Abstract
Background
Many modern pharmaceutical researchers continue to focus on the discovery and evaluation of natural compounds for possible therapies for obesity, diabetes, infections, cancer, and oxidative stress. Extraction of Ocimum basilicum seed essential oil and evaluation of its antioxidant, anti-obesity, antidiabetic, antibacterial, and cytotoxic activities were the goals of the current study.
Method
O. basilicum seed essential oil was extracted and evaluated for its anticancer, antimicrobial, antioxidant, anti-obesity, and anti-diabetic properties utilizing standard biomedical assays.
Results
O. basilicum seed essential oil showed good anticancer activity against Hep3B (IC50 56.23 ± 1.32 µg/ml) and MCF-7 (80.35 ± 1.17 µg/ml) when compared with the positive control, Doxorubicin. In addition, the essential oil showed potent antibacterial (against Klebsiella pneumoniae, Escherichia coli, Staphylococcus aureus, Proteus mirabilis, and Pseudomonas aeruginosa) and antifungal (against Candida albicans) activities. Moreover, as for the anti-amylase test, IC50 was 74.13 ± 1.1 µg/ml, a potent effect compared with the IC50 of acarbose, which was 28.10 ± 0.7 µg/ml. On the other hand, for the anti-lipase test, the IC50 was 112.20 ± 0.7 µg/ml a moderate effect compared with the IC50 of orlistat, which was 12.30 ± 0.8 µg/ml. Finally, the oil had a potent antioxidant effect with an IC50 of 23.44 ± 0.9 µg/ml compared with trolox (IC50 was 2.7 ± 0.5 µg/ml).
Conclusion
This study has provided initial data that supports the importance of O. basilcum essential oil in traditional medicine. The extracted oil not only exhibited significant anticancer, antimicrobial, and antioxidant properties but also antidiabetic and anti-obesity effects, which provided a foundation for future research.
Keywords: Ocimum basilicum, Anticancer, Antimicrobial, Antioxidant, Anti-diabetic, Anti-obesity
Introduction
Herbal medicine is still practiced today due to its biomedical benefits as well as cultural beliefs in many parts of the world [1]. In Palestine, traditional herbal therapy has a long history of usage and continues to provide significant benefits in the treatment of many disorders [2]. However, scientific explorations of the therapeutic and chemical qualities of plants utilized in traditional Palestinian medicine are trivial [3]. According to reports, a number of traditional Palestinian medicinal herbs possess remarkable bioactivities that may contribute to the enhancement of community health and lifestyle. Since ancient times, people have relied on the healing and preservation powers of medicinal aromatic herbs [4, 5]. Essential oils, which are secondary metabolites of plants, are responsible for some of these activities [6]. These substances have the physical aim of protecting plants against bacteria, fungi, viruses, insects, and even herbivores by lowering their desire for these plants [7]. Antimicrobial, sedative, food additive, and cosmetic uses for essential oils are just a few of the many essential oil based products on the market [7–9]. The result is that a number of research teams are investigating the fundamental components of essential oils and their antioxidant, anthelmintic, and antibacterial properties, as well as the primary components of essential oils and their antifungal properties [10–12].
Oxidative stress causes reactive oxygen species (ROS), which are known to contribute to a number of cardiovascular disorders, including heart failure, atherosclerosis, and ventricular remodeling. Antioxidants and pro-oxidants may have contradictory effects on the development of cancer; they can prevent oxidative stress to DNA and inhibit tumorigenesis [13]. It is the usual cellular signaling pathways that are interrupted when this delicate equilibrium is upset, resulting in unchecked cell multiplication. Due to the overproduction of reactive oxygen, oxidative stress plays a vital role in atherosclerotic lesion formation. Through the activity of several enzymes, endothelial cells and smooth muscle cells are capable of producing oxidants. The majority of reactive oxygen in the vascular wall is generated by NADPH oxidases, a group of membrane-associated enzymes typical of cells of mesodermal origin [13, 14]. A greater level of oxidative stress is seen in these situations and in the tumor environment, so the increased antioxidant system can take advantage of the upregulated antioxidant system [13, 15]. A number of antioxidants, including coenzyme Q10, beta-carotene, lycopene, quercetin, and vitamins C and E, have been found to be effective in the prevention and treatment of various forms of cardiovascular disease (CVD) [13].
Worldwide, the prevalence of obesity has roughly tripled since 1975, making it a growing health concern. There has been an increase in obesity in low- and middle-income nations, particularly in metropolitan areas, since the 1970s [16]. Overweight and obese children in Palestine accounted for 6% of the population in children and 18% of the population in adults, respectively [17]. Increased BMI is connected with non-communicable diseases such as cardiovascular disease, diabetes, and numerous types of cancer [18]. Along with obesity, diabetes mellitus and its consequences are serious health issues that pressure the healthcare system and people. The frequency is rising worldwide, especially in low- and middle-income nations. Approximately 422 million people globally have diabetes, according to a recent estimate [19]. In Palestine, diabetes prevalence was estimated in 2000 at 9.7%, rising to 15.3% by 2010, and it is anticipated to reach 20.8% by 2020 and 23.4% by 2030 for persons aged 25 and over in the country [20].
The biggest global risk to effectively treating infections caused by harmful pathogens right now is antimicrobial resistance. Antimicrobial agent resistance has been shown to have negative effects on both clinical and therapeutic outcomes, with costs ranging from longer hospital stays, higher rates of morbidity and mortality, and more expensive healthcare to treatment failures and the need for more expensive and safe alternative medications. In the ongoing fight against microbial infections, there is an urgent need for new antimicrobial agents. Antimicrobial resistance is unavoidable, and pharmaceutical firms consistently show little interest in funding novel antibiotic research [21–24]. The abuse and misuse of antimicrobials has led to an increase in the number of “antibiotic-resistant bacteria,“ which can no longer be treated with current antimicrobials because of the rise in antimicrobial resistance. To combat the rise of antibiotic-resistant bacteria and the negative effects of synthetic antimicrobials, researchers have turned to natural remedies [25].
Great basil, also known as Saint-Joseph’s wort or Ocimum basilicum (O. basilicum), is a major member of the Lamiaceae family, which is often known as the mint family [26, 27]. Previous scientific studies revealed many pharmacological effects in curing several health problems, this plant showed potent antioxidant, anticancer, antiviral, anti-aging, and antimicrobial properties [28–30]. O. basilicum seeds can be described as tiny black, ellipsoid seeds, as shown in Fig. 1 [31]. These seeds are widely used in traditional medicine to treat colic ulcers, dyspepsia, and diarrhea [1, 32]. O. basilicum seeds have a remarkable capacity for hydration because of their adherent seed mucilage, which is known to be produced in testa cells during seed development [33, 34]. Research has shown that rosmarinic acid is the main biologically active component in O. basilicum that is related to these activities [35].
Fig. 1.

