml TGF-β1 for 48 h. (A) Immunoblots showing total p65 and p-p65 levels (left). Quantified protein levels were displayed as a bar graph (right). (B) Immunoblots showing protein levels of p65 in the nuclear and cytosolic extracts (left). Quantified protein levels in the nucleus were displayed as a bar graph (right). (C) Confocal images showing nuclear translocation of p65 (red). The nucleus was stained with DAPI (blue). Scale bar, 50 μm. Data are presented as mean ± SD from three independent experiments (*P < 0.05, **P < 0.01, ***P < 0.001 vs. scramble control group; one-way ANOVA followed by Bonferroni post hoc test). (D–F) Primary ConFB were stimulated with 1 ng/ml TGF-β1 for 48 h, with co-treatment of 2 μM rapamycin or serum starvation (0.5% FBS) for 18 h. (D) Immunoblots showing total p65 and p-p65 levels (left). Quantified protein levels were displayed as a bar graph (right). (E) Immunoblots showing protein levels of p65 in the nuclear and cytosolic extracts (left). Quantified protein levels in the nucleus were displayed as a bar graph (right). (F) Confocal images showing nuclear translocation of p65 (red). The nucleus was stained with DAPI (blue). Scale bar, 50 μm. Data are presented as mean ± SD from three independent experiments (*P < 0.05, ***P < 0.001 vs. control group; #P < 0.05, ###P < 0.001 vs. TGF-β1 treatment group; one-way ANOVA followed by Bonferroni post hoc test). SC, scramble control; KD, knockdown; Rapa, rapamycin.
It has been reported that SQSTM1 activated the NF-κB signaling pathway via interacting with aPKCs (Moscat et al., 2006). The two members of aPKCs, namely PKCι and PKCζ, were knocked down in ConFB, respectively, in our work. Knockdown of PKCι significantly inhibited the phosphorylation of p65 induced by SQSTM1 overexpression (Supplementary Figure S8A), while knockdown of PKCζ had a mild but not significant effect (Supplementary Figure S8B).
Together, these data revealed that autophagy modulated TGF-β1-induced fibrosis by inhibiting the SQSTM1–PKCι–NF-κB signaling cascades.
Discussion
The major histological components of pterygium are hyperproliferative fibrovascular tissues (Kim et al., 2016). The importance of fibrotic process for pterygium occurrence has been well established in recent years. The positive correlation between α-SMA protein level and pterygium grade was reported (Zhao et al., 2018). The expression level of the profibrogenic cytokine TGF-β was upregulated in primary pFB (Bianchi et al., 2012). In accordance with the previous studies, the present work found the increased expression levels of α-SMA and FN in primary cultured pFB (Figure 1C), demonstrating the formation of myofibroblasts and the deposit of ECM in pterygium.
Our work compared the autophagic activity between pterygium and normal conjunctival tissues from the same patient, so that the confounding from individual discrepancy was effectively ruled out. Compared with the normal conjunctival tissue, decreased LC3 staining was observed in the pterygium connective tissue, but not in the epithelium (Figure 1A and B). Our data also confirmed that the autophagic activity of primary cultured pFB was significantly lower than that of normal ConFB (Figure 1C and D; Supplementary Figure S1).
During the TGF-β1-induced fibrotic process in normal ConFB, TGF-β1 obviously downregulated the autophagic activity in a dose-dependent manner. Similar to pFB, normal ConFB displayed overactive proliferative and invasive abilities after TGF-β1 treatment. UV radiation, the major stimuli for pterygium onset, also decreased autophagy level and induced myofibroblast transdifferentiation in normal ConFB (Supplementary Figure S2). The autophagy inhibition was closely associated with the fibrotic process and pterygium development.
The exact relation between autophagy and fibrosis has raised concerns, yet is still uncertain. Of note, TGF-β1-induced fibrosis of normal ConFB was almost blocked after autophagy was activated by rapamycin or starvation (Figure 3). Rapamycin activates autophagy by targeting the mTOR complex, an evolutionarily conserved serine/threonine kinase involved in growth and proliferation by regulating various downstream pathways. Although the role of mTOR signaling in fibrosis has been documented (Liu et al., 2017; Zhao et al., 2018; Kim et al., 2019), whether mTOR regulates the fibrotic process via inhibiting autophagy is still unclear. On the other hand, inhibition of autophagy by knocking down ATG3 or ATG7 or by chemical autophagy inhibitors can aggravate fibrosis following TGF-β1 treatment (Figure 4; Supplementary Figure S4), suggesting the negative regulatory effect of autophagy on fibrosis in human ConFB. These data strongly indicated the role of autophagy in the fibrotic process.
