Abstract
Purpose
This is a systematic review and meta(regression) analysis to assess the performance of custom triflange acetabular components (CTAC) in total hip arthroplasty (THA) revision surgery. Implant-related complications, failure rate, functional outcomes and implant and surgical technique-related predictors for outcome were assessed.
Methods
This systematic review was performed according to PRISMA guidelines and registered with PROSPERO (2020 CRD42020209700). PubMed, Embase, Web of Science, COCHRANE Library and Emcare were searched. Studies on Paprosky type 3A and 3B or AAOS type 3 and 4 acetabular defects with a minimum follow-up of 12 months and cohorts > 10 patients were included.
Results
Thirty-three studies were eligible for inclusion (n = 1235 hips, 1218 patients). The methodological quality of the studies was moderate (AQUILA: 7.4/11 points). Considerable heterogeneity was observed in terms of complications, re-operations and implant failure reporting. The total incidence of implant-related complications was 24%. The incidence of re-operation for any reason was 15%, and the implant failure rate was 12% at a mean of 46.9 months and the post-operative Harris Hip Score improved by a mean of 40 points. Several predictors for outcome were found, such as implant generation, follow-up length and study start date.
Conclusions
The use of CTAC in revision THA has satisfactory complication and implant failure rates. The CTAC technique improves post-operative clinical outcomes and the meta-regression analysis showed that there is a clear association between improvements in the CTAC performance and the evolvement of this technique over time.
Keywords: custom triflange acetabular component, total hip arthroplasty revision, large acetabular defects
Introduction
The management of large acetabular defects in total hip arthroplasty (THA) revision surgery is challenging. Large defects with and even without pelvic discontinuity are notoriously difficult to reconstruct. Paprosky type 3A and 3B and AAOS type 3 and 4 defects demonstrate severe bone loss of the acetabular rim and supporting structures, such as the medial wall and the anterior/posterior column with possible pelvic discontinuity (1, 2, 3). Over the past decades, multiple reconstruction techniques have been proposed, including structural allografts, bone impaction grafting, jumbo cups, anti-protrusion cages, trabecular metal augments, cup-cage combinations, oblong cups and custom-made cages (4, 5, 7, 6, 7, 8, 9, 10, 11). Nevertheless, none of these surgical techniques has been proven to be superior. For that matter, custom triflange acetabular components (CTAC) aimed at reconstructing large defects with a mono-block, patient-specific implant which fits into the defect and can also address stabilisation of pelvic discontinuity, have also been proposed. These CTAC implants have evolved over the past years with improvements in rapid prototyping and three-dimensional (3D) printing techniques (12). Improvements have also been made in surgical pre-planning including 3D visualisation of the defects, interaction between surgeon and manufacturer on the design and intra-operative (navigation) tools, as well as the implant characteristics itself. Thus, these developments may have an effect on the outcome of this technique. Several studies have reported on the use of CTAC for hip revision arthroplasty, reporting on small numbers, with large heterogeneity in patient characteristics, outcome measures, complication reporting and end-point definition (e.g. implant survival, failure). This heterogeneity makes valid comparisons between data unreliable (13, 14).
Therefore, our primary aim was to perform a systematic review, meta-analysis and meta-regression analysis on the outcome of CTAC for pelvic reconstructions in THA revision surgery for Paprosky type 3A and 3B and AAOS type 3 and 4 acetabular defects. The main objectives were to assess CTAC performance by means of identifying implant-related complications, implant failure and functional outcomes. The secondary aim was to evaluate the predictors of outcome for CTAC surgical techniques and implant characteristics.
Methods
The reporting of this systematic review is in accordance with the PRISMA 2020 guidelines (15) and was registered under PROSPERO number 2020 CRD42020209700. All studies on THA revision surgery in patients older than 18 years using a CTAC to reconstruct Paprosky type 3A and 3B and AAOS type 3 and 4 acetabular defects, with or without revision of the femoral component, were included. For analysis, inclusion was limited to studies with a minimum follow-up of 12 months and reporting on implant survival or complication rates. Exclusion criteria were as follows: studies on non-custom acetabular implants, reviews or expert opinion articles and case series including less than ten patients. Articless in a language other than English, such as Dutch or German were not considered.
We adopted a search strategy up to 28th of January 2022 using the following bibliographic databases: PubMed, Embase (OVID version), Web of Science, Cochrane Library and Emcare (OVID version). Meeting abstracts were searched in Embase, Web of Science and Cochrane Library. The full search strategy (composed by DB, ZV and JS) for all databases can be found in Appendix 1 (see section on supplementary materials given at the end of this article). Additionally, reference lists of papers included in the systematic review and systematic review reports on similar topics were examined.
Two independent reviewers (ZV and DB) reviewed all titles and abstracts of the retrieved articles. Next, full texts of the selected papers were screened by two independent reviewers (RT and DB) for inclusion, applying the pre-defined in- and exclusion criteria. Disagreement on inclusion was resolved by discussion, and if no consensus was reached, a third independent reviewer was consulted (RN) until consensus was reached.
Two reviewers (RT and DB) individually extracted data from the selected studies into two separate electronic databases (IBM SPSS Statistics, Version 23.0., IBM Corp). Next, both databases were compared and inconsistencies were identified and resolved by re-evaluating the specific studies until consensus was reached. Variables/study-level factors collected in the database included: study title, year of publication, author, study design, number of patients and hips included, study period, patient demographics (age, gender, BMI), follow-up in months, amount of previous surgical revisions on the affected hip, acetabular defect type (Paprosky/AAOS), clinical data (surgical time, time to full weight bare), implant-related complications, re-operations (for any reason), implant failure (defined as partial or full surgical revision of the CTAC or associated THA components; that is, open reduction without component revision for dislocation will not classify as implant failure), functional outcome scores, surgical approach, implant manufacturer, liner type and fixation method into the CTAC, bone interface type (hydroxyapatite, porous, combinations), screw type (locking/non-locking) and CTAC generation. CTAC generation was defined as either old or new. New generation was defined as 3D titanium printed implants, developed with rapid prototyping and/or the availability of patient-specific drill guides and digital or 3D printed bone models to aid the surgical implantation. Old-generation implant was defined as older CTAC manufacturing techniques such as machining or casting without the use of rapid prototyping, also lacking patient-specific drill guides and digital or 3D printed bone models to aid the surgical procedure.
Quality assessment
The methodological quality of all included studies was assessed using the Assessment of Quality In Lower Limb Arthroplasty (AQUILA) checklist, a tool specifically designed to appraise the quality of observational studies concerning total hip and knee arthroplasty (16, 17). Two authors (RT and DB) independently assessed the quality of all included studies using predefined data extraction sheets. Inconsistencies between the two authors were resolved by consensus.
