Abstract
Ischemic insult stimulates proliferation of neural stem cells (NSCs) in the subventricular zone (SVZ) after stroke. However, only a fraction of NSC‐derived neuroblasts from SVZ migrate toward poststroke brain region. We have previously reported that direct‐current stimulation guides NSC migration toward the cathode in vitro. Accordingly, we set up a new method of transcranial direct‐current stimulation (tDCS), in which the cathodal electrode is placed on the ischemic hemisphere and anodal electrode on the contralateral hemisphere of rats subjected to ischemia–reperfusion injury. We show that the application of this bilateral tDCS (BtDCS) promotes the migration of NSC‐derived neuroblasts from SVZ toward the cathode direction into poststroke striatum. Reversing the position of the electrodes blocks the effect of BtDCS on the migration of neuroblasts from SVZ. BtDCS protects against neuronal death and improves the functional recovery of stroke animals. Thus, the migration of NSC‐derived neuroblasts from SVZ toward poststroke brain region contributes to the effect of BtDCS against ischemia‐induced neuronal death, supporting a potential development of noninvasive BtDCS as an endogenous neurogenesis‐based stroke therapy.
Keywords: ischemic stroke, neural stem cells, stem cell migration, subventricular zone, transcranial direct‐current stimulation
Ischemic insult stimulates proliferation of neural stem cells (NSCs) in the subventricular zone (SVZ) after stroke. We set up a new method of transcranial direct‐current stimulation (tDCS), in which the cathodal electrode is placed on the ischemic hemisphere and anodal electrode on the contralateral hemisphere of rats subjected to ischemia–reperfusion injury. This bilateral tDCS (BtDCS) promotes the migration of NSC‐derived neuroblasts from SVZ toward the cathode direction into poststroke striatum. BtDCS protects against ischemic neuronal death of stroke animals.
1. INTRODUCTION
Neurogenesis in the subventricular zone (SVZ) is greatly enhanced after cerebral ischemia, which promotes the proliferation of neural stem cells (NSCs) in the SVZ and induces NSC‐derived neuroblast migration from SVZ toward the damaged brain region. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 However, only a fraction of NSC‐derived neuroblasts are found to migrate from SVZ to the damaged brain region, 1 which is a critical barrier to prevent the potential of endogenous neurogenesis‐based brain repair. While much investigations have been focused on the development of exogenous stem cell‐based stroke therapy, little effort has been made toward the development of therapeutic approaches that utilize the regenerative potential of endogenous neurogenesis in ischemic stroke. 11 While promoting the mobilization of endogenous stem cells seems beneficial to ischemic brain repair, effort needs to increase the stem cell pool in focal ischemic stroke. 11 As the first step, we sought to develop a noninvasive strategy that promotes NSC‐derived neuroblast migration from SVZ toward damaged brain in ischemic stroke. We have previously provided the first evidence that direct‐current stimulation guides and facilitates the migration of neuroblasts toward the cathode in cultured embryonic explants. 12 Recent study shows that electrical stimulation guides the migration of transplanted human neural stem cells in rat brain. 13 These studies support a notion that direct‐current stimulation could be developed as a practical approach to repair brain damage by directing NSC‐derived neuroblast migration from SVZ to the injured brain regions. 14
Experimental evidences show that transcranial direct‐current stimulation (tDCS) exerts a measurable neuroprotective effect in an animal model of ischemic stroke. 15 , 16 , 17 It has been reported that tDCS exerts its beneficial effects through multiple neurobiological mechanisms, including the modulation of cortical excitability, expression of growth factors, neuroinflammation, and neural stem cell activation, and the long‐term changes in cortical excitability by tDCS mediates changes in N‐methyl‐D‐aspartate receptors and Ca++ channels. 15 , 18 , 19 , 20 , 21 , 22 , 23 , 24 Clinical trials have investigated the potential efficacy of tDCS in poststroke motor recovery. 25 , 26 , 27 , 28 , 29 , 30 , 31 Based on recent findings by us and others, we reasoned that the efficacy of tDCS in poststroke recovery could be enhanced by promoting NSC‐derived neuroblast migration from SVZ toward the damaged brain region. As tDCS is a noninvasive and interference‐controllable technique, investigating the effect of tDCS on NSC‐derived neuroblasts may lead to the development of novel therapeutic strategies for stroke patients.
In this study, we test the effect of tDCS on NSC‐derived neuroblast migration in the rat model of middle cerebral artery occlusion (MCAO). We show that application of bilateral tDCS (BtDCS), in which the cathodal electrode is placed on the ischemic hemisphere and anodal electrode on the contralateral hemisphere, promotes migration of the neuroblasts from SVZ toward the direction of the cathode to the poststroke striatum. Reversing the position of cathodal and anodal electrodes prevents BtDCS‐induced neuroblast migration. Application of BtDCS reduces the number of neuronal deaths in the damaged brain region and improves functional outcomes in stroke animals. This study provides direct evidence for the effect of direct‐current stimulation on endogenous neuroblast migration in poststroke brain and supports a potential application of noninvasive BtDCS as a biophysical therapy in ischemic stroke.
2. MATERIALS AND METHODS
2.1. Animals and MCAO
The adult male Sprague–Dawley rats weighing 270–300 g were employed to create 60‐min MCAO ischemia model. The standard method of the MCAO model has been described by us in detail previously. 32 , 33 Animals were anesthetized with 4% isoflurane in 70% N2O and 30% O2 using a mask. A midline incision was made in the neck, the right external carotid artery (ECA) was carefully exposed and dissected, and a 3–0 monofilament nylon suture was inserted from the ECA into the right internal carotid artery to occlude the origin of the right middle cerebral artery (MCA) (~ 22 mm). After 90 min of occlusion, the suture was removed to allow reperfusion, the ECA was ligated, and the wound was closed. Sham‐operated rats underwent identical surgery except that the suture was inserted and withdrawn immediately. Rectal temperature was maintained at 37.0 ± 0.5°C using a heating pad and heating lamp. At 24 h after MCAO, rats were decapitated after reperfusion with ice‐cold 0.9% saline after being anesthetized with 4% isoflurane in 70% N2O and 30% O2, and the brains were rapidly removed. The stroke animals were observed for recovery for 22 h after ischemia onset, and the BtDCS was applied at 2 days after ischemia–reperfusion injury. All animal experiments were approved and carried out in compliance with the IACUC guidelines of Wuhan University School of Medicine and Qingdao University School of Medicine. All animal use and experimental protocols were approved and carried out in compliance with the IACUC guidelines and the Animal Care and Ethics Committee of Wuhan University School of Medicine and Qingdao University School of Medicine.
