Abstract
Objective
Gout flares during urate-lowering therapy (ULT) initiation are common, but predictors of these flares are poorly understood. The aim of this study was to determine whether serum CA72-4 is an independent predictor for gout flares during ULT initiation.
Methods
A prospective cohort study was conducted between March 2021 and January 2022. Men with gout, at least one gout flare in the past year, and at least three serum CA72-4 measurements in the previous six months were enrolled. Participants were grouped according to their highest recorded serum CA72-4 levels (above or within the normal range). All participants took oral febuxostat 20 mg daily without flare prophylaxis therapy, and attended face-to-face visits every four weeks until 24 weeks. The incidence of gout flare was compared between the two groups. Backward stepwise logistic regression analyses were used to identify risk factors associated with flares. Receiver operating characteristic curve analysis was used to evaluate prediction efficacy.
Results
A total of 193 completed the study (79 with high CA72-4; 114 with normal CA72-4). The cumulative incidence of at least one gout flare was 48.1% (62.1% in the high CA72-4 group, 38.4% in the normal CA72-4 group, P = 0.001), and recurrent (≥2) flares was 33.0% (47.1% in the high CA72-4 group, 23.2% in the normal CA72-4, P < 0.001). High CA72-4, disease duration, intra-articular tophus size, glucose, high-density lipoprotein-cholesterol and ESR were independent risk factors for gout flares. Serum CA72-4 alone predicted recurrent flares with an area under the curve of 0.63 (95% CI = 0.54, 0.71), and 0.78 (95% CI = 0.71, 0.85) when combined with other independent variables.
Conclusion
High serum CA72-4 predicts the risk of gout flares during ULT initiation.
Trial registration
ChiCTR; https://www.chictr.org.cn/; ChiCTR2100043573.
Keywords: CA72-4, gout flare, prediction
Rheumatology key messages.
Elevated baseline serum CA72-4 was independently associated with recurrent flares.
Serum CA72-4 alone predicted recurrent flares with an AUC of 0.63, and 0.78 when combined with the other five clinical variables.
Monitoring the dynamic changes of CA72-4 may allow more rational use of anti-inflammatory prophylaxis.
Introduction
Gout flares occur in over one half of patients with gout at the time of initiating urate-lowering therapy (ULT) [1]. The risk of these flares can be reduced with gradual dose escalation of urate-lowering medication and use of anti-inflammatory prophylaxis for the first 3–6 months of ULT [2, 3]. However, many gout patients have contraindications or side effects to anti-inflammatory medications (colchicine, NSAIDs, oral steroids) for flares [4]. Except for intensity of serum urate lowering, risk factors for gout flares during initiation of ULT are not well defined. Identifying which patients are at higher risk for these flares may allow more rational use of anti-inflammatory prophylaxis.
CA72-4, a glycoprotein identified by two monoclonal antibodies CC49 and B72.3, is a biomarker for gastrointestinal, pancreatic, ovary, breast and non-small cell lung cancer [5–7]. Emerging studies showed that serum CA72-4 is also elevated in several inflammatory diseases including gout and FMF [8–11]. The epitope antigen of CA72-4 is the sialosyl-a(2–6)-N-acetylgalactosamine-a-serine structure, which is carried by the unknown mucin core protein [12, 13].
There is increasing evidence that CA72-4 may be a useful biomarker in gout diagnosis and prognosis. Two limited observational studies reported that serum CA72-4 was elevated in patients with gout [8, 9]. Our group reported the highest level of serum CA72-4 in patients with gout among 37 diseases [14]. In an observational study in 833 gout patients (in comparisons with 120 hyperuricemia, 1292 non-gouty arthritis and 541 heathy controls), we reported that serum CA72-4 was specifically elevated in gout and that high CA72-4 level (exceeding the upper limit of normal) was a strong predictor of gout flares over a six-month period [11]. A key outstanding question following this study was whether serum CA72-4 is an independent predictor for gout flare, or whether other confounding variables account for the observed association.
To address if serum CA72-4 is an independent predictor for gout flare, we conducted a 24-week prospective cohort study of gout patients initiating ULT with fixed low-dose febuxostat.
Methods
Study design and procedures
A prospective ULT cohort study was conducted with the primary aim to determine whether serum CA72-4 is an independent predictor for gout flare. Men with gout visiting the clinic were screened consecutively. Eligible patients were recruited and underwent a two-week washout period. All participants were then treated with fixed low-dose febuxostat and attended face-to-face visits every 4 weeks until 24 weeks. Gout flare incidence over the preceding 4 weeks was recorded at each study visit. A gout flare was defined as an episode with more than two signs/symptoms of joint swelling, tenderness and redness, which was deemed (by the participants and/or the investigator) to require treatment with an anti-inflammatory medication [2, 15].
This study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University and registered in Chinese Clinical Trial Registration Center (#ChiCTR2100043573). All participants provided written informed consent.
Participants
Eligibility criteria were male sex, age ≥18 years old, meeting the 2015 American College of Rheumatology/European League Against Rheumatism classification criteria for gout [16], serum CA72-4 measurement at least three times during the preceding 6 months, and at least one gout flare within the past 12 months. As serum CA72-4 levels can fluctuate over time, this approach was taken to identify patients with a persistently normal CA72-4 level. Key exclusion criteria were baseline serum urate <7 mg/dl, estimated glomerular filtration (eGFR) <45 mL/min/1.73m2, and transaminases >2-fold of the upper normal limit. In this study, participants with CA72-4 >6.9 U/ml (the upper limit of normal) at least once were included in the high CA72-4 group, and participants with all CA72-4 measurements within the normal range were included in the normal CA72-4 group.
Urate-lowering therapy
All participants underwent a two-week washout period with discontinuation of drugs affecting serum urate level or occasional ULT and anti-inflammatory drugs, and a low-purine diet at least 5 days before the baseline visit. Participants then took oral febuxostat 20 mg once every morning for 24 weeks. No anti-inflammatory gout flare prophylaxis was used. If a gout flare occurred during the trial period, participants were additionally given etoricoxib 120 mg per day for 3–5 days as a regular treatment. Polyene phosphatidylcholine was given for liver protection if the serum transaminases exceeded two times of the normal upper limit. Participants who discontinued medication for three consecutive days during the study were identified as withdrawn from the study.