Statistical analysis
The data were combined for the meta-analysis and a random effects model was applied using the metafor package for R version 3.5 (18). Heterogeneity between studies was tested using the I2 statistic, which describes the variation across studies as a result of heterogeneity. Possible sources of heterogeneity were explored using meta-regression: the random-effects regression model, which has previously been used to study the effectiveness of the BCG vaccine against tuberculosis, was employed (19). The random-effects regression model identifies modifying variables that affect the outcome of interest between studies, therefore helping to resolve contradictory outcomes of different studies. To assess publication bias, a funnel plot for studies reporting the primary outcome was constructed. In the case of asymmetry in the funnel plot, a trim-and-fill method and cumulative meta-analysis were used to explore the magnitude and direction of the publication bias.
Results
Literature review
The flow chart of study selection is shown in Fig. 1. The search strategy resulted in a total of 1428 references. One additional study was identified through screening of study reference lists. After removing duplicates, a total of 623 studies remained. Screening of title and abstract identified 140 papers eligible for inclusion. Three meeting abstracts that matched the inclusion criteria were not published and therefore could not be reviewed/included, leaving 137 papers for full-text assessment. In total, 104 studies were excluded for the following reasons: 36 papers were review papers or expert opinions; 27 papers concerned other techniques or the cohort existed of CTAC and standard implants without sufficient individual patient data to extract the CTAC patients; 27 papers included a cohort of less than 10 patients and 13 papers did not meet the language requirements. One paper was excluded after verification with the author that the cohort was included in a more recent published study by the same group (20). A total of 33 papers remained for final analysis, including a total of 1218 patients and 1235 hips. Details of the included studies are summarized in Table 1.
Figure 1.
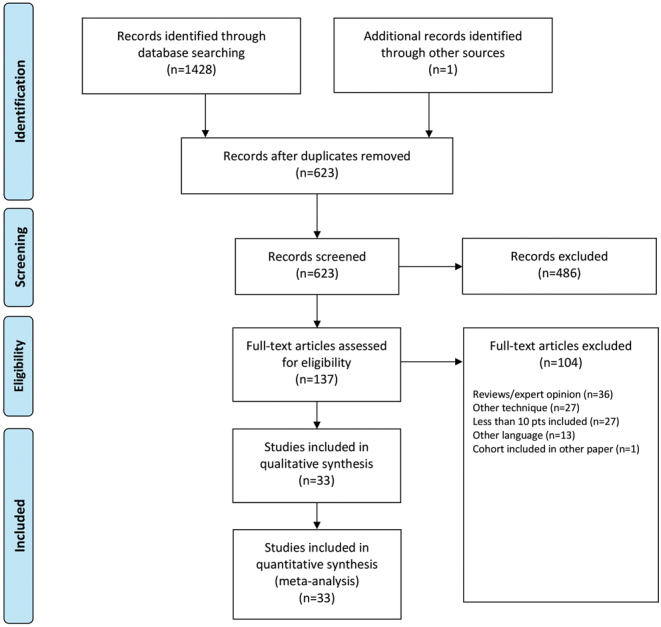
Flow chart for study selection.
Table 1.
Details of included studies.
| Study | Publication year | Study design | Patients (n) | Hips (n) | Mean age (years)* | Male/female | BMI (kg/m²)* | Follow-up (months)* | Year study start | CTAC generation |
|---|---|---|---|---|---|---|---|---|---|---|
| Christie et al. (12) | 2001 | RCS | 76 | 78 | 59 (29–87) | 20/56 | NA | 53 (24–107) | 1992 | Old |
| Joshi et al. (21) | 2002 | RCS | 27 | 27 | 68 (55–77) | 9/18 | NA | 40 (25–56) | 1993 | Old |
| Dennis (22) | 2003 | RCS | 24 | 24 | 68 (44–82) | 7/17 | NA | 48 (24–78) | NA | Old |
| Holt & Dennis (23) | 2004 | RCS | 26 | 26 | 69 (44–82) | 8/18 | NA | 54 (24–85) | NA | Old |
| DeBoer et al. (24) | 2007 | RCS | 18 | 20 | 56 (30–77) | 3/15 | NA | 123 (89–157) | 1992 | Old |
| Taunton et al. (25) | 2012 | RCS | 57 | 57 | 61 (35–81) | 6/51 | 27.0 (21–40) | 76 (24–215) | 1992 | Old |
| Wind et al. (26) | 2013 | RCS | 19 | 19 | 58 (42–79) | 7/12 | NA | 31 (16–59) | 2001 | Old |
| Friedrich et al. (27) | 2014 | RCS | 18 | 18 | 68 (26–79) | 7/11 | NA | 30 (17–62) | 2007 | New |
| Berasi et al. (28) | 2015 | RCS | 23 | 24 | 67 (47–85) | 7/17 | 28.0 (23–39) | 57 (28–108) | 2003 | Old |
| Mao et al. (29) | 2015 | RCS | 22 | 23 | 61 (38–80) | NA | NA | 82 (NA) | 2001 | New |
| Barlow et al. (30) | 2016 | RCS | 63 | 63 | 63 (NA) | NA | 27.0 (NA) | 52 (NA) | NA | New |
| Li et al. (31) | 2016 | RCS | 24 | 24 | 65 (54–79) | 8/16 | NA | 67 (24–120) | 2003 | New |
| Baauw et al. (32) | 2017 | RCS | 12 | 12 | 66 (33–79) | 3/9 | NA | 25 (18–39) | 2011 | New |
| Myncke et al. (33) | 2017 | RCS | 21 | 21 | 67 (50–83) | 6/15 | NA | 25 (NA) | 2009 | New |
| Berend et al. (34) | 2018 | RCS | 94 | 95 | 66 (38–85) | 34/61 | 29.0 (18–51) | 43 (4–128) | 2004 | Old |
| Gladnick et al. (35) | 2018 | RCS | 73 | 73 | 59 (32–83) | 21/52 | 29.6 (30–48) | 90 (60–144) | 2000 | Old |
| Gorianov et al. (20) | 2018 | RCS | 11 | 11 | 72 (48–82) | 5/6 | NA | 20 (6–37) | NA | New |
| Kieser et al. (36) | 2018 | RCS | 20 | 20 | 67 (50–89) | 8/12 | NA | 38 (34–110) | 2007 | New |
| Moore et al. (37) | 2018 | RCS | 35 | 35 | 60 (38–80) | 21/14 | 33.0 (23–42) | Min. 120 (NA) | 2001 | Old |
| Weber et al. (38) | 2018 | RCS | 11 | 11 | 76 (60–85) | 3/8 | 27.6 (24–40) | 21 (6–36) | 2013 | New |
| Jones et al. (39) | 2019 | RCS | 91 | 96 | 62 (31–85) | 22/74 | 27.0 (NA) | 25 (0–116) | 2004 | Old |
| Burastero et al. (40) | 2020 | RCS | 19 | 20 | 60 (NA) | 11/8 | 25.1 (NA) | 42 (NA) | 2014 | New |
| Durand-Hill et al. (41) | 2020 | RCS | 20 | 20 | 66 (NA) | 7/13 | 28.0 (NA) | 26 (12–40) | 2016 | New |
| Fröschen et al. (42) | 2020 | RCS | 68 | 68 | 70 (27–89) | NA | 27.3 (19–39) | 43 (2–120) | 2008 | Unknown |
| Matar et al. (43) | 2020 | RCS | 17 | 17 | 72 (61–83) | 3/14 | NA | 43 (24–84) | 2013 | Combination |
| Walter et al. (44) | 2020 | RCS | 54 | 58 | 70 (NA) | 14/44 | 27.3 (NA) | 56 (24–120) | 2008 | Unknown |
