Abstract
In post-stroke aphasia, language improvements following speech therapy are variable and can only be partially explained by the lesion. Brain tissue integrity beyond the lesion (brain health) may influence language recovery and can be impacted by cardiovascular risk factors, notably diabetes. We examined the impact of diabetes on structural network integrity and language recovery. Seventy-eight participants with chronic post-stroke aphasia underwent six weeks of semantic and phonological language therapy. To quantify structural network integrity, we evaluated the ratio of long-to-short-range white matter fibers within each participant’s whole brain connectome, as long-range fibers are more susceptible to vascular injury and have been linked to high level cognitive processing. We found that diabetes moderated the relationship between structural network integrity and naming improvement at 1 month post treatment. For participants without diabetes (n = 59), there was a positive relationship between structural network integrity and naming improvement (t = 2.19, p = 0.032). Among individuals with diabetes (n = 19), there were fewer treatment gains and virtually no association between structural network integrity and naming improvement. Our results indicate that structural network integrity is associated with treatment gains in aphasia for those without diabetes. These results highlight the importance of post-stroke structural white matter architectural integrity in aphasia recovery.
Keywords: aphasia, brain health, connectome, diabetes, stroke
Introduction
Aphasia is an acquired language disorder that affects approximately one third of those who experience a stroke (Berthier 2005). Although aphasia therapy is an effective way to rehabilitate language deficits for many individuals, it is difficult to predict the extent to which patients will recover, as well as which patients are likely to maintain improvements. Currently, the best prognostic indicators of aphasia rehabilitation are pre-treatment performance/aphasia severity, lesion size and location, age, and education (Johnson et al. 2022). Nonetheless, there is still considerable unexplained variance in recovery beyond these factors, which has been extensively acknowledged in the literature (Lazar and Antoniello 2008; Thye and Mirman 2018; Sul et al. 2019; Osa García et al. 2020; Johnson et al. 2022). Moreover, general cognitive functions such as nonverbal working memory, attention, executive function, and nonlinguistic cognitive function might also account for some of the variance in recovery (Harnish and Lundine 2015; Harnish et al. 2018; Gilmore et al. 2019; Diedrichs et al. 2022), but even with the inclusion of these additional factors, the interindividual variability in clinical trajectories remains large.
Recent quantitative neuroimaging studies have supported the idea that the integrity and connectivity status of the remaining neural tissue could be a central determinant of recovery in aphasia (Wardlaw et al. 2013; Basilakos et al. 2015; den Ouden et al. 2019; Gilmore et al. 2019; Klingbeil et al. 2019; Chang et al. 2021; Keator et al. 2021; Busby et al. 2022; Johnson et al. 2022; Kristinsson et al. 2022). For example, white matter hyperintensities or premature aging of the residual brain tissue independently predicted response to language therapy while controlling for age, lesion volume, and severity in aphasia (Varkanitsa et al. 2020; Kristinsson et al. 2022).
Importantly, stroke survivors commonly have cardiovascular risk factors such as diabetes, hypertension, hyperlipidemia, tobacco use, and reduced physical activity (Di Liegro et al. 2019; Johnson et al. 2019; Kelly and Rothwell 2020), which can negatively affect overall brain health and white matter integrity. Among older adults without history of stroke, cerebro-cardio-vascular risk factors, including diabetes, are associated with cognitive decline, language deficits, and impaired memory performance (Waldstein et al. 2005; Biessels and Reijmer 2014; Sadanand et al. 2016; Johnson et al. 2019; Olaya et al. 2019; Lee et al. 2021). Among these, uncontrolled diabetes is also independently associated with progressive decline in cognitive ability among older adults after stroke, with one recent meta-analysis showing that approximately 28% of stroke survivors have diabetes, and that this condition is associated with worse neurological outcomes (Lau et al. 2019).
Currently, the impact of diabetes per se on neuroplasticity in the context of aphasia recovery remains unclear. In general, diabetes can lead to decrements in neuroplasticity and cause microstructural changes in white matter, thus suggesting that it may have an effect on aphasia recovery (Ho et al. 2013; Ryan et al. 2016; Ma et al. 2018; Krinock and Singhal 2021; Yang et al. 2021). Given (i) the relationship between diabetes and general cognitive decline, (ii) the high prevalence of diabetes among stroke survivors with aphasia, and (iii) the broad cognitive demands of language, it is reasonable to hypothesize that diabetes could significantly affect aphasia recovery through impacting brain health.
Cardiovascular risk factors are associated with white matter hyperintensities, disrupted white matter connectivity, and proportion of axonal projections (i.e. fiber length) (Debette and Markus 2010; Voss et al. 2010; Moroni et al. 2018). The literature shows that diabetes is also associated with disrupted white matter networks, decreased gray matter volume (both globally and regionally), and altered functional connectivity (Musen et al. 2006; van Elderen et al. 2010; Zhang et al. 2016; Wang et al. 2019; Zhou et al. 2022). The mechanisms supporting the loss of white matter integrity are multiple, and it is well established that white matter is more susceptible to injury compared to gray matter (Hamner et al. 2011). For instance, anaerobic resistance (the ability of cells to survive in the absence of sufficient oxygen) is known to decline with age (Hamner et al. 2011). Within the white matter, long-range axonal projections are particularly susceptible to injury due to their higher metabolic demand because of their length (Buzsáki 2006; Ju et al. 2016). Studies outside of the aphasia literature have shown the susceptibility of long-range fibers to cardiovascular risk factors such as high blood pressure (Carnevale et al. 2018) and diabetes (Huang et al. 2022) in the context of long-range fiber preservation and cognitive aging (Hilal et al. 2021), multiple sclerosis (Meijer et al. 2020), and dementia (Savard et al. 2022). Long-range fiber loss due to cardiovascular risk factors has been shown to be predictive of cognitive performance in a non-stroke population (Marebwa et al. 2018), as well as among stroke survivors with aphasia (Wilmskoetter et al. 2019).