Previous studies illustrated the dual effect of autophagy on fibrosis, depending on the different disease models. Defective autophagy dramatically promoted the heart failure progression and interstitial fibrosis (Del Principe et al., 2013; Nishida and Otsu, 2016). Li et al. (2016) found that autophagy in proximal epithelial cells was crucial in preventing renal fibrosis. Mice with ATG5 depletion were more susceptible to liver fibrosis (Lodder et al., 2015). However, autophagy can also be profibrotic, as increased autophagic activity in hepatic stellate cells was found to be associated with activation of hepatic stellate cells and hepatic fibrogenesis (Nwosu et al., 2016). In this study, our data demonstrated the antifibrotic role of autophagy in pterygium pathogenesis.
It is noticeable that the fibrosis was further enhanced following TGF-β1 treatment, after knockdown of ATG3/ATG7 (Figure 4). These results indicated that TGF-β1-induced fibrosis might be partially dependent on autophagy. TGF-β1 activates multiple intracellular signaling pathways to induce fibrosis, including the Smad2/3, PI3K/AKT, MAPK, and MEK/ERK pathways (Zhang et al., 2016; Nuwormegbe et al., 2017; Chen et al., 2019). Further investigations are desirable to elucidate whether TGF-β1 triggers the fibrotic process of human ConFB via additional mechanisms other than autophagy.
Autophagy is closely related to the protein level of SQSTM1, the autophagy receptor for degradation of ubiquitinated substrates. Enhanced autophagy leads to degradation of SQSTM1, while impairment of autophagy results in accumulation of SQSTM1. SQSTM1 protein consists of multiple domains and interacts with various binding partners to participate in diverse important signaling cascades, including the NF-κB, NRF2, and mTOR signaling cascades (Sanchez-Martin et al., 2019). SQSTM1 can serve as a signaling hub to link autophagy and downstream pathways (Katsuragi et al., 2015). Using a lentiviral-shRNA or lentiviral-overexpression system, our work demonstrated that knockdown of SQSTM1 significantly alleviated the fibrosis of ConFB induced by TGF-β1, while overexpression of SQSTM1 accelerated the fibrotic process. Importantly, overexpression of SQSTM1 abrogated the negative effect of autophagy activation on the fibrotic process. These findings suggested that autophagy negatively regulated the fibrosis of ConFB through the clearance of SQSTM1 protein and hence contributed to the occurrence of pterygium.
NF-κB regulates the expression of several pro-survival and pro-inflammatory genes. Enhanced NF-κB activation was associated with epithelial–mesenchymal transition (EMT) and fibrosis (Sun et al., 2017; Hill et al., 2019; Peng et al., 2019). SQSTM1 can activate the IκB kinase complex, which then releases NF-κB from its inhibitor IκB (Sanchez-Martin et al., 2019). SQSTM1 also contributes to the non-canonical mechanism of NRF2 activation by directly binding to KEAP1, resulting in the release and nuclear translocation of NRF2 (Jiang et al., 2015; Katsuragi et al., 2015). The role of the NRF2 signaling pathway in fibrosis is controversial. Acute activation of NRF2 was responsible for attenuation of fibrosis in diverse organs (Walters et al., 2008; Chung et al., 2019; Liu et al., 2019; Pompili et al., 2019), whereas persistent activation of NRF2 resulted in fibrosis and tumorigenesis in the liver (Ni et al., 2014). In this work, the phosphorylation and nuclear translocation of NF-κB subunit p65 were elevated in primary ConFB treated with TGF-β1, but the activation of NRF2 remained unchanged. Moreover, the critical role of NF-κB in TGF-β1-induced fibrosis was confirmed by p65 knockdown, as p65 gene silencing resulted in reduced expression levels of fibrotic markers and impaired cellular proliferative and invasive abilities (Figure 6).
