Summary
Long non-coding RNAs (lncRNAs) are implicated in a plethora of cellular processes, but an in-depth understanding of their functional features or their mechanisms of action is currently lacking. Here we study Meteor, a lncRNA transcribed near the gene encoding EOMES, a pleiotropic transcription factor implicated in various processes throughout development and in adult tissues. Using a wide array of perturbation techniques, we show that transcription elongation through the Meteor locus is required for Eomes activation in mouse embryonic stem cells, with Meteor repression linked to a change in the subpopulation primed to differentiate to the mesoderm lineage. We further demonstrate that a distinct functional feature of the locus—namely, the underlying DNA element—is required for suppressing Eomes expression following neuronal differentiation. Our results demonstrate the complex regulation that can be conferred by a single locus and emphasize the importance of careful selection of perturbation techniques when studying lncRNA loci.
Keywords: long non-coding RNAs, lncRNAs, embryonic stem cells, pluripotency, differentiation, EOMES
Graphical abstract
Highlights
-
•
An array of perturbations targeting the Meteor long non-coding RNA locus
-
•
Transcription elongation through Meteor is required for Eomes activation in mESCs
-
•
Meteor is dispensable for the formation of beating cardiomyocytes
-
•
Meteor promoter DNA is required for suppressing Eomes during neuronal differentiation
Using a wide array of perturbation techniques, Gil et al. dissect the locus encoding the Meteor long non-coding RNA and show that distinct cell-type-specific functional features within the locus fine-tune the spatiotemporal expression patterns of the nearby gene Eomes in mouse embryonic stem cells and during differentiation.
Introduction
Long non-coding RNAs (lncRNAs) are RNA polymerase II transcription products emanating from thousands of loci in mammalian genomes.1,2,3 Concomitantly with the rise in the number of annotated lncRNAs, they have been implicated in a wide array of cellular processes. In particular, multiple lncRNAs have been shown to contribute to fine-tuning of exit from pluripotency and developmental programs.4,5
A substantial number of lncRNAs are transcribed from regions with enhancer characteristics6,7,8 and, accordingly, multiple studies suggested lncRNA contributions to enhancer functionality, for example through bridging enhancer-promoter chromatin loops,9,10 recruiting activating proteins,11 or affecting nuclear localization.12 Such lncRNA loci can operate through the use of distinct functional features within the lncRNA gene, with the functionality of most such lncRNAs apparently relying not on the mature RNA product but rather on the underlying DNA element or various aspects of the transcriptional process,13,14,15 consistent with the generally poor conservation of these loci.8,16
The study of enhancer-transcribed lncRNAs is proving to be particularly challenging, as the reciprocal interactions and interdependencies between transcription, chromatin modifications, chromatin architecture, and nuclear localization complicate efforts to separate direct and indirect effects of lncRNA activity. Crucially, these processes vary in their sensitivity to both the on-target and side effects of different perturbation techniques. This is exemplified by the sometimes contradictory effects noted when using different perturbation techniques to study particular loci,17,18 emphasizing the need to interpret the consequences of lncRNA perturbations with great care.
One enhancer-transcribed lncRNA of substantial interest is Meteor, also known as Platr11, Gm26975, or linc1405.5,19,20 Meteor is transcribed from the MesEndoderm Transcriptional Enhancer Organizing Region, located ∼70 kb downstream of the gene encoding the EOMES transcription factor (TF).14,20 EOMES, also known as TBR2, is a member of the T-box family of TFs, and is a master regulator of many developmental processes.21,22 Eomes is first expressed in the trophectoderm lineage, where it is required for self-renewal and early differentiation of trophoblast stem cells, so that Eomes-null embryos arrest shortly after implantation.23,24,25 Within the inner cell mass, Eomes expression initially marks the region that will become the primitive streak, making it one of the earliest markers of gastrulation onset. While Eomes is downregulated prior to the end of gastrulation, it is again detected in the developing forebrain,26,27 and has been attributed functions in various neuronal cell types both during development and in the adult brain21,28 as well as in several types of immune cells.29,30,31 Owing to its involvement in and regulation of these eclectic developmental processes, Eomes expression levels and domains are themselves subject to tight regulation through cis-regulatory elements, some of which have been characterized.32
As befits its initial identification as a pluripotency-associated transcript,5,19 Meteor expression is highly associated with the pluripotent state,14,20 although one study indicated that its expression is upregulated in the embryonic heart and in mouse embryonic stem cell (mESC)-derived mesoderm precursors.33 Meteor’s function is also subject to conflicting evidence. Two studies found that Meteor knockout (KO) caused reduction in Eomes expression in mESCs,14,34 suggesting that Eomes is the direct target of the Meteor locus. In contrast, another study, using a similar deletion scheme (Figure S1A), did not note any effect on Eomes expression in mESCs, but rather changes in expression of other genes, possibly indicating an Eomes-independent function of the Meteor locus.20 Nonetheless, all three studies agree that in mESCs, targeting of Meteor’s transcription or RNA product does not have any noticeable effects on Eomes expression or otherwise,14,20,34 pointing to an RNA-independent function of the Meteor locus. In contrast, two additional studies argue in favor of an RNA-dependent Meteor function, showing that Meteor repression by either insertion of an early polyadenylation site (pAS)20,33 or short hairpin RNAs (shRNAs)33 caused significant reduction in the ability of mESCs to differentiate to functional cardiac mesoderm, though with conflicting evidence as to whether or not Eomes expression was the mediator of this activity. Specifically, one report suggested that during the process of cardiac mesoderm differentiation, Meteor functions in trans, as overexpression of one of the Meteor exons could rescue the phenotype of Meteor repression.33 The reduced ability to differentiate to cardiac mesoderm was suggested to prime the mESCs to differentiate to the ectoderm lineage, exemplified by increased efficiency of neuronal differentiation.20
In this study, we set out to address some of the key unanswered questions regarding Meteor biology. What is the effect of Meteor repression in mESCs, and to what extent is it mediated through Eomes? What is the role of the Meteor locus in priming cardiac mesoderm differentiation, and what is the contribution of the lncRNA to this activity? What are the roles, if any, of the Meteor locus during neurogenesis? Finally, what is the functional feature within Meteor that carries out these activities? We use a variety of perturbation techniques to show that two distinct functional features within the Meteor locus—transcriptional elongation through the Meteor locus, and the underlying DNA element—modulate Eomes expression levels in opposing manners in distinct cellular states, illustrating the complexity of the roles played by lncRNA loci within gene-regulatory networks.
Results
A suite of perturbation methods for interrogating the Meteor locus
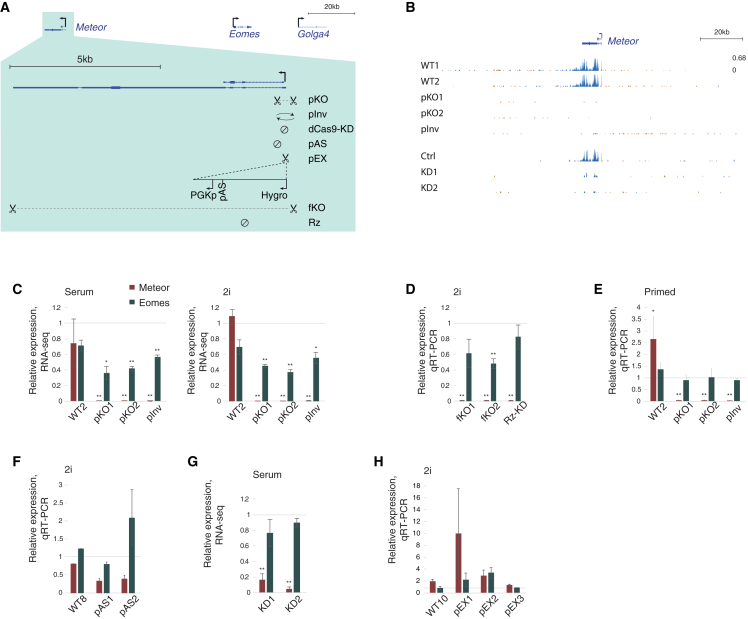
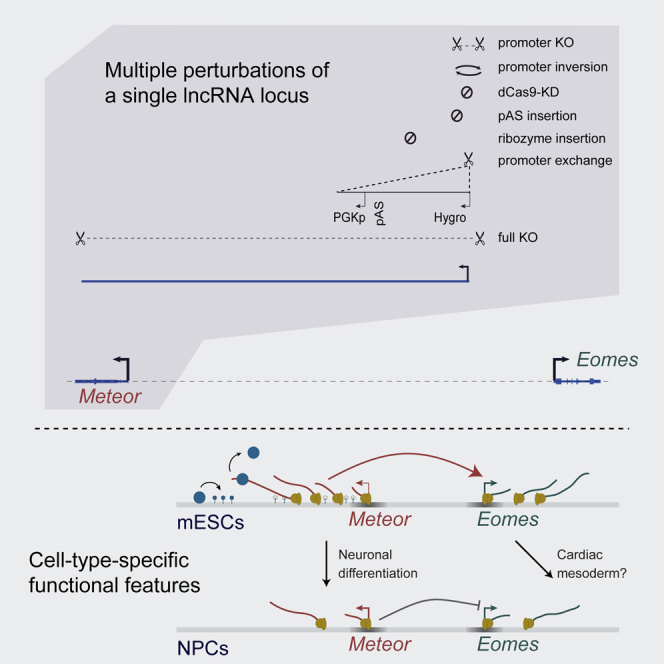
To probe Meteor functionality and identify its functional features, we employed a suite of methods for perturbation of the Meteor locus in mESCs (Figures 1A and S1A–S1D). First, we used CRISPR-Cas9 and a pair of guide RNAs (gRNAs) targeted to the two sides of the Meteor transcription start site (TSS). This generated clones with homozygous KO of the Meteor promoter (pKO) that resulted in complete ablation of Meteor expression, and a clone with a homozygous inversion of the promoter (pInv) that showed very low and inefficient transcription to the other side of the Meteor promoter (Figures 1A–1C). The deletion/inversion encompassed ∼550 bp, a more targeted region than the 1- to 3-kb region deleted in previous Meteor KO models (Figure S1A).14,20 We also received cell lines in which the entire Meteor locus was removed (full KO, or fKO lines)34 (Figure 1A). As a complementary approach that targets Meteor transcription without changing the DNA sequence, we infected vectors expressing gRNAs targeting the Meteor TSS into mESCs stably expressing a catalytically dead Cas9 (dCas9) protein (dCas9-KD [knockdown]),35 which reduced Meteor levels by ∼90% (Figure 1B). In addition, we generated cell lines which introduced a polyadenylation site (pAS) into the first intron of Meteor so as to reduce transcription through the Meteor locus, and similarly employed a cell line in which Meteor expression was destabilized by insertion of a self-cleaving ribozyme (Rz) sequence in its second exon34 (Rz-KD, Figure 1A). Lastly, to separate the effects of locus transcription from the DNA sequence at the promoter, we generated promoter exchange (pEX) cell lines in which the constitutive PGK promoter was knocked in downstream of the Meteor promoter, so that the transcription from the Meteor promoter is blocked by a pAS and the Meteor region is transcribed from the PGK promoter instead (Figure 1A).
Figure 1.
Meteor transcriptional elongation promotes Eomes expression in naive mESCs
(A) Perturbations used to analyze the Meteor locus in this study. Shown are the locations of gRNAs used for deleting or inverting the Meteor promoter (pKO) or gene body (fKO) or for inhibiting transcription (dCas9-KD); the locations of insertion of ribozyme (Rz) or polyadenylation (pAS) sequences; and the scheme for knocking in the PGK promoter downstream of the Meteor promoter (pEX). The fKO and Rz-KD mESC lines are the same as in Tuck et al.34
(B) RNA-seq tracks showing Meteor expression in the various pKO and pInv lines (grown in serum-free 2i/LIF conditions) and dCas9-KD lines (serum/LIF conditions). All tracks are normalized to the same scale. Orange denotes transcription on the plus strand, and blue denotes transcription on the minus strand.
(C) RNA-seq quantifications of Meteor and Eomes in Meteor pKO and pInv cell lines grown in serum/LIF (left) or serum-free 2i/LIF (right) conditions. Amounts normalized to WT1. Bars represent standard errors; n = 3. ∗p < 0.05, ∗∗p < 0.005.
(D) qRT-PCR quantifications of Meteor and Eomes levels in Meteor fKO and Rz-KD mESCs grown in serum-free 2i/LIF conditions. Levels were normalized to WT4 and Ppib for internal control. Bars represent standard errors; n = 8. ∗p < 0.05, ∗∗p < 0.005.