In the current study, we examine brain health and diabetes in the context of post-stroke aphasia to predict language recovery following treatment. Specifically, we aim to examine the interaction between structural network integrity (proportion of long- to short-range fibers normalized by total number of fibers) with diabetes to predict treatment gains.
Materials and methods
The study was approved by the Institutional Review Boards at the Medical University of South Carolina and the University of South Carolina. All procedures, including informed consent, were performed within the guidelines and regulations at each institution and according to the Declaration of Helsinki.
Participants
Participants included 78 adults with (> 12 months) post-stroke aphasia due to a left-hemisphere stroke. Participants did not have a history of other neurological or psychiatric disorders. Participants were 59% male and 41% female and on average 59.92 years old (SD = 11.38). Most participants had Broca’s aphasia (48%), followed by Anomic aphasia (27%), Conduction aphasia (13%), Wernicke’s aphasia (6%), Global aphasia (5%), and Transcortical motor aphasia (1%), classified based on Western Aphasia Battery-Revised (WAB-R) score distributions and clinician assessment (Kertesz et al. 2007). Participants were on average 45.24 months post-stroke at the time of baseline evaluation (SD = 44.25), and 67% of participants experienced an ischemic stroke, followed by hemorrhagic (25%), or other (“Other” etiology refers to cases in which it was unclear whether the stroke was ischemic or hemorrhagic) (8%).
Design and instruments
All participants were part of the Predicting Outcomes of Language Rehabilitation in aphasia (POLAR) clinical trial (Kristinsson et al. 2021). They completed a 3-week block of speech therapy with one form of therapy (semantic or phonological), followed by a 4-week rest period, and then another 3-week block with the other form of therapy (semantic or phonological). The order of therapy approaches was randomized. Semantically focused therapy included three tasks: (i) semantic feature analysis, (ii) a semantic barrier task, and (iii) Verb Network Strengthening Treatment (VNeST) (Edmonds 2014). In turn, phonologically focused therapy included three tasks as well: (i) phonological component analysis, (ii) a phonological production task, and (iii) a phonological judgment task. Each therapy session was one hour.
The WAB-R was administered at baseline to identify type and severity of aphasia (Kertesz et al. 2007). Cardiovascular risk factors were also collected at baseline, including a yes/no question about diabetes (diabetes absent: n = 59, diabetes present: n = 19). Participants were administered the Philadelphia Naming Test (PNT) at baseline and 1 month following therapy (Fig. 1).
Fig. 1.
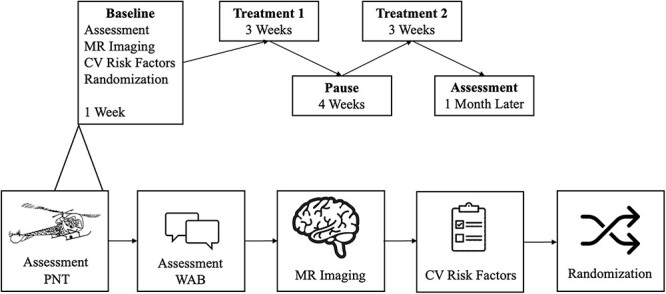
Schematic of study design (note that treatment 1 and 2 were randomly assigned order).
In this study, we opted to evaluate naming treatment results based on proportion of potential maximal gain (PMG), which can provide information about improvement while considering the available magnitude of potential improvement. However, PMG can be numerically influenced by baseline performance and possibly mask gains if not fully controlled for baseline deficits (Bonkhoff et al. 2019; Bowman et al. 2021). Therefore, PMG was used as a dependent measure controlling for baseline impairments in our moderation analysis.
MRI acquisition and analyses
High-resolution structural MRI data were obtained from all 78 participants using a Siemens 3 T Trio or a Prisma Systems scanner with 20-channel head-neck (16/4) coil located at the University of South Carolina or at the Medical University of South Carolina, respectively. The images were acquired with the following parameters: T1-weighted images—MR-RAGE sequence with 1 mm3 isotropic voxels, FOV matrix of 256 mm × 256 mm, 9-degree flip angle, and 192 sagittal slice sequence with TR = 2,250 ms, T1 = 925 ms, and TE = 4.15 ms, with parallel imaging (GRAPPA = 2, 80 reference lines); T2-weighted images -3D SPACE voxel size of 1 mm3, 256 mm × 256 mm FOV matrix, 160 sagittal slice sequence, variable flip angle, TR = 3,200 ms, TE = 352 ms, with no slice acceleration. Diffusion echo planar imaging (EPI) data were also obtained from all participants in accordance with the following parameters: diffusion EPI scan using 36 directions with b = 1000 (60 volumes), 2,000 (60), and 0 s/mm2 (11), TR = 6,100 ms, TE = 101 ms, 82 × 82 matrix, 222 mm × 222 mm FOV, with parallel imaging GRAPPA = 2, 45 contiguous 2.7 mm axial slices, TA = 853 s.
