Abstract
Event segmentation is a spontaneous part of perception, important for processing continuous information and organizing it into memory. Although neural and behavioral event segmentation show a degree of inter-subject consistency, meaningful individual variability exists atop these shared patterns. Here we characterized individual differences in the location of neural event boundaries across four short movies that evoked variable interpretations. Event boundary alignment across subjects followed a posterior-to-anterior gradient that was tightly correlated with the rate of segmentation: slower-segmenting regions that integrate information over longer time periods showed more individual variability in boundary locations. This relationship held irrespective of the stimulus, but the degree to which boundaries in particular regions were shared versus idiosyncratic depended on certain aspects of movie content. Furthermore, this variability was behaviorally significant in that similarity of neural boundary locations during movie-watching predicted similarity in how the movie was ultimately remembered and appraised. In particular, we identified a subset of regions in which neural boundary locations are both aligned with behavioral boundaries during encoding and predictive of stimulus interpretation, suggesting that event segmentation may be a mechanism by which narratives generate variable memories and appraisals of stimuli.
Keywords: event segmentation, fMRI, individual differences, naturalistic stimuli, stimulus properties
Introduction
Event segmentation refers to the spontaneous chunking of continuous experiences into meaningful distinct units or events as part of everyday perception (Zacks et al. 2007; Zacks and Swallow 2007). This cognitive mechanism is an automatic and adaptive part of perceptual processing, optimizing attention and facilitating the subsequent organization of experiences into memory (Zacks et al. 2007; Zacks and Swallow 2007; Kurby and Zacks 2008; DuBrow and Davachi 2016; McGatlin et al. 2018).
Event boundaries, typically reported behaviorally by pressing a button to indicate transitions between events (Newtson 1973), are to some extent “normative,” or consistent across people. Boundary locations not only have high intersubject reliability, but also are meaningfully structured, corresponding to moments of decreased contextual stability where the prediction of the immediate future fails, such as changes in action, goals, or locations (Newtson et al. 1976; Speer et al. 2009; Zacks et al. 2009; Raccah et al. 2022). Neural responses at normative event boundaries have been reported in several regions including the hippocampus, lateral frontal cortex, precuneus, cingulate, and lateral parietal cortex (Zacks et al. 2001; Speer et al. 2003, 2007; DuBrow and Davachi 2013, 2016; Ezzyat and Davachi 2014; Baldassano et al. 2017; Ben-Yakov and Henson 2018).
Despite this degree of consistency in how people segment events, meaningful individual differences exist atop these shared tendencies. Individual differences in behavioral segmentation are stable over time (Speer et al. 2009), correlate with age and other cognitive abilities such as working memory capacity, long-term memory retrieval, and performance on other tasks (Zacks et al. 2006; Sargent et al. 2013; Bailey et al. 2017; Jafarpour et al. 2022), and are disrupted in certain clinical conditions such as schizophrenia (Zalla et al. 2004). Yet despite this ample behavioral evidence, investigations into individual differences in neural event segmentation have been very limited. This is due, in part, to the challenge of capturing individual boundaries (as opposed to relying on normative boundaries or using stimuli with pre-defined boundaries) while also preserving natural viewing (i.e. avoiding having subjects segment during encoding, which could alter the viewing experience and introduce unwanted effects on brain activity, or segment upon a second, biased viewing). However, recently developed algorithms for identifying latent brain state changes (Baldassano et al. 2017; Geerligs et al. 2021) allow us to infer neural event boundaries from fMRI data acquired during passively experienced continuous “naturalistic” stimuli (e.g. movies) without prior knowledge of boundary locations.
Characterizing individual differences in neural event segmentation during natural, passive viewing can extend our understanding of both cortical variability in event segmentation and the functional role of segmentation in cataloging experiences. Individual neural activity at normative event boundaries during a narrative reading task has been shown to predict the organization of information into long-term memory (Ezzyat and Davachi 2011). Here we sought to extend this work: above and beyond differences in objective recall accuracy, complex narrative stimuli generate personalized, idiosyncratic memories and appraisals (Black and Bower 1980), which could arise in part from individual differences in event segmentation during encoding—in other words, how the same (presented) events are (uniquely) segmented. We hypothesized that an individual’s pattern of neural event boundaries during movie-watching would predict how they ultimately remembered and appraised the stimulus. Further, these effects might be somewhat stimulus-dependent: different stimuli (in our case, movies) might evoke more or less individual variability overall, more or less individual variability in specific cortical regions, and/or differences in the specific regions where neural event boundaries relate to ultimate appraisal. We investigated these questions in a dataset of healthy adults who freely viewed and appraised four short movies during fMRI scanning.
To preview our findings, we first demonstrated that a recently developed algorithm for automatically inferring neural event boundaries (Baldassano et al. 2017) can operate reliably at the individual level. Using individually defined neural boundaries, we found that across-subject variability followed a posterior-to-anterior cortical gradient: sensory processing regions were most consistent across individuals, whereas higher-order association regions were most variable. We found evidence for a relationship between individual neural event boundaries and the ultimate recall and appraisal of narratives in a subset of regions that also showed normative alignment with behavioral boundaries in our movies. The specific regions showing these relationships were different across movies, suggesting that depending on the stimulus, distinct regions support aspects of online “chunking” that impact how the stimulus is later remembered and appraised.
Materials and methods
Experiment 1 (main fMRI experiment)
We recruited and scanned a total of 48 subjects (all native English speakers; 27F, median age 24.5, range = (19,64)) at the National Institutes of Health (NIH). All subjects provided informed written consent prior to the start of the study in accordance with experimental procedures approved by the Institutional Review Board of the NIH. We discarded incomplete datasets without all four movies (described below), leaving 43 subjects whose data we analyzed here. Subjects were compensated $50 per hour for their time.
Subjects watched four movies (ranging from 7:27–12:27 min each) while we collected fMRI data using a 3 T Siemens Prisma scanner with a 64-channel head coil. We refer to these movies here by four short titles: Iteration, Defeat, Growth, and Lemonade. The movie order was pseudorandomized for each subject such that order was counterbalanced at the group level. Functional images were acquired using a T2*-weighted multiband, multi-echo echo-planar imaging (EPI) pulse sequence with the following parameters: TR = 1,000 ms, echo times (TE) = [13.6, 31.86, 50.12 ms], flip angle = 60°, field of view = 216 × 216 mm2, in-plane resolution = 3.0 mm2, slice thickness 3.0 mm, number of slices = 52 (whole-brain coverage), multiband acceleration factor = 4). Anatomical images were acquired using a T1-weighted MPRAGE pulse sequence with the following parameters: TR = 2,530 ms, TE = 3.30 ms, flip angle = 7°, field of view = 256 × 256 mm2, in-plane resolution = 1.0 mm2, slice thickness = 1.0 mm).
The movies were projected onto a rear-projection screen located in the magnet bore and viewed with an angled mirror. The experiment was presented using PsychoPy (Peirce et al. 2019). Following each movie (i.e. while still in the scanner), the subjects completed a task battery designed to probe their interpretations and reactions to the movie, including the following: (i) a 3-min free recall/appraisal task in which subjects spoke freely about their memories and impressions of the movie, during which their speech was captured with a noise-canceling microphone; (ii) multiple-choice comprehension questions designed to ensure they had been paying attention (e.g: “At what holiday dinner does the father yell at the older son?” for Growth); (iii) multiple-choice and Likert-style items assessing reactions to various characters and to the movie overall. See section “Measuring cross-subject similarity in movie recall/appraisal” for the experimental instructions for the free recall/appraisal task and see study GitHub repository for all of the memory comprehension questions.
During a separate behavioral visit, subjects completed a battery of tasks and questionnaires including selected instruments from the NIH Cognition and Emotion toolboxes as well as other psychological and psychiatric self-report scales. These data are not analyzed or reported here.
MRI data preprocessing
Following the conversion of the original DICOM images to NIFTI format, we used AFNI (Cox 1996) to preprocess MRI data. Preprocessing included the following steps: despiking, head motion correction, affine alignment with subject-specific anatomical (T1-weighted) image, nonlinear alignment to a group MNI template (MNI152_2009), combination of data across the three echoes using AFNI’s “optimally combine” method, and smoothing with an isotropic full-width half-maximum of 4 mm. Each subject’s six motion time series, their derivatives, and linear polynomial baselines for each of the functional runs were regressed from the data prior to further analysis. All analyses were conducted in volume space and projected to the surface for visualization purposes.
We used mean framewise displacement (MFD), a per-subject summary metric, to assess the amount of head motion in the sample. MFD was overall relatively low [Iteration: mean = 0.08, SD = 0.03, range = (0.03, 0.18); Defeat: mean = 0.08, SD = 0.03, range = (0.04, 0.19); Growth:mean = 0.07, SD = 0.03, range = (0.04, 0.17); Lemonade:mean = 0.08, SD = 0.03, range = (0.04, 0.17)] and did not differ across movies (repeated-measures ANOVA, F(3,126) = 1.44, P = 0.23).