(E) Same as (D), for Meteor pKO and pInv mESCs grown in primed conditions, normalized to WT1. n = 4.
(F) Same as (D), for pAS clones grown in serum-free 2i/LIF conditions. Levels were normalized to WT7. n = 3.
(G) Same as (C), for Meteor dCas9-KD lines grown in serum/LIF conditions. Amounts normalized to Ctrl. n = 3. The dCas9-KD efficiencies of Meteor were 85% and 94% for KD1 and KD2, respectively.
(H) Same as (D), for pEX clones grown in serum-free 2i/LIF conditions. Levels were normalized to WT9. n = 3.
See also Figure S1.
Transcription into the Meteor gene body enhances Eomes expression in mESCs
As previously noted,14,34 KO of either the entire Meteor gene body (fKO) or only the promoter region (pKO) caused a significant reduction of ∼50% in Eomes levels (Figures 1C and 1D). However, it is noteworthy that here and throughout, we saw a wide variability in both Meteor and Eomes expression levels among wild type (WT) and (in the case of Eomes) Meteor KO clones, especially when the cells were grown in serum, as can also be noted from the expression levels of these genes in the ESpresso single-cell RNA-sequencing (scRNA-seq) dataset (Figure S1E). This hindered our ability to detect a significant change in Eomes levels when assayed by qRT-PCR, which might explain contradicting previous reports.20 While alkaline phosphatase staining confirmed that the Meteor pKO lines were pluripotent (Figure S1F), we suspected that even small changes in pluripotency state could affect expression of these genes and therefore cultured the cells in serum-free medium supplemented with PD0325901 and CHIR99021, selective inhibitors of MEK and GSK3 (“2i” medium). 2i conditions shield the cells from differentiation signals, resulting in a morphologically and transcriptionally uniform “ground state” of pluripotency,36,37 which indeed resulted in more uniform reduction in Eomes expression upon Meteor KO (Figures 1C and 1D). This effect of Meteor on Eomes expression appears to be specific to the naive state of pluripotency, as Meteor pKO cells cultured in the presence of fibroblast growth factor and activin, conditions which favor the generation of a “primed” epiblast stem cell-like state, did not exhibit changes in Eomes levels (Figure 1E). These cells are found at a more advanced state of differentiation representative of the post-implantation embryo, in which lineage-specific markers, including Eomes, are induced compared with naive mESCs.38,39 The lack of effect of Meteor repression on Eomes levels in primed cells suggests that Eomes induction at the onset of differentiation is not directly dependent on Meteor but rather is likely a consequence of altered cell state at the naive mESC stage (see below).
We were not successful in achieving substantial silencing of Meteor RNA levels using infections of shRNA-expressing vectors, possibly due to the strong chromatin association of Meteor lncRNA (Figure S1G). Nonetheless, no significant changes in Eomes expression were noted when Meteor expression became undetectable through Rz-KD (Figure 1D), nor when a partially effective pAS was inserted into the first intron of Meteor (Figure 1F), suggesting that the Meteor RNA product likely does not contribute to Eomes regulation in mESCs. Furthermore, Eomes expression was not substantially affected when Meteor transcription was reduced by 80%–90% using dCas9-KD (Figure 1G). Interestingly, however, inversion of the Meteor promoter caused significantly reduced expression of Eomes, albeit to a slightly lower extent than the pKO (Figure 1C). As transcription from the Meteor promoter is initiated in this cell line, with evidence for inefficient elongation to the opposite direction (Figure 1B), this could indicate that transcription elongation into the Meteor locus—and specifically, the minimal level of elongation that still occurs in the Meteor dCas9-KD cells—is required for proper Eomes regulation in mESCs. Alternatively, the functional feature of the Meteor enhancer could be the underlying DNA sequence, which is left intact in the Rz-KD, pAS, and dCas9-KD cells lines, with the promoter inversion interfering with proper functionality of this DNA, for example by disrupting the integrity, arrangement, or orientation of TF binding sites (TFBSs). To examine this possibility, we used the JASPAR database40 and FIMO41 to systematically compare the predicted TFBSs in the WT and the inverted sequence. While only few differences were identified (Figure S1H), we cannot exclude the possibility that disruption of binding of one of these TFs plays a role in regulation of Eomes levels. Replacing the Meteor promoter with another constitutively expressed promoter increased expression of Meteor, albeit with a variable effect between different clones, but had no consistent effect on Eomes levels (Figure 1H). Combined, results from these various cell lines and perturbations suggest that the functional feature of the Meteor locus in mESCs is minimal transcription initiation or elongation into the Meteor gene body.
Reduced Eomes expression is associated with altered chromatin organization in mESCs
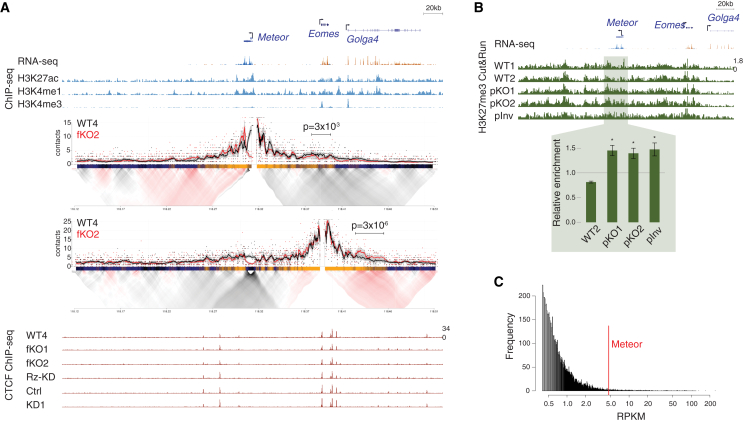
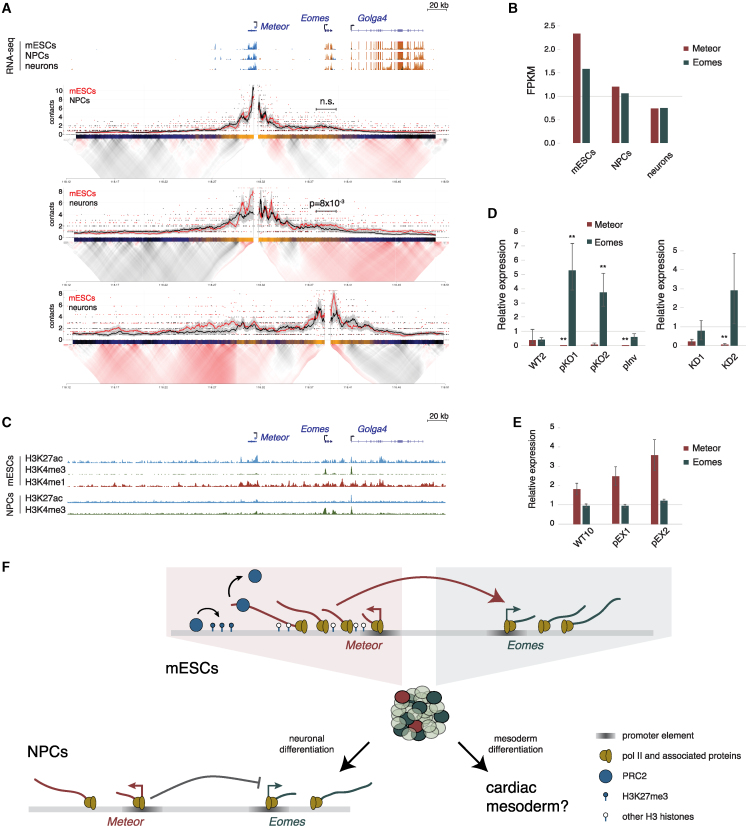
As Meteor is transcribed from an enhancer,20 we next wanted to examine whether Meteor perturbations that are associated with Eomes downregulation are also manifested in an altered chromatin organization of the locus. We therefore probed the topology of the Meteor locus using targeted chromosome conformation capture (4C)42 and CCCTC-binding factor (CTCF) chromatin immunoprecipitation sequencing (ChIP-seq). 4C analysis using either the Meteor or Eomes promoters as bait demonstrated spatial proximity between their respective loci, as has been shown previously14; deletion of either the entire Meteor gene body or the promoter region resulted in reduced contacts between the region surrounding Meteor and the Eomes locus, as did inversion of the Meteor promoter (Figures 2A, S2A, and S2B). In accordance with their lack of effect on Eomes levels, no significant changes between the Meteor and Eomes loci were noted in the dCas9-KD or Rz-KD mESC lines (Figures S2A and S2C).
Figure 2.
Meteor depletion induces chromatin changes in mESCs
(A) (Top) Genome browser image of the region surrounding the Meteor locus. Shown are representative transcript models; RNA-seq tracks where orange denotes transcription on the plus strand and blue denotes transcription on the minus strand; and ENCODE mESC ChIP-seq tracks. (Middle) 4C analysis in the indicated mESC lines using the Meteor or Eomes promoters as viewpoints. Domainograms showing mean contact per fragment end for a series of window sizes are placed below smoothed trend lines and raw counts of the contact profiles. (Bottom) ChIP-seq tracks of CTCF in the indicated mESC lines. All tracks are normalized to the same scale.
(B) (Top) Genome browser image of the region surrounding the Meteor locus. RNA-seq track is the same as shown in (A); Cut&Run analysis of H3K27me3 levels in the indicated mESC lines grown in serum-free 2i/LIF conditions. (Bottom) Bar plot shows quantification of signal in the highlighted region, normalized to WT1 and to a H3K27me3-rich region near the Ppib gene (see STAR Methods). Bars represent standard errors; n = 3. ∗p < 0.05, one-sided t test.
(C) Distribution of reads per kilobase per million (RPKM) of all transcripts identified in an EZH2 RNA immunoprecipitation (RIP) dataset taken from Zhao et al.43 RPKM of Meteor indicated by a red line.
See also Figure S2.
These altered contacts between the Eomes and Meteor loci were not associated with any evident differential CTCF occupancy in the region (Figure 2A). It was shown that in mESCs, PRC2, the chromatin remodeler responsible for H3K27me3 deposition, modulates chromatin interactions between poised enhancers and their targets, thus promoting a chromatin topology required for proper induction of the target genes throughout differentiation processes.44,45,46 Indeed, while the Meteor promoter region is associated with active chromatin, the region downstream to the Meteor gene body displays chromatin marks associated with both active (H3K27ac) and repressive (H3K27me3) states (Figure S2D). We therefore employed Cut&Run to map H3K27me3 deposition around the Meteor locus in mESCs carrying various Meteor perturbations. We noted an increase in H3K27me3 levels surrounding Meteor and its downstream enhancer region in the Meteor pKO lines (Figures 2B and S2E). These increased H3K27me3 levels upon Meteor repression would suggest that Meteor acts in preventing, rather than promoting, PRC2 recruitment and H3K27me3 deposition, in line with some indications that the binding of PRC2 to DNA and RNA is mutually antagonistic.47,48,49,50 Accordingly, analysis of previously published PRC2 RNA immunoprecipitation (RIP) data43 revealed that Meteor is within the top 5% of transcripts bound by EZH2, the catalytic subunit of PRC2 (Figure 2C), with data from an independent mESC RIP experiment51 confirming an above-background association between Meteor and EZH2 but not with SUZ12, another PRC2 subunit (Figure S2F). The increased H3K27me3 deposition was also noted when the Meteor promoter was inverted (Figure 2B), altogether supporting a negative correlation between H3K27me3 deposition at the Meteor locus and Eomes expression in mESCs (Figures 1B and 2B). Combined, these results suggest that transcription through the Meteor gene body is required for restricting the spread of H3K27me3 in the Meteor enhancer region and enabling proper expression of Eomes in naive mESCs, with the nascent RNA likely mediating this function during its transcription while being dispensable after its production (see discussion).
Meteor repression does not alter the cardiac mesoderm differentiation potential of mESCs
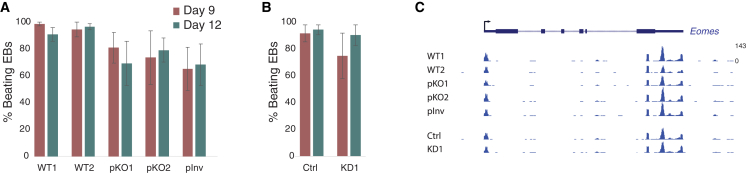
We next wanted to examine the effects of Meteor transcription on the cardiac mesoderm differential potential of mESCs. To this end, we utilized the hanging-drop embryoid body (EB) differentiation system.52 Consistent with its pluripotency-specific expression and with one previous study,20 Meteor expression was quickly reduced in the early days of the EB differentiation protocol (Figure S3A). Markers for the three germ layers were induced throughout the differentiation, with a relatively low induction of ectoderm markers such as Pax6 and Otx2, consistent with reduced efficiency of differentiation to the ectodermal lineage previously reported in this system for differentiation in leukemia inhibitory factor (LIF)-free and retinoic acid-free medium (Figure S3B).53,54,55 Expression of Eomes, as well as additional early mesoderm markers such as T (Brachyury) and FoxA2, peaked at day 4 (Figure S3B).