Basil (Ocimum basilicum) plant
As previously noted, basil has been shown to help digestion, and when combined with camphor, it can also be used to treat epistaxis. An internal infusion of O. basilicum is used to treat a variety of ailments, including cephalalgia, high fever, cough, gouty joint pain, otitis media, snake bites, gastroenteritis, and piles. gonorrhea, chronic dysentery, diarrhea, and diabetes mellitus can be treated with an infusion of basil seed [36, 37]. This research focused on the study of the anticancer, antimicrobial, antioxidant, anti-obesity, and antidiabetic effects of the O. basilicum essential oil since no previous studies have investigated these healthcare problems with the O. basilicum seeds essential oil from Palestine.
Materials and methods
Chemical reagents and instruments
2,2-Diphenyl-1-picrylhydrazyl (DPPH) (Sigma Aldrich, Germany) and ((s)-(-)-6 hydroxy-2,5,7,8-tetramethychroman-2-carboxylic acid) (Sigma Aldrich, Denmark). n-hexane (Frutarom Ltd, Israel). Methanol (Lobachemie, India), The solvents used for the extraction of plant material were of HPLC grade. Sigma-Aldrich (USA) provided porcine pancreatic lipase, starch, acarbose, DPPH (2,2-diphenyl-1-picrylhydrazyl), PNPP (p-nitrophenyl palmitate), and orlistat, while MP Biochemicals (Illkirch, France) provided porcine pancreatic α-amylase. All the chemicals used in the experiments were of analytical grade. O. basilicum seeds were obtained from different regions in Palestine. The plant seeds were characterized in the Pharmacy Department at An-Najah National University. The plant material was collected in accordance with the WHO Guidelines for the Assessment of Herbal Medicines and Legislation.
The rotary evaporator (Heidolph OB2000, VV2000, Germany), the grinder (Moulinex model, Uno, China), the balance (Radwag, AS 220/c/2, Poland), and the filter papers (Machrery-Nagel, MN 617, Whatman no. 1, USA) were all used.
Collection and extraction of O. basilicum essential oil
The seeds of the plant were gathered from various areas in Palestine and then processed into a fine powder using a mechanical blender. This yielded 25 g of powdered plant material, which was then extracted utilizing the hydrodistillation Clevenger technique for 4 h based on the method described in the British Pharmacopoeia. The resulting oil was chemically dried by utilizing calcium carbonate and stored at 4–8 °C in a dark bottle until further use [38].
Antioxidant activity of O. basilicum essential oil in terms of free radical scavenging
At first, the O. basilicum essential oil and the Trolox standard were mixed together to make a stock solution with a concentration of 1 mg/ml that was dissolved in methanol. Stock solutions were used to prepare working solutions with the following concentrations (2, 3, 5, 10, 20, 30, 50, 80, and 100 µg/ml) by serial dilution in methanol. The following concentrations were achieved by diluting stock solutions sequentially in methanol. A newly made solution of DPPH with a concentration of 0.002% weight-per-volume was created. After that, it was combined with methanol and each of the working concentrations in a proportion of 1:1:1, starting with the lowest concentration. Methanol was used as a blank solution for the spectrometer assay for the DPPH assay. The DPPH solution mixed with just methanol was the starting point for the series of concentrations. Around thirty minutes were spent incubating the solutions at room temperature in a completely dark cabinet. The absorbance of the samples was then measured with the spectrophotometer set to a wave length of 517 nm [39].
Using the following formula, we were able to determine the proportion of antioxidant activity contained within the extracted oil in comparison to the Trolox standard:
DPPH inhibition activity (%) = (A-B)/A ×100%.
Where A is the absorbance of the blank and B is the absorbance of the sample. The data was analyzed and reported as the percentage of inhibition. The inhibition of the extracted oil and the Trolox standard at different concentrations, were plotted and tabulated.
Antidiabetic evaluation of O. basilicum essential oil using the α-amylase inhibitory method
Following a scientifically adopted protocol in the enzymatic assay of amylase inhibitory action [40], the O. basilicum essential oil was dissolved in a few milliliters of 10% DMSO and mixed with buffer (0.02 M Na2HPO4/NaH2PO4, 0.006 M NaCl, pH 6.9). Different dilutions were prepared (50, 100, 200, 400, and 1000 µg/ml) using 10% DMSO as the solvent for the dilution. Then, 0.2 ml of porcine pancreatic-amylase enzyme solution (2 units/ml) was prepared and mixed with 0.2 ml of O. basilicum essential oil before incubating this sample for 10 min at 30 •C. Following the previous step, 0.2 ml of the freshly prepared 1% starch aqueous solution was incubated for 3 min and then added to each tube. To stop the reaction after diluting each sample by 5 ml of distilled water, 0.2 ml of DNSA solution was then added. Finally, each tube was warmed at 90 •C in a water bath for 10 min and then allowed to cool down, with the spectrophotometer adjusted after that at 540 nm to take absorbance for each tube. The blank was made using the same previous steps without adding the essential oil. As a standard reference, acarbose was used with the same previous steps for comparison as a standard.
We used the formula: amylase inhibition% = (Ab - As)/Ab * 100%.
Ab: blank absorbance, As: sample absorbance.
Anti-obesity evaluation O. basilicum essential oil using the lipase inhibitory method
The porcine pancreatic lipase inhibitory experiment was performed on O. basilicum essential oil using modified methods from Zheng et al. (2010) and Bustanji et al. (2010) [16, 17]. At the beginning, a stock solution of O. basilicum essential oil in 10% DMSO at a concentration of 1 mg/ml (1000 µg/mL) was employed. From this solution, five different solutions with the following concentrations were prepared: 50, 100, 200, 300, and 400 µg/ml respectively. Just before its application, a stock solution of pancreatic lipase enzyme at a concentration of 1 mg/ml was made. To make a stock solution of PNPB (p-nitrophenyl butyrate), 20.9 mg of PNPB were dissolved in 2 mL of acetonitrile. This produced the stock solution. In test tubes that already contained 0.2 mL of O. basilicum at one of five different concentrations (50, 100, 200, 300, or 400 µg/ml), 0.1 ml of porcine pancreatic lipase at a concentration of 1 mg/mL was added. After that, the resultant mixtures were diluted to a volume of 1 ml with a Tri-HCl solution (pH 7.4) and left to incubate at a temperature of 25 •C for 15 min. When the period of incubation had concluded, 0.1 milliliter of PNPB solution was poured into each test tube. The combination went back into the incubator for another half an hour at 37 •C. An UV-visible spectrophotometer was used to evaluate the level of pancreatic lipase activity by monitoring the rate at which p-nitrophenyl butyrate was converted into p-nitrophenol at a wavelength of 405 nm. The exact same process was carried out using orlistat, which served as a positive control, utilizing the identical concentrations that were described before.
We used the formula: lipase inhibition% = (Ab - As)/Ab * 100%.
Ab: blank absorbance, As: sample absorbance.