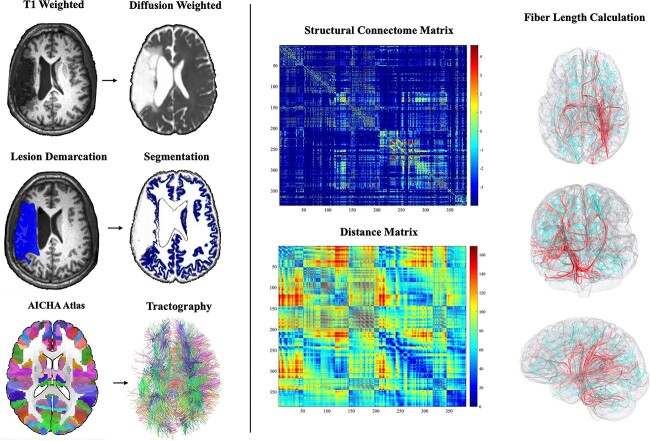
DICOM to NIfTI format conversion was performed using dcm2niix (Li et al. 2016). The stroke lesions were manually delineated on the T2 weighted images by trained personnel under supervision of a neurologist (LB) who were blinded to behavioral scores. The stroke lesions were normalized to standard space using SPM12 and open source MATLAB scripts developed in-house (Rorden et al. 2012) using an enantiomorphic approach (Nachev et al. 2008) within SPM12’s unified segmentation-normalization (Ashburner and Friston 2005) to avoid lesion-related distortions in spatial normalization (Fig. 2). Diffusion images were undistorted using a TOPUP sequence (Andersson et al. 2003), and movement and Eddy motion correction were applied using FSL’s -eddy tool (Bodammer et al. 2004; Andersson and Sotiropoulos 2016).
Fig. 2.
Schematic of imaging methods used from using the T1 weighted, diffusion weighted, and AICHA atlas to constructing a whole brain connectome and tractography, then calculating the distance of each area, followed by categorizing fiber length.
Brain health: Structural network integrity
We employed a whole-brain structural connectome approach to measure the integrity of white matter networks and the global impact of diabetes. The whole-brain structural connectome was reconstructed from all individuals using probabilistic diffusion tensor imaging. We computed a reverse normalization to warp the Atlas of Intrinsic Connectivity of Homotopic Areas (AICHA; Joliot et al. 2015) brain atlas in standard space to the individual’s native diffusion image using SPM12’s “oldnorm” function. Probabilistic gray and white matter maps were obtained during SPM12’s unified segmentation-normalization of T1 images and also registered into diffusion space to guide tractography. Pairwise connectivity between all possible gray matter regions from the atlas was measured through probabilistic tractography using FDT’s Bedpost and FDT’s probtrackX (5,000 individual pathways drawn through the probability distributions on principal fiber direction, curvature threshold set at 0.2, 200 maximum steps, step length 0.5 mm, and distance correction (Behrens et al. 2007)). The probabilistic white-matter map excluding the stroke lesion was used as a waypoint mask. For each possible pair of regions, we computed the number of probable streamlines arriving in one region when another region was seeded (averaged with the vice-versa direction given the undirected nature of diffusion tensor imaging data). The weighted connectivity between regions was corrected based on the distance traveled by the streamlines (“distance correction” built into probtrackX) and the sum of volume of the regions to account for space and length biases. As a result, an individualized connectivity matrix was obtained from each individual. Since these were based on probabilistic tractography, connections with very low probability (lower than the 20th percentile) were considered likely spurious and transformed into zero weight.
To quantify proportion of long-range fibers, we used a similar approach as in Wilmskoetter et al. (2019). Proportions were computed by measuring the Euclidean distance between the centroids of ROIs in standard Montreal Neurological Institute (MNI space). Connections whose distance was within the first quartile (lowest 25%) were identified as “short distance” fibers, those within the second and third quartiles (25–75%) were identified as “middle distance” fibers, and connections within the fourth quartile (75% and above) were identified as “long range” fibers (Fig. 2). Hence, structural network integrity was measured as the proportion of long-range to short-range fibers normalized to the total number of fibers:
 |
Accordingly, better structural network integrity was characterized by a larger proportion of long-range to short-range fibers normalized to total number of fibers. We will refer to this calculation as “structural network integrity” from this point on.
Statistical analyses
To address our hypotheses, we used t-tests to compare structural network integrity and improvements in naming accuracy for those with versus without diabetes. We then used a moderation analysis for naming improvement at 1 month following treatment, controlling for age, lesion volume, and baseline PNT performance in SPSS 27 with PROCESS (Hayes 2013) to investigate the relationship of diabetes, structural network integrity, and treatment gains.
Results
Statistical analyses
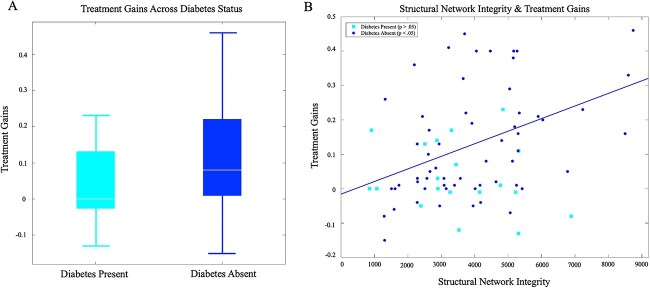
A series of t-tests were used to compare baseline naming performance, naming improvement at 1 month post-treatment, structural network integrity, lesion volume, and age for participants with and without diabetes. The diabetes present and diabetes absent groups did not differ on any of variables except for recovery at 1 month, which was significantly lower for those with diabetes (t = −3.08, p = 0.003; see Table 1 and Fig. 3A).
Table 1.
Comparison of baseline Philadelphia naming test (PNT), treatment gains, lesion volume, and structural network integrity across those with and without diabetes.
| Diabetes (n = 19) | No Diabetes (n = 59) | |||
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | t-value | p-value | |
| Baseline PNT | 67.92 (68.00) | 81.21 (61.10) | −.80 | .43 |
| Treatment Gains (1 Month) | .034 (.10) | .13 (.16) | −3.08 | .003 |
| Age | 61. 32 (8.81) | 59.36 (12.00) | .66 | .51 |
| Lesion Volume | 125,268 (109,667) | 124,600 (85,433) | .028 | .98 |
| Structural Network Integrity | 3496 (1630) | 3955 (1801) | −.99 | .33 |
Fig. 3.
