Abstract
Objectives
In some patients with RA, joint pain is more severe than expected based on the amount of joint swelling [referred to as disproportionate articular pain (DP)]. We assessed DP prevalence and the effects of sarilumab, an IL-6 inhibitor, on DP.
Methods
Data from RA patients treated with placebo or 200 mg sarilumab in the phase 3 randomized controlled trials (RCTs) MOBILITY and TARGET, adalimumab 40 mg or sarilumab 200 mg in the phase 3 RCT MONARCH and sarilumab 200 mg in open-label extensions (OLEs) were used. DP was defined as an excess tender 28-joint count (TJC28) over swollen 28-joint count (SJC28) of ≥7 (TJC28 − SJC28 ≥ 7). Treatment response and disease activity were determined for patients with and without DP.
Results
Of 1531 sarilumab 200 mg patients from RCTs, 353 (23%) had baseline DP. On average, patients with DP had higher 28-joint DAS using CRP (DAS28-CRP) and pain scores than patients without DP, whereas CRP levels were similar. After 12 and 24 weeks, patients with baseline DP treated with sarilumab were more likely to be DP-free than those treated with placebo or adalimumab. In RCTs, more sarilumab-treated patients achieved low disease activity vs comparators, regardless of baseline DP status. In OLEs, patients were more likely to lose rather than gain DP status.
Conclusion
About one-quarter of patients with RA experienced DP, which responded well to sarilumab. These data support the concept that other mechanisms (potentially mediated via IL-6) in addition to inflammation may contribute to DP in RA.
Trial registrations
Keywords: RA, IL-6, sarilumab, pain, analgesia
Rheumatology key messages.
Disproportionate articular pain affects a sizeable proportion of patients with rheumatoid arthritis.
IL-6 may be a potential mediator of pain in rheumatoid arthritis.
Sarilumab treatment was associated with improved disproportionate articular pain and clinical outcomes in rheumatoid arthritis.
Introduction
RA is estimated to affect up to 1% of the global population [1, 2]. Pain is a central symptom of RA and is the strongest predictor of psychosocial health [3]. Consequently, pain is commonly described by patients as their most important symptom [4].
Although inflammation and joint damage are known causes of pain in RA [5, 6], the level of pain and pain-associated disability reported by some patients is greater than would be expected based on examination of the extent of joint damage [7] and inflammation [8, 9]. This is defined here as disproportionate articular pain (DP) and it is a clinically relevant issue for patients with RA. Additionally, in some patients, pain remains constant despite treatment with anti-inflammatory, disease-modifying, anti-rheumatic drugs [10]. Patients with RA have been shown to experience enhanced pain sensitivity to a variety of stimuli [11] and this sensitivity may be increased with a longer duration of RA [12]. Thus the greater than expected level of pain experienced by some patients with RA may be a result of hyperalgesia.
Both peripheral and central mechanisms of pain may be involved in hyperalgesia in patients with RA [13]. Peripheral mechanisms of pain in RA are generally well described. Pro-inflammatory cytokines such as TNF-α, IL-6, IL-1β and IL-17 play a dual role in the peripheral mechanism of pain by promoting inflammation but also by rapidly changing the excitability, ion currents and second messenger systems of nociceptive neurons [14].
The role of the central mechanism of pain in RA is less well established. The mechanism underlying central pain dysregulation might include systemic circulation of cytokines and other neuromodulatory factors [15]. Additionally, circulating cytokines may directly access the central nervous system through the blood–brain barrier, which is compromised in systemic inflammation [16].
A greater understanding of the different mechanisms of pain may lead to more targeted approaches to analgesia in RA. When pain is a result of a central mechanism, medications that are targeted to this mechanism may be more effective than drugs (e.g. opioids) that treat peripheral or nociceptive pain [17]. Although opioid prescriptions for pain in RA doubled in the period 2011–2016 [18], they are not considered an ideal long-term treatment option for chronic pain [19].
IL-6 is a key component of inflammation and RA [20] and may be of particular importance in mechanical hyperalgesia [14]. Sarilumab, a fully human monoclonal antibody directed against the IL-6 receptor antagonist, is approved for the treatment of moderate–severe active RA [21]. In phase 3 randomized controlled trials (RCTs) of sarilumab in patients with RA, meaningful improvements in pain were demonstrated for sarilumab 150 mg or 200 mg administered every 2 weeks (q2w) compared with placebo [22, 23] or adalimumab [24]. Given that IL-6 may play a role in both peripheral and central pain mechanisms, it is hypothesized that blocking the effects of IL-6 may reduce both pain pathways, and consequently may reduce inappropriate opioid use in RA patients. However, no analysis to date has been conducted about the potential relationship between sarilumab treatment, disease activity and DP in patients with RA.