Antimicrobial evaluation of O. basilicum essential oil
The antibacterial activity of O. basilicum essential oil was tested using five strains obtained from the American Type Culture Collection (ATCC), including Klebsiella pneumoniae, Escherichia coli, Staphylococcus aureus, Proteus mirabilis, and Pseudomonas aeruginosa, in addition to the clinical isolate Methicillin-Resistant Staphylococcus aureus (MRSA). For the antifungal test, Candida albicans was employed.
The essential oil of O. basilicum has been tested for antimicrobial activity using the broth microdilution technique. O. basilicum essential oil stock solution was prepared with a concentration of 200 µg/mL 20%, dissolved in 20% DMSO and 60% Muller-Hinton broth. The prepared O. basilicum essential oil solution was serially diluted (2-folds) with sterile Muller-Hinton broth to achieve serial dilutions of 50, 25, 12.5, 6.25, 3.125, etc. µg/mL (RPMI medium was used for the C. albicans strain); DMSO concentration was 5% at the first well and was then serially diluted two-fold to exclude its antimicrobial effect. In 96-well plates, the dilution procedure was conducted aseptically. Micro-well 11 contained O. basilicum essential oil-free media (inoculated with the microbe), which served as a positive control for microbial growth in the micro-wells used to evaluate the antibacterial activity of the essential oil. On the other hand, the Mueller-Hinton broth in microwell 12 was oil- and microbial-free, which serves as a negative control for the proliferation of microorganisms. There were no microbes found in this well when it was utilized as a negative control. The test microorganisms were injected into micro-wells 1–11. The antibacterial activity of O. basilicum essential oil was evaluated three times. All inoculation plates were kept at 35 •C. Plates containing test bacterial strains were incubated for approximately 18–24 h, whereas plates containing C. albicans were incubated for approximately 48 h. When no microbial growth could be seen in the micro-wells after adding a modest dose of O. basilicum essential oil, the MIC was determined to be that concentration. It was decided to use antimicrobial drugs with established antibacterial activity to test the approach with the microorganisms, such as ampicillin and ciprofloxacin for bacteria and fluconazole for molds [40].
Cytotoxicity assay of O. basilicum essential oil
A cytotoxicity assay was performed for O. basilicum essential oil and doxorubicin (Positive control). HeLa, MCF-7, and Hep3B cells were grown in RPMI-1640 medium (Sigma-Norwich, United Kingdom) that was supplemented with 1% L-glutamine (Sigma, United Kingdom), 1% penicillin/streptomycin antibiotics (BI, India), and 10% fetal bovine serum. It was kept at 37 •C in a humidified environment with 5% CO2 in order to develop cancer cells. In a 96-well plate, cells were planted at a density of 2.6104 cells per well. O. basilicum essential oil doses of 300, 120, 60, 30, and 10 µg/ml were incubated for 24 h with cancer cells after 48 h of incubation with the tested O. basilicum essential oil. The CellTilter 96® Aqueous One Solution Cell Proliferation (MTS) Assay was used to determine the viability of the cells in accordance with the manufacturer’s instructions (Promega Corporation, Madison, USA). MTS solution was added to each well after the treatment, and the well plates were incubated at 37 •C for two hours. Four hundred and ninety-nine-nanometer absorbance. The same procedure was carried out for doxorubicin (positive control) [39].
Data analysis
The cytotoxic, antimicrobial, antioxidant, anti-obesity, and antidiabetic activities were tested for O. basilicum essential oil and were performed in triplicate. The data were presented as the mean and standard deviation (± SD). The results were considered significant only when the p values were less than 0.005.
Results
Antioxidant activity of O. basilicum essential oil
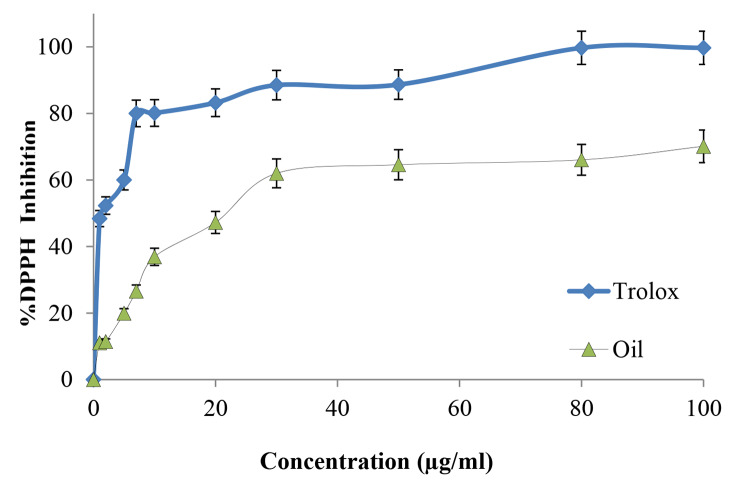
The free radical scavenging activity of the extracted O. basilicum essential oil was tested by the DPPH radical method using trolox as a reference standard and shown in Fig. 2. In this study, O. basilicum essential oil showed good antioxidant activity (IC50 = 23.44 ± 0.9 µg/ml) when compared to the standard antioxidant compound trolox (IC50 = 2.7 ± 0.5 µg/ml).
Fig. 2.
%DPPH inhibition profile by trolox and O. basilicum essential oil
Antidiabetic activity of O. basilicum essential oil
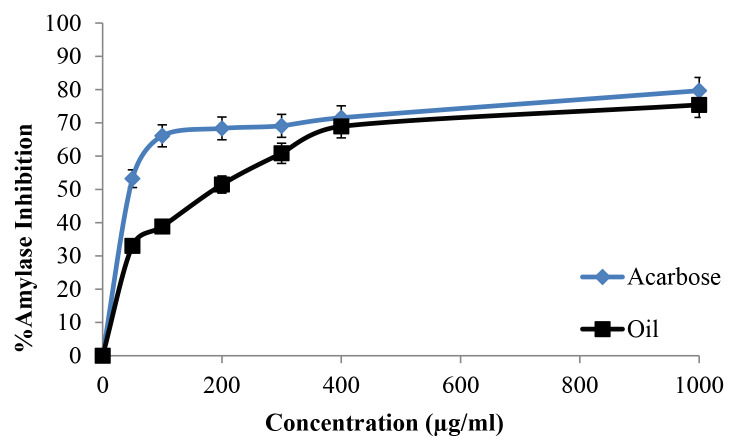
The α-amylase inhibitory activity of the extracted O. basilicum essential oil was evaluated against porcine pancreatic α-amylase. The result is shown in Fig. 3. When compared to the standard acarbose (IC50 = 28.10 ± 0.7 µg/ml), O. basilicum essential oil demonstrated strong α -amylase inhibitory activity with an IC50 = 74.13 ± 1.1 µg/ml.
Fig. 3.