Variables and data collection
Parameters obtained at the baseline visit included age, height, body weight, blood pressure, comorbidities, medication, gout history (age at onset, disease duration, family history and gout flares in the last 6 months), palpable and ultrasonographic tophi. Ultrasonography of the knees, ankles and feet were performed by the same physician. Crystal deposition or aggregation was recorded, including the double-contour sign, the number and size of tophi inside the articular cavity or para-articular. The diameter of the largest tophus was selected for evaluating size of the intra-articular tophi. BMI ≥28 kg/m2 was defined as obesity [17, 18]. Laboratory assessment included serum CA72-4, serum urate, creatinine (Cr), glucose, triglyceride, total cholesterol, high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), alanine aminotransferase (ALT), aspartate aminotransferase (AST), CRP and ESR.
Serum CA72-4 was measured by an electrochemiluminescence method using Elecsys kits (CA72-4/11776258 122, Roche Diagnostics GmbH, Mannheim, Germany), CRP by the latex-enhanced turbidimetric method, and ESR by Widmanstaten natural sedimentation method. Biochemical parameters were measured by an automatic biochemical analyser (TBA-40FR, Toshiba Company, Tokyo, Japan). The eGFR was used to assess kidney function and calculated as follows: Cr ≤0.9 mg/dl, eGFR = 141 × (Cr/0.9)–0.411 × (0.993)age; Cr >0.9 mg/dl, eGFR = 141 × (Cr/0.9)−1.209 × (0.993)age [19].
Sample size
According to our previous study, the incidence of gout flare was 70.91% in patients with high serum CA72-4 level and 60.81% in patients with normal level, difference in rate of gout flare was 10.10% [11]. Based on these data, it was estimated that 65 in the high CA72-4 group and 98 in the normal CA72-4 group (1:1.5 ratio) would provide >85% power at a significance level of 0.05. Considering an attrition rate of ∼20%, at least 88 in the high CA72-4 group and 122 in the normal CA72-4 group participants were needed, and finally 87 and 125 participants included in two groups, respectively.
Statistical analyses
All analyses were performed using SPSS 26.0 (IBM, Armonk, NY, USA). P-value <0.05 was considered statistically significant. Continuous variables were expressed as mean (S.D.) or median (IQR), and categorical variables were expressed as number (percentage). Independent sample t-test or Wilcoxon sign-rank test were used to compare continuous variables and chi-squared test was used for categorical variables between the two groups. The flare-free and recurrent gout flares-free survival proportions of participants in different groups were estimated by Kaplan–Meier curves. Generalized estimating equations (GEE) test was also used to test associations between flares and serum CA72-4.
A backward stepwise logistic regression was used to identify possible predictors of recurrent gout flares out of the following covariates based on their biological relevance: age, BMI, comorbidities, disease duration, positive family history, palpable tophus, intra-articular tophus size, at least one flare in the last 6 months, high CA72-4, serum urate, eGFR, glucose, triglyceride, total cholesterol, HDL-C, LDL-C, ALT, AST, CRP, ESR. Comorbidities, positive family history, palpable tophus, at least one flare in the last 6 months and high CA72-4 were treated as dichotomous variables and others were continuous variables. Variables with P-values <0.1 were accepted in the model. Results are expressed as odds ratio (OR) and 95% confidence interval (95% CI). Subsequently, the receiver operating characteristic (ROC) curve analyses were performed with each independent variable included in the multivariate logistic regression to evaluate the prediction efficacy of recurrent gout flares.
Results
Participants and baseline characteristics
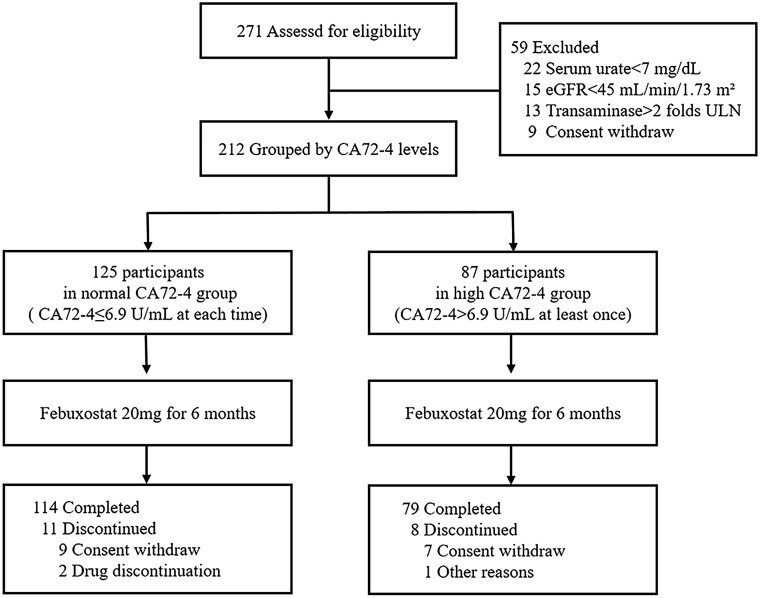
The study commenced in March 2021, and the last participant was enrolled in July 2021. Follow-up was completed in January 2022. A total of 271 patients were screened and 212 eligible participants were enrolled; of these 87 were classified as high CA72-4 and 125 as normal CA72-4. There were 193 (91.0%) participants who completed the 24-week study (Fig. 1). Of the 19 participants who dropped out, the leading cause was withdrawal of consent, one in the high CA72-4 group was lost to follow-up, and two in the normal CA72-4 group was due to adverse effects of febuxostat.
Figure 1.
