Abstract
The human cerebral cortex is one of the most evolved regions of the brain, responsible for most higher-order neural functions. Since nerve cells (together with synapses) are the processing units underlying cortical physiology and morphology, we studied how the human neocortex is composed regarding the number of cells as a function of sex and age. We used the isotropic fractionator for cell quantification of immunocytochemically labeled nuclei from the cerebral cortex donated by 43 cognitively healthy subjects aged 25–87 years old. In addition to previously reported sexual dimorphism in the medial temporal lobe, we found more neurons in the occipital lobe of men, higher neuronal density in women’s frontal lobe, but no sex differences in the number and density of cells in the other lobes and the whole neocortex. On average, the neocortex has ~10.2 billion neurons, 34% in the frontal lobe and the remaining 66% uniformly distributed among the other 3 lobes. Along typical aging, there is a loss of non-neuronal cells in the frontal lobe and the preservation of the number of neurons in the cortex. Our study made possible to determine the different degrees of modulation that sex and age evoke on cortical cellularity.
Keywords: aging, cerebral cortex, isotropic fractionator, number of neurons, sexual dimorphism
Introduction
The human cerebral cortex comprises the outermost surface of the cerebrum, usually with a thickness of 2–4 mm and a complex, folded architecture. Histologically, it contains the somata of neuronal, glial, and vascular cells, and the intricate net of their processes. The human cortex is essential for the most complex sensory, motor, emotional, and cognitive information processing. Integrating information from different sources in multimodal cortex regions allows us to make decisions and direct our behavior based on experience and sensory inputs. The adequate interaction between billions of neurons and glial cells is fundamental for proper cortical performance. At this microscale level, the quantitative characterization of the cellular composition is crucial to understand how the human cortex microstructure is related to function and how cellularity can be influenced by factors such as sex and age in cognitively healthy subjects.
The influence of sex on the cerebral cortex
Understanding the brain morphological and functional differences among sexes is a frequent and controversial topic in the literature. Several questions arise from the sex differences observed in behavioral and cognitive functions and the prevalence of some neurological or psychiatric diseases. Some studies suggest men perform better in visuospatial and women in language tasks (Bourisly et al. 2017). On the other hand, major depressive disorder, anxiety, eating disorders, and Alzheimer's disease (AD) are more frequent in women, whereas autism spectrum disorders, schizophrenia, attention deficit, and hyperactivity disorder are more frequent in men (Ritchie et al. 2018). When do these differences appear? Do they have structural correlates? Are men’s and women’s brains structurally different? There is still no consensus, and contradictory answers are common in the literature (McCarthy 2016; Grabowska 2017; DeCasien et al. 2022). For this reason, understanding how the brain is structured in men and women can help to reveal the mechanisms behind these differences.
One of the most consistent findings is that men’s brains are larger than women’s: according to postmortem studies, brain mass is 12% higher in men (Pakkenberg and Gundersen 1997). An extensive cross-sectional neuroimaging study with ~5,000 adult subjects found that men have greater volumes and cortical surface area, whereas women have a greater cortical thickness (Ritchie et al. 2018). According to Kaczkurkin et al. (2019), the brain size difference among sexes persists during childhood, adolescence, and adulthood, and remains even after correcting for height, which is necessary because men are, on average, taller than women (Sacher et al. 2013). A recent paper evaluated more than 100,000 Magnetic Resonance images throughout the lifespan from 115 days postconception to 100 years of age, showing the differences in the trajectories for gray and white matter volumes, cortical thickness, and cerebrum volume between males and females (Bethlehem et al. 2022).
Nopoulos et al. (2000) showed that the volumes of brain lobes are greater in men. Similar results were found by Allen et al. (2003), who reported a larger volume of gray and white matters in the frontal, parietal, temporal, and occipital lobes, as well as the cingulate gyrus and insula in men. Another neuroimaging study showed that men have a larger volume of white matter in the different lobes, with only the postcentral gyrus white matter being larger in women (Bourisly et al. 2017). Using relative metrics, Nopoulos et al. (2000) found that the proportion of gray matter is higher in men, whereas Allen et al. (2003) found higher values in women.
There are also controversies regarding brain functional differences. However, these results must be carefully interpreted since they are strongly influenced by environmental conditions and may be associated with educational, social, and public policy factors (Reilly et al. 2016). The most replicated results show that men perform better in visuospatial memory tasks such as mental rotation, perception, and spatial visualization. On the other hand, women exceed in language tasks, such as verbal fluency and grammar tasks, and olfaction abilities, such as odor discrimination and identification (Allen et al. 2003; Andreano and Cahill 2009; Sorokowski et al. 2019).
The neurobiological basis behind these structural and functional differences needs to be better understood. It is increasingly accepted that they cannot be derived from a single factor (genetic, hormonal, structural, etc.) but from a combination of biological and sociocultural aspects that impact brain function and structure. The representation of schemes imposed by society, such as paternal expectations, gender stereotypes, cultural beliefs, and socialization experiences, according to gender also modifies the brain. However, we do not know how much genes and environment explain, each one separately, structural and functional brain differences between men and women.
The influence of age on the cerebral cortex
World populational aging, expressed by the increasing proportion of people aged 60+ years, makes the study about the influence of age on the human brain structure particularly relevant. Cerebral aging can occur in 2 forms: healthy aging or senescence and pathological aging or senility. Postmortem studies show a decrease of 0.5% per year in the volume and weight of the brain after the fifth decade of life (Peters 2006). Other expected changes related to aging are the atrophy of the cortex, widening and deepening of the sulci, increase in the size of ventricles, calcifications of the dura mater, and thinning of the leptomeninges (Allen et al. 2005; Shankar 2010). Neuroimaging studies reported a decrease in total brain, white and gray matter volumes (Allen et al. 2005), whereas another study showed the preservation of gray matter volume (Vernooij and Smits 2012). It was also observed that hippocampus volume decreased by ~1.6% per year, and the hyperintense lesions in the subcortical white matter increase in number (Vernooij and Smits 2012).
Along with aging, astrocytes show increased reactivity in rodents as well as human and nonhuman primates (Matias et al. 2019). In addition, 20–30% of healthy older adults display amyloid deposits in the brain parenchyma (Rodrigue et al. 2009), as well as increases in mitochondrial iron and reactive oxygen species, changes of intracellular calcium homeostasis and mitochondrial dysfunction, and altered cell responsiveness to repair the damage, leading to neuronal dysfunction (Yankner et al. 2008; Shankar 2010). Accumulation of lipofuscin, an autofluorescent and insoluble pigment, is also frequent in the somata of neurons and some glial cells of the cortex, inferior olivary nucleus, and thalamus (Mattson and Arumugam 2018).
Some changes in brain functioning during cognitively healthy aging are considered physiological since they are subtle and do not interfere with daily activities. Thus, the decline in processing speed and working memory can be evident in neuropsychological tests after the third decade of life (Harada et al. 2013). Vocabulary tends to be more resilient and usually increases with age through gain from experience. Semantic memory remains preserved and declines only in later ages, and episodic memory is also preserved throughout life with a decline around the age of 70 (Juan and Adlard 2019). The decrease in reasoning and cognitive flexibility can also be characteristic of physiological aging. Sustained attention shows a decline after 40 years old (Anderson and Craik 2017). Because these changes are mild and often hardly perceived by the subjects, it is a great challenge for clinicians to differentiate when they are part of a typical course of aging or represent initial signs of dementia. In a Brazilian cohort with 1,095 participants, 665 (61%) had normal cognition, of which 22% met the criteria for a neuropathological diagnosis, with AD being the most frequent in 57% of cases (Suemoto et al. 2017). In this work, the association between neuropathological lesions and dementia status of subjects with different levels of dementia was also established.
On the other hand, several authors have tried to understand how histological changes could be related to the atrophy of the cortex and functional changes observed during aging (Erten-Lyons et al. 2013; Armstrong et al. 2020; Cox et al. 2021). In particular, it seemed relevant to verify if there are changes in the quantitative cellular composition of the brain in subjects of different ages during cognitively healthy aging (Andrade-Moraes et al. 2013; Oliveira-Pinto et al. 2016). The cerebral cortex, however, has still not been investigated as related to cellularity employing the isotropic fractionator (IF) technique, despite its great relevance for the most complex and elaborate neuropsychological functions. We here report the results of a quantitative study of the cerebral cortex of human subjects aged between 25 and 87 years to analyze the similarities and differences in cell composition between men and women during aging.
Materials and methods
Participants
Forty-three brains were collected by the Biobank for Aging Studies of the University of São Paulo (Grinberg et al. 2007). The selection criteria for this study included subjects 20–100 years old with no history of cognitive decline and normal neuropathological diagnosis. Subjects were excluded if they had a history of neurological diseases, psychiatric diagnosis, alcohol or drug abuse, unsuitable tissue for neuropathological analyses (e.g. cerebrospinal fluid pH < 6.5, or findings of major acute brain lesions, such as hemorrhages or tumors), and inconsistent clinical data provided by the informant. All procedures were approved by institutional ethics committees (N° 588/2010, Federal University of Rio de Janeiro; N° 458.272/2013, São Paulo Medical School; N°032/2015, N° 25000.050648/2014-2061, CONEP).
Clinical and functional postmortem assessment
A knowledgeable informant underwent a semi-structured interview, previously adapted and validated for use in postmortem studies (Ferretti et al. 2010), with detailed assessments of demographic data and relevant clinical history. Cognitive evaluation includes The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE; Jorm and Jacomb 1989; Jorm 1994; Zevallos Bustamante et al. 2003) and the Clinical Dementia Rating (CDR; Morris 1993). Previous cognition was considered normal when CDR was equal to 0 and IQCODE < 3.41. To assure reliable data, subjects were included in the study if the informant had at least weekly contact with the donor within the last 6 months before death. All informants signed written informed consent for the study.
