Abstract
Stress-related disorders such as depression and anxiety exhibit sex differences in prevalence and negatively impact both mental and physical health. Affective illness is also frequently accompanied by changes in ventromedial prefrontal cortical (vmPFC) function. However, the neurobiology that underlies sex-specific cortical processing of affective stimuli is poorly understood. Although rodent studies have investigated the prefrontal impact of chronic stress, postmortem studies have focused largely on males and yielded mixed results. Therefore, genetically defined population recordings in behaving animals of both sexes were used to test the hypothesis that chronic variable stress (CVS) impairs the neural processing of affective stimuli in the rodent infralimbic region. Here, we targeted expression of a calcium indicator, GCaMP6s, to infralimbic pyramidal cells. In males, CVS reduced infralimbic responses to social interaction and restraint stress but increased responses to novel objects and food reward. In contrast, females did not have CVS-induced changes in infralimbic activity, which was partially dependent on the ovarian status. These results indicate that both male and female vmPFC cells encode social, stress, and reward stimuli. However, chronic stress effects are sex-dependent and behavior-specific. Ultimately, these findings extend the understanding of chronic stress-induced prefrontal dysfunction and indicate that sex is a critical factor for cortical processing of affective stimuli.
Keywords: chronic variable stress, food reward, infralimbic cortex, ovariectomy, photometry, social
Introduction
Major depressive disorder (MDD), characterized by negative mood, anhedonia, and avoidance behaviors, is the leading cause of years lived with disability worldwide (Friedrich 2017). Furthermore, females are disproportionately impacted by depressive disorders, with twice the prevalence of males (Kuehner 2017). Epidemiological studies implicate life stressors as a primary risk factor for the onset of mood disorders (Grippo and Johnson 2009; Binder and Nemeroff 2010; Myers et al. 2014; Sgoifo et al. 2015). Additionally, patients experiencing depression have exaggerated physiological responses to novel stressors and impaired habituation to repeated stressors (Gianferante et al. 2014; Morris and Rao 2014), contributing to poor cardiometabolic health outcomes (Chida and Steptoe 2010). However, the mechanisms that integrate the impacts of biological sex and stress history on neural processing of affective stimuli are largely unexplored.
The ventromedial prefrontal cortex (vmPFC) is important for cognitive and emotional processes; furthermore, altered structure and function of vmPFC associates with mood disorders (Wood and Grafman 2003; Krishnan and Nestler 2008; Myers-Schulz and Koenigs 2012; McKlveen et al. 2015; Wallace and Myers 2021). In particular, the Brodmann Area 25 (BA25) subregion of vmPFC is activated by sadness-provoking stimuli, responds to social isolation, and has reduced volume in MDD (Liotti et al. 2000; Beckmann et al. 2009; Vijayakumar et al. 2017). Although, studies of the relationship between BA25 activity and affective illness have yielded mixed results. Combined-sex studies have reported both decreased activity in MDD (Drevets et al. 1997) as well as increased activity in treatment-resistant depression (Mayberg et al. 2005). Thus, although vmPFC function relates to mood, the role of defined cell populations in regulating emotion is unclear.
The rodent infralimbic cortex (IL), a homolog of BA25, contains glutamatergic pyramidal neurons as well as a heterogenous network of interneurons (McKlveen et al. 2015, 2016). Collectively, IL local circuitry regulates activity of the pyramidal cell projections that target the limbic system to modulate behavior and physiology (Vertes 2004; Wood et al. 2019). In fact, acute optogenetic stimulation of rat IL pyramidal cells regulates socio-affective behaviors and physiological stress responses in a sex-dependent manner (Wallace et al. 2021). Further, chronic stress exposure induces dendritic atrophy of male IL pyramidal cells (Cook and Wellman 2004; Cerqueira et al. 2005; Goldwater et al. 2009; Shansky et al. 2009; Czéh et al. 2018), an effect prevented in females by ovarian hormones (Shansky et al. 2010; Wei et al. 2014). Although multiple studies provide histological evidence for altered pyramidal morphology, functional studies have yielded conflicting results. To date, ex vivo slice recordings have focused on males and found evidence of chronic stress both increasing pyramidal cell inhibition (McKlveen et al. 2016) and decreasing inhibition (Czéh et al. 2018). Combined with reports of chronic stress-induced interneuronal plasticity and altered gene expression (Gilabert-Juan et al. 2013; McKlveen et al. 2016, 2019; Shepard et al. 2016; Czéh et al. 2018; Page et al. 2019), it is unclear how prolonged adversity impacts the endogenous activity of vmPFC pyramidal cells.
To determine how vmPFC neural populations represent affective stimuli in vivo, we expressed the fluorescent calcium indicator GCaMP6 under the calcium/calmodulin-dependent protein kinase type II α (CaMKIIα) promoter. Photometric recordings of ILCaMKIIα pyramidal neuron activity were carried out during exposure to novel, social, stress, and reward stimuli in control rats, as well as animals exposed to chronic variable stress (CVS). To test the hypothesis that chronic stress-induced shifts in excitatory/inhibitory balance are sex-specific, experiments included male, female, and ovariectomized (OVX) rats. Altogether, these studies provide evidence that behavioral context, stress history, and biological sex are pivotal factors affecting the real-time activity of ILCaMKIIα cells in behaving animals.
Methods
Animals
Age-matched adult male (250–300 g), female (150–200 g), and ovariectomized (OVX) female (250–300 g) Sprague–Dawley rats were obtained from Envigo (Denver, CO). Ovariectomy occurred after puberty (approximately 7 weeks), two weeks prior to shipping. Animals then acclimated to the facility for 1 week prior to stereotaxic surgery. After stereotaxic surgery, rats were housed individually in shoebox cages with cardboard tubes for enrichment in a temperature- and humidity-controlled room with a 12-h light–dark cycle (lights on at 07:00 h, off at 19:00 h) and food and water ad libitum. Per ARRIVE guidelines, all treatments were randomized, and experimenters were blinded. All procedures and protocols were approved by the Colorado State University Institutional Animal Care and Use Committee (protocol: 2129) and complied with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Signs of poor health and/or weight loss ≥20% of pre-surgical weight were a priori exclusion criteria. These criteria were not met by any animals in the current experiments.
Microinjection and cannulation
Microinjections and cannulations were performed as previously described (Wallace et al. 2021). Briefly, rats were anesthetized with isoflurane (1–5%) and administered analgesic (0.6 mg/kg buprenorphine-SR, subcutaneous) and antibiotic (5 mg/kg gentamicin, intramuscular). Rats received unilateral microinjections (1–1.5 μL) of adeno-associated virus (AAV) into the IL (males: 2.7 mm anterior to bregma, 0.6 mm lateral to midline, and 4.2 mm ventral from dura; females: 2.3 mm anterior to bregma, 0.5 mm lateral to midline, and 4 mm ventral from dura) in randomized fashion so that right and left IL were equally represented. AAV9-packaged constructs (Addgene, virus # 107790) expressed GCaMP6s under the CaMKIIα promoter to achieve pyramidal cell-predominant expression (Wood et al. 2019). All microinjections were carried out with a 25-gauge, 2-μL microsyringe (Hamilton, Reno, NV) using a microinjection unit (Kopf, Tujunga, CA) at a rate of 5 min/μL. Following injection, unilateral fiber-optic cannulas (flat tip 400/430 μm, NA = 0.57, 5 mm protrusion; Doric Lenses, Québec, Canada) were aligned with the IL injection sites and lowered to the IL. Cannulas were secured to the skull with metal screws (Plastics One) and dental cement (Stoelting, Wood Dale, IL). Skin was sutured and, following 1 week of recovery, rats were handled daily and acclimated to the recording procedure for another week before experiments began. Rat handling and cannula habituation continued daily throughout experiments.
Experimental design
Multiple experiments were conducted with results displayed collectively given the normalization of photometry data. Experiment 1a was carried out in No CVS and CVS males (n = 15/group) according to the timeline in Fig. 1(B). After 14 days of CVS, responses to social (day 15), stress (days 16–18), and reward stimuli (day 21) were assessed. Due to the possibility of body composition affecting ILCaMKIIα responses to food reward, an additional experiment (experiment 1b) was carried out with weight-matched No CVS males (n = 7). Experiment 2 included intact cycling females that were either No CVS controls or exposed to CVS (n = 10/group). A final experiment was run in OVX females to determine whether ovarian hormones modulate ILCaMKIIα neural activity in No CVS controls and CVS-exposed animals (n = 11/group). Experiments 2 and 3 followed the same experimental timeline outlined in Fig. 1(B) with tissue collected at the conclusion of all experiments to verify fiber optic placement.
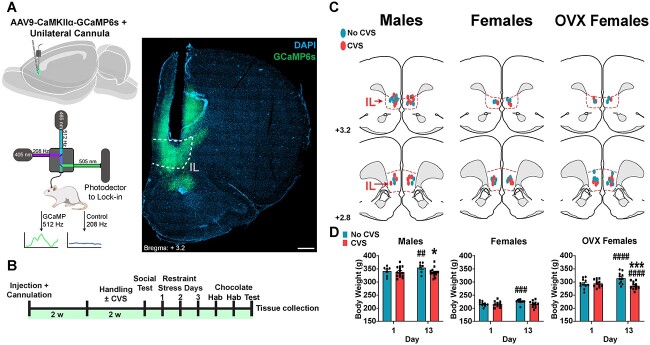
Fig. 1.