Using qRT-PCR and RNA-seq, we monitored the effect of Meteor repression on mesodermal gene induction and cardiac mesoderm formation in this differentiation system. Perturbation of Meteor expression by either KO of its promoter or dCas9-KD did not lead to any significant reduction in the formation of beating foci in EBs at either day 9 or day 12 (Figures 3A and 3B). This is in stark contrast to previous reports in which Meteor repression led to severe inhibition of functional cardiac mesoderm, with only 0%–20% of EBs exhibiting beating foci.20,33 Accordingly, and likewise in contrast to previous reports, we did not note any consistent changes in expression of Eomes or other mesodermal marker genes in either the pKO or dCas9-KD cells throughout the differentiation process (Figures 3C, S3C, and S3D). Specifically, no decrease in Eomes expression was noted at day 4, which is the peak of Eomes induction during differentiation of WT cells.
Figure 3.
Embryoid body differentiation of Meteor-depleted cells
(A) Percentage of EBs that have beating foci at day 9 and day 12 of EB differentiation in the indicated cell lines. Bars represent standard errors; n = 3.
(B) Same as (A), for Meteor dCas9-KD cells.
(C) 3′ RNA-seq tracks of the Eomes locus on day 4 of EB differentiation in the indicated cell lines. All tracks are normalized to the same scale.
See also Figure S3.
This deviation from previous reports might suggest that Meteor regulation of Eomes throughout EB differentiation depends on the genetic background of the mESCs. However, we could not find any consistent effect on either the levels or timing of activation of Eomes or additional mesoderm marker genes in mESCs derived from another background (129-C57Bl/6 mESCs), in which the full Meteor gene body was removed (fKO) or Meteor RNA was destabilized by insertion of a self-cleaving ribozyme sequence (Rz-KD)34 (Figures 1A and S3E). Nevertheless, we cannot rule out that accumulated genomic or karyotypic aberrations in our cell lines affect their pluripotency status or differentiation potential, a general concern with long-term culture of pluripotent stem cells in general and those undergoing clonal selection in particular.56,57
The most likely explanation for the inconsistency with previous reports stems from the difference in the growth medium in which the mESCs were kept prior to onset of the differentiation. Specifically, while in previous reports the mESCs were cultured in serum-based medium,20,33 we cultured the cells in 2i medium pre-differentiation. mESCs grown in either serum or 2i conditions are considered to be found in a “naive” state of pluripotency and retain the ability to differentiate into the three germ layers, although both protocols are associated with some alterations to differentiation potential. Specifically, serum-cultured cells exhibit high transcriptomic variability, with a subset of the population found at a more differentiated state with elevated expression of lineage-determination genes.36,37,58,59 Therefore, and given the discrepancy in cardiac mesoderm differentiation when Meteor is depleted despite similar effects on Eomes levels in mESCs, we reasoned that Meteor repression affects cardiac mesoderm differentiation indirectly through affecting the cellular state at the naive mESC stage, an effect that is particularly apparent when mESCs are cultured in serum.
Meteor and Eomes are expressed in distinct mESC subpopulations
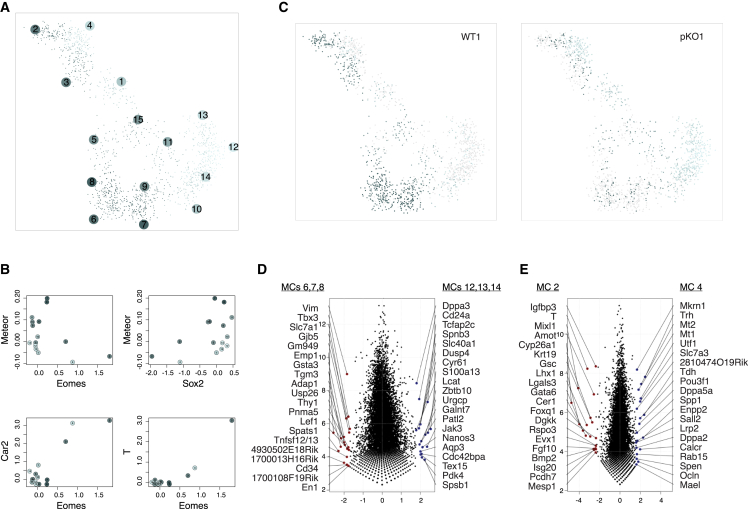
To test this hypothesis, we performed scRNA-seq to characterize the effect of Meteor KO on Eomes expression and cellular state in serum-grown mESCs. Approximately 750 cells from either one WT or one Meteor pKO line were assayed. scRNA-seq data showed that both Meteor and Eomes are variably expressed in mESCs, with Meteor detected (≥1 reads) in just ∼9% of the WT cells (Figure S4A). About a quarter of WT cells had detectable expression of Eomes, mostly at low levels of 1–4 reads/cell, although a small population (∼2.5% of all cells) expressed Eomes at higher levels (Figure S4A).
We analyzed the scRNA-seq data using the MetaCell package,60 which partitions the cells into groups of metacells (MCs) that represent a single putative cellular state. Interestingly, despite their spatial proximity and the regulatory link offered by the reduced expression of Eomes in Meteor KO cells (Figure 1C), Meteor and Eomes were predominantly expressed in non-overlapping MCs (Figures 4A, 4B, S4B, and S4C) (p = 0.046; one-sided Fisher’s test considering only WT cells). Eomes was enriched primarily in MCs that express genes associated with a more differentiated state, such as T, Lhx1, and Otx2.61,62,63 Accordingly, gene ontology (GO) term analysis of the top 100 genes correlated with Eomes expression in this dataset yielded terms such as “anterior/posterior axis specification” and “gastrulation” (Figure S4D). Meteor, on the other hand, was enriched in a subset of MCs that are associated with high expression of pluripotency genes, such as Esrrb and Sox2 (Figures 4B, S4B, and S4C).
Figure 4.
Distinct subpopulations of mESCs in Meteor WT and KO mESCs
(A) 2D projection of the MetaCell60 adjacency graph. Cells are shown as small dots, whose location indicates similarity to the adjacent cells and metacells (MCs). Color was assigned to each MC according to the ratio of WT and pKO cells that comprise it, with darker shades representing MCs comprised mostly of WT cells and lighter shades representing MCs comprised mostly of pKO cells.
(B) Correlation between the log of the fold enrichment values (expression enrichment over the median MC) for the indicated gene pairs, separated by MCs.
(C) Same as (A), separately for WT1 and pKO1 cells.
(D) Scatterplot comparing gene expression between the indicated groups of MCs. Highlighted are the 20 most differentially expressed genes in each group.
(E) Same as (D), for MCs 2 and 4.
See also Figure S4.
Altered expression of pluripotency-associated programs of Meteor-depleted mESCs
scRNA-seq was performed in parallel on Meteor WT and pKO cells. The total number of cells occupying MCs associated with high levels of pluripotency markers (MCs 5–15; Figures 4B, S4B, and S4C) was similar in the WT and pKO line (Figure S4E). However, among those MCs, there was a shift of pKO cells from MCs 6–8 to MCs 12–14 (Figures 4C and S4E). Examination of the genes differentially expressed between these MC groups indicates that MCs 12–14, which are more dominant in the Meteor pKO cells, preferentially expressed pluripotency-related and primordial germ cell-related genes such as Dppa3, Zbtb10, and Nanos3, whereas MCs 6–8, which are more prominent in WT cells, preferentially expressed genes related to differentiation and development processes, such as Emp1, Tgm3, and Adap1 (Figure 4D). Accordingly, while we did not note any general effect of Meteor KO on the levels of classic pluripotency markers such as Oct4, Sox2, and Nanog in bulk RNA-seq, we did observe upregulation of genes normally associated with the pluripotent state and downregulation of genes that are normally associated with more differentiated states (Figure S4F).
The total number of cells occupying the more differentiated subset of the Meteor pKO population (MCs 2–4; Figure S4B) did not significantly change. However, similarly to the shift within the pluripotency-related population, there was a shift within these MCs, with Meteor pKO cells occupying MC 4 rather than MCs 2–3, which were populated predominantly by WT cells (Figures 4C, S4B, and S4E). The expression of Eomes itself was highest in MC 2 (Figures 4B and S4B), which is associated with high expression of genes involved in mesoderm specification such as T, Mesp1, Mixl1, and Gsc (Figure 4E). The reduction of cells occupying MC 2 was thus the likely cause for both the reduction of total Eomes levels seen in the bulk RNA-seq (Figure 1C) as well as the reduced capacity of serum-grown mESCs to differentiate into cardiac mesoderm genes previously noted.20,33 The fact that Meteor KO caused a decrease in cells from MCs in which Meteor itself was not expressed at significant levels (Figures 4B, 4C, S4B, and S4E) suggests that either Meteor RNA is dispensable for the establishment of the cellular states represented by these MCs—making the DNA element the functional feature of Meteor in this system—or, alternatively, that cells cycle back and forth between the different states, with Meteor repression in the pluripotency-related states preventing their entering the state represented by MCs 2 and 3, possibly mediated by epigenetic changes that we observed in the Meteor locus (Figures 2A and 2B) (see discussion).
A distinct functional feature in Meteor represses Eomes in NPCs
As opposed to MC 2, MC 4, which was substantially more populated in the Meteor KO population compared with WT cells, did not express predominantly mesoderm-related genes but was rather defined by an enrichment of genes associated with neurogenesis such as Pou3f1, Sall2, Trh, and Rab15 (Figure 4E). It was previously suggested that the reduced mesodermal differentiation capacity of Meteor KO cells drives them toward the neuronal lineage.20 We therefore examined whether Meteor repression had an effect on the ability of mESCs to differentiate into neurons. In contrast to the EB differentiation system, where Meteor was rapidly reduced to undetectable levels (Figure S3A), Meteor continued to be expressed throughout neuronal differentiation (using a two-step differentiation protocol described by Ying et al.64), albeit with reduced expression levels, an expression pattern that correlated with Eomes expression in this system (Figures 5A and 5B). The chromatin organization of the locus also changed throughout the differentiation system, with Meteor-Eomes contacts remaining stable in neural progenitor cells (NPCs) but decreasing at day 8 of neuronal differentiation (Figure 5A). Active regions within the locus also shifted throughout the differentiation system, with the Golga4 promoter—which is found in significant spatial proximity to Eomes in neurons (Figure 5A)—being the most active region in NPCs, as indicated by H3K27ac ChIP-seq we performed in these cells (Figure 5C). This draws a correlation between Eomes expression and spatial proximity to the Meteor locus throughout neuronal differentiation and suggests that distinct cis elements regulate Eomes expression in this system, with the Meteor lncRNA or its underlying enhancer possibly contributing to this regulation.
Figure 5.
A distinct functional feature of the Meteor locus represses Eomes throughout neuronal differentiation
(A) (Top) Genome browser image of the region surrounding the Meteor locus. Shown are representative transcript models, and RNA-seq tracks taken from Hezroni et al.65 where orange denotes transcription on the plus strand and blue denotes transcription on the minus strand. All tracks are normalized to the same scale. (Bottom) 4C analysis in the indicated cells using the Meteor or Eomes promoters as viewpoints. Domainograms showing mean contact per fragment end for a series of window sizes are placed below smoothed trend lines and raw counts of the contact profiles.
(B) RSEM quantifications of Meteor and Eomes expression levels in the indicated cell types; RNA-seq data are the same as shown in (A).
(C) ENCODE ChIP-seq tracks in mESCs (top), and H3K27ac ChIP-seq and H3K4me3 Cut&Run tracks in NPCs (bottom). Genomic coordinates are aligned to (A).
(D) DESeq2 quantifications of Meteor and Eomes in NPCs derived from the indicated cell lines. Amounts normalized to WT1/Ctrl. Bars represent standard errors; n = 3. ∗∗padj < 0.005.
(E) qRT-PCR quantifications of Meteor and Eomes in NPCs derived from the indicated cell lines. Levels were normalized to WT9 and Ppib for internal control. Bars represent standard errors; n = 3.