To date, there is no standard way of assessing DP. While specialized assessments that may identify specific pain mechanisms, such as quantitative sensory testing and neuroimaging, are available [25], they are not commonly included in clinical trials. Patient and physician assessment of disease activity has been used in trials in a number of conditions, including RA [26]. In RA, a discrepancy between patient global assessment (PtGA) and physician global assessment (PhGA) may be driven by the influence of pain, with patients with higher levels of pain reporting more disease activity than physicians [27], and thus could provide an opportunity to identify the presence of hyperalgesia. Measures of the number of tender and swollen joints are a standard measurement in RA trials, and a large difference between the tender joint count (TJC) and swollen joint count (SJC) [28] and the SJC:TJC ratio [29] have each statistically predicted pain. Pollard et al. [28] observed that a 28-joint tender joint count (TJC28) − 28-joint swollen joint count (SJC28) ≥7 predicts RA with high levels of pain.
In this post hoc analysis of sarilumab clinical trials we investigated the prevalence of DP, the effect of sarilumab on DP and the association between sarilumab treatment, disease activity and DP status. In addition, the change in opioid use over time according to DP status and pain status using a visual analogue scale (VAS) was also evaluated.
Methods
Data sources
Patient data were collected from three phase 3 trials [placebo-controlled MOBILITY [30] (patients with inadequate response to methotrexate; NCT01061736) and TARGET [31] (patients with intolerance or an inadequate response to TNF inhibitors; NCT01709578) and adalimumab-controlled MONARCH [24] (patients with intolerance or inadequate response to methotrexate; NCT02332590)] and their open-label extensions (OLEs). We selected patients who received placebo or the recommended sarilumab dose of 200 mg q2w in MOBILITY and TARGET and adalimumab 40 mg or sarilumab 200 mg q2w in MONARCH. In the extension studies EXTEND (for patients from MOBILITY and TARGET; NCT01146652) and MONARCH OLE, all patients were assigned to receive open-label sarilumab 200 mg q2w. The protocols for the included studies were approved by the appropriate ethics committees/institutional review boards and each patient gave written consent before participation in the study. The studies were conducted in compliance with institutional review board regulations, the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki.
Outcomes
DP was defined as a TJC28 that exceeded the SJC28 by at least 7 (i.e. TJC28 − SJC28 ≥ 7), according to established criteria [28, 32]. Other definitions were also explored: SJC:TJC ratio <0.5 [29] and a difference between PtGA and PhGA scores of at least 2 (PtGA − PhGA ≥ 2) [33, 34]. The former was abandoned because, mathematically, it could not be used for assessment of patients with a TJC of 0 without imputing data for a sizeable number of patients. The latter was abandoned because the baseline characteristics of that subset were substantially different from the characteristics of patients selected using the other two definitions.
For the RCTs, the proportions of patients with DP and demographic and disease characteristics according to DP status were reported at baseline. Outcomes assessed included persistence of DP after 12 and 24 weeks of treatment, clinical response at week 24 by baseline DP status [ACR 20/50/70 criteria, Clinical Disease Activity Index (CDAI) ≤10 low disease activity (LDA), 28-joint DAS using CRP (DAS28-CRP) ≤3.2 LDA and Simple Clinical Disease Activity Index (SDAI) ≤11 LDA] and mean change from baseline to week 24 in efficacy and patient-reported outcomes by baseline DP status (CDAI, CRP, PtGA, SJC28 and TJC28).
For the OLEs, demographic and disease characteristics at baseline and weeks 24 and 48 according to DP status and the proportion of patients with DP over time were reported. Outcomes assessed included a shift in DP status in the treatment groups over the OLE period, as well as pain levels in the treatment groups, measured using a pain visual analogue scale (VAS), over the combined RCT and OLE periods according to DP status. Finally, for the MONARCH OLE, the number of patients using opioids was assessed over 4 years according to DP at OLE baseline.
Statistical analysis
Due to the post hoc nature of this analysis, data were analysed using observed cases and descriptive statistics only; no inferential statistical analyses were performed. This article focuses on patients who were assigned the 200 mg q2w dose of sarilumab at RCT baseline, irrespective of dose changes during follow-up.
Results
Prevalence of DP
Of the 1531 participants in the sarilumab 200 mg or placebo/adalimumab arms of the three RCTs, 353 (23%) had DP at baseline [MOBILITY, 198 (25%); TARGET, 65 (18%); MONARCH, 90 (24%)]. The prevalence of DP at RCT baseline was similar across the trials and treatment groups (Table 1).
