Abstract
Objectives
To investigate the expression of type I IFN (IFN-I) and neutrophil transcripts in kidney tissue from patients with different classes of LN and their association with distinct clinical and histopathological features.
Methods
Quantitation of IFN-I, defensin-α3 and formyl peptide receptor-like 1 (FPRL-1) transcripts was performed in kidney biopsy tissue from 24 patients with various classes of LN (6 class III, 14 class IV, 4 class V) and 3 control samples. Patient demographics, glomerular filtration rate (eGFR) and histopathological characteristics, including activity and chronicity indices, were analysed.
Results
IFNα2 and IFNβ transcripts were overexpressed in renal tissues from patients with proliferative forms of LN (III/IV) compared with patients with membranous nephritis and control kidneys. Patients with LN and impaired renal function, attested by eGFR, displayed higher relative expression of IFNα2 transcripts in renal tissues compared with those with normal renal function (23.0 ± 16.2 vs 12.0 ± 14.8, P = 0.04). Defensin-α3, but not FPRL-1, transcripts were overexpressed in LN tissues, particularly those with segmental necrotizing lesions, and were correlated with higher renal pathological activity indices (r = 0.61, P = 0.02), urinary protein levels (r = 0.44, P = 0.048) and IFNα2 expression (r = 0.50, P = 0.01).
Conclusion
IFN-I transcripts are expressed locally in kidneys from patients with proliferative LN and are associated with impaired renal function. Elevated defensin-α3 transcripts, a neutrophil product associated with neutrophil extracellular traps, may identify a driver of local IFN-I expression. These findings provide insights into the mechanisms of proliferative LN and may inform therapeutic decisions regarding selection of IFN-I pathway inhibitors.
Keywords: type I IFN, α-defensins, granulopoiesis signature, neutrophils, proliferative lupus nephritis
Rheumatology key messages.
IFN-I transcripts are overexpressed in renal tissues from patients with proliferative forms of lupus nephritis.
IFNα2 transcripts are higher in renal tissue from lupus nephritis patients with impaired renal function.
Defensin-α3 transcripts in lupus nephritis renal tissues correlate with renal pathologic activity, urine protein levels and IFNα2 expression.
Introduction
SLE is the prototype systemic autoimmune disease and is characterized by a wide array of clinical manifestations and hallmarked by overexpression of both IFN-I–induced and neutrophil-related transcripts in peripheral blood [1, 2]. LN is a significant clinical manifestation of SLE, with activation of the IFN-I pathway and impaired degradation of neutrophil extracellular traps (NETs) previously identified as potential pathogenetic contributors [3, 4]. Εarlier studies revealed an association of a peripheral blood IFN-I signature with LN [5, 6], and higher expression of Long Interspersed Nuclear Element-1 retroelements (LINE-1; shown to induce IFN-I) in renal tissues from LN patients was associated with proliferative forms of LN (III/IV) [7]. Moreover, human neutrophil peptides 1–3 (also called α-defensins) levels were reported to be elevated in sera from patients with LN, in association with urinary protein excretion and activity index in renal tissues [8]. Though IFN-I–induced genes have been previously found to be overexpressed in cellular populations of renal biopsies from LN patients [9], data regarding the local expression of IFN-Is themselves (as well as neutrophil-related peptides in renal tissues and across distinct pathologic classes) is rather limited [10]. In the present study, we wished to determine whether IFN-I and neutrophil-derived defensin-α3 (DEFA3) and formyl peptide receptor-like 1 (FPRL-1) transcripts are overexpressed in kidney tissues from patients with different classes of LN and associated with distinct clinical and histopathological characteristics.
Patients and methods
Patients
Kidney biopsies from 24 patients with LN were obtained from the Department of Pathology and Laboratory Medicine at New York Presbyterian Hospital, New York, NY, and were classified according to the current revision of the International Society of Nephrology ISN/RPS classification criteria for LN (6 class III, 14 class IV, 4 class V) [11], following analysis by light microscopy, IF and EM. Eight patients with proliferative forms of nephritis also had histopathological features compatible with membranous type V (Supplementary Table S1, available at Rheumatology online). The activity and chronicity indices were evaluated and the presence of active glomerular lesions, tubulointerstitial or vascular involvement were recorded for all biopsies. Serum creatinine levels at the time of renal biopsy were available for the majority of patients, and the estimated glomerular filtration rate (eGFR) was calculated.