Brains
During necropsy, the brain was removed from the skull by a transverse section using the foramen magnum as the caudal limit of the medulla oblongata. After removal, a section was performed along the sagittal plane to split the 2 hemispheres. The time between death and brain fixation (postmortem interval) was <24 h, except in one case (25 h).
Histopathological analysis and diagnostic criteria
Histopathological analysis was systematically done on the right hemisphere. This hemisphere was fixed by immersion in buffered paraformaldehyde (PF) solution at 4% and used for histopathological analysis (Fig. 1A). Following fixation, selected brain fragments were extracted from the middle frontal gyrus, middle and superior temporal gyri, angular gyrus, superior frontal, and anterior cingulate gyrus, visual cortex, hippocampal formation, amygdala, basal ganglia, thalamus, midbrain, pons, medulla oblongata, and cerebellum. Each fragment was embedded in paraffin, cut into 5-μm thick sections and each section mounted onto glass slides, sequentially deparaffinized, rehydrated, and finally stained with hematoxylin and eosin. Immunohistochemistry was performed (Fig. 1B and C) using antibodies against β-amyloid (4G8, 1:10,000; Signet Pathology Systems, Dedham, Massachusetts), phosphorylated tau (PHF-1, 1:2,000, gift of Pater Davis), TDP-43 (1:500, Proteintech, Chicago, Illinois), and α-synuclein (EQV-1, 1:10,000, gift of Kenji Ishida). Internationally accepted histopathological criteria were employed to diagnose brain pathologies, especially for the detection of AD—CERAD (Mirra et al. 1991) and Braak and Braak (Braak and Braak 1991), Frontotemporal Lobar Degeneration (FTLD; Cairns et al. 2007), Limbic-predominant Aged-related TDP-43 Encephalopathy (LATE; Nelson et al. 2019), Lewy Body Disease (Braak et al. 2003), as well as synucleinopathies, hippocampal sclerosis, and granulovacuolar degeneration. All cases did not meet the criteria for AD neuropathological diagnosis and also lacked signs of synucleinopathy, LATE, or FTLD. We also excluded cases with minimal areas available for neuropathological diagnosis (Fig. S1). In sum, this study included only brains without any neuropathologic diagnoses.
Fig. 1.
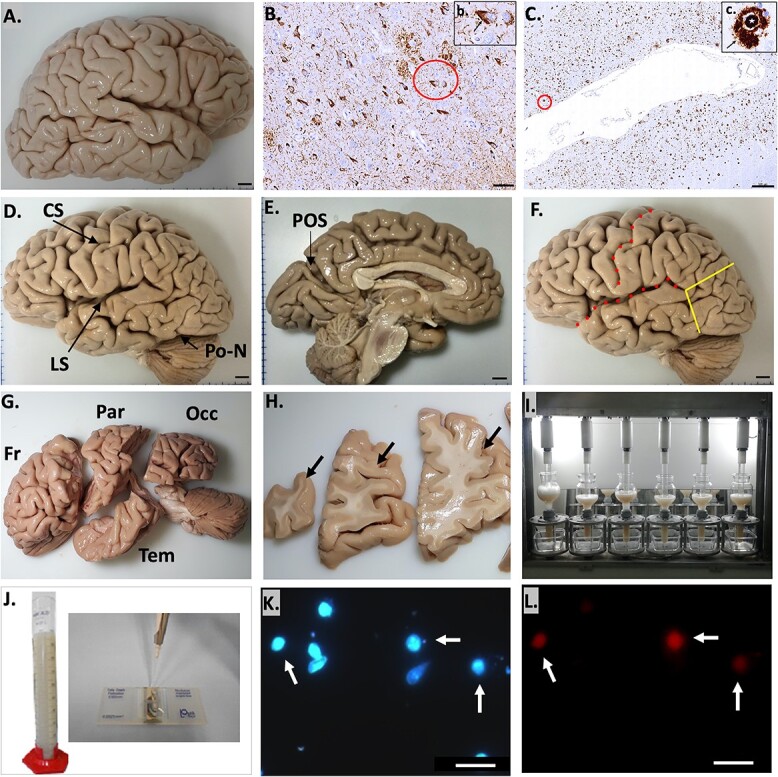
A) Right hemisphere. B and C) Histopathological assessment. B) Phospho-tau neurofibrillary pathology in CA1 of the hippocampus. A neurofibrillary tangle is identified by a red circle, and amplified in the insert b (arrow) in the same region. Scale bar: 50 μm (insert, 20 μm). C) β-amyloid deposits in the middle temporal cortex. A neuritic plaque (red circle) is shown comprising a dense core (white star in the insert c) surrounded by a clear halo (arrow in the insert c). Scale bar: 500 μm (insert, 20 μm). D) Left hemisphere, with anatomical landmarks for cortex segmentation in the dorsal surface. E) Medial surface, POS. F) Brain dissection landmarks. Red dotted lines: CS and LS, yellow line: anterior landmark to separate the occipital lobe. G) Dissected lobes. Fr, frontal lobe; Par, parietal lobe; Tem, temporal lobe; Occ, occipital lobe. H) Anatomical differentiation of gray and white matters in coronal sections. Arrows: gray matter. I) Semi-automatic isotropic fractionation. J) Isotropic suspension and Neubauer chamber for nuclei counting. K) Cell nuclei in suspension stained with DAPI. Arrows: NeuN-positive nuclei, as seen in L). Scale bar: 20 μm. L) Immunocytochemistry to NeuN. Scale bar: 20 μm.
Isotropic fractionation
All cell counting results were obtained from the left hemispheres using the IF. After its creation (Herculano-Houzel and Lent 2005), this technique has been validated independently by other groups (Bahney and Von Bartheld 2014; Miller et al. 2014; Ngwenya et al. 2017) by comparison with unbiased stereology, flow cytometry and cell quantification by DNA extraction, demonstrating it as a reliable, simple, and fast method to arrive at absolute cell numbers in brain regions. It was recently implemented to estimate the cellularity of animals as diverse as Drosophila (Raji and Potter 2021), Hymenoptera (Godfrey et al. 2021), lizards (Storks et al. 2020), and rodents (Farrow et al. 2021; Grigoletti-Lima et al. 2022; Torres et al. 2022).
The left hemisphere was fixed in 2% PF in phosphate buffer saline (PBS) for 36–40 h at 4°C. The cerebrum was dissected into the 4 major lobes using consistent anatomical landmarks as criteria for dissection: the central sulcus (CS), the lateral sulcus (LS), the preoccipital notch (Po-N), and the parieto-occipital sulcus (POS; Fig. 1D and E). The frontal lobe was identified as anterior to the CS and superior to the LS. Delimitation among the other lobes was done by standard cuts (Fig. 1F, yellow lines) between the Po-N (Fig. 1D) and the caudal point of the LS, and between the latter and the POS (Fig. 1E). The temporal lobe was inferior to the LS and anterior to the ventral standard cut described above. The parietal lobe was posterior to the CS and anterior to the dorsal standard cut, and the occipital lobe was posterior to the standard cuts. The frontal lobe was divided into the prefrontal lobe and the precentral gyrus, using the precentral sulcus as a landmark. The medial temporal lobe was removed from the whole temporal lobe, and included in another study (Oliveira-Pinto et al. 2016). Thus, this study’s analysis of the temporal lobe did not include the medial temporal lobe. Each cerebral lobe (Fig. 1G) was sliced into coronal sections about 1 cm thick (Fig. 1H) and later dissected with a scalpel to separate the white from the gray matter. Each region was separately processed by the IF technique following the protocol previously described (Herculano-Houzel and Lent 2005; Azevedo et al. 2009, 2013). In brief, tissue samples were inserted into glass homogenizers containing a standard solution (40 mM sodium citrate and 1% Triton X-100). Homogenizers were controlled electronically by a semi-automated machine (Azevedo et al. 2013; Fig. 1I) that performed rotational and vertical movements with controlled torque and speed. This procedure allowed a chemomechanical dissociation of the tissue and was ended when all visible fragments were dissolved, plasmalemmas were destroyed and nuclei became free, as ascertained later by microscopic examination. A final suspension of nuclei was obtained, and homogenizers were washed with PBS to collect all tissue remnants and avoid loss of nuclei during the procedure. The nuclear suspension was transferred into a graduated cylinder to provide the final, most convenient volume (Fig. 1J). This suspension was maintained homogeneous by continuous agitation to prevent sedimentation.
Aliquots of 10 μL were previously reacted with 2% 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, 20 mg/dl in PBS, Invitrogen) and used for counting. They were loaded onto a hemocytometer or Neubauer chamber (Fig. 1J) for counting under a fluorescence microscope (Zeiss Axioimager) at ×20 magnification (Fig. 1K). Counts were made at least 4 times and the suspension was considered homogeneous when counts have a coefficient of error < 15%. The average density was calculated and the total number of cells of the original region was estimated by multiplying mean nuclear density by total suspension volume, assuming all neural cells have only one nucleus.
We identified neuronal nuclei with NeuN marker (splicing factor Fox3: Kim et al. 2009) using immunohistochemistry (Fig. 1L). NeuN has been shown as a universal and specific marker for neuronal nuclei suitable to be used in formalin-fixed human tissue (Wolf et al. 1996). Most neuronal cells of the central and peripheral nervous system express NeuN except Purkinje cells of the cerebellum, olfactory bulb mitral cells, and retinal photoreceptor cells in rats (Mullen et al. 1992). So far, no neuronal type in the adult cerebral cortex has been identified without reactivity to NeuN.