α-Amylase inhibitory activity profile by acarbose standard and O. basilicum essential oil
Anti-obesity activity of O. basilicum essential oil
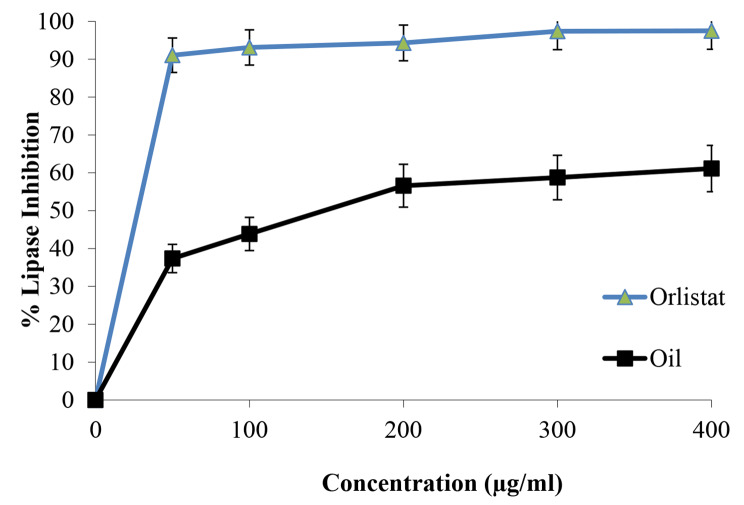
The porcine pancreatic lipase inhibitory activity of the extracted O. basilicum essential oil was evaluated and shown in Fig. 4. The results revealed that O. basilicum essential oil has moderate to good activity against porcine pancreatic lipase enzyme (IC50 = 112.20 ± 0.7 µg/ml) when compared with the orlistat standard (IC50 = 12.30 ± 0.8 µg/ml).
Fig. 4.
Porcine pancreatic lipase inhibitory activity profile by orlistat standard and O. basilicum essential oil
Antimicrobial activity of O. basilicum essential oil
The antibacterial test of O. basilicum essential oil was performed on different strains of gram-positive and gram-negative bacteria using broth microdilution and showed broad-spectrum antibacterial and antifungal effects against all the screened bacterial and fungal strains in comparison with control positive antibiotics and antifungals, such as ampicillin, ciprofloxacin, and fluconazole, respectively. The O. basilicum essential oil MIC values are demonstrated in Table 1.
Table 1.
MIC values (µg/mL ± 0.05) of O. basilicum essential oil compared with Ampicillin, Ciprofloxacin, and Fluconazole antibiotics,
| Microorganisms | O. basilicum oil | Ampicillin | Ciprofloxacin | Fluconazole |
|---|---|---|---|---|
| S. aureus(ATCC 25,923) | 2.3 | 6.25 | 0.78 | - |
| MRSA | 2.3 | 32 | 12.5 | - |
| E. coli (ATCC 25,922) | 1.0 | 3.12 | 0.78 | - |
| P. vulgaris(ATCC 8427) | 1.5 | 3.25 | 0.06 | - |
| K. pneumonia (ATCC 13,883) | 1.0 | 12.5 | 0.06 | - |
| P. aeruginosa (ATCC 9027) | 1.0 | 100 | 3.12 | - |
| C. albicans (ATCC 90,028) | 1.3 | - | - | 3.12 |
Cytotoxicity effect of O. basilicum essential oil
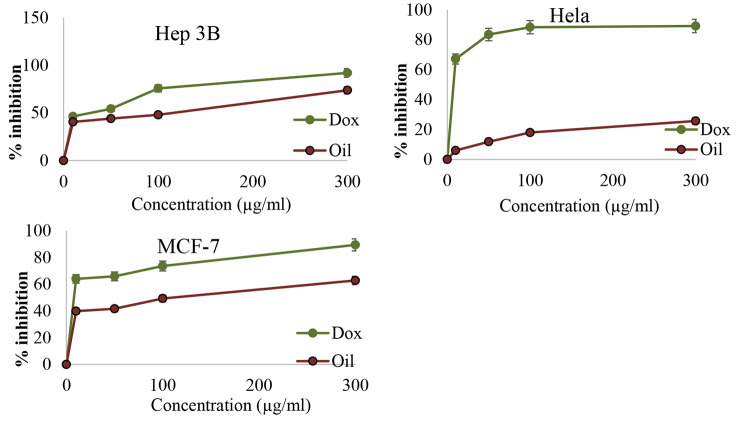
After treatment of HeLa, MCF-7, and Hep3B tumor cells with O. basilicum essential oil, the MTS assay results showed an interesting result (Fig. 5), which explains the relationship between the concentration of O. basilicum essential oil and doxorubicin plotted against the inhibition percent of cancer cell growth. When the concentrations of oil and doxorubicin increased, the inhibition of the growth of cancer cells also increased, which means that there is an effect of oil against these cancer cells. O. basilicum essential oil had a good cytotoxic effect against MCF-7 and Hep3B cancer cell lines that showed an IC50 value of 80.35 ± 1.17 µg/ml and 56.23 ± 1.32 µg/ml respectively. However, its activity against Hela was inactive. These results were compared with the IC50 of doxorubicin, as shown in Table 2.
Fig. 5.
The IC50 values (µg/mL) of O. basilicum essential oil and Doxorubicin against different cancer cells line
Table 2.
The IC50 values (µg/mL) of O. basilicum essential oil and Doxorubicin against different cancer cells line
| Hep 3B | Hela | MCF-7 | |
|---|---|---|---|
| O. basilicum essential oil IC 50 (µg/ml) | 56.23 ± 1.32 | Inactive (53.70 mg/ml) | 80.35 ± 1.17 |
| Doxorubicin IC 50 (µg/ml) | 21.37 ± 0.62 | 10.11 ± 1.17 | 15.02 ± 0.72 |
Discussion
The well-known O. basilicum (basil plant) includes a number of bioactive secondary metabolites, including polyphenols, flavonoids, and terpenes, which have the potential to have a wide range of biological effects. Its essential oil is mainly composed of linalool, methyl estragole, methyl cinnamate, and methyl chavicol [41, 42]. The fact that there are over 25 distinct varieties of O. basilicum, each with a unique constitution, has made it hard for previous researchers to create a uniform fundamental composition for basil extract or oil [43]. Since the DPPH radical is stable in its radical state, it is one of the most frequently utilized substrates for rapid evaluation of antioxidant activity. It was determined whether or not the extracts studied may function as hydrogen or electron donors in the transformation of DPPH into its reduced form DPPH-H by the use of the DPPH assay [44]. DPPH is a free radical that may be used to evaluate the effectiveness of pharmaceutical or herbal items to scavenge free radicals. Following both electron transfer and hydrogen atom transfer processes, it provides an evaluation of antioxidants that includes the ability to follow any or both of these processes [45]. The O. basilicum essential oil in the present work showed significant power to scavenge free radicals with a potent antioxidant effect, having an IC50 23.44 ± 0.9 µg/ml compared with trolox (IC50 was 2.7 ± 0.5 µg/ml). O. basilicum essential oil demonstrated a higher content of phenols and maximal levels of DNA protection and free radical scavenging against toxicity induced by cadmium chloride [46]. As found in other research, the antioxidant property of this plant oil may be due to the polyphenoid rosmarinic acid, which is a derivative of cinnamic acid [47].