All but two patients were females between the ages of 11 and 55 at the time of biopsy, fulfilled the EULAR/ACR classification criteria for SLE [12] and provided informed consent. Supplementary Table S1, available at Rheumatology online, displays pertinent demographic, clinical and histopathological characteristics of the study participants. In addition, control renal tissue was obtained commercially from Ambion and from two patients who presented with proteinuria/hematuria and whose renal biopsies showed no pathology (male individuals ages 8 and 12, respectively). The study complies with the Declaration of Helsinki. The Institutional Review Boards of the Hospital for Special Surgery and Weill Cornell Medicine approved the study and the use of patient tissue samples.
Methods
RNA extraction and gene expression analysis
Total RNA was isolated from stored frozen kidney biopsy specimens using TRIzol reagent from Invitrogen. All samples were treated with DNAase I (Qiagen) prior to cDNA synthesis. The TaqMan reverse transcription kit (Applied Biosystems) was used to synthesize first-strand cDNA from RNA samples using 0.5 nM of oligo dT primers. Real-time PCR was performed using a Bio-Rad iCycler system with iTaq SYBR Green supermix (Bio-Rad). The primers that were used for the quantitation of the IFN-I (IFNα2 and IFNβ), DEFA3 and FPRL-1 (current gene name FPR2) transcripts are listed in Supplementary Table S2, available at Rheumatology online. Data are expressed as relative expression (RE) compared with a housekeeping gene control.
Statistics
Two-group comparisons of continuous data were assessed using t-tests, or the Mann–Whitney test when data were not normally distributed. Correlation between gene expression data and activity index was determined using the non-parametric Spearman’s test. Differences were considered statistically significant for P < 0.05.
Results
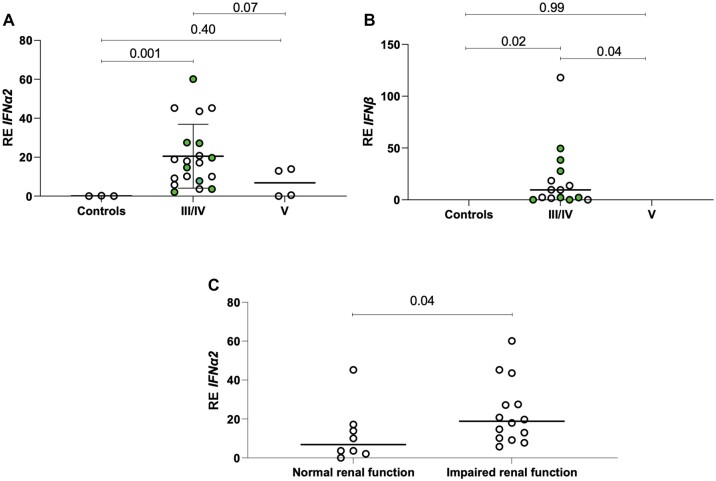
As shown in Fig. 1A and B, both IFNα2 and IFNβ transcripts were overexpressed in kidney biopsies from patients with proliferative forms of LN (III/IV) compared with both patients with membranous LN and control kidneys (RE 20.5 ± 16.4 vs 6.9 ± 7.6 vs 0.09 ± 0.10, P-values 0.07 and 0.001, respectively, for IFNα2). Such a difference was not detected between patients with membranous LN and controls (P-values 0.40 and 0.99 for IFNα2 and IFNβ, respectively). It was of interest that LN patients with impaired renal function, as attested by eGFR < 60 ml/min/1.73 m2, displayed higher IFNα2 transcript levels in renal tissues compared with those with normal renal function (RE 23.0 ± 16.2 vs 12.0 ± 14.8, P = 0.04) (Fig. 1C).
Figure 1.