Experimental design. (A) Top left: schematic of AAV injections and cannulation. Bottom left: schematic of fiber photometry recording system. Right: photomicrograph of GCaMP6s expression at the IL cannulation site; scale bar: 500 mm. (B) Experimental timeline; CVS: chronic variable stress. (C) Mapped fiber optic cannula positions within, or at the dorsal margin of, the IL (red outline); numbers are mm rostral to Bregma, OVX: ovariectomized. (D) Body weight of animals before and after stress condition, CVS or No CVS. Within-treatment comparisons across time: ##P < 0.01, ###P < 0.001, ####P < 0.0001. Within-time comparisons between treatments: *P < 0.05, ***P < 0.001.
Chronic variable stress
CVS was conducted as previously described (Myers et al. 2017; Schaeuble et al. 2019; Pace et al. 2020; Wallace et al. 2021) with 14 days of twice daily (AM and PM) heterotypic stressors presented in a randomized manner including: exposure to a brightly-lit open field (28.5″ × 18″, 13″ deep, 1 h), cold room (4°C, 1 h), cage tilt (45°, 1 h), forced swim (23° to 27°C, 10 min), predator odor (fox or coyote urine, 1 h), and shaker stress (100 rpm, 1 h). Additionally, overnight stressors were variably included, comprised of damp bedding (400 mL water) or overnight light. Rats were weighed every 3–4 days to monitor body weight over the course of experimentation.
Food restriction
A cohort of male rats (experiment 1b) was food restricted for 14 days to control for the reduced food intake and body weight gain of CVS-exposed males. Two weeks after injection and cannulation, male rats received 16 g of standard chow at lights off and 2 g of standard chow at lights on to prevent fasting, as previously described (Flak et al. 2011; Schaeuble et al. 2019). Similar to all other experiments, food-restricted rats were given chocolate chips for 2 days prior to the test day with food reward testing performed on day 15.
Estrous cycle
All intact cycling female rats were housed concurrently and followed the same experimental design. Immediately following all tests, vaginal cytology was analyzed to approximate the estrous cycle stage as previously described (Wallace et al. 2021). Briefly, a cotton swab dipped in deionized water was used to collect and transfer cells onto a glass slide. Dried slides were viewed via light microscopy (10× objective) by a minimum of two blind observers to categorize as proestrus, estrus, metestrus, or diestrus (Cora et al. 2015; Solomon et al. 2015).
Fiber photometry
GCaMP6s fluorescence was recorded to measure activity-dependent changes in intracellular calcium (Gunaydin et al. 2014). Implanted fiber optic cannulas were connected via patch cord to a fiber photometry system (Doric: 1-site 2-color Fiber Photometry System). Excitation was achieved through a 465-nm wavelength LED modulated at 512 Hz. Movement control was achieved with 405-nm wavelength LED modulated at 208 Hz. The power delivery was approximately 3 mW at cannula tip, measured with a photodiode sensor (PM160, Thorlabs Inc, Newton, NJ). To allow for rodent movement, rats were connected to the photometry system through a pigtailed rotary joint patch cord. Emitted fluorescence was captured with a photon detector (Newport Model: 2151). To separate the components of the 465- and 405-nm excitation, a lock-in amplifier filtered the incoming fluorescence into the 512- and 208-Hz components, respectively, with a 12-Hz band filter. Autofluorescence was reduced by running the 465- and 405-nm LEDs at full power for 30 min prior to testing. Further, the optical interface between the implanted fiber optic and the patch cord was cleaned with 10% ethanol and dried immediately before testing.
Photometry recordings were acquired at 1,200 Hz and down sampled to 30 Hz for analysis (MATLAB). Behavior was recorded by a camera mounted above the arena for automated optic hardware control (Noldus Information Technologies). A transistor-transistor logic pulse at the beginning of video acquisition was used to timeclock with photometry recordings. Prior to analysis, 465- and 405-nm components of the excited 512-nm fluorescence were assessed by a treatment-blind observer for movement artifacts. If movement artifacts were detected, the recorded trials were excluded from the analysis. Photometry signal analysis and Z-score calculation were performed using MATLAB (version 2019b, MathWorks).
Novel object and social interaction
A modified version of a social behavior arena was used to accommodate optic patch cords (Felix-Ortiz and Tye 2014; Moy et al. 2004). To reduce environmental novelty, experimental rats were habituated to the interaction arena for 3 days (10 min/day) prior to testing. Additionally, interactor rats were habituated to enclosures in the arena for 3 days (20 min/day) prior to testing. On testing day, the 14th day of CVS, rats were connected to fiber optic patch cords and placed in the center of a black rectangular Plexiglas arena (36″ × 23″, 15.8″ deep). Initially, the arena was empty and experimental rats were allowed to explore. After 5 min, an empty enclosure, novel to the experimental animals, was placed in the center of the arena. Rats were then allowed to explore the novel object for 5 min, after which the empty enclosure was removed. After 2 min, a new enclosure containing a novel conspecific rat was placed in the center of the arena for 5 min. Although this non-random sequence does not control for the factor of time in the assay, the design was chosen to control for stimulus specificity. Acclimatization to the environment followed by interaction with the novel enclosure controlled for the specificity of the social stimulus relative to other aspects of the behavioral context. Interactions were scored by a treatment-blind observer as nose pokes onto the empty enclosure (object interaction) or enclosure containing a conspecific (social interaction).
For analysis of ILCaMKIIα GCaMP fluorescence during interactions, the 465-nm component signal was median Z-scored with a rolling 5-min median and median absolute deviation (MAD), 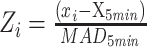 , then aligned to interactions and averaged within subjects. The within-subject mean signals were then averaged within groups for statistical analysis. Object interactions were filtered to identify interactions that were ≥ 1 s and preceded by 3 s of non-interaction. Social behaviors were filtered to detect interactions ≥ 2 s and preceded by 3 s of non-interaction. The first object and social interactions were analyzed separately from subsequent interactions to examine contextual novelty. To determine if the GCaMP signal differed during interactions from non-specific behavior, 7-s intervals without interactions were randomly selected with the average GCaMP signal calculated during the middle 2 s of these periods. To isolate ILCaMKIIα activity prior to interaction, social interactions were filtered to only include interactions with a preceding ≥10-sec period of non-interaction.
, then aligned to interactions and averaged within subjects. The within-subject mean signals were then averaged within groups for statistical analysis. Object interactions were filtered to identify interactions that were ≥ 1 s and preceded by 3 s of non-interaction. Social behaviors were filtered to detect interactions ≥ 2 s and preceded by 3 s of non-interaction. The first object and social interactions were analyzed separately from subsequent interactions to examine contextual novelty. To determine if the GCaMP signal differed during interactions from non-specific behavior, 7-s intervals without interactions were randomly selected with the average GCaMP signal calculated during the middle 2 s of these periods. To isolate ILCaMKIIα activity prior to interaction, social interactions were filtered to only include interactions with a preceding ≥10-sec period of non-interaction.
Restraint stress and endocrine analysis
To examine ILCaMKIIα cellular responses to novel and repeated stressors, animals underwent 3 days of restraint. To measure baseline neural activity, rats were connected to the photometry system in the home cage for 5 min each day. Rats were then restrained in plastic film decapicones and reconnected to the photometry system for 30 min. To assess endocrine stress responses, blood samples (approximately 250 μL) were collected by tail clip at the end of restraint (Vahl et al. 2005). Although there are sex differences in basal hormone levels, as well as the magnitude and timing of endocrine stress responses (Oyola and Handa 2017; Heck and Handa 2019; Dearing et al. 2021; Dearing et al. 2022), the current design did not examine these dynamics. Instead, all samples were collected following the recording session so that neural responses were specific to restraint stress and not confounded by blood collection.
For analysis, the 465-nm photometry signal was median Z-scored with a rolling 1-min window within 5-min bins to match the home cage period. Transient peaks in calcium fluorescence were defined as values exceeding 2.91 MADs above the median for every 5-min period (Gunaydin et al. 2014; Muir et al. 2018). The transient frequency for each 5-min bin was then averaged within groups. To restrict detection to absolute peaks, a minimum of 0.3 s between peaks and prominence of 1 MAD above the surrounding signal was required (MATLAB).
Blood glucose mobilization in response to behavioral stress was assessed as a measure of sympathetic activation (Bialik et al. 1988). Contour Next EZ glucometers (Bayer, Parsippany, NJ) were used to generate duplicate readings for each time point that were then averaged. For plasma analysis of corticosterone, the primary product of the rat hypothalamic–pituitary–adrenal axis (Herman et al. 2016; Ghosal et al. 2017b), blood samples were centrifuged at 3,000 g for 15 minutes at 4°C with plasma stored at −20°C until ELISA. Plasma corticosterone was measured with an ENZO Corticosterone ELISA (ENZO Life Sciences, Farmingdale, NY) with an intra-assay coefficient of variation of 8.4% and an inter-assay coefficient of variation of 8.2% (Bekhbat et al. 2018; Dearing et al. 2021). Samples were analyzed in duplicate with all time points included in the same assay.