Two aliquots of 1,000 μL were collected from the nuclear suspension, centrifuged, and washed 3 times with PBS to estimate the total neurons number. The first sample was incubated with the primary antibody anti-mouse NeuN (MAB377) diluted 1:200 in PBS for 24 h at 4°C overnight, and the second was used as a negative control. In sequence, aliquots were washed 3 times with PBS and incubated with the secondary antibody (Alexa-Fluor 546 at 1:300 PBS in a solution containing 10% NGS and 3% DAPI) for 2 h at 4°C. Later, 3 new washes were performed and resuspended with PBS for observation in the Neubauer counting chamber at the fluorescence microscope. A minimum of 500 DAPI-stained nuclei were counted for NeuN co-staining in each sample. The proportion of NeuN-positive nuclei was determined separately for each region and multiplied by the total number of nuclei to yield the absolute number of neurons. The absolute number of non-neuronal cells was calculated by subtracting the number of NeuN-positive nuclei from the total number of nuclei. Absolute cells numbers were obtained from the left hemisphere, and multiplied by 2 to yield whole-brain numbers.
The total number of each cell group was divided by the sample weight to obtain the number of nuclei (and, by extension, the number of cells) per gram of tissue, which we denominated “cell density.” DAPI and NeuN counts showed adequate inter and intraindividual variation (Fig. S2).
Statistical analysis
The software used for these analyses was: SPSS 25 (International Business Machines Corporation, Armonk, NY, USA) and GraphPad Prism 6 (GraphPad Software Inc.). In all cases, normality was checked using the Shapiro–Wilk and D'Agostino and Pearson test. After proving the Gaussian distribution, parametric tests were justified in subsequent analyses. The detection of outlier cases was performed using GraphPad Prism’s ROUT tool.
For the study of brain dimorphism, we tested both groups using the 2-way Student’s t-test, considering P < 0.05 as the significance limit for this and all analyses. As we found that age was a confounding variable in some dimorphism analyses, we needed to redo the analyses including age as a covariate, using ANCOVA test. For the correlation of variables as a function of age, a Pearson correlation test was used and its correlation index (r) was shown for all analyses and its significance level (P-value). Additionally, a linear regression test using the least squares method was used to further characterize the data. In all cases, the P-value was calculated. Since sex proved to be an influencing variable, it was included as a covariate in the correlation tests.
Results
Fifty-five subjects were eligible for this study, of which 12 cases were excluded because of abnormal macroscopic findings or a neuropathological diagnosis (Fig. S1). Among the included cases, 32 were males and 11 were females (Tables 1 and 2 present the demographic and clinical data). The age interval of the male group was 29–85 years, and that of the female group was 25–87 years. The mean age of each group was 56.6 ± 11.7 years old for men and 63.3 ± 20.7 years old for women, with no significant age difference among groups (P = 0.33).
Table 1.
Clinical and demographic variables of male subjects.
| ID | Age (y) | Ed (y) | Hd | Brain mass (g) |
Braak AD stage |
CERAD AD score |
Other NP findings | PMI (h) | Cause of death |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 29 | 12 | R | 1,136 | 0 | 0 | None | 15 | Left hemothorax |
| 2 | 34 | 4 | R | 1,320 | 1 | 0 | None | 18 | PTE |
| 3 | 35 | 11 | R | 1,506 | 0 | 0 | None | 17 | Pulmonary Infarction |
| 4 | 44 | 12 | R | 1,340 | 0 | 0 | None | 15 | Hemopericardium |
| 5 | 47 | 15 | R | 1,530 | 0 | 0 | None | 17 | Pulmonary edema |
| 6 | 47 | 8 | R | 1,582 | 0 | 0 | None | 18 | Pulmonary edema |
| 7 | 47 | 16 | R | 1,775 | 1 | 0 | None | 14 | Bronchopneumonia |
| 8 | 49 | 4 | R | 1,221 | 1 | 0 | None | 11 | CPD |
| 9 | 53 | 7 | L | 1,435 | 3 | 0 | None | 18 | Acute MI |
| 10 | 53 | 2 | R | 1,282 | 3 | 0 | None | 13 | Pulmonary TB |
| 11 | 54 | 15 | R | 1,310 | 0 | 0 | None | 8 | PTE |
| 12 | 55 | 4 | R | 1,150 | 1 | Scarce | None | 12 | Pulmonary edema |
| 13 | 55 | 12 | R | 1,249 | 0 | 0 | None | 19 | PTE |
| 14 | 56 | 12 | R | 1,298 | 1 | 0 | AGD | 11 | Pulmonary edema |
| 15 | 56 | 5 | R | 1,246 | 2 | 0 | None | 9 | Pulmonary edema |
| 16 | 58 | 4 | R | 1,210 | 2 | 0 | None | 15 | Sepsis |
| 17 | 58 | 8 | L | 1,210 | 0 | 0 | None | 17 | Alveolar hemorrhage |
| 18 | 58 | 11 | R | 1,382 | 1 | 0 | AGD | 13 | Pulmonary infarction |
| 19 | 58 | 11 | R | 1,351 | 2 | 0 | None | 15 | PTE |
| 20 | 59 | 4 | R | 1,271 | 2 | 0 | None | 14 | Acute kidney injury |
| 21 | 61 | 8 | R | 1,544 | 1 | 0 | None | 9 | Pulmonary edema |
| 22 | 62 | 8 | R | 1,220 | 2 | 0 | None | 11 | Acute MI |
| 23 | 62 | 0 | R | 1,209 | 1 | 0 | None | 17 | Septic shock |
| 24 | 63 | 8 | NA | 1,238 | 1 | 0 | None | 18 | Bronchopneumonia |
| 25 | 63 | 11 | R | 1,176 | 1 | 0 | None | 12 | RP hemorrhage |
| 26 | 64 | 4 | R | 1,200 | 0 | 0 | None | 16 | Bronchopneumonia |
| 27 | 64 | 11 | R | 1,453 | 1 | 0 | None | 19 | Acute MI |
| 28 | 65 | 4 | NA | 1,413 | 4 | 0 | AGD | 25 | Acute MI |
| 29 | 65 | 11 | L | 1,242 | 1 | 0 | None | 8 | Pulmonary edema |
| 30 | 75 | 11 | R | 1,368 | 0 | 0 | None | 11 | Pulmonary edema |
| 31 | 76 | 4 | NA | 1,275 | 4 | 0 | AGD | 17 | Bronchopneumonia |
| 32 | 85 | 8 | R | 1,124 | 2 | Scarce | None | 12 | Acute anemia |
AGD, argyrophilic grain disease; CPD, chronic pulmonary disease; Ed, education; h, hours; Hd, handedness; MI, myocardial infarction; NA, not available; PMI, postmortem interval; PTE, pulmonary thromboembolism; RP, retroperitoneal; TB, tuberculosis; y, years.
Table 2.
Clinical and demographic variables of female subjects.
| ID | Age (y) | Ed (y) | Hd | Brain mass (g) |
Braak AD stage |
CERAD AD score |
Other NP findings | PMI (h) | Cause of death |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 25 | 11 | R | 1,184 | 0 | 0 | None | 14 | PTE |
| 2 | 40 | 3 | R | 1,142 | 0 | 0 | None | 15 | Pulmonary edema |
| 3 | 48 | 8 | R | 1,286 | 1 | 0 | None | 20 | PTE |
| 4 | 49 | 8 | R | 1,166 | 2 | 0 | None | 21 | Pulmonary edema |
| 5 | 57 | 4 | R | 1,146 | 0 | Scarce | None | 22 | Pulmonary edema |
| 6 | 71 | 4 | NA | 1,094 | 4 | 0 | AGD | 15 | PTE |
| 7 | 74 | 4 | R | 1,270 | 2 | 0 | None | 13 | Cardiac tamponade |
| 8 | 77 | 0 | R | 1,060 | 3 | Moderate | None | 13 | RDS |
| 9 | 84 | 0 | R | 1,002 | NA | 0 | AGD | 12 | Hemoperitoneum |
| 10 | 84 | 4 | NA | 1,084 | 4 | 0 | AGD | 14 | Hemopericardium |
| 11 | 87 | 4 | NA | 976 | 4 | 0 | AGD | 15 | Pulmonary edema |
AGD, argyrophilic grain disease; Ed, education; h, hours; Hd, handedness; NA, not available; NP, neuropathological; PMI, postmortem interval; PTE, pulmonary thromboembolism; RDS, respiratory distress syndrome; y, years.
The mean brain mass of men (1,304 ± 120 g) was higher (P < 0.001) than that of women (1,135 ± 104 g). No significant statistical difference was found for cortex mass (Fig. 2A). For brain mass comparison, we included more cases because of data availability. Comparison of normalized mass (cortical region mass/cortical mass) was similar between sexes (Fig. 2B).
Fig. 2.