2.2. BtDCS application
The BtDCS was applied in rats without anesthesia by a constant current stimulator purchased from Schneider Electronics that was specifically designed for the application of low‐intensity currents in small mammals. 34 , 35 , 36 The currents were applied transcranially to the temporal area of the animals. The electrodes were fixed directly onto the cranium to ensure a defined contact area over the cortex. The epicranial electrode, composed of a tubular plastic jacket, was fixed onto the skull 7 days before the ischemia onset. One plastic jacket was positioned on each side of the cranium over the dorsolateral prefrontal cortex in a symmetrical way and fixed with nontoxic glass ionomer cement. The plastic jacket was filled with saline before stimulation. The contact area of the electrode toward the skull was 3.5 mm2. 34 , 35 , 36 The electrode over the ischemic cortex was connected to the cathodal terminal and the other electrode was connected to anodal terminal. The animals in the I/R group as control received the same BtDCS‐setup as the stimulated animals with the stimulator turned off and these animals went through the same procedure with the implantation of epicranial electrodes.
2.3. BrdU labeling
To label the proliferative cells, the S‐phase marker BrdU (Sigma‐Aldrich) was intraperitoneally injected (50 mg/body weight) 1 , 37 and given at 8 h intervals twice daily for 13 days starting 1 day after MCAO. Animals were killed for BrdU staining on 14 days after MCAO.
2.4. Lentivirus‐GFP labeling
To determine whether the new neurons detected in the ischemic region were immigrated from SVZ, we labeled the cells in the SVZ by injecting self‐inactivating lentiviruses expressing GFP (lentivirus‐GFP; GeneChem) into the SVZ 1 day before inducing stroke. 38 , 39 The animals were anesthetized, and 2 μL of high titers (1 × 109 units/mL) lentivirus‐GFP was injected stereotaxically in 2 coordinates for each SVZ (distance relative to bregma, anterior, +2.0 mm; lateral, ±1.1 mm; depth, 4.6 mm; and anterior, +0.2 mm; lateral, ±2.0 mm; depth, 3.4 mm).
2.5. Immunocytochemical staining and microscopical analysis
The immunocytochemical staining was performed as described previously. 1 , 40 Animals were decapitated under anesthesia with a Ketamine/Xylazine mix (K: 100 mg/kg and X: 10 mg/kg; i.p.). After transcardial perfusion with 4% ice‐cold phosphate‐buffered paraformaldehyde, brains were postfixed in 4% paraformaldehyde and sectioned in the coronal plane at 30 μm. The sections were then double stained for BrdU and Dcx. Dcx was also stained in lentivirus‐GFP‐labeled brain sections. Sections were denatured by incubation in 1 M hydrochloric acid at 65°C for 30 min before BrdU staining. Sections were then incubated for 36 h with antibody against BrdU (Sigma) and anti‐Dcx antibody (Santa Cruz Biotechnology). Microscopical analyses were performed according to the method described previously. 1 The analyses were performed with a confocal laser scanning microscope. Dcx‐labeled cells were counted in three 30‐μm sections from rostrocaudal levels. Double labeling of Dcx with BrdU or GFP was determined by random sampling of 100 or more labeled cells for analysis.
2.6. Nissl staining and infarct volume measurement
At 14 days after MCAO, animals were decapitated under anesthesia with a Ketamine/Xylazine mix (K: 100 mg/kg and X: 10 mg/kg; i.p.), and perfused transcardially with 4% paraformaldehyde and 0.9% saline at 4°C. The Nissl staining was performed with thionin according to the protocol reported previously. 41 Twelve sequential 10 μm coronal Nissl sections were prepared every 500 μm. Infarct volume was calculated by integrating the cross‐sectional area of damage on each section and the distance between the various levels. In each stained section, the necrotic area was identified and outlined at a magnification of × 2.5 and measured using an image analysis software (Image‐Pro Plus Version 6.0). The infarct volume (V) was calculated by the following formula: V = Σ (A i × T S × n), where A i is the ischemic area measured at the section, T S is the section thickness (10 μm), and n is the number of sections between two adjacent levels. 41
2.7. Neurobehavioral tests
The modified neurological severity score (mNSS) test for evaluating neurological function, 42 the Beam walk test for measuring animals' complex neuromotor function 43 , 44 and the adhesive‐removal test, a modified sticky tape (MST) test, for evaluating forelimb function 45 were described in detail in our previous study. 32 , 33
2.8. Statistical analysis
SPSS and Microsoft Excel served for statistical analyses. Values are means ± SEM. Numbers of labeled cells were analyzed with the ANOVA test. The data of infarct volumes were analyzed using ANOVA followed by the Fisher's least significant difference test. The data for neurobehavioral tests were evaluated using ANOVA and the subsequent post hoc Bonferroni's test.
3. RESULTS
3.1. BtDCS increases the number of neuroblasts in the ischemic brain
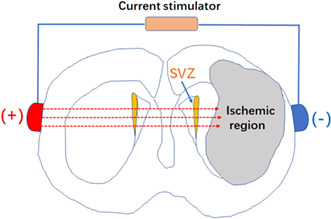
According to our previous finding that direct‐current stimulation guides the migration of neuroblasts toward the cathode in vitro, 12 we set up to test in vivo the effect of BtDCS on NSC‐derived neuroblast migration in the injured rat brain subjected to MCAO. A constant current stimulator that was specifically designed for current stimulation in small mammals was used. 34 , 35 , 36 The electrodes were positioned on each side of the cranium over the temporal area in a symmetrical manner, with the cathodal electrode placed on the ischemic hemisphere and the anodal electrode on the contralateral hemisphere, which allowed the currents to pass through the SVZ. The contact area of the electrode toward the skull was 3.5 mm2 (Figure 1).
FIGURE 1.