We included an additional preprocessing step in which we used a shared response model (Chen et al. 2015) as implemented in BrainIAK (Kumar et al. 2020) to account for different functional topographies across individuals. This step was performed for each of the four movies separately. First, we fit a model to capture the reliable whole-brain responses to the movie across subjects in a lower dimensional feature space (features = 50). We then applied this model to reconstruct the individual voxelwise time courses for each participant. This procedure serves as an additional denoising step and makes spatiotemporal patterns more consistent across subjects.
Experiment 2 (auxiliary behavioral experiment)
We collected an auxiliary behavioral dataset (i.e. outside the MRI scanner) at Dartmouth College to further refine and support results from Experiment 1. 44 subjects (21F, median age 20, range = 18–32) were presented with the same paradigm as in Experiment 1, except that while watching each film, individuals were instructed to press a button each time they perceived that a new scene is starting (i.e. points in the movie when there is a “major change in topic, location, time, etc.”). They were also told to expect scenes to be between 10 s and 3 min long. These were the same instructions used in Baldassano et al. (2017). We discarded subjects who did not complete all four movies, leaving n = 40 for our analysis. The paradigm was hosted and presented using JsPsych (de Leeuw 2015). Subjects were compensated $15 an hour for their time or given participation credit, and the study was approved by the Institutional Review Board of Dartmouth College.
Movie overview
Our stimuli were four short films made by independent filmmakers that were chosen because they were rich and engaging, yet they depicted ambiguous scenarios that provoked divergent reactions and interpretations in different individuals. Three of the movies were “social” in nature and followed different narratives of humans taking actions and interacting, whereas the fourth depicted purely mechanical information (a long, complex Rube Goldberg machine that traversed a house). We chose independent films so that subjects would be less likely to have experienced the material before. In a debriefing questionnaire, three subjects in Experiment 1 reported having seen one of the movies (Growth) prior to the experiment.
Here we provide brief descriptions of each movie along with YouTube links. (Note that the versions presented to subjects were edited to remove credits and title pages; these edited versions are available upon request.) Iteration (https://youtu.be/c53fGdK84rc; 12:27 min:s) is a sci-fi movie that follows a female character as she goes through multiple iterations of waking up and trying to escape a facility. A male character appears toward the end to help her. Defeat (https://youtu.be/6yN9VH_4GSQ; 7:57 min:s) follows a family of three (mother, two children) as the brother bullies his sister and she builds a time machine to go back and get revenge. Growth (https://youtu.be/JyvFXBA3O8o; 8:27 min:s) follows a family of four (mother, father, two brothers) as the children grow up and eventually move out amid some family conflict. Lemonade (https://youtu.be/Av07QiqmsoA; 7:27 min:s) is a Rube-Goldberg machine consisting of a series of objects that move throughout a house and ends in the pouring of a cup of lemonade. This movie was lightly edited to remove fleeting shots of human characters. Iteration and Defeat both contained screen cuts (continuity editing), whereas Growth and Lemonade were shot in a continuous fashion with the camera panning smoothly from one scene to the next.
Automatic neural event boundary detection
To automatically identify neural event boundaries from fMRI data, we fit a series of Hidden Markov Models (HMMs) (split-merge option; Baldassano et al. 2017) as implemented in the BrainIAK toolbox (Kumar et al. 2020), adapting code made available on the Naturalistic Data tutorial website (Chang et al. 2020). The HMM approach does not rely on annotations or hand-demarcated events, but rather infers event boundaries from shifts in stable patterns of brain activity. It relies on voxelwise patterns within regions. We restricted our analyses to the neocortex and used the 100-parcel, 7-network Schaefer parcellation (Schaefer et al. 2018), in keeping with past work using HMMs to identify event boundaries (Cohen and Baldassano 2021).
The HMM approach assumes that each event has a distinct signature of activity that shifts at event boundaries within region. Specifically, the model assumes that (i) each subject starts in an event and then each forthcoming timepoint is either in the same event (state) or in the next state, and (ii) that the voxelwise pattern of activity in a region is similar across timepoints within the same event. The model identifies both the optimal number of events and the transitions between these events. We were ultimately interested in individual variability in the temporal location of event boundaries. Although we acknowledge that there is likely interesting individual variability in the number of events (i.e. segmentation rate) in each region, this is a somewhat different question than variability in the location of neural event boundaries: consider that even if two individuals have the same number of events in a region, the specific locations (timestamps) of their event boundaries could still be completely different. The location of boundaries is more directly related to the movie content onscreen at any given moment than the overall number of events, and therefore of greater scientific interest to us here, given that our goals were to investigate (i) how moment-to-moment segmentation patterns relate to ultimate appraisals, and (ii) how movie content affects the anatomical locations and degree of this variability. In addition to this theoretical justification, there are two related methodological justifications to fixing the number of events within a region while allowing locations to vary across subjects. First, we are using an inherently noisy method to infer neural event boundaries, which was created to work well at the group level. Therefore, by fixing the number of events using group-average data, we constrain the individual HMM solutions to a reasonable number of events. Second, it is not clear how to reliably estimate the optimal number of events at the individual level, as training and test data need to be independent.
Therefore, to focus on individual variability in boundary locations, we first determined the optimal number of events (k) for each region at the group level using a train-test split procedure (with different subjects in the train and test groups). We used a fairly liberal range of possible values for k: the minimum allowed k was 6, and the maximum (90–150) differed across movies owing to their different lengths, but always reflected a minimum average event length of 5 s. For each movie and each region, we split the subjects into train/test groups (each with 21/22 subjects) and averaged voxelwise time courses in each group, resulting in one average voxel-by-time array each for the training and test data. We then fit a series of models to the average training data using each possible value of k and tested each model on the average test data, assessing model fit using the log-likelihood. After repeating this procedure for each k, we took the k value with the maximum log-likelihood as the optimal number of events within this region for this train/test split. We repeated this procedure 100 times with different train/test splits within each region. For each region, we then calculated the median optimal k value across these 100 iterations and used this as the k value for our subsequent analyses.
Using these fixed values of k, for each region, subject, and movie, we then fit an HMM to that subject’s individual time series to identify the location of implicit neural event boundaries. Therefore, we had one set of boundary locations for each brain region for each subject for each movie.
We evaluated the fit of the HMM to individual neural data across movies to ensure that any across-movie differences in degree of variability were not due to differences in quality of model fit. Notably, the fit of the HMM (as measured by the log-likelihood) is tightly coupled with the number of events and our movies do differ in length (such that the model fit is best with the shortest movie and worst with the longest movie). When accounting for the difference in movie length, the model fit does not differ across movies. Model fit was also highest in sensory regions (e.g., V1) and decreased on a cortical gradient in multimodal regions; this illustrates that regions where there is more consistency across people (cf. Fig. 1), are also regions where the model is performing better, implying that more shared boundaries are not due to poor model performance.
Fig. 1.
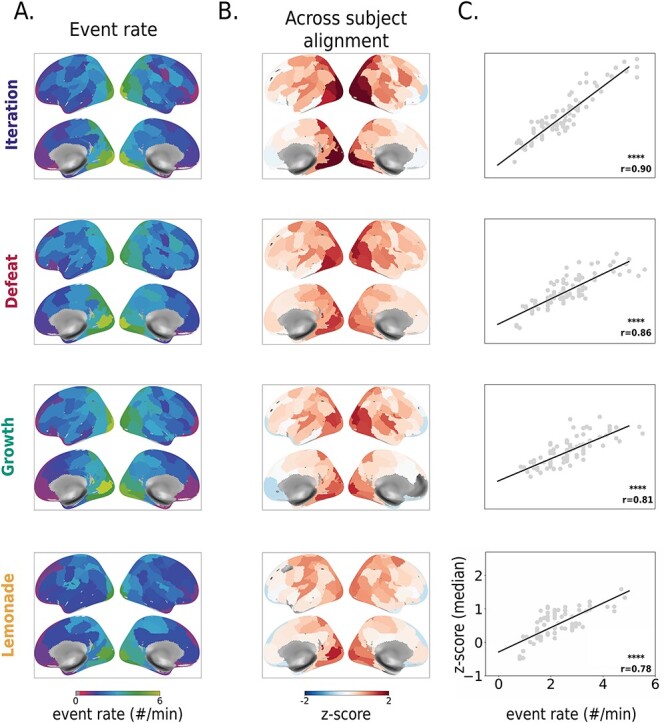
Segmentation rate and degree of idiosyncrasy in boundary locations covary across the cortex. (A) Event rate. The optimal number of events for each region (determined at the group level) follows the expected cortical gradient: faster segmentation (more events) in posterior, sensory regions and slower segmentation (fewer events) in anterior, higher-order regions. (B) Across-subject alignment within the movie. There is generally above-chance alignment in the location of event boundaries among individuals across the cortex (n = 10,000 bootstraps, q < 0.05, FDR-corrected across 100 regions). Alignment is highest in posterior sensory regions and lowest in anterior association regions. The degree of alignment in each region varies slightly across movies. Although greater-than-chance alignment was found in the majority of regions (>89/100) in each movie, regions that did not show significant alignment tended to be in higher-order regions such as the prefrontal cortex. (C) Correlation between event rate and within-movie alignment. In all four movies, the event rate and degree of alignment were strongly correlated across regions (Spearman r = 0.78–0.90, P < 0.001) such that fast transitioning regions were more closely aligned across subjects, whereas slower regions were less aligned (i.e. showed more individual variability). Each gray dot indicates a region; we highlight a visual region in blue and a prefrontal region in red. Legend:**** P < 0.0001.