Table 1.
Prevalence of DP at RCT baseline
| MOBILITY, n (%) | ||
|---|---|---|
| Placebo (n = 398) | Sarilumab 200 mg (n = 399) | Both arms (N = 797) |
| 106 (27) | 92 (23) | 198 (25) |
| TARGET, n (%) | ||
|---|---|---|
| Placebo (n = 181) | Sarilumab 200 mg (n = 184) | Both arms (N = 365) |
| 30 (17) | 35 (19) | 65 (18) |
| MONARCH, n (%) | ||
|---|---|---|
| Adalimumab 40 mg (n = 185) | Sarilumab 200 mg (n = 184) | Both arms (N = 369) |
| 47 (25) | 43 (23) | 90 (24) |
DP was defined as TJC28 − SJC28 ≥ 7.
At RCT baseline, sarilumab 200 mg patients with and without DP had similar demographic characteristics overall. However, as expected, patients with DP had higher TJC28, CDAI, DAS28-CRP and pain VAS scores than patients without DP. SJC28 levels were somewhat lower in patients with DP, and CRP levels were similar between the two subsets (Table 2).
Table 2.
Baseline demographic and disease characteristics: sarilumab 200 mg patients (pooled MOBILITY, TARGET and MONARCH data)
| Parameter | With DP (n = 170, 22% of total) | Without DP (n = 597, 78% of total) |
|---|---|---|
| Age, mean (s.d.), years | 54 (11) | 51 (13) |
| Women, n (%) | 143 (84) | 502 (84) |
| Race, n (%) | ||
| White | 149 (88) | 495 (83) |
| Black | 3 (2) | 11 (2) |
| Asian | 3 (2) | 33 (6) |
| Other | 15 (9) | 154 (10) |
| Hispanic ethnicity, n (%) | 55 (32) | 230 (39) |
| Weight, mean (s.d.), kg | 77 (18)a | 74 (20) |
| BMI group, n (%) | ||
| <25 kg/m2 | 50 (30)a | 210 (35) |
| ≥25–<30 kg/m2 | 56 (33)a | 195 (33) |
| ≥30 kg/m2 | 63 (37)a | 192 (32) |
| NSAIDs use, n (%) | 112 (66) | 399 (67) |
| Glucocorticoids use, n (%) | 97 (57) | 366 (61) |
| Opioid use, n (%) | 23 (14) | 72 (12) |
| Duration of RA, years, mean (s.d.) | 9.1 (8.7) | 9.5 (8.0) |
| TJC28, mean (s.d.) | 21.4 (4.9) | 14.7 (6.3) |
| SJC28, mean (s.d.) | 10.3 (4.3) | 13.2 (6.2) |
| CRP, mg/L, mean (s.d.) | 22.9 (26.3) | 23.2 (24.4) |
| HAQ-DI (range 0–3), mean (s.d.) | 1.8 (0.6) | 1.7 (0.6) |
| DAS28-CRP, mean (s.d.) | 6.4 (0.7) | 6.0 (0.9) |
| CDAI, mean (s.d.) | 45.3 (9.6) | 41.1 (13.4) |
| Pain VAS (range 1–100), mean (s.d.) | 75 (18) | 68 (21) |
DP was defined as TJC28 − SJC28 ≥ 7.
n = 169.
At OLE baseline, patients with DP also had higher levels of TJC28, CDAI and DAS28-CRP (and in addition attained higher scores for SJC28, HAQ-DI and CRP) than patients without DP (Supplementary Table S1, available at Rheumatology online).
Effect of treatment on DP
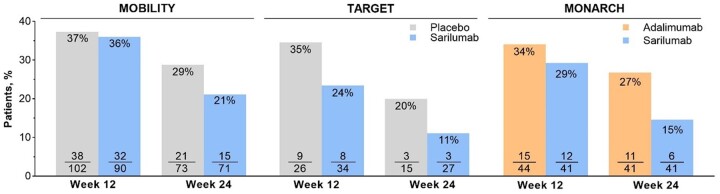
In the RCTs, the reduction in the proportion of patients with DP was numerically larger in patients treated with sarilumab than in those who were treated with placebo or adalimumab (Fig. 1). In all three studies, the relative difference in proportions of patients with DP between sarilumab and control treatments appeared to increase with treatment duration.
Figure 1.