Expression of type I IFN transcripts in proliferative and membranous classes of LN and clinical/histopathological associations. (A and B) IFNα2 and IFNβ transcript expression in renal biopsy tissues from patients with proliferative forms of LN (III/IV) compared with patients with membranous nephritis (V) and control kidneys; filled circles identify the concomitant presence of membranous nephritis. (C) IFNα2 transcript levels in renal tissues from LN patients with impaired renal function, as attested by eGFR < 60 ml/min/1.73 m2, compared with those with normal renal function
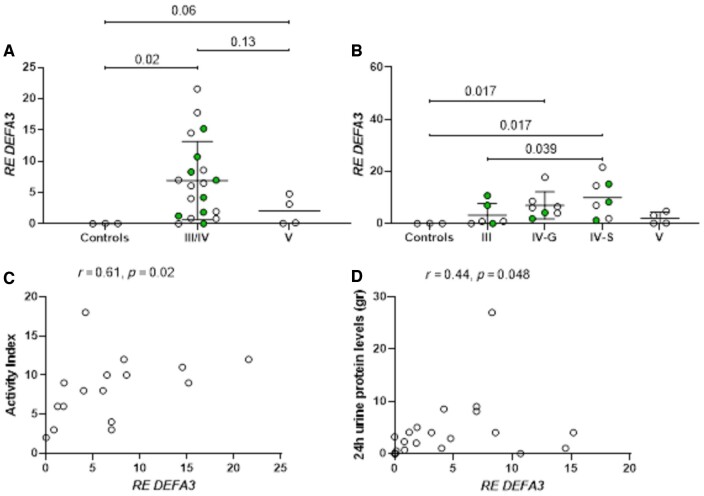
To investigate potential drivers of IFN-I expression in renal tissues of patients with LN, DEFA3 transcripts, encoding defensin-α3, a neutrophil antimicrobial product associated with NETs, as well as transcripts encoding a low-affinity neutrophil receptor for N-formyl methionyl peptides (FPRL-1), were assessed. DEFA3 transcripts were higher in biopsy tissues from patients with proliferative and membranous forms of LN compared with control tissues (RE 6.9 ± 6.3 vs 2.0 ± 2.3 vs 0.03 ± 0.03, P-values 0.02 and 0.06, respectively) (Fig. 2A), with expression in proliferative LN biopsies tending to increase from class III to class IV-G (mainly global lesions) to class IV-S (mainly segmental lesions) (Fig. 2B). The marginally significant difference detected between patients with membranous LN and control kidneys might be related to the relatively small number of patients studied, or possibly the low activity. No significant differences were detected in DEFA3 transcripts (or IFN-I transcripts) between pure proliferative (III/IV) and mixed forms (concomitant presence of III/IV and V) of LN (Supplementary Fig. S1, available at Rheumatology online), and no significant differences were observed in FPRL-1 mRNA expression among distinct LN classes (Supplementary Fig. S2, available at Rheumatology online).
Figure 2.
Expression of defensin-α3 (DEFA3) transcripts across distinct lupus nephritis classes and clinical/histopathological associations. (A) DEFA3 mRNA expression in patients with proliferative (III and IV) and membranous (V) forms of LN compared to controls; filled circles identify the concomitant presence of membranous nephritis. (B) DEFA3 mRNA expression based on pathologic features of proliferative nephritis; IV-G: Class IV LN with predominant global lesions; IV-S: Class IV LN with predominant segmental lesions. (C) Correlation between activity index in renal biopsy tissues and DEFA3 transcript expression. (D) Correlation between 24-hour urinary protein levels and DEFA3 transcript expression in renal tissues.
Of note, there was a strong association between higher activity index and DEFA3 transcripts in the renal tissue (Fig. 2C, r = 0.61, P = 0.02), suggesting a potential contribution of neutrophil products to active LN. In addition, a positive correlation between DEFA3 and IFNα2 transcripts was noted (r = 0.50, P = 0.01, Supplementary Fig. S3, available at Rheumatology online). While no other meaningful associations were detected between IFN-I or DEFA3 transcript levels and other histopathological parameters, including tubulointerstitial inflammation, vascular lesions, vascular sclerosis or tubuloreticular inclusions (data not shown), a significant correlation between DEFA3 transcripts and urinary 24-h protein levels was detected (r = 0.44, P = 0.048, Fig. 2D). No significant association was seen between IFNα2/IFNβ/DEFA3 transcript levels and low C3 (data not shown).