| Zampelis & Flivik (45) | 2020 | PCS | 10 | 10 | 64 (36–87) | 5/5 | 26.0 (19–34) | 12 (NA) | 2014 | New |
| Fröschen et al. (46) | 2021 | RCS | 70 | 70 | 70 (27–89) | 19/51 | 27.4 (19–27) | 42 (1–120) | 2008 | Combination |
| Goriainov et al. (47) | 2021 | RCS | 19 | 19 | 77 (52–91) | 8/11 | NA | 53 (17–88) | 2014 | New |
| Kawalkar et al. (48) | 2021 | RCS | 13 | 13 | 69 (57–86) | 5/8 | 27 (NA) | 50 (18–70) | 2011 | New |
| Scharff-Baauw et al. (49) | 2021 | PCS | 49 | 49 | 68 (41–89) | 8/41 | 27.0 (19–44) | 24 (NA) | 2013 | New |
| Sershon et al. (50) | 2021 | RCS | 50 | 50 | 60 (33–83) | 16/34 | 28.4 (17–48) | NA | 2000 | Unknown |
| von Hertzberg-Boelch et al. (51) | 2021 | RCS | 14 | 14 | 70 (55–83) | 5/9 | 28 (25–31) | 35 (14–94) | 2010 | New |
| Tikhilov et al. (52) | 2022 | RC | 61 | 61 | 57 (NA) | 9/52 | 28.1 (15–44) | 37 (12–59) | 2013 | New |
| Total | 1218 | 1235 | 65.4 | 29/71% | 27.8 |
*Values in parentheses are range.
Min., minimum; NA, not available; PCS, prospective case series; RC, retrospective cohort; RCS, retrospective case series.
Quality assessment
The mean AQUILA methodological quality score was 7.4 points, ranging from 4 to 10 points (out of a maximum of 11 points). The majority of methodological flaws were related to the follow-up period (AQULA item 4): in 8 of 33 studies the follow-up was predefined; in 22 of 33 studies the follow-up was performed when patients had complaints or a chart review (of non-predefined follow-up) and in 3 of 33 studies it was unclear how the follow-up was performed. Methodological quality related to follow-up (AQUILA item 4) was not an effect modifier. Table 2 shows the methodological scores for each item.
Table 2.
Overview of AQUILA study methodological scores.
| AQUILA methodological quality item | Number of studies |
|---|---|
| 1. Is there a clear primary research question/hypothesis? | Yes: 25 of 33 |
| 2. How were the cohorts constructed? | |
| A: Consecutively | 21 of 33 |
| B: Non-consecutively | 5 of 33 |
| C: Unknown | 7 of 33 |
| 3. How adequate was the follow-up? | |
| A: Fully completed FU | 21 of 33 |
| B: ≤5% lost-to-FU or FU quotient is ≤1 | 6 of 33 |
| C: >5% lost-to-FU or FU quotient is >1 | 6 of 33 |
| D: Unknown | 0 of 33 |
| 4. How was the follow-up performed? | |
| A: Predefined (e.g. yearly) | 8 of 33 |
| B: When the patient had complaints or chart review (of non-predefined FU) | 22 of 33 |
| C: Unknown | 3 of 33 |
| 5. How many arthroplasties are at risk at the FU of interest? | |
| A: ≥20 | 20 of 33 |
| B: <20 | 13 of 33 |
| C: Unknown | 0 of 33 |
| 6. Has a worst-case analysis or competing risk analysis for competing endpoints been performed? | Yes: 0 of 33 |
Surgical details, implant characteristics and CTAC generation
Sixteen of 33 studies reported the number of prior THA revisions before CTAC usage. The mean prior revision amount was 2.8 (s.d. 1.3). The surgical approach was described in the majority of the selected studies (30 of 33). The posterolateral approach was used in the majority of patients (22 studies, 720 patients, 731 hips). Others described a mix of approaches used (six studies). The direct lateral and triradiate-transtrochantaric approaches were used in one study each. Different fixation techniques for the THA liner into the CTAC were described. The liner was cemented into the CTAC in 390 hips (15 studies, 386 patients) and the liner attached/combined into the CTAC in an uncemented fashion in 468 hips (11 studies, 461 patients). The type of liner fixation into the CTAC was not reported in five studies. The majority of studies (17 studies, 772 patients, 782 hips) reported a mix of standard, bipolar or constrained liners used in their cohorts. Constrained liners were mainly applied if the revision of the liner in the CTAC was needed due to THA instability. Six studies (119 patients, 120 hips) reported the exclusive use of standard liners. Only four studies reported the sole use of bipolar liners (61 patients, 62 hips) and six studies (266 patients, 271 hips) did not report liner type. In most cases, non-locking screws were used for CTAC primary fixation (18 studies, 514 patients, 519 hips), whereas a combination of locking and non-locking screws were used in 381 hips (6 studies, 374 patients). Screw type was unknown in nine studies (330 patients, 335 hips). The CTAC had porous bone interfaces in 383 hips (13 studies, 382 patients); hydroxyapatite coatings in 194 hips (3 studies, 189 patients) and a combination of porous surfaces and hydroxyapatite coating in 347 hips (8 studies, 340 patients). Nine studies did not report bone interface type (307 patients, 311 hips). In 448 hips (16 studies, 442 patients), 3D metal printing was used to manufacture the CTAC, whereas in 491 hips (12 studies, 485 patients) the CTAC was machined. After applying the criteria for old and new generation as stated earlier, 574 hips (12 studies, 563 patients) were classified as old-generation CTAC and 398 hips (16 studies, 396 patients) were classified as new-generation CTAC. Due to insufficient or missing information on implant/technique characteristics in several papers, it was not possible to classify implant generation (old or new) for 263 hips (5 studies, 259 patients).