Computing alignment across subjects
We used permutation testing to quantitatively assess the consistency of neural event boundary locations across individuals. For each movie, region, and pair of subjects, we permuted boundary locations n = 1000 times to derive a z-score for the match between true boundaries relative to the null distribution. In both the true and permuted data, boundaries that were within 3 TRs (seconds) of one another were counted as a match, consistent with past work (Baldassano et al. 2017; Williams et al. 2022). We then created subject-by-subject matrices of these z-scores in every region. Importantly, depending on which member of a pair of subjects was permuted (versus treated as the “ground truth”), there were slight differences in the resulting z-score; we took the mean of the upper and lower triangle of these matrices for subsequent analyses. We used the same permutation-based approach to assess the consistency of behavioral event boundaries acquired in Experiment 2.
Controlling for possible confounds
Several factors outside the ongoing cognitive processes of interest could contribute to higher or lower alignment in detected boundary locations between a given pair of subjects. The factors detailed below were used as regressors of no interest to control for these unwanted influences in the ensuing analyses, as described in subsequent sections.
Inter-subject correlation of head motion
It is possible that shared head motion at similar moments in the movie could lead the HMM to (perhaps falsely) detect similar neural event boundaries in a given pair of subjects. To control for this possibility, we computed the inter-subject correlation (ISC) of the framewise displacement (FD) across time for each subject pair. We then used this subject-by-subject motion-ISC matrix as a nuisance regressor in subsequent analyses.
Overall head motion
Similarly, subjects with high overall levels of head motion likely have lower-quality fMRI data, which could bias the detection of neural event boundaries altogether (though importantly, as detailed in “MRI data preprocessing”, absolute levels of head motion were relatively low in our sample). To control for this possible confound, we used each subject’s median FD across all timepoints and generated a subject-by-subject similarity matrix using the Anna Karenina principle (“all low (or high) motion scorers are alike; each high (or low) motion scorer is different in their own way”) by taking the mean score between each subject pair (Finn et al. 2020).
Memory performance
Some subjects may simply have been paying better attention throughout the scan session and/or during certain movies, which could generate stronger stimulus-driven neural responses and, in turn, drive up similarity in event boundaries among these subjects. We controlled for this possibility using subjects’ performance on the four multiple-choice memory recall questions presented at the end of each movie, which were designed to be challenging and require subjects to have paid close attention to answer correctly. We computed each subject’s memory performance score as the fraction of correct responses on these questions, and generated a subject-by-subject similarity matrix of these scores also according to the aforementioned Anna Karenina principle (“all low (or high) memory scorers are alike; each high (or low) scorer is different in their own way”) by taking the mean score between each subject pair (Finn et al. 2020). The same approach was applied to the behavioral boundaries collected in Experiment 2.
Across-subject alignment within movies
The goal of this analysis was to assess the degree to which event boundary locations were aligned across subjects within each movie. Subject-by-subject matrices of alignment values (z-scores) in every region, generated using the permutation method described above (see section “Computing alignment across subjects”), were used.
Experiment 1
We fit a linear regression to regress out (i) head-motion ISC, (ii) overall head motion, and (iii) memory performance (see “Controlling for possible confounds” for more detail) from the subject-by-subject boundary alignment matrix in each region. Using the residuals of this regression, we took the median alignment value (z-score) across subject pairs as the summary statistic for each region. To determine whether alignment was significantly above chance (i.e. greater than 0), we performed this same calculation in a subject-wise bootstrapping framework (n = 10,000 bootstraps) to create a non-parametric null distribution and compared our observed median residual z-score to this distribution to calculate a P-value. P-values were corrected for multiple comparisons using the false discovery rate (FDR) based on the number of regions in our parcellation (100) using an alpha of 0.05.
Experiment 2
We fit a linear regression to regress out memory performance (see “Controlling for possible confounds” for more detail) from the subject-by-subject matrix of alignment in behaviorally reported event boundaries. Using the residuals of this regression, we took the median alignment value (z-score) across subject pairs as the summary statistic. To determine whether alignment was significantly above chance (i.e. greater than 0), we performed this same calculation in a subject-wise bootstrapping framework (n = 10,000 bootstraps) to create a non-parametric null distribution and compared our observed median residual z-score to this distribution to calculate a P-value.
Identifying stimulus-dependent properties by taking the difference in alignment across movies
The goal of this analysis was to assess the degree to which stimulus content influences across-subject alignment in event boundary locations by comparing subject-by-subject alignment matrices between pairs of movies.
Experiment 1
For a given region and pair of movies, we fit a linear regression to model the difference between the two subject-by-subject alignment matrices as a function of the following regressors of no interest: (i) difference in head-motion ISC, (ii) the difference in overall head motion, and (iii) the difference in memory performance between the movies (see “Controlling for possible confounds” for more detail). We then took the median value from this residual difference matrix and compared it with a non-parametric null distribution (n = 10,000 bootstraps) to calculate a P-value. P-values were corrected for multiple comparisons using the FDR based on the number of regions in our parcellation (100) using an alpha of 0.05. To investigate the overall (i.e. whole-brain) variation in alignment across movies, we employed a linear mixed-effects model, implemented using lme4 and lmerTest (Bates et al. 2015) in R. Estimated marginal means (EMMs) were calculated using the emmeans package (Lenth 2023). We used the intercept from the within-movie alignment regression (accounting for the regressors of no interest, see Methods section—“Across-subject alignment within movies”), treating region as a random effect.
Experiment 2
For a given pair of movies, we fit a linear regression to model the difference between the two subject-by-subject alignment matrices as a function of the following regressor of no interest: the difference in memory performance between the movies (see “Controlling for possible confounds” for more detail). We then took the median value from this residual difference matrix and compared it to a non-parametric null distribution (n = 10,000 bootstraps) to extract a P-value. To investigate the variation in behavioral alignment across movies, we adopted the method used by Chen et al. (2017b) and fit a linear mixed-effects model predicting alignment by movie and memory (our regressor of no interest) with crossed random effects. This approach allowed us to account for non-independence of the pairwise alignment in the data from repeated observations for each participant. To account for redundancy, we manually adjusted the degrees of freedom and standard error, as suggested by Chen et al. (2017b).
Measuring cross-subject similarity in movie recall/appraisal
We used inter-subject representational similarity analysis (IS-RSA) (Glerean et al. 2016; Chen et al. 2020; Finn et al. 2020) to investigate whether pairs of subjects that were similar in their neural event boundaries in a region also had similar interpretations of the movie.
To elicit appraisals, we presented the following prompt to subjects immediately following each movie: “During this section, you will have three minutes to say what you remember about the video. You can talk about characters, events, your opinions, or anything else that comes to mind. Try to fill the whole three minutes once the timer appears and remember- there are no wrong answers!” Subjects spoke freely while their speech was recorded using a noise-canceling microphone. These recordings were professionally transcribed and minimally cleaned to remove interjections, such as “um” or “uh,” and repeated words. One subject’s recall data were not able to be transcribed due to being corrupted by scanner noise and was discarded from this analysis, leaving n = 42.
The text from each individual’s speech data was then embedded into a 512-dimensional space via Google’s Universal Sentence Encoder [USE; (Cer et al. 2018); implemented via https://www.tensorflow.org/hub/tutorials/semantic_similarity_with_tf_hub_universal_encoder]. We chose the USE model in part because it was trained to identify similarities between pairs of sentences, and, as a sanity check, because it was best able to differentiate appraisals from different movies (i.e. recall between Growth-Growth was more similar than recall between Growth-Defeat, etc.), whereas this sensitivity was absent or weaker with other pre-trained context-sensitive models that we tested (including BERTBASE, MiniLM, and MPNet; Devlin et al. 2019; Song et al. 2020; Wang et al. 2020). USE was also used on event descriptions in a recent publication (Lee and Chen 2022). We computed the cosine similarity between the 512-dimensional vectors generated by USE to measure the semantic similarity between pairs of subjects' interpretations, resulting in one subject-by-subject appraisal similarity matrix per movie. In an IS-RSA, we then used a Spearman correlation to compare the lower triangle of these appraisal similarity matrices to the lower triangle of the boundary alignment matrices in each region. A partial Mantel test (n = 10,000 permutations; q < 0.05, FDR-corrected across regions) was used to assess the relationship between event boundary alignment and appraisal similarity while controlling for memory performance (see “Controlling for possible confounds” for more detail). By controlling for memory performance, we were able to bias our findings towards relationships driven more by subjective impressions and interpretations than objective recall per se.