Discussion
The present study addresses potential mechanisms of LN in relation to the patterns of glomerular lesions in renal biopsy tissue. We found that IFN-I transcripts (both IFNα2 and IFNβ) were elevated in renal tissues from patients with proliferative forms of LN, in association with impaired renal function. Moreover, DEFA3 transcripts encoding a neutrophil-derived peptide associated with NETs were overexpressed, mainly in the proliferative forms of glomerular disease, where the presence of neutrophils and/or karyorrhexis are parameters included in the LN activity index, and were associated with 24-h urinary protein levels.
Our findings are in accord with previous observations suggesting that proliferative classes III/IV of LN are more often identified in patients displaying high IFN-I activity in their blood compared with those with low IFN-I activity [13], and that peripheral blood IFN-I scores from patients with LN based on IFN-I–induced transcript expression correlate well with IFN-I scores in renal tissues [9]. Moreover, previous transcriptomic data from kidney biopsies indicated that patients with proliferative classes of LN display activation of the IFN-I system in both their renal tubular cells and in keratinocytes in the skin, in contrast to patients with membranous LN (class V) [14]. Proteomic analysis of paraffin-embedded tissues showed that the most significant upregulated pathway involved in membranous LN was the histone deacetylase class I pathway, while in proliferative forms of LN, type I and II IFN pathways were among the highest upregulated pathways [15]. However, expression of IFN-I transcripts in renal tissues was not documented in that report or in other studies of proliferative and membranous LN samples characterized using single-cell transcriptomic analysis [9, 14, 16], potentially due to transient expression and low mRNA concentration for those transcripts. In contrast, IFNα at both mRNA and protein levels has been previously detected in tubular epithelial cells of patients with class IV LN by in situ hybridization and immunohistochemistry analysis, respectively [7, 17]. Of note, glomerular and tubulointerstitial IFN-related transcripts, along with other immune mediators such as complement, monocytes and T cell transcripts, were shown to differentiate treatment non-responders and complete clinical responders following standard therapeutic regimens for LN [18].
IFN-I plays an essential role in the host immune response to infection with viruses, but the IFN-I cytokine family is also central to the immunopathogenesis of SLE, altering the function of cells of the innate and adaptive immune systems and promoting autoimmunity [1]. Among its actions, IFN-I promotes chemokine expression and neutrophil chemotaxis as well as B cell differentiation and autoantibody production [1]. IFN-I can induce apoptosis, and it is associated with collagen production and fibrosis. In view of IFN-I’s protean biologic effects, our documentation of local IFNα2 and IFNβ expression in the kidney tissues of patients with proliferative lupus glomerulonephritis supports its likely contribution to the immunopathogenesis of LN. Current candidate therapeutics have the goal of reducing IFN-I production, blocking its receptor, or inhibiting signalling downstream from the IFN-I receptor [19]. Expression of IFNα or IFNβ in renal tissue, as we have demonstrated, may be an important determinant of the efficacy of these therapeutic strategies for LN. The preferential expression of IFN-I transcripts in kidney biopsy specimens from patients with proliferative, compared with membranous, forms of LN indicates that consideration of local IFN-I production in relation to pathologic class may be important in the design of clinical trials of therapeutics as well as for defining mechanisms of disease. As IFN-I–induced transcripts in peripheral blood cells and in renal tissue are nicely correlated [9], assessment of IFN-I pathway activation in a blood sample may be useful for identification of those patients most likely to respond to therapeutic agents targeting the IFN-I pathway.