Health problems such as diabetes and obesity are becoming more prevalent throughout the globe [48]. They have been linked to an increase in the mortality rates of cancer, cardiovascular, respiratory, hepatic, and renal illnesses in several studies [49]. A high frequency of these metabolic illnesses has been evident and alarming for the past three decades in Palestine and many other nations worldwide [50]. To put it another way, any pharmacological drug that interferes with the activity of metabolic enzymes like lipase and amylase is guaranteed to be effective in curing these life-threatening conditions. Aromatic herbs have been utilized for a long time to cure obesity, overweightness, dyslipidemia, and diabetes in several global medical systems [51].
According to previous research findings, O. basilicum inhibits human pancreatic α-amylase in the small intestine, suppressing carbohydrate digestion and thus controlling the entry of glucose into the human body to maintain a reasonable postprandial glucose level [52–54]. Malapermal et al. examined the α-amylase inhibitory activity of aqueous, 60% ethanolic, and 70% ethanolic extracts of the leaves of O. basilicum, and the extracts exhibited nearly equivalent efficacy to acarbose [55]. Noor et al. evaluated the anti-diabetic potential of O. basilicum using porcine pancreatic α-amylase inhibitory activities. The results revealed that O. basilicum exhibited α-amylase inhibitory activity almost equivalent to the drug acarbose [54]. As we obtained in this study, the anti-amylase test confirmed these previous results as the IC50 was 74.13 ± 1.1 µg/ml which meant a potent effect compared with the IC50 of acarbose (the reference compound), which was 28.10 ± 0.7 µg/ml. The hydrolysis of lipids by pancreatic lipase into glycerol and fatty acids is well known. As a result, inhibiting this enzyme prevents lipids from being converted into absorbable compounds in the body. As a result, lipase inhibitory medicines or herbal items can be used to manage obesity [54]. O. basilicum essential oil anti-lipase activity was tested using orlistat as a reference control because of its similar mode of action. As stablished in other studies, O. basilicum essential oil significantly lowered both plasma triglycerides and cholesterol in acute hyperlipidemia induced by Triton WR-1339 in rats [28]. Irondi et al. examined the ethyl acetate leaf extract of O. basilicum. The extract exhibited excellent lipase inhibitory action, but it was lower compared to orlistat [56]. A similar finding was reported by Noor et al. who discovered that extracts of O. basilicum flowers and leaves possess significant lipase inhibitory action [54]. This recent study also showed an anti-lipase result in which the IC50 was 112.20 ± 0.7 µg/ml which can be described as a moderate effect compared with the IC50 of orlistat, which was 12.30 ± 0.8 µg/ml.
Antimicrobial resistance has recently been an issue in global healthcare systems, particularly in hospitals, and can impact anybody, anywhere, at any age. Many infectious disorders caused by fungus, viruses, parasites, and bacteria are at risk because of antimicrobial resistance [57, 58]. In fact, MRSA, K. pneumoniae, E. coli, S. aureus, P. mirabilis, and P. aeruginosa bacterial species can cause very harmful and lethal infectious diseases and have resistance to the most commonly used antibiotics currently [59, 60]. In addition, the most prevalent isolated yeast from bloodstream infections is C. albicans. This infection remains a serious problem in intensive care units all around the world despite impressive advancements in diagnostic and treatment techniques. This fungus species has resistance to the majority of currently utilized antifungal commercial drugs [61].
As a result, the healthcare system has recently turned to the use of phytomedicines to treat microbial diseases. The results of the antimicrobial test demonstrated that the essential oil of O. basilicum had a broad-spectrum antibacterial activity and suppressed the development of all tested microbial strains, in contrast to the antimicrobial medications ampicillin, ciprofloxacin, and fluconazole. The oil showed a range of minimum inhibitory concentrations (MICs) between 1 and 2.3 µg/mL for all tested bacterial strains. Intriguingly, the essential oil suppressed the development of the fungus strain C. albicans far more than the prospective antifungal medication fluconazole, with a MIC of 1.3 µg/ml. These results were consistent with Joshi (2014), who measured the minimal bactericidal concentration against different microbial strains and found that O. basilicum essential oil was active against gram-positive, gram-negative bacteria, and fungi [42]. The majority of essential oils tested for their antibacterial qualities had a more dramatic effect on gram-positive bacteria than other types of bacteria. The resistance of gram-negative bacteria to essential oil has been attributed to their hydrophilic outer membrane, which can prevent hydrophobic chemicals from penetrating the target cell membrane [62]. The presence of phenolic components in the essential oil may contribute to its antibacterial action by triggering intracellular ATP and potassium ion leakage, which results in cell death [63, 64].
Drug resistance against cancer is still a significant barrier in medical oncology. Resistance can develop clinically either before or after cancer treatment. Due to the fact that using natural plant products alone or in combination with chemotherapeutics may reduce resistance or limit the proliferation of cancer cells [65].
The anticancer activity of O. basilicum essential oil was tested against three kinds of cancer cells, namely HeLa, Hep3B, and MCF-7 cancer cells. Cervical cancer tissues were used to get HeLa cells, which are the most frequent kind of cancer in women [66]. This form of cancer can be caused by a variety of factors, including smoking, oral contraceptives, and HPV, which is a sexually transmitted infection [67]. Even though screening, diagnosis, and immunization programs have improved over the last few decades, this kind of cancer is still not under control. Breast cancer, one of the most common cancers in women, is the source of the MCF-7 cell line, which has a high death rate around the globe [68]. One of the most prominent risk factors for this form of cancer is estrogen [69]. Other risk factors, such as a history of the disease in one’s family, may also be significant. Cancer of the liver, or hepatocellular carcinoma, gave rise to the Hep3B cells. Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are the most common causes of hepatocellular cancer. Hepatomas develop as a result of the altered liver matrix caused by these diseases [70].
O. basilicum essential oil showed significant cytotoxic activity against liver cancer (Heb3B) (IC50 = 56.23 ± 1.32 µg/ml) and breast cancer (MCF7) (IC50 = 80.35 ± 1.17 µg/ml) cell lines when compared with doxorubicin (IC50 = 21.37 ± 0.62 and 15.02 ± 0.72 µg/ml respectivly). The obtained results were confirmed by many studies involving a variety of basil extracts and their anticancer effects on different cancer cell lines. Elansary and Mahmoud compared the anticancer activity of several compositions on multiple human cancer lines to see which combination had the highest effect. The O. basilicum species had the most potent anticancer effects among the investigated concentrations and varieties. All basil extracts promoted apoptosis and cell cycle progression, resulting in antiproliferation and cell death [71]. The presence of bioactive substances, such as rosmarinic, chicoric, and caftaric acids, was suggested to be associated with these properties [71, 72].