The total number of words spoken varied across subjects and movies: Iteration: mean = 386, SD = 80, range = 213–547; Defeat: mean = 362, SD = 78, range = 153–533; Growth: mean = 368, SD = 72, range = 243–520; Lemonade: mean = 359, SD = 84, range = 180–576. The number of words differed significantly between movies (repeated-measures ANOVA, F(3,123) = 2.87, P = 0.04). Post hoc tests showed that the number of words used in Iteration was significantly greater than the number of words used in Defeat (paired t-test, t = 2.81, P = 0.01) and Lemonade (paired t-test, t = 2.12, P = 0.04), which is not surprising, given that Iteration was the longest movie (~12 min compared to ~ 8 min for the other three movies). As our goal in this analysis was to compare similarity in neural event boundary locations to similarity in appraisal within a movie as opposed to across movies, differences in the number of words used across movies should not influence our results in a meaningful way. Importantly, the degree of similarity in the appraisal of Iteration is not significantly higher compared to the other movies. In fact, the highest similarity was in Growth, which was greater than Iteration, Defeat, and Lemonade (paired t-tests, t ≥ 11.84, P < 0.001) (median similarity—Iteration—0.58, Defeat 0.59, Growth 0.63, and Lemonade 0.58; repeated-measures ANOVA, F(3,2580) = 86.06, P < 0.0001). Therefore, there does not seem to be a direct relationship between the number of words used to appraise a movie and the degree of similarity in appraisals across subjects.
Comparison of normative neural event boundaries and normative behavioral event boundaries
We used data from Experiment 2 to identify a set of group-level or “normative” behavioral boundaries for each movie. Individuals’ button presses were first rounded to the nearest second. To make the behavioral boundaries commensurate with the HMM-derived neural boundaries, we needed to account for the delay in the hemodynamic response, which may be partially offset by the delay in behavioral motor responses (i.e. button presses) after a boundary is detected. Therefore, we added 3 TRs/seconds to each button press (without allowing for events past the end of the movie).
We took two approaches, a density-based and a peak-based method (described in turn below), to compute the similarity between normative HMM-derived neural boundaries and normative behavioral boundaries.
Density alignment method
We generated a density distribution of “button presses” to indicate, at each timepoint (second), what percentage of subjects in the behavioral study marked a boundary location at that timepoint (or within +/− 3 s of that timepoint). Specifically, a binary distribution (event or no event) over time was generated for each subject. These subject-level distributions were combined to derive a density distribution reflecting the proportion of subjects indicating an event per timepoint. We then computed a Pearson correlation between this behavioral density distribution and the density distribution of individual-subject HMM-derived boundaries for each region (calculated in the same way) and compared this true correlation to a null distribution of correlations that were generated by “rolling” (or circle-shifting) the HMM-derived boundaries at each TR (thus, the number of permutations was limited to the number of TRs for each movie). This generation of a proportion-based density distribution is similar to the “segmentation agreement” previously used to compare individuals with the group average (Zacks et al. 2006; Bailey et al. 2013a, 2013b).
Peak alignment method
We used our permutation-based alignment metric (see “Computing alignment across subjects”), treating the brain as the “ground-truth” (i.e. permuting behavioral boundaries) to compare the behaviorally-derived boundaries to the group-average HMM-derived boundaries for each region. Normative (group-average) HMM-derived boundaries were computed by averaging voxelwise activity across subjects and then fitting an HMM to data within each region using the pre-determined number of events for that region (see “Automatic neural event boundary detection”). To determine the normative boundaries from the behavioral study for this approach, we needed “peak” shared behavioral boundaries. For this, we defined the peaks as timepoints when > 50%, or at least 21/40, subjects marked a boundary and enforced local sparsity by limiting peaks to timepoints that were more than 3 s apart. (If two peaks were within 3 s of one another, we took the higher “peak” [more agreement]; if they were of equal height, we took the median timepoint.) Importantly, we used 3 s as our tolerance for small deviations in button-press times across subjects to be in line with the fMRI data, where we had used 3 TRs (= 3 s).
Combining methods
To declare significant alignment between normative behavioral and normative neural boundaries in a region, that region had to show above-chance alignment with behavioral boundaries (P < 0.05) in both the density and peak methods.
Code accessibility
Data analysis, including links to code and other supporting material, can be found at: https://github.com/thefinnlab/individual_event_seg/.
Data accessibility
Data from this study, including raw MRI data, is available on OpenNeuro at https://openneuro.org/datasets/ds004516/. Other data including the behaviorally reported boundaries and the full transcribed recall and appraisal (text) can be found at: https://github.com/thefinnlab/individual_event_seg.
Results
In this work, our main goal was to investigate variability in neural event segmentation at the individual level and its behavioral consequences. We automatically detected region-wise event boundaries in individual subjects and used these to quantify the degree of variability across the cortex and how this variability changes with stimulus content. We also showed how and where similarity between individuals’ neural event boundaries during encoding can predict the similarity of recall and interpretation, thereby highlighting a functional role for event segmentation in an individual’s ultimate appraisal of an experience.
Slower-segmenting regions show more individual variability
Forty-three subjects watched four movies during fMRI scanning. These movies differed in meaningful and important ways (see Methods—“Movie overview” and Results—“Degree of across-subject alignment varies with movie content.”). Although we hypothesized that these differences would impact individual variability (an idea we return to in the following section), our goal with this first analysis was to demonstrate that cortical patterns of event segmentation, and individual variability therein, are generally consistent irrespective of the stimulus used.
To define neural event boundaries, we used a recently developed algorithm for detecting event boundaries in fMRI data: the Hidden Markov Model (HMM) proposed by Baldassano et al. (2017), which does not rely on annotations or hand-demarcated events but rather detects event boundaries as shifts in stable patterns of brain activity within a region. Although this algorithm yields valid neural event boundaries at the group level, to our knowledge, it had not been previously applied to individual-subject data. Therefore, we first undertook an analysis to assess whether this model can stably and reliably detect event boundaries at the individual level. All fMRI data were parcellated into 100 cortical regions using the Schaefer atlas (Schaefer et al. 2018).
We first sought to replicate past work (Baldassano et al. 2017; Geerligs et al. 2022) showing that at the group level, the number of events (i.e. the granularity of segmentation) is higher in sensory regions and lower in higher-order association areas that are sensitive to narrative information at longer timescales (Lerner et al. 2011; Honey et al. 2012). Using a train-test procedure to determine the optimal number of events (k) for each region from group-average data, we demonstrate that this relationship is maintained irrespective of the stimulus: event rate (number of events per minute) follows a posterior-to-anterior gradient (Fig. 1A).
We then investigated to what degree segmentation varied across individuals. Event segmentation can vary both at the level of the number of events (k) and the location of boundaries; we focus here on the latter, both for methodological reasons and because it is more directly related to movie content (see Methods section “Automatic neural event boundary detection” for more details). We used the fixed value of k for each region determined at the group level (cf. Fig. 1A) to fit an HMM to each individual subjects’ data from that region (see Methods section “Automatic neural event boundary detection”). We then assessed to what degree event boundary locations were aligned across subjects using a permutation-based method that generates a z-score for observed alignment relative to an appropriate null distribution for the total number of boundaries in each region (see Methods sections “Computing alignment across subjects” and “Across-subject alignment within movies: Experiment 1”).
Across all four movies, event boundaries were significantly aligned across subjects in most regions of the brain (>89/100), but this degree of alignment varied on another posterior to anterior gradient such that event boundary locations were more shared in sensory regions and more idiosyncratic in higher-order regions (Fig. 1B). We established strong correlations (r = 0.78–.90) between event rate and the degree of alignment across all four movies: faster-segmenting regions showed higher alignment across subjects (less individual variability), whereas slower-segmenting regions showed less alignment across subjects (more individual variability; Fig. 1C).
Notably, insignificant or negative across-subject alignment was seen in 20 unique regions across the four movies (1 for Defeat, 9 for Iteration, 11 for Growth, and 10 for Lemonade; negative across-subject alignment would indicate that permuted boundaries have a higher chance of showing alignment than the true boundaries). These regions were located in the prefrontal cortex (14/20), orbitofrontal cortex (2/20), temporal pole (3/20), and the cingulate (1/20). Of these, the majority were higher order “default mode” (40%) or limbic regions (25%) that have the slowest timescales of information processing (Hasson et al. 2008; Geerligs et al. 2022) and show more idiosyncratic anatomy and function (Hill et al. 2010; Mueller et al. 2013), including during naturalistic stimulation (Vanderwal et al. 2017; Finn et al. 2020; Gao et al. 2020; Chang et al. 2021). (It should also be noted, however, that these regions are also most commonly affected by signal drop-out.) No parcel showed negative or insignificant alignment across all four movies. (All analyses in this section were controlled for the effects of head motion and overall memory performance; see Methods section “Controlling for possible confounds”).