Food reward
To measure ILCaMKIIα neural activity during reward acquisition, animals were fed chocolate. Chocolate was used as a food reward as it promotes anticipatory behavior (Angeles-Castellanos et al. 2008) and induces c-Fos expression in reward-related brain areas (Blancas et al. 2014). Furthermore, both male and female rats have a motivational preference for chocolate and develop binge-like eating with free access (Giuliano et al. 2012). Chocolate chips (Ghiradelli, semi-sweet chocolate morsels) were chosen to facilitate temporal alignment of neural activity with discrete reward acquisition events. The chips were high in fat (51% of caloric value) and sugar (46% of caloric value) indicating palatability. For 2 days at both lights on and off, animals were acclimated to being moved to an adjacent behavioral testing room with a chocolate chip placed in the home cage. On the testing day, rats were connected to the fiber photometry recording system and placed in their home cage for 3 min prior to adding the chocolate chip to the cage. Animals taking > 30 min to consume the chocolate were excluded from analysis. Neural activity during chocolate interactions was analyzed as described above for object and social interactions.
Tissue collection
At the conclusion of experiments, rats were given an overdose of sodium pentobarbital and perfused transcardially with 0.9% saline followed by 4.0% paraformaldehyde in 0.1-M PBS. Brains were removed and post-fixed in 4.0% paraformaldehyde for 24 h at room temperature, followed by storage in 30% sucrose in PBS at 4°C. Coronal sections were made on a freezing microtome at 30 μm thickness and then stored in cryoprotectant solution at −20°C prior to localizing viral injections and optic cannula placement.
Statistical analysis
Data are expressed as mean ± standard error of the mean. Data were analyzed using Prism 8 (GraphPad, San Diego, CA), with statistical significance set at P < 0.05 for rejection of null hypotheses. Weights by treatment (CVS) and day were analyzed using repeated-measure two-way analysis of variance (ANOVA), followed by Fisher’s post hoc if significant main or interaction effects were present. Object, social, and reward latencies, interactions, and Z-scores were analyzed via unpaired t-tests between stress or interaction condition. Data collected from the left and right hemispheres were also compared with unpaired t-tests. Peak neural activity preceding social interaction, as well as glucose and corticosterone across restraint days were analyzed using mixed-effects analysis with stress and time (repeated) as factors, followed by Fisher’s post hoc if significant main or interaction effects were present. Transient frequency during restraint was analyzed using mixed-effects analysis with home cage/restraint (repeated) and CVS as factors followed by Sidak’s post hoc if significant main or interaction effects were present. Male body weight change and mean chocolate Z-score comparisons across male conditions were analyzed by one-way ANOVA, followed by Fisher’s LSD post hoc. Pearson's coefficients of correlation were used to examine potential associations between ILCaMKIIα activity and behavior as well as post-stress glucose and corticosterone. The data that support the findings of this study are available from the corresponding author upon request.
Results
Design and validation
AAV viral injections were targeted to the IL (Fig. 1A) for expression of GCaMP6s under CaMKIIα promoter regulation (ILCaMKIIα). Fiber optic cannulas were targeted to the IL to allow excitation and collection of GCaMP6s fluorescence (Gunaydin et al. 2014; Muir et al. 2018). All experiments (male, female, and OVX) underwent the same experimental design (Fig. 1B). Only animals with cannula placement verified to be within or immediately (≤ 0.2 mm) dorsal to the IL were included in analyses (Fig. 1C). In all experiments, No CVS day 13 weight was higher than day 1. This effect was not present in any CVS group and male and OVX females had lower body weight than No CVS controls (males: repeated-measures two-way ANOVA: Time × Stress F(1, 19) = 10.66, P < 0.01; females: repeated-measures two-way ANOVA: Time × Stress F(1, 18) = 8.15, P < 0.05; OVX females: repeated-measures two-way ANOVA: Time × Stress F(1, 20) = 209.2, P < 0.0001) (Figs Fig. 1D and S1). As previously reported (Chen and Heiman 2001; Fang et al. 2015), OVX females had higher body weight before CVS than cycling females (n = 20–22/group, unpaired t-test: Female vs OVX Female weight t(40) = 18.61, P < 0.0001). Intact female estrous phase was determined following each testing session (Table 1); however, sample sizes did provide the statistical power required to analyze phase-dependent effects on neural activity.
Table 1.
Distribution of estrous cycle phase during testing. M: Metestrus, D: Diestrus: E: Estrus, P: Proestrus.
| Assay | Group | Estrous phase (% within group) | |||
|---|---|---|---|---|---|
| M | D | E | P | ||
| Object | No CVS | 29.0% | 0.0% | 14.0% | 57.0% |
| CVS | 25.0% | 25.0% | 13.0% | 38.0% | |
| Social | No CVS | 22.0% | 22.0% | 11.0% | 44.0% |
| CVS | 22.0% | 22.0% | 11.0% | 44.0% | |
| Restraint Day 1 | No CVS | 10.0% | 50.0% | 20.0% | 20.0% |
| CVS | 0.0% | 20.0% | 60.0% | 20.0% | |
| Restraint Day 3 | No CVS | 40.0% | 30.0% | 0.0% | 30.0% |
| CVS | 40.0% | 50.0% | 0.0% | 10.0% | |
| Chocolate | No CVS | 57.0% | 0.0% | 29.0% | 14.0% |
| CVS | 50.0% | 13.0% | 25.0% | 13.0% | |
Object interactions
To determine how ILCaMKIIα neurons encode interactions with a novel object, an empty enclosure was placed in the arena (Fig. 2A). During the first object interaction (Fig. 2B), CVS males had a greater GCaMP signal than No CVS males (n = 8–10/group, unpaired t-test: No CVS vs CVS t(16) = 2.483, P < 0.05; Fig. 2C). All No CVS groups had ILCaMKIIα responses (Fig. 2D) during subsequent object interactions (Random vs Object unpaired t-test: males: n = 8, t(14) = 4.75, P < 0.001; females: n = 7, t(12) = 5.8, P < 0.0001; OVX: n = 5, t(8) = 3.1, P < 0.05; Fig. 2E), there was no effect of CVS in any experiment to alter the encoding of a familiar object. Although, activity during the initiation (first 0.5 s) of interactions trended upward in intact CVS females (Females unpaired t-test: n = 7–8/group, t(13) = 2.12, P = 0.054; Fig. 2F). Behaviorally, CVS reduced the average length of male object interactions (unpaired t-test n = 7–8/group, t(13) = 3.083; Fig. S2). Furthermore, CVS-exposed males had significant lateralization effects during object interactions (unpaired t-test n = 3–4/group, t(5) = 3.338; Fig. S3). Overall, chronic stress increases male ILCaMKIIα neuronal responses to novelty, an effect not present in females regardless of ovarian status.
Fig. 2.

ILCaMKIIα neurons responded to object interactions. (A) Schematic of experimental approach with a representative median-corrected 465-nm signal trace (green). (B) Group-averaged Z-scores aligned to the first object interaction, the dashed line represents interaction start; OVX: ovariectomized. (C) CVS exposure increased neural activity during the first object interaction in males only. (D) Group-averaged Z-scores aligned to subsequent object interactions. (E) All No CVS groups had higher mean Z-scores during object interactions compared to randomly selected non-specific behaviors. (F) CVS trended (P = 0.054) toward increased signal at the start of object interactions only in cycling females. Comparisons between treatments: //P < 0.06, *P < 0.05, ***P < 0.001, ****P < 0.0001.