Complications
All complications extracted from the included studies are summarised in Table 3. Meta-analysis on 31 studies including 1069 hips showed a total incidence of implant-related complications of 24% (95% CI: 18–29%; I2 of 81%), indicating considerable heterogeneity. Two studies did not report on complications. THA dislocation was the most frequently reported implant-related complication: the meta-analysis on 30 studies, comprising 1006 hips, showed an incidence of hip dislocation of 6.6% (95% CI: 4.7–8.6%; I2 of 39%). The meta-regression did not indicate any study-level factors, such as liner type or liner fixation method, CTAC generation or surgical approach associated with the incidence of dislocation. Results of the meta-regression indicated that CTAC generation did have an association with the incidence of total implant-related complications: 29% (95% CI: 21–37%) complications for the old generation compared to 16% (95% CI: 9.2–22%) for the new-generation CTAC. Meta-regression did not identify any other study-level factors that influenced complication rates.
Table 3.
Summary of implant-related complications, re-operations and failure rates.
| Number of | Incidence | 95% CI | ||
|---|---|---|---|---|
| Studies | Hips | |||
| Implant-related complications | ||||
| Prosthetic joint infection | 31 | 1069 | 4.9% | 3.3–6.5% |
| Dislocation | 30 | 1006 | 6.6% | 4.7–8.6% |
| Nerve lesion | 21 | 743 | 2.0% | 0.9–3.1% |
| Screw failure | 23 | 714 | 2.1% | 1.0–3.1% |
| Aseptic loosening | 24 | 810 | 2.2% | 1.3–3.3% |
| Periprosthetic fracture | 27 | 902 | 2.5% | 1.5–3.5% |
| Total implant-related complications | 31 | 1069 | 24% | 18–29% |
| Re-operations | ||||
| Prosthetic joint infection | 32 | 1157 | 5.3% | 3.6–7.1% |
| Dislocation | 31 | 1148 | 2.9% | 1.8–4.0% |
| Screw failure | 25 | 877 | 1.1% | 0.4–1.8% |
| Aseptic loosening | 26 | 976 | 2.5% | 1.5–3.4% |
| Periprosthetic fracture | 25 | 914 | 1.4% | 0.7–2.2% |
| Total re-operations | 33 | 1235 | 15% | 10–19% |
| Total CTAC failures | 33 | 1235 | 12% | 8.2–16% |
Re-operations and implant failure
The meta-analysis of 33 studies including 1235 hips found an incidence of re-operation for any reason of 15% (95% CI 10–19%; I2 of 85%) at a mean follow-up of 46.9 months (range 12–123 months). The meta-analysis found a large heterogeneity between studies. All reasons for re-operations extracted from the included studies are summarised in Table 3. The results of the meta-regression indicated that the mean follow-up (months) and study start date (i.e. the time frame during which the implants were probably introduced) were associated with the incidence of re-operation for any reason. For every month increase in mean study follow-up time, the incidence of re-operation for any reason increased by 0.24% (95% CI 0.04–0.44%). The incidence of implant failure decreased by 0.64% (95% CI 0.1–1.3%) each subsequent year after 1992 (the start date of the first study that was included in this meta-analysis). There were no other study-level factors associated with re-operation for any reason, such as bone interface type, mean number of prior revisions, implant manufacturing process, CTAC generation, screw type, study publication year or methodological quality.
The meta-analysis on CTAC failure included 33 studies and 1235 hips and showed a mean implant failure rate of 12% (95% CI 8.2–16%; I2 of 83%) at a mean follow-up of 46.9 months (range 12–123 months). The results of the meta-regression indicated that follow-up time, number of prior revisions, CTAC generation and start year of the study were associated with the incidence of implant failure. For every month increase in mean follow-up time, the incidence of failure increased by 0.2% (95% CI 0.03–0.4%). For every additional prior THA revision, the CTAC failure rate increased by 4.2% (95% CI 1.0% to 7.5%). Regarding the evolution of the CTAC technique, the year in which the study started was positively associated with implant survival: implant failure rate decreased by 0.6% (95% CI: 0.04–1.1%) for each year the study started after 1992. Regarding CTAC generation, implant failure rates were higher for the old generation; 16% (95% CI: 10–21%), compared to 6.4% (95% CI: 1.6–11%) for the new generation. This result remained similar after correcting for study follow-up time. There were no other study-level factors associated with a change in the incidence of implant failure, such as bone interface type, mean number of prior revisions, implant manufacturing process, screw type, liner type or liner fixation method, study publication year or methodological quality.
Functional outcomes
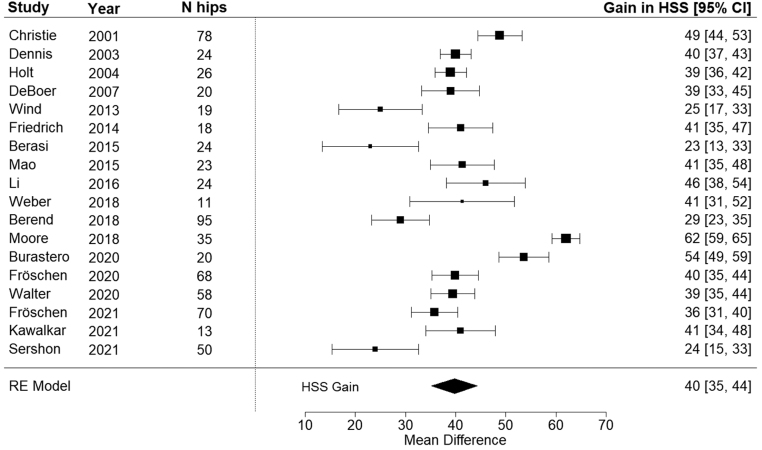
The majority of the included studies reported on clinical outcome scores assessed by the physicians (29 of 33 studies). Harris hip score (HHS) was the most frequently used outcome measure (21 of 33 studies). Seven studies reported on the Oxford hip score (OHS) as a patient outcome measure. For 676 hips (18 studies, 664 patients) the pre- and post- operative HHS were available. A mean 40 points (95% CI: 35–44 points; I2 of 93%) increase in the post-operative HHS was found (Fig. 2). Meta-regression indicated that bone interface type influenced the post-operative gain in HSS, showing favourable HHS results for hydroxyapatite-coated implants over porous surfaced implants or implants with combinations of porous surfaces and hydroxyapatite coatings. For hydroxyapatite coated implants, the post-operative HHS increased by 62 points (95% CI: 48–76 points), compared to a 44-point (95% CI: 38–51 points) increase for porous coated implants and a 38-point (95% CI: 32–44 points) increase for implants with porous and hydroxyapatite coatings.