Degree of across-subject alignment varies with movie content
Having established that there is significant across-subject alignment that follows a cortical gradient in all movies, we next aimed to quantify how the degree of individual variability in event segmentation within region differed across the four movies. To help validate any differences across movies in neural event segmentation, we conducted an auxiliary behavioral experiment (Experiment 2—see Methods) in which a separate set of 40 subjects at Dartmouth College performed the same task as Experiment 1 outside the scanner, except that while watching each movie, individuals were instructed to press a button each time they thought there was an event boundary (i.e. points in the movie when there is a major change in topic, location, time, etc.). This allowed us to investigate whether similar patterns of movie-dependent variability in neural event segmentation were also present with behavioral event segmentation.
The movies differed in numerous and important ways. For the purposes of this project, we focus on two a prioriselected dimensions: (i) the presence or absence of screen cuts, and (ii) the presence or absence of social and affective information. Three of our movies followed a character-driven narrative trajectory complete with social and affective information (Iteration, Defeat, and Growth), whereas the fourth (Lemonade) followed a series of mechanical events (Rube-Goldberg machine) as opposed to a human-driven plot line. Two—Iteration and Defeat—used continuity editing (i.e. screen cuts), which serve as a strong cue for the start of a new event (Schwan et al. 2000; Zacks et al. 2010; Magliano and Zacks 2011; Smith et al. 2012; Loschky et al. 2020). We hypothesized that both dimensions would facilitate segmentation and increase the degree of across-subject alignment. First, continuity editing (such as screen cuts) is a well-documented driver of event boundaries; it often indicates a scene change and is highly correlated with situational changes that indicate the start of a new event (Schwan et al. 2000; Zacks et al. 2010; Magliano and Zacks 2011; Smith et al. 2012; Loschky et al. 2020). Second, socio-affective content often engages shared schemas of social interactions (e.g. family dinners in Growth; (Dunbar 1998; Wood et al. 2003; Adolphs et al. 2016) and is often more engaging than its non-social counterpart. Both social and affective information induce shared neural responses during movie-watching (Finn et al. 2018; Chen et al. 2020; Song et al. 2021) and recall (Chen et al. 2017a; Tomita et al. 2021) and may aid in segmentation (Boggia and Ristic 2015; Ristic and Capozzi 2022). It is notable, however, that, somewhat paradoxically, along with these shared responses, such dynamic, emotional content is also better suited at pulling out nuanced individual differences (Finn and Bandettini 2021), which we explore in the next section.
In our dataset, Iteration, a social movie with screen cuts, had significantly higher self-reported arousal (which we take as a proxy for affect/emotion) than the other three movies (repeated-measures ANOVA, F(3,126) = 7.49, P = 0.0001; Iteration > Defeat, Growth, Lemonade; t ≥ 3.68, P < 0.001). Overall, self-reported engagement levels did not differ between movies (repeated-measures ANOVA, F(3,126) =1.67, P = 0.18), although in pairwise comparisons, Iteration had mildly higher self-reported engagement levels than Defeat (pairwise comparisons, t = 2.2, P = 0.03). Thus, we expected that we would see the following rank-order from highest to lowest in across-subject consistency: (i) Iteration (social information, screen cuts, and most emotionally arousing), (ii) Defeat (social information and screen cuts), (iii) Growth (social information, but no screen cuts), and (iv) Lemonade (no social information and no screen cuts).
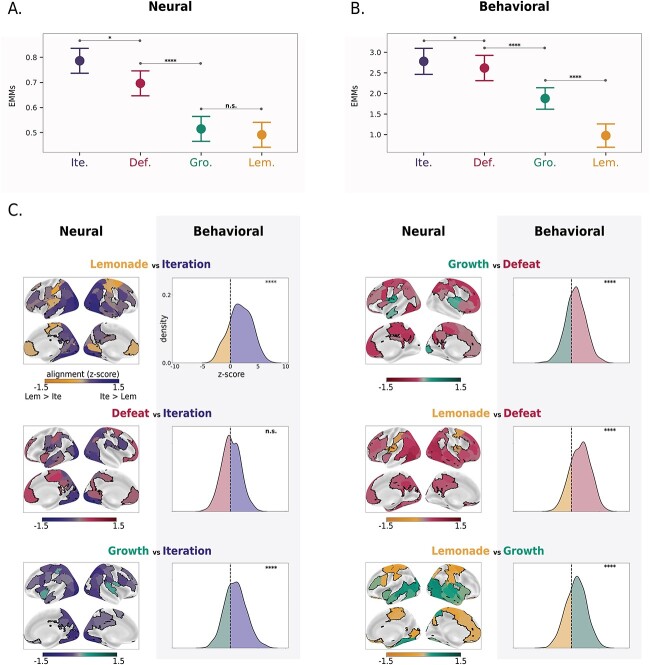
We tested these hypotheses by comparing alignment in each cortical region between each pair of movies (see Methods section “Identifying stimulus-dependent properties by taking the difference in alignment across movies.”) (All analyses in this section were controlled for effects of head motion [in neural data] and overall memory performance [in both behavioral and neural data]; see Methods section “Controlling for possible confounds”.) We used a linear mixed-effects model to assess the effect of movie on across-subject alignment, with region treated as a random effect. The analysis revealed a significant effect of movie (F(3, 300) = 39.5, P < 0.001). Post hoc tests were conducted to compare the estimated marginal means (EMMs) of the four movies. The results showed that alignment in Iteration was significantly higher than Defeat (estimate = 0.09, P = 0.029), Growth (estimate = 0.27, P < 0.001), and Lemonade (estimate = 0.29, P < 0.001). Additionally, alignment in Defeat was significantly higher than in Growth (estimate = 0.18, P < 0.001) and Lemonade (estimate = 0.20, P < 0.001), whereas Growth and Lemonade did not differ significantly (estimate = 0.02, P = 0.89). Thus, we observed the following rank order of movies, from highest to lowest neural alignment: [Iteration] > [Defeat] > [Growth and Lemonade] (Fig. 2A). Critically, variability in behavioral alignment largely mirrored this rank order of movies: although we found significant overall alignment in behavioral event boundaries within all movies (P = 0.0001; nbootstraps = 10,000—see Methods section “Across-subject alignment within movies—Experiment 2”), we further observed that movies that evoked more variability in neural event segmentation (across all brain regions) also tended to evoke more variability in behavioral event segmentation ([Iteration] > [Defeat] > [Growth] > [Lemonade]; linear mixed effects modeling with movie as fixed effect; pairwise comparisons of the EMMs are shown in Fig. 2B and bootstrapped pairwise comparisons are shown in Fig. 2C, behavioral (right) columns).
Fig. 2.
Degree of individual variability in neural and behavioral event segmentation depends on stimulus content. (A) Degree of overall alignment in neural event boundaries differs by movie.Plots depict the distribution of the estimated marginal means (EMMs) for the alignment across all regions (n = 100) for each movie. Whole-brain alignment shows the following rank order across movies: [Iteration] > [Defeat] > [Growth and Lemonade]. (B) Degree of alignment in behavioral event boundaries differs by movie.Plots depict the distribution of marginal means for the behavioral alignment across subjects. Across-subject behavioral alignment shows a rank order across movies that largely correspond to the rank order of neural alignment: [Iteration] > [Defeat] > [Growth] > [Lemonade]. (C) Cortical variability in degree of alignment across movies and comparison with degree of behavioral alignment. Left columns show pairwise comparisons between movies, indicating regions where the neural alignment is significantly higher in one movie versus another. Coloring indicates the movie where there is higher alignment and shade reflects the magnitude of the difference (maps are thresholded at q < 0.05; FDR-corrected to 100 regions within movie pair). Significance was determined using the residuals after regressing the effects of head motion and memory performance. Right columns show pairwise comparisons between movies to indicate cases where behavioral alignment is significantly higher in one movie versus another. For visualization purposes, we show the distribution of the difference in alignment values (z-scores) between pairs of subjects (the movie on the left was subtracted from the movie on the right). Significance was determined using the residuals after regressing the effects of memory performance (bootstraps = 10,000). Note that the dominant directionality of each pairwise comparison (i.e. which movie shows higher alignment) is similar in the neural and behavioral data. Legend: * P < 0.05; **** P < 0.0001; n.s., not significant.
Again, these four movies differ along many dimensions that could influence the degree of individual variability in boundary locations. To formally assess whether our two a priori hypothesized features account for significant variance in across-subject alignment, we modeled these features (instead of movie identity itself) as fixed effects in a series of follow-up analyses. For the neural data, we ran two independent linear mixed effects models: one with social information (present/absent) and one with screen cuts/continuity editing (present/absent) as the fixed effect. Each model contained region as the random effect. Both social information and continuity editing were significant predictors of neural alignment in their respective models (social: beta 0.17, P < 0.0001; continuity editing: beta 0.24, P < 0.0001). However, running a single model that included both social information and continuity editing revealed that when considered jointly, only continuity editing was a significant predictor of alignment (beta 0.23, P < 0.0001). For the behavioral data, both social information and continuity editing were significant predictors of behavioral alignment when treated as fixed effects in a single model (social: beta 0.91, P < 0.0001; continuity editing: beta 0.80, P < 0.0001) that also included subject-level crossed-random effects and memory scores as a fixed effect.