A) Treatment gains for those with and without diabetes (p < 0.05); B) plot of proportion of structural network integrity and treatment gains at 1 month for those with and without diabetes (note that the relationship between treatment gains and structural network integrity is significant for “diabetes absent” while the “diabetes present” group is not significant).
Moderation: treatment gains at 1 month
With naming improvement at 1 month as the dependent variable, with structural network integrity as the independent variable, with diabetes as the moderating variable (where diabetes = 1 and no diabetes = 2), and with age, lesion volume, and baseline performance as covariates, the model summary for the regression predicting naming improvement at 1 month was significant, R = 0.73, R2 = 0.53 F(6,71) = 13.26, p < 0.001. The interaction between diabetes and structural network integrity was significant, and R2 = 0.043, F(1,71) = 6.49, p = 0.013 (i.e., the interaction explains 4.3% of the variance in treatment gains; see Table 2, Fig. 3). This interaction indicated that diabetes moderates the relationship between structural network integrity and naming improvement at 1 month. Although the overall interaction was significant, the conditional effects showed that the relationship between structural network integrity and naming improvement at 1 month was only significant for those without diabetes (i.e. not significant for those with diabetes), for whom there was a positive relationship between structural network integrity and naming improvement (t = 2.19, p = 0.032; see Table 2, Fig. 3B). Again, the effect was absent for participants with diabetes.
Table 2.
Moderation model: 1 month follow-up.
| Overall Model | ||||||||
|---|---|---|---|---|---|---|---|---|
| Standardized Coefficient | Unstandardized Coefficient | Unstandardized Coefficient Standard Error | t-value | p-value | LLCI | ULCI | ||
| Constant | .31 | .14 | 2.15 | .035 | .023 | .60 | ||
| Structural Network Integrity | −.84 | −.0001 | .0000 | −2.19 | .032 | −.0001 | .0000 | |
| Lesion Volume | .069 | .0000 | .0000 | .77 | .44 | .0000 | .0000 | |
| Diabetes | −.27 | −.095 | .070 | −1.35 | .18 | −.23 | .05 | |
| Baseline | .52 | .001 | .0002 | 5.22 | <.001 | .0008 | .002 | |
| Age | −.23 | −.003 | .001 | −2.57 | .012 | −.0054 | −.0007 | |
| Interaction | 1.12 | .0000 | .0000 | 2.55 | .013 | .0000 | .0001 | |
| Conditional Effects of the Focal Predictor at Values of the Moderator | ||||||||
| Coefficient | Standard Error | t-value | p-value | LLCI | ULCI | |||
| Diabetes Present | .0000 | .0000 | −1.66 | .101 | −.0001 | .0000 | ||
| Diabetes Absent | .0000 | .0000 | 2.19 | .032 | .0000 | .0000 | ||
An analysis including scanner site can be found in the supplementary materials, which shows that it is not a significant covariate (Supplementary Analysis 1). A linear regression with and without the interaction terms can also be found in the Supplementary Materials (Supplementary Analysis 2).
Discussion
In the current study, we examined the relationship among structural network integrity, presence of diabetes, and treatment gains in post-stroke aphasia. Our results show that diabetes moderates the relationship between structural network integrity and naming improvement at 1 month after treatment, meaning that the strength of the relationship between structural network integrity and treatment gains is influenced by diabetes. More specifically, our findings reveal that structural network integrity and the absence of diabetes interact (i.e. diabetes status × structural network integrity) to predict treatment gains.
Our results highlight the importance of considering cardiovascular health and the health of non-lesioned tissue in aphasia recovery trajectories, which is in line with previous research on residual network integrity and aphasia (van Elderen et al. 2010; Zhang et al. 2016; den Ouden et al. 2019; Keator et al. 2021; Busby et al. 2022; Johnson et al. 2022; Kristinsson et al. 2022). More specifically, we found that those with diabetes recover less than those without diabetes, which is similar to work showing diabetes as associated with cognitive decline (Waldstein et al. 2005; Biessels and Reijmer 2014; Sadanand et al. 2016; Johnson et al. 2019; Olaya et al. 2019; Lee et al. 2021). Taken together, these observations support the inclusion of cerebro-cardio-vascular profile in models of cognitive recovery (Marebwa et al. 2018; Song et al. 2020; Zanon Zotin et al. 2021).
There are a number of important caveats in the interpretation of our findings. First, we recognize that brain health can be defined in many ways. Here, we focused specifically on structural network integrity, defined as the proportion of long to short range fibers normalized by total fibers because (i) this is a biomarker that can be derived at the individual level in a straight-forward fashion in a research setting, making it good for generalization; and (ii) prior studies have shown the relevance of this measure as a marker for brain integrity, particularly in the context of cardiovascular risk factors, such as hypertension and diabetes or aging and dementia (Pires et al. 2013; Gao et al. 2014; Huang et al. 2022; Savard et al. 2022). Although, clinically, this biomarker might not be currently feasible for treatment planning, we urge the field to focus on cardiovascular risk factors in relation to both brain health and aphasia recovery in order to create more clinically relevant tools and ultimately improve prognostic evaluation. Yet, the mere finding of an association between structural network integrity and aphasia recovery is an encouraging avenue, and we propose further exploration of this marker and its applications in translational neuroimaging research and clinical practice. In our study, this measure accounted for ~ 4.3% of the variability in treatment gains despite strictly controlling for other well-established contributors to such variability (i.e. age, lesion volume, baseline PNT performance). Although our measure of structural network integrity seems to be a useful measure, ideal quantification of brain health will likely rely on a multi-pronged and multi-modal approach rather than a single measure.