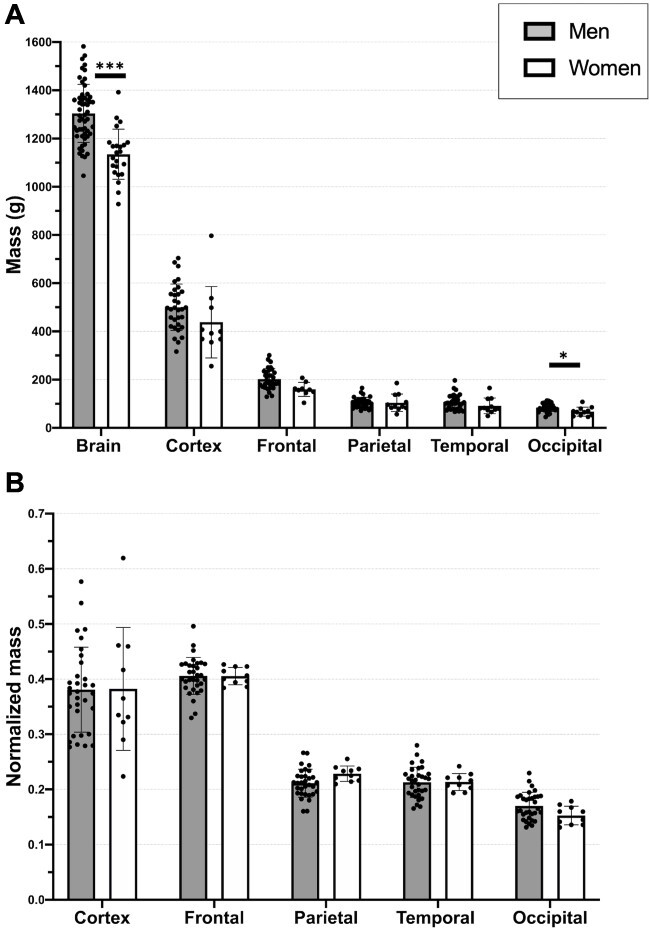
A) Mass comparison of different regions of interest among sexes. B) Normalized mass. The cortex measures were normalized for brain mass, and brain lobes for cortex mass. Temporal lobe mass did not include the medial temporal lobe. Bars indicate mean, and error bars indicate standard deviation. *P < 0.05, ***P < 0.001.
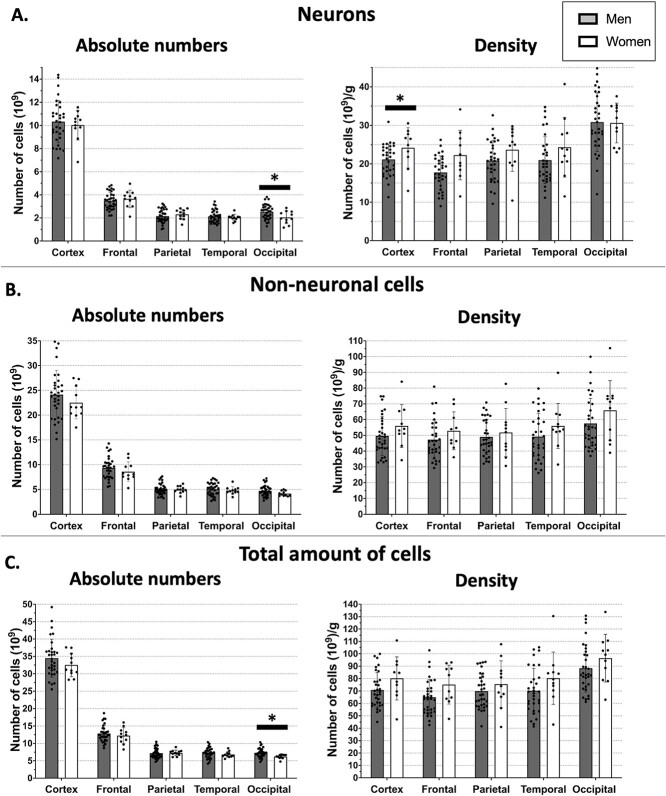
As no significant difference has previously been reported by our group for neuronal, non-neuronal, and total cell numbers between right and left hemispheres (Azevedo et al. 2009), all numbers were derived from left hemispheres and multiplied by 2 to obtain all cortex cell composition. Concerning the analysis of the number of neurons, we found no significant difference between men and women for the cortex as a whole (P = 0.53). No difference was found for the cortical lobes (Fig. 3). The mean total number of neurons in the cortex was 10.3 billion in men and 10.0 billion in women. For cortical lobes, the number of neuronal cells within the occipital cortex was greater in men (P = 0.01).
Fig. 3.
Absolute cell numbers and density among sexes. A) Neurons. B) Non-neuronal cells. C) All cells. The temporal lobe did not include the medial temporal lobe. Bars indicate mean, and error bars indicate standard deviation. *P < 0.05.
The number of non-neuronal cells and the total amount of cortical cells were also similar between men and women (P = 0.54 and P = 0.44, respectively). The mean number of non-neuronal cells was 24.1 billion in men and 22.5 in women. For cortical lobes, there was a significant difference in the total amount of cells in the occipital cortex (P = 0.02; Fig. 3).
Regarding cell density (number of cells/mass), neuronal density in the frontal lobe was greater in women (P = 0.02). No other differences were found in cell density between the sexes.
We found a negative correlation between brain mass and increasing age for both sexes (r = −0.31, P = 0.02). These results indicate a progressive decrease in brain mass during cognitively healthy aging. The entire cortex mass and subregions, however, showed no correlation with age. The same result was obtained when we normalized the cortical mass by brain mass (Fig. 4).
Fig. 4.
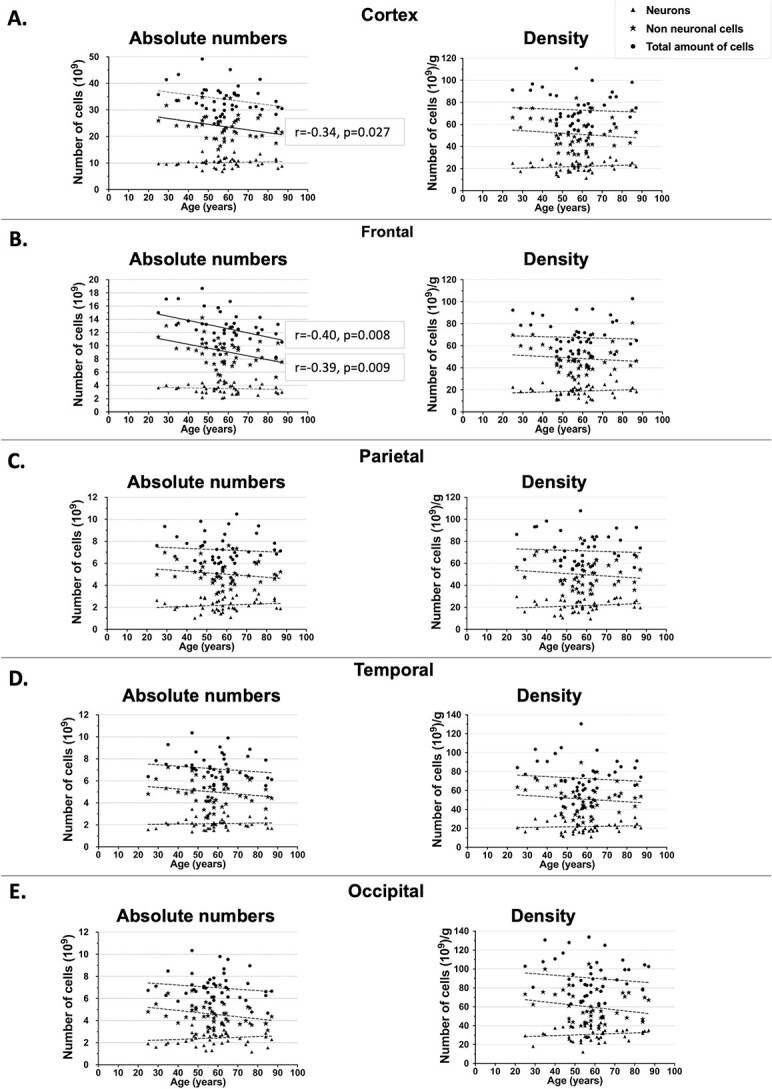
Absolute cell numbers (left) and density (right) during aging. Circles: total amount of cells. Stars: non-neuronal cells. Triangles: neurons. A) Cortex. B) Frontal. C) Parietal. D) Temporal. E) Occipital. Temporal lobe did not include the medial temporal lobe. r: coefficient of Pearson, P: P-value. Dotted lines: nonsignificant linear regression. Continuous line: significant linear regression.
The results of the correlation between age and number of cells showed the preservation of the number of neurons across the cortex with increasing age (r = −0.08, P = 0.59) and a decrease in the number of non-neuronal cells (r = −0.34, P = 0.027).
When we analyzed each lobe separately, we did not find a correlation between increasing age and the number of neurons in the frontal lobe (r = −0.10, P = 0.54; Fig. 4B), whereas we found a negative correlation for non-neuronal cells (r = −0.40, P = 0.008) and the total amount of cells (r = −0.39, P = 0.009). The frontal cortex was the region most affected by physiological aging, showing a loss in the number of non-neuronal cells, mostly represented by glia. This loss was close to 25%.
Regarding cell density of the cortex as a function of age, we observed no correlation with increasing age, suggesting that density is maintained along cognitively healthy aging (Fig. 4). We also did not observe a correlation between cell density in each cortical lobe and increase of age (Fig. 4).
With all the data obtained for the entire cerebral cortex (right and left combined, without the medial temporal lobe), from both sexes of all ages, we were able to regionally compare the cell composition throughout the adult human cerebral cortex, addressing more specifically how the mass and number of neuronal and non-neuronal cells are distributed in the different lobes of the cortex (Fig. 5).
Fig. 5.