Proportions of patients with baseline DP who still had DP at weeks 12 and 24
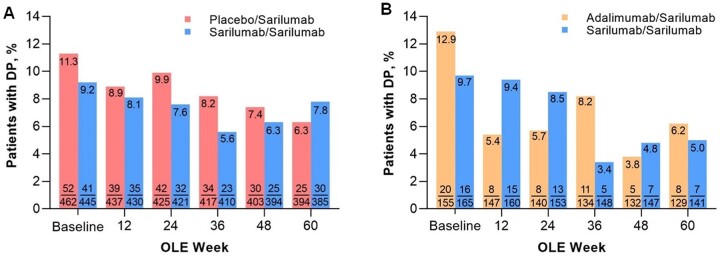
As expected, at OLE baseline, patients switching from placebo or adalimumab had a higher prevalence of DP than patients previously treated with sarilumab. By week 48 of the OLEs, the proportions of patients who had switched to sarilumab and reported DP were similar to those who had been receiving sarilumab continuously (Fig. 2).
Figure 2.
Proportion of patients with DP over time in the OLEs. (A) EXTEND (MOBILITY/TARGET) and (B) MONARCH OLE. DP was defined as TJC28 − SJC28 ≥ 7. For MOBILITY and TARGET, data are shown only for patients who received sarilumab 200 mg
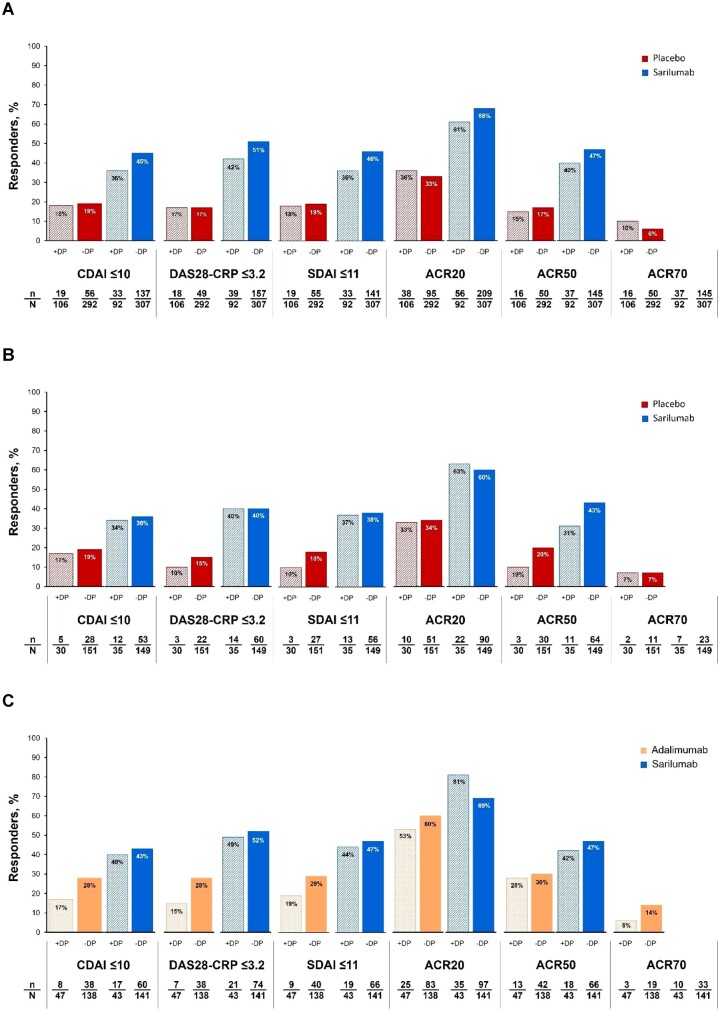
In all three RCTs, a higher proportion of sarilumab-treated than control patients achieved LDA (CDAI, DAS28 or SDAI) or ACR20, 50 or 70 responses at week 24, regardless of the presence of baseline DP (Fig. 3).
Figure 3.
Clinical responses in the RCTs at week 24. (A) MOBILITY, (B) TARGET and (C) MONARCH. DP was defined as TJC28 − SJC28 ≥ 7. For MOBILITY and TARGET, data are shown only for patients who received sarilumab 200 mg
In all three RCTs, the mean reduction in pain level was greater with sarilumab than with the comparators, irrespective of baseline DP status. In the OLEs, the sarilumab continuation subgroups maintained the improvement in pain, and the switch groups improved to similar levels (Supplementary Fig. S1, available at Rheumatology online).
Over 4 years in the MONARCH OLE, patients with and without DP reported a decrease in opioid use, regardless of the presence of DP (Supplementary Fig. S2, available at Rheumatology online).