(F) Model of Meteor function. In mESCs, the Meteor locus activates Eomes expression. Perturbing elongation through the locus is associated with increased H3K27me3 deposition and decreased Eomes expression, likely through decreasing the Eomes-expressing mESC subpopulation. As a function of the growth conditions of the cells, this might result in reduced efficiency of cardiac mesoderm formation. Following neuronal differentiation, the Meteor locus now represses Eomes levels, with the DNA element or transcription initiation serving as the functional feature.
See also Figure S5.
Accordingly, we tested the ability of Meteor KO mESCs to undergo neuronal differentiation. Both Meteor pKO and pInv mESCs were able to differentiate to neurons, as seen by their ability to form Nestin-expressing NPCs and Tuj1-expressing neurons (Figures S5A and S5B). Surprisingly, however, when Meteor pKO cells were differentiated to NPCs, a strong upregulation of Eomes expression was noted (Figure 5D). Many genes associated with Eomes were also upregulated in the Meteor KO NPCs, including genes related to differentiation (such as Nodal, Fgf4, Fgf8, and FoxA2). The only GO term category significantly associated with Eomes upregulation in these cells was neuronal differentiation, in accordance with the known roles of EOMES in the regulation of neuronal development and differentiation.21
Eomes was upregulated in NPCs only when the Meteor promoter was deleted, but not inverted (Figure 5D). This is in contrast to the reduced level of Eomes seen in mESCs with an inverted Meteor promoter (Figure 1C), suggesting that a distinct functional feature mediates Meteor activity in mESCs as compared with NPCs, and also indicating that altered Eomes expression in NPCs is not a consequence of the differential Eomes expression in mESCs, which was noted in both pKO and pInv cells. Furthermore, Eomes levels were not increased when the pEX lines, in which transcription into the Meteor gene body was initiated at a constitutive PGK promoter rather than the Meteor promoter, were differentiated to NPCs (Figure 5E). A plausible explanation for this observation is competition between the Eomes and Meteor promoters over the activity of a shared enhancer (see discussion). Consistent with this model, Meteor dCas9-KD cells also did not show significant upregulation of Eomes upon differentiation to NPCs (Figure 5D), although it is noteworthy that there was some non-significant upregulation of Eomes compared with the control in one of the cell lines in which the KD was somewhat more efficient. It thus appears that the DNA element underlying the Meteor transcriptional activity is important for proper regulation of Eomes levels in NPCs.
Discussion
The low sequence conservation of enhancer-transcribed lncRNAs8,16,66 suggests that as a group, the RNA products of these genes likely do not play an important role in enhancer activity. Rather, if functional, these loci are proposed to act mostly via their underlying DNA element or by the act of their transcription.15 Distinguishing between these levels of functionality is notoriously difficult, complicating efforts to reconcile results from different studies on the same gene. Here, we show that the Meteor locus encodes for two distinct, cell-type-specific functions, one of which is DNA mediated and the other dependent on the act of transcription through the locus (Figure 5F). In naive mESCs, elongation through the Meteor gene body appears to be necessary for maintaining Eomes levels, as KO or inversion of the Meteor promoter are sufficient to reduce Eomes levels by ∼50%, whereas dCas9-KD, introduction of Rz or pAS sequences, or promoter replacement—all of which allow some level of transcription through the locus—do not have this effect on Eomes levels (Figures 1C–1H). It is noteworthy that the effects of “the act of transcription” may be specific to transcription through a particular region within the lncRNA gene, as has been demonstrated for the Airn lncRNA.67 The effect of Rz-mediated cleavage on the distance and dynamics of transcription after the Rz sequence has been encountered is not yet clear and might depend on local sequence attributes that dictate folding dynamics. Similarly, the transcription machinery has been suggested to continue transcribing for a few kilobases after encountering a pAS.68 Thus, the lack of a significant effect on Eomes levels caused by insertion of a pAS or Rz sequence into the first intron or second exon of Meteor, respectively (Figures 1A, 1D, and 1F) does not necessarily indicate that transcription through the remainder of the locus is dispensable for full functionality, nor does it contradict the phenotype observed upon insertion of a pAS further downstream of the Meteor TSS.20
The reduced Eomes expression in Meteor pKO lines is accompanied by H3K27me3 accumulation around the broad enhancer region within the Meteor locus (Figures 2B and S2E). Combined with the association of Meteor lncRNA with PRC2 (Figures 2C and S2F), this suggests that as Meteor is being transcribed its association with PRC2 evicts the latter from the DNA and prevents H3K27me3 deposition, in accordance with previous findings that the binding of PRC2 to DNA and RNA is mutually exclusive.47,48,49,50 Importantly, functionality of the act of transcription may also include the nascent RNA produced in the process—for example, by recruiting or evicting proteins such as PRC2 from the chromatin, as has been proposed69—with the lingering RNA present at the end of the transcriptional process being dispensable for the activity. It is not yet clear in what way the increased H3K27me3 accumulation around the Meteor enhancer is translated into reduced Eomes expression and whether this involves PRC2’s role in mediating spatial chromatin organization in mESCs,44,45,46 as might be suggested by the reduced contacts between the Eomes and Meteor regions in Meteor KO cells (Figures 2A and S2B). Regardless of the exact mechanism, however, the association of PRC2 with Meteor does not necessarily imply a sequence-specific function of Meteor, as the selectivity and specificity of PRC2’s interaction with RNA is unclear.70,71,72,73,74
Intriguingly, inhibition of Meteor transcription elongation in mESCs causes reduction in Eomes levels despite the two transcripts being expressed in largely distinct cells within the mESC population (Figures 4B, S4B, and S4C), suggesting that Meteor transcription indirectly regulates Eomes by affecting the state at which the cells are found. This model can also explain the apparently different effects of Meteor inhibition on the ability of mESCs to differentiate to beating EBs, which was unaffected in our hands when growing mESCs in 2i but severely affected in Meteor-null cells grown in serum.20,33 This indicates that Meteor repression affects the differentiation potential of the population when grown under specific conditions rather than the hindered differentiation ability being a direct consequence of reduced Eomes levels. This altered differentiation potential can be linked to the altered epigenetic landscape in Meteor pKO and pInv cells (Figures 2A and 2B), which could itself prevent mESCs from entering or remaining in a cellular state that requires Eomes expression, affecting the overall level of Eomes expression detected in bulk RNA-seq. This exemplifies how transcription of lncRNA loci could participate in priming differentiation or developmental processes during which they are no longer required to be expressed. Interestingly, an additional pluripotency-associated lncRNA, Platr4, was recently shown to act in trans to promote mesoderm differentiation in vitro and in vivo while itself being rapidly downregulated during differentiation, suggesting that such priming activities by lncRNAs might be common in early development.75
In contrast to the situation in mESCs, during neuronal differentiation the Meteor locus acts to repress, rather than enhance, Eomes expression (Figures 5D and 5F). Also contrary to its function in mESCs, the functional feature of Meteor in this system appears to be the DNA element—specifically, the short region that encompasses the TSS and first exon of the lncRNA—rather than elongation through the Meteor enhancer, as no changes in Eomes expression in NPCs are noted when the Meteor promoter is inverted (Figure 5D). The mechanism through which Meteor represses Eomes in this system is not yet clear, although considering the dependency of the phenotype on the DNA rather the RNA element and the lack of effect when the promoter sequence is severely disrupted by insertion of an alternative promoter and a hygromycin gene (Figure 5E), a particularly appealing possibility is competition between the Eomes and Meteor loci over binding to enhancers, as has been shown for other lncRNA loci.76 A possible candidate for the limiting enhancer is the region downstream of Eomes with which the Eomes locus is associated following neuronal differentiation (Figure 5A). Regardless of the mechanism, the consequence of the heightened Eomes expression in Meteor-null NPCs, and specifically whether it affects the efficiency of neuronal differentiation, remains to be seen. Alternatively, as Eomes is expressed only at specific times and compartments within the developing brain,21 increased Eomes expression in NPCs could affect the composition of the neuronal types acquired throughout the differentiation process, implicating the Meteor enhancer in fine-tuning neuronal differentiation.
The study of Meteor illustrates the importance of careful selection of perturbation techniques for the study of lncRNA loci, as both the suggested function and functional feature of the locus appear to depend on the employed perturbation method. Taken together, the emerging picture is that Meteor controls Eomes levels through distinct, cell-type-specific functional features, but not through its RNA product. While our findings do not exclude the possibility that the Meteor locus has additional functions in other systems or through additional functional features, as has been suggested,33 we could not detect any substantial Meteor expression in systems other than mESCs, testes, and throughout neuronal differentiation (Figures S3A, 5A, and 5B; https://bis.zju.edu.cn/MCA/search3.html), which makes an RNA-dependent Meteor function in other cell types unlikely. The lack of an RNA-based function is also consistent with our inability to identify sequence-similar homologs of the Meteor lncRNA outside of rodents. The human lncRNA LINC01980 is transcribed from a syntenic region and has a similar expression pattern,20 but when its sequence is projected to the mouse genome through a whole-genome alignment it does not overlap with the Meteor locus.
The intricate, cell-type-specific regulation of Eomes through distinct features of the Meteor locus serves as an illustration of how complex regulation of master regulator TFs can be fine-tuned through the use of lncRNA transcriptional units. This can be achieved through combinatorial activity of multiple lncRNA units, as has been described for the regulatory network of the Hand2 TF17,77 or, as described here, through distinct functions mediated by the same locus in different cellular contexts. It is expected that in-depth study of additional lncRNA loci, using multiple perturbations in various biological systems, will uncover additional loci with complex phenotypical output and unveil other mechanisms through which lncRNA loci contribute to regulation of gene expression.
Limitations of the study
In contrast to several previous studies of Meteor lncRNA, we show here that Meteor-null mESCs can efficiently differentiate to cardiac mesoderm. To ascertain whether different initial growth conditions of the mESCs, and not clonal selection, indeed explain these variations, further experiments would be needed, including differentiation of our KO cells after growing them in serum-based media. Furthermore, we currently compare the effects of Meteor depletion on early mesoderm differentiation and on NPCs, which comprise a more differentiated state along the ectoderm lineage. In the future, it would be interesting to look at earlier stages of ectoderm differentiation, potentially with single-cell resolution, so as to directly compare the results with findings relating to mesoderm differentiation and to ascertain whether the changes in the Eomes-expressing subpopulation of the mESCs have an effect on differentiation to additional germ layers.