Flow chart
Demographic and clinical characteristics were shown in Table 1. Participants were predominantly middle-aged with a mean (s.d.) age of 45.3 (12.4) years, and a mean (s.d.) BMI of 27.2 (3.6) kg/m2. The median duration of gout was 7.0 years, 13.2% of participants had palpable tophi and 87.7% had at least one comorbidity. Most baseline features were comparable between the high and normal CA72-4 groups, except for more intra-articular tophus (70.1% vs 56.0%, P = 0.037), larger intra-articular tophus size (9.0 mm vs 7.0 mm, P = 0.029), more participants had at least one flare in the last 6 months (54.0% vs 40.0%, P = 0.005) and higher median CRP (1.6 mg/l vs 0.9 mg/l, P = 0.001) in the high CA72-4 group.
Table 1.
Baseline characteristics
| Normal CA72-4 (n = 87) | High CA72-4 (n = 125) | P-value | |
|---|---|---|---|
| Demographic and general characteristics | |||
| Age (years) | 44.0 (12.1) | 47.3 (12.6) | 0.057 |
| Height (cm) | 175.5 (6.5) | 174.8 (5.1) | 0.361 |
| Body weight (kg) | 84.5 (13.9) | 82.3 (12.2) | 0.230 |
| BMI (kg/m2) | 27.4 (3.9) | 26.9 (3.3) | 0.336 |
| Systolic BP (mmHg) | 135.1 (15.8) | 138.2 (17.3) | 0.190 |
| Diastolic BP (mmHg) | 87.8 (10.6) | 90.0 (11.5) | 0.173 |
| Comorbidities | |||
| Obesity | 48 (38.4%) | 26 (29.9%) | 0.201 |
| Hypertension | 34 (27.2%) | 30 (34.5%) | 0.256 |
| Cardiovascular disease | 2 (1.6%) | 2 (2.3%) | 0.722 |
| Fatty liver | 68 (54.4%) | 50 (57.5%) | 0.658 |
| Hyperlipidemia | 59 (47.2%) | 43 (49.4%) | 0.750 |
| Diabetes | 17 (13.6%) | 12 (13.8%) | 0.968 |
| Kidney stones | 16 (12.8%) | 12 (13.8%) | 0.834 |
| Gout history and examination | |||
| Onset age (years) | 36.6 (10.4) | 38.5 (11.1) | 0.209 |
| Disease duration (years) | 6.0 (3.0-10.0) | 8.0 (3.0-12.0) | 0.193 |
| Family history of gout | 28 (22.4%) | 21 (24.1%) | 0.768 |
| Palpable tophus | 14 (11.2%) | 14 (16.1%) | 0.301 |
| Intra-articular tophus | 70 (56.0%) | 61 (70.1%) | 0.037 |
| Intra-articular tophus size (mm) | 8.0 (0.0-12.0) | 9.0 (0.0-14.0) | 0.029 |
| At least one flare in the last 6 months | 50 (40.0%) | 47 (54.0%) | 0.005 |
| Laboratory measures | |||
| CA72-4 (U/mL) | 1.3 (1.0-1.8) | 5.5 (3.2-9.2) | <0.001 |
| Serum urate (mg/dL) | 9.5 (1.3) | 9.6 (1.5) | 0.552 |
| eGFR (mL/min/1.73 m2) | 88.4 (17.7) | 83.8 (18.4) | 0.072 |
| Glucose (mmol/L) | 5.9 (5.5-6.2) | 5.8 (5.6-6.3) | 0.685 |
| Triglyceride (mmol/L) | 1.7 (1.3-2.4) | 1.9 (1.3-2.7) | 0.521 |
| Total cholesterol (mmol/L) | 5.0 (1.0) | 5.0 (0.9) | 0.778 |
| HDL-C (mmol/L) | 1.2 (1.0-1.4) | 1.2 (1.0-1.4) | 0.279 |
| LDL-C (mmol/L) | 3.5 (0.9) | 3.5 (0.8) | 0.997 |
| ALT (U/L) | 25.0 (16.0-39.0) | 24.0 (16.0-31.0) | 0.282 |
| AST (U/L) | 20.0 (17.0-27.0) | 20.0 (17.0-25.0) | 0.866 |
| CRP (mg/L) | 0.9 (0.5-1.6) | 1.6 (1.0-3.5) | 0.001 |
| ESR (mm/h) | 6.0 (3.0-9.0) | 7.0 (3.8-14.0) | 0.064 |
Data are n (%), mean (s.d.), or median (IQR).
ALT: alanine aminotransferase; AST: aspartate aminotransferase; eGFR: estimated glomerular filtration rate; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol.
During the 24-week follow-up, there was no difference in adverse events between the two groups (Supplementary Data S1, available at Rheumatology online).
Gout flares according to CA72-4 groups
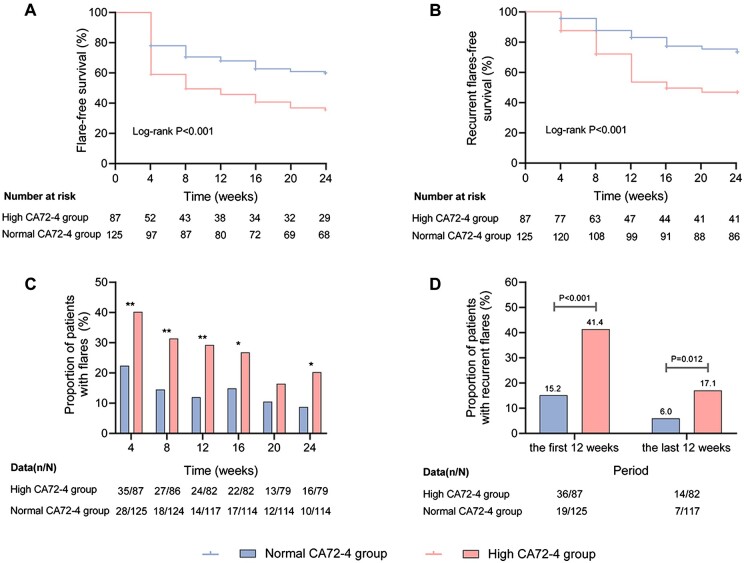
Over the 24 weeks of follow-up, the cumulative incidence of gout flares was 48.1% in all participants (62.1% in the high CA72-4 group, 38.4% in the normal CA72-4 group, P = 0.001). A single gout flare occurred in 15.1% participants (14.9% in the high CA72-4 group, 15.2% in the normal CA72-4 group, P > 0.05), and recurrent (≥2) gout flares in 33.0% participants (47.1% in the high CA72-4 group, 23.2% in the normal CA72-4 group, P < 0.001) (Fig. 2A, B).