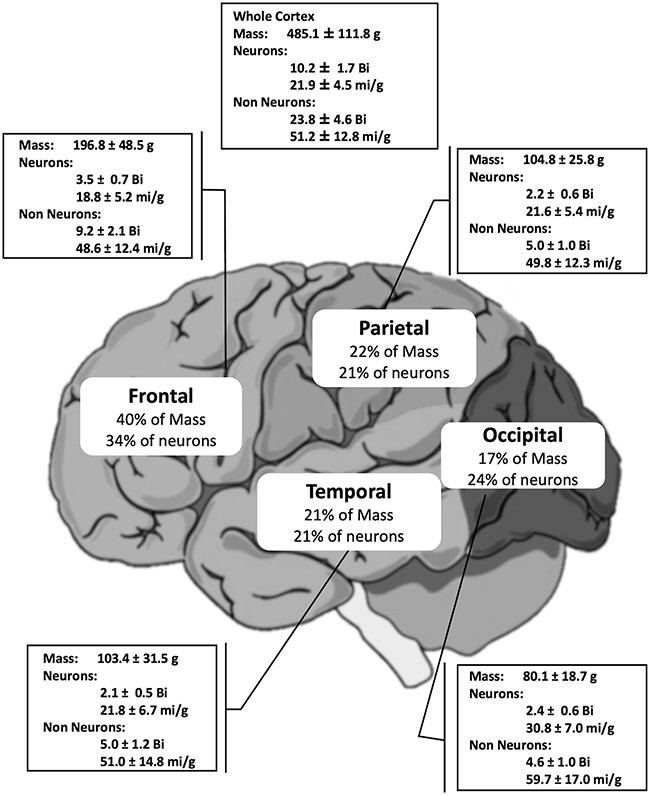
Human brain cortex in numbers. Bi, billion; mi, million. Temporal lobe did not include the medial temporal lobe.
We found that the human cerebral cortex of subjects (both sexes) between 20 and 90 years of age has on average 485.1 ± 111.8 g of mass, 10.2 ± 1.7 billion neurons (21.9 ± 4.5 million/g), and 23.8 billion non-neuronal cells (51.2 ± 12.8 million/g). The frontal cortex contains 34% of all these cortical neurons; the parietal cortex, 21%; the temporal cortex (without the medial temporal lobe), 21%; and the occipital cortex, 24%. These percentages are close to each region’s relative gray matter mass: 40%, frontal cortex; 22%, parietal cortex; 21%, temporal cortex; and 17%, occipital cortex (Fig. 5).
We observed that the density of neurons (number of neurons per gram of tissue) varied across the cortex. The lowest density values (18.8 million/g) were found in the frontal cortex, and the highest in the occipital cortex (30.8 million/g). The density of non-neuronal cells also showed the same profile, higher in the posterior region: 59.7 mi/g in the occipital lobe, against 48.6 mi/g in the frontal lobe.
Regarding the frontal lobe gray matter, we found that the prefrontal cortex contains 85% neurons (3.00 ± 0.71 billion) and 82% non-neuronal cells (7.26 ± 1.79 billion). In comparison, precentral (motor) cortex has 15% neurons (0.51 ± 0.09 billion) and 18% non-neuronal cells (1.57 ± 0.23 billion; Fig. S3).
Discussion
Why is counting neural cells relevant for neuroscience?
Understanding how brain morphology varies among different species according to sex, developmental stage, and aging, as well as in pathological conditions, is a great challenge. The study of cellularity is an important level of analysis to understand brain structure and is useful to reveal the quantitative profile of the brain processing units (neurons and glial cells). Quantitative neuroscience offers several techniques to achieve this goal (Schmitz and Hof 2005; Herculano-Houzel et al. 2015; von Bartheld et al. 2016; von Bartheld 2018; Neves et al. 2019); it is essential that the technique used be accurate, precise, observer-independent, and easily reproducible to obtain reliable results. In addition, research is benefitted from fast and low-cost techniques.
In this work, we studied how cerebral cortex cellularity in humans varies according to sex and age. This topic has been previously studied with a different cellular quantification approach, namely, stereology (Brody 1955; Haug 1987; Pakkenberg and Gundersen 1997; Freeman et al. 2008; Pelvig et al. 2008). In this study, we used the IF for the first time to answer this question. This technique fulfills the features listed above and proved capable of revisiting various dogmas of quantitative neuroscience, revealing interesting data about how the human brain is made up in numbers, and unveiling its changes in physiological and pathological conditions (Azevedo et al. 2009; Lent et al. 2012; Andrade-Moraes et al. 2013; Oliveira-Pinto et al. 2014, 2016).
The IF has proved a fast, easy, inexpensive technique, independent of histological sectioning or tissue volume. Since its description in 2005, it has been employed to obtain precise and accurate results. Reliability has been tested by comparing cell quantifications performed by 3 different experimenters in a non-isogenic (Swiss) mouse strain (Neves et al. 2019). Despite cell quantities, counters, and immunostaining variations, the authors found high test–retest reliability coefficients and offered suggestions to increase the reliability of the IF. On the other hand, some numbers obtained with stereology can vary from 100 to 300%, e.g. Purkinje cells in rats and humans, and neurons in specific regions of mice hippocampus and human thalamus (reviewed by Herculano-Houzel et al. 2015). On the other hand, despite the still low quantity of publications of human brain cellularity obtained with the recent IF technique, they show a smaller variation of data, around 25% (von Bartheld 2018).
As a main limitation, the IF can only be used for standardized dissectible brain regions and requires a complete tissue dissolution, making it impossible to employ histological sections or any other region delimited microscopically (Lent et al. 2012). Since the architecture of the tissue is be dissociated, other morphological quantifications as soma size, and dendritic arborization, are not viable.
The influence of sex on cortical cellularity
Our first findings deal with the possibility of a sex dimorphism on several morphometric parameters of the brain and cerebral cortex, namely, mass, number of cells, and cell density. Different authors have shown morphological and functional differences between men and women for the whole brain (Kruggel 2006) and for specific brain regions (Goldstein et al. 2001). We here report a 15% difference in brain mass, in agreement with findings already consolidated in the literature, in which the brains of men are reported larger than those of women. Furthermore, several autopsy and neuroimaging studies showed that the size of the brain is correlated with the height of the subject (Kruggel 2006). Men tend to have higher heights and larger brains on average (Ferretti-Rebustini et al. 2015).
Regarding cortical cell composition, our results showed no significant difference in the number of neurons and total cells between men and women, except for the occipital cortex, where higher numbers were found in men. Using stereology, Pakkenberg and Gundersen (1997) found 23 billion neurons in men and 19 billion in women, a difference of 16%. As for the number of non-neuronal cells, we also found no statistical differences. Pelvig et al. (2008) found an average of 39 billion glial cells in men and 28 billion in women. We did not show a significant dimorphism in human brain cortex cellularity, since we observed similar values between sexes, with more data dispersion in men. Our results agree with an early study by Haug (1987) that also failed to find a sex dimorphism in the cortical number of neurons in humans. Also, in agreement with our results, he found a higher density of neurons in women: around 4,000 more neurons per mm3 in their cortex. We should consider that the variability of the human brain numbers, in addition to the different number of cases, and low n in our female group, may affect the statistical tests capacity to detect sex differences.
We observed a positive correlation between the number of neurons and non-neuronal cells with lobes mass, suggesting that bigger brains tend to have more neurons, independent of sex. Using the IF, previous studies from our group have shown that other brain regions are sexually dimorphic in cell composition. Women were shown to have ~50% more neurons and 40% more non-neuronal cells in the olfactory bulb (Oliveira-Pinto et al. 2014), whereas men have 34% more neurons and ~20% more non-neuronal cells in the medial temporal lobe. No differences were recognized in the cerebellum (Oliveira-Pinto et al. 2016).
The influence of age on cortex cellularity
The present results have shown that brain mass decreases with increasing age, confirming Ferretti-Rebustini et al. (2015) for absolute values, adjusted for head circumference. They found that other variables such as education and comorbidities, e.g. hypertension and coronary disease, were also correlated with the mass of the brain.
For the entire cortex, the number of cells showed no loss of neurons with aging but revealed a reduction of glial cell numbers. Pioneering works since the 1950s showed a decreased number and density of neurons with increasing age (Brody 1955; Hanley 1974; Devaney and Johnson 1980; Henderson et al. 1980; von Bartheld 2018). Brody (1955) reported an extensive neuronal loss of 20–60% with aging, using 2D counts in histological sections of the cortex (precentral, postcentral, superior temporal, and inferior temporal gyri). Cragg (1975) used stereology and found that the number of neurons in 7 regions of the cortex was similar between older and younger adults.
In 1980s, using stereological techniques, Haug found no loss of neurons with physiological aging in 81 subjects between 20 and 110 years old (Haug 1987). Another study (Terry et al. 1987) employed a combination of manual and automatic cell counting methods, and also found no changes in the total number or density of neurons in small areas of the prefrontal, temporal, and parietal cortexes of 51 subjects aged 24–100 years. The authors found a decrease in the number of large neurons (>90 μm2 soma size) and an increase in small neurons (41–90 μm2) in these regions. They attributed these findings to a specific numerical reduction of large neurons with aging, in parallel with a similar increase of small neurons, resulting in an unchanged absolute number of neurons as a whole. Another work employed serial sections of the primary visual cortex of 46 subjects with different prenatal ages up to 93 years old: results showed great inter-individual variability in the number of neurons, with no evidence of any reduction in the number of cells with increasing age (Leuba and Kraftsik 1994).