Discussion
In general, most physicians would expect joint pain due to RA to be associated with synovitis. There are accumulating data however that a proportion of pain experienced by patients with RA may not be directly ascribable to the magnitude of joint inflammation. Therefore DP can be considered as joint pain with little sign of inflammation and it is a clinically relevant issue for patients with RA. In our data, nearly one in four patients may have had a component of DP at baseline, a prevalence that was consistent across three sarilumab phase 3 trials. Similar findings were recently reported in a study of 243 Japanese patients with RA (NCT02293902), of whom 15% had DP at baseline. After 24 weeks, sarilumab treatment was associated with a reduction in the prevalence of DP and with better clinical outcomes in patients with DP compared with placebo [35]. Similarly, in another analysis of these trials, sarilumab-treated patients with unacceptable pain (VAS ≥40 mm) despite control of inflammation had lower odds of unacceptable or refractory pain vs comparators [36]. Improvements in total pain scores, regardless of whether inflammation was reduced, have also been observed with tocilizumab [37]. However, in the current analysis, some patients continued to have DP, which may suggest a local articular effect. Therefore the causes of DP may be multifactorial.
Given that the duration of nociceptive pain may be a factor in central pain dysregulation (neuroplasticity), there is a compelling argument to selectively address active pain in RA early and robustly [38]. It seems reasonable to assume that the window of opportunity for improvement in DP (and perhaps all pain) would narrow with prolonged disease duration. In RA, DP may be partly due to peripheral sensitization, which in turn may lead to central pain dysregulation [38]. If this hypothesis is correct, early, adequate and targeted treatment of pain could prevent central pain dysregulation and the spread of pain to non-articular locations, which would be of interest for further research.
Data from the MONARCH OLE suggest that sarilumab treatment may have an opioid-sparing effect, since treatment with sarilumab over 4 years was associated with a decline in opioid use, regardless of DP status or general pain level. However, interpretation of these results is limited by the small number of patients with DP.
Our data suggest that sarilumab may be a better option than adalimumab for reducing DP and achieving LDA in patients with DP, but this difference in reducing DP would need to be confirmed in prospective, adequately designed trials. It is possible that some patients with DP have a particular phenotype with a greater concentration of fibroblasts and more neovascularization, which is more sensitive to IL-6 inhibition, whereas other patients may have a higher level of T-cells and macrophages and may be equally susceptible to TNF and IL-6 inhibitors. Regardless, these observations provide support for early intervention to reverse IL-6-dependent nerve sensitization.
This study has the usual limitations associated with open-label extensions and post hoc analyses, such as patient selection bias, inadequate sample size, unmasking bias and a non-randomized sample. These limitations may result in differences that may not be due to treatment alone. There was a slight imbalance in the proportion of patients with a BMI >30 between patients with and without DP in the RCTs. While the difference is small, it is possible that this may have had an effect on results, as pain complaints are common in obese patients [39] and circulating IL-6 levels are known to be higher in these patients [40]. The greater reduction from baseline in TJC28 observed in patients with DP compared with those without DP may be due to the former group of patients having lower TJC at baseline. In addition, the prevalence of DP may not be generalizable to the general population of patients with RA, because all participants in these trials had high disease activity, which may have predisposed them to meeting the DP criterion that we used. It is also possible that a component of the residual pain is inflammatory: in some cases, the pain could be related to tendinopathy [41], which might account for some of the improvement over time. This hypothesis is supported by observations that subclinical inflammation in both joints and tendons has been discovered using ultrasound and MRI in a substantial proportion of RA patients who are in clinical remission [42, 43]. Despite these limitations, it is notable that these clinical findings are consistent with preclinical studies of IL-6 and pain [38]. These data provide further support for the hypothesis that other mechanisms (mediated via IL-6 signalling) in addition to direct inflammation contribute to pain in RA.
In conclusion, our data support the view that DP is common in patients with RA who have active disease. These data support the concept that other mechanisms (potentially mediated via IL-6) in addition to inflammation may contribute to DP in RA.
Supplementary Material
Acknowledgements
The authors and Sanofi thank the patients for their participation in the trials, as well as the MOBILITY, MONARCH and TARGET Steering Committees and investigators. Gregory St John, an employee of Regeneron Pharmaceuticals, Inc. (current affiliation: Intercept Pharmaceuticals), contributed to the design and analysis of this study when it was undertaken. Medical writing assistance was provided by Richard J. Hogan, Jonathon Gibbs and Vojislav Pejović, of Elevate Scientific Solutions, a division of Envision Pharma Group, and editorial and graphics assistance was provided by Elevate Scientific Solutions, funded by Sanofi. Some of the data included in this manuscript were presented previously at the Annual European Congress of Rheumatology, 3–6 June 2020 (Virtual Congress; Choy et al., SAT0102) and the American College of Rheumatology Annual Meeting, 5–9 November 2020 (Virtual Congress; Choy et al., 1234).