An important limitation to consider is the determination of diabetes solely as present or absent. Due to the inherent biases in retrospective analyses, we did not have a richer approach to quantify this risk factor. The binary measurement of diabetes may have led us to find null effects in the diabetes present group due to differing variability in this group. Additional measurement of diabetes severity/management has the potential to uncover patterns of structural integrity in relation to treatment response for individuals with diabetes. However, we argue that our findings make expanding on definitions and measurement of diabetes worthy of further exploration. In particular, we propose studying multifaceted variables to include hemoglobin A1c as a continuous measure, the use of anti-diabetes insulinic and non-insulinic agents, duration of disease, and the presence or absence of diabetes-related end-organ damage, as including these factors may reveal what is occurring in the brain of those with comorbid diabetes and aphasia. One of the main implications of the current study is emphasizing the importance of prospectively gathering these clinical variables in detail, which will enrich models and help better define the mechanistic pathways regarding how brain health integrity supports cognitive rehabilitation. Additionally, we did not examine diabetes maintenance (i.e. medication, diet, exercise) in relation to brain health or aphasia recovery. This should certainly be examined in future work.
Conclusion
Our results support the importance of brain health in aphasia recovery. Specifically, structural network integrity is a mechanistic factor related to treatment gains for those without diabetes. Although this relationship was not present for those with diabetes, we did find that there were fewer treatment gains in the diabetes present group. In sum, the current study supports a network integrity/brain health perspective to aphasia recovery and the influence of diabetes on recovery.
Funding
The current study was supported by the National Institute on Deafness and Other Communication Disorders (R01 014021 PI: LB, P50 DC014664 PI: JF; R01 DC05375 to AEH). This study was also supported by research grants from the National Institutes of Health/National Institute on Deafness and Other Communication Disorders (grant number T32DC014435 to Trainee: Schwen Blackett).
Conflict of interest statement: None declared.
Data availability
The data and analysis scripts that support the findings of this study are available upon reasonable request from the corresponding author, although restrictions may apply to adhere to participant consent and anonymity.
Competing interest statement
The authors all declare that they have no competing interests that could have influenced their work in the current study.
CRediT author statement
Rebecca Roth (Formal analysis, Methodology, Visualization, Writing—original draft, Writing—review and editing), Natalie Busby (Formal analysis, Writing—review and editing), Janina Wilmskoetter (Conceptualization, Formal analysis, Visualization, Writing—review and editing), Deena Schwen Blackett (Writing—review and editing), Ezequiel Gleichgerrcht (Writing—review and editing), Lisa Johnson (Writing—review and editing), Chris Rorden (Software), Roger Newman-Norlund (Writing—review and editing), Argye Hillis (Writing—review and editing), Dirk den Ouden (Writing—review and editing), Julius Fridriksson (Funding acquisition, Supervision, Writing—review and editing), Leonardo Bonilha (Conceptualization, Funding acquisition, Investigation, Methodology, Supervision, Visualization, Writing—review and editing).
Supplementary Material
Contributor Information
Rebecca Roth, Department of Neurology, Emory University, Atlanta, GA 30322, USA.
Natalie Busby, Department of Communication Sciences and Disorders, University of South Carolina, Columbia, SC 29208, USA.
Janina Wilmskoetter, Department of Rehabilitation Sciences, Medical University of South Carolina, Charleston, SC 29425, USA.
Deena Schwen Blackett, Department of Neurology, Medical University of South Carolina, Charleston, SC 29425, USA.
Ezequiel Gleichgerrcht, Department of Neurology, Medical University of South Carolina, Charleston, SC 29425, USA.
Lisa Johnson, Department of Communication Sciences and Disorders, University of South Carolina, Columbia, SC 29208, USA.
Chris Rorden, Department of Psychology, University of South Carolina, Columbia, SC 29208, USA.
Roger Newman-Norlund, Department of Psychology, University of South Carolina, Columbia, SC 29208, USA.
Argye E Hillis, Department of Neurology, School of Medicine, Johns Hopkins University, Baltimore, MD 21218 USA.
Dirk B den Ouden, Department of Communication Sciences and Disorders, University of South Carolina, Columbia, SC 29208, USA.
Julius Fridriksson, Department of Communication Sciences and Disorders, University of South Carolina, Columbia, SC 29208, USA.
Leonardo Bonilha, Department of Neurology, Emory University, Atlanta, GA 30322, USA.