Characterizing the relevant inducers of IFN-I transcription in LN should provide new understanding of pathogenic mechanisms and may identify novel therapeutic targets. To investigate the hypothesis that neutrophil-derived products might contribute to induction of IFN-I expression in LN, we measured two neutrophil-related transcripts in the renal tissues: DEFA3, encoding an antimicrobial protein associated with NETs, and FPRL-1, a receptor involved in neutrophil activation. DEFA3, but not FPRL-1, transcripts were significantly elevated in proliferative LN tissues and showed a moderate correlation with IFNα2 transcripts. One interpretation of these results is that augmented NETosis or impaired clearance of NETs may provide a stimulus for IFN-I production. Though the association of DEFA3 expression levels with renal disease activity will require further investigation, we hypothesize that NETs, in association with lupus autoantibodies, could represent an active inducer of IFN-I in lupus kidneys, as has been previously shown in in vitro studies, and thus contribute to renal activity [20]. Serum NET levels are heightened in incident lupus and LN and correlate with hallmarks such as anti-dsDNA autoantibodies, low complement levels and proteinuria [21]. The highest level of DEFA3 was seen in renal biopsies showing proliferative class IV LN with mainly segmental lesions, the samples most likely to be associated with tubular atrophy and fibrosis, suggesting that neutrophils may play a particularly important role in those pathologic lesions that tend to show more necrotizing features and lesser involvement of immune complex deposits [22].
This study has several limitations. Renal biopsy tissues were retrieved as frozen stored samples, with incomplete demographic and clinical data available for some samples. The lack of detailed clinical information, and the relatively small number of samples included in each class, particularly for class V, warrants caution regarding any conclusions related to clinical associations and distinctions between class III/IV and class V LN.
In conclusion, IFN-I transcripts, of both IFNα2 and IFNβ, are expressed in renal biopsy tissue from patients with proliferative forms of LN in association with impaired renal function. Moreover, the increased expression of the neutrophil-derived transcript DEFA3 points to a potential role for neutrophil NETs in IFN-I expression and LN pathogenesis. These findings justify further analyses of local IFN-I expression in renal biopsy samples and could provide guidance in clinical practice, allowing tailored therapeutic approaches based on the distinct underlying pathogenetic features of LN classes.
Supplementary Material
Contributor Information
Clio P Mavragani, Department of Medicine, Mary Kirkland Center for Lupus Research, Hospital for Special Surgery and Weill Cornell Medicine, New York, NY, USA; Department of Physiology, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece; Joint Academic Rheumatology Program, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece.
Kyriakos A Kirou, Department of Medicine, Mary Kirkland Center for Lupus Research, Hospital for Special Surgery and Weill Cornell Medicine, New York, NY, USA.
Surya V Seshan, Department of Pathology and Laboratory Medicine, Weill Cornell Medicine, New York, NY, USA.
Mary K Crow, Department of Medicine, Mary Kirkland Center for Lupus Research, Hospital for Special Surgery and Weill Cornell Medicine, New York, NY, USA.
Supplementary data
Supplementary data are available at Rheumatology online.
Data availability statement
The primary data underlying this article will be shared on reasonable request to the corresponding author.
Funding
This work was supported by the Arthritis Foundation, New York Chapter, NY (S. Niarchos Exchange Fellowship Program award to C.P.M); the Alliance for Lupus Research (Target Identification in Lupus Grant to M.K.C.); and the National Institutes of Health (R01 AI059893 to M.K.C.).
Disclosure statement: C.P.M. has received unrestricted research support from Abbvie, Pfizer and Genesis. M.K.C. serves as a consultant for AMPEL BioSolutions, Astra Zeneca, Bristol Myers Squibb, Glaxo Smith Kline, and Lilly. She has received a research grant from Gilead Pharmaceuticals. She owns public stock in Amgen, Johnson and Johnson, and Regeneron. The remaining authors have declared no conflicts of interest.