Blocking autophagy induced SQSTM1 accumulation and promoted p65 phosphorylation. Knockdown of SQSTM1 significantly inhibited p65 activation and TGF-β1-induced fibrosis of ConFB. Knocking down PKCι rescued the effect of SQSTM1 overexpression on p65 activation. These results suggested that autophagy negatively regulated TGF-β1-induced fibrosis through degrading SQSTM1 protein and subsequently suppressing the SQSTM1–PKCι–NF-κB signaling pathway. Our results are corroborated by the recent studies. Hill et al. (2019) reported that blockage of autophagy promoted EMT via the SQSTM1–NF-κB pathway in alveolar epithelial cells and in RAS-mutated cancer cells. Reduced autophagy and increased SQSTM1 accumulation in lung fibroblasts were found to trigger NF-κB activation and profibrotic gene expression (Tsoyi et al., 2021).
Collectively, the present work revealed the close interaction between autophagy, SQSTM1, and the NF-κB signaling pathway in the fibrotic process of pterygium. Autophagy modulated TGF-β1-induced fibrosis of human ConFB by regulating the SQSTM1–PKCι–NF-κB signaling pathway. Our work contributed to elucidating the role of autophagy in pterygium occurrence. Modulation of autophagy might be a potential and effective therapeutic target for pterygium. Further studies are desirable to unveil the exact mechanism of the fibrotic process in pterygium regulated by autophagy.
Materials and methods
Tissue sampling
The human pterygium and normal conjunctival tissues were obtained from 10 pterygium patients aged 63.2 ± 5.2 years (4 males and 6 females) who received pterygium excision surgery in the Department of Ophthalmology, the First Affiliated Hospital of Zhejiang University. The exclusion criteria for these patients were (i) the head of pterygium invading inside the limbus for <2 mm, (ii) any history of previous ocular surgery or trauma, and (iii) concomitant ocular surface disorders including pinguecula, symblepharon, etc. The severity of pterygium was graded as follows (Zhong et al., 2012): grade 0 (no pterygium), grade 1 (head of pterygium at the limbus), grade 2 (head of pterygium between the limbus and the undilated pupil margin), grade 3 (head of pterygium within the pupil margin), and grade 4 (head of pterygium crossing the pupil). The clinical data of the pterygium patients are shown in Supplementary Table S1. Signed informed consents were obtained after the nature of the research was explained to each patient. This study was conducted in accordance with the tenets of the Declaration of Helsinki and was approved by the Research Ethics Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University.
For each pterygium patient, pterygium excision followed by conjunctiva autograft transplantation was performed. After the pterygium tissue was removed, a piece of normal conjunctiva beside the superior limbus was cut off and trimmed for transplantation. The normal conjunctiva clippings (at least 1 mm × 1 mm) and the removed pterygium tissues were collected. For immunohistochemistry, the tissues were immediately fixed in 4% paraformaldehyde. For primary cell culture, the tissues were washed in phosphate-buffered saline (PBS) three times and placed in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS (10099-141, Gibco), 100 U/ml penicillin, and 0.1 mg/ml streptomycin (15140-122, Gibco). Tissues were transferred to the laboratory within 1 h before further processing.
Immunohistochemistry
Pterygium tissues and normal conjunctival tissues were fixed and embedded in paraffin for immunohistochemistry analysis. Tissues were sliced, de-waxed, rehydrated, and incubated with hydrogen peroxide for 10 min. Sections were then blocked with 5% normal goat serum and incubated with primary antibody (LC3B rabbit pAb, ab48394, Abcam; SQSTM1/p62 mouse mAb, ab56416, Abcam; phospho-NF-κB p65 (Ser 536) rabbit mAb, 3033, Cell Signaling Technology) overnight at 4°C, followed by biotinylated secondary antibody for 2 h at room temperature. Images were acquired using an Olympus BX61 microscope. To determine the staining intensity index of LC3, SQSTM1, and p-p65 proteins, five randomly selected images from each sample were analyzed. The cells were categorized and scored according to the intensity of staining: (i) negatively stained, each cell scored 0; (ii) mildly stained, each cell scored 1; (iii) moderately stained, each cell scored 2; and (iv) strongly stained, each cell scored 3. The staining intensity index was calculated as the aggregate score divided by the cell number.