Contributor Information
Ernest Choy, CREATE Centre, Division of Infection and Immunity, Cardiff University School of Medicine, Cardiff, UK.
Vivian Bykerk, Inflammatory Arthritis Centre, Hospital for Special Surgery, New York, NY, USA.
Yvonne C Lee, Division of Rheumatology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Hubert van Hoogstraten, Neurology and Immunology, Sanofi, Bridgewater, NJ, USA.
Kerri Ford, Medical Affairs Immunology and Inflammation-Rheumatology, Rare Inflammatory Disorders, Sanofi, Bridgewater, NJ, USA.
Amy Praestgaard, Biostatistics, Immunology, Sanofi, Cambridge, MA, USA.
Serge Perrot, Pain Center, Cochin Hospital, Paris University, Paris, France.
Janet Pope, Division of Rheumatology, Schulich School of Medicine, University of Western Ontario, St. Joseph’s Health Care, London, ON, Canada.
Anthony Sebba, Department of Rheumatology, University of South Florida, Tampa, FL, USA.
Supplementary data
Supplementary data are available at Rheumatology online.
Data availability
Qualified researchers may request access to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and data set specifications. Patient-level data will be anonymised, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies and process for requesting access can be found at: https://www.vivli.org/.
Authors’ contributions
The analysis was designed by B.A.H.O., K.F. and E.C. Data were verified and statistical analysis was done by A.P. The manuscript was drafted by a writing agency. All authors provided interpretation of data and critical revision of the drafts. The final version of the manuscript was approved by all authors, who had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding
Funding was provided by Sanofi (Cambridge, MA, USA) and Regeneron Pharmaceuticals, INC. (Tarrytown, NY, USA).
Disclosure statement: E.C. reports grant/research support from Amgen, Bio-Cancer, Chugai Pharma, Ferring Pharmaceuticals, Novimmune, Pfizer, Roche and UCB; consultant fees from AbbVie, Amgen, AstraZeneca, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Chelsea Therapeutics, Chugai Pharma, Daiichi Sankyo, Eli Lilly, Ferring Pharmaceuticals, Gilead, GlaxoSmithKline, Hospira, Ionis, Janssen, Jazz Pharmaceuticals, MedImmune, Merck Sharp & Dohme, Merrimack Pharmaceutical, Napp, Novartis, Novimmune, ObsEva, Pfizer, R-Pharm, Regeneron Pharmaceuticals, Inc., Roche, SynAct Pharma, Sanofi Genzyme, Tonix and UCB; and is a member of speakers bureau for Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai Pharma, Eli Lilly, Galapagos, Gilead, Hospira, Merck Sharp & Dohme, Novartis, Pfizer, Regeneron Pharmaceuticals, Inc., Roche, Sanofi-Aventis and UCB. V.B. received consulting fees from Amgen, Bristol-Myers Squibb, Gilead, Pfizer, Regeneron Pharmaceuticals, Inc., Sanofi and UCB and her host institution has received research grants from Amgen, the Cedar Hill Foundation and the National Institutes of Health. Y.L. has received research grants and/or consulting fees from Highland Instruments and Pfizer and holds stock and/or stock options in Cigna-Express Scripts. H.v.H., K.F. and A.P. are employees of Sanofi and may hold stock and/or stock options in the company. S.P. has received speaker fees/honoraria from Grunenthal, Menarini, MSD, Pfizer, Sanofi and UPSA. J.P. reports grant/research support from AbbVie, Bristol-Myers Squibb, Merck, Pfizer, Roche, UCB and Seattle Genetics; consultant fees from AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Janssen, Eli Lilly, Medexus, Merck, Novartis, Pfizer, Roche, Sanofi, Sandoz, Teva and UCB. A.S. has received consulting fees from or participated in speakers’ bureaus for Genentech, Gilead, Eli Lilly, Regeneron Pharmaceuticals, Roche and Sanofi.