Furthermore, additional cellular models can help validate and identify other functional features of the Meteor locus. Specifically, replacing the Meteor promoter with a different, sequence-diverged yet transcriptionally active promoter might help validate our findings regarding the DNA element being the functional feature of Meteor in NPCs. Nonetheless, as clean separation of the effects of a DNA from any transcriptional activity is challenging, and as another promoter might contain some sequence elements in common with the Meteor promoter, a library of mutated Meteor promoter sequences would enable zeroing in on the exact sequence elements that confer the promoter activity. Lastly, this study focused on the roles of different functional features within the Meteor locus in vitro; further studies are needed to delineate their contribution to early embryonic development in vivo.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit monoclonal anti-H3K27ac (D5E4) | Cell Signaling Technology | Cat#8173; RRID AB_10949503 |
| Rabbit monoclonal anti-CTCF (D31H2) | Cell Signaling Technology | Cat#3418; RRID AB_2086791 |
| Rabbit monoclonal anti-H3K27me3 (C36B11) | Cell Signaling Technology | Cat#9733; RRID AB_2616029 |
| Rabbit monoclonal anti-H3K4me3 (C42D8) | Cell Signaling Technology | Cat#9751; RRID AB_2616028 |
| Critical commercial assays | ||
| SENSE mRNA-Seq Library Prep Kit V2 | Lexogen | Cat#001.96 |
| QuantSeq 3′ mRNA-Seq Library Prep Kit FWD | Lexogen | Cat#015.96 |
| Xfect mESC Transfection Reagent | Takara Bio | Cat#631320 |
| Deposited data | ||
| Raw and analyzed RNA-seq data | This study | GEO: GSE223445 |
| Raw and analyzed 3′ RNA-seq data | This study | GEO: GSE223449 |
| Raw and analyzed scRNA-seq data | This study | GEO: GSE223452 |
| Raw and analyzed 4C data | This study | GEO: GSE223451 |
| Raw and analyzed ChIP-seq data | This study | GEO: GSE223447 |
| Raw and analyzed Cut&Run data | This study | GEO: GSE223448 |
| Analyzed data of gene expression levels in different mESC compartments | GEO record GSE99366 | GEO: GSE99366 |
| Raw data of control mESCs undergoing neural differentiation | Stryjewska et al.78 | GEO: GSE75616 |
| Raw data of EZH2 RIP-seq | Zhao et al.43 | GEO: GSE17064 |
| Raw data of EZH2 and SUZ12 RIP-seq in WT mESCs | Garland et al.51 | GEO: GSE137491 |
| Experimental models: Cell lines | ||
| M. musculus: WT1: R1 wild type | This study | N/A |
| M. musculus: WT2: R1, wild type | This study | N/A |
| M. musculus: WT3: R1, wild type | This study | N/A |
| M. musculus: pKO1: R1, homozygous KO of Meteor promoter | This study | N/A |
| M. musculus: pKO2: R1, homozygous KO of Meteor promoter | This study | N/A |
| M. musculus: pInv: R1, homozygous inversion of Meteor promoter | This study | N/A |
| M. musculus: WT4: 129-C57Bl/6, wild type | Laboratory of Marc Bühler34 | N/A |
| M. musculus: WT5: 129-C57Bl/6, wild type | Laboratory of Marc Bühler34 | N/A |
| M. musculus: WT6: 129-C57Bl/6, wild type | Laboratory of Marc Bühler34 | N/A |
| M. musculus: fKO1: 129-C57Bl/6, homozygous KO of Meteor gene | Laboratory of Marc Bühler34 | N/A |
| M. musculus: fKO2: 129-C57Bl/6, homozygous KO of Meteor gene | Laboratory of Marc Bühler34 | N/A |
| M. musculus: Rz-KD: 129-C57Bl/6, homozygous insertion of a Rz sequence into Meteor | Laboratory of Marc Bühler34 | N/A |
| M. musculus: Ctrl: R1 stably expressing dCas9 | This study | N/A |
| M. musculus: KD1: R1 stably expressing dCas9 and gRNA for KD1 | This study | N/A |
| M. musculus: KD2: R1 stably expressing dCas9 and gRNA for KD2 | This study | N/A |
| M. musculus: WT7: R1, wild type | This study | N/A |
| M. musculus: WT8: R1, wild type | This study | N/A |
| M. musculus: pAS1: R1, homozygous insertion of pAS/MAZ into Meteor | This study | N/A |
| M. musculus: pAS2: R1, homozygous insertion of pAS/MAZ into Meteor | This study | N/A |
| M. musculus: WT9: R1, wild type | This study | N/A |
| M. musculus: WT10: R1, wild type | This study | N/A |
| M. musculus: pEX1: R1, homozygous insertion of a Hyg/pAS/PGKp into Meteor | This study | N/A |
| M. musculus: pEX2: R1, homozygous insertion of a Hyg/pAS/PGKp into Meteor | This study | N/A |
| M. musculus: pEX3: R1, homozygous insertion of a Hyg/pAS/PGKp into Meteor | This study | N/A |
| Oligonucleotides | ||
| For a list of oligonucleotides used in this study see Table S1 | This study | N/A |
| Recombinant DNA | ||
| pCas9-GFP | Ding et al.79 | Addgene plasmid #44719 |
| pKLV-U6gRNA | Koike-Yusa et al.80 | Addgene plasmid #50946 |
| lenti MS2-P65-HSF1_Hygro | Konermann et al.81 | Addgene plasmid #61426 |
| CSII-U6-gRNA-CBh-3xFLAG-PA-dCas9-P2A-Puro | Deposited by the laboratory of Tohru Kimura | Addgene plasmid #83306 |
| Software and algorithms | ||
| STAR | Dobin et al.82 | https://github.com/alexdobin/STAR |
| RSEM | Li and Dewey83 | https://deweylab.github.io/RSEM/ |
| DEseq2 | Love et al.84 | https://bioconductor.org/packages/release/bioc/html/DESeq2.html |
| MetaCell | Baran et al.60 | https://tanaylab.github.io/metacell/ |
| KentUtils | Kent et al.85 | https://github.com/ENCODE-DCC/kentUtils |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Igor Ulitsky (igor.ulitsky@weizmann.ac.il).
Materials availability
This study did not generate new unique reagents.
Experimental model and study participant details
R1 and 129-C57Bl/6 mESCs were routinely grown on gelatin-coated plates on MEFs, in one of the following media:
Serum-based medium: DMEM (Gibco) containing 15% ES-grade fetal calf serum (Biological Industries), 1X Sodium pyruvate (Gibco), 1X Nonessential amino acids (Gibco), 0.1mM β-mercaptoethanol (Sigma), 100U/ml Penicillin and 0.1 mg/mL Streptomycin (Biological Industries), and 10 ng/ml LIF (Weizmann Proteomics Unit). Cells were passaged with 0.25% Trypsin (Biological Industries) every 2–3 days.
2i medium: a 1:1 mixture of Neurobasal medium (Gibco) and Knockout DMEM (Gibco), supplemented with 1X B27 (Gibco), 1X N2 (Gibco), 1X Glutamax (Gibco), 1X Sodium pyruvate (Gibco), 1X Nonessential amino acids (Gibco), 0.1mM β-mercaptoethanol (Sigma), 52.5 μg/ml BSA, 100U/ml Penicillin and 0.1 mg/mL Streptomycin (Biological Industries), 1μM PD 0325901 (Axon Medchem), 3μM CHIR 99021 (Axon Medchem), and 10 ng/ml LIF (Weizmann Proteomics Unit). Cells were passaged with 0.25% Trypsin (Biological Industries) every 2–3 days. Trypsin was quenched with serum-based medium, which was removed prior to re-plating.
Primed medium: DMEM/F12 (Sigma) supplemented with 1% Knockout serum replacement (Gibco), 1X B27 (Gibco), 1X N2 (Gibco), 1X Glutamax (Gibco), 1X Sodium pyruvate (Gibco), 1X Nonessential amino acids (Gibco), 0.1mM β-mercaptoethanol (Sigma), 100U/ml Penicillin and 0.1 mg/mL Streptomycin (Biological Industries), 12 ng/ml FGF2 (Peprotech), and 20 ng/ml Activin A (R&D). Cells were passaged with 0.05% Trypsin (Biological Industries) every 3 days. Trypsin was quenched with serum-based medium, which was removed prior to re-plating. 10μm ROCK inhibitor (Axon Medchem) was added to the medium the day before and after each split.
Method details
Generation of promoter KO (pKO) and inversion (pInv) cell lines
To generate Meteor promoter KO lines, mESCs were depleted from MEFs by 20 min incubation on gelatin-coated plates and seeded on 6-wells (5x105 cells/well). mESCs were transfected with pCas9-GFP and two pKLV-U6gRNA plasmids containing gRNAs targeting the two sides of the Meteor TSS (see Table S1). Transfections were carried out using Xfect mESC Transfection Reagent (Takara Bio) according to manufacturer’s instructions. MEFs were added 3h post-transfection. 0.8 μg/μL Puromycin was added to the medium 24h after transfection for selection. Six days after transfection, mESCs were seeded at a very low density on a 10cm plate. Colonies were picked, expanded, and the genomic deletion was verified using PCR and single molecule FISH (see Figures S1B–S1C and Table S1). Lack of Meteor expression was verified by qRT-PCR and RNA-seq. Clones that underwent a similar process but did not exhibit the genomic deletion were expanded alongside and used as WT controls.
Generation of polyA insertion (pAS) cell lines
Meteor polyA insertion mESCs were generated by transfection with a pKLV-U6gRNA plasmid containing gRNA 2 that was used for generating Meteor pKO lines (see Table S1), pCas9-GFP, and a ssODN (200nt) containing two homology arms (50nt each), a short poly(A) site (49nt), and two MAZ sites.86 Colonies were picked, expanded, and the poly(A)/MAZ insertion was confirmed by PCR amplifications and sequencing (Table S1). Clones without an insertion were expanded alongside and used as WT controls.
Generation of PGK promoter exchange (pEX) cell lines
The PGK promoter knock in vector (pBlueScript-KS+ vector (Stratagene)) was generated by insertion of Meteor 5′ homology arm (Meteor promoter, 985 bp in length, mm9 coordinates chr9:118,315,278-118,316,262) and 3′ homology arm (Meteor first exon, 362 bp, mm9 coordinates chr9:118,314,889-118,315,250) upstream of a Hygromycin resistance gene and a pAS (taken from Addgene plasmid #61426) and downstream of a PGK promoter (taken from pKLV-U6gRNA), respectively (see Figure 1A).87 The targeting vector was co-transfected with pCas9-GFP and pKLV-U6gRNA plasmids containing gRNA pEX (see Table S1) which targets immediately upstream of Meteor TSS. 0.8 μg/μL Puromycin and 4 μg/μL Hygromycin were added to the medium 24h after transfection. Six days after transfection, mESCs were seeded at a very low density on a 10cm plate. Colonies were picked, expanded, and the Hygromycin resistance and PGK insertion was verified using PCR (Table S1). Clones without an insertion were expanded alongside and used as WT controls.
Generation of dCas9-KD cell lines
Viruses were produced by transfecting HEK293T cells with a 1:0.65:0.35:0.25 ratio of CSII-U6-gRNA-CBh-3xFLAG-PA-dCas9-P2A-Puro, pMDL, pVSVG, and pRev plasmids, respectively, using PEI transfection.88 Medium was collected 48h after transfection, filtered, and kept at −80°C.
These viruses were used to create mESCs stably expressing dCas9: mESCs were depleted from MEFs by 20 min incubation on gelatin-coated plates, and 6x105 cells were incubated in 1.5mL of virus-containing medium for 30 min at 37°C, and then seeded on two 6-wells. 0.8 μg/μL Puromycin was added to the medium 24h after transfection. Surviving colonies were seeded at a very low density on a 10cm plate. Colonies were picked, expanded, and the presence and expression of dCas9 was assayed by PCR and Western Blot.
Meteor dCas9-KD mESCs were created by infecting the dCas9-expressing mESCs with viruses prepared as described above from pKLV-U6gRNA plasmids containing gRNAs targeting the vicinity of the Meteor TSS (see Table S1). 48h post-infection, mESCs were sorted for BFP expression using FACSAria II (BD Biosciences), and screened for Meteor KD using qRT-PCR and RNA-seq.
EB differentiation
mESCs were depleted from MEFs by 20 min incubation on gelatin-coated plates, and seeded at 500cells/27μL drop in differentiation medium (serum-based mESC medium without LIF), on the lids of 10cm plates with PBS. On d2 drops were collected, combined, and transferred to non-adherent 10cm petri dishes in differentiation medium. On d7 individual EBs were transferred to separate gelatin-coated 24-wells and grown in differentiation medium for five additional days. EBs were screened for beating foci at day 9 and day 12 of differentiation. RNA expression throughout the differentiation was assayed using 3′ mRNA-Seq or qRT-PCR (see Table S1 for primer sequences).
Neuronal differentiation
Neuronal differentiation was based on a protocol taken from.89 mESCs were grown for two passages in serum-based medium on MEF-free, gelatin-coated plates. Cells were then seeded on gelatin-coated plates (2x105 cells/6-well) in neuronal differentiation medium: 1:1 mixture of DMEM/F12 (Sigma) and Neurobasal medium (Gibco), supplemented with 0.5X B27 (Gibco), 0.5X N2 (Gibco), 1X Glutamax (Gibco), 0.1mM β-mercaptoethanol (Sigma), 25 μg/ml BSA, and 100U/ml Penicillin and 0.1 mg/mL Streptomycin (Biological Industries). After four days, the cells were dissociated with 0.05% Trypsin (Biological Industries) and re-plated (3.5x105/6-well) on Poly-D-Lysine (Sigma) and Laminin (Life)-coated plates, in neuronal differentiation medium also containing 20 ng/ml FGF2 (Peprotech). FGF2 was removed after 24hr and the cells were grown for an additional three days.
For stainings,65 cells were fixed in 4% paraformaldehyde for 15 min, and incubated in blocking buffer (5% donkey serum, 2% BSA, and 0.1% Triton X-100 in PBS). Primary antibodies (mouse anti-Nestin (Abcam ab6142) or rabbit anti-Beta III Tubulin (Tuj1, Abcam ab18207)) were diluted in permeabilization buffer (same as above, without Triton X-100) and incubated with the cells at 4°C overnight. Secondary antibodies (donkey anti-mouse Alexa 594 (Molecular Probes A21203) or goat anti-rabbit Alexa 594 (Abcam ab150080)) were diluted in permeabilization buffer and incubated with fixed cells for 2h at RT. Imaging was done using the EVOS FL Cell Imaging System.