Primary cell culture and treatment
Pterygium tissues and normal conjunctival tissues were cut into 1 mm × 1 mm pieces under the microscope and placed in 24-well cell culture plates without culture medium. The tissue pieces were incubated at 37°C for 2 h. Subsequently, 1 ml of cell culture medium (DMEM supplemented with 10% FBS, 100 U/ml penicillin, and 0.1 mg/ml streptomycin) was added carefully to prevent the tissue pieces from floating. The incubation continued for 3–7 days before primary fibroblasts migrated from the explants.
To collect primary fibroblasts, the tissue explants were removed by microforceps, and the remaining cells were washed by PBS and digested with 0.05% trypsin–EDTA at 37°C for 2 min. The digestion was stopped by adding cell culture medium and centrifuging at 300× g for 5 min. Then, the cells were incubated in a humidified atmosphere enriched with 5% CO2 at 37°C and passaged every 3 days. Cells passaged 3–7 times were used for experiments.
Primary fibroblasts were inoculated in 6-well cell culture plates at a density of 1 × 105 cells per well at 18 h before treatment. Cells were treated with 1 ng/ml TGF-β1 (100-21, PeproTech) for 48 h, with or without co-treatment of 2 μM rapamycin (S1039, Selleck), serum starvation (0.5% FBS), 2 mM 3-MA (GC10710, GLPBIO), or 2 μM CQ (GC19549, GLPBIO) for 18 h. For UV treatment, the culture medium was removed and cells were washed by PBS three times. The cell culture plates were put 5 cm away from the UV lamp for the indicated duration, and the cells were then cultured for 24 h in fresh culture medium.
Lentiviral transduction
For RNA interference, oligonucleotides were synthesized and ligated into the digested pLVX-shRNA vector to construct recombinant plasmids. The shRNA sequences of target genes are listed in Supplementary Table S2. The p65 E39I mutant, resistant to RNA interference, was designed according to the silent mutations in the coding sequence. The recombinant plasmid expressing the p65 E39I mutant was synthesized by Genewis Co. HEK293T cells (purchased from the Cell Bank of Chinese Academy of Sciences) were applied for lentivirus package. HEK293T cells were cultured in DMEM supplemented with 10% FBS, 100 U/ml penicillin, and 0.1 mg/ml streptomycin. The recombinant plasmids as well as three lentiviral packaging plasmids were transfected into HEK293T cells using PolyJet reagent (SL100688, SignaGen) according to the manufacturer's protocol. At 30 h after transfection, the cell culture medium containing lentiviral particles was collected and filtered using a 0.45-μm cell strainer. The lentivirus expressing human SQSTM1 protein was purchased from GeneCopoeia Co. (LPP-M0245-Lv105-100).
For lentiviral transduction, primary fibroblasts were inoculated in 6-well cell culture plates at a density of 1 × 105 cells per well and incubated for 18 h before infection. The culture medium containing lentiviral particles was added with a multiplicity of infection at 100. Polybrene (HB-PB-1000, HANBIO) was added at a final concentration of 6 μg/ml. Cells were centrifuged at 1500× g for 90 min at 32°C. After centrifugation, the lentiviral supernatant was replaced with fresh complete cell culture medium, and the cells were incubated for 48 h before further experiments.
Western blot analysis
Cultured cells were collected and lysed with iced NP-40 lysis buffer (P0013F, Beyotime) containing phosphatase inhibitors (4906837001, Roche) and phenylmethylsulfonyl fluoride (PMSF) (ST506, Beyotime). The protein samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Immobilon‐P, IPVH00010). The membranes were blocked for 1 h at room temperature in Tris-buffered saline containing 5% skim milk and 0.05% Tween-20 and then incubated with primary antibodies overnight at 4°C. The membranes were then washed and incubated with secondary antibodies for 2 h at room temperature. The primary and secondary antibodies are summarized in Supplementary Table S3. Subsequently, the membranes were scanned using an Alpha Chemiluminescence Gel Imaging System (FluorChem E, Cell Biosciences). All experiments were repeated at least three times, and the images were analyzed using ImageJ software.
Nuclear and cytoplasmic protein extraction
Nuclear and cytoplasmic protein extraction was performed at 4°C using a Nuclear and Cytoplasmic Extraction Kit (C500009, Sangon Biotech) according to the manufacturer's instructions. Briefly, primary fibroblasts were collected and incubated in hypotonic buffer containing 1 mM DTT, 1 mM PMSF, and Phosphatase Inhibitor Cocktail for 20 min. After centrifugation at 800× g for 10 min, the supernatant containing cytoplasmic protein was collected. The mucoid sediment containing nuclei was re-suspended in lysis buffer containing 1 mM DTT, 1 mM PMSF, and Phosphatase Inhibitor Cocktail for 20 min incubation. The supernatant containing nuclear protein was collected after centrifugation at 12000× g for 10 min.