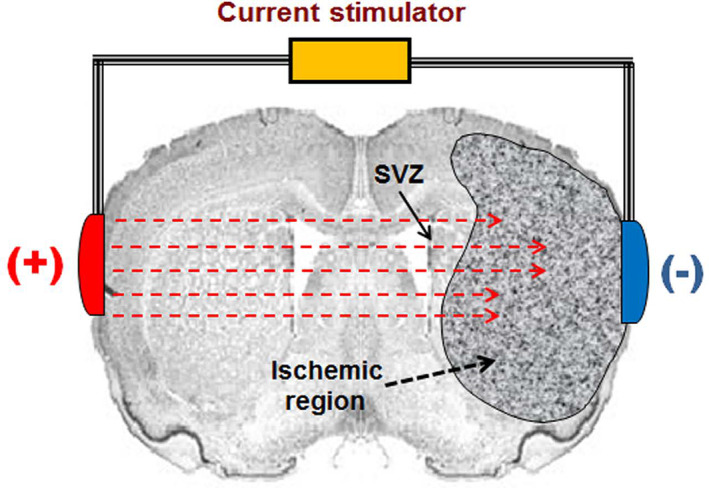
A schematic diagram showing the setup of BtDCS application. The effect of BtDCS on NSC‐derived neuroblast migration was tested in the injured rat brain subjected to MCAO. A constant current stimulator was used. The electrodes were positioned on each side of the cranium over the temporal area in a symmetrical manner, with the cathodal electrode placed on the ischemic hemisphere and the anodal electrode on the contralateral hemisphere, which allowed the currents to pass through the SVZ. The contact area of the electrode toward the skull is 7.0 mm2.
The adult male Sprague–Dawley rats were used to create a 60‐min MCAO ischemia–reperfusion model. 32 , 33 Based on the established safe strength of tDCS and stimulation time‐point tested in rats previously, 15 , 35 , 46 the MCAO animals were applied with 150 μA (current density: 42.9 A/m2) of BtDCS for 30 min of continuous exposure per day for 12 days starting at 2 days after ischemia–reperfusion injury in nonanesthesia conditions. The rats in the ischemia–reperfusion group received the same BtDCS‐setup as the stimulated animals with the stimulator turned off (sham stimulation) and these animals went through the same procedure with the implantation of epicranial electrodes.
An antibody against the S‐phase marker BrdU (5‐bromr‐2′‐deoxyuridine) was used to label the proliferative cells and an antibody against Dcx (doublecortin, a marker of neuroblast) was used to label neuroblasts. The colocalization of BrdU/Dcx was measured to determine whether BtDCS application altered the number of neuroblasts in the poststroke brain region. As shown in Figures 2 and 3, the application of BtDCS increases the colocalization of BrdU/Dcx in the ischemic striatum at 14 days after ischemia–reperfusion. These data suggest that BtDCS increases the number of neuroblasts in the ischemic brain region.
FIGURE 2.
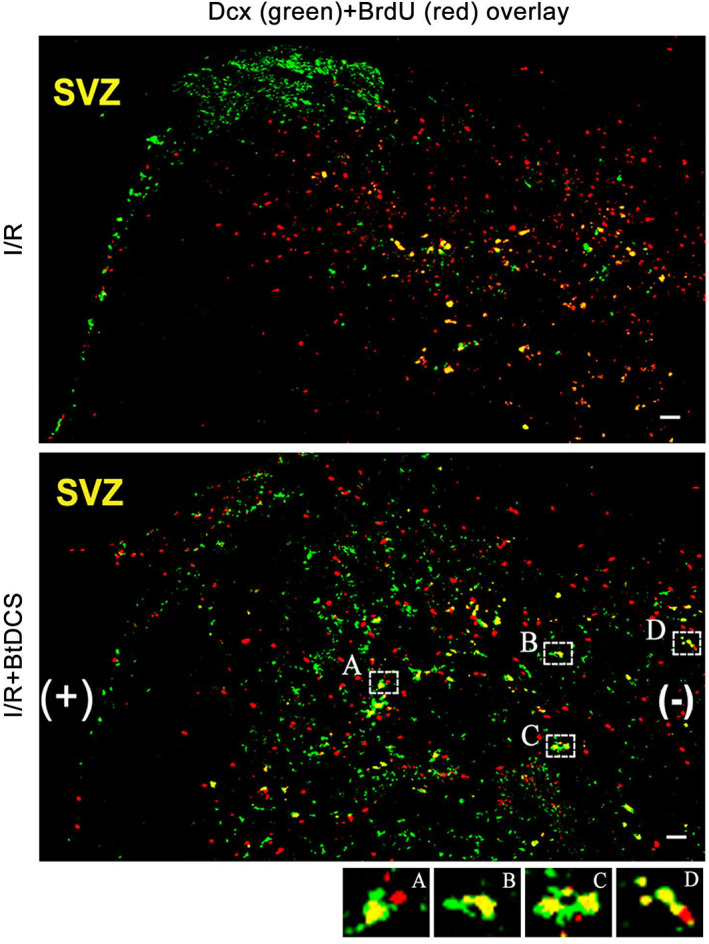
Colabeling of Dcx and BrdU in the ipsilateral striatum of MCAO rat after BtDCS treatment. Representative images show that BtDCS application for 150 μA for 30 min per day for 12 days at 2 days after MCAO in nonanesthesia conditions increases the number of colabeling of Dcx and BrdU in ipsilateral striatum at 14 days after ischemia–reperfusion injury. Scale bar = 100 μm.
FIGURE 3.
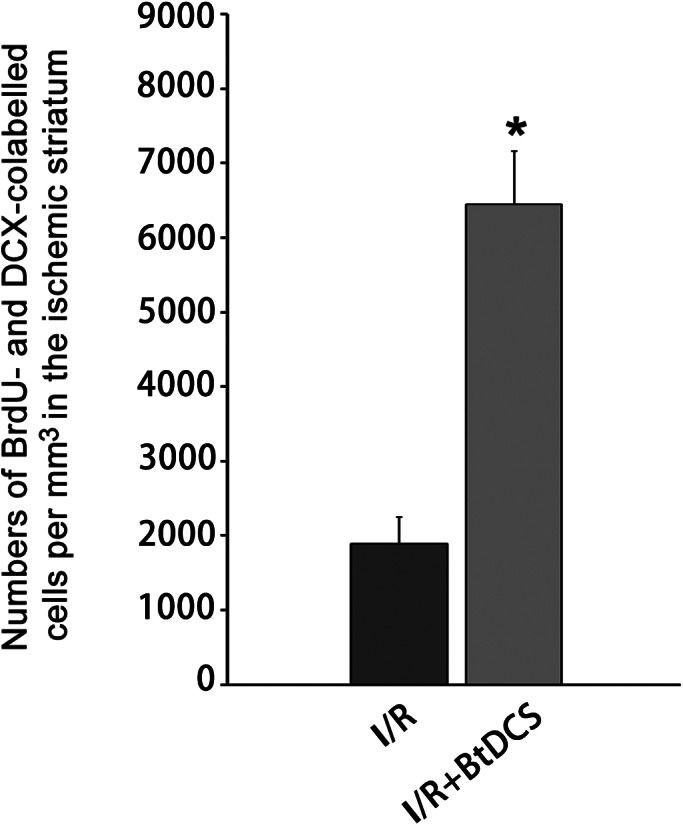
BtDCS treatment increases the number of colabeled Dcx and BrdU in the ipsilateral striatum of MCAO rat. Summarized data show that BtDCS increases the colocalization of BrdU/Dcx in the ischemic striatum at 14 days after ischemia–reperfusion (n = 7, *p < 0.05 vs. I/R, Student t test).