Thus, consistent with our hypotheses, we found that both behavioral and neural alignment were higher in movies in which there was a human-driven, social, and emotionally-engaging narrative trajectory, especially when there were screen cuts cueing new scenes (Iteration, Defeat). In movies where there were no screen cuts (Growth and Lemonade), across-subject alignment was lower. This supports the inference that segmentation is more straightforward for certain types of stimuli irrespective of the modality (neural/HMM-derived or behaviorally-derived).
Next, we took a region-specific approach to determine where in the cortex showed the biggest differences in alignment between movies (in other words, to identify brain regions where the degree of alignment is most content-dependent). Results are shown in Fig. 2C (neural (left) columns; nbootstraps = 10,000). We identified a trend such that certain movie content (social information) drives more alignment in neural event boundaries in mid-level regions of association-cortex. For instance, although regions on either end of the cortical hierarchy showed consistently low (PFC) or high (V1) alignment across movies (cf. Fig. 1B), canonical social-processing regions such as the superior temporal sulcus showed higher alignment in movies with social interactions compared to non-social movies ([Iteration, Defeat, Growth] > [Lemonade]). Our non-social movie, Lemonade, showed primarily higher alignment in somatomotor processing regions, likely reflecting neural state changes in response to the continuous switches in the location and object-driven motion that are specific to this movie.
Shared neural event boundaries lead to shared interpretations
We next tested the hypothesis that individual variability in neural event segmentation would have behavioral consequences, such that individuals who were more similar in their neural event boundaries during encoding would also be more similar in their interpretation of the movie. To do so, we used free-speech data acquired immediately following each of the four movies in which subjects were prompted to speak for 3 minutes about what they remembered and how they felt about the events and characters (see Methods section “Measuring cross-subject similarity in movie recall/appraisal” for exact instructions). Crucially, subjects did not simply recall the events objectively as they might do in a typical episodic memory task, but also shared subjective interpretations and overall reflections on the movies, often including links to their own personal lives. Thus, we henceforth refer to this task as the appraisal task.
We recorded and transcribed each subject’s speech and submitted the transcripts to Google’s Universal Sentence Encoder (USE), a tool from natural language processing (NLP) that encodes text into high-dimensional vectors that reflect semantic content (Cer et al. (2018); see Methods section “Measuring cross-subject similarity in movie recall/appraisal”). Language embeddings provide a relatively unbiased way to quantify similarity in appraisal content. For each movie, we then calculated a subject-by-subject similarity matrix from these vectors and compared it to the subject-by-subject similarity matrix of neural event boundaries in each region while controlling for objective memory performance (as measured by performance on memory questions) using an inter-subject representational similarity analysis (Fig. 3A,inset).
Fig. 3.
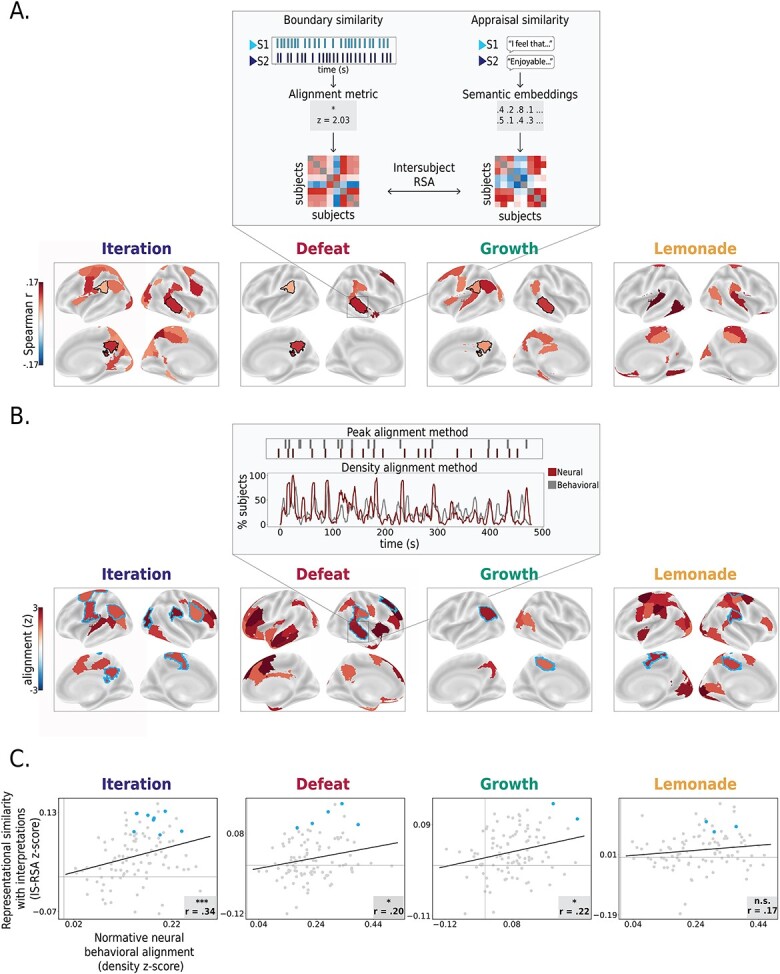
Relationship between neural event segmentation and behavior. (A) Inter-subject representational similarity between neural event boundary locations and ultimate appraisals. Maps depict regions where pairs of subjects that were more similar in their neural event boundaries also had more similar interpretations of the movie (partial Mantel test controlling for objective memory performance; q < 0.05, FDR-corrected to 100 regions within the movie). Black contours indicate regions that showed significant representational similarity across all three social movies: the superior temporal sulcus (partial Mantel test, q < 0.05, corrected within the movie), the precuneus, and the angular gyrus (partial Mantel test, P < 0.05 uncorrected across all three social movies). (B) Brain regions where normative (group-level) neural boundaries align with normative (group-level) behavioral boundaries. (q < 0.05, FDR-corrected across 100 regions within each movie). Importantly, though for each movie there are numerous regions where this relationship holds, specific regions vary across movies, suggesting that this relationship is somewhat movie-specific. Alignment between normative behavioral and neural boundaries was computed using two approaches (see Methods; inset depicts an example region in the superior temporal sulcus) Blue contours indicate regions that also show significant alignment with appraisal (cf. panel A). (C) At the movie level, similar brain regions show relationships with both normative behavioral segmentation and individual-level appraisals. Specifically, the degree of alignment between neural and behavioral normative boundaries (“Density alignment”) is correlated with the relationship between individual neural event boundaries and individual interpretations. Gray dots represent each region. Regions that show a significant relationship in both analyses (blue contours in B) are demarcated with blue dots. Values reflect Fisher z-transformed correlation coefficients. A Spearman correlation was used. Legend:*P < 0.05; *** P < 0.001.
Results (Fig. 3A) showed that in several brain regions, the degree of alignment in neural event boundaries during movie-watching predicted similarity in appraisal: in other words, subjects who were similar in event boundaries in these regions also tended to speak similarly about the movie afterwards. The specific regions showing these effects differed for each movie, but roughly corresponded to regions sensitive to each movie’s respective high-level content (e.g., humans versus objects; Iteration, Growth, Defeat versus Lemonade; Speer et al. 2009). In the three movies with social information (Iteration, Defeat, and Growth), these regions included high-order social cognition areas that are often associated with complex narrative processing, some of which are considered part of the default-mode network (DMN; for review, see Yeshurun et al. 2021). Three regions—the superior temporal sulcus, the angular gyrus, and the precuneus—emerged in all three social movies (Fig. 3A; black contours). Significant regions in the non-social movie (Lemonade) had a different spatial pattern from the social movies. These regions included mostly somatomotor, dorsal-attention, and limbic regions, including a posterior temporal, ventral stream object-category selective region, the lingual gyrus, the temporal parietal junction, cingulate, orbitofrontal cortex, and a left somatomotor region previously linked to motor imagery (Chen et al. 2009; Chinier et al. 2014).
Relationships between neural boundaries and interpretations are strongest in regions reflecting normative behavioral segmentation
As noted above, the different spatial patterns of relationships between neural event segmentation and appraisal across movies that we identified in the previous analysis are likely due to differences in movie content. For a particular movie, are the regions most closely tied to group-level behavioral segmentation during encoding also those most likely to show individual-difference relationships between segmentation and ultimate appraisal?
To answer this question, we needed to independently quantify the degree to which neural event boundaries in a region reflect behavioral event boundaries. Although past work has shown that automatically detected neural event boundaries tend to align with behaviorally reported event boundaries in certain regions of association cortex such as the angular gyrus or the posterior medial cortex (Baldassano et al. 2017; Lee et al. 2021; Williams et al. 2022; Yates et al. 2022), this work has typically been limited to single stimuli, so the extent to which the regions showing behavioral-neural event alignment holds across stimuli has yet to be explored.
Toward this goal, we, again, used our auxiliary behavioral study (Experiment 2) and identified “normative” behavioral boundaries where subjects agreed, on average, that there was an event boundary. We then compared these normative behavioral boundaries with normative neural boundaries determined by fitting an HMM to the fMRI data averaged across subjects. We did this neural-behavioral normative boundary comparison using two approaches (Fig. 3B inset, see Methods section “Comparison of neural event boundaries and behavioral event boundaries”) and only considered regions that were significantly aligned to behavior (P < 0.05) in both methods.