Figure 2.
Gout flares in the high and normal CA72-4 group over the 24-week study period. (A) Kaplan Meier analysis of the accumulated flare-free survival curves. (B) Kaplan Meier analysis of the accumulated recurrent (≥2) flares-free survival curves. (C) The proportion of patients with flares. (D) The proportion of patients with recurrent (≥2) flares. *means comparisons between the two groups P < 0.05; **means comparisons between the two groups P < 0.01
The incidence of flares in the preceding 4 weeks reduced gradually in both groups (for the high CA72-4 group: 40.2% in week 4 and 20.3% in week 24; for the normal CA72-4 group: 22.4% in week 4 and 8.8% in week 24), and was higher in the high CA72-4 group than in the normal CA72-4 group at every visit, except for week 20 (P < 0.01 at the first 12 weeks, P < 0.05 at week 16 and week 24) (Fig. 2C). Recurrent flares were more common in the high CA72-4 group in the first 12 weeks (41.4% vs 15.2%, P < 0.001) and the last 12 weeks of follow-up (17.1% vs 6.0%, P = 0.012) (Fig. 2D). GEE analysis confirmed that high baseline CA72-4 was associated with gout flare over the 24-week period (B Coefficient = 0.970, P = 0.025).
Clinical variables and their associations with the incidence of recurrent gout flares
Table 2 reports the univariate analysis for recurrent gout flares as well as the multivariate logistic regression analysis. Starting with 20 covariates that might theoretically be predictors of recurrent gout flares, the stepwise logistic regression model reduced them to 7. Among these independent variables, high CA72-4 level (OR = 2.34, 95% CI = 1.14, 4.82), larger intra-articular tophus size (OR = 1.97, 95% CI = 1.19, 3.24), longer disease duration (OR = 1.08, 95% CI = 1.01, 1.16) and increasing ESR (OR = 1.06, 95% CI = 1.01, 1.12) were associated with an increased likelihood of recurrent gout flares, while increasing glucose (OR = 0.51, 95% CI = 0.27, 0.97) and HDL-C (OR = 0.13, 95% CI = 0.03, 0.61) were associated with its reduction (P < 0.05). In addition, at least one flare in the last 6 months was also included in the multiple regression model, but it was not statistically significant (OR = 1.87, 95% CI = 0.91, 3.82, P = 0.087).
Table 2.
Logistic regression models for factors associated with recurrent flares
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
|||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Age (years) | 1.02 (0.99, 1.04) | 0.235 | ||||
| BMI (kg/m2) | 1.00 (0.92, 1.09) | 0.958 | ||||
| ≥1 Comorbidities | 1.63 (0.57, 4.71) | 0.364 | ||||
| Disease duration (years) | 1.07 (1.01, 1.12) | 0.013 | 1.01 (1.08, 1.15) | 0.032 | 1.08 (1.01, 1.16) | 0.026 |
| Positive family history | 1.24 (0.61, 2.53) | 0.548 | ||||
| Palpable tophus | 2.84 (1.21, 6.67) | 0.017 | ||||
| Intra-articular tophus size (mm) | 2.83 (1.55, 3.68) | <0.001 | 2.00 (1.22, 3.28) | 0.006 | 1.97 (1.19, 3.24) | 0.008 |
| At least one flare in the last 6 months | 1.97 (1.08, 3.59) | 0.028 | 1.86 (0.92, 3.76) | 0.085 | 1.87 (0.91, 3.82) | 0.087 |
| High CA72-4a | 2.85 (1.54, 5.26) | 0.001 | 2.41 (1.18, 4.91) | 0.016 | 2.34 (1.14, 4.82) | 0.021 |
| Serum urate (mg/dL) | 1.31 (1.05, 1.63) | 0.017 | ||||
| eGFR(mL/min/1.73m2) | 0.98 (0.97, 1.00) | 0.034 | ||||
| Glucose (mmol/L) | 0.67 (0.41, 1.10) | 0.116 | 0.55 (0.31, 0.98) | 0.041 | 0.51 (0.27, 0.97) | 0.040 |
| Triglyceride (mmol/L) | 1.23 (0.99, 1.53) | 0.056 | ||||
| Total cholesterol (mmol/L) | 1.15 (0.84, 1.58) | 0.391 | ||||
| HDL-C (mmol/L) | 0.25 (0.07, 0.87) | 0.029 | 0.12 (0.03, 0.54) | 0.006 | 0.13 (0.03, 0.61) | 0.010 |
| LDL-C (mmol/L) | 1.05 (0.75, 1.49) | 0.761 | ||||
| ALT (U/L) | 0.99 (0.97, 1.01) | 0.377 | ||||
| AST (U/L) | 0.99 (0.96, 1.03) | 0.740 | ||||
| CRP (mg/L) | 1.04 (0.98, 1.11) | 0.204 | ||||
| ESR (mm/h) | 1.07 (1.02, 1.12) | 0.003 | 1.06 (1.01, 1.11) | 0.025 | 1.06 (1.01, 1.12) | 0.020 |
Model 1 followed the backward stepwise selection rule. Model 2 was adjusted for the usage of anti-hypertensive drugs, glucose-lowering drugs, liver and kidney protecting drugs during the 24-week follow-up, and irregular ULT without a prescription in the preceding six months before enrolment.
CA72-4 exceeded the normal upper limit at least once in the last 6 months.
95% CI: 95% confidence interval; ALT: alanine aminotransferase; AST: aspartate aminotransferase; eGFR: estimated glomerular filtration rate; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; OR: odds ratio.