CCK8 assay
The CCK8 assay was performed to assess cell viability. Primary fibroblasts were inoculated in 96-well cell culture plates at a density of 2 × 103 cells per well for 18 h before exposed to stimulus. Each well was added with 10 μl CCK8 solution (CK04, Dojindo) and incubated at 37°C for 2 h. The absorbance was measured by a Model 680 Microplate Reader (Bio-Rad) at 450 nm. All experiments were performed in triplicate and repeated at least three times.
Transwell invasion assay
The transwell assay was performed to assess cell invasive ability. The 8.0-μm-pore transwell inserts (3422, Corning) were placed in 24-well plates. The Matrigel (356234, BD Biosciences) was diluted at 1:8 by DMEM supplemented with 0.5% FBS on ice. Each insert was coated with 50 μl diluted Matrigel and incubated at 37°C for 2 h. Then, primary fibroblasts were collected and re-suspended in DMEM supplemented with 0.5% FBS at a density of 5 × 105/ml. Each insert was added with 100 μl cell suspension, so that 5 × 104 cells were inoculated. Each well was added with 600 μl DMEM supplemented with 10% FBS. The 24-well plates with inserts were incubated at 37°C for 24 h. Subsequently, the culture medium inside the inserts and wells was removed. The cells and Matrigel inside the inserts were carefully wiped by cotton swabs. The inserts were then fixed in methanol for 15 min and dyed with DAPI (D1306, ThermoFisher) for 30 min. The inserts were washed by PBS three times. The cells invading the membrane of the inserts were visualized using a fluorescence microscope (IX53, Olympus). All experiments were performed in triplicate and repeated at least three times, and at least five randomly selected images per sample were taken for further analysis.
Annexin V–PI assay
The Annexin V-FITC/PI apoptosis kit (70-AP101-100, Multi Sciences) was used to assess cell apoptosis, according to the manufacturer's protocol. Numbers of Annexin V- and PI-positive cells were detected by ACEA Novocyte flow cytometry (ACEA Biosciences), and data were processed with FlowJo software (v10.4.0).
Immunofluorescence microscopy
Primary fibroblasts were seeded on coverslips placed in 24-well plates. After treated with stimulus for the indicated time, the primary fibroblasts were fixed in 4% paraformaldehyde for 15 min at room temperature and washed three times with PBS. The fixed cells were then permeabilized with 0.1% saponin (47036-50G-F, Sigma) in PBS for 20 min and blocked with 10% bovine serum albumin in PBS for 1 h. The cells were incubated with primary antibody (LC3 mouse mAb, CAC-CTB-LC3-2-IC, Cosmo Bio; SQSTM1/p62 rabbit pAb, 18420-1-AP, Proteintech; NF-κB p65 rabbit mAb, 8242, Cell Signaling Technology) for 16 h at 4°C, and then incubated with secondary antibody [goat anti-mouse IgG (H&L) (Alexa Fluor 546), A-11003, Invitrogen; goat anti-rabbit IgG (H&L) (Cyanine5), A10523, Invitrogen; goat anti-rabbit IgG (H&L) (Alexa Fluor 647), A-21245, Invitrogen] for 1 h at room temperature. The cells were rinsed again and stained with DAPI (D1306, ThermoFisher). Samples were observed using a confocal microscope system (IX83-FV3000-OSR, Olympus).
Transmission electron microscopy
Primary fibroblasts were collected and fixed in 2.5% glutaraldehyde (G5882, Sigma) for 2 h at room temperature. Following rinsing with 0.1 M PBS, the cells were postfixed with 1% osmium acid (365092, Sigma) for 1 h. After rinsing three times in water, the cells were stained with 2% uranyl acetate for 0.5 h and dehydrated through a graded series of ethanol. After embedding, the cells were sliced using an ultra-thin trimming machine. The slices were observed under a Tecnai transmission electron microscope (FEI).