3.2. BtDCS promotes migration of neuroblasts from SVZ toward the direction of cathode to the ischemic brain area
To determine whether the BtDCS‐induced increase of neuroblasts in the ischemic striatum is immigrated from SVZ, we labeled the NSCs in the SVZ by injecting self‐inactivating lentiviruses expressing GFP (lentivirus‐GFP) into the SVZ at 1 day before rat stroke onset. The colocalization of GFP/Dcx is measured to detect the immigrated neuroblasts in the ischemic region. The animals in the ischemia–reperfusion group received sham stimulation with the stimulator turned off and these animals went through the same procedure with the implantation of epicranial electrodes. Our data show that BtDCS treatment for 12 days increases the colocalization of GFP/Dcx in the ischemic striatum at 14 days after ischemia–reperfusion (Figures 4 and 5).
FIGURE 4.
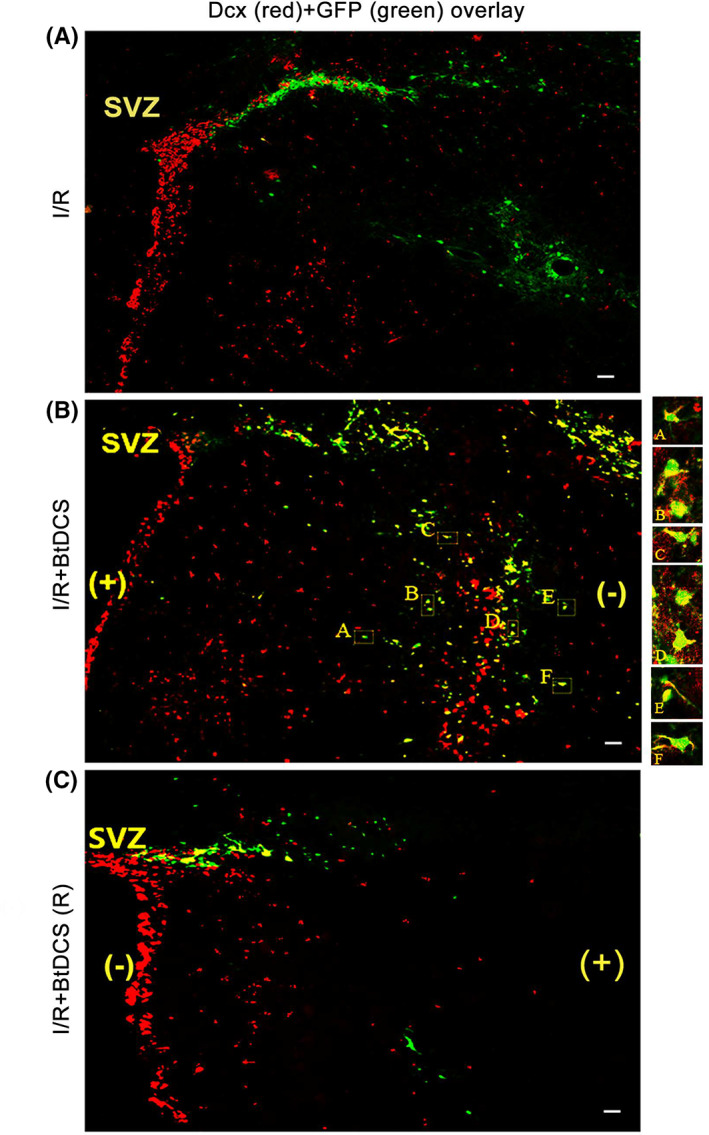
Colabeling of Dcx with GFP‐labeled cells in the ipsilateral striatum of MCAO rat after BtDCS treatment. (A–B) Representative images show that BtDCS exposure of 150 μA for 30 min per day for 2 days after MCAO for 12 days, increases the colocalization of Dcx with GFP in ipsilateral striatum at 14 days after ischemia–reperfusion injury. Scale bar = 100 μm. (C) BtDCS exposure of 150 μA for 30 min per day for 2 days after MCAO for 12 days, with the anodal electrode placed on the ischemic hemisphere, decreases the colocalization of GFP with Dcx in the ischemic striatum at 14 days after ischemia–reperfusion injury. Scale bar = 100 μm.
FIGURE 5.
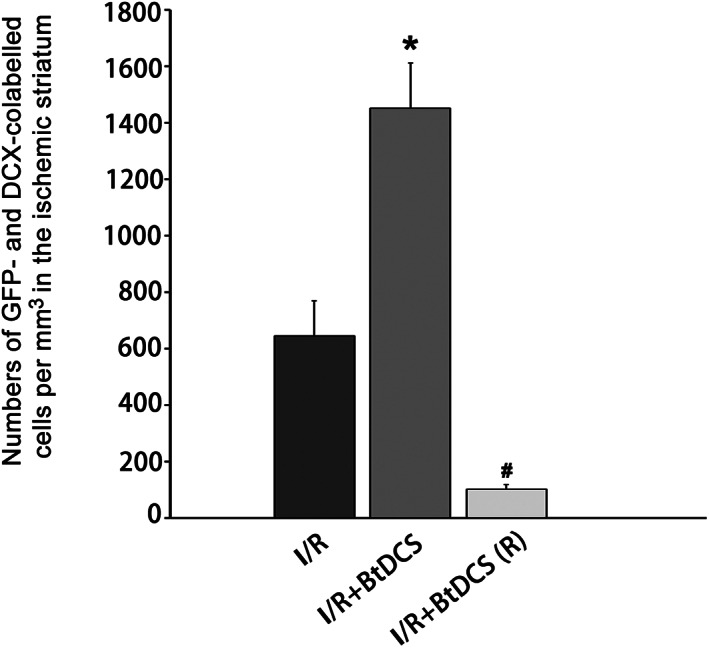
BtDCS increases the colocalization of Dcx with GFP‐labeled cells in ipsilateral striatum. Summarized data show that BtDCS treatment for 12 days increases the colocalization of GFP/Dcx in the ischemic striatum at 14 days after ischemia–reperfusion injury (n = 8, *p < 0.05 vs. I/R, # p < 0.05 vs. I/R + BtDCS, ANOVA test).