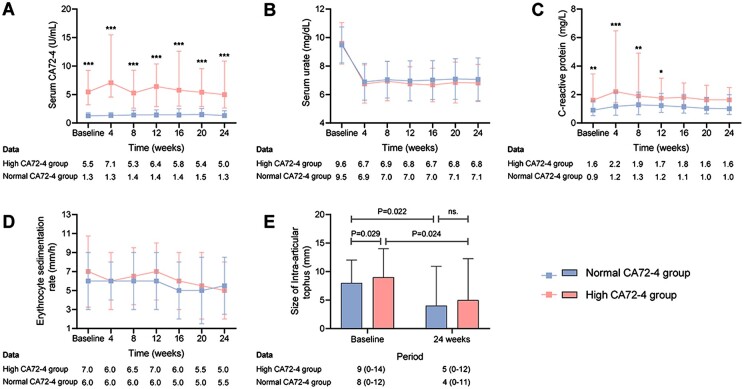
During the 24-week period, the serum CA72-4 level in the high CA72-4 group remained higher than in the normal CA72-4 group (P < 0.001 at every visit, Fig. 3A). Serum urate reduced in both groups, with no between-group differences throughout the study period (Fig. 3B). For inflammatory markers, CRP was higher in the high CA72-4 group at the first 12 weeks (Fig. 3C), while ESR did not differ between the two groups (Fig. 3D). Intra-articular tophus size reduced in both groups (P < 0.05), and did not differ between groups at week 24 (5.0 mm vs 4.0 mm, P > 0.05) (Fig. 3E).
Figure 3.
Other clinical and laboratory variables in the high and normal CA72-4 group over the 24-week study period. (A) Serum CA72-4. (B) Serum urate. (C) C-reactive protein. (D) Erythrocyte sedimentation rate. (E) Size of intra-articular tophus. Data are expressed as median (IQR), except serum urate as mean (s.d.). *means comparisons between the two groups P <0.05; **means comparisons between the two groups P < 0.01; ***means comparisons between the two groups P < 0.001
Prediction efficacy for recurrent flares
High CA72-4 (>6.9U/ml) could predict recurrent gout flares during initiation of ULT with the AUC of 0.63 (95% CI = 0.54, 0.71), with a sensitivity of 0.58 and a specificity of 0.68. When CA72-4 was included as a continuous variable in the ROC analysis, the cut-off value was 24.58, and the AUC was 0.67 (95% CI = 0.58, 0.75), with the sensitivity 0.32 and the specificity 0.96 (Supplementary Fig. S1. available at Rheumatology online). Considering the poor sensitivity of the latter, we chose high CA72-4 combined with other independent variables identified by the stepwise logistic regression model, including disease duration, intra-articular tophus size, glucose, HDL-C and ESR to predict recurrent gout flares, with the AUC of 0.78 (95% CI = 0.71, 0.85) (Fig. 4). Meanwhile, of these six independent variables, high CA72-4 had the highest AUC for future recurrent gout flares among easily measurable serum parameters, and was second only to intra-articular tophus size (Supplementary Table S1, available at Rheumatology online).
Figure 4.
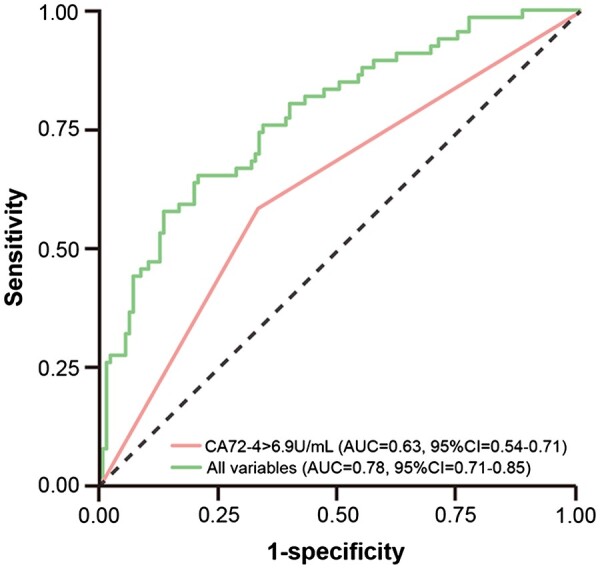
ROC curves for recurrent gout flares. All variables including CA72-4 >6.9U/mL, disease duration, intra-articular tophus size, glucose, high-density lipoprotein cholesterol and ESR. AUC: area under the curve; ROC: receiver operating characteristic.
Discussion
Here, we report the first prospective cohort study to examine the association between serum CA72-4 and gout flares following ULT initiation. These findings provide evidence that baseline elevated serum CA72-4 is associated with gout flare incidence independent of other risk factors, and can assist with prediction of gout flares in the first 6 months of ULT. The ROC curve analysis of recurrent gout flares showed that AUC was 0.63 for serum CA72-4 >6.9 U/ml, and the AUC was 0.78 when combined with disease duration, intra-articular tophus size, glucose, HDL-C and ESR. These novel findings have identified patients who are most likely to flare during initiation of ULT, and suggest the potential to stratify patients who may benefit from gout flare prophylaxis drug treatments.
A variety of risk factors for recurrent flares have been reported, including periods of alcohol consumption, high diet-purine consumption, high serum urate, rapid drop of serum urate, longer disease duration, obesity and hypertriglyceridemia, male sex, and cardiometabolic comorbidities [20–26]. However, those risk factors, alone or in a composite manner, have not satisfactorily identified the patients with the highest frequency of flares over a prolonged period of time following ULT initiation [25]. A prior study developed a prediction model for gout flares in hospitalized patients with comorbid gout using nine clinical variables (pre-admission urate >0.36 mmol/l, tophus, no pre-admission ULT, no pre-admission gout prophylaxis, acute kidney injury, surgery, initiation or increase of gout prophylaxis, adjustment of ULT and diuretics prior to flare), and achieved adequate accuracy [27]. Given the information to date, and the assumption that aetiology of gout flare is heterogeneous, reliable markers of gout flare susceptibility remain an unmet need. In this study, participants with high concentration of baseline serum CA72-4 experienced more gout flares in terms of monthly incidence, cumulative incidence or recurrent gout during ULT initiation. Among all parameters with clinical importance, CA72-4 was an independent risk factor for recurrent gout flares, verified by the multivariate logistic model. The AUC was 0.63 for CA72-4 >6.9U/ml solely and up to 0.78 by combining CA72-4 >6.9U/ml with other five associated clinical variables. The findings suggest that serum CA72-4 is a potential marker for identifying those with recurrent gout flares who might benefit from anti-inflammatory prophylaxis treatment. A prospective controlled interventional study using anti-inflammatory prophylaxis selectively in those with high baseline CA72-4 could test this hypothesis.