Social interactions
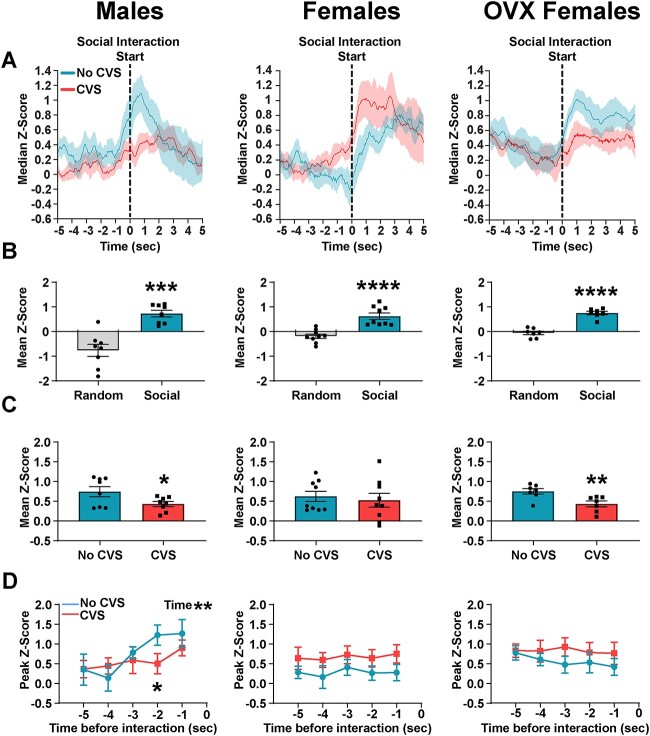
Following object interaction, a novel conspecific was added to the arena to examine IL representation of social interaction (Figs. S4 and S5). There were no CVS effects in any experiment on the GCaMP signal during the first social interaction (data not shown). For subsequent social interactions (Fig. 3A), No CVS males, females, and OVX females all had higher mean ILCaMKIIα neural activity during social interactions than random intervals (Random vs Social unpaired t-test: males: n = 8, t(14) = 5.3, P < 0.0001; females: n = 9, t(16) = 5.28, P < 0.0001; OVX females: n = 7, t(12) = 8.05, P < 0.0001; Fig. 3B). Further, CVS reduced the mean GCaMP signal during social interaction in males (Fig. 3C). Females were resistant to CVS effects on social representation, which was dependent on ovarian hormones (No CVS vs CVS unpaired t-tests: males: n = 8/group, t(14) = 2.19, P < 0.05; OVX females: n = 7/group, t(12) = 3.09, P < 0.01). Neural activity immediately preceding social interaction was assessed to examine the neural basis of social motivation. In males, the ILCaMKIIα GCaMP signal increased prior to the start of social interaction (Males mixed-effects model: Time F(2.648, 32.43) = 5.2, P < 0.01; Fig. 3D), which CVS reduced (P < 0.05). In contrast, there were no time-dependent changes in either intact or OVX females. Taken together, chronic stress reduced the prefrontal representation of social interaction in males and OVX females without altering responses in intact females. Further, male IL activity increased prior to social interaction, an effect that was reduced by chronic stress.
Fig. 3.
CVS reduced ILCaMKIIα neural activity during social interactions in males and OVX females. (A) Group-averaged Z-scores aligned to social interactions, the dashed line represents interaction start; OVX: ovariectomized. (B) All No CVS groups had higher mean Z-scores during social interactions compared to randomly selected non-interaction periods. (C) CVS exposure reduced mean Z-scores during social interactions in males and OVX females, without affecting cycling females. (D) Male GCaMP signal increased prior to social interactions, which was reduced by CVS. Females and OVX females showed no change in neural activity preceding interactions. Comparisons between treatments: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Restraint stress
Restraint was used to examine ILCaMKIIα neuronal responses to an acute novel stressor (Fig. 4A). Restraint stress was then repeated (a total of 3 days) to query neural encoding after repeated stress (Fig. S6). After each restraint session, blood was sampled to measure glucose and corticosterone as indices of the physiological stress response. In males, CVS increased corticosterone after restraint on the second and third days (mixed-effects model: Stress F(1, 27) = 11.99, P < 0.01; Fig. 4B). In cycling females, there was an interaction between day and CVS (repeated-measures two-way ANOVA: Day × Stress F(2, 32) = 3.57, P < 0.05) where CVS increased corticosterone on day 1 (P < 0.05). Similarly, OVX females had a day and CVS interaction (mixed-effects model: Day × Stress F(2, 39) = 4.66, P < 0.05; Fig. 4B), with CVS increasing corticosterone on day 1 (P < 0.01). Additionally, OVX No CVS females had higher corticosterone on day 2 than day 1 (P < 0.05). Consistent with previous research (Viau and Meaney 1991), cycling No CVS females had higher corticosterone stress responses than No CVS OVX females, (unpaired t-test Female vs OVX Female: n = 9–11/group, t(18) = 2.76, P < 0.05). Altogether, corticosterone data indicate that, relative to No CVS controls, CVS caused a progressive HPA axis sensitization in males contrasted by a transient facilitation in both female experiments.
Fig. 4.

ILCaMKIIα neurons responded to acute and repeated stress. (A) Left: schematic of restraint approach. Right: representative transient peak quantification during home cage and restraint. (B) CVS increased corticosterone responses to restraint in males on days 2 and 3, whereas females and OVX females had day 1 increases; OVX: ovariectomized. (C) CVS exposure increased male glucose responses on days 2 and 3. There was no CVS effect in females or OVX females. (D) Acute novel restraint. In males, only No CVS animals had higher transient frequency during restraint than home cage. In intact and OVX females, both No CVS and CVS rats had higher transient frequency during restraint. (E) Day 3 repeated restraint. No CVS males had higher transient frequency during restraint than home cage. Intact No CVS females had higher transient frequency during restraint, whereas OVX CVS females had higher transient frequency. Treatment effects: *P < 0.05, **P < 0.01. Within-treatment time comparisons: #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001.
There were main effects of CVS and day on male glucose responses with CVS-exposed animals having higher glucose mobilization on days 2 and 3 than No CVS controls (mixed-effects model: Day F(1.588, 39.69) = 4.912, P < 0.05; Stress F(1, 28) = 5.13, P < 0.05; Fig. 4C). Also, No CVS males had lower glucose on day 3 than day 1 (P < 0.01), a habituation that was prevented by CVS. In both intact and OVX females there was a main effect of restraint day (mixed-effects model females: Day F(1.882,31.05) = 13.66, P < 0.0001; OVX females: Day F(1.887,33.97) = 4.43, P < 0.05) where cycling No CVS females had higher glucose on day 2 than day 1 (P < 0.05). Thus, chronic stress prevented habituation of sympathetic glucose mobilization in males without altering glucose responses in intact or OVX females.
Neural activity was measured as ILCaMKIIα transient peak frequency using rolling 1-minute medians and MAD Z-scores. Recordings occurred in the home cage and immediately following in restraint. On day 1 in males, No CVS animals had higher transient frequency during restraint compared to home cage, whereas CVS animals did not (mixed-effects model: Restraint F(1, 13) = 19.50, P < 0.001 Sidak’s: No CVS: P < 0.01; Fig. 4D). In contrast, both female and OVX female stress conditions had higher transient frequency during restraint than home cage (mixed-effects model: Females Restraint F(1, 15) = 36.35, P < 0.0001, Sidak’s: No CVS: P < 0.001, CVS: P < 0.01; OVX females Restraint F(1, 16) = 70.07, P < 0.0001, Sidak’s: No CVS: P < 0.0001, CVS: P < 0.0001).
On the third day of repeated restraint, male responses were similar to day 1 with a main effect of restraint that was significant only in the No CVS group (mixed-effects model: Males Restraint F(1, 19) = 20.16, No CVS P < 0.001; Fig. 4E). Cycling females had population transient frequencies similar to males with an effect of restraint in the No CVS group only (mixed-effects model: Restraint F(1, 16) = 24.85, P < 0.001, No CVS P < 0.001). However, OVX females had an effect of restraint where CVS animals had higher transient frequency (mixed-effects model: Restraint F(1, 17) = 14.56, CVS P < 0.01). Altogether, the data suggest that stress increased ILCaMKIIα calcium transients only in No CVS males. Regardless of the stress condition, both intact and OVX females had increased calcium transients during novel restraint, followed by diverging condition-specific responses on day 3.
To assess associations between ILCaMKIIα neural activity during stress and endocrine responses, Pearson’s correlations were calculated between transient frequency and glucose or corticosterone (Table 2). Only in males, No CVS ILCaMKIIα population activity positively correlated with glucose (day 2; P < 0.05; day 3; P < 0.01) and corticosterone (day 3; P < 0.001) during repeated restraint. In contrast, acute novel restraint (day 1; P < 0.05) led to a negative correlation between male CVS neural activity and stressor-evoked glucose. There were no significant correlations in any female condition between ILCaMKIIα transient frequency during restraint and glucose or corticosterone.
Table 2.