Figure 2.
Forrest plot of studies analysing gained post-operative HHS. The black diamond shows overall effect size.
As for the surgical approach, the posterolateral approach resulted in a mean HHS improvement of 44 points (95% CI: 39–49 points), compared to a mean increase in HHS of 37 points (95% CI: 29–46 points) for the studies reporting various surgical approaches. The use of only the direct lateral approach was reported in one study, which showed a mean increase of HHS of 23 points (95% CI: 4–42 points). Age, follow-up time, mean number of prior revisions, implant manufacturing process, screw type, liner type and fixation method or CTAC generation were not associated with the post-operative HSS.
Discussion
Our meta-analysis showed an overall CTAC implant-related complication rate of 24% in reconstructing large acetabular defects (Paprosky type 3A and 3B and AAOS type 3 and 4 acetabular defects). Dislocation and prosthetic joint infection were the most frequent reported complications. The new CTAC generation of implants was associated with a lower overall implant-related complication rate (16%) compared to the old generation (29%). This difference remained, also after correction for possible modifiers such as follow-up time. The latter stresses the important positive evolution over time of this complex acetabular revision technique with CTAC. We could not identify any other study-level predictors (such as CTAC generation, liner type, surgical approach, surgical time, number of prior revisions) that influenced the rates of dislocation, prosthetic joint infection or any other type of implant-related complication. Furthermore, our analysis shows that at a mean follow-up of nearly 4 years, the overall CTAC implant failure rate was 12%. Interestingly, implant failure rates decrease as the start date of the study increases, and the incidence of CTAC failure is higher for older generation implants (16%) as compared to new-generation CTACs (6.4%). We were unable to identify any other associations between study-level factors and the incidence of implant failure. Finally, the result of our meta-analysis showed that the CTAC technique resulted in a clinically relevant increase in post-operative functional outcomes, with an overall HHS gain of 40 points, although with large variability between studies. No relation between HHS and study-level factors was found, apart from bone interface type; for hydroxyapatite-coated implants, the post-operative HHS increased the most, by 62 points (95% CI: 48–76 points).
We are not aware of any other meta-analysis performed on this specific subject on extensive acetabular defect reconstruction. Two systematic reviews focussing on CTAC for THA revision in the presence of Paprosky 3A, 3B, AAOS III or IV defects are available in the literature (13, 14). However, these systematic reviews lack appropriate meta-analytic techniques, do not take into account the between-study variation and have not evaluated sources of possible heterogeneity. The systematic review by de Martino et al. (13) published in 2019, reports on 17 papers including 579 hips. They report a 17.3% overall re-operation rate, which is in line with our study results (15%). The overall complication rate of 29% reported in their study is higher compared to our analysis (24%). CTAC revision-free survival reported in this study was 82.7% at a mean follow-up of 57 months. It was unclear, however, how the authors classified implant failure. We classified implant failure as full or partial hip revision and have found a 12% failure rate, resulting in an 88% survival rate at 47 months. Martino et al. present a mean gain in post-operative HHS of 38.6 points, based on eight studies (293 hips), which is consistent with our results that show a mean of 40 points gained based on 676 hips. A systematic review by Chiarlone et al. (14) published in 2020, including 18 papers and 634 hips, is very similar to the review published by de Martino et al., with almost identical included papers (15/18) and comparable study results. In contrast to our study, both the abovementioned reviews lack a meta-analysis and the presented outcomes of pooled data do not account for between-study variation (heterogeneity), nor do they try to explain it. In the period after these reviews, a significant additional amount of studies have been published which we were able to include in our systematic review, to a total of 33 papers and 1235 hips.
Our results revealed substantial between-study variation, indicating that it is important to use the appropriate meta-analytic and meta-regression techniques. We found that the following study-level factors were associated with the study’s primary and secondary outcomes: CTAC generation, study start date, follow-up time and bone interface type.
The new CTAC generation was associated with a lower overall implant-related complication rate compared to the old generation. It is possible that improved patient selection, increased experience and improved techniques in general (surgical tools, implant designs, 3D printing, etc.) could (partially) explain this improvement, but the available data did not allow to explore these factors in more detail. CTAC generation was also associated with implant failure, favouring the new-generation implants. Since studies with new-generation implants were published more recently and had a shorter follow-up, a correction for the duration of follow-up was performed and the results remained similar with a remarkable lower implant failure rates for new-generation implants as compared to the old-generation implants. Therefore, based on our data, we observed that CTAC technique performance (i.e. lower failure rates) has improved over time.
We observed an association between the type of bone interface and improved functional outcomes, favouring hydroxyapatite-coated implants over porous surfaced implants and implants combining porous and hydroxyapatite interfaces. Nevertheless, care must be taken to interpret these results, as this association does not imply causality. Data on bone interface type supplied in the included studies were heterogeneous and often lacked specifics on details such as total contact area, HA/porous ratios, pore geometry, pore diameter or porosity percentage. Also, new-generation CTACs often incorporate highly porous 3D printed titanium interfaces, which could be seen as a completely separate bone interface entity, but this could not be differentiated in our analysis. Other unidentified factors may have played a role in the superior clinical outcome of hydroxyapatite-coated CTAC. Hydroxyapatite promotes osteointegration, and it might be that hydroxyapatite produces a more stable implant, resulting in better functional outcomes, as has been shown in primary THA literature (53, 54, 55). Nevertheless, the latter is also seen in early and mid-term results of the latest generation non-custom highly porous 3D printed titanium acetabular components used for primary and revision THA (56, 57).
We should consider some limitations. First, most papers in this analysis are observational studies with a high between-study heterogeneity and a moderate level of methodologic quality. Identified associations between outcomes and factors such as CTAC generation, study start date and bone interface type that were found in our meta-regression analysis are based on study-level associations and do not imply association on a patient level. Secondly, many studies lacked detailed information on CTAC designs, manufacturing processes, surgical techniques or peri-operative tools such as patient-specific instruments. Therefore, for several studies, only a distinction between an old- and new-generation CTAC could be made based on general or indirect implant characteristic information based on, implant manufacturer.