To provide further evidence that the neuroblasts identified in the ischemic striatum are immigrated from SVZ, we reversed the electrode placement with the anodal electrode placed on the ischemic hemisphere and the cathodal electrode on the contralateral hemisphere. A continuous exposure of BtDCS (150 μA) for 30 min per day is applied in ischemic animals for 12 days after ischemia–reperfusion injury. We show that the reversed BtDCS decreases the colocalization of GFP/Dcx in the ischemic striatum at 14 days after ischemia–reperfusion injury (Figures 4 and 5). Together, these results indicate that noninvasive BtDCS guides and promotes the migration of NSC‐derived neuroblasts, from SVZ toward cathode direction, to the ischemic brain region.
3.3. BtDCS protects against neuronal death after ischemia–reperfusion injury
To explore the therapeutic potential of BtDCS in ischemic stroke, we tested the effect of BtDCS on the infarct volume of stroke animals. The Nissl staining was performed to measure the infarct volume as previously described. 41 The animals in the I/R group as control received the same BtDCS‐setup as the stimulated animals with the stimulator turned off and these animals went through the same procedure with the implantation of epicranial electrodes. We demonstrated that BtDCS treatment for 12 days reduced the infarct volume of stroke animals at 14 days after rat ischemia–reperfusion injury (Figure 6A‐B, D).
FIGURE 6.
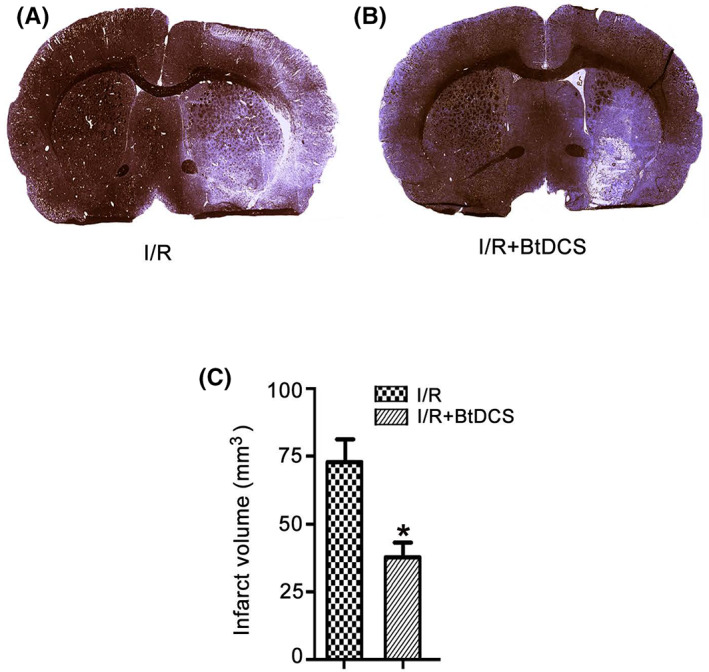
BtDCS treatment reduces the infarct volume. (A‐B) Representative images of Nissl staining show that BtDCS application for 150 μA for 30 min per day for 12 days at 2 days after MCAO reduces the infarct volume of MCAO animals at 14 days after ischemia–reperfusion injury. IR = ischemia–reperfusion injury. (C) Summarized data of Nissl staining indicate that BtDCS application for 150 μA for 30 min per day for 12 days at 2 days after MCAO reduces the infarct volume of MCAO animals at 14 days after ischemia–reperfusion injury (n = 8 rats for each group, *p < 0.05 vs. I/R, Student t test).
3.4. BtDCS promotes functional recovery of stroke animals
To determine the functional consequence of BtDCS in stroke animals, we performed a battery of neurobehavioral tests including modified neurological severity scores (mNSS) test, beam‐walking test, and modified sticky tape (MST) test on 1 day before MCAO, and at 3, 7, 14 and 28 days after MCAO. 32 , 33 The animals in the sham and I/R group as experimental control received the same BtDCS‐setup as the stimulated animals with the stimulator turned off and these animals went through the same procedure with the implantation of epicranial electrodes. Our data show that rats treated with BtDCS for 12 days have significantly lower scores of mNSS test on day 14 and 28 after MCAO (Figure 7A), lower scores of beam‐walking test on day 14 and 28 after MCAO (Figure 7B), and higher ratio of modified sticky tape (MST) test on day 14 and 28 after MCAO (Figure 7C). Together, these results provide functional evidence for the possible role of BtDCS in promoting endogenous neurogenesis‐mediated neurobehavioral effects in stroke animals.
FIGURE 7.