Pearson's coefficients of correlation between mean daily ILCaMKIIα calcium transients during restraint and post-stress glucose and corticosterone. * indicates significant (P < 0.05) correlation.
| Males | Females | OVX Females | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucose | Corticosterone | Glucose | Corticosterone | Glucose | Corticosterone | ||||||||
| Day | Group | r | p | r | p | r | p | r | p | r | p | r | p |
| 1 | No CVS | 0.60 | 0.11 | 0.45 | 0.26 | −0.60 | 0.12 | 0.30 | 0.46 | −0.21 | 0.59 | 0.24 | 0.54 |
| 1 | CVS | −0.63 | 0.04* | −0.56 | 0.09 | −0.13 | 0.75 | −0.42 | 0.30 | −0.49 | 0.15 | −0.07 | 0.85 |
| 2 | No CVS | 0.66 | 0.04* | 0.48 | 0.16 | −0.03 | 0.94 | 0.14 | 0.72 | −0.25 | 0.48 | 0.54 | 0.11 |
| 2 | CVS | 0.49 | 0.07 | −0.14 | 0.64 | 0.25 | 0.49 | −0.39 | 0.26 | 0.53 | 0.10 | −0.04 | 0.91 |
| 3 | No CVS | 0.71 | 0.01* | 0.84 | 0.001* | 0.58 | 0.10 | −0.22 | 0.58 | 0.07 | 0.86 | 0.39 | 0.30 |
| 3 | CVS | 0.32 | 0.29 | 0.26 | 0.39 | −0.19 | 0.65 | −0.02 | 0.97 | −0.21 | 0.56 | −0.28 | 0.43 |
Food reward
Chocolate chips were given as a palatable food to assess ILCaMKIIα neural responses to reward. To reduce novelty, rats were acclimated to chocolate chips in the home cage for 2 days prior to recording. Controlling for potential effects of energy balance, Experiment 1 had an additional group of males that were food restricted to match the body weight change of CVS males (Fig. 5A). Food-restricted males had 2-week body weight changes similar to CVS-exposed rats, with both groups reduced compared to No CVS males (one-way ANOVA: n = 7–14/group, F(2,26) = 11.07, P < 0.001, Tukey post hoc; Fig. 5B). Photometry data were centered on grabbing events when the chocolate reward was acquired (Fig. 5C). No CVS rats in all experiments had higher neural activity during the first half-second of grabbing chocolate than randomly selected periods (unpaired t-test random vs chocolate: males: n = 7/group, t(12) = 3.55, P < 0.01; females: n = 9/group, t(16) = 5.154, P < 0.0001; OVX females: n = 6/group, t(10) = 2.664, P < 0.05: Fig. 5D). Further, intact No CVS females had higher ILCaMKIIα GCaMP signal at chocolate acquisition than No CVS males or OVX females (one-way ANOVA: n = 6–9, F(2,19) = 3.822, P < 0.05). Although CVS males had increased GCaMP fluorescence with chocolate acquisition, food-restricted males did as well (one-way ANOVA: n = 7–11, F(2,20) = 3.466, P = 0.05; Fig. 5E). There were no CVS effects on ILCaMKIIα neural responses in either female or OVX female experiments. However, after CVS, OVX females had decreased latency to eat chocolate (unpaired t-test: n = 6/group, t(10) = 2.488, P < 0.05; Fig. S7). Correlations were performed between the behavioral latency to eat chocolate and ILCaMKIIα neural activity during chocolate acquisition (Table 3). Interestingly, No CVS males had a positive correlation between behavioral latency and ILCaMKIIα activity (P < 0.01), while CVS reversed this relationship (P < 0.05). Overall, CVS increased ILCaMKIIα neural activity during food reward in males, an effect that was also produced by weight matching. However, CVS did not alter ILCaMKIIα reward processing in either female experiment (Fig. S8).
Fig. 5.
CVS and food restriction increased male ILCaMKIIα neuronal responses to food reward. (A) Mapped fiber-optic cannula positions within the IL (red outline). (B) Food restriction reduced body weight gain similar to CVS. (C) Group-averaged Z-scores were aligned to chocolate acquisition, dashed line represents rat grabbing chocolate; OVX: ovariectomized. (D) All groups had higher mean Z-scores during the first half-second of grabbing the chocolate compared to randomly selected non-interaction periods. Cycling females had greater IL pyramidal cell responses than males or OVX females. (E) CVS exposure and food restriction increased GCaMP signaling in males, with no effect in cycling or OVX females. Treatment effects: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Table 3.
Pearson's coefficients of correlation between latency to eat and ILCaMKIIα GCaMP signal during chocolate acquisition. * indicates significant (P < 0.05) correlation.
| Males | Females | OVX Females | |||||
|---|---|---|---|---|---|---|---|
| No CVS | CVS | Food restriction | No CVS | CVS | No CVS | CVS | |
| r | 0.88 | −0.63 | 0.10 | −0.57 | −0.02 | −0.37 | 0.18 |
| p | 0.009* | 0.049* | 0.870 | 0.108 | 0.967 | 0.473 | 0.728 |
Discussion
The current studies examined the effects of chronic stress on vmPFC pyramidal neuron activity in male and female rats. Recordings of neural activity occurred during real-time behavior as animals engaged affective stimuli. Both negatively-valanced stimuli (e.g. stressors) and positively-valanced paradigms (including food reward) assessed cortical processing of emotion-relevant behaviors. The collective results indicate that, in previously unstressed rats, both male and female ILCaMKIIα neurons increase activity during acute stress as well as novel, social, and reward interactions compared to non-specific behaviors in the testing environment. However, only males had time-dependent neural activity during social approach. Moreover, we examined the consequences of CVS on in vivo ILCaMKIIα neural activity during behavior. Our results indicate that chronic stress-induced outcomes are dependent on both sex and behavioral contexts. Specifically, CVS increased male GCaMP signaling during interactions with a novel, but not familiar, object. Male ILCaMKIIα neural activity also increased during acquisition of chocolate food reward, an effect reproduced by weight matching. In contrast, CVS reduced male responses during social approach and interaction. CVS in males also prevented stressor-induced increases in ILCaMKIIα transient frequency during novel and repeated restraint, as well as increasing endocrine stress responses over the course of repeated restraint.
Compared with No CVS controls, chronically-stressed cycling female rats did not have significant changes in ILCaMKIIα calcium signaling during behavior. Sex differences in the consequences of CVS were partially accounted for by ovarian hormones. Similar to males, CVS reduced social interaction encoding in OVX females. Thus, ovarian factors prevented the effects of CVS on ILCaMKIIα representation of social interaction. However, the prevention of other CVS effects was independent of the ovarian status. For instance, neither cycling nor OVX females were susceptible to CVS effects on social approach or novel object encoding. Although CVS facilitated female glucocorticoid responses to novel restraint relative to No CVS controls, this effect was not dependent on ovarian status. In addition, novel restraint after CVS increased ILCaMKIIα calcium transients in both female conditions. Altogether, the results point toward multiple sex differences in chronic stress-induced outcomes that are independent of the activational effects of ovarian hormones. Although male metabolic state accounted for CVS effects on food reward and ovarian status explained sex differences in social encoding after CVS, the biological bases underlying broader sex differences in novelty and stress responses remain to be determined. Ovariectomy prevents the activational effects of ovarian hormones, yet the potential remains for developmental effects as well as hormone-independent chromosomal effects (Sheng et al. 2021). Further, gonadal hormone receptor modulation by neurosteroids (Wei et al. 2014; Brann et al. 2021) and/or ligand-independent signaling (Pak et al. 2006; Pinceti et al. 2015) may also contribute. Ultimately, multiple interacting factors may account for sex differences in prefrontal activity after chronic stress.
Models of chronic heterotypic stress (e.g. CVS, chronic mild stress, chronic unpredictable stress) are widely used to study the neural, behavioral, and physiological impacts of chronic stress. Numerous rodent studies report that CVS and related paradigms promote novelty avoidance (Muir et al. 2020; Pace et al. 2020), reduced sociability (Franceschelli et al. 2014; Muir et al. 2020), anhedonia (Willner 2005; Hersey et al. 2022), and dysregulated stress responses (Myers et al. 2017), core features of affective disorders. However, these paradigms and subsequent behavioral assessments were largely developed and validated in male animals. With more research investigating chronic stress in females, sex differences are frequently reported in the outcomes of chronic heterotypic stress. Temporal factors such as the duration of stress paradigms seem to be involved (Johnson et al. 2021). Thus, some studies have altered chronic stress paradigms so that females have similar behavioral outcomes to males. This typically involves shorter (e.g. female 4 days vs male 21 days) periods of stress exposure for females (Muir et al. 2020). Alternatively, others have used identical paradigms for males and females and reported the sex differences in outcomes (Franceschelli et al. 2014). For instance, our prior studies indicate that males undergoing CVS have increased passive coping behaviors and hypersecrete glucocorticoids during the forced swim test. While females also have enhanced HPA axis responses, they do not exhibit increased passive coping behaviors (Dearing et al. 2021). However, females, but not males, have glucose intolerance after CVS (Dearing et al. 2021), suggesting that the sexes may be differentially susceptible to the deleterious effects of chronic stress.
During the current studies we quantified behavioral responses to stimulus presentation to examine neuro-behavioral associations. However, it is important to emphasize that the paradigms were significantly altered from conventional tests of behavior to isolate the effects of stimulus presentation on neural activity. In males, CVS reduced time interacting with the novel object. CVS also reduced the latency to acquire chocolate in OVX females. Given the additional modifications such as presenting object and social stimuli sequentially as opposed to simultaneously, we were not able to detect more widespread changes in behavior. Although CVS enhanced glucocorticoid responses to stress in cycling females, it is possible that there is a relationship between the lack of effect on female ILCaMKIIα activity after CVS and the absence of detected chronic stress-induced behaviors.