Based on this study, several recommendations for future research can be made. In general, it is important to provide detailed CTAC specifications and surgical techniques in future studies in order to perform more valid comparisons. Secondly, considering the innovation phase of the CTAC technique as such, it is important to evaluate implant safety. One of the areas of potential safety concern for custom acetabular components would be long-term implant fixation/osseointegration. Since no long-term data on the fixation of any new type of implant can be available, radiostereometric analysis (RSA) can be of value for CTAC implant evaluation. RSA can be used as an adjunct to identify early migration patterns in order to predict long-term implant fixation and survival, as demonstrated before in primary total hip arthroplasty RSA studies (58, 59). Lastly, the custom shape of a CTAC necessitates precise implant positioning for biomechanical hip/pelvis reconstruction as well as an adequate implant-to-bone apposition and screw placement. Intra-operative difficulties can arise when the pre-operative plan and the custom implant shape do not match the peri-operative situation. Nevertheless, positioning accuracy studies on new-generation CTAC do demonstrate very high levels of accuracy between the pre-operative plan and the achieved position in regards to cup anteversion, cup inclination, implant rotation and hip centre of rotation restoration (38, 41, 45, 60, 61). As a sequelae to implant positioning analysis, studying the usage of intra-operative navigation can be beneficial to assess if navigation could improve implant positioning and thereby implant performance.
The results of this study are of value for orthopaedic hip surgeons in clinical decision-making and in informing patients. Identifying which aspects of this technique, and if changes to this technique seen over the past decade, have resulted in improvements in CTAC performance and patients’ functional outcomes is of importance for clinicians and implant manufacturers, seeking to use and/or improve the CTAC technique.
Conclusion
The use of CTAC in revision THA for large peri-acetabular bone defects demonstrates satisfactory complication and implant failure rates and the CTAC technique improves post-operative clinical outcomes. Our meta-regression analysis showed that there is a clear association between improvements in CTAC performance (such as lower complication and implant failure rates) and the evolution of this technique over time. Nevertheless, it is still unclear which part of the technique evolution contributed most to this improvement. The quality of evidence of the included studies was moderate and further research is important, either to assess long-term outcomes and in understanding which innovations have, and will lead to further optimization of this technique, for the better of patient outcomes.
Supplementary Materials
ICMJE conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding statement
This work did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- 1.Paprosky WG Perona PG & Lawrence JM. Acetabular defect classification and surgical reconstruction in revision arthroplasty. A 6-year follow-up evaluation. Journal of Arthroplasty 1994933–44. ( 10.1016/0883-5403(9490135-x) [DOI] [PubMed] [Google Scholar]
- 2.D’antonio JA Capello WN Borden LS Bargar WL Bierbaum BF Boettcher WG Steinberg ME Stulberg SD & Wedge JH. Classification and management of acetabular abnormalities in total hip arthroplasty. Clinical Orthopaedics and Related Research 1989243126–137. ( 10.1097/00003086-198906000-00019) [DOI] [PubMed] [Google Scholar]
- 3.Telleria JJM & Gee AO. Classifications in brief: Paprosky classification of acetabular bone loss. Clinical Orthopaedics and Related Research 20134713725–3730. ( 10.1007/s11999-013-3264-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baauw M Hooff van ML & Spruit M. Current construct options for revision of large acetabular defects: A systematic review. JBJS Reviews 20164. ( 10.2106/JBJS.RVW.15.00119) [DOI] [PubMed] [Google Scholar]
- 5.Blumenfeld TJ. Implant choices, technique, and results in revision acetabular surgery: a review. Hip International 201222235–247. ( 10.5301/HIP.2012.9281) [DOI] [PubMed] [Google Scholar]
- 6.Chen AF & Hozack WJ. Component selection in revision total hip arthroplasty. Orthopedic Clinics of North America 201445275–286. ( 10.1016/j.ocl.2014.03.001) [DOI] [PubMed] [Google Scholar]
- 7.Issack PS Nousiainen M Beksac B Helfet DL Sculco TP & Buly RL. Acetabular component revision in total hip arthroplasty. Part II: management of major bone loss and pelvic discontinuity. American Journal of Orthopedics 200938550–556. [PubMed] [Google Scholar]
- 8.Jain S Grogan RJ & Giannoudis PV. Options for managing severe acetabular bone loss in revision hip arthroplasty. A systematic review. Hip International 201424109–122. ( 10.5301/hipint.5000101) [DOI] [PubMed] [Google Scholar]
- 9.Sheth NP Nelson CL Springer BD Fehring TK & Paprosky WG. Acetabular bone loss in revision total hip arthroplasty: Evaluation and management. Journal of the American Academy of Orthopaedic Surgeons 201321128–139. ( 10.5435/JAAOS-21-03-128) [DOI] [PubMed] [Google Scholar]
- 10.Villanueva M Rios-Luna A Pereiro De Lamo J Fahandez-Saddi H & Böstrom MPG. A review of the treatment of pelvic discontinuity. HSS Journal 20084128–137. ( 10.1007/s11420-008-9075-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volpin A Konan S Biz C Tansey RJ & Haddad FS. Reconstruction of failed acetabular component in the presence of severe acetabular bone loss: a systematic review. Musculoskeletal Surgery 20191031–13. ( 10.1007/s12306-018-0539-7) [DOI] [PubMed] [Google Scholar]
- 12.Christie MJ Barrington SA Brinson MF Ruhling ME & DeBoer DK. Bridging massive acetabular defects with the triflange cup: 2- to 9-year results. Clinical Orthopaedics and Related Research 2001393216–227. ( 10.1097/00003086-200112000-00024) [DOI] [PubMed] [Google Scholar]
- 13.Martino ID Strigelli V Cacciola G Gu A Bostrom MP & Sculco PK. Survivorship and clinical outcomes of custom triflange acetabular components in revision total hip arthroplasty: a systematic review. Journal of Arthroplasty 2019342, 51–2. ( 10.1016/j.arth.2019.05.032) [DOI] [PubMed] [Google Scholar]