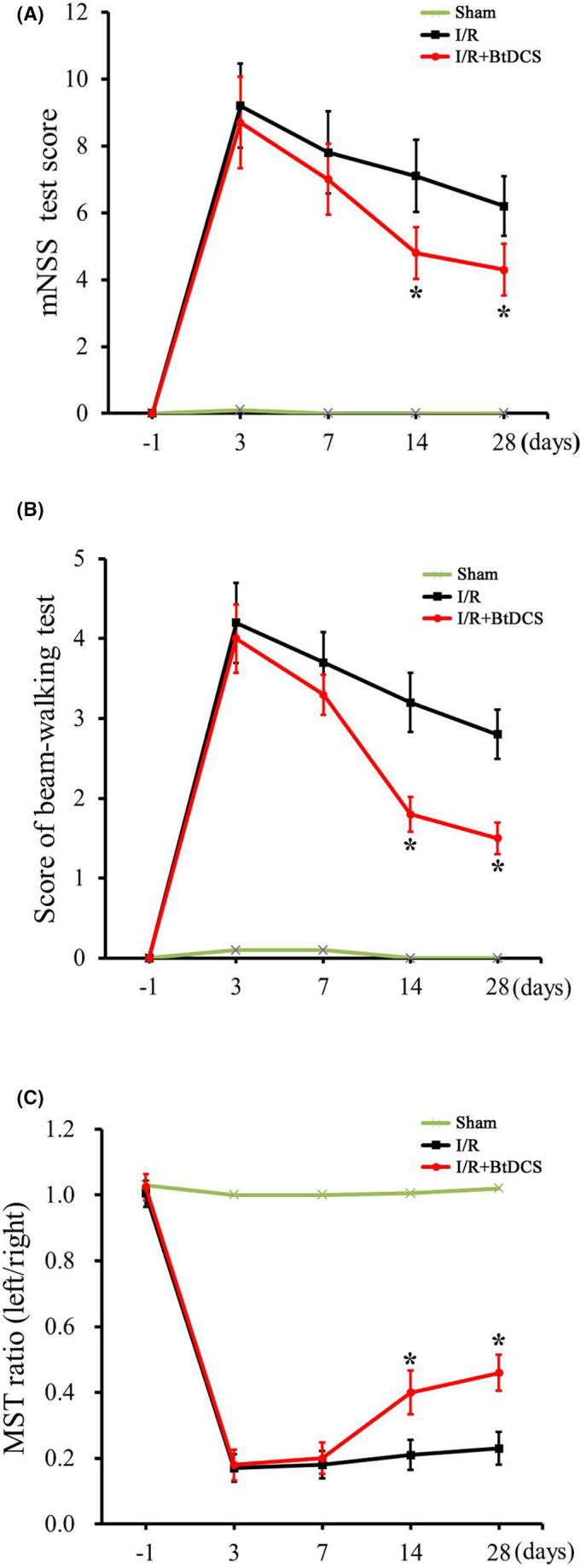
BtDCS improves the neurobehavioral function of stroke animals. (A) Summarized data show that rats treated with BtDCS for 150 μA for 30 min per day for 12 days have significantly lower scores of mNSS tests on day 14 (I/R group score = 7.2 ± 1.02; I/R + BtDCS score = 4.8 ± 0.77) and day 28 (I/R group score = 6.2 ± 0.85; I/R + BtDCS score = 4.0 ± 0.67) after MCAO, (n = 7 rats for each group, *p < 0.05 vs. I/R; ANOVA and subsequent post hoc Bonferroni's test). (B) Summarized data show that rats treated with BtDCS for 150 μA for 30 min per day for 12 days have significantly lower scores of beam‐walking test on day 14 (I/R group score = 3.2 ± 0.48; I/R + BtDCS score = 2.1 ± 0.35) and day 28 (I/R group score = 2.8 ± 0.43; I/R + BtDCS score = 1.7 ± 0.22) after MCAO, (n = 9 rats for each group, *p < 0.05 vs. I/R; ANOVA and subsequent post hoc Bonferroni's test). (C) Summarized data show that rats treated with BtDCS for 150 μA for 30 min per day for 12 days have a significantly higher ratio of a modified sticky tape test on day 14 (I/R group score = 0.21 ± 0.03; I/R + BtDCS score = 0.40 ± 0.06) and day 28 (I/R group score = 0.24 ± 0.03; I/R + BtDCS score = 0.46 ± 0.06) after MCAO (n = 7 rats for each group, *p < 0.05 vs. I/R; ANOVA and subsequent post hoc Bonferroni's test).
4. DISCUSSION
Endogenous neurogenesis‐mediated therapeutic potential in ischemic stroke remains unexplored. 6 , 7 Although ischemia injury is known to stimulate the neurogenesis in the SVZ, it is noticed that there are only a small amount of NSC‐derived neuroblasts that can migrate from SVZ toward poststroke brain region, 1 which apparently reduces the possibility of endogenous neurogenesis‐mediated recovery. We sought to identify the therapeutic strategy to promote the migration of SVZ‐derived neuroblasts toward ischemic brain region to protect against the brain damage. 12 We previously provided the first evidence that direct‐current stimulation guides NSC‐derived neuroblast migration toward the cathode in cultured embryonic explants. 12 This finding led us to hypothesize that direct‐current stimulation guides and promotes migration of adult NSC‐derived neuroblasts from SVZ toward ischemic brain region in vivo.
In this study, we demonstrated that BtDCS promoted the migration of NSC‐derived neuroblasts from SVZ toward the cathode direction into poststroke striatum. Reversing the position of cathodal and anodal electrodes prevents BtDCS‐induced neuroblast migration. Application of BtDCS reduces the number of neuronal deaths in the damaged brain region and improves functional outcomes in stroke animals. This study provides direct evidence for the effect of direct‐current stimulation on endogenous neuroblast migration in poststroke brain, suggesting that immigration of NSC‐derived neuroblasts from SVZ at least in part leads to the neuroprotective effect of BtDCS against neuronal death after cerebral ischemia–reperfusion injury.
An important reason that leads us to develop an interference‐controllable biophysical approach in regulating NSC migration in the ischemic brain is because it is apparently hard to apply chemical therapy to guide NSC migration in the ischemic brain. However, chemical therapy could be used to enhance the neurogenesis in the SVZ after ischemic stroke. It is possible that BtDCS application, combined with chemical therapy to promote neurogenesis, would enhance the migration of NSC‐derived neuroblasts from SVZ toward the poststroke brain region.
While our data suggest that BtDCS‐induced neuroprotection is in part mediated through immigrated neuroblasts from SVZ after ischemia–reperfusion injury, other mechanisms underlying the neuroprotective effect of BtDCS need to be explored in the future. The following possibilities are proposed: (1) Immigrated new neurons replace the lost neurons in the injured brain region to repair the brain damage 1 ; (2) immigrated new neurons, new glial cells or neuroblasts release cell survival‐promoting factors in the injured brain region to protect against ischemic neuron death 11 ; (3) BtDCS treatment may increase the release and/or enhance the activity of cell survival‐promoting signals in the existing neurons and/or glial cells of the injured brain, thus protecting against ischemic brain damage 47 ; (4) immigrated new neurons enhances the local cerebral blood flow (LCBF) in the peri‐infarct. 11
Clinical trials have investigated the potential efficacy of tDCS in poststroke motor recovery. 25 , 26 , 28 Although tDCS is proved safe for stroke patients, the functional outcomes are inconsistent. 29 The discrepancy is possibly due to multiple reasons. 31 In this study, we establish a new setup of tDCS, the BtDCS, by positioning the electrode on each side of the cranium over the dorsolateral prefrontal cortex with the cathodal electrode placed on the ischemic hemisphere and the anodal electrode on the contralateral hemisphere, which promotes the migration of NSC‐derived neuroblasts from SVZ toward the damaged brain region. Based on our findings, we reason that the efficacy of tDCS in poststroke recovery could be enhanced in part by promoting NSC‐derived neuroblast migration from SVZ toward the damaged brain region. Since BtDCS is a noninvasive and interference‐controllable technique, uncovering the effect of BtDCS on NSC‐derived neuroblasts would lead to the development of novel therapy for ischemic stroke patients.