As expected, regions with significant neural-behavioral segmentation alignment varied across movies (Fig. 3B), but generally showed anatomical consistency with previous reports of regions that are sensitive to event changes (Zacks et al. 2001; Speer et al. 2003, 2007; DuBrow and Davachi 2016; Baldassano et al. 2017; Masís-Obando et al. 2022); namely, the temporoparietal junction, prefrontal cortex, insula, parietal cortex, and cingulate (though note, interestingly, that Lemonade, the non-social movie, showed neural-behavioral segmentation alignment in earlier visual regions).
Finally, we correlated, across regions, the degree of cross-modal alignment between normative neural and behavioral boundaries (density metric; Fig. 3B) and the representational similarity r-value between individual neural event boundaries and appraisal (cf. Fig. 3A). Indeed, these two properties were positively correlated in three of the four movies (Fig. 3C), indicating that, for a given movie, segmentation in regions that mirror behaviorally reported event boundaries at the group level are also relevant for how that movie is ultimately remembered and appraised at the individual level. Regions that showed significant cross-modal normative alignment, as well as a significant relationship between individual boundary locations and appraisal, are highlighted with blue contours and blue dots in Fig. 3B and C, respectively. We suggest that the same regions that show neural segmentation at moments corresponding to behaviorally reported boundaries were likely involved in appraising and translating these events into memory. Future work should investigate this in a more causal manner.
Discussion
Individuals segment incoming information into events in different ways. Here, using four different short-film stimuli, we investigated individual differences in neural event boundaries and how they relate to behavior. Results showed that there is a posterior-to-anterior gradient for between-subject alignment in neural event boundaries that is tightly correlated with the rate of segmentation, such that regions that segment more slowly also show less alignment (i.e. more individual variability). Notably, we found strong movie effects for regions in the middle of this gradient: although alignment was high in low-level sensory regions and low in high-order narrative processing regions across movies, regions that are more tuned to a certain type of input showed variable alignment depending on the movie. We also describe one mechanism by which narratives may generate variable interpretations across people: individuals with more similar neural event boundaries in certain regions during movie-watching tended to have more similar appraisals of the movie.
Our first goal was to validate the use of automated event segmentation algorithms on fMRI data from individual subjects, and to characterize how much individuals vary in their neural event boundary locations. We found that although subjects show above-chance alignment in event boundaries in the majority of the cortex irrespective of the stimulus, the degree of alignment decreases from unimodal to transmodal association regions. This finding is in line with reports using ISC to show that synchrony of activity decreases from posterior to anterior regions (Yeshurun et al. 2017a; Haxby et al. 2020; Chang et al. 2022). Thus, regardless of measurement modality—ISC (continuous activity timecourses) or event segmentation (discrete state switches)—dynamic neural responses to external stimuli are more idiosyncratic in higher-order regions including the temporoparietal junction, superior temporal sulcus, precuneus, dorsal medial and ventral medial prefrontal cortex, and the medial frontal gyrus. Notably, these regions are often considered part of the default mode network with reported involvement in social cognition, self-referential processing, and the consolidation of autobiographical memory (for a review, see Yeshurun et al. 2021).
We also found that the degree of alignment is tightly correlated with segmentation rate across regions. This broadens recent findings on neural event rates (Baldassano et al. 2017; Geerligs et al. 2022; Yates et al. 2022) and on the intrinsic process memory and temporal receptive windows (i.e., the length of time before a brain area’s response in which information will impact that response), which are all thought to follow a hierarchical posterior-to-anterior gradient as well (Lerner et al. 2011; Honey et al. 2012; Hasson et al. 2015). We suggest that information integration (as measured by event boundary alignment) is more stereotyped across subjects in sensory, unimodal cortex and becomes less standardized in higher-order regions with slower dynamics due, in part, to idiosyncratic processing strategies, experiences, and memories.
To support the link between idiosyncratic neural event boundaries and variable information integration, we demonstrate that patterns of neural event segmentation in certain regions relate to an individual’s memory of the segmented experience. Using ISC, it has been shown that individuals with shared context (Yeshurun et al. 2017b), shared traits (e.g. paranoia; (Finn et al. 2018)), or shared experimentally manipulated perspectives (Lahnakoski et al. 2014) have similar neural responses while experiencing a narrative. We aimed to extend this past work and capture meaningful differences in how idiosyncratic neural activity during encoding relates to endogenously generated interpretations and recalls. Past work has theorized that current perceptual information interacts with long-term knowledge about event categories (schemas and scripts) when forming and updating event models (Radvansky and Zacks 2014). These stereotyped changes likely underlie shared boundaries (Baldassano et al. 2018; Masís-Obando et al. 2022) and reflect central hubs in the narrative (Lee and Chen 2022). We suggest that individual-specific boundaries—i.e., those not shared among the majority of subjects—may reflect moments with more idiosyncratic meanings (for example, a moment activating one’s own autobiographical memory) that lead to variable interpretations of a stimulus. To test this, we leveraged complex narratives that generated variable appraisals across people and had subjects freely discuss the movies. We then used inter-subject representational similarity analysis to show that pairs of subjects with more similar event boundaries in numerous higher-order regions, including the angular gyrus, superior temporal sulcus, and precuneus, also had more similar appraisals of each stimulus. A large subset of these regions also reflect behaviorally reported event boundaries. Altogether, these findings uphold the functional role of these regions in the high-level encoding of an experience and its ultimate behavioral consequences, including its organization into memory.
Although the idea that stimulus features systematically affect group-level segmentation is not novel—Newtson et al. (1977) were among the first to consider how a movie’s features encourage segmentation of continuous streams of information into units—here, we extend this to characterize how movie content affects neural and behavioral segmentation at the individual level. We demonstrated that although the spatial patterns of relative alignment were consistent across movies, the absolute degree of alignment differed with the specific movie and its content. Although these four movies differed along numerous dimensions that could have affected degree of alignment, our two a priori hypothesized features—namely, the presence of both continuity editing and a social, character-driven, emotional narrative—drove both higher overall alignment across the cortex (cf. Fig. 2) and in behaviorally reported boundaries (cf. Fig. 3).
There are some limitations to this work. First, although our choice of movies was somewhat principled in that it was based in existent theories about the effects of content on segmentation (Grall and Finn 2022), our ultimate stimulus set is still arbitrary and there are plenty of other low-, mid-, and high-level features that vary across these movies. We hope that through the release of our dataset, others can build on this work and investigate additional stimulus features that may affect individual segmentation patterns and variability therein. Second, our sample size (n = 43) is relatively low for individual-differences work. However, the strength of our design is that we compared the same subjects across four movies. Furthermore, we did not attempt to link individual neural event segmentation patterns to trait-level behavioral measurements, an analysis for which we would likely be underpowered; rather, we focused on quantifying the overall degree of individual variability and how this differs as a function of both brain region and stimulus content, for which the sample size should be a less limiting factor.
In sum, our work shows that individual differences in the location of neural event boundaries (i) meaningfully relate to behavior, and (ii) vary with stimulus content in both their strength and spatial patterns, thereby emphasizing the importance of considering stimulus content in naturalistic neuroimaging paradigms. Although numerous studies have explored individual differences in event segmentation at the behavioral level, here, we extend existing methods to identify individual differences at the neural level during encoding and their consequences for how a stimulus is ultimately remembered and appraised. Future work should investigate individual variability not only in the temporal locations of event boundaries as we did here, but also in the rate of segmentation. Future work should also explore whether personality or clinical traits (see Zacks and Sargent 2010)) that are stable over time are associated with particular styles of neural segmentation that impact ongoing cognition and behavior.
Acknowledgments
The authors thank Peter Bandettini, Peter Molfese, Daniel Handwerker, and Javier Gonzalez-Castillo for helpful discussions about experimental design and support with data collection. We thank Gang Chen for statistical advice and help implementing the LME-CRE analyses. We thank Tory Benson for help with figure preparation. We also thank Sofia Yawand-Wossen and Payton Weiner for their assistance with movie labeling and appraisal transcript preparation, and the Undergrad Research Assistantships at Dartmouth program for funding their support.
Contributor Information
Clara Sava-Segal, Department of Psychological and Brain Sciences, Dartmouth College, Hanover, NH 03748, USA.
Chandler Richards, Laboratory of Brain and Cognition, National Institute of Mental Health, Bethesda, MD 20892-9663, USA.
Megan Leung, Department of Psychological and Brain Sciences, Dartmouth College, Hanover, NH 03748, USA.
Emily S Finn, Department of Psychological and Brain Sciences, Dartmouth College, Hanover, NH 03748, USA.
Funding
This work was supported by a National Science Foundation Graduate Research Fellowship (to C.S.S.) and by the National Institutes of Health (grants K99MH120257 and R00MH120257 to E.S.F.).
Conflict of interest statement: None declared.