The variables contributing to gout flares in ROC analysis are clinically meaningful. The natural history of gout includes the progression from asymptomatic hyperuricemia to first flare, recurrent flares and chronic gout and tophi, especially when the disease is undertreated by ULT over time. MSU crystals induce the activation of NLRP3 inflammasome in macrophages and monocytes, triggering a gout flare and the recruitment of neutrophils. The formation of adequate neutrophil extracellular traps contributes to the resolution of a flare, as well as the formation of tophi [28]. Chronic inflammation induced by sustained flare or active tophi involves the activation of both innate immune and adaptive immune [29]. In that case, ESR may increase as reported by several studies [30]. In prolonged disease, both continuing flare activity and elevated ESR predicted those predisposed to more flares going forward. A reciprocal association between dyslipidaemia (hypertriglyceridemia and low HDL-C) and chronic low-grade inflammation has been established by several studies [31–33]. In this study, lower HDL-C predicted recurrent gout flares during ULT, indicating that dyslipidaemia might facilitate gouty inflammation, which corroborated a previous study [34]. Moreover, results of this study showed that tophus sizes were robust predictors for recurrent gout flares, consistent with previous studies [35, 36]. In this study, higher glucose levels are associated with lower incidence of recurrent gout flares, potentially because the majority of urate removal from the body is dependent on renal urate excretion, and a high urinary excretion rate of glucose level is inversely associated with serum urate level [37].
The reasons why serum CA72-4 is elevated in gout patients remain unclear. We speculate that increased CA72-4 in gout patients could be a consequence of urate priming or MSU crystal induced phagocyte or mucosal cell activation, as in other cases with innate immune activation induced by intrinsic abnormality like FMF, or an agonist such as ganoderma lucidium spore [8–11, 38]. CA72–4 is a saccharide structure of an unknown protein [12, 13]. Glycosylation is the most diverse and common posttranslational modification of proteins. In some inflammatory settings, cell membrane glycan structures often exhibit dramatic changes and incomplete synthesis followed by accumulation of precursor structures, which may be responsible for the epitope generation [14]. It appears that CA72-4 may be more predisposed than other cancer biomarkers to be elevated in those with active innate immune inflammatory states. CA72-4 is not only expressed in tumour tissues but also in normal tissues such as the secretory endometrium and transitional colonic mucosa, which indicates that CA72-4 is not a unique product of cancer cells. It is still unclear what stimuli trigger CA72-4 production.
The main limitation of this study is that all subjects in the study were male and Chinese, so it is uncertain whether the association of CA72-4 with gout flares can be extended to females and other ethnicities. In addition, our ULT regimen used a standardized fixed low dose of febuxostat, rendering the control of serum urate levels incomplete in some participants. Therefore, the study findings may not be generalizable to patients treated with other febuxostat doses, other urate-lowering drugs, or with no urate lowering at all. Our study only recruited participants with relatively normal renal function, and the results may not be generalized to patients with severe kidney disease.
In conclusion, this study demonstrated that elevated serum CA72-4 predicts the risk of gout flares during ULT initiation, especially when combined with other variables. Monitoring the dynamic changes of CA72-4 may have potential to identify which patients may benefit from gout flare prophylaxis during ULT initiation.
Supplementary Material
Acknowledgements
We thank our patients, as well as the doctors and nurses who assisted in this study. R.T. was supported by the NIH (#AR060772, #AR075990) and the VA Research Service (#I01BX001660).
Contributor Information
Shuhui Hu, Shandong Provincial Key Laboratory of Metabolic Diseases and Qingdao Key Laboratory of Gout, The Affiliated Hospital of Qingdao University, Qingdao, China; Department of Endocrinology and Metabolism, The Affiliated Hospital of Qingdao University, Qingdao, China; Institute of Metabolic Diseases, Qingdao University, Qingdao, China; Shandong Provincial Clinical Research Center for Immune Diseases and Gout, Qingdao, China.
Mingshu Sun, Shandong Provincial Clinical Research Center for Immune Diseases and Gout, Qingdao, China; Department of Rheumatology, The Affiliated Hospital of Qingdao University, Qingdao, China.
Maichao Li, Shandong Provincial Key Laboratory of Metabolic Diseases and Qingdao Key Laboratory of Gout, The Affiliated Hospital of Qingdao University, Qingdao, China; Department of Endocrinology and Metabolism, The Affiliated Hospital of Qingdao University, Qingdao, China.
Xiaomei Xue, Shandong Provincial Key Laboratory of Metabolic Diseases and Qingdao Key Laboratory of Gout, The Affiliated Hospital of Qingdao University, Qingdao, China; Department of Endocrinology and Metabolism, The Affiliated Hospital of Qingdao University, Qingdao, China.
Robert Terkeltaub, VA San Diego VA Healthcare Center, University of California San Diego, San Diego, USA.
Can Wang, Shandong Provincial Key Laboratory of Metabolic Diseases and Qingdao Key Laboratory of Gout, The Affiliated Hospital of Qingdao University, Qingdao, China.
Ming Wang, Shandong Provincial Key Laboratory of Metabolic Diseases and Qingdao Key Laboratory of Gout, The Affiliated Hospital of Qingdao University, Qingdao, China; Department of Endocrinology and Metabolism, The Affiliated Hospital of Qingdao University, Qingdao, China.
Jie Lu, Shandong Provincial Key Laboratory of Metabolic Diseases and Qingdao Key Laboratory of Gout, The Affiliated Hospital of Qingdao University, Qingdao, China; Department of Endocrinology and Metabolism, The Affiliated Hospital of Qingdao University, Qingdao, China; Institute of Metabolic Diseases, Qingdao University, Qingdao, China; Shandong Provincial Clinical Research Center for Immune Diseases and Gout, Qingdao, China.