References
- Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage. 2016:125:1063–1078. 10.1016/j.neuroimage.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage. 2003:20(2):870–888. 10.1016/s1053-8119(03)00336-7. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation [research support, non-U.S. Gov't]. NeuroImage. 2005:26(3):839–851. 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Basilakos A, Rorden C, Bonilha L, Moser D, Fridriksson J. Patterns of poststroke brain damage that predict speech production errors in apraxia of speech and aphasia dissociate. Stroke. 2015:46(6):1561–1566. 10.1161/strokeaha.115.009211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? NeuroImage. 2007:34(1):144–155. 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthier ML. Poststroke aphasia: epidemiology, pathophysiology and treatment. Drugs Aging. 2005:22(2):163–182. 10.2165/00002512-200522020-00006. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Reijmer YD. Brain changes underlying cognitive dysfunction in diabetes: what can we learn from MRI? Diabetes. 2014:63(7):2244–2252. 10.2337/db14-0348. [DOI] [PubMed] [Google Scholar]
- Bodammer N, Kaufmann J, Kanowski M, Tempelmann C. Eddy current correction in diffusion-weighted imaging using pairs of images acquired with opposite diffusion gradient polarity. Magn Reson Med. 2004:51(1):188–193. 10.1002/mrm.10690. [DOI] [PubMed] [Google Scholar]
- Bonkhoff A, Hope T, Bzdok D, Guggisberg A, Hawe R, Dukelow S, Rehme A, Fink G, Grefkes C, Bowman H. Bringing proportional recovery into proportion: Bayesian hierarchical modelling of post-stroke motor performance. 2019. https://www.medrxiv.org/content/10.1101/19009159v1. [DOI] [PubMed]
- Bowman H, Bonkhoff A, Hope T, Grefkes C, Price C. Inflated estimates of proportional recovery from stroke. Stroke. 2021:52(5):1915–1920. 10.1161/STROKEAHA.120.033031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busby N, Wilmskoetter J, Gleichgerrcht E, Rorden C, Roth R, Newman-Norlund R, Hillis AE, Keller SS, de Bezenac C, Kristinsson S, et al. Advanced brain age and chronic Poststroke aphasia severity. Neurology. 2022:100(11):e1166–e1176. 10.1212/wnl.0000000000201693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Rhythms of the brain. Oxford University Press, 2006. https://academic.oup.com/book/11166?login=false [Google Scholar]
- Carnevale L, D'Angelosante V, Landolfi A, Grillea G, Selvetella G, Storto M, Lembo G, Carnevale D. Brain MRI fiber-tracking reveals white matter alterations in hypertensive patients without damage at conventional neuroimaging. Cardiovasc Res. 2018:114(11):1536–1546. 10.1093/cvr/cvy104. [DOI] [PubMed] [Google Scholar]
- Chang AJ, Wilmskoetter J, Fridriksson J, McKinnon ET, Johnson LP, Basilakos A, Jensen JH, Rorden C, Bonilha L. Cortical microstructural changes associated with treated aphasia recovery. Ann Clin Transl Neurol. 2021:8(9):1884–1894. 10.1002/acn3.51445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010:341(jul26 1):c3666. 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Liegro CM, Schiera G, Proia P, Di Liegro I. Physical activity and brain health. Genes (Basel). 2019:10(9). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6770965/pdf/genes-10-00720.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichs VA, Jewell CC, Harnish SM. A scoping review of the relationship between nonlinguistic cognitive factors and aphasia treatment response. Top Lang Disord. 2022:42(3):212–235. 10.1097/tld.0000000000000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds LA. Tutorial for verb network strengthening treatment (VNeST): detailed description of the treatment protocol with corresponding theoretical rationale. Perspectives on Neurophysiology and Neurogenic Speech and Language Disorders. 2014:24(3):78–88. 10.1044/nnsld24.3.78. [DOI] [Google Scholar]
- van Elderen SG, de Roos A, de Craen AJ, Westendorp RG, Blauw GJ, Jukema JW, Bollen EL, Middelkoop HA, van Buchem MA, van der Grond J. Progression of brain atrophy and cognitive decline in diabetes mellitus: a 3-year follow-up. Neurology. 2010:75(11):997–1002. 10.1212/WNL.0b013e3181f25f06. [DOI] [PubMed] [Google Scholar]
- Gao J, Cheung RTF, Chan Y-S, Chu L-W, Mak HKF, Lee TMC. The relevance of short-range Fibers to cognitive efficiency and brain activation in aging and dementia. PLoS One. 2014:9(4):e90307. 10.1371/journal.pone.0090307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore N, Meier EL, Johnson JP, Kiran S. Nonlinguistic cognitive factors predict treatment-induced recovery in chronic Poststroke aphasia. Arch Phys Med Rehabil. 2019:100(7):1251–1258. 10.1016/j.apmr.2018.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamner MA, Möller T, Ransom BR. Anaerobic function of CNS white matter declines with age. J Cereb Blood Flow Metab. 2011:31(4):996–1002. 10.1038/jcbfm.2010.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnish SM, Lundine JP. Nonverbal working memory as a predictor of anomia treatment success. Am J Speech Lang Pathol. 2015:24(4):S880–S894. 10.1044/2015_ajslp-14-0153. [DOI] [PubMed] [Google Scholar]
- Harnish SM, Schwen Blackett D, Zezinka A, Lundine JP, Pan X. Influence of working memory on stimulus generalization in anomia treatment: a pilot study. J Neurolinguistics. 2018:48:142–156. 10.1016/j.jneuroling.2018.02.003. [DOI] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. New York, NY, US: Guilford Press; 2013 [Google Scholar]