Statistical analysis
Statistical analysis was performed by SPSS software (version 26.0, IBM Corp.). Data were presented as mean ± standard deviation (SD). For comparisons between two groups, Student's t-test was performed. For comparisons among more than two groups, one‐way analysis of variance (ANOVA) followed by Bonferroni post hoc test was performed. All statistical tests were two‐sided. P < 0.05 was considered statistically significant. All experiments were carried out at least three times unless otherwise stated.
Supplementary Material
Acknowledgements
We thank the Key Laboratory of Immunity and Inflammatory Diseases of Zhejiang Province for the experimental platform of autophagy. We thank Chenyu Yang in the Center of Cryo-Electron Microscopy, Zhejiang University for her technical assistance on transmission electron microscopy.
Contributor Information
Qin He, D epartment of Ophthalmology, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou 310003, China.
Yiting Cai, Institute of Immunology, School of Medicine, Zhejiang University, Hangzhou 310058, China.
Jiani Huang, Eye Center of the Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou 310009, China.
Xiaoying He, Eye Center of the Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou 310009, China.
Wei Han, Eye Center of the Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou 310009, China.
Wei Chen, Institute of Immunology, School of Medicine, Zhejiang University, Hangzhou 310058, China.
Funding
This work was supported by the National Natural Science Foundation of China (81670842 and 31370879), the China Postdoctoral Science Foundation (2020M671752), the State Natural Science Foundation of Zhejiang Province (LQ20H120006 and LY17C080003), and the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (2020KY121).
Conflict of interest: none declared.
References
- Bianchi E., Scarinci F., Grande C.et al. (2012). Immunohistochemical profile of VEGF, TGF-β and PGE₂ in human pterygium and normal conjunctiva: experimental study and review of the literature. Int. J. Immunopathol. Pharmacol. 25, 607–615. [DOI] [PubMed] [Google Scholar]
- Boya P., Esteban-Martinez L., Serrano-Puebla A.et al. (2016). Autophagy in the eye: development, degeneration, and aging. Prog. Retin. Eye Res. 55, 206–245. [DOI] [PubMed] [Google Scholar]
- Bradley J.C., Yang W., Bradley R.H.et al. (2010). The science of pterygia. Br. J. Ophthalmol. 94, 815–820. [DOI] [PubMed] [Google Scholar]
- Chen K., Lai K., Zhang X.et al. (2019). Bromfenac inhibits TGF-β1-induced fibrotic effects in human pterygium and conjunctival fibroblasts. Invest. Ophthalmol. Vis. Sci. 60, 1156–1164. [DOI] [PubMed] [Google Scholar]
- Chu W.K., Choi H.L., Bhat A.K.et al. (2020). Pterygium: new insights. Eye 34, 1047–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S., Kim S., Son M.et al. (2019). Inhibition of p300/CBP-associated factor attenuates renal tubulointerstitial fibrosis through modulation of NF-κB and Nrf2. Int. J. Mol. Sci. 20, 1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clearfield E., Muthappan V., Wang X.et al. (2016). Conjunctival autograft for pterygium. Cochrane Database Syst. Rev. 2, CD011349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codogno P., Mehrpour M., Proikas-Cezanne T. (2011). Canonical and non-canonical autophagy: variations on a common theme of self-eating? Nat. Rev. Mol. Cell Biol. 13, 7–12. [DOI] [PubMed] [Google Scholar]
- Del Principe D., Lista P., Malorni W.et al. (2013). Fibroblast autophagy in fibrotic disorders. J. Pathol. 229, 208–220. [DOI] [PubMed] [Google Scholar]
- Deretic V. (2021). Autophagy in inflammation, infection, and immunometabolism. Immunity 54, 437–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Girolamo N., Chui J., Coroneo M.T.et al. (2004). Pathogenesis of pterygia: role of cytokines, growth factors, and matrix metalloproteinases. Prog. Retin. Eye Res. 23, 195–228. [DOI] [PubMed] [Google Scholar]
- Dikic I., Elazar Z. (2018). Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 19, 349–364. [DOI] [PubMed] [Google Scholar]