Conclusion
For the first time, numerous biological investigations of the Palestinian O. basilicum seed essential oil were carried out in this study. The findings demonstrated that it has potent antioxidant, anti-α-glucosidase, and antimicrobial effects against gram-positive and gram-negative bacteria, as well as C. albicans fungus. In addition, it demonstrated possible cytotoxic activity against the cancer cell lines MCF-7 and Hep3B. These results showed that the essential oil of O. basilicum might be a significant source of natural antioxidants that are potentially effective in the detoxification mechanisms of living organisms, particularly against illnesses caused by oxidative stress. Additionally, it might be utilized as a complementary and alternative treatment for infectious disorders caused by pathogens. Consequently, this essential oil’s potential usage as a beneficial novel medicinal agent with functional properties for food, food supplements, and pharmaceutical items should be investigated.
Acknowledgements
The authors would like to acknowledge the Faculty of Medicine and Health Sciences at An-Najah National University.
Authors’ contributions
Ahmad M Eid: Conceptualization, Methodology, Writing?original draft, Writing?review & editing, Supervision, Validation, Formal analysis. The rest of the authors: Writing, Formal analysis.
Funding
None.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The plant is widely accessible, locals utilize it as a traditional medicine, and there are no restrictions on its collection by the government. The plant was identified by a pharmacognosist Dr. Nidal Jaradat at the Pharmacy Department of An-Najah National University. Collected plants were authenticated by Herbal Products Laboratory,
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ganesan K, Xu B. Ethnobotanical studies on folkloric medicinal plants in Nainamalai, Namakkal District, Tamil Nadu, India. Trends in Phytochemical Research. 2017;1(3):153–68. [Google Scholar]
- 2.Jaradat N, Zaid AN. Herbal remedies used for the treatment of infertility in males and females by traditional healers in the rural areas of the West Bank/Palestine. BMC Complement Altern Med. 2019;19(1):1–12. doi: 10.1186/s12906-019-2617-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali-Shtayeh M, Jamous R, Abu Zaitoun S. A comprehensive science-based field assessment of bioactive properties of the native plants of Palestine. J Biodivers Biopros Dev. 2015;2(151):2376–021410001. [Google Scholar]
- 4.Barata AM, Rocha F, Lopes V, Carvalho AM. Conservation and sustainable uses of medicinal and aromatic plants genetic resources on the worldwide for human welfare. Ind Crops Prod. 2016;88:8–11. doi: 10.1016/j.indcrop.2016.02.035. [DOI] [Google Scholar]
- 5.Singh J, Singh J, Sharma S, Dhupper R, Nagar RD. Ethnobotanical uses of medicinal and aromatic plants in Chopal Forest Division of Himachal Pradesh, India. Med Plants-International J Phytomedicines Relat Industries. 2019;11(3):265–78. doi: 10.5958/0975-6892.2019.00035.2. [DOI] [Google Scholar]
- 6.Hussein RA, El-Anssary AA. Plants secondary metabolites: the key drivers of the pharmacological actions of medicinal plants. Herb Med. 2019;1(3):12–30. [Google Scholar]
- 7.Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils–a review. Food Chem Toxicol. 2008;46(2):446–75. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 8.Abelan US, de Oliveira AC, Cacoci ÉSP, Martins TEA, Giacon VM, Velasco MVR. Lima CRRdC: potential use of essential oils in cosmetic and dermatological hair products: a review. J Cosmet Dermatol. 2022;21(4):1407–18. doi: 10.1111/jocd.14286. [DOI] [PubMed] [Google Scholar]
- 9.Naja F, Hamadeh R, Alameddine M. Regulatory frameworks for a safe and effective use of essential oils: a critical appraisal. Adv Biomedical Health Sci. 2022;1(1):7–12. [Google Scholar]
- 10.Sharma R, Rao R, Kumar S, Mahant S, Khatkar S. Therapeutic potential of citronella essential oil: a review. Curr Drug Discov Technol. 2019;16(4):330–9. doi: 10.2174/1570163815666180718095041. [DOI] [PubMed] [Google Scholar]
- 11.Al-Aamri MS, Al-Abousi NM, Al-Jabri SS, Alam T, Khan SA. Chemical composition and in-vitro antioxidant and antimicrobial activity of the essential oil of Citrus aurantifolia L. leaves grown in Eastern Oman. J Taibah Univ Med Sci. 2018;13(2):108–12. doi: 10.1016/j.jtumed.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohammedi H, Mecherara-Idjeri S, Hassani A. Variability in essential oil composition, antioxidant and antimicrobial activities of Ruta montana L. collected from different geographical regions in Algeria. J Essent Oil Res. 2020;32(1):88–101. doi: 10.1080/10412905.2019.1660238. [DOI] [Google Scholar]
- 13.K Jain A, Mehra N K, Swarnakar K. Role of antioxidants for the treatment of cardiovascular diseases: challenges and opportunities. Curr Pharm Design. 2015;21(30):4441–55. doi: 10.2174/1381612821666150803151758. [DOI] [PubMed] [Google Scholar]
- 14.Khosravi M, Poursaleh A, Ghasempour G, Farhad S, Najafi M. The effects of oxidative stress on the development of atherosclerosis. Biol Chem. 2019;400(6):711–32. doi: 10.1515/hsz-2018-0397. [DOI] [PubMed] [Google Scholar]
- 15.Thyagarajan A, Sahu RP. Potential contributions of antioxidants to cancer therapy: immunomodulation and radiosensitization. Integr Cancer Ther. 2018;17(2):210–6. doi: 10.1177/1534735416681639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blüher M. Obesity: global epidemiology and pathogenesis. Nat Reviews Endocrinol. 2019;15(5):288–98. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- 17.Elessi K, Albaraqouni L. Prevalence of obesity and overweight in Palestine: a systematic review. The Lancet. 2019;393:20. doi: 10.1016/S0140-6736(19)30606-3. [DOI] [Google Scholar]
- 18.Nyberg ST, Batty GD, Pentti J, Virtanen M, Alfredsson L, Fransson EI, Goldberg M, Heikkilä K, Jokela M, Knutsson A. Obesity and loss of disease-free years owing to major non-communicable diseases: a multicohort study. The Lancet Public Health. 2018;3(10):e490–7. doi: 10.1016/S2468-2667(18)30139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lovic D, Piperidou A, Zografou I, Grassos H, Pittaras A, Manolis A. The growing epidemic of diabetes mellitus. Curr Vasc Pharmacol. 2020;18(2):104–9. doi: 10.2174/1570161117666190405165911. [DOI] [PubMed] [Google Scholar]