- Hilal S, Liu S, Wong TY, Vrooman H, Cheng CY, Venketasubramanian N, Chen CL, Zhou JH. White matter network damage mediates association between cerebrovascular disease and cognition. J Cereb Blood Flow Metab. 2021:41(8):1858–1872. 10.1177/0271678x21990980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho N, Sommers MS, Lucki I. Effects of diabetes on hippocampal neurogenesis: links to cognition and depression. Neurosci Biobehav Rev. 2013:37(8):1346–1362. 10.1016/j.neubiorev.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Ma X, Yue X, Kang S, Li Y, Rao Y, Feng Y, Wu J, Long W, Chen Y, et al. White matter characteristics of damage along Fiber tracts in patients with type 2 diabetes mellitus. Clin Neuroradiol. 2022. https://link.springer.com/article/10.1007/s00062-022-01213-7#citeas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L, Basilakos A, Yourganov G, Cai B, Bonilha L, Rorden C, Fridriksson J. Progression of aphasia severity in the chronic stages of stroke. Am J Speech Lang Pathol. 2019:28(2):639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L, Nemati S, Bonilha L, Rorden C, Busby N, Basilakos A, Newman-Norlund R, Hillis AE, Hickok G, Fridriksson J. Predictors beyond the lesion: health and demographic factors associated with aphasia severity. Cortex. 2022:154:375–389. 10.1016/j.cortex.2022.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliot M, Jobard G, Naveau M, Delcroix N, Petit L, Zago L, Crivello F, Mellet E, Mazoyer B, Tzourio-Mazoyer N. AICHA: an atlas of intrinsic connectivity of homotopic areas. J Neurosci Methods. 2015:254:46–59. https://neuro.unboundmedicine.com/medline/citation/26213217/AICHA:_An_atlas_of_intrinsic_connectivity_of_homotopic_areas_. [DOI] [PubMed] [Google Scholar]
- Ju H, Hines ML, Yu Y. Cable energy function of cortical axons. Sci Rep. 2016:6(1):29686. 10.1038/srep29686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keator LM, Yourganov G, Basilakos A, Hillis AE, Hickok G, Bonilha L, Rorden C, Fridriksson J. Independent contributions of structural and functional connectivity: evidence from a stroke model. Netw Neurosci. 2021:5(4):911–928. 10.1162/netn_a_00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DM, Rothwell PM. Blood pressure and the brain: the neurology of hypertension. Pract Neurol. 2020:20(2):100–108. 10.1136/practneurol-2019-002269. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Kertesz A, Raven JC. WAB-R: western aphasia battery-revised. In: Western aphasia battery. Revised. ed. San Antonio, TX: Harcourt Assessment, Inc.; 2007 [Google Scholar]
- Klingbeil J, Wawrzyniak M, Stockert A, Saur D. Resting-state functional connectivity: an emerging method for the study of language networks in post-stroke aphasia. Brain Cogn. 2019:131:22–33. 10.1016/j.bandc.2017.08.005. [DOI] [PubMed] [Google Scholar]
- Krinock MJ, Singhal NS. Diabetes, stroke, and neuroresilience: looking beyond hyperglycemia. Ann N Y Acad Sci. 2021:1495(1):78–98. 10.1111/nyas.14583. [DOI] [PubMed] [Google Scholar]
- Kristinsson S, Basilakos A, Elm J, Spell LA, Bonilha L, Rorden C, den Ouden DB, Cassarly C, Sen S, Hillis A, et al. Individualized response to semantic versus phonological aphasia therapies in stroke. Brain Communications. 2021:3(3):1–18. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6770965/pdf/genes-10-00720.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristinsson S, Busby N, Rorden C, Newman-Norlund R, den Ouden DB, Magnusdottir S, Hjaltason H, Thors H, Hillis AE, Kjartansson O, et al. Brain age predicts long-term recovery in post-stroke aphasia. Brain Communications. 2022:4(5). https://academic.oup.com/braincomms/article/4/5/fcac252/6750030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau LH, Lew J, Borschmann K, Thijs V, Ekinci EI. Prevalence of diabetes and its effects on stroke outcomes: a meta-analysis and literature review. J Diabetes Investig. 2019:10(3):780–792. 10.1111/jdi.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar RM, Antoniello D. Variability in recovery from aphasia. Curr Neurol Neurosci Rep. 2008:8(6):497–502. 10.1007/s11910-008-0079-x. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim TD, Kim RY, Joo Y, Chung YA, Lim SM, Lyoo IK, Kim J, Yoon S. Hippocampal subregional alterations and verbal fluency in the early stage of type 2 diabetes mellitus. Eur J Neurosci. 2021:54(10):7550–7559. 10.1111/ejn.15505. [DOI] [PubMed] [Google Scholar]
- Li X, Morgan PS, Ashburner J, Smith J, Rorden C. The first step for neuroimaging data analysis: DICOM to NIfTI conversion. J Neurosci Methods. 2016:264:47–56. 10.1016/j.jneumeth.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Ma S, Wang J, Wang Y, Dai X, Xu F, Gao X, Johnson J, Xu N, Leak RK, Hu X, et al. Diabetes mellitus impairs White matter repair and Long-term functional deficits after cerebral ischemia. Stroke. 2018:49(10):2453–2463. 10.1161/strokeaha.118.021452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marebwa BK, Adams RJ, Magwood GS, Basilakos A, Mueller M, Rorden C, Fridriksson J, Bonilha L. Cardiovascular risk factors and brain health: impact on Long-range cortical connections and cognitive performance. J Am Heart Assoc. 2018:7(23):e010054. 10.1161/JAHA.118.010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer KA, Steenwijk MD, Douw L, Schoonheim MM, Geurts JJG. Long-range connections are more severely damaged and relevant for cognition in multiple sclerosis. Brain. 2020:143(1):150–160. 10.1093/brain/awz355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni F, Ammirati E, Rocca MA, Filippi M, Magnoni M, Camici PG. Cardiovascular disease and brain health: focus on white matter hyperintensities. Int J Cardiol Heart Vasc. 2018:19:63–69. 10.1016/j.ijcha.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musen G, Lyoo IK, Sparks CR, Weinger K, Hwang J, Ryan CM, Jimerson DC, Hennen J, Renshaw PF, Jacobson AM. Effects of type 1 diabetes on Gray matter density as measured by voxel-based morphometry. Diabetes. 2006:55(2):326–333. 10.2337/diabetes.55.02.06.db05-0520. [DOI] [PubMed] [Google Scholar]