Pakkenberg and Gundersen (1997) found, in a cohort of 94 subjects, a neuronal loss of 9.5% between 18 and 93 years, with the preservation of neuronal density. They used the optical dissector, a robust stereological counting technique in 3 dimensions, but the postmortem material did not undergo histopathological analyses to exclude neurodegenerative disorders. Freeman et al. (2008), also using stereology, studied 17–19 cortical sections of older subjects (between 56 and 103 years old, n = 27) without cognitive changes, finding the preservation of neuronal density. More recent studies also report a maintenance of the number of neurons and a decrease in the number of oligodendrocytes even at later ages (between 65 and 105 years old; Fabricius et al. 2013). They suggest that neuronal preservation is a biological advantage related to the longevity of these subjects.
Older conceptions based on early studies, which found a massive loss of neurons, could have been overestimated because of the techniques limitations. Haug suggested that previous conflicting results could be because of technical artifacts, as different patterns of brain shrinkage between young and aged subjects after the process of fixation could interfere with the final cell number estimations (Haug 1987; von Bartheld 2018).
More recent studies in humans, including ours, the first to use the IF technique for this purpose, suggest that there is no neuronal loss when there is no cognitive decline and neuropathology in the cerebral cortex (Peters 2002; Dickstein et al. 2007; Shankar 2010). On the other hand, we detected that glial cells decrease by about 25%, which is a relatively large number. Since the time of Virchow when glia was conceived only as the glue of the nervous system (Chvátal and Verkhratsky 2018), many concepts have been revisited. Today, it is well understood that these cells participate actively in processing neural information and are key to the proper operation of the nervous system. Our results refer to non-neuronal cells broadly, including glial cells, but do not identify each type of glial cell separately for the lack of specific and universal nuclear markers at the time the experiments were done. More recently, our lab and others have tested and validated nuclear markers for oligodendrocytes (Valério-Gomes et al. 2018) and astrocytes (Sun et al. 2017) for use with the IF. Future work will now be possible to clarify the specific cellularity of glial subtypes.
Oligodendrocytes seem to be the most frequent glial subtype in the cortex as seen in other species and humans. Pelvig et al. (2008) showed a decrease of ~ 27% in the number of oligodendrocytes, the preservation of the number of astrocytes, and a slight increase of microglia in women in a sample of 31 brains from subjects aged between 18 and 93 years.
Interestingly, although a decrease of glial numbers was detected as a function of age, these individuals remained cognitively healthy. We did not explore synapse quantification, but it has already been shown that a reduction occurs during senescence and in neurodegenerative disease such as AD (Anderson and Rutledge 1996; Scheff and Price 2003; Scheff et al. 2006; Yankner et al. 2008; McGinnis et al. 2011). The cerebral reserve can be a possible mechanism explaining cognitive brain function preservation despite morphological changes.
Human brain cortex in numbers
We found that, on average, the human cerebral cortex contains around 10.2 billion neurons with great inter-individual variability. This result is in accordance with other studies using the IF technique: in a single female case, Ribeiro et al. (2013) found 9.26 billion neurons; in 4 middle-aged male cases, Azevedo et al. (2009) reported 12.36 billion for the gray matter, and including the medial temporal lobe, and in 6 women older than 60 years old, Andrade-Moraes et al. (2013) found 11.8 billion, around 13 billion in 81 subjects (Haug 1987), much lower than the numbers found by Pakkenberg and Gundersen (1997; 19 billion for women, 23 billion for men).
The present results concerning the regional distribution of cells throughout the human cerebral cortex are relevant to understand how our brain is structured. The occipital lobe has the highest density of cells and the frontal cortex the lowest, indicating an increase in density along the anterior–posterior axis. This finding is in agreement with Ribeiro et al. (2013). It could be attributed to the smaller size of cells and a decrease in dendritic arborization in caudal regions, or a higher level of intrinsic connectivity frontally.
The dissection employed here to divide brain lobes used as reference main cortical sulci with known low inter-individual variability. Thus, this delimitation and results can be reproduced for future comparisons and used as a baseline in studies evaluating changes in cellularity in individuals with diseases affecting the cerebral cortex, such as neurodegenerative diseases, tumors, or stroke, and in comparative studies among species to study cortical organization rules.
All analyses of cell counting were systematically done for the left hemispheres. Functional interhemispheric asymmetries are well established and especially represented by unilateral language and motor dominance (Sperry 1984; Corballis 2014). Qualitative and quantitative differences between left and right hemispheres have been reported, including asymmetrical sulci, different gyri volumes, and different dendritic arborization (Simic et al. 2005). Besides, some specific regions as Brocas’ area exhibited higher number of neurons in the left side (Uylings et al. 2006). Despite these differences, and the reported small proportion of right dominance in left-handers, we had only a small number of left-handed (3 cases) in our sample to reveal any significant differences that could impact the main conclusions. Besides, no differences in the total amount of neuronal, non-neuronal, and total amount of cells have been previously observed among left and right hemispheres (Azevedo et al. 2009).
In sum, in this work, we examined whether sex and age impact on the cellularity of the human neocortex of cognitively healthy subjects with no neuropathological diseases. We found no differences in the frontal, parietal, and temporal lobes between men and women, except in the occipital lobe, with a greater number of neurons in men. We also found a statistical difference in neuronal density in the frontal lobe, greater in women. We did not find changes in the number of neurons associated with age, what may contribute to the maintenance of most cognitive abilities during senescence. Our results show that the neocortex has ~10.2 billion neurons; most of them (34%) are in the frontal lobe, whereas the remaining 66% are similarly distributed among the other lobes. These results serve as a baseline for quantitative research that investigates the composition of the cerebral cortex and can help compare typical conditions with different neurological impairments, such as neurodegenerative diseases.
Supplementary Material
Acknowledgments
We thank our lab technicians, Ludmilla Ribeiro and Camila Lopes, for their help during all stages of this work.
Contributor Information
Emily Castro-Fonseca, Institute of Biomedical Sciences, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil; D’Or Institute for Research and Education, Rio de Janeiro, Brazil.
Viviane Morais, Institute of Biomedical Sciences, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil.
Camila G da Silva, Institute of Biomedical Sciences, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil.
Juliana Wollner, Institute of Biomedical Sciences, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil.
Jaqueline Freitas, Institute of Biomedical Sciences, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil.
Arthur F Mello-Neto, Institute of Biomedical Sciences, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil.
Luiz E Oliveira, Institute of Biomedical Sciences, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil.
Vilson C de Oliveira, Institute of Biomedical Sciences, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil.
Renata E P Leite, Biobank for Aging Studies, LIM 22, University of São Paulo Medical School, São Paulo, Brazil; Laboratory of Medical Research in Aging (LIM-66), University of São Paulo Medical School, São Paulo, Brazil.
Ana T Alho, Biobank for Aging Studies, LIM 22, University of São Paulo Medical School, São Paulo, Brazil.
Roberta D Rodriguez, Biobank for Aging Studies, LIM 22, University of São Paulo Medical School, São Paulo, Brazil; Department of Pathology, University of São Paulo Medical School, São Paulo, Brazil.
Renata E L Ferretti-Rebustini, Biobank for Aging Studies, LIM 22, University of São Paulo Medical School, São Paulo, Brazil; Department of Medical Surgical Nursing, University of São Paulo School of Nursing, São Paulo, Brazil.
Claudia K Suemoto, Biobank for Aging Studies, LIM 22, University of São Paulo Medical School, São Paulo, Brazil; Laboratory of Medical Research in Aging (LIM-66), University of São Paulo Medical School, São Paulo, Brazil.
Wilson Jacob-Filho, Biobank for Aging Studies, LIM 22, University of São Paulo Medical School, São Paulo, Brazil; Laboratory of Medical Research in Aging (LIM-66), University of São Paulo Medical School, São Paulo, Brazil.
Ricardo Nitrini, Biobank for Aging Studies, LIM 22, University of São Paulo Medical School, São Paulo, Brazil; Department of Neurology, University of São Paulo Medical School, São Paulo, Brazil.
Carlos A Pasqualucci, Biobank for Aging Studies, LIM 22, University of São Paulo Medical School, São Paulo, Brazil; Department of Pathology, University of São Paulo Medical School, São Paulo, Brazil.
Lea T Grinberg, Biobank for Aging Studies, LIM 22, University of São Paulo Medical School, São Paulo, Brazil; Department of Pathology, University of São Paulo Medical School, São Paulo, Brazil; Memory and Aging Center, Department of Neurology, University of California, San Francisco, CA, United States.
Fernanda Tovar-Moll, Institute of Biomedical Sciences, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil; D’Or Institute for Research and Education, Rio de Janeiro, Brazil.
Roberto Lent, Institute of Biomedical Sciences, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil; D’Or Institute for Research and Education, Rio de Janeiro, Brazil; National Institute of Translational Neuroscience, Ministry of Science and Technology, São Paulo, Brazil.
Author contributions
Emily Castro-Fonseca (Conceptualization, Formal analysis, Investigation, Methodology, Writing—original draft, Writing—review & editing), Viviane Morais (Investigation, Writing—review & editing), Camila G. da Silva (Investigation), Juliana Wollner (Investigation), Jaqueline Freitas (Investigation), Arthur F. Mello-Neto (Investigation), Luiz E. Oliveira (Investigation), Vilson C. de Oliveira (Investigation), Renata E.P. Leite (Investigation), Ana T. Alho (Investigation), Roberta D. Rodriguez (Investigation), Renata E.L. Ferretti-Rebustini (Investigation), Claudia K. Suemoto (Formal analysis, Funding acquisition, Investigation, Methodology, Writing—review & editing), Wilson Jacob-Filho (Conceptualization, Funding acquisition, Resources, Writing—review & editing), Ricardo Nitrini (Conceptualization, Writing—review & editing), Carlos Pasqualucci (Resources), Lea T. Grinberg (Conceptualization, Funding acquisition, Resources, Writing—review & editing), Fernanda F. Tovar-Moll (Formal analysis, Funding acquisition, Methodology, Resources, Writing—review & editing), and Roberto Lent (Conceptualization, Methodology, Project administration, Resources, Supervision, Writing—original draft, Writing—review & editing)
Funding
This work was supported by grants from the Brazilian Council for Science and Technology Development (CNPq, Proc. 402990/2016-1), the Rio de Janeiro Foundation for the Support of Science (FAPERJ, Proc. E-26/203.024/2015, E-26/202.892/2018), and the Brazilian Ministry of Science, Technology, and Innovation (Program of National Institutes of Science and Technology, MCTI-INCTs, Proc. 465346/2014-6). CKS receives a scholarship because of research productivity from the Brazilian National Council for Scientific and Technological Development (CNPq 303883/2021-9). LTG is funded by the National Institutes of Health (K24 AG043435).