Zijing Ran, Shandong Provincial Key Laboratory of Metabolic Diseases and Qingdao Key Laboratory of Gout, The Affiliated Hospital of Qingdao University, Qingdao, China; Department of Endocrinology and Metabolism, The Affiliated Hospital of Qingdao University, Qingdao, China.
Hailong Li, Institute of Metabolic Diseases, Qingdao University, Qingdao, China.
Aichang Ji, Institute of Metabolic Diseases, Qingdao University, Qingdao, China.
Wenyan Sun, Shandong Provincial Key Laboratory of Metabolic Diseases and Qingdao Key Laboratory of Gout, The Affiliated Hospital of Qingdao University, Qingdao, China.
Xinde Li, Shandong Provincial Key Laboratory of Metabolic Diseases and Qingdao Key Laboratory of Gout, The Affiliated Hospital of Qingdao University, Qingdao, China.
Yuwei He, Shandong Provincial Key Laboratory of Metabolic Diseases and Qingdao Key Laboratory of Gout, The Affiliated Hospital of Qingdao University, Qingdao, China.
Zhen Liu, Shandong Provincial Key Laboratory of Metabolic Diseases and Qingdao Key Laboratory of Gout, The Affiliated Hospital of Qingdao University, Qingdao, China.
Hui Zhang, Institute of Metabolic Diseases, Qingdao University, Qingdao, China.
Xuefeng Wang, Shandong Provincial Key Laboratory of Metabolic Diseases and Qingdao Key Laboratory of Gout, The Affiliated Hospital of Qingdao University, Qingdao, China.
Xiaopeng Ji, Shandong Provincial Key Laboratory of Metabolic Diseases and Qingdao Key Laboratory of Gout, The Affiliated Hospital of Qingdao University, Qingdao, China.
Nicola Dalbeth, Department of Medicine, University of Auckland, Auckland, New Zealand.
Changgui Li, Shandong Provincial Key Laboratory of Metabolic Diseases and Qingdao Key Laboratory of Gout, The Affiliated Hospital of Qingdao University, Qingdao, China; Department of Endocrinology and Metabolism, The Affiliated Hospital of Qingdao University, Qingdao, China; Institute of Metabolic Diseases, Qingdao University, Qingdao, China; Shandong Provincial Clinical Research Center for Immune Diseases and Gout, Qingdao, China.
Supplementary material
Supplementary data are available at Rheumatology online.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Contribution statement
S.H., M.S., N.D. and C.L. contributed to study design. S.H., M.S., M.L., X.X., C.W., M.W., J.L., Z.R., H.L., A.J., W.S., X.L., Y.H., Z.L., H.Z., X.W., X.J. and C.L. contributed to study analysis. All authors contributed to interpretation of results. S.H., M.S., M.L., X.X., R.T. and N.D. drafted the manuscript, and all authors reviewed and revised the manuscript for content.
Funding
This work was supported by the National Natural Science Foundation of China (#81871288, #31900413), Shandong Provincial Key Research and Development Plan (Major Scientific and Technological Innovation Project, #2021CXGC011103) and Shandong Provincial Science Foundation for Outstanding Youth Scholars (#ZR2021YQ56).
Disclosure statement: N.D. reports grants and personal fees from AstraZeneca, grants from Amgen, personal fees from Dyve BioSciences, personal fees from JW Pharmaceuticals, personal fees from Selecta, personal fees from Arthrosi, personal fees from Horizon, personal fees from Abbvie, personal fees from Janssen, personal fees from PK Med, outside the submitted work. R.T. has received research funding from Astra-Zeneca, and has consulted with Horizon, Selecta, SOBI, and Astra-Zeneca, Allena, Fortress Bio and LG Life Sciences.
References
- 1. Shiozawa A, Szabo SM, Bolzani A, Cheung A, Choi HK.. Serum uric acid and the risk of incident and recurrent gout: a systematic review. J Rheumatol 2017;44:388–96. [DOI] [PubMed] [Google Scholar]
- 2. Yamanaka H, Tamaki S, Ide Y. et al. Stepwise dose increase of febuxostat is comparable with colchicine prophylaxis for the prevention of gout flares during the initial phase of urate-lowering therapy: results from FORTUNE-1, a prospective, multicentre randomised study. Ann Rheum Dis 2018;77:270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borstad GC, Bryant LR, Abel MP. et al. Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis. J Rheumatol 2004;31:2429–32. [PubMed] [Google Scholar]
- 4. Pascart T, Lioté F.. Gout: state of the art after a decade of developments. Rheumatology 2019;58:27–44. [DOI] [PubMed] [Google Scholar]
- 5. Nuti M, Teramoto YA, Mariani-Costantini R. et al. A monoclonal antibody (B72.3) defines patterns of distribution of a novel tumor-associated antigen in human mammary carcinoma cell populations. Int J Cancer 1982;29:539–45. [DOI] [PubMed] [Google Scholar]
- 6. Xu Y, Zhang P, Zhang K, Huang C.. The application of CA72-4 in the diagnosis, prognosis, and treatment of gastric cancer. Biochim Biophys Acta Rev Cancer 2021;1876:188634. [DOI] [PubMed] [Google Scholar]
- 7. Mariampillai AI, Cruz JPD, Suh J. et al. Cancer antigen 72-4 for the monitoring of advanced tumors of the gastrointestinal tract, lung, breast and ovaries. Anticancer Res 2017;37:3649–56. [DOI] [PubMed] [Google Scholar]
- 8. Zhao B, Zhang M, Liang Y, Yang Z.. An abnormal elevation of serum CA72-4 rather than other tumor markers can be caused by use of colchicine. Int J Biol Markers 2019;34:318–21. [DOI] [PubMed] [Google Scholar]
- 9. Zhao B, Zhang M, Xie J. et al. An abnormal elevation of serum CA72-4 due to taking colchicine. Clin Chem Lab Med 2017;56:e13–5. [DOI] [PubMed] [Google Scholar]
- 10. Balaban YH, Simsek H, Yilmaz R. et al. Tumor markers in familial Mediterranean fever and their correlation with the frequency of attacks. Clin Exp Rheumatol 2008;26:S114–6. [PubMed] [Google Scholar]