- Nachev P, Coulthard E, Jager HR, Kennard C, Husain M. Enantiomorphic normalization of focally lesioned brains. NeuroImage. 2008:39(3):1215–1226. 10.1016/j.neuroimage.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaya B, Moneta MV, Bobak M, Haro JM, Demakakos P. Cardiovascular risk factors and memory decline in middle-aged and older adults: the English longitudinal study of ageing. BMC Geriatr. 2019:19(1):337. 10.1186/s12877-019-1350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osa García A, Brambati SM, Brisebois A, Désilets-Barnabé M, Houzé B, Bedetti C, Rochon E, Leonard C, Desautels A, Marcotte K. Predicting early post-stroke aphasia outcome from initial aphasia severity. Front Neurol. 2020:11:120. 10.3389/fneur.2020.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Ouden DB, Malyutina S, Basilakos A, Bonilha L, Gleichgerrcht E, Yourganov G, Hillis AE, Hickok G, Rorden C, Fridriksson J. Cortical and structural-connectivity damage correlated with impaired syntactic processing in aphasia. Hum Brain Mapp. 2019:40(7):2153–2173. 10.1002/hbm.24514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires PW, Dams Ramos CM, Matin N, Dorrance AM. The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol. 2013:304(12):H1598–H1614. 10.1152/ajpheart.00490.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Bonilha L, Fridriksson J, Bender B, Karnath HO. Age-specific CT and MRI templates for spatial normalization. NeuroImage. 2012:61(4):957–965. 10.1016/j.neuroimage.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CM, van Duinkerken E, Rosano C. Neurocognitive consequences of diabetes. Am Psychol. 2016:71(7):563–576. 10.1037/a0040455. [DOI] [PubMed] [Google Scholar]
- Sadanand S, Balachandar R, Bharath S. Memory and executive functions in persons with type 2 diabetes: a meta-analysis. Diabetes Metab Res Rev. 2016:32(2):132–142. 10.1002/dmrr.2664. [DOI] [PubMed] [Google Scholar]
- Savard M, Pascoal TA, Servaes S, Dhollander T, Iturria-Medina Y, Kang MS, Vitali P, Therriault J, Mathotaarachchi S, Benedet AL, et al. Impact of long- and short-range fibre depletion on the cognitive deficits of fronto-temporal dementia. elife. 2022:11:e73510. 10.7554/eLife.73510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Xu H, Dintica CS, Pan K-Y, Qi X, Buchman AS, Bennett DA, Xu W. Associations between cardiovascular risk, structural brain changes, and cognitive decline. J Am Coll Cardiol. 2020:75(20):2525–2534. 10.1016/j.jacc.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sul B, Lee KB, Hong BY, Kim JS, Kim J, Hwang WS, Lim SH. Association of Lesion Location with Long-term recovery in post-stroke aphasia and language deficits. Front Neurol. 2019:10:776. 10.3389/fneur.2019.00776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thye M, Mirman D. Relative contributions of lesion location and lesion size to predictions of varied language deficits in post-stroke aphasia. Neuroimage Clin. 2018:20:1129–1138. 10.1016/j.nicl.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varkanitsa M, Peñaloza C, Charidimou A, Caplan D, Kiran S. White matter Hyperintensities predict response to language treatment in Poststroke aphasia. Neurorehabil Neural Repair. 2020:34(10):945–953. 10.1177/1545968320952809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Erickson KI, Prakash RS, Chaddock L, Malkowski E, Alves H, Kim JS, Morris KS, White SM, Wójcicki TR, et al. Functional connectivity: a source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia. 2010:48(5):1394–1406. 10.1016/j.neuropsychologia.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldstein SR, Brown JR, Maier KJ, Katzel LI. Diagnosis of hypertension and high blood pressure levels negatively affect cognitive function in older adults. Ann Behav Med. 2005:29(3):174–180. 10.1207/s15324796abm2903_3. [DOI] [PubMed] [Google Scholar]
- Wang YF, Gu P, Zhang J, Qi R, de Veer M, Zheng G, Xu Q, Liu Y, Lu GM, Zhang LJ. Deteriorated functional and structural brain networks and normally appearing functional-structural coupling in diabetic kidney disease: a graph theory-based magnetic resonance imaging study. Eur Radiol. 2019:29(10):5577–5589. 10.1007/s00330-019-06164-1. [DOI] [PubMed] [Google Scholar]
- Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O'Brien JT, Barkhof F, Benavente OR, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013:12(8):822–838. 10.1016/s1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmskoetter J, Marebwa B, Basilakos A, Fridriksson J, Rorden C, Stark BC, Johnson L, Hickok G, Hillis AE, Bonilha L. Long-range fibre damage in small vessel brain disease affects aphasia severity. Brain. 2019:142(10):3190–3201. 10.1093/brain/awz251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Boudier-Revéret M, Kwon S, Lee MY, Chang MC. Effect of diabetes on post-stroke recovery: a systematic narrative review. Front Neurol. 2021:12:747878. 10.3389/fneur.2021.747878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanon Zotin MC, Sveikata L, Viswanathan A, Yilmaz P. Cerebral small vessel disease and vascular cognitive impairment: from diagnosis to management. Curr Opin Neurol. 2021:34(2):246–257. 10.1097/wco.0000000000000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Liu Z, Li Z, Wang Y, Chen Y, Li X, Chen K, Shu N, Zhang Z. Disrupted White matter network and cognitive decline in type 2 diabetes patients. J Alzheimers Dis. 2016:53(1):185–195. 10.3233/jad-160111. [DOI] [PubMed] [Google Scholar]
- Zhou B, Wang X, Yang Q, Wu F, Tang L, Wang J, Li C. Topological alterations of the brain functional network in type 2 diabetes mellitus patients with and without mild cognitive impairment. Front Aging Neurosci. 2022:14:834319. 10.3389/fnagi.2022.834319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and analysis scripts that support the findings of this study are available upon reasonable request from the corresponding author, although restrictions may apply to adhere to participant consent and anonymity.