Conflict of interest statement. None declared.
Data availability
Please contact the corresponding author.
References
- Allen JS, Damasio H, Grabowski TJ, Bruss J, Zhang W. Sexual dimorphism and asymmetries in the gray-white composition of the human cerebrum. NeuroImage. 2003:18:880–894. [DOI] [PubMed] [Google Scholar]
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol Aging. 2005:26:1245–1260. [DOI] [PubMed] [Google Scholar]
- Anderson ND, Craik FIM. 50 years of cognitive aging theory. J Gerontol Ser B Psychol Sci Soc Sci. 2017:72:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B, Rutledge V. Age and hemisphere effects on dendritic structure. Brain. 1996:119:1983–1990. [DOI] [PubMed] [Google Scholar]
- Andrade-Moraes CH, Oliveira-Pinto AV, Castro-Fonseca E, da Silva CG, Guimarães DM, Szczupak D, Parente-Bruno DR, Carvalho LRB, Polichiso L, Gomes BV, et al. Cell number changes in Alzheimer’s disease relate to dementia, not to plaques and tangles. Brain. 2013:136:3738–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learn Mem. 2009:16:248–266. [DOI] [PubMed] [Google Scholar]
- Armstrong NM, An Y, Shin JJ, Williams OA, Doshi J, Erus G, Davatzikos C, Ferrucci L, Beason-Held LL, Resnick SM. Associations between cognitive and brain volume changes in cognitively normal older adults. NeuroImage. 2020:223:117289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo FAC, Carvalho LRB, Grinberg LT, Farfel JM, Ferretti REL, Leite REP, Filho WJ, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009:513:532–541. [DOI] [PubMed] [Google Scholar]
- Azevedo FAC, Andrade-Moraes CH, Curado MR, Oliveira-Pinto AV, Guimarães DM, Szczupak D, Gomes BV, Alho ATL, Polichiso L, Tampellini E, et al. Automatic isotropic fractionation for large-scale quantitative cell analysis of nervous tissue. J Neurosci Methods. 2013:212:72–78. [DOI] [PubMed] [Google Scholar]
- Bahney J, Von Bartheld CS. Validation of the isotropic fractionator: comparison with unbiased stereology and DNA extraction for quantification of glial cells. J Neurosci Methods. 2014:222:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethlehem RAI, Seidlitz J, White SR, Vogel JW, Anderson KM, Adamson C, Adler S, Alexopoulos GS, Anagnostou E, Areces-Gonzalez A, et al. Brain charts for the human lifespan. Nature. 2022:604:525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourisly AK, Gejo G, Hayat AA, Alsarraf L, Dashti FM, Di Paola M. White matter sexual dimorphism of the adult human brain. Transl Neurosci. 2017:8:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991:82:239–259. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rüb U, De Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003:24:197–211. [DOI] [PubMed] [Google Scholar]
- Brody H. Organization of the cerebral cortex. III. A study of aging in the human cerebral cortex. J Comp Neurol. 1955:102:511–556. [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Bigio EH, Mackenzie IRA, Neumann M, Lee VM-Y, Hatanpaa KJ, White CL, Schneider JA, Grinberg LT, Halliday G, et al. Neuropathologic diagnostic and nosologic criteria for Frontotemporal Lobar Degeneration: consensus of the consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007:114:5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvátal A, Verkhratsky A. An early history of Neuroglial research: personalities. Neuroglia. 2018:1:245–281. [Google Scholar]
- Corballis MC. Left brain, right brain: facts and fantasies. PLoS Biol. 2014:12:e1001767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox SR, Harris MA, Ritchie SJ, Buchanan CR, Valdés Hernández MC, Corley J, Taylor AM, Madole JW, Harris SE, Whalley HC, et al. Three major dimensions of human brain cortical ageing in relation to cognitive decline across the eighth decade of life. Mol Psychiatry. 2021:26:2651–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg BG. The density of synapses and neurons in normal, mentally defective and ageing human brains. Brain. 1975:98:81–90. [DOI] [PubMed] [Google Scholar]
- DeCasien AR, Guma E, Liu S, Raznahan A. Sex differences in the human brain: a roadmap for more careful analysis and interpretation of a biological reality. Biol Sex Differ. 2022:13:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaney KO, Johnson HA. Neuron loss in the aging visual cortex of man. J Gerontol. 1980:35:836–841. [DOI] [PubMed] [Google Scholar]
- Dickstein DL, Kabaso D, Rocher AB, Luebke JI, Wearne SL, Hof PR. Changes in the structural complexity of the aged brain. Aging Cell. 2007:6:275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erten-Lyons D, Dodge HH, Woltjer R, Silbert LC, Howieson DB, Kramer P, Kaye JA. Neuropathologic basis of age-associated brain atrophy. JAMA Neurol. 2013:70:616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricius K, Jacobsen JS, Pakkenberg B. Effect of age on neocortical brain cells in 90+ year old human females-a cell counting study. Neurobiol Aging. 2013:34:91–99. [DOI] [PubMed] [Google Scholar]
- Farrow LF, Andronicos NM, McDonald PG, Hamlin AS. Quantitative determination of neuronal size and density using flow cytometry. J Neurosci Methods. 2021:352:109081. [DOI] [PubMed] [Google Scholar]
- Ferretti REL, Damin AE, SMD B, Morillo LS, Perroco TR, Campora F, Moreira EG, Balbino ÉS, do C de A Lima M, Battela C, et al. Post-mortem diagnosis of dementia by informant interview. Dement Neuropsychol. 2010:4:138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti-Rebustini REL, Jacob-Filho W, Suemoto CK, Farfel JM, REP L, Grinberg LT, Pasqualucci CA, Nitrini R. Factors associated with morphometric brain changes in cognitively normal aging. Dement Neuropsychol. 2015:9:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SH, Kandel R, Cruz L, Rozkalne A, Newell K, Frosch MP, Hedley-Whyte ET, Locascio JJ, Lipsitz LA, Hyman BT. Preservation of neuronal number despite age-related cortical brain atrophy in elderly subjects without Alzheimer disease. J Neuropathol Exp Neurol. 2008:67:1205–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey RK, Swartzlander M, Gronenberg W. Allometric analysis of brain cell number in hymenoptera suggests ant brains diverge from general trends. Proc R Soc B Biol Sci. 2021:288:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001:11:490–497. [DOI] [PubMed] [Google Scholar]
- Grabowska A. Sex on the brain: are gender-dependent structural and functional differences associated with behavior? J Neurosci Res. 2017:95:200–212. [DOI] [PubMed] [Google Scholar]
- Grigoletti-Lima GB, Lopes MG, Franco ATB, Damico AM, Boer PA, Rocha Gontijo JA. Severe gestational low-protein intake impacts hippocampal cellularity, tau, and amyloid-β levels, and memory performance in male adult offspring: an Alzheimer-simile disease model? J Alzheimer’s Dis Rep. 2022:6:17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinberg LT, Lucena Ferretti RE, Farfel JM, Leite R, Pasqualucci CA, Rosemberg S, Nitrini R, Saldiva PHN, Jacob FW. Brain bank of the Brazilian aging brain study group - a milestone reached and more than 1,600 collected brains. Cell Tissue Bank. 2007:8:151–162. [DOI] [PubMed] [Google Scholar]
- Hanley T. “Neuronal fall-out” in the ageing brain: a critical review of the quantitative data. Age Ageing. 1974:3:133–151. [DOI] [PubMed] [Google Scholar]
- Harada CN, Natelson Love MC, Triebel KL. Normal cognitive aging. Clin Geriatr Med. 2013:29:737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug H. Brain sizes, surfaces, and neuronal sizes of the cortex cerebri: a stereological investigation of man and his variability and a comparison with some mammals (primates, whales, marsupials, insectivores, and one elephant). Am J Anat. 1987:180:126–142. [DOI] [PubMed] [Google Scholar]
- Henderson G, Tomlinson BE, Gibson PH. Cell counts in human cerebral cortex in normal adults throughout life using an image analysing computer. J Neurol Sci. 1980:46:113–136. [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S, Lent R. Isotropic fractionator: a simple, rapid method for the quantification of total cell and neuron numbers in the brain. J Neurosci. 2005:25:2518–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S, von Bartheld CS, Miller DJ, Kaas JH. How to count cells: the advantages and disadvantages of the isotropic fractionator compared with stereology. Cell Tissue Res. 2015:360:29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994:24:145–153. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989:19:1015–1022. [DOI] [PubMed] [Google Scholar]
- Juan SMA, Adlard PA. Ageing and cognition BT. In: Harris JR, Korolchuk VI, editors. Biochemistry and cell biology of ageing: part II clinical science. Springer Singapore: Singapore; 2019. pp. 107–122 [Google Scholar]
- Kaczkurkin AN, Raznahan A, Satterthwaite TD. Sex differences in the developing brain: insights from multimodal neuroimaging. Neuropsychopharmacology. 2019:44:71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK, Adelstein RS, Kawamoto S. Identification of neuronal nuclei (NeuN) as Fox-3, a new member of the Fox-1 gene family of splicing factors. J Biol Chem. 2009:284:31052–31061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruggel F. MRI-based volumetry of head compartments: normative values of healthy adults. NeuroImage. 2006:30:1–11. [DOI] [PubMed] [Google Scholar]