- 11. Bai X, Sun M, He Y. et al. Serum CA72-4 is specifically elevated in gout patients and predicts flares. Rheumatology 2020;59:2872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnson VG, Schlom J, Paterson AJ. et al. Analysis of a human tumor-associated glycoprotein (TAG-72) identified by monoclonal antibody B72.3. Cancer Res 1986;46:850–7. [PubMed] [Google Scholar]
- 13. Thor A, Ohuchi N, Szpak CA, Johnston WW, Schlom J.. Distribution of oncofetal antigen tumor-associated glycoprotein-72 defined by monoclonal antibody B72.3. Cancer Res 1986;46:3118–24. [PubMed] [Google Scholar]
- 14. Zhang Y, Zhang M, Bai X, Li C, Zhang L.. Increased serum CA724 levels in patients suffering gout vs cancers. Prog Mol Biol Transl Sci 2019;162:177–86. [DOI] [PubMed] [Google Scholar]
- 15. Sundy JS, Schumacher HR, Kivitz A. et al. Rilonacept for gout flare prevention in patients receiving uric acid-lowering therapy: results of RESURGE, a phase III, international safety study. J Rheumatol 2014;41:1703–11. [DOI] [PubMed] [Google Scholar]
- 16. Neogi T, Jansen TLTA, Dalbeth N. et al. 2015 Gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2015;74:1789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou B-F; Cooperative Meta-Analysis Group of the Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci 2002;15:83–96. [PubMed] [Google Scholar]
- 18. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63. [DOI] [PubMed] [Google Scholar]
- 19. Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rothenbacher D, Primatesta P, Ferreira A, Cea-Soriano L, Rodríguez LAG.. Frequency and risk factors of gout flares in a large population-based cohort of incident gout. Rheumatology 2011;50:973–81. [DOI] [PubMed] [Google Scholar]
- 21. Singh JA, Reddy SG, Kundukulam J.. Risk factors for gout and prevention: a systematic review of the literature. Curr Opin Rheumatol 2011;23:192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Neogi T, Chen C, Niu J. et al. Alcohol quantity and type on risk of recurrent gout attacks: an internet-based case-crossover study. Am J Med 2014;127:311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abhishek A, Valdes AM, Jenkins W, Zhang W, Doherty M.. Triggers of acute attacks of gout, does age of gout onset matter? A primary care based cross-sectional study. PLoS One 2017;12:e0186096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Becker MA, MacDonald PA, Hunt BJ, Lademacher C, Joseph-Ridge N.. Determinants of the clinical outcomes of gout during the first year of urate-lowering therapy. Nucleosides Nucleotides Nucleic Acids 2008;27:585–91. [DOI] [PubMed] [Google Scholar]
- 25. Abhishek A, Valdes AM, Zhang W, Doherty M.. Association of serum uric acid and disease duration with frequent gout attacks: a case-control study. Arthritis Care Res 2016;68:1573–7. [DOI] [PubMed] [Google Scholar]
- 26. Chen J-H, Pan W-H, Hsu C-C. et al. Impact of obesity and hypertriglyceridemia on gout development with or without hyperuricemia: a prospective study. Arthritis Care Res 2013;65:133–40. [DOI] [PubMed] [Google Scholar]
- 27. Jatuworapruk K, Grainger R, Dalbeth N, Taylor WJ.. Development of a prediction model for inpatient gout flares in people with comorbid gout. Ann Rheum Dis 2020;79:418–23. [DOI] [PubMed] [Google Scholar]
- 28. Dalbeth N, Gosling AL, Gaffo A, Abhishek A.. Gout. Lancet 2021;397:1843–55. [DOI] [PubMed] [Google Scholar]
- 29. Dalbeth N, Pool B, Gamble GD. et al. Cellular characterization of the gouty tophus: a quantitative analysis. Arthritis Rheum 2010;62:1549–56. [DOI] [PubMed] [Google Scholar]
- 30. Xu G, Lin J, Liang J. et al. Entheseal involvement of the lower extremities in gout: an ultrasonographic descriptive observational study. Clin Rheumatol 2021;40:4649–57. [DOI] [PubMed] [Google Scholar]
- 31. Hutchinson AN, Tingö L, Brummer RJ.. The potential effects of probiotics and ω-3 fatty acids on chronic low-grade inflammation. Nutrients 2020;12:2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hansen SEJ, Madsen CM, Varbo A, Nordestgaard BG.. Low-grade inflammation in the association between mild-to-moderate hypertriglyceridemia and risk of acute pancreatitis: a study of more than 115000 individuals from the general population. Clin Chem 2019;65:321–32. [DOI] [PubMed] [Google Scholar]
- 33. Spartalis M, Spartalis E, Tzatzaki E. et al. The beneficial therapy with colchicine for atherosclerosis via anti-inflammation and decrease in hypertriglyceridemia. Cardiovasc Hematol Agents Med Chem 2018;16:74–80. [DOI] [PubMed] [Google Scholar]
- 34. Mak A, Ho RCM, Tan JYS. et al. Atherogenic serum lipid profile is an independent predictor for gouty flares in patients with gouty arthropathy. Rheumatology 2009;48:262–5. [DOI] [PubMed] [Google Scholar]
- 35. Ebstein E, Forien M, Norkuviene E. et al. UltraSound evaluation in follow-up of urate-lowering therapy in gout phase 2 (USEFUL-2): Duration of flare prophylaxis. Joint Bone Spine 2020;87:647–51. [DOI] [PubMed] [Google Scholar]
- 36. Zou Z, Yang M, Wang Y, Zhang B.. Association of urate deposition shown by ultrasound and frequent gout attacks. Z Rheumatol 2021;80:565–9. [DOI] [PubMed] [Google Scholar]
- 37. Qin Y, Zhang S, Cui S. et al. High urinary excretion rate of glucose attenuates serum uric acid level in type 2 diabetes with normal renal function. J Endocrinol Invest 2021;44:1981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yan B, Meng X, Shi J. et al. Ganoderma lucidum spore induced CA72-4 elevation in gastrointestinal cancer: a five-case report. Integr Cancer Ther 2014;13:161–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.