In addition to the sex-specific impacts of chronic stress on prefrontal cortex activity reported here, male and female ILCaMKIIα neurons also divergently modulate behavioral and physiological responses to stress (Wallace and Myers 2021). Glutamate output from the male IL is necessary for chronic stress-induced avoidance behaviors (Pace et al. 2020) and constrains physiological stress responses (Myers et al. 2017; Schaeuble et al. 2019). Furthermore, optogenetic stimulation of male ILCaMKIIα neurons increases place preference and social motivation, in addition to reducing sympathetic and HPA axis responses to stressors (Wallace et al. 2021). In contrast, female ILCaMKIIα stimulation increases sympathetic stress responding without modulating social or motivational behaviors. Combined with the results of the current experiments, these findings point toward an interesting female divergence of behavioral regulation and behavior processing where female ILCaMKIIα neurons respond to social interaction without modulating social motivation. Additionally, the differential impact of male and female IL activity on physiological regulation may contribute to the male-specific associations between ILCaMKIIα GCaMP signaling and homeostatic responses. In No CVS males, there were positive associations between neural activity and glucose, corticosterone, and latency to eat chocolate. Intriguingly, CVS reversed these associations, leading to negative correlations of neural activity with glucose and feeding latency. As correlations do not inform directionality, it is unclear whether these associations relate more to modulation or interoception. Although ILCaMKIIα stimulation can broadly modulate homeostatic processes, little is known about prefrontal sensation of internal physiology.
Histological studies have examined the impact of chronic stress on vmPFC morphology with multiple reports of male pyramidal cell dendritic hypotrophy (Cerqueira et al. 2005; Goldwater et al. 2009; Shansky et al. 2009; Luczynski et al. 2015; Czéh et al. 2018). However, changes in male local inhibitory networks are mixed. Chronic stress increases dendritic arborization of GAD67-positive interneurons while reducing the number of GABAergic interneurons (Gilabert-Juan et al. 2013). Additional evidence for stress-induced male vmPFC reorganization comes from studies identifying both elevated (Shepard et al. 2016) and reduced GAD67 mRNA (Ghosal et al. 2020). The functional consequences of altered interneuronal morphology and gene expression have been queried with ex vivo slice physiology. To date, this approach has also yielded reports of both increased (McKlveen et al. 2016) and decreased (Czéh et al. 2018) inhibitory currents on male IL pyramidal cells after CVS. Accordingly, hypotheses have developed of vmPFC hyper- or hypo-inhibition after chronic stress (Ghosal et al. 2017a; Fogaça and Duman 2019; McKlveen et al. 2019; Page and Coutellier 2019). The current studies use genetically-targeted population recordings to advance this ex vivo single-cell framework and show that, during in vivo behavior, chronic stress dynamically shifts male excitatory neuron activity in a context-dependent manner. In this case, responses to novelty and reward are increased while social and stress responses are decreased. Ultimately, both hypo- and hyper-function of the male vmPFC may produce context-inappropriate behaviors and underlie multiple aspects of stress pathology.
Regarding females, no studies to our knowledge have investigated chronic heterotypic stress effects on IL-specific pyramidal cell structure or function. Investigations of pyramidal morphology in other PFC regions have yielded a variety of outcomes, likely as a function of stress paradigm. For instance, the prelimbic cortex has similar reductions in pyramidal spine density in males and females after CVS but fewer impairments after homotypic chronic restraint stress (Anderson et al. 2019). However, shorter durations of chronic restraint stress produce estradiol-dependent female dendritic proliferation in prelimbic cortex (Garrett and Wellman 2009). Interestingly, chronic restraint stress affects IL pyramidal cells in a circuit-specific and hormone-dependent manner. Here, OVX females have general increases in IL spine density after chronic restraint that are prevented by estradiol replacement (Shansky et al. 2010). However, amygdala-projecting IL neurons have increased spine density following chronic restraint regardless of hormonal status (Shansky et al. 2010). Female PFC interneurons are also susceptible to structural changes following prolonged stress. In a CVS-like paradigm, females, but not males, have increased parvalbumin mRNA in the PFC (Shepard et al. 2016). More specific to the IL, chronic stress also increases the density of female parvalbumin-positive interneurons (Shepard et al. 2016). It is unclear how increased female IL parvalbumin cells might impact the function of local circuits. However, broad PFC slice recordings after chronic restraint indicate that females do not have the reduced glutamatergic transmission described in males (Yuen et al. 2012; Wei et al. 2014). This sex difference is accounted for by estradiol signaling at estrogen receptors in the PFC. Although the source of estradiol remains to be determined as the signaling is ovary-independent and reliant on aromatase (Wei et al. 2014). In our studies, females did not exhibit CVS-induced changes in ILCaMKIIα activity. Ovarian hormones prevented the impact of CVS on social encoding but the absence of CVS effects in other contexts was ovary independent. Given that female ILCaMKIIα cells facilitate sympathetic stress responses (Wallace and Myers 2021), it is unclear whether this resistance to CVS is a beneficial or deleterious outcome.
Although the current studies significantly advance understanding of in vivo neural activity in chronically stressed males and females, there are multiple methodological limitations worth noting. One limitation of bulk population recordings is the inability to identify neural ensembles that may be stimulus- or behavior-specific. Within the large-scale population activity measured here, there are likely numerous heterogenous populations with divergent activity profiles. The degree of cellular diversity in response characteristics across stimuli remains unknown, as does the potential overlap of cellular populations encoding social, stress, and reward stimuli. Further heterogeneity may arise from connectivity-defined ensembles. Thus, specific cortical afferent and efferent circuits may give rise to sex-dependent heterogeneity of cellular function as well as differential sensitivity to chronic stress. Another interpretive consideration is the relatively slow kinetics of the GCaMP fluorescent indicator. Previous studies have demonstrated that photometry recordings are reflective of summated neural activity, yet the kinetics of GCaMP do not permit action potential-level resolution (London et al. 2018). Furthermore, the photometry approach is a normalized measure of relative changes in calcium signaling and does not quantify absolute neural activity. Thus, the required baseline corrections for event-centered analyses may limit detection of sex or stress effects on total neural activity. Another factor that may contribute to the current results is the need for single housing. Although unavoidable following cannulation, social isolation is a stressor for rodents. However, daily handling of male rats, as in the current study, prevents the consequences of isolation and produces similar behavior and physiology to social housing (Manouze et al. 2019). It is unclear if handling reduces the stress of social isolation in females. There are additional understudied aspects of female physiology that may affect interpretation of the current results. For instance, our data suggest that body composition impacts ILCaMKIIα neural activity. Prior reports of male rats undergoing chronic stress indicate increased body fat percentage, particularly in mesenteric depots (Rebuffé-Scrive et al. 1992; Karagiannides et al. 2014). Yet, to our knowledge, it is unknown how CVS affects lean or fat mass in cycling and OVX females. Although CVS did not alter ILCaMKIIα activity in intact females, chronic stress may impact cyclicity relative to No CVS female rats. There are reports that chronic mild stress increases the average cycle length (Grippo et al. 2005) without affecting estradiol or progesterone levels (Brooks et al. 2018).
In summary, the current experiments provide in vivo recordings of IL pyramidal cell activity in males and females. Response characteristics were similar across sexes with the exceptions of social approach and food reward. However, chronic stress exposure predominately altered male ILCaMKIIα neural activity. Notably, CVS did not globally increase or decrease male neural activity, instead chronic stress altered activity depending on the behavioral context. Thus, the effects of chronic stress on male ILCaMKIIα population activity are highly context-dependent. Although male metabolic and female hormonal status partially explain sex differences in responses to chronic stress, other outcomes arise from different mechanisms. Ultimately, the data shed light on the importance of behavioral context and sex for understanding cortical processing of affective stimuli after chronic stress and suggest that these factors require consideration in the effort to understand and treat affective illness.
Supplementary Material
Acknowledgments
The authors have no conflicts of interest to disclose. Addgene viral prep #107790-AAV9 was made available by a gift from James M. Wilson.
Contributor Information
Tyler Wallace, Department of Biomedical Sciences, Colorado State University, Fort Collins, CO 80523, USA.
Brent Myers, Department of Biomedical Sciences, Colorado State University, Fort Collins, CO 80523, USA.
CRediT authors statement
Tyler Wallace (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing—original draft), Brent Myers (Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing—original draft, Writing—review and editing).
Funding
This work was supported by the College of Veterinary Medicine and Biomedical Sciences Research Council and National Institutes of Health (grant HL150559 to B.M.).
Conflict of interest statement: None declared.