As the first step of this study, we focused our investigation on the effect of BtDCS on the migration of SVZ‐derived neuroblasts toward ischemic brain. In future, it is important to examine the effect of BtDCS on the proliferation and/or differentiation of the neuronal progenitor populations in our experimental model. Whether or not the immigrated new neurons replace the lost neurons in the injured brain region to repair the brain damage. Whether or not the immigrated new neurons and/or new glial cells releases cell survival‐promoting factors in the injured brain region to protect against ischemic neuron death. Whether or not BtDCS treatment may increase the release and/or enhance the activity of cell survival‐promoting signals in the existing neurons and/or glial cells of the injured brain, thus protecting against ischemic brain damage. Whether or not the immigrated new neurons enhance the local cerebral blood flow (LCBF) in the peri‐infarct. Whether or not the immigrated new glial cells contribute to BtDCS‐induced neuroprotection. It is also necessary to examine whether BtDCS is neuroprotective if BtDCS is applied at 24 h, 48 h, or 72 h after ischemia injury.
In summary, this study provides direct evidence for the effect of direct‐current stimulation on regulating endogenous neuroblast migration in poststroke brain, and supports a potential application of noninvasive BtDCS as an endogenous neurogenesis‐based biophysical therapy in ischemic stroke.
AUTHOR CONTRIBUTIONS
RL and SW performed the experiment, analyzed the data, and wrote the manuscript. AL, JC, ZZ, JR, XY, XK, and WM performed the experiments and analyzed the data. FC, JC, and QW involved in conception and design.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by the American Heart Association (AHA: 12GRNT9560012), the Canadian Institutes of Health Research (CIHR: RMA147354), the Natural Science Foundation of China (NSFC: 82071385), the Key Research and Development Project of Shandong (2019JZZY021010), and the TaiShan Industrial Experts Programme (No. tscy20200412) to Q.W. This work was also supported by NSFC (81701291) to J.C.
Lei R, Wang S, Liu A, et al. Bilateral transcranial direct‐current stimulation promotes migration of subventricular zone‐derived neuroblasts toward ischemic brain. FASEB BioAdvances. 2023;5:277‐286. doi: 10.1096/fba.2023-00017
Ruixue Lei and Shu Wang contributed equally to this work.
Contributor Information
Juan Chen, Email: chenjuan0828@163.com.
Qi Wan, Email: qiwan1@hotmail.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963‐970. [DOI] [PubMed] [Google Scholar]
- 2. Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33‐41. [DOI] [PubMed] [Google Scholar]
- 3. Zhang RL, Zhang ZG, Chopp M. Ischemic stroke and neurogenesis in the subventricular zone. Neuropharmacology. 2008;55:345‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jin K, Minami M, Lan JQ, et al. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 2001;98:4710‐4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Felling RJ, Levison SW. Enhanced Neurogenesis following stroke. J Neurosci Res. 2003;73:277‐283. [DOI] [PubMed] [Google Scholar]
- 6. Kruger GM, Morrison SJ. Brain repair by endogenous progenitors. Cell. 2002;110:399‐402. [DOI] [PubMed] [Google Scholar]
- 7. Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399‐421. [DOI] [PubMed] [Google Scholar]
- 8. Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Dent Neurol. 2002;52:802‐813. [DOI] [PubMed] [Google Scholar]
- 9. Sundholm‐Peters NL, Yang HK, Goings GE, Walker AS, Szele FG. Subventricular zone neuroblasts emigrate toward cortical lesions. J Neuropathol Exp Neurol. 2005;64:1089‐1100. [DOI] [PubMed] [Google Scholar]
- 10. Yamashita T, Ninomiya M, Hernandez Acosta P, et al. Subventricular zone‐derived neuroblasts migrate and differentiate into mature neurons in the post‐stroke adult striatum. J Neurosci. 2006;26:6627‐6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wei ZZ, Zhang JY, Taylor TM, Gu X, Zhao Y, Wei L. Neuroprotective and regenerative roles of intranasal Wnt‐3a administration after focal ischemic stroke in mice. Journal of Cerebral Blood Flow and Metabolism : Official Journal of the International Society of Cerebral Blood Flow and Metabolism. 2018;38:404‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li L, el‐Hayek YH, Liu B, et al. Direct‐current electrical field guides neuronal stem/progenitor cell migration. Stem Cells. 2008;26:2193‐2200. [DOI] [PubMed] [Google Scholar]
- 13. Feng JF, Liu J, Zhang L, et al. Electrical guidance of human stem cells in the rat brain. Stem Cell Reports. 2017;9:177‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Russo C, Souza Carneiro MI, Bolognini N, Fregni F. Safety review of transcranial direct current stimulation in stroke. Neuromodulation : Journal of the International Neuromodulation Society. 2017;20:215‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peruzzotti‐Jametti L, Cambiaghi M, Bacigaluppi M, et al. Safety and efficacy of transcranial direct current stimulation in acute experimental ischemic stroke. Stroke. 2013;44:3166‐3174. [DOI] [PubMed] [Google Scholar]
- 16. Zhang K, Guo L, Zhang J, et al. A safety study of 500 muA cathodal transcranial direct current stimulation in rat. BMC Neurosci. 2019;20:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ahn SM, Jung DH, Lee HJ, et al. Contralesional application of transcranial direct current stimulation on functional improvement in ischemic stroke mice. Stroke. 2020;51:2208‐2218. [DOI] [PubMed] [Google Scholar]
- 18. Nitsche MA, Fricke K, Henschke U, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003;553:293‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Braun R, Klein R, Walter HL, et al. Transcranial direct current stimulation accelerates recovery of function, induces neurogenesis and recruits oligodendrocyte precursors in a rat model of stroke. Exp Neurol. 2016;279:127‐136. [DOI] [PubMed] [Google Scholar]