References
- 1. Crow MK. Type I interferon in the pathogenesis of lupus. J Immunol 2014;192:5459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bennett L, Palucka AK, Arce E. et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med 2003;197:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peterson KS, Huang JF, Zhu J. et al. Characterization of heterogeneity in the molecular pathogenesis of lupus nephritis from transcriptional profiles of laser-captured glomeruli. J Clin Invest 2004;113:1722–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hakkim A, Fürnrohr BG, Amann K. et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci USA 2010;107:9813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kirou KA, Lee C, George S. et al. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum 2005;52:1491–503. [DOI] [PubMed] [Google Scholar]
- 6. Feng X, Wu H, Grossman JM. et al. Association of increased interferon-inducible gene expression with disease activity and lupus nephritis in patients with systemic lupus erythematosus. Arthritis Rheum 2006;54:2951–62. [DOI] [PubMed] [Google Scholar]
- 7. Mavragani CP, Sagalovskiy I, Guo Q. et al. Expression of long interspersed nuclear element 1 retroelements and induction of type I interferon in patients with systemic autoimmune disease. Arthritis Rheumatol 2016;68:2686–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng FJ, Zhou XJ, Zhao YF. et al. Human neutrophil peptide 1–3, a component of the neutrophil extracellular trap, as a potential biomarker of lupus nephritis. Int J Rheum Dis 2015;18:533–40. [DOI] [PubMed] [Google Scholar]
- 9. Arazi A, Rao DA, Berthier CC. et al. ; Accelerating Medicines Partnership in SLE network. The immune cell landscape in kidneys of patients with lupus nephritis. Nat Immunol 2019;20:902–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gilmore AC, Wilson HR, Cairns TD. et al. ; MASTERPLANS Consortium. Immune gene expression and functional networks in distinct lupus nephritis classes. Lupus Sci Med 2022;9:e000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bajema IM, Wilhelmus S, Alpers CE. et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int 2018;93:789–96. [DOI] [PubMed] [Google Scholar]
- 12. Aringer M, Costenbader K, Daikh D. et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis 2019;78:1151–9. [DOI] [PubMed] [Google Scholar]
- 13. Iwamoto T, Dorschner JM, Selvaraj S. et al. High systemic type I interferon activity is associated with active class III/IV lupus nephritis. J Rheumatol 2022;49:388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Der E, Suryawanshi H, Morozov P. et al. ; Accelerating Medicines Partnership Rheumatoid Arthritis and Systemic Lupus Erythematosus (AMP RA/SLE) Consortium. Tubular cell and keratinocyte single-cell transcriptomics applied to lupus nephritis reveal type I IFN and fibrosis relevant pathways. Nat Immunol 2019;20:915–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen YY, Ding Y, Li LL. et al. Proteomic profiling of kidney samples in patients with pure membranous and proliferative lupus nephritis. Lupus 2022;31:837–47. [DOI] [PubMed] [Google Scholar]
- 16. Fava A, Rao DA, Mohan C. et al. ; Accelerating Medicines Partnership in Rheumatoid Arthritis and Systemic Lupus Erythematosus Network. Urine proteomics and renal single-cell transcriptomics implicate interleukin-16 in lupus nephritis. Arthritis Rheumatol 2022;74:829–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Castellano G, Cafiero C, Divella C. et al. Local synthesis of interferon-alpha in lupus nephritis is associated with type I interferons signature and LMP7 induction in renal tubular epithelial cells. Arthritis Res Ther 2015;17:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Parikh SV, Malvar A, Song H. et al. Molecular profiling of kidney compartments from serial biopsies differentiate treatment responders from non-responders in lupus nephritis. Kidney Int 2022;102:845–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paredes JL, Niewold TB.. Type I interferon antagonists in clinical development for lupus. Expert Opin Investig Drugs 2020;29:1025–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lande R, Ganguly D, Facchinetti V. et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med 2011;3:73ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bruschi M, Bonanni A, Petretto A. et al. Neutrophil extracellular traps profiles in patients with incident systemic lupus erythematosus and lupus nephritis. J Rheumatol 2020;47:377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hill GS, Delahousse M, Nochy D. et al. Class IV-S versus class IV-G lupus nephritis: clinical and morphologic differences suggesting different pathogenesis. Kidney Int 2005;68:2288–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The primary data underlying this article will be shared on reasonable request to the corresponding author.