References
- Anderson RM, Johnson SB, Lingg RT, Hinz DC, Romig-Martin SA, Radley JJ. Evidence for similar prefrontal structural and functional alterations in male and female rats following chronic stress or glucocorticoid exposure. Cereb Cortex. 2019:30(1):353–370. 10.1093/cercor/bhz092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeles-Castellanos M, Salgado-Delgado R, Rodríguez K, Buijs RM, Escobar C. Expectancy for food or expectancy for chocolate reveals timing systems for metabolism and reward. Neuroscience. 2008:155(1):297–307. 10.1016/j.neuroscience.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MFS. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci. 2009:29:1175–1190. 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhbat M, Glasper ER, Rowson SA, Kelly SD, Neigh GN. Measuring corticosterone concentrations over a physiological dynamic range in female rats. Physiol Behav. 2018:194:73–76. 10.1016/j.physbeh.2018.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialik RJ, Smythe JM, Roberts DCS. Alpha 2-adrenergic receptors mediate the increase in blood glucose levels induced by epinephrine and brief footshock stress. Prog Neuro-Psychopharmacol Biol Psychiatry. 1988:12:307–314. 10.1016/0278-5846(88)90049-8. [DOI] [PubMed] [Google Scholar]
- Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety insights from human genetic studies. Mol Psychiatry. 2010:15:574–588. 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blancas A, González-García SD, Rodríguez K, Escobar C. Progressive anticipation in behavior and brain activation of rats exposed to scheduled daily palatable food. Neuroscience. 2014:281:44–53. 10.1016/j.neuroscience.2014.09.036. [DOI] [PubMed] [Google Scholar]
- Brann DW, Lu Y, Wang J, Sareddy GR, Pratap UP, Zhang Q, Tekmal RR, Vadlamudi RK. Neuron-derived estrogen-a key neuromodulator in synaptic function and memory. Int J Mol Sci. 2021:22(24):13242. 10.3390/IJMS222413242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SD, Hileman SM, Chantler PD, Milde SA, Lemaster KA, Frisbee SJ, Shoemaker JK, Jackson DN, Frisbee JC. Protection from vascular dysfunction in female rats with chronic stress and depressive symptoms. Am J Physiol Heart Circ Physiol. 2018:314(5):H1070–H1084. 10.1152/ajpheart.00647.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira JJ, Pêgo JM, Taipa R, Bessa JM, Almeida OFX, Sousa N. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci. 2005:25:7792–7800. 10.1523/JNEUROSCI.1598-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Heiman ML. Increased weight gain after ovariectomy is not a consequence of leptin resistance. Am J Physiol - Endocrinol Metab. 2001:280(2):E315–E322. 10.1152/ajpendo.2001.280.2.E315. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status. Hypertension. 2010:55:1026–1032. 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004:60:236–248. 10.1002/NEU.20025. [DOI] [PubMed] [Google Scholar]
- Cora MC, Kooistra L, Travlos G. Vaginal cytology of the laboratory rat and mouse: review and criteria for the staging of the estrous cycle using stained vaginal smears. Toxicol Pathol. 2015:43:776–793. 10.1177/0192623315570339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czéh B, Vardya I, Varga Z, Febbraro F, Csabai D, Martis LS, Højgaard K, Henningsen K, Bouzinova EV, Miseta A, et al. Long-term stress disrupts the structural and functional integrity of GABAergic neuronal networks in the medial prefrontal cortex of rats. Front Cell Neurosci. 2018:12:148. 10.3389/fncel.2018.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearing C, Morano R, Ptaskiewicz E, Mahbod P, Scheimann JR, Franco-Villanueva A, Wulsin L, Myers B. Glucoregulation and coping behavior after chronic stress in rats: sex differences across the lifespan. Horm Behav. 2021:136:105060. 10.1016/J.YHBEH.2021.105060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearing C, Handa RJ, Myers B. Sex differences in autonomic responses to stress: implications for cardiometabolic physiology. Am J Physiol Endocrinol Metab. 2022:323(3):E281–E289. 10.1152/ajpendo.00058.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997:386(6627):824–827. 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Fang J, Yang L, Zhang R, Zhu X, Wang P. 2015. Are there differences between Sprague-Dawley and Wistar rats in long-term effects of ovariectomy as a model for postmenopausal osteoporosis? Int J Clin Exp Pathol 8:1491. Available at: /pmc/articles/PMC4396247/. [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Tye KM. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J. Neurosci. 2014:34:586–595. 10.1523/JNEUROSCI.4257-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flak JN, Jankord R, Solomon MB, Krause EG, Herman JP. Opposing effects of chronic stress and weight restriction on cardiovascular, neuroendocrine and metabolic function. Physiol Behav. 2011:104:228–234. 10.1016/j.physbeh.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogaça MV, Duman RS. Cortical GABAergic dysfunction in stress and depression: new insights for therapeutic interventions. Front Cell Neurosci. 2019:13:87. 10.3389/fncel.2019.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschelli A, Herchick S, Thelen C, Papadopoulou-Daifoti Z, Pitychoutis PM. Sex differences in the chronic mild stress model of depression. Behav Pharmacol. 2014:25:372–383. 10.1097/FBP.0000000000000062. [DOI] [PubMed] [Google Scholar]
- Friedrich MJ. Depression is the leading cause of disability around the world. JAMA. 2017:317:1517. 10.1001/jama.2017.3826. [DOI] [PubMed] [Google Scholar]
- Garrett JE, Wellman CL. Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience. 2009:162:195–207. 10.1016/J.NEUROSCIENCE.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal S, Hare BD, Duman RS. Prefrontal cortex GABAergic deficits and circuit dysfunction in the pathophysiology and treatment of chronic stress and depression. Curr Opin Behav Sci. 2017a:14:1–8. 10.1016/J.COBEHA.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal S, Packard AEB, Mahbod P, McKlveen JM, Seeley RJ, Myers B, Ulrich-Lai Y, Smith EP, D'Alessio DA, Herman JP. Disruption of glucagon-like peptide 1 signaling in sim1 neurons reduces physiological and behavioral reactivity to acute and chronic stress. J Neurosci. 2017b:37(1):184–193. 10.1523/JNEUROSCI.1104-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal S, Duman CH, Liu RJ, Wu M, Terwilliger R, Girgenti MJ, Wohleb E, Fogaca MV, Teichman EM, Hare B, et al. Ketamine rapidly reverses stress-induced impairments in GABAergic transmission in the prefrontal cortex in male rodents. Neurobiol Dis. 2020:134:104669. 10.1016/j.nbd.2019.104669. [DOI] [PubMed] [Google Scholar]
- Gianferante D, Thoma MV, Hanlin L, Chen X, Breines JG, Zoccola PM, Rohleder N. Post-stress rumination predicts HPA axis responses to repeated acute stress. Psychoneuroendocrinology. 2014:49:244–252. 10.1016/J.PSYNEUEN.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilabert-Juan J, Castillo-Gomez E, Guirado R, Moltó MD, Nacher J. Chronic stress alters inhibitory networks in the medial prefrontal cortex of adult mice. Brain Struct Funct. 2013:218:1591–1605. 10.1007/S00429-012-0479-1. [DOI] [PubMed] [Google Scholar]
- Giuliano C, Robbins TW, Nathan PJ, Bullmore ET, Everitt BJ. Inhibition of opioid transmission at the μ-opioid receptor prevents both food seeking and binge-like eating. Neuropsychopharmacology. 2012:37(12):2643–2652. 10.1038/npp.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, Morrison JH. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009:164(2):798–808. 10.1016/j.neuroscience.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Johnson AK. Stress, depression and cardiovascular dysregulation: a review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress. 2009:12:1–21. 10.1080/10253890802046281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Sullivan NR, Damjanoska KJ, Crane JW, Carrasco GA, Shi J, Chen Z, Garcia F, Muma NA, Van de Kar LD. Chronic mild stress induces behavioral and physiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psychopharmacology. 2005:179(4):769–780. 10.1007/s00213-004-2103-4. [DOI] [PubMed] [Google Scholar]
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, et al. Natural neural projection dynamics underlying social behavior. Cell. 2014:157(7):1535–1551. 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck AL, Handa RJ. Sex differences in the hypothalamic-pituitary-adrenal axis' response to stress: an important role for gonadal hormones. Neuropsychopharmacology. 2019:1:45–58. 10.1038/s41386-018-0167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, Scheimann J, Myers B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016:6(2):603–621. 10.1002/cphy.c150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersey M, Reneaux M, Berger SN, Mena S, Buchanan AM, Ou Y, Tavakoli N, Reagan LP, Clopath C, Hashemi P. A tale of two transmitters: serotonin and histamine as in vivo biomarkers of chronic stress in mice. J Neuroinflammation. 2022:19(1):1–20. 10.1186/S12974-022-02508-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, Rainville JR, Rivero-Ballon GN, Dhimitri K, Hodes GE. Testing the limits of sex differences using variable stress. Neuroscience. 2021:454:72–84. 10.1016/j.neuroscience.2019.12.034. [DOI] [PubMed] [Google Scholar]