References
- 1. Cross M, Smith E, Hoy D. et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014;73:1316–22. [DOI] [PubMed] [Google Scholar]
- 2. Hunter TM, Boytsov NN, Zhang X. et al. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004–2014. Rheumatol Int 2017;37:1551–7. [DOI] [PubMed] [Google Scholar]
- 3. Courvoisier DS, Agoritsas T, Glauser J. et al. Pain as an important predictor of psychosocial health in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:190–6. [DOI] [PubMed] [Google Scholar]
- 4. ten Klooster PM, Veehof MM, Taal E, van Riel PL, van de Laar MA.. Changes in priorities for improvement in patients with rheumatoid arthritis during 1 year of anti-tumour necrosis factor treatment. Ann Rheum Dis 2007;66:1485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boyden SD, Hossain IN, Wohlfahrt A, Lee YC.. Non-inflammatory causes of pain in patients with rheumatoid arthritis. Curr Rheumatol Rep 2016;18:30. [DOI] [PubMed] [Google Scholar]
- 6. Choy EHS, Calabrese LH.. Neuroendocrine and neurophysiological effects of interleukin 6 in rheumatoid arthritis. Rheumatology (Oxford) 2018;57:1885–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Young-Min S, Cawston T, Marshall N. et al. Biomarkers predict radiographic progression in early rheumatoid arthritis and perform well compared with traditional markers. Arthritis Rheum 2007;56:3236–47. [DOI] [PubMed] [Google Scholar]
- 8. McWilliams DF, Zhang W, Mansell JS. et al. Predictors of change in bodily pain in early rheumatoid arthritis: an inception cohort study. Arthritis Care Res (Hoboken) 2012;64:1505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Druce KL, Jones GT, Macfarlane GJ, Basu N.. Determining pathways to improvements in fatigue in rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Arthritis Rheumatol 2015;67:2303–10. [DOI] [PubMed] [Google Scholar]
- 10. Wolfe F, Michaud K.. Assessment of pain in rheumatoid arthritis: minimal clinically significant difference, predictors, and the effect of anti-tumor necrosis factor therapy. J Rheumatol 2007;34:1674–83. [PubMed] [Google Scholar]
- 11. Edwards RR, Wasan AD, Bingham CO 3rd. et al. Enhanced reactivity to pain in patients with rheumatoid arthritis. Arthritis Res Ther 2009;11:R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leffler AS, Kosek E, Lerndal T, Nordmark B, Hansson P.. Somatosensory perception and function of diffuse noxious inhibitory controls (DNIC) in patients suffering from rheumatoid arthritis. Eur J Pain 2002;6:161–76. [DOI] [PubMed] [Google Scholar]
- 13. Meeus M, Vervisch S, De Clerck LS. et al. Central sensitization in patients with rheumatoid arthritis: a systematic literature review. Semin Arthritis Rheum 2012;41:556–67. [DOI] [PubMed] [Google Scholar]
- 14. Schaible HG. Nociceptive neurons detect cytokines in arthritis. Arthritis Res Ther 2014;16:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McWilliams DF, Walsh DA.. Pain mechanisms in rheumatoid arthritis. Clin Exp Rheumatol 2017;35(Suppl 107):94–101. [PubMed] [Google Scholar]
- 16. Nishioku T, Yamauchi A, Takata F. et al. Disruption of the blood-brain barrier in collagen-induced arthritic mice. Neurosci Lett 2010;482:208–11. [DOI] [PubMed] [Google Scholar]
- 17. Lee YC, Nassikas NJ, Clauw DJ.. The role of the central nervous system in the generation and maintenance of chronic pain in rheumatoid arthritis, osteoarthritis and fibromyalgia. Arthritis Res Ther 2011;13:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang Y, Rege S, Chatterjee S, Aparasu RR.. Opioid prescribing among outpatients with rheumatoid arthritis. Pain Med 2021;22:2224–34. [DOI] [PubMed] [Google Scholar]
- 19. Bullock J, Rizvi SAA, Saleh AM. et al. Rheumatoid arthritis: a brief overview of the treatment. Med Princ Pract 2018;27:501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fonseca JE, Santos MJ, Canhao H, Choy E.. Interleukin-6 as a key player in systemic inflammation and joint destruction. Autoimmun Rev 2009;8:538–42. [DOI] [PubMed] [Google Scholar]
- 21.KEVZARA (sarilumab) prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2017/761037s000lbl.pdf (5 December 2022, date last accessed).