- Fan L., Yin S., Zhang E.et al. (2018). Role of p62 in the regulation of cell death induction. Apoptosis 23, 187–193. [DOI] [PubMed] [Google Scholar]
- Hill C., Li J., Liu D.et al. (2019). Autophagy inhibition-mediated epithelial-mesenchymal transition augments local myofibroblast differentiation in pulmonary fibrosis. Cell Death Dis. 10, 591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T., Harder B., Rojo de la Vega M.et al. (2015). p62 links autophagy and Nrf2 signaling. Free Radic. Biol. Med. 88, 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuragi Y., Ichimura Y., Komatsu M. (2015). p62/SQSTM1 functions as a signaling hub and an autophagy adaptor. FEBS J. 282, 4672–4678. [DOI] [PubMed] [Google Scholar]
- Kim K.K., Sheppard D., Chapman H.A. (2018). TGF-β1 signaling and tissue fibrosis. Cold Spring Harb. Perspect. Biol. 10, a022293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.W., Park S.H., Kim J.C. (2016). Fibroblast biology in pterygia. Exp. Eye Res. 142, 32–39. [DOI] [PubMed] [Google Scholar]
- Kim K.W., Park S.H., Wee S.W.et al. (2013). Overexpression of angiogenin in pterygium body fibroblasts and its association with proliferative potency. Invest. Ophthalmol. Vis. Sci. 54, 6355–6362. [DOI] [PubMed] [Google Scholar]
- Kim S.W., Kim H.I., Thapa B.et al. (2019). Critical role of mTORC2–Akt signaling in TGF-β1-induced myofibroblast differentiation of human pterygium fibroblasts. Invest. Ophthalmol. Vis. Sci. 60, 82–92. [DOI] [PubMed] [Google Scholar]
- Lee B.H., Park S.Y., Kang K.B.et al. (2002). NF-κB activates fibronectin gene expression in rat hepatocytes. Biochem. Biophys. Res. Commun. 297, 1218–1224. [DOI] [PubMed] [Google Scholar]
- Lee H., Jeong H., Lee C.M.et al. (2019). Antifibrotic effects of sakuraso-saponin in primary cultured pterygium fibroblasts in comparison with mitomycin C. Invest. Ophthalmol. Vis. Sci. 60, 4784–4791. [DOI] [PubMed] [Google Scholar]
- Li H., Peng X., Wang Y.et al. (2016). Atg5-mediated autophagy deficiency in proximal tubules promotes cell cycle G2/M arrest and renal fibrosis. Autophagy 12, 1472–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Gao Y., Ci X. (2019). Role of Nrf2 and its activators in respiratory diseases. Oxid. Med. Cell Longev. 2019, 7090534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R.M., Gaston Pravia K.A. (2010). Oxidative stress and glutathione in TGF-β-mediated fibrogenesis. Free Radic. Biol. Med. 48, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Xu H., An M. (2017). mTORC1 regulates apoptosis and cell proliferation in pterygium via targeting autophagy and FGFR3. Sci. Rep. 7, 7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston M.J., Ding H.F., Huang S.et al. (2016). Persistent activation of autophagy in kidney tubular cells promotes renal interstitial fibrosis during unilateral ureteral obstruction. Autophagy 12, 976–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder J., Denaes T., Chobert M.N.et al. (2015). Macrophage autophagy protects against liver fibrosis in mice. Autophagy 11, 1280–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai W., Chen M., Huang M.et al. (2019). Targeting platelet-derived growth factor receptor beta inhibits the proliferation and motility of human pterygial fibroblasts. Expert Opin. Ther. Targets 23, 805–817. [DOI] [PubMed] [Google Scholar]
- Moscat J., Diaz-Meco M.T., Albert A.et al. (2006). Cell signaling and function organized by PB1 domain interactions. Mol. Cell 23, 631–640. [DOI] [PubMed] [Google Scholar]
- Ni H.M., Woolbright B.L., Williams J.et al. (2014). Nrf2 promotes the development of fibrosis and tumorigenesis in mice with defective hepatic autophagy. J. Hepatol. 61, 617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida K., Otsu K. (2016). Autophagy during cardiac remodeling. J. Mol. Cell Cardiol. 95, 11–18. [DOI] [PubMed] [Google Scholar]
- Nuwormegbe S.A., Sohn J.H., Kim S.W. (2017). A PPAR-gamma agonist rosiglitazone suppresses fibrotic response in human pterygium fibroblasts by modulating the p38 MAPK pathway. Invest. Ophthalmol. Vis. Sci. 58, 5217–5226. [DOI] [PubMed] [Google Scholar]