- 20.Abu-Rmeileh NM, Husseini A, Capewell S, O’Flaherty M. Preventing type 2 diabetes among Palestinians: comparing five future policy scenarios. BMJ Open. 2013;3(12):e003558. doi: 10.1136/bmjopen-2013-003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan J, Yuan W, Guo Y, Wu Q, Wang F, Xuan H. Anti-Biofilm Activities of Chinese Poplar Propolis essential oil against Streptococcus mutans. Nutrients. 2022;14(16):3290. doi: 10.3390/nu14163290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spengler G, Gajdács M, Donadu MG, Usai M, Marchetti M, Ferrari M, Mazzarello V, Zanetti S, Nagy F, Kovács R. Evaluation of the antimicrobial and antivirulent potential of essential oils isolated from Juniperus oxycedrus L. ssp. macrocarpa aerial parts. Microorganisms. 2022;10(4):758. doi: 10.3390/microorganisms10040758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossi C, Chaves-López C, Serio A, Casaccia M, Maggio F, Paparella A. Effectiveness and mechanisms of essential oils for biofilm control on food-contact surfaces: an updated review. Crit Rev Food Sci Nutr. 2022;62(8):2172–91. doi: 10.1080/10408398.2020.1851169. [DOI] [PubMed] [Google Scholar]
- 24.Chinemerem Nwobodo D, Ugwu MC, Oliseloke Anie C, Al-Ouqaili MT, Chinedu Ikem J, Victor Chigozie U, Saki M. Antibiotic resistance: the challenges and some emerging strategies for tackling a global menace. J Clin Lab Anal. 2022;36(9):e24655. doi: 10.1002/jcla.24655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Combarros-Fuertes P, Fresno JM, Estevinho MM, Sousa-Pimenta M, Tornadijo ME, Estevinho LM. Honey: another alternative in the fight against antibiotic-resistant bacteria? Antibiotics. 2020;9(11):774. doi: 10.3390/antibiotics9110774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miraj S, Kiani S. Study of pharmacological effect of Ocimum basilicum: a review. Der Pharmacia Lettre. 2016;8(9):276–80. [Google Scholar]
- 27.Antora RA, Salleh RM. Antihyperglycemic effect of Ocimum plants: a short review. Asian Pac J Trop Biomed. 2017;7(8):755–9. doi: 10.1016/j.apjtb.2017.07.010. [DOI] [Google Scholar]
- 28.Ch MA, Naz SB, Sharif A, Akram M, Saeed MA. Biological and pharmacological properties of the sweet basil (Ocimum basilicum) Br J Pharm Res. 2015;7:330–9. doi: 10.9734/BJPR/2015/16505. [DOI] [Google Scholar]
- 29.Güez CM, Souza ROd, Fischer P, Leão MFdM, Duarte JA, Boligon AA, Athayde ML, Zuravski L, Oliveira LFSd, Machado MM. Evaluation of basil extract (Ocimum basilicum L.) on oxidative, anti-genotoxic and anti-inflammatory effects in human leukocytes cell cultures exposed to challenging agents. Brazilian J Pharm Sci 2017, 53.
- 30.Rahayu S, Sirait LI, Aritonang T, Natzir R, Massi MN, Rauf S, Hatta M, Kamelia E. Ocimum Basilicum as Alternative Natural Cancer Care. Int J Sci: Basic Appl Res. 2017;34(3):302–8. [Google Scholar]
- 31.Nazir S, Wani IA. Physicochemical characterization of basil (Ocimum basilicum L.) seeds. J Appl Res Med Aromatic Plants. 2021;22:100295. [Google Scholar]
- 32.Poddar S, Sarkar T, Choudhury S, Chatterjee S, Ghosh P. Indian traditional medicinal plants: a concise review. Int J Bot Stud. 2020;5(5):174–90. [Google Scholar]
- 33.Nazir S, Wani IA, Masoodi FA. Extraction optimization of mucilage from Basil (Ocimum basilicum L.) seeds using response surface methodology. J Adv Res. 2017;8(3):235–44. doi: 10.1016/j.jare.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y, Liu Z, Zhu X, Hu X, Zhang H, Guo Q, Yada RY, Cui SW. Seed coat mucilages: Structural, functional/bioactive properties, and genetic information. Compr Rev Food Sci Food Saf. 2021;20(3):2534–59. doi: 10.1111/1541-4337.12742. [DOI] [PubMed] [Google Scholar]
- 35.Touiss I, Ouahhoud S, Harnafi M, Khatib S, Bekkouch O, Amrani S, Harnafi H. Toxicological Evaluation and Hepatoprotective Efficacy of Rosmarinic Acid-Rich Extract from Ocimum basilicum L. Comprehensive Reviews in Food Science and Food Safety 2021, 2021:10. [DOI] [PMC free article] [PubMed]
- 36.Kadan S, Saad B, Sasson Y, Zaid H. In vitro evaluation of anti-diabetic activity and cytotoxicity of chemically analysed Ocimum basilicum extracts. Food Chem. 2016;196:1066–74. doi: 10.1016/j.foodchem.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 37.Etsassala NG, Hussein AA, Nchu F. Potential application of some lamiaceae species in the management of diabetes. Plants. 2021;10(2):279. doi: 10.3390/plants10020279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pharmacopoeia B. HMSO: London; 1998.
- 39.Eid AM, Hawash M. Biological evaluation of Safrole oil and safrole oil nanoemulgel as antioxidant, antidiabetic, antibacterial, antifungal and anticancer. BMC Complement Med Ther. 2021;21(1):1–12. doi: 10.1186/s12906-021-03324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ali H, Houghton P, Soumyanath A. α-Amylase inhibitory activity of some malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J Ethnopharmacol. 2006;107(3):449–55. doi: 10.1016/j.jep.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Hashim M, Ahmad B, Drouet S, Hano C, Abbasi BH, Anjum S. Comparative effects of different light sources on the production of key secondary metabolites in plants in vitro cultures. Plants. 2021;10(8):1521. doi: 10.3390/plants10081521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joshi RK. Chemical composition and antimicrobial activity of the essential oil of Ocimum basilicum L.(sweet basil) from western ghats of North West Karnataka, India. Anc Sci Life. 2014;33(3):151–6. doi: 10.4103/0257-7941.144618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Güez CM, Souza ROd, Fischer P, Leão MFdM, Duarte JA, Boligon AA, Athayde ML, Zuravski L, Oliveira LFSd, Machado MM. Evaluation of basil extract (Ocimum basilicum L.) on oxidative, anti-genotoxic and anti-inflammatory effects in human leukocytes cell cultures exposed to challenging agents. Brazilian J Pharm Sci. 2017;53(1):5098. [Google Scholar]
- 44.Kaurinovic B, Popovic M, Vlaisavljevic S, Trivic S. Antioxidant capacity of Ocimum basilicum L. and Origanum vulgare L. extracts. Molecules. 2011;16(9):7401–14. doi: 10.3390/molecules16097401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie J, Schaich K. Re-evaluation of the 2, 2-diphenyl-1-picrylhydrazyl free radical (DPPH) assay for antioxidant activity. J Agric Food Chem. 2014;62(19):4251–60. doi: 10.1021/jf500180u. [DOI] [PubMed] [Google Scholar]