RNA sequencing
0.5–1μg total RNA was used to prepare RNA-seq libraries using either the SENSE mRNA-Seq Library Prep Kit V2 (Lexogen) or the QuantSeq 3′ mRNA-Seq Library Prep Kit for Illumina (FWD) (Lexogen), according to the manufacturer’s protocols, and sequenced on either a NextSeq 500 or Novaseq 6000 machine. Reads were mapped to the mouse genome (mm9) using STAR.82 Expression levels were quantified using RSEM,83 and differential expression analysis was performed using DESeq2.84 For 3′ RNA-seq, RSEM was run on a gene annotation GTF file where the last exon of each gene was extended by 2Kb to the 3′ direction if no other gene was found on the same strand within that distance, to ensure that reads falling outside the annotated genes were assigned correctly.
For scRNA-seq, cells were sorted into 384-well plates containing 2nM barcodes and lysis buffer. After MARS-seq barcoding and library preparation90 the libraries were sequenced on a NextSeq 500 machine. UMI matrices were generated, summarizing the expression levels of all genes across all WT and Meteor pKO cells,91,92 and metacells were constructed and analyzed using the MetaCell package.60
Chromatin immunoprecipitation (ChIP)
ChIP was performed93 by crosslinking 1x107 cells in 1% formaldehyde for 10 min at RT, and the reaction was quenched by addition of 125mM glycine and 5 min incubation at RT. Cells were centrifuged 5 min at 2000xg, 4°C, washed three times in PBS containing protease inhibitors (PI), and lysed in 1mL lysis buffer (5mM PIPES pH 8.0, 85mM KCl, 1% Igepal, and 1X PI) for 15 min on ice. Cells were centrifuged 5 min at 250Xg, 4°C, supernatant was discarded, and nuclei were lysed by incubation in nuclei lysis buffer (50mM Tris-HCl pH 8.1, 10mM EDTA pH 8.0, 1% SDS, and 1X PI) for 30 min on ice, followed by flash freezing in liquid nitrogen and re-thawing. Pellets were sonicated using a Bioruptor for either 12 (mESCs) or 15 (NPCs) cycles in high setting, 30 s ON, 30 s OFF, centrifuged 10 min at 14,000rpm, 4°C, and supernatant was transferred to a new tube and diluted ×10 in IP dilution buffer (50mM Tris-HCl pH 7.4, 150mM NaCl, 1% Igepal, 0.25% Deoxycholic acid, 1mM EDTA pH 8.0, and 1X PI). 50μL protein A/G magnetic beads (GenScript, Cat #L00277) per IP were washed twice in binding/washing buffer (0.5% BSA and 0.5% Tween 20 in PBS), resuspended in 1 mL binding/washing buffer with either anti-H3K27ac (D5E4) antibody (Cell Signaling #8173) or anti-CTCF (D31H2) antibody (Cell Signaling, #3418), and incubated >1h at RT with rotation. Beads were washed with 1.5mL binding/washing buffer, and ∼60μg chromatin per IP was added and incubated overnight at 4°C rotation. IPs were washed on ice six times with RIPA buffer (10mM Tris-HCl pH 8.0, 140mM NaCl, 1mM EDTA pH 8.0, 1% Triton X-100, 0.1% SDS, 0.1% Deoxycholic acid; first wash also contained 1X PI), twice with RIPA-500 buffer (same, except with 500mM NaCl), twice with LiCl wash buffer (10mM Tris-HCl pH 8.0, 1mM EDTA pH 8.0, 250mM LiCl, 0.5% NP-40, and 0.5% Deoxycholic acid), once with TE (10mM Tris-HCl pH 8.0 and 1mM EDTA pH 8.0), and then resuspended in 50μL of direct elution buffer (10mM Tris-HCl pH 8.0, 5mM EDTA pH 8.0, 300mM NaCl, and 0.5% SDS). Decrosslinking was done by incubating with 1μg RNAse for 30 min at 37°C, followed by addition of 20μg Glycogen and 50μg proteinase K and incubation for 2h at 37°C and overnight at 65°C. DNA was cleaned with 2.3x AMPure XP breads and eluted in 60μL of 10mM Tris-HCl pH 8.0.
Library preparation was done by end repair, A-tailing, and adapter ligation,94 followed by sequencing on either a NextSeq 500 or NovaSeq 6000 instrument.
Cut&Run
For Cut&Run analysis,95 2x105 cells were pelleted 3 min at 600xg, washed twice in 1.5mL Wash buffer (20mM Hepes-Naoh pH 7.5, 150mM NaCl, 0.5mM Spermidine, and 1X protease inhibitors), and resuspended in 300μL Wash buffer. 10μL of BioMag Plus Concanavalin A beads (#BP531, WeisScientific) were washed twice in Binding buffer (20mM Hepes-KOH pH 7.9, 10mM KCl, 1mM CaCl2, and 40mM MnCl2) and added to the cells. Cell-bead mixture was rotated 5–10 min at RT, magnetized, resuspended in 150μL Antibody buffer (Wash buffer with 0.15% Digitonin, 2mM EDTA, and 1.5μL of either H3K27me3 antibody (C36B11, Cell Signaling #9733) or H3K4me3 antibody (C42D8, Cell Signaling #9751), and incubated overnight at 4°C.
Cells were washed twice in Dig-Wash buffer (Wash buffer also containing 0.15% Digitonin), resuspended in 150μL Dig-Wash buffer containing 350 ng/ml pA-MNAse (Weizmann Proteomics Unit), and rotated 1h at 4°C. Cells were then washed twice with Dig-Wash buffer, resuspended in 100μL Dig-Wash buffer, and placed in ice water for a few minutes. MNAse was activated by adding 2μL of 100mM CaCl2 and incubating 30 min on ice. Reaction was stopped by adding 100μL 2X STOP buffer (340mM NaCl, 20mM EDTA, 4mM EGTA, 0.15% Digitonin, 100 μg/ml RNAse A, and 50 μg/ml glycogen). Chromatin fragments were released by incubating 30 min at 37°C, transferring the supernatant to new tubes, and incubating 1h at 50°C with 1μL 20% SDS and 2.5μL 20 mg/ml Proteinase K. DNA was isolated using phenol-chloroform extraction. Library construction was done by end repair, A-tailing, adaptor ligation, and amplification95,96 and libraries were sequenced on either a NextSeq 500 or NovaSeq 6000 instrument.
Targeted chromosome conformation capture (4C)
mESC cultures were depleted from MEFs by 20 min incubation on gelatin-coated plates. 3C92 was carried out on 5x106 cells, with the following slight modification from the protocol.42,97 permeabilization buffer constitution was 10 mM Tris-HCl pH 8, 10 mM NaCl, 0.5% NP-40, supplemented with protease inhibitors. For a list of primers used for the 4C analysis, see Table S1. Libraries were sequenced on either a NextSeq 500 or NovaSeq 6000 instrument, and analyzed using the UMI-4C package.42
Quantification and statistical analysis
Statistical analyses
The statistical analyses and software used are indicated in the Method Details section. Statistical tests used, including replicate numbers, are indicated in the figure legends, and all computed p values are indicated in the figures.
Cut&Run data analysis
H3K27me3 signal was quantified using the bigWigAverageOverBed tool of the kentUtils package as mean signal of three repeats at the following regions: the Meteor gene body (mm9 coordinates chr9:118,306,226–118,315,298), a region encompassing the Meteor locus and downstream enhancer (mm9 coordinates chr9:118,286,700–118,314,400), and an H3K27me3-rich control region at the HoxB locus (mm9 coordinates chr11:96,149,799–96,216,130). Signal was normalized to the WT1 cell line and to a control region near the Ppib gene (mm9 coordinates chr9:65,914,278–65,932,710).
Acknowledgments
We thank the Weizmann Institute Stem Cells facility for assistance with generating the Meteor pEX and pAS cell lines. We thank Yonatan Stelzer and Phillip Grote for helpful discussions and members of the Ulitsky lab for helpful discussions and comments on the manuscript. We thank Amos Tanay and members of the Tanay lab for discussions on 4C and on analysis of scRNA-seq and Eyal David for discussions on analysis of 3′ RNA-seq. We thank Jacob Hanna, Nofar Mor, and members of the Hanna lab, as well as Yossi Buganim and Mufeed Abdeen, for discussions on mESC growth conditions. We thank Liat Fellus Alyagor and Dana Hirsch for their help with single-molecule fluorescence in situ hybridization experiments. This study was supported by grants to I.U. from the European Research Council Consolidator grant program (lncIMPACT project) and from the Helen and Martin Kimmel Institute for Stem Cell Research. Z.M. is the incumbent of the anonymous Europe Endowment for Research Fellow Chair in artificial intelligence, and R.B.-T.P. is the incumbent of the Arlyn Imberman Research Fellow Chair.
Author contributions
N.G. and I.U. conceived the study. N.G. and R.B.-T.P. carried out experiments. N.G. analyzed all high-throughput data. Z.M. assisted with generating scRNA-seq libraries. A.T. and M.B. generated the Meteor fKO and Rz-KD model. N.G. and I.U. wrote the manuscript with input from the other authors.
Declaration of interests
I.U. is a member of the Cell Reports advisory board.
Published: May 31, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2023.112569.