- 14.Chiarlone F Zanirato A Cavagnaro L Alessio-Mazzola M Felli L & Burastero G. Acetabular custom-made implants for severe acetabular bone defect in revision total hip arthroplasty: a systematic review of the literature. Archives of Orthopaedic and Trauma Surgery 2020140415–424. ( 10.1007/s00402-020-03334-5) [DOI] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ Publishing Group British Medical Journal Publishing Group 2021372 n71. ( 10.1136/bmj.n71) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pijls BG Dekkers OM Middeldorp S Valstar ER Heide van der HJ Van der Linden-Van der Zwaag HM & Nelissen RG. Aquila: assessment of quality in lower limb arthroplasty. An expert Delphi consensus for total knee and total hip arthroplasty. BMC Musculoskeletal Disorders 201112 173. ( 10.1186/1471-2474-12-173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cowan JB Mlynarek RA Nelissen RGHH Pijls BGCW & Gagnier JJ. Evaluation of quality of lower limb arthroplasty observational studies using the assessment of quality in lower limb arthroplasty (Aquila) checklist. Journal of Arthroplasty 2015301513–1517. ( 10.1016/j.arth.2015.03.020) [DOI] [PubMed] [Google Scholar]
- 18.Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. Journal of Statistical Software 2010361–48. ( 10.18637/jss.v036.i03) [DOI] [Google Scholar]
- 19.Colditz GA Brewer TF Berkey CS Wilson ME Burdick E Fineberg HV & Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 1994271698–702. ( 10.1001/jama.1994.03510330076038) [DOI] [PubMed] [Google Scholar]
- 20.Goriainov V McEwan JK Oreffo RO & Dunlop DG. Application of 3D-printed patient-specific skeletal implants augmented with autologous skeletal stem cells. Regenerative Medicine 201813283–294. ( 10.2217/rme-2017-0127) [DOI] [PubMed] [Google Scholar]
- 21.Joshi AB Lee J & Christensen C. Results for a custom acetabular component for acetabular deficiency. Journal of Arthroplasty 200217643–648. ( 10.1054/arth.2002.32106) [DOI] [PubMed] [Google Scholar]
- 22.Dennis DA. Management of massive acetabular defects in revision total hip arthroplasty. Journal of Arthroplasty 200318(3) 121–125. ( 10.1054/arth.2003.50105) [DOI] [PubMed] [Google Scholar]
- 23.Holt GE & Dennis DA. Use of custom triflanged acetabular components. Clinical Orthopaedics 2004429209–214. ( 10.1097/01.blo.0000150252.19780.74) [DOI] [PubMed] [Google Scholar]
- 24.DeBoer DK Christie MJ Brinson MF & Morrison JC. Revision total hip arthroplasty for pelvic discontinuity. Journal of Bone and Joint Surgery. American Volume 200789835–840. ( 10.2106/JBJS.F.00313) [DOI] [PubMed] [Google Scholar]
- 25.Taunton MJ Fehring TK Edwards P Bernasek T Holt GE & Christie MJ. Pelvic discontinuity treated with custom triflange component: a reliable option. Clinical Orthopaedics and Related Research 2012470428–434. ( 10.1007/s11999-011-2126-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wind MA Swank ML & Sorger JI. Short-term results of a custom triflange acetabular component for massive acetabular bone loss in revision THA. Orthopedics 201336e260–e265. ( 10.3928/01477447-20130222-11) [DOI] [PubMed] [Google Scholar]
- 27.Friedrich MJ Schmolders J Michel RD Randau TM Wimmer MD Kohlhof H Wirtz DC & Gravius S. Management of severe periacetabular bone loss combined with pelvic discontinuity in revision hip arthroplasty. International Orthopaedics 2014382455–2461. ( 10.1007/s00264-014-2443-6) [DOI] [PubMed] [Google Scholar]
- 28.Berasi CC Berend KR Adams JB Ruh EL & Lombardi AV. Are custom triflange acetabular components effective for reconstruction of catastrophic bone loss? Clinical Orthopaedics and Related Research 2015473528–535. ( 10.1007/s11999-014-3969-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao Y Xu C Xu J Li H Liu F Yu D & Zhu Z. The use of customized cages in revision total hip arthroplasty for Paprosky type III acetabular bone defects. International Orthopaedics 2015392023–2030. ( 10.1007/s00264-015-2965-6) [DOI] [PubMed] [Google Scholar]
- 30.Barlow BT Oi KK Lee YY Carli AV Choi DS & Bostrom MP. Outcomes of custom flange acetabular components in Revision Total Hip Arthroplasty and Predictors of Failure. Journal of Arthroplasty 2016311057–1064. ( 10.1016/j.arth.2015.11.016) [DOI] [PubMed] [Google Scholar]
- 31.Li H Qu X Mao Y Dai K & Zhu Z. Custom acetabular cages offer stable fixation and improved hip scores for revision THA with severe bone defects. Clinical Orthopaedics and Related Research 2016474731–740. ( 10.1007/s11999-015-4587-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baauw M Hellemondt van GG & Spruit M. A custom-made acetabular implant for Paprosky Type 3 defects. Orthopedics 201740e195–e198. ( 10.3928/01477447-20160902-01) [DOI] [PubMed] [Google Scholar]
- 33.Myncke I Schaik D & Scheerlinck T. Custom-made triflanged acetabular components in the treatment of major acetabular defects. Short-Term results and clinical experience. Acta Orthopaedica Belgica 201783341–350. [PubMed] [Google Scholar]
- 34.Berend ME Berend KR Lombardi AV Cates H & Faris P. The patient-specific Triflange acetabular implant for revision total hip arthroplasty in patients with severe acetabular defects: planning, implantation, and results. Bone and Joint Journal 2018100–B(Supplement A) 50–54. ( 10.1302/0301-620X.100B1.BJJ-2017-0362.R1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gladnick BP Fehring KA Odum SM Christie MJ DeBoer DK & Fehring TK. Midterm survivorship after revision total hip arthroplasty with a custom triflange acetabular component. Journal of Arthroplasty 201833500–504. ( 10.1016/j.arth.2017.09.026) [DOI] [PubMed] [Google Scholar]
- 36.Kieser DC Ailabouni R Kieser SCJ Wyatt MC Armour PC Coates MH & Hooper GJ. The use of an ossis custom 3D-printed tri-flanged acetabular implant for major bone loss: minimum 2-year follow-up. Hip International 201828668–674. ( 10.1177/1120700018760817) [DOI] [PubMed] [Google Scholar]
- 37.Moore KD McClenny MD & Wills BW. Custom triflange acetabular components for large acetabular defects: minimum 10-year follow-up. Orthopedics 201841e316–e320. ( 10.3928/01477447-20180213-11) [DOI] [PubMed] [Google Scholar]
- 38.Weber M Witzmann L Wieding J Grifka J Renkawitz T & Craiovan B. Customized implants for acetabular Paprosky III defects may be positioned with high accuracy in revision hip arthroplasty. International Orthopaedics 2019432235–2243. ( 10.1007/s00264-018-4193-3) [DOI] [PubMed] [Google Scholar]
- 39.Jones CW Choi DS Sun P Chiu YF Lipman JD Lyman S Bostrom MPG & Sculco PK. Clinical and design factors influence the survivorship of custom flange acetabular components. Bone and Joint Journal 2019101–B68–76. ( 10.1302/0301-620X.101B6.BJJ-2018-1455.R1) [DOI] [PubMed] [Google Scholar]