- 46.Thirugnanasampandan R, Jayakumar R. Protection of cadmium chloride induced DNA damage by Lamiaceae plants. Asian Pac J Trop Biomed. 2011;1(5):391–4. doi: 10.1016/S2221-1691(11)60086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jebur AB, El-Sayed RA, El-Demerdash FM. Ocimum basilicum essential oil modulates hematotoxicity, oxidative stress, DNA damage, and cell cycle arrest induced by β-cyfluthrin in rat liver. Front Pharmacol. 2021;12:784281. doi: 10.3389/fphar.2021.784281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhupathiraju SN, Hu FB. Epidemiology of obesity and diabetes and their cardiovascular complications. Circul Res. 2016;118(11):1723–35. doi: 10.1161/CIRCRESAHA.115.306825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lega IC, Lipscombe LL. Diabetes, obesity, and cancer—pathophysiology and clinical implications. Endocr Rev. 2020;41(1):33–52. doi: 10.1210/endrev/bnz014. [DOI] [PubMed] [Google Scholar]
- 50.Awuchi CG. The biochemistry, toxicology, and uses of the pharmacologically active phytochemicals: alkaloids, terpenes, polyphenols, and glycosides. J Food Pharm Sci. 2019;7(3):131–50. [Google Scholar]
- 51.Jugran AK, Rawat S, Devkota HP, Bhatt ID, Rawal RS. Diabetes and plant-derived natural products: from ethnopharmacological approaches to their potential for modern drug discovery and development. Phytother Res. 2021;35(1):223–45. doi: 10.1002/ptr.6821. [DOI] [PubMed] [Google Scholar]
- 52.Manach C, Regerat F, Texier O, Agullo G, Demigne C, Remesy C. Bioavailability, metabolism and physiological impact of 4-oxo-flavonoids. Nutr Res. 1996;16(3):517–44. doi: 10.1016/0271-5317(96)00032-2. [DOI] [Google Scholar]
- 53.Venthodika A, Chhikara N, Mann S, Garg MK, Sofi SA, Panghal A. Bioactive compounds of Aegle marmelos L., medicinal values and its food applications: a critical review. Phytother Res. 2021;35(4):1887–907. doi: 10.1002/ptr.6934. [DOI] [PubMed] [Google Scholar]
- 54.Noor ZI, Ahmed D, Rehman HM, Qamar MT, Froeyen M, Ahmad S, Mirza MU. In vitro antidiabetic, anti-obesity and antioxidant analysis of Ocimum basilicum aerial biomass and in silico molecular docking simulations with alpha-amylase and lipase enzymes. Biology. 2019;8(4):92. doi: 10.3390/biology8040092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malapermal V, Botha I, Krishna SBN, Mbatha JN. Enhancing antidiabetic and antimicrobial performance of Ocimum basilicum, and Ocimum sanctum (L.) using silver nanoparticles. Saudi J Biol Sci. 2017;24(6):1294–305. doi: 10.1016/j.sjbs.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Irondi EA, Agboola SO, Oboh G, Boligon AA. Inhibitory effect of leaves extracts of Ocimum basilicum and Ocimum gratissimum on two key enzymes involved in obesity and hypertension in vitro. J Intercultural Ethnopharmacol. 2016;5(4):396–402. doi: 10.5455/jice.20160814112756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hussain AI, Anwar F, Nigam PS, Sarker SD, Moore JE, Rao JR, Mazumdar A. Antibacterial activity of some Lamiaceae essential oils using resazurin as an indicator of cell growth. LWT-Food Sci Technol. 2011;44(4):1199–206. doi: 10.1016/j.lwt.2010.10.005. [DOI] [Google Scholar]
- 58.Chandler CI. Current accounts of antimicrobial resistance: stabilisation, individualisation and antibiotics as infrastructure. Palgrave Commun. 2019;5(1):1–13. doi: 10.1057/s41599-019-0263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bao L, Peng R, Ren X, Ma R, Li J, Wang Y. Analysis of some common pathogens and their drug resistance to antibiotics. Pakistan J Med Sci. 2013;29(1):135. doi: 10.12669/pjms.291.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Oliveira Santos JV, da Costa Júnior SD, de Fátima Ramos dos Santos Medeiros SM, Cavalcanti IDL, de Souza JB, Coriolano DL, da Silva WRC, Alves MHME, Cavalcanti IMF. Panorama of bacterial infections caused by epidemic resistant strains. Curr Microbiol. 2022;79(6):175. doi: 10.1007/s00284-022-02875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Costa-de-Oliveira S, Rodrigues AG. Candida albicans antifungal resistance and tolerance in bloodstream infections: the triad yeast-host-antifungal. Microorganisms. 2020;8(2):154. doi: 10.3390/microorganisms8020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wan J, Wilcock A, Coventry M. The effect of essential oils of basil on the growth of Aeromonas hydrophila and Pseudomonas fluorescens. J Appl Microbiol. 1998;84(2):152–8. doi: 10.1046/j.1365-2672.1998.00338.x. [DOI] [PubMed] [Google Scholar]
- 63.Nazzaro F, Fratianni F, De Martino L, Coppola R, De Feo V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals. 2013;6(12):1451–74. doi: 10.3390/ph6121451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tariq S, Wani S, Rasool W, Shafi K, Bhat MA, Prabhakar A, Shalla AH, Rather MA. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb Pathog. 2019;134:103580. doi: 10.1016/j.micpath.2019.103580. [DOI] [PubMed] [Google Scholar]
- 65.Zahreddine H, Borden KL. Mechanisms and insights into drug resistance in cancer. Front Pharmacol. 2013;4:28. doi: 10.3389/fphar.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vu M, Yu J, Awolude OA, Chuang L. Cervical cancer worldwide. Curr Probl Cancer. 2018;42(5):457–65. doi: 10.1016/j.currproblcancer.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 67.Tsikouras P, Zervoudis S, Manav B, Tomara E, Iatrakis G, Romanidis C, Bothou A, Galazios G. Cervical Cancer: screening, diagnosis and staging. J BUON. 2016;21(2):320–5. [PubMed] [Google Scholar]
- 68.Li J, Guo Y, Duan L, Hu X, Zhang X, Hu J, Huang L, He R, Hu Z, Luo W. AKR1B10 promotes breast cancer cell migration and invasion via activation of ERK signaling. Oncotarget. 2017;8(20):33694–703. doi: 10.18632/oncotarget.16624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tian J, Ran B, Zhang C, Yan D, Li X. Estrogen and progesterone promote breast cancer cell proliferation by inducing cyclin G1 expression. Braz J Med Biol Res. 2018;51(3):1–7. doi: 10.1590/1414-431x20175612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156(2):477–91. doi: 10.1053/j.gastro.2018.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elansary HO, Mahmoud EA. In vitro antioxidant and antiproliferative activities of six international basil cultivars. Nat Prod Res. 2015;29(22):2149–54. doi: 10.1080/14786419.2014.995653. [DOI] [PubMed] [Google Scholar]
- 72.Perna S, Alawadhi H, Riva A, Allegrini P, Petrangolini G, Gasparri C, Alalwan TA, Rondanelli M. In Vitro and in vivo anticancer activity of Basil (Ocimum spp.): current insights and future prospects. Cancers. 2022;14(10):2375. doi: 10.3390/cancers14102375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.