- 20. Rueger MA, Keuters MH, Walberer M, et al. Multi‐session transcranial direct current stimulation (tDCS) elicits inflammatory and regenerative processes in the rat brain. PLoS One. 2012;7:e43776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pikhovych A, Stolberg NP, Jessica Flitsch L, et al. Transcranial direct current stimulation modulates neurogenesis and microglia activation in the mouse brain. Stem Cells International. 2016;2016:2715196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rabenstein M, Unverricht‐Yeboah M, Keuters MH, et al. Transcranial current stimulation alters the expression of immune‐mediating genes. Front Cell Neurosci. 2019;13:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lieberman DN, Mody I. Regulation of NMDA channel function by endogenous Ca(2+)‐dependent phosphatase. Nature. 1994;369:235‐239. [DOI] [PubMed] [Google Scholar]
- 24. Monai H, Ohkura M, Tanaka M, et al. Calcium imaging reveals glial involvement in transcranial direct current stimulation‐induced plasticity in mouse brain. Nat Commun. 2016;7:11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Achacheluee ST, Rahnama L, Karimi N, Abdollahi I, Arslan SA, Jaberzadeh S. The effect of Unihemispheric concurrent dual‐site transcranial direct current stimulation of primary motor and dorsolateral prefrontal cortices on motor function in patients with sub‐acute stroke. Front Hum Neurosci. 2018;12:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hamoudi M, Schambra HM, Fritsch B, et al. Transcranial direct current stimulation enhances motor skill learning but not generalization in chronic stroke. Neurorehabil Neural Repair. 2018;32:295‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feng W, Kautz SA, Schlaug G, Meinzer C, George MS, Chhatbar PY. Transcranial direct current stimulation for poststroke motor recovery: challenges and opportunities. PM R. 2018;10:S157‐S164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fregni F, Boggio PS, Mansur CG, et al. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport. 2005;16:1551‐1555. [DOI] [PubMed] [Google Scholar]
- 29. Lefebvre S, Liew SL. Anatomical parameters of tDCS to modulate the motor system after stroke: a review. Front Neurol. 2017;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hummel FC, Cohen LG. Non‐invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 2006;5:708‐712. [DOI] [PubMed] [Google Scholar]
- 31. Chhatbar PY, Chen R, Deardorff R, et al. Safety and tolerability of transcranial direct current stimulation to stroke patients ‐ a phase I current escalation study. Brain Stimul. 2017;10:553‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang ZF, Chen J, Han X, et al. Bisperoxovandium (pyridin‐2‐squaramide) targets both PTEN and ERK1/2 to confer neuroprotection. Br J Pharmacol. 2017;174:641‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen J, Hu R, Liao H, et al. A non‐ionotropic activity of NMDA receptors contributes to glycine‐induced neuroprotection in cerebral ischemia‐reperfusion injury. Sci Rep. 2017;7:3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liebetanz D, Klinker F, Hering D, et al. Anticonvulsant effects of transcranial direct‐current stimulation (tDCS) in the rat cortical ramp model of focal epilepsy. Epilepsia. 2006;47:1216‐1224. [DOI] [PubMed] [Google Scholar]
- 35. Liebetanz D, Koch R, Mayenfels S, König F, Paulus W, Nitsche MA. Safety limits of cathodal transcranial direct current stimulation in rats. Clin Neurophysiol. 2009;120:1161‐1167. [DOI] [PubMed] [Google Scholar]
- 36. Liebetanz D, Fregni F, Monte‐Silva KK, et al. After‐effects of transcranial direct current stimulation (tDCS) on cortical spreading depression. Neurosci Lett. 2006;398:85‐90. [DOI] [PubMed] [Google Scholar]
- 37. Deierborg T, Staflin K, Pesic J, Roybon L, Brundin P, Lundberg C. Absence of striatal newborn neurons with mature phenotype following defined striatal and cortical excitotoxic brain injuries. Exp Neurol. 2009;219:363‐367. [DOI] [PubMed] [Google Scholar]
- 38. Consiglio A, Gritti A, Dolcetta D, et al. Robust in vivo gene transfer into adult mammalian neural stem cells by lentiviral vectors. Proc Natl Acad Sci USA. 2004;101:14835‐14840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rogelius N, Ericson C, Lundberg C. In vivo labeling of neuroblasts in the subventricular zone of rats. J Neurosci Methods. 2005;142:285‐293. [DOI] [PubMed] [Google Scholar]
- 40. Ning K et al. Circadian regulation of GABA(a) receptor function by CKIepsilon‐CKIdelta in the rat suprachiasmatic nuclei. Nat Neurosci. 2004;7:489‐490. [DOI] [PubMed] [Google Scholar]
- 41. Mastroiacovo F, Moyanova S, Cannella M, et al. Genetic deletion of mGlu2 metabotropic glutamate receptors improves the short‐term outcome of cerebral transient focal ischemia. Mol Brain. 2017;10:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke; a Journal of Cerebral Circulation. 2001;32:1005‐1011. [DOI] [PubMed] [Google Scholar]
- 43. Petullo D, Masonic K, Lincoln C, Wibberley L, Teliska M, Yao DL. Model development and behavioral assessment of focal cerebral ischemia in rats. Life Sci. 1999;64:1099‐1108. [DOI] [PubMed] [Google Scholar]
- 44. Aronowski J, Samways E, Strong R, Rhoades HM, Grotta JC. An alternative method for the quantitation of neuronal damage after experimental middle cerebral artery occlusion in rats: analysis of behavioral deficit. Journal of Cerebral Blood Flow and Metabolism : Official Journal of the International Society of Cerebral Blood Flow and Metabolism. 1996;16:705‐713. [DOI] [PubMed] [Google Scholar]
- 45. Sughrue ME, Mocco J, Komotar RJ, et al. An improved test of neurological dysfunction following transient focal cerebral ischemia in rats. J Neurosci Methods. 2006;151:83‐89. [DOI] [PubMed] [Google Scholar]
- 46. Boonzaier J, van Tilborg GAF, Neggers SFW, Dijkhuizen RM. Noninvasive brain stimulation to enhance functional recovery after stroke: studies in animal models. Neurorehabil Neural Repair. 2018;32:927‐940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baba T, Kameda M, Yasuhara T, et al. Electrical stimulation of the cerebral cortex exerts antiapoptotic, angiogenic, and anti‐inflammatory effects in ischemic stroke rats through phosphoinositide 3‐kinase/Akt signaling pathway. Stroke. 2009;40:e598‐e605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