- 22. Strand V, Kosinski M, Chen CI. et al. Sarilumab plus methotrexate improves patient-reported outcomes in patients with active rheumatoid arthritis and inadequate responses to methotrexate: results of a phase III trial. Arthritis Res Ther 2016;18:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Strand V, Reaney M, Chen CI. et al. Sarilumab improves patient-reported outcomes in rheumatoid arthritis patients with inadequate response/intolerance to tumour necrosis factor inhibitors. RMD Open 2017;3:e000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burmester GR, Lin Y, Patel R. et al. Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy for the treatment of patients with active rheumatoid arthritis (MONARCH): a randomised, double-blind, parallel-group phase III trial. Ann Rheum Dis 2017;76:840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang A, Lee YC.. Mechanisms for joint pain in rheumatoid arthritis (RA): from cytokines to central sensitization. Curr Osteoporos Rep 2018;16:603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Felson DT, Anderson JJ, Boers M. et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. Arthritis Rheum 1993;36:729–40. [DOI] [PubMed] [Google Scholar]
- 27. Nicolau G, Yogui MM, Vallochi TL. et al. Sources of discrepancy in patient and physician global assessments of rheumatoid arthritis disease activity. J Rheumatol 2004;31:1293–6. [PubMed] [Google Scholar]
- 28. Pollard LC, Kingsley GH, Choy EH, Scott DL.. Fibromyalgic rheumatoid arthritis and disease assessment. Rheumatology (Oxford) 2010;49:924–8. [DOI] [PubMed] [Google Scholar]
- 29. Kristensen LE, Bliddal H, Christensen R. et al. Is swollen to tender joint count ratio a new and useful clinical marker for biologic drug response in rheumatoid arthritis? Results from a Swedish cohort. Arthritis Care Res (Hoboken) 2014;66:173–9. [DOI] [PubMed] [Google Scholar]
- 30. Genovese MC, Fleischmann R, Kivitz AJ. et al. Sarilumab plus methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate: results of a phase III study. Arthritis Rheumatol 2015;67:1424–37. [DOI] [PubMed] [Google Scholar]
- 31. Fleischmann R, van Adelsberg J, Lin Y. et al. Sarilumab and nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis and inadequate response or intolerance to tumor necrosis factor inhibitors. Arthritis Rheumatol 2017;69:277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Durán J, Combe B, Niu J. et al. The effect on treatment response of fibromyalgic symptoms in early rheumatoid arthritis patients: results from the ESPOIR cohort. Rheumatology (Oxford) 2015;54:2166–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Desthieux C, Hermet A, Granger B, Fautrel B, Gossec L.. Patient-physician discordance in global assessment in rheumatoid arthritis: a systematic literature review with meta-analysis. Arthritis Care Res (Hoboken) 2016;68:1767–73. [DOI] [PubMed] [Google Scholar]
- 34. Khan NA, Spencer HJ, Abda E. et al. Determinants of discordance in patients’ and physicians’ rating of rheumatoid arthritis disease activity. Arthritis Care Res (Hoboken) 2012;64:206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tanaka Y, Takahashi T, van Hoogstraten H, Praestgaard A, Kato N.. Effect of sarilumab treatment on noninflammatory pain in Japanese patients with rheumatoid arthritis. Mod Rheumatol 2021;31:S227. [Google Scholar]
- 36. Bykerk V, Wei W, Boklage S. et al. Impact of sarilumab on unacceptable pain and inflammation control in moderately-to-severely active rheumatoid arthritis (RA) patients in 3 phase 3 studies [abstract] . Arthritis Rheumatol 2019;71(Suppl 10):abstract 1393. [Google Scholar]
- 37. Sebba A, Han J, Mohan S.. Pain and other patient-reported outcomes in patients with rheumatoid arthritis who did or did not achieve treatment response based on improvement in swollen joints in tocilizumab clinical trials. Ann Rheum Dis 2020;79:994–5. [Google Scholar]
- 38. Sebba A. Pain: a review of interleukin-6 and its roles in the pain of rheumatoid arthritis. Open Access Rheumatol 2021;13:31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Okifuji A, Hare BD.. The association between chronic pain and obesity. J Pain Res 2015;8:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Khaodhiar L, Ling PR, Blackburn GL, Bistrian BR.. Serum levels of interleukin-6 and C-reactive protein correlate with body mass index across the broad range of obesity. JPEN J Parenter Enteral Nutr 2004;28:410–5. [DOI] [PubMed] [Google Scholar]
- 41. Hernández-Díaz C, Sánchez-Bringas G, Ventura-Ríos L, Robles-San Román M, Filippucci E.. Ankle pain in rheumatoid arthritis: comparison of clinical and sonographic findings. Clin Rheumatol 2019;38:2891–5. [DOI] [PubMed] [Google Scholar]
- 42. Zhang H, Xu H, Chen S, Mao X.. The application value of MRI in the diagnosis of subclinical inflammation in patients with rheumatoid arthritis in remission. J Orthop Surg Res 2018;13:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sundin U, Sundlisater NP, Aga A-B. et al. Value of MRI and ultrasound for prediction of therapeutic response and erosive progression in patients with early rheumatoid arthritis managed by an aggressive treat-to-target strategy. RMD Open 2021;7:e001525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request access to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and data set specifications. Patient-level data will be anonymised, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies and process for requesting access can be found at: https://www.vivli.org/.