- Karagiannides I, Golovatscka V, Bakirtzi K, Sideri A, Salas M, Stavrakis D, Polytarchou C, Iliopoulos D, Pothoulakis C, Bradesi S. Chronic unpredictable stress regulates visceral adipocyte-mediated glucose metabolism and inflammatory circuits in male rats. Physiol Rep. 2014:2(5):e00284. 10.14814/phy2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008:455:894–902. 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehner C. Why is depression more common among women than among men? Lancet Psychiatry. 2017:4:146–158. 10.1016/S2215-0366(16)30263-2. [DOI] [PubMed] [Google Scholar]
- Liotti M, Mayberg HS, Brannan SK, McGinnis S, Jerabek P, Fox PT. Differential limbic-cortical correlates of sadness and anxiety in healthy subjects: implications for affective disorders. Biol Psychiatry. 2000:48:30–42. 10.1016/S0006-3223(00)00874-X. [DOI] [PubMed] [Google Scholar]
- London TD, Licholai JA, Szczot I, Ali MA, LeBlanc KH, Fobbs WC, Kravitz AV. Coordinated ramping of dorsal striatal pathways preceding food approach and consumption. J Neurosci. 2018:38(14):3547–3558. 10.1523/JNEUROSCI.2693-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczynski P, Moquin L, Gratton A. Chronic stress alters the dendritic morphology of callosal neurons and the acute glutamate stress response in the rat medial prefrontal cortex. Stress. 2015:18:654–667. 10.3109/10253890.2015.1073256. [DOI] [PubMed] [Google Scholar]
- Manouze H, Ghestem A, Poillerat V, Bennis M, Ba-M’hamed S, Benoliel JJ, Becker C, Bernard C. Effects of single cage housing on stress, cognitive, and seizure parameters in the rat and mouse pilocarpine models of epilepsy. eNeuro. 2019:6(4):ENEURO.0179–ENEU18.2019. 10.1523/ENEURO.0179-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005:45(5):651–660. 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McKlveen JM, Myers B, Herman JP. The medial prefrontal cortex: coordinator of autonomic, neuroendocrine and behavioural responses to stress. J Neuroendocrinol. 2015:27:446–456. 10.1111/jne.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKlveen JM, Morano RL, Fitzgerald M, Zoubovsky S, Cassella SN, Scheimann JR, Ghosal S, Mahbod P, Packard BA, Myers B, et al. Chronic stress increases prefrontal inhibition: a mechanism for stress-induced prefrontal dysfunction. Biol Psychiatry. 2016:80(10):754–764. 10.1016/j.biopsych.2016.03.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKlveen JM, Moloney RD, Scheimann JR, Myers B, Herman JP. “Braking” the prefrontal cortex: the role of glucocorticoids and interneurons in stress adaptation and pathology. Biol Psychiatry. 2019:86:669–681. 10.1016/j.biopsych.2019.04.032. [DOI] [PubMed] [Google Scholar]
- Morris MC, Rao U. Cortisol response to psychosocial stress during a depressive episode and remission. Stress. 2014:17:51–58. 10.3109/10253890.2013.857398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: An approach to assess autistic-like behavior in mice. Genes, Brain Behav. 2004:3:287–302. 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Muir J, Lorsch ZS, Ramakrishnan C, Deisseroth K, Nestler EJ, Calipari ES, Bagot RC. In vivo fiber photometry reveals signature of future stress susceptibility in nucleus accumbens. Neuropsychopharmacology. 2018:43(2):255–263. 10.1038/npp.2017.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir J, Tse YC, Iyer ES, Biris J, Cvetkovska V, Lopez J, Bagot RC. Ventral hippocampal afferents to nucleus accumbens encode both latent vulnerability and stress-induced susceptibility. Biol Psychiatry. 2020:88(11):843–854. 10.1016/J.BIOPSYCH.2020.05.021. [DOI] [PubMed] [Google Scholar]
- Myers B, McKlveen JM, Herman JP. Glucocorticoid actions on synapses, circuits, and behavior: implications for the energetics of stress. Front Neuroendocrinol. 2014:35:180–196. 10.1016/j.yfrne.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B, McKlveen JM, Morano R, Ulrich-Lai YM, Solomon MB, Wilson SP, Herman JP. Vesicular glutamate transporter 1 knockdown in infralimbic prefrontal cortex augments neuroendocrine responses to chronic stress in male rats. Endocrinology. 2017: 158(10):3579–3591. 10.1210/en.2017-00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers-Schulz B, Koenigs M. Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Mol Psychiatry. 2012:17:132–141. 10.1038/mp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyola MG, Handa RJ. Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity. Stress. 2017:20(5):476–494. 10.1080/10253890.2017.1369523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace SA, Christensen C, Schackmuth MK, Wallace T, McKlveen JM, Beischel W, Morano R, Scheimann JR, Wilson SP, Herman JP, et al. Infralimbic cortical glutamate output is necessary for the neural and behavioral consequences of chronic stress. Neurobiol Stress. 2020:13:100274. 10.1016/j.ynstr.2020.100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page CE, Coutellier L. Prefrontal excitatory/inhibitory balance in stress and emotional disorders: evidence for over-inhibition. Neurosci Biobehav Rev. 2019:105:39–51. 10.1016/j.neubiorev.2019.07.024. [DOI] [PubMed] [Google Scholar]
- Page CE, Shepard R, Heslin K, Coutellier L. Prefrontal parvalbumin cells are sensitive to stress and mediate anxiety-related behaviors in female mice. Sci Rep. 2019:9:19772. 10.1038/s41598-019-56424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak TR, Chung WC, Roberts JL, Handa RJ. Ligand-independent effects of estrogen receptor beta on mouse gonadotropin-releasing hormone promoter activity. Endocrinology. 2006:147:1924–1931. 10.1210/EN.2005-1297. [DOI] [PubMed] [Google Scholar]
- Pinceti E, Shults CL, Rao YS, Mott NN, Pak TR. Phosphorylation alters oestrogen receptor β-mediated transcription in neurons. J Neuroendocrinol. 2015:27:861. 10.1111/JNE.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebuffé-Scrive M, Walsh UA, McEwen B, Rodin J. Effect of chronic stress and exogenous glucocorticoids on regional fat distribution and metabolism. Physiol Behav. 1992:52(3):583–590. 10.1016/0031-9384(92)90351-2. [DOI] [PubMed] [Google Scholar]
- Schaeuble D, Packard AEB, McKlveen JM, Morano R, Fourman S, Smith BL, Scheimann JR, Packard BA, Wilson SP, James J, et al. Prefrontal cortex regulates chronic stress-induced cardiovascular susceptibility. J Am Heart Assoc. 2019:8(24):e014451. 10.1161/JAHA.119.014451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgoifo A, Carnevali L, Pico Alfonso MDLA, Amore M. Autonomic dysfunction and heart rate variability in depression. Stress. 2015:18:343–352. 10.3109/10253890.2015.1045868. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb Cortex. 2009:19:2479–2484. 10.1093/cercor/bhp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, Morrison JH. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb Cortex. 2010:20:2560–2567. 10.1093/cercor/bhq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng JA, Bales NJ, Myers SA, Bautista AI, Roueinfar M, Hale TM, Handa RJ. The hypothalamic-pituitary-adrenal axis: development, programming actions of hormones, and maternal-fetal interactions. Front Behav Neurosci. 2021:14:601939. 10.3389/FNBEH.2020.601939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard R, Page CE, Coutellier L. Sensitivity of the prefrontal GABAergic system to chronic stress in male and female mice: relevance for sex differences in stress-related disorders. Neuroscience. 2016:332:1–12. 10.1016/j.neuroscience.2016.06.038. [DOI] [PubMed] [Google Scholar]
- Solomon MB, Loftspring M, de Kloet AD, Ghosal S, Jankord R, Flak JN, Wulsin AC, Krause EG, Zhang R, Rice T, et al. Neuroendocrine function after hypothalamic depletion of glucocorticoid receptors in male and female mice. Endocrinology. 2015:156(8):2843–2853. 10.1210/en.2015-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D’Alessio DA, Herman JP. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am. J. Physiol. Endocrinol. Metab. 2005:289:E823–8. 10.1152/ajpendo.00122.2005. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004:51:32–58. 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991:129:2503–2511. 10.1210/ENDO-129-5-2503. [DOI] [PubMed] [Google Scholar]
- Vijayakumar N, Cheng TW, Pfeifer JH. Neural correlates of social exclusion across ages: a coordinate-based meta-analysis of functional MRI studies. NeuroImage. 2017:153:359–368. 10.1016/j.neuroimage.2017.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace T, Myers B. Effects of biological sex and stress exposure on ventromedial prefrontal regulation of mood-related behaviors. Front Behav Neurosci. 2021:15:202. 10.3389/FNBEH.2021.737960/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace T, Schaeuble D, Pace SA, Schackmuth MK, Hentges ST, Chicco AJ, Myers B. Sexually divergent cortical control of affective-autonomic integration. Psychoneuroendocrinology. 2021: 129:105238. 10.1016/j.psyneuen.2021.105238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Yuen EY, Liu W, Li X, Zhong P, Karatsoreos IN, McEwen BS, Yan Z. Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition. Mol Psychiatry. 2014:19(5):588–598. 10.1038/mp.2013.83. [DOI] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005:52:90–110. 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Wood JN, Grafman J. Human prefrontal cortex: processing and representational perspectives. Nat Rev Neurosci. 2003:4:139–147. 10.1038/nrn1033. [DOI] [PubMed] [Google Scholar]
- Wood M, Adil O, Wallace T, Fourman S, Wilson SP, Herman JP, Myers B. Infralimbic prefrontal cortex structural and functional connectivity with the limbic forebrain: a combined viral genetic and optogenetic analysis. Brain Struct Funct. 2019:1–25(1):73–97. 10.1007/s00429-018-1762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012:73:962–977. 10.1016/J.NEURON.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.