Supplemental information
Data and code availability
-
•
All RNA-seq, 3′RNA-seq, scRNA-seq, 4C, ChIP-seq, and Cut&Run data generated in this study have been deposited at GEO and are publicly available as of the date of publication. Accession numbers are listed in the key resources table. This paper also analyzes existing, publicly available data. These accession numbers for the datasets are listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Guttman M., Amit I., Garber M., French C., Lin M.F., Feldser D., Huarte M., Zuk O., Carey B.W., Cassady J.P., et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mercer T.R., Gerhardt D.J., Dinger M.E., Crawford J., Trapnell C., Jeddeloh J.A., Mattick J.S., Rinn J.L. Targeted RNA sequencing reveals the deep complexity of the human transcriptome. Nat. Biotechnol. 2011;30:99–104. doi: 10.1038/nbt.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carninci P., Kasukawa T., Katayama S., Gough J., Frith M.C., Maeda N., Oyama R., Ravasi T., Lenhard B., Wells C., et al. The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 4.Sherstyuk V.V., Medvedev S.P., Zakian S.M. Noncoding RNAs in the regulation of pluripotency and reprogramming. Stem Cell Rev. Rep. 2018;14:58–70. doi: 10.1007/s12015-017-9782-9. [DOI] [PubMed] [Google Scholar]
- 5.Guttman M., Donaghey J., Carey B.W., Garber M., Grenier J.K., Munson G., Young G., Lucas A.B., Ach R., Bruhn L., et al. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature. 2011;477:295–300. doi: 10.1038/nature10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vučićević D., Corradin O., Ntini E., Scacheri P.C., Ørom U.A. Long ncRNA expression associates with tissue-specific enhancers. Cell Cycle. 2015;14:253–260. doi: 10.4161/15384101.2014.977641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Santa F., Barozzi I., Mietton F., Ghisletti S., Polletti S., Tusi B.K., Muller H., Ragoussis J., Wei C.-L., Natoli G. A large fraction of extragenic RNA pol II transcription sites overlap enhancers. PLoS Biol. 2010;8:e1000384. doi: 10.1371/journal.pbio.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marques A.C., Hughes J., Graham B., Kowalczyk M.S., Higgs D.R., Ponting C.P. Chromatin signatures at transcriptional start sites separate two equally populated yet distinct classes of intergenic long noncoding RNAs. Genome Biol. 2013;14:R131. doi: 10.1186/gb-2013-14-11-r131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiang J.-F., Yin Q.-F., Chen T., Zhang Y., Zhang X.-O., Wu Z., Zhang S., Wang H.-B., Ge J., Lu X., et al. Human colorectal cancer-specific CCAT1-L lncRNA regulates long-range chromatin interactions at the MYC locus. Cell Res. 2014;24:513–531. doi: 10.1038/cr.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai F., Orom U.A., Cesaroni M., Beringer M., Taatjes D.J., Blobel G.A., Shiekhattar R. Activating RNAs associate with Mediator to enhance chromatin architecture and transcription. Nature. 2013;494:497–501. doi: 10.1038/nature11884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fanucchi S., Fok E.T., Dalla E., Shibayama Y., Börner K., Chang E.Y., Stoychev S., Imakaev M., Grimm D., Wang K.C., et al. Immune genes are primed for robust transcription by proximal long noncoding RNAs located in nuclear compartments. Nat. Genet. 2019;51:138–150. doi: 10.1038/s41588-018-0298-2. [DOI] [PubMed] [Google Scholar]
- 12.Isoda T., Moore A.J., He Z., Chandra V., Aida M., Denholtz M., Piet van Hamburg J., Fisch K.M., Chang A.N., Fahl S.P., et al. Non-coding transcription instructs chromatin folding and compartmentalization to dictate enhancer-promoter communication and T cell fate. Cell. 2017;171:103–119.e18. doi: 10.1016/j.cell.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gil N., Ulitsky I. Production of spliced long noncoding RNAs specifies regions with increased enhancer activity. Cell Syst. 2018;7:537–547.e3. doi: 10.1016/j.cels.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engreitz J.M., Haines J.E., Perez E.M., Munson G., Chen J., Kane M., McDonel P.E., Guttman M., Lander E.S. Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature. 2016;539:452–455. doi: 10.1038/nature20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gil N., Ulitsky I. Regulation of gene expression by cis-acting long non-coding RNAs. Nat. Rev. Genet. 2020;21:102–117. doi: 10.1038/s41576-019-0184-5. [DOI] [PubMed] [Google Scholar]
- 16.Ulitsky I. Evolution to the rescue: using comparative genomics to understand long non-coding RNAs. Nat. Rev. Genet. 2016;17:601–614. doi: 10.1038/nrg.2016.85. [DOI] [PubMed] [Google Scholar]
- 17.Anderson K.M., Anderson D.M., McAnally J.R., Shelton J.M., Bassel-Duby R., Olson E.N. Transcription of the non-coding RNA upperhand controls Hand2 expression and heart development. Nature. 2016;539:433–436. doi: 10.1038/nature20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han X., Zhang J., Liu Y., Fan X., Ai S., Luo Y., Li X., Jin H., Luo S., Zheng H., et al. The lncRNA Hand2os1/Uph locus orchestrates heart development through regulation of precise expression of Hand2. Development. 2019;146:dev176198. doi: 10.1242/dev.176198. [DOI] [PubMed] [Google Scholar]
- 19.Bergmann J.H., Li J., Eckersley-Maslin M.A., Rigo F., Freier S.M., Spector D.L. Regulation of the ESC transcriptome by nuclear long noncoding RNAs. Genome Res. 2015;25:1336–1346. doi: 10.1101/gr.189027.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexanian M., Maric D., Jenkinson S.P., Mina M., Friedman C.E., Ting C.-C., Micheletti R., Plaisance I., Nemir M., Maison D., et al. A transcribed enhancer dictates mesendoderm specification in pluripotency. Nat. Commun. 2017;8:1806. doi: 10.1038/s41467-017-01804-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mihalas A.B., Hevner R.F. Control of neuronal development by T-box genes in the brain. Curr. Top. Dev. Biol. 2017;122:279–312. doi: 10.1016/bs.ctdb.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Probst S., Arnold S.J. Eomesodermin-at dawn of cell fate decisions during early embryogenesis. Curr. Top. Dev. Biol. 2017;122:93–115. doi: 10.1016/bs.ctdb.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Kidder B.L., Palmer S. Examination of transcriptional networks reveals an important role for TCFAP2C, SMARCA4, and EOMES in trophoblast stem cell maintenance. Genome Res. 2010;20:458–472. doi: 10.1101/gr.101469.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russ A.P., Wattler S., Colledge W.H., Aparicio S.A., Carlton M.B., Pearce J.J., Barton S.C., Surani M.A., Ryan K., Nehls M.C., et al. Eomesodermin is required for mouse trophoblast development and mesoderm formation. Nature. 2000;404:95–99. doi: 10.1038/35003601. [DOI] [PubMed] [Google Scholar]
- 25.Strumpf D., Mao C.-A., Yamanaka Y., Ralston A., Chawengsaksophak K., Beck F., Rossant J. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development. 2005;132:2093–2102. doi: 10.1242/dev.01801. [DOI] [PubMed] [Google Scholar]
- 26.Ciruna B.G., Rossant J. Expression of the T-box gene Eomesodermin during early mouse development. Mech. Dev. 1999;81:199–203. doi: 10.1016/s0925-4773(98)00243-3. [DOI] [PubMed] [Google Scholar]
- 27.Hancock S.N., Agulnik S.I., Silver L.M., Papaioannou V.E. Mapping and expression analysis of the mouse ortholog of Xenopus Eomesodermin. Mech. Dev. 1999;81:205–208. doi: 10.1016/s0925-4773(98)00244-5. [DOI] [PubMed] [Google Scholar]
- 28.Lv X., Ren S.-Q., Zhang X.-J., Shen Z., Ghosh T., Xianyu A., Gao P., Li Z., Lin S., Yu Y., et al. TBR2 coordinates neurogenesis expansion and precise microcircuit organization via Protocadherin 19 in the mammalian cortex. Nat. Commun. 2019;10:3946. doi: 10.1038/s41467-019-11854-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordon S.M., Chaix J., Rupp L.J., Wu J., Madera S., Sun J.C., Lindsten T., Reiner S.L. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity. 2012;36:55–67. doi: 10.1016/j.immuni.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearce E.L., Mullen A.C., Martins G.A., Krawczyk C.M., Hutchins A.S., Zediak V.P., Banica M., DiCioccio C.B., Gross D.A., Mao C.-A., et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 31.Zhu Y., Ju S., Chen E., Dai S., Li C., Morel P., Liu L., Zhang X., Lu B. T-bet and eomesodermin are required for T cell-mediated antitumor immune responses. J. Immunol. 2010;185:3174–3183. doi: 10.4049/jimmunol.1000749. [DOI] [PubMed] [Google Scholar]
- 32.Simon C.S., Downes D.J., Gosden M.E., Telenius J., Higgs D.R., Hughes J.R., Costello I., Bikoff E.K., Robertson E.J. Functional characterisation of cis-regulatory elements governing dynamic Eomes expression in the early mouse embryo. Development. 2017;144:1249–1260. doi: 10.1242/dev.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo X., Xu Y., Wang Z., Wu Y., Chen J., Wang G., Lu C., Jia W., Xi J., Zhu S., et al. A linc1405/eomes complex promotes cardiac mesoderm specification and cardiogenesis. Cell Stem Cell. 2018;22:893–908.e6. doi: 10.1016/j.stem.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Tuck A.C., Natarajan K.N., Rice G.M., Borawski J., Mohn F., Rankova A., Flemr M., Wenger A., Nutiu R., Teichmann S., Bühler M. Distinctive features of lincRNA gene expression suggest widespread RNA-independent functions. Life Sci. Alliance. 2018;1:e201800124. doi: 10.26508/lsa.201800124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilbert L.A., Larson M.H., Morsut L., Liu Z., Brar G.A., Torres S.E., Stern-Ginossar N., Brandman O., Whitehead E.H., Doudna J.A., et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013;154:442–451. doi: 10.1016/j.cell.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo G., Pinello L., Han X., Lai S., Shen L., Lin T.-W., Zou K., Yuan G.-C., Orkin S.H. Serum-based culture conditions provoke gene expression variability in mouse embryonic stem cells as revealed by single-cell analysis. Cell Rep. 2016;14:956–965. doi: 10.1016/j.celrep.2015.12.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marks H., Kalkan T., Menafra R., Denissov S., Jones K., Hofemeister H., Nichols J., Kranz A., Stewart A.F., Smith A., Stunnenberg H.G. The transcriptional and epigenomic foundations of ground state pluripotency. Cell. 2012;149:590–604. doi: 10.1016/j.cell.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joo J.Y., Choi H.W., Kim M.J., Zaehres H., Tapia N., Stehling M., Jung K.S., Do J.T., Schöler H.R. Establishment of a primed pluripotent epiblast stem cell in FGF4-based conditions. Sci. Rep. 2014;4:7477. doi: 10.1038/srep07477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kojima Y., Kaufman-Francis K., Studdert J.B., Steiner K.A., Power M.D., Loebel D.A.F., Jones V., Hor A., de Alencastro G., Logan G.J., et al. The transcriptional and functional properties of mouse epiblast stem cells resemble the anterior primitive streak. Cell Stem Cell. 2014;14:107–120. doi: 10.1016/j.stem.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 40.Castro-Mondragon J.A., Riudavets-Puig R., Rauluseviciute I., Lemma R.B., Turchi L., Blanc-Mathieu R., Lucas J., Boddie P., Khan A., Manosalva Pérez N., et al. Jaspar 2022: the 9th release of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2022;50:D165–D173. doi: 10.1093/nar/gkab1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bailey T.L., Boden M., Buske F.A., Frith M., Grant C.E., Clementi L., Ren J., Li W.W., Noble W.S. Meme suite: tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwartzman O., Mukamel Z., Oded-Elkayam N., Olivares-Chauvet P., Lubling Y., Landan G., Izraeli S., Tanay A. UMI-4C for quantitative and targeted chromosomal contact profiling. Nat. Methods. 2016;13:685–691. doi: 10.1038/nmeth.3922. [DOI] [PubMed] [Google Scholar]
- 43.Zhao J., Ohsumi T.K., Kung J.T., Ogawa Y., Grau D.J., Sarma K., Song J.J., Kingston R.E., Borowsky M., Lee J.T. Genome-wide identification of polycomb-associated RNAs by RIP-seq. Mol. Cell. 2010;40:939–953. doi: 10.1016/j.molcel.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cruz-Molina S., Respuela P., Tebartz C., Kolovos P., Nikolic M., Fueyo R., van Ijcken W.F.J., Grosveld F., Frommolt P., Bazzi H., Rada-Iglesias A. PRC2 facilitates the regulatory topology required for poised enhancer function during pluripotent stem cell differentiation. Cell Stem Cell. 2017;20:689–705.e9. doi: 10.1016/j.stem.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 45.Denholtz M., Bonora G., Chronis C., Splinter E., de Laat W., Ernst J., Pellegrini M., Plath K. Long-range chromatin contacts in embryonic stem cells reveal a role for pluripotency factors and polycomb proteins in genome organization. Cell Stem Cell. 2013;13:602–616. doi: 10.1016/j.stem.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ngan C.Y., Wong C.H., Tjong H., Wang W., Goldfeder R.L., Choi C., He H., Gong L., Lin J., Urban B., et al. Chromatin interaction analyses elucidate the roles of PRC2-bound silencers in mouse development. Nat. Genet. 2020;52:264–272. doi: 10.1038/s41588-020-0581-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beltran M., Tavares M., Justin N., Khandelwal G., Ambrose J., Foster B.M., Worlock K.B., Tvardovskiy A., Kunzelmann S., Herrero J., et al. G-tract RNA removes Polycomb repressive complex 2 from genes. Nat. Struct. Mol. Biol. 2019;26:899–909. doi: 10.1038/s41594-019-0293-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beltran M., Yates C.M., Skalska L., Dawson M., Reis F.P., Viiri K., Fisher C.L., Sibley C.R., Foster B.M., Bartke T., et al. The interaction of PRC2 with RNA or chromatin is mutually antagonistic. Genome Res. 2016;26:896–907. doi: 10.1101/gr.197632.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hosogane M., Funayama R., Shirota M., Nakayama K. Lack of transcription triggers H3K27me3 accumulation in the gene body. Cell Rep. 2016;16:696–706. doi: 10.1016/j.celrep.2016.06.034. [DOI] [PubMed] [Google Scholar]