- 40.Burastero G Pianigiani S Zanvettor C Cavagnaro L Chiarlone F & Innocenti B. Use of porous custom-made cones for meta-diaphyseal bone defects reconstruction in knee revision surgery: a clinical and biomechanical analysis. Archives of Orthopaedic and Trauma Surgery 20201402041–2055. ( 10.1007/s00402-020-03670-6) [DOI] [PubMed] [Google Scholar]
- 41.Durand-Hill M Henckel J Di Laura A & Hart AJ. Can custom 3D printed implants successfully reconstruct massive acetabular defects? A 3D-CT assessment. Journal of Orthopaedic Research 2020382640–2648. ( 10.1002/jor.24752) [DOI] [PubMed] [Google Scholar]
- 42.Fröschen FS Randau TM Hischebeth GTR Gravius N Wirtz DC Gravius S & Walter SG. Outcome of repeated multi-stage arthroplasty with custom-made acetabular implants in patients with severe acetabular bone loss: a case series. Hip International 202030(1_suppl) 64–71. ( 10.1177/1120700020928247) [DOI] [PubMed] [Google Scholar]
- 43.Matar HE Selvaratnam V Shah N & Wynn Jones H. Custom triflange revision acetabular components for significant bone defects and pelvic discontinuity: early UK experience. Journal of Orthopaedics 20202125–30. ( 10.1016/j.jor.2020.01.053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walter SG Randau TM Gravius N Gravius S & Fröschen FS. Monoflanged custom-made acetabular components promote biomechanical restoration of severe acetabular bone defects by metallic defect reconstruction. Journal of Arthroplasty 202035831–835. ( 10.1016/j.arth.2019.10.040) [DOI] [PubMed] [Google Scholar]
- 45.Zampelis V & Flivik G. Custom-made 3D-printed cup-cage implants for complex acetabular revisions: evaluation of pre-planned versus achieved positioning and 1-year migration data in 10 patients. Acta Orthopaedica 20209223–28. ( 10.1080/17453674.2020.1819729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fröschen FS Randau TM Gravius N Wirtz DC Gravius S & Walter SG. Risk factors for implant failure of custom-made acetabular implants in patients with Paprosky III acetabular bone loss and combined pelvic discontinuity. Technology and Health Care 202230703–711. ( 10.3233/THC-202236) [DOI] [PubMed] [Google Scholar]
- 47.Goriainov V King LJ Oreffo ROC & Dunlop DG. Custom 3D-printed triflange implants for treatment of severe acetabular defects, with and without pelvic discontinuity: early results of our first 19 consecutive cases. JB and JS Open Access 20216. ( 10.2106/JBJS.OA.21.00057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawalkar AC Kalanie A & Neil MJ. Excellent midterm results of triflange patient matched implants for extensive acetabular bone defect. Hip Pelvis 20213387–95. ( 10.5371/hp.2021.33.2.87) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scharff-Baauw M Van Hooff ML Van Hellemondt GG Jutte PC Bulstra SK & Spruit M. Good results at 2-year follow-up of a custom-made triflange acetabular component for large acetabular defects and pelvic discontinuity: a prospective case series of 50 hips. Acta Orthopaedica 202192297–303. ( 10.1080/17453674.2021.1885254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sershon RA McDonald JF Nagda S Hamilton WG Engh CA & Cups CT. Custom Triflange Cups: 20-year experience. Journal of Arthroplasty 2021363264–3268. ( 10.1016/j.arth.2021.05.005) [DOI] [PubMed] [Google Scholar]
- 51.Hertzberg-Boelch von SP Wagenbrenner M Arnholdt J Frenzel S Holzapfel BM & Rudert M. Custom made monoflange acetabular components for the treatment of Paprosky Type III defects. Journal of Personalized Medicine 202111. ( 10.3390/jpm11040283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tikhilov RM Dzhavadov AA Kovalenko AN Bilyk SS Denisov AO & Shubnyakov II. Standard versus custom-made acetabular implants in Revision Total Hip Arthroplasty. In Journal of Arthroplasty 202237119–125. ( 10.1016/j.arth.2021.09.003) [DOI] [PubMed] [Google Scholar]
- 53.Valancius K Søballe K Nielsen PT & Laursen MB. No superior performance of hydroxyapatite-coated acetabular cups over porous-coated cups. Acta Orthopaedica 201384544–548. ( 10.3109/17453674.2013.854665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gottliebsen M Rahbek O Ottosen PF Søballe K & Stilling M. Superior 11-year survival but higher polyethylene wear of hydroxyapatite-coated Mallory-Head cups. Hip International 20122235–40. ( 10.5301/HIP.2012.9075) [DOI] [PubMed] [Google Scholar]
- 55.Jørgensen PB Daugaard H Jakobsen SS Lamm M Søballe K & Stilling M. Higher early proximal migration of hemispherical cups with electrochemically applied hydroxyapatite (BoneMaster) on a porous surface compared with porous surface alone: a randomized RSA study with 53 patients. Acta Orthopaedica 20209126–32. ( 10.1080/17453674.2019.1687860) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malahias MA Kostretzis L Greenberg A Nikolaou VS Atrey A & Sculco PK. Highly porous titanium acetabular components in primary and revision total hip arthroplasty: A systematic review. Journal of Arthroplasty 2020351737–1749. ( 10.1016/j.arth.2020.01.052) [DOI] [PubMed] [Google Scholar]
- 57.Castagnini F Bordini B Yorifuji M Giardina F Natali S Pardo F & Traina F. Highly porous titanium Cups versus hydroxyapatite-Coated Sockets: midterm Results in metachronous bilateral total hip arthroplasty. Medical Principles and Practice 201928559–565. ( 10.1159/000500876) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pijls BG Nieuwenhuijse MJ Fiocco M Plevier JW Middeldorp S Nelissen RG & Valstar ER. Early proximal migration of cups is associated with late revision in THA: a systematic review and meta-analysis of 26 RSA studies and 49 survivalstudies. Acta Orthopaedica 201283583–591. ( 10.3109/17453674.2012.745353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nieuwenhuijse MJ Valstar ER Kaptein BL & Nelissen RGHH. Good diagnostic performance of early migration as a predictor of late aseptic loosening of acetabular cups: results from ten years of follow-up with Roentgen stereophotogrammetric analysis (RSA). Journal of Bone and Joint Surgery. American Volume 201294874–880. ( 10.2106/JBJS.K.00305) [DOI] [PubMed] [Google Scholar]
- 60.Baauw M Hellemondt van GG Hooff van ML & Spruit M. The accuracy of positioning of a custom-made implant within a large acetabular defect at revision arthroplasty of the hip. Bone and Joint Journal 201597–B780–785. ( 10.1302/0301-620X.97B6.35129) [DOI] [PubMed] [Google Scholar]
- 61.Wessling M, Gebert C, Hakenes T, Dudda M, Hardes J, Frieler S, et al. Reconstruction of Paprosky III Defects with custom-made implants: do we get them in the correct position? The British Editorial Society of Bone & Joint Surgery 20221041110–1117. ( 10.1302/0301-620X.104B10.BJJ-2022-0508.R1) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a