- Lent R, Azevedo FAC, Andrade-Moraes CH, Pinto AVO. How many neurons do you have? Some dogmas of quantitative neuroscience under revision. Eur J Neurosci. 2012:35:1–9. [DOI] [PubMed] [Google Scholar]
- Leuba G, Kraftsik R. Changes in volume, surface estimate, three-dimensional shape and total number of neurons of the human primary visual cortex from midgestation until old age. Anat Embryol. 1994:190:351–366. [DOI] [PubMed] [Google Scholar]
- Matias I, Morgado J, Gomes FCA. Astrocyte heterogeneity: impact to brain aging and disease. Front Aging Neurosci. 2019:11:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Arumugam TV. Hallmarks of brain aging: adaptive and pathological modification by metabolic states. Cell Metab. 2018:27:1176–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM. Multifaceted origins of sex differences in the brain. Philos Trans R Soc B Biol Sci. 2016:371:20150106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis SM, Brickhouse M, Pascual B, Dickerson BC. Age-related changes in the thickness of cortical zones in humans. Brain Topogr. 2011:24:279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DJ, Balaram P, Young NA, Kaas JH. Three counting methods agree on cell and neuron number in chimpanzee primary visual cortex. Front Neuroanat. 2014:8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, Belle G, v., Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991:41:479–479. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993:43:2412–2414. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebratesxs. Development. 1992:116:201–211. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Dickson DW, Trojanowski JQ, Jack CR, Boyle PA, Arfanakis K, Rademakers R, Alafuzoff I, Attems J, Brayne C, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019:142:1503–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves K, Menezes Guimarães D, Rayêe D, Valério-Gomes B, Meneses Iack P, Lent R, Mota B. The reliability of the isotropic fractionator method for counting total cells and neurons. J Neurosci Methods. 2019:326:108392. [DOI] [PubMed] [Google Scholar]
- Ngwenya A, Nahirney J, Brinkman B, Williams L, Iwaniuk AN. Comparison of estimates of neuronal number obtained using the isotropic fractionator method and unbiased stereology in day old chicks (Gallus domesticus). J Neurosci Methods. 2017:287:39–46. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Flaum M, O’Leary D, Andreasen NC. Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Res. 2000:98:1–13. [DOI] [PubMed] [Google Scholar]
- Oliveira-Pinto AV, Santos RM, Coutinho RA, Oliveira LM, Santos GB, Alho ATL, Leite REP, Farfel JM, Suemoto CK, Grinberg LT, et al. Sexual dimorphism in the human olfactory bulb: females have more neurons and glial cells than males. PLoS One. 2014:9:e111733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Pinto AV, Andrade-Moraes CH, Oliveira LM, Parente-Bruno DR, Santos RM, Coutinho RA, Alho ATL, Leite REP, Suemoto CK, Grinberg LT, et al. Do age and sex impact on the absolute cell numbers of human brain regions? Brain Struct Funct. 2016:221:3547–3559. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans. J Comp Neurol. 1997:384:312–320. [PubMed] [Google Scholar]
- Pelvig DP, Pakkenberg H, Stark AK, Pakkenberg B. Neocortical glial cell numbers in human brains. Neurobiol Aging. 2008:29:1754–1762. [DOI] [PubMed] [Google Scholar]
- Peters A. Structural changes that occur during normal aging of primate cerebral hemispheres. Neurosci Biobehav Rev. 2002:26:733–741. [DOI] [PubMed] [Google Scholar]
- Peters R. Ageing and the brain. Postgrad Med J. 2006:82:84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raji JI, Potter CJ. The number of neurons in Drosophila and mosquito brains. PLoS One. 2021:16:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly D, Neumann DL, Andrews G. Sex and sex-role differences in specific cognitive abilities. Intelligence. 2016:54:147–158. [Google Scholar]
- Ribeiro PFM, Ventura-Antunes L, Gabi M, Mota B, Grinberg LT, Farfel JM, Ferretti-Rebustini REL, Leite REP, Filho WJ, Herculano-Houzel S. The human cerebral cortex is neither one nor many: neuronal distribution reveals two quantitatively different zones in the gray matter, three in the white matter, and explains local variations in cortical folding. Front Neuroanat. 2013:7:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie SJ, Cox SR, Shen X, Lombardo MV, Reus LM, Alloza C, Harris MA, Alderson HL, Hunter S, Neilson E, et al. Sex differences in the adult human brain: evidence from 5216 UK biobank participants. Cereb Cortex. 2018:28:2959–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue KM, Kennedy KM, Park DC. Beta-amyloid deposition and the aging brain. Neuropsychol Rev. 2009:19:436–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J, Neumann J, Okon-Singer H, Gotowiec S, Villringer A. Sexual dimorphism in the human brain: evidence from neuroimaging. Magn Reson Imaging. 2013:31:366–375. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA. Synaptic pathology in Alzheimer’s disease: a review of ultrastructural studies. Neurobiol Aging. 2003:24:1029–1046. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2006:27:1372–1384. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Hof PR. Design-based stereology in neuroscience. Neuroscience. 2005:130:813–831. [DOI] [PubMed] [Google Scholar]
- Shankar S. Biology of aging brain. Indian J Pathol Microbiol. 2010:53:595. [DOI] [PubMed] [Google Scholar]
- Simic G, Bexheti S, Kelovic Z, Kos M, Grbic K, Hof PR, Kostovic I. Hemispheric asymmetry, modular variability and age-related changes in the human entorhinal cortex. Neuroscience. 2005:130:911–925. [DOI] [PubMed] [Google Scholar]
- Sorokowski P, Karwowski M, Misiak M, Marczak MK, Dziekan M, Hummel T, Sorokowska A. Sex differences in human olfaction: a meta-analysis. Front Psychol. 2019:10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry R. Consciousness, personal identity and the divided brain. Neuropsychologia. 1984:22:661–673. [DOI] [PubMed] [Google Scholar]
- Storks L, Powell BJ, Leal M. Peeking inside the lizard brain: neuron numbers in Anolis and its implications for cognitive performance and vertebrate brain evolution. Integr Comp Biol. 2020:icaa129. 10.1093/icb/icaa129. [DOI] [PubMed] [Google Scholar]
- Suemoto CK, Ferretti-Rebustini REL, Rodriguez RD, Leite REP, Soterio L, Brucki SMD, Spera RR, Cippiciani TM, Farfel JM, Chiavegatto Filho A, et al. Neuropathological diagnoses and clinical correlates in older adults in Brazil: a cross-sectional study. PLoS Med. 2017:14:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XW, Cornwell XA, Li J, Peng XS, Osorio MJ, Aalling XN, Wang S, Benraiss A, Lou N, Goldman SA, et al. SOX9 is an astrocyte-specific nuclear marker in the adult brain outside the neurogenic regions. J Neurosci. 2017:37:4493–4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RD, DeTeresa R, Hansen LA. Neocortical cell counts in normal human adult aging. Ann Neurol. 1987:21:530–539. [DOI] [PubMed] [Google Scholar]
- Torres DB, Lopes A, Rodrigues AJ, Lopes MG, Ventura-Silva AP, Sousa N, Gontijo JAR, Boer PA. Gestational protein restriction alters early amygdala neurochemistry in male offspring. Nutr Neurosci. 2022:4:1–17. [DOI] [PubMed] [Google Scholar]
- Uylings HBM, Jacobsen AM, Zilles K, Amunts K. Left-right asymmetry in volume and number of neurons in adult Broca’s area. Cortex. 2006:42:652–658. [DOI] [PubMed] [Google Scholar]
- Valério-Gomes B, Guimarães DM, Szczupak D, Lent R. The absolute number of oligodendrocytes in the adult mouse brain. Front Neuroanat. 2018:12:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooij MW, Smits M. Structural neuroimaging in aging and Alzheimer’s disease. Neuroimaging Clin N Am. 2012:22:33–55. [DOI] [PubMed] [Google Scholar]
- von Bartheld CS. Myths and truths about the cellular composition of the human brain: a review of influential concepts. J Chem Neuroanat. 2018:93:2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bartheld CS, Bahney J, Herculano-Houzel S. The search for true numbers of neurons and glial cells in the human brain: a review of 150 years of cell counting. J Comp Neurol. 2016:524:3865–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf HK, Buslei R, Schmidt-Kastner R, Schmidt-Kastner PK, Pietsch T, Wiestler OD, Blümcke I. NeuN: a useful neuronal marker for diagnostic histopathology. J Histochem Cytochem. 1996:44:1167–1171. [DOI] [PubMed] [Google Scholar]
- Yankner BA, Lu T, Loerch P. The aging brain. Annu Rev Pathol Mech Dis. 2008:3:41–66. [DOI] [PubMed] [Google Scholar]
- Zevallos Bustamante SE, Bottino CMC, Lopes MA, Azevedo D, Hototian SR, Litvoc J, Filho WJ. Combined instruments on the evaluation of dementia in the elderly: preliminary results. Arq Neuropsiquiatr. 2003:61:601–606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Please contact the corresponding author.