- 50.Kaneko S., Son J., Bonasio R., Shen S.S., Reinberg D. Nascent RNA interaction keeps PRC2 activity poised and in check. Genes Dev. 2014;28:1983–1988. doi: 10.1101/gad.247940.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garland W., Comet I., Wu M., Radzisheuskaya A., Rib L., Vitting-Seerup K., Lloret-Llinares M., Sandelin A., Helin K., Jensen T.H. A functional link between nuclear RNA decay and transcriptional control mediated by the polycomb repressive complex 2. Cell Rep. 2019;29:1800–1811.e6. doi: 10.1016/j.celrep.2019.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maltsev V.A., Wobus A.M., Rohwedel J., Bader M., Hescheler J. Cardiomyocytes differentiated in vitro from embryonic stem cells developmentally express cardiac-specific genes and ionic currents. Circ. Res. 1994;75:233–244. doi: 10.1161/01.res.75.2.233. [DOI] [PubMed] [Google Scholar]
- 53.Guan K., Rohwedel J., Wobus A.M. Embryonic stem cell differentiation models: cardiogenesis, myogenesis, neurogenesis, epithelial and vascular smooth muscle cell differentiation in vitro. Cytotechnology. 1999;30:211–226. doi: 10.1023/A:1008041420166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He Z., Li J.-J., Zhen C.-H., Feng L.-Y., Ding X.-Y. Effect of leukemia inhibitory factor on embryonic stem cell differentiation: implications for supporting neuronal differentiation. Acta Pharmacol. Sin. 2006;27:80–90. doi: 10.1111/j.1745-7254.2006.00254.x. [DOI] [PubMed] [Google Scholar]
- 55.Gajović S., St-Onge L., Yokota Y., Gruss P. Retinoic acid mediates Pax6 expression during in vitro differentiation of embryonic stem cells. Differentiation. 1997;62:187–192. doi: 10.1046/j.1432-0436.1998.6240187.x. [DOI] [PubMed] [Google Scholar]
- 56.Gaztelumendi N., Nogués C. Chromosome instability in mouse embryonic stem cells. Sci. Rep. 2014;4:5324. doi: 10.1038/srep05324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keller A., Spits C. The impact of acquired genetic abnormalities on the clinical translation of human pluripotent stem cells. Cells. 2021;10 doi: 10.3390/cells10113246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hayashi K., de Sousa Lopes S.M.C., Tang F., Lao K., Surani M.A. Dynamic equilibrium and heterogeneity of mouse pluripotent stem cells with distinct functional and epigenetic states. Cell Stem Cell. 2008;3:391–401. doi: 10.1016/j.stem.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi J., Huebner A.J., Clement K., Walsh R.M., Savol A., Lin K., Gu H., Di Stefano B., Brumbaugh J., Kim S.-Y., et al. Prolonged Mek1/2 suppression impairs the developmental potential of embryonic stem cells. Nature. 2017;548:219–223. doi: 10.1038/nature23274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baran Y., Bercovich A., Sebe-Pedros A., Lubling Y., Giladi A., Chomsky E., Meir Z., Hoichman M., Lifshitz A., Tanay A. MetaCell: analysis of single-cell RNA-seq data using K-nn graph partitions. Genome Biol. 2019;20:206. doi: 10.1186/s13059-019-1812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perea-Gomez A., Lawson K.A., Rhinn M., Zakin L., Brûlet P., Mazan S., Ang S.L. Otx2 is required for visceral endoderm movement and for the restriction of posterior signals in the epiblast of the mouse embryo. Development. 2001;128:753–765. doi: 10.1242/dev.128.5.753. [DOI] [PubMed] [Google Scholar]
- 62.Perea-Gómez A., Shawlot W., Sasaki H., Behringer R.R., Ang S. HNF3beta and Lim1 interact in the visceral endoderm to regulate primitive streak formation and anterior-posterior polarity in the mouse embryo. Development. 1999;126:4499–4511. doi: 10.1242/dev.126.20.4499. [DOI] [PubMed] [Google Scholar]
- 63.Rivera-Pérez J.A., Magnuson T. Primitive streak formation in mice is preceded by localized activation of Brachyury and Wnt3. Dev. Biol. 2005;288:363–371. doi: 10.1016/j.ydbio.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 64.Ying Q.-L., Stavridis M., Griffiths D., Li M., Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat. Biotechnol. 2003;21:183–186. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- 65.Hezroni H., Ben-Tov Perry R., Gil N., Degani N., Ulitsky I. Regulation of neuronal commitment in mouse embryonic stem cells by the Reno1/Bahcc1 locus. EMBO Rep. 2020;21:e51264. doi: 10.15252/embr.202051264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hezroni H., Koppstein D., Schwartz M.G., Avrutin A., Bartel D.P., Ulitsky I. Principles of long noncoding RNA evolution derived from direct comparison of transcriptomes in 17 species. Cell Rep. 2015;11:1110–1122. doi: 10.1016/j.celrep.2015.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Latos P.A., Pauler F.M., Koerner M.V., Şenergin H.B., Hudson Q.J., Stocsits R.R., Allhoff W., Stricker S.H., Klement R.M., Warczok K.E., et al. Airn transcriptional overlap, but not its lncRNA products, induces imprinted Igf2r silencing. Science. 2012;338:1469–1472. doi: 10.1126/science.1228110. [DOI] [PubMed] [Google Scholar]
- 68.Nojima T., Gomes T., Grosso A.R.F., Kimura H., Dye M.J., Dhir S., Carmo-Fonseca M., Proudfoot N.J. Mammalian NET-seq reveals genome-wide nascent transcription coupled to RNA processing. Cell. 2015;161:526–540. doi: 10.1016/j.cell.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Long Y., Hwang T., Gooding A.R., Goodrich K.J., Rinn J.L., Cech T.R. RNA is essential for PRC2 chromatin occupancy and function in human pluripotent stem cells. Nat. Genet. 2020;52:931–938. doi: 10.1038/s41588-020-0662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cifuentes-Rojas C., Hernandez A.J., Sarma K., Lee J.T. Regulatory interactions between RNA and polycomb repressive complex 2. Mol. Cell. 2014;55:171–185. doi: 10.1016/j.molcel.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Colognori D., Sunwoo H., Kriz A.J., Wang C.-Y., Lee J.T. Xist deletional analysis reveals an interdependency between xist RNA and polycomb complexes for spreading along the inactive X. Mol. Cell. 2019;74:101–117.e10. doi: 10.1016/j.molcel.2019.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davidovich C., Zheng L., Goodrich K.J., Cech T.R. Promiscuous RNA binding by Polycomb repressive complex 2. Nat. Struct. Mol. Biol. 2013;20:1250–1257. doi: 10.1038/nsmb.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang X., Goodrich K.J., Gooding A.R., Naeem H., Archer S., Paucek R.D., Youmans D.T., Cech T.R., Davidovich C. Targeting of polycomb repressive complex 2 to RNA by short repeats of consecutive guanines. Mol. Cell. 2017;65:1056–1067.e5. doi: 10.1016/j.molcel.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 74.Mattick J.S., Amaral P.P., Carninci P., Carpenter S., Chang H.Y., Chen L.-L., Chen R., Dean C., Dinger M.E., Fitzgerald K.A., et al. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023 doi: 10.1038/s41580-022-00566-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hazra R., Brine L., Garcia L., Benz B., Chirathivat N., Shen M.M., Wilkinson J.E., Lyons S.K., Spector D.L. Platr4 is an early embryonic lncRNA that exerts its function downstream on cardiogenic mesodermal lineage commitment. Dev. Cell. 2022;57:2450–2468.e7. doi: 10.1016/j.devcel.2022.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cho S.W., Xu J., Sun R., Mumbach M.R., Carter A.C., Chen Y.G., Yost K.E., Kim J., He J., Nevins S.A., et al. Promoter of lncRNA gene PVT1 is a tumor-suppressor DNA boundary element. Cell. 2018;173:1398–1412.e22. doi: 10.1016/j.cell.2018.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ritter N., Ali T., Kopitchinski N., Schuster P., Beisaw A., Hendrix D.A., Schulz M.H., Müller-McNicoll M., Dimmeler S., Grote P. The lncRNA locus Handsdown regulates cardiac gene programs and is essential for early mouse development. Dev. Cell. 2019;50:644–657.e8. doi: 10.1016/j.devcel.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 78.Stryjewska A., Dries R., Pieters T., Verstappen G., Conidi A., Coddens K., Francis A., Umans L., van IJcken W.F.J., Berx G., et al. Zeb2 regulates cell fate at the exit from epiblast state in mouse embryonic stem cells. Stem. Cells. 2017;35:611–625. doi: 10.1002/stem.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ding Q., Regan S.N., Xia Y., Oostrom L.A., Cowan C.A., Musunuru K. Enhanced efficiency of human pluripotent stem cell genome editing through replacing TALENs with CRISPRs. Cell Stem Cell. 2013;12:393–394. doi: 10.1016/j.stem.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koike-Yusa H., Li Y., Tan E.-P., Velasco-Herrera M.D.C., Yusa K. Genome-wide recessive genetic screening in mammalian cells with a lentiviral CRISPR-guide RNA library. Nat. Biotechnol. 2014;32:267–273. doi: 10.1038/nbt.2800. [DOI] [PubMed] [Google Scholar]
- 81.Konermann S., Brigham M.D., Trevino A.E., Joung J., Abudayyeh O.O., Barcena C., Hsu P.D., Habib N., Gootenberg J.S., Nishimasu H., et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 2015;517:583–588. doi: 10.1038/nature14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li B., Dewey C.N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kent W.J., Zweig A.S., Barber G., Hinrichs A.S., Karolchik D. BigWig and BigBed: enabling browsing of large distributed datasets. Bioinformatics. 2010;26:2204–2207. doi: 10.1093/bioinformatics/btq351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ballarino M., Cipriano A., Tita R., Santini T., Desideri F., Morlando M., Colantoni A., Carrieri C., Nicoletti C., Musarò A., et al. Deficiency in the nuclear long noncoding RNA Charme causes myogenic defects and heart remodeling in mice. EMBO J. 2018;37:e99697. doi: 10.15252/embj.201899697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yin Y., Yan P., Lu J., Song G., Zhu Y., Li Z., Zhao Y., Shen B., Huang X., Zhu H., et al. Opposing roles for the lncRNA Haunt and its genomic locus in regulating HOXA gene activation during embryonic stem cell differentiation. Cell Stem Cell. 2015;16:504–516. doi: 10.1016/j.stem.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 88.Tiscornia G., Singer O., Verma I.M. Production and purification of lentiviral vectors. Nat. Protoc. 2006;1:241–245. doi: 10.1038/nprot.2006.37. [DOI] [PubMed] [Google Scholar]
- 89.Ying Q.-L., Smith A.G. Defined conditions for neural commitment and differentiation. Methods Enzymol. 2003;365:327–341. doi: 10.1016/s0076-6879(03)65023-8. [DOI] [PubMed] [Google Scholar]
- 90.Mittnenzweig M., Mayshar Y., Cheng S., Ben-Yair R., Hadas R., Rais Y., Chomsky E., Reines N., Uzonyi A., Lumerman L., et al. A single-embryo, single-cell time-resolved model for mouse gastrulation. Cell. 2021;184:2825–2842.e22. doi: 10.1016/j.cell.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jaitin D.A., Kenigsberg E., Keren-Shaul H., Elefant N., Paul F., Zaretsky I., Mildner A., Cohen N., Jung S., Tanay A., Amit I. Massively parallel single-cell RNA-seq for marker-free decomposition of tissues into cell types. Science. 2014;343:776–779. doi: 10.1126/science.1247651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Keren-Shaul H., Kenigsberg E., Jaitin D.A., David E., Paul F., Tanay A., Amit I. MARS-seq2.0: an experimental and analytical pipeline for indexed sorting combined with single-cell RNA sequencing. Nat. Protoc. 2019;14:1841–1862. doi: 10.1038/s41596-019-0164-4. [DOI] [PubMed] [Google Scholar]
- 93.Rom A., Melamed L., Gil N., Goldrich M.J., Kadir R., Golan M., Biton I., Perry R.B.-T., Ulitsky I. Regulation of CHD2 expression by the Chaserr long noncoding RNA gene is essential for viability. Nat. Commun. 2019;10:5092. doi: 10.1038/s41467-019-13075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Blecher-Gonen R., Barnett-Itzhaki Z., Jaitin D., Amann-Zalcenstein D., Lara-Astiaso D., Amit I. High-throughput chromatin immunoprecipitation for genome-wide mapping of in vivo protein-DNA interactions and epigenomic states. Nat. Protoc. 2013;8:539–554. doi: 10.1038/nprot.2013.023. [DOI] [PubMed] [Google Scholar]
- 95.Meers M.P., Bryson T.D., Henikoff J.G., Henikoff S. Improved CUT&RUN chromatin profiling tools. Elife. 2019;8:e46314. doi: 10.7554/eLife.46314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Janssens, D., and Henikoff, S.: CUT&RUN: Targeted in situ genome-wide profiling with high efficiency for low cell numbers v3 (protocols.io.zcpf2vn). Protocols.io. 10.17504/protocols.io.zcpf2vn. [DOI] [PubMed]
- 97.Olivares-Chauvet P., Mukamel Z., Lifshitz A., Schwartzman O., Elkayam N.O., Lubling Y., Deikus G., Sebra R.P., Tanay A. Capturing pairwise and multi-way chromosomal conformations using chromosomal walks. Nature. 2016;540:296–300. doi: 10.1038/nature20158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All RNA-seq, 3′RNA-seq, scRNA-seq, 4C, ChIP-seq, and Cut&Run data generated in this study have been deposited at GEO and are publicly available as of the date of publication. Accession numbers are listed in the key resources table. This paper also analyzes existing, publicly available data. These accession numbers for the datasets are listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.