- Nwosu Z.C., Alborzinia H., Wolfl S.et al. (2016). Evolving insights on metabolism, autophagy, and epigenetics in liver myofibroblasts. Front. Physiol. 7, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeckinghaus A., Hayden M.S., Ghosh S. (2011). Crosstalk in NF-κB signaling pathways. Nat. Immunol. 12, 695–708. [DOI] [PubMed] [Google Scholar]
- Peng X., Wang Y., Li H.et al. (2019). ATG5-mediated autophagy suppresses NF-κB signaling to limit epithelial inflammatory response to kidney injury. Cell Death Dis. 10, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompili S., Sferra R., Gaudio E.et al. (2019). Can Nrf2 modulate the development of intestinal fibrosis and cancer in inflammatory bowel disease? Int. J. Mol. Sci. 20, 4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Martin P., Komatsu M. (2018). p62/SQSTM1—steering the cell through health and disease. J. Cell Sci. 131, jcs222836. [DOI] [PubMed] [Google Scholar]
- Sanchez-Martin P., Saito T., Komatsu M. (2019). p62/SQSTM1: ‘Jack of all trades’ in health and cancer. FEBS J. 286, 8–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seet L.F., Tong L., Su R.et al. (2012). Involvement of SPARC and MMP-3 in the pathogenesis of human pterygium. Invest. Ophthalmol. Vis. Sci. 53, 587–595. [DOI] [PubMed] [Google Scholar]
- Shu D.Y., Lovicu F.J. (2017). Myofibroblast transdifferentiation: the dark force in ocular wound healing and fibrosis. Prog. Retin. Eye Res. 60, 44–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisavljevic J., Porta-de-la-Riva M., Batlle R.et al. (2011). The p65 subunit of NF-κB and PARP1 assist Snail1 in activating fibronectin transcription. J. Cell Sci. 124, 4161–4171. [DOI] [PubMed] [Google Scholar]
- Sun K., Xu L., Jing Y.et al. (2017). Autophagy-deficient Kupffer cells promote tumorigenesis by enhancing mtROS–NF-κB–IL1α/β-dependent inflammation and fibrosis during the preneoplastic stage of hepatocarcinogenesis. Cancer Lett. 388, 198–207. [DOI] [PubMed] [Google Scholar]
- Touhami A., Di Pascuale M.A., Kawatika T.et al. (2005). Characterisation of myofibroblasts in fibrovascular tissues of primary and recurrent pterygia. Br. J. Ophthalmol. 89, 269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoyi K., Liang X., De Rossi G.et al. (2021). CD148 deficiency in fibroblasts promotes the development of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 204, 312–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters D.M., Cho H.Y., Kleeberger S.R. (2008). Oxidative stress and antioxidants in the pathogenesis of pulmonary fibrosis: a potential role for Nrf2. Antioxid. Redox Signal. 10, 321–332. [DOI] [PubMed] [Google Scholar]
- Wu S.Q., Xu Q.B., Sheng W.Y.et al. (2020). A novel role for Livin in the response to ultraviolet B radiation and pterygium development. Int. J. Mol. Med. 45, 1103–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zada M., Pattamatta U., White A. (2018). Modulation of fibroblasts in conjunctival wound healing. Ophthalmology 125, 179–192. [DOI] [PubMed] [Google Scholar]
- Zhang J., Tian X.J., Xing J. (2016). Signal transduction pathways of EMT induced by TGF-β, SHH, and WNT and their crosstalks. J. Clin. Med. 5, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X.R., Zhang M.C., Xie H.T.et al. (2017). Expression of mTOR in primary pterygium and its correlation with α-smooth muscle actin. Eur. J. Ophthalmol. 27, 664–669. [DOI] [PubMed] [Google Scholar]
- Zhao X.R., Zhang M.C., Xie H.T.et al. (2018). p70S6K activation promotes the transdifferentiation of fibroblasts to myofibroblasts in pterygium tissue growth on the cornea. Biotechnol. Lett. 40, 437–444. [DOI] [PubMed] [Google Scholar]
- Zhong H., Cha X., Wei T.et al. (2012). Prevalence of and risk factors for pterygium in rural adult chinese populations of the Bai nationality in Dali: the Yunnan Minority Eye Study. Invest. Ophthalmol. Vis. Sci. 53, 6617–6621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.