Author contributions
Clara Sava-Segal (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing—original draft, Writing—review and editing), Chandler Richards (Data curation, Investigation, Project administration), Megan Leung (Data curation, Project administration), Emily Finn (Conceptualization, Data curation, Funding acquisition, Investigation, Project administration, Supervision, Writing—original draft, Writing—review and editing).
References
- Adolphs R, Nummenmaa L, Todorov A, Haxby JV. Data-driven approaches in the investigation of social perception. Philos Trans R Soc B. 2016:371(1693):20150367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey HR, Kurby CA, Giovannetti T, Zacks JM. Action perception predicts action performance. Neuropsychologia. 2013a:51(11):2294–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey HR, Zacks JM, Hambrick DZ, Zacks RT, Head D, Kurby CA, Sargent JQ. Medial temporal lobe volume predicts elders’ everyday memory. Psychol Sci. 2013b:24(7):1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey HR, Kurby CA, Sargent JQ, Zacks JM. Attentional focus affects how events are segmented and updated in narrative reading. Mem Cogn. 2017:45(6):940–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassano C, Chen J, Zadbood A, Pillow JW, Hasson U, Norman KA. Discovering event structure in continuous narrative perception and memory. Neuron. 2017:95(3):709–721.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassano C, Hasson U, Norman KA. Representation of real-world event schemas during narrative perception. J Neurosci. 2018:38(45):9689–9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015:67(1):1–48. [Google Scholar]
- Ben-Yakov A, Henson RN. (Eds.)The hippocampal film editor: sensitivity and specificity to event boundaries in continuous experience. J Neurosci. 2018:38(47):10057–10068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JB, Bower GH. Story understanding as problem-solving. Poetics. 1980:9:223–250. [Google Scholar]
- Boggia J, Ristic J. Social event segmentation. Q J Exp Psychol. 2015:68:731–744. [DOI] [PubMed] [Google Scholar]
- Cer D, Yang Y, Kong S, Hua N, Limtiaco N, John RS, Constant N, Guajardo-Cespedes M, Yuan S, Tar C, et al. Universal Sentence Encoder. 2018:arXiv:180311175 [cs].
- Chang L, Fleetwood G, Manning J, Parkinson C, Finn E, Wager T, Haxby J, Geerligs L, Vega A de la, Lahnakoski J, Yeshurun Y, Shappell H, Yarkoni T, Baldassano C, Shim WM. 2020. Neuroimaging analysis methods for naturalistic data ( No. FZJ-2020-04493). Presented at the Annual meeting of the Organization for Human Brain Mapping 2020, Zenodo. [Google Scholar]
- Chang LJ, Jolly E, Cheong JH, Rapuano KM, Greenstein N, Chen P-HA, Manning JR. Endogenous variation in ventromedial prefrontal cortex state dynamics during naturalistic viewing reflects affective experience. Sci Adv. 2021:7:eabf7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CHC, Nastase SA, Hasson U. Information flow across the cortical timescale hierarchy during narrative construction. Proc Natl Acad Sci. 2022:119(51):e2209307119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P-H(C), Chen J, Yeshurun Y, Hasson U, Haxby J, Ramadge PJ. A reduced-dimension fMRI shared response model. In: Advances in neural information processing systems. New York: Curran Associates, Inc. 2015:28. [Google Scholar]
- Chen H, Yang Q, Liao W, Gong Q, Shen S. Evaluation of the effective connectivity of supplementary motor areas during motor imagery using Granger causality mapping. NeuroImage. 2009:47(4):1844–1853. [DOI] [PubMed] [Google Scholar]
- Chen J, Leong YC, Honey CJ, Yong CH, Norman KA, Hasson U. Shared memories reveal shared structure in neural activity across individuals. Nat Neurosci. 2017a:20(1):115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Taylor PA, Shin Y-W, Reynolds RC, Cox RW. Untangling the relatedness among correlations, part II: inter-subject correlation group analysis through linear mixed-effects modeling. NeuroImage. 2017b:147:825–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P-HA, Jolly E, Cheong JH, Chang LJ. Intersubject representational similarity analysis reveals individual variations in affective experience when watching erotic movies. NeuroImage. 2020:216:116851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinier E, N’Guyen S, Lignon G, Ter Minassian A, Richard I, Dinomais M. Effect of motor imagery in children with unilateral cerebral palsy: fMRI study. PLoS One. 2014:9(4):e93378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SS, Baldassano C. Developmental changes in story-evoked responses in the neocortex and hippocampus. 2021. [DOI] [PMC free article] [PubMed]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996:29:162–173. [DOI] [PubMed] [Google Scholar]
- Devlin J, Chang M-W, Lee K, Toutanova K. BERT: pre-training of deep bidirectional transformers for language understanding. 2019: arXiv:181004805 [cs].
- de Leeuw JR. jsPsych: a JavaScript library for creating behavioral experiments in a web browser. Behav Res Methods. 2015:47(1):1–12. [DOI] [PubMed] [Google Scholar]
- DuBrow S, Davachi L. The influence of context boundaries on memory for the sequential order of events. J Exp Psychol Gen. 2013:142(4):1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBrow S, Davachi L. Temporal binding within and across events. Neurobiol Learn Mem. 2016:134:107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar RIM. The social brain hypothesis. Evol Anthropol. 1998:6(5):178–190. [Google Scholar]
- Ezzyat Y, Davachi L. What constitutes an episode in episodic memory? Psychol Sci. 2011:22(2):243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzyat Y, Davachi L. Similarity breeds proximity: pattern similarity within and across contexts is related to later mnemonic judgments of temporal proximity. Neuron. 2014:81(5):1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn ES, Bandettini PA. Movie-watching outperforms rest for functional connectivity-based prediction of behavior. NeuroImage. 2021:235:117963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn ES, Corlett PR, Chen G, Bandettini PA, Constable RT. Trait paranoia shapes inter-subject synchrony in brain activity during an ambiguous social narrative. Nat Commun. 2018:9(1):2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn ES, Glerean E, Khojandi AY, Nielson D, Molfese PJ, Handwerker DA, Bandettini PA. Idiosynchrony: from shared responses to individual differences during naturalistic neuroimaging. NeuroImage. 2020:215:116828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Chen G, Wu J, Wang Y, Hu Y, Xu T, Zuo X-N, Yang Z. Reliability map of individual differences reflected in inter-subject correlation in naturalistic imaging. NeuroImage. 2020:223:117277. [DOI] [PubMed] [Google Scholar]
- Geerligs L, van Gerven M, Güçlü U. Detecting neural state transitions underlying event segmentation. NeuroImage. 2021:236:118085. [DOI] [PubMed] [Google Scholar]
- Geerligs L, Gözükara D, Oetringer D, Campbell KL, van Gerven M, Güçlü U. A partially nested cortical hierarchy of neural states underlies event segmentation in the human brain. eLife. 2022:11:e77430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glerean E, Pan RK, Salmi J, Kujala R, Lahnakoski JM, Roine U, Nummenmaa L, Leppämäki S, Nieminen-von Wendt T, Tani P, et al. Reorganization of functionally connected brain subnetworks in high-functioning autism. Hum Brain Mapp. 2016:37(3):1066–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grall C, Finn ES. Leveraging the power of media to drive cognition: a media-informed approach to naturalistic neuroscience. Soc Cogn Affect Neurosci. 2022:17(6):598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Yang E, Vallines I, Heeger DJ, Rubin N. A hierarchy of temporal receptive windows in human cortex. J Neurosci. 2008:28(10):2539–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Chen J, Honey CJ. Hierarchical process memory: memory as an integral component of information processing. Trends Cogn Sci. 2015:19(6):304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Guntupalli JS, Nastase SA, Feilong M. Hyperalignment: modeling shared information encoded in idiosyncratic cortical topographies. eLife. 2020:9:e56601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J, Dierker D, Neil J, Inder T, Knutsen A, Harwell J, Coalson T, Van Essen D. A surface-based analysis of hemispheric asymmetries and folding of cerebral cortex in term-born human infants. J Neurosci. 2010:30:2268–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Thesen T, Donner TH, Silbert LJ, Carlson CE, Devinsky O, Doyle WK, Rubin N, Heeger DJ, Hasson U. Slow cortical dynamics and the accumulation of information over long timescales. Neuron. 2012:76(2):423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafarpour A, Buffalo EA, Knight RT, Collins AGE. Event segmentation reveals working memory forgetting rate. iScience. 2022:25(3):103902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Anderson M, Antony J, Baldassano C, Brooks P, Cai MB, Chen P-HC, Ellis CT, Henselman-Petrusek G, Huberdeau D, et al. BrainIAK: the brain imaging analysis kit. Aperture Neuro. 2021:2021(4):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurby CA, Zacks JM. Segmentation in the perception and memory of events. Trends Cogn Sci. 2008:12(2):72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahnakoski JM, Glerean E, Jääskeläinen IP, Hyönä J, Hari R, Sams M, Nummenmaa L. Synchronous brain activity across individuals underlies shared psychological perspectives. NeuroImage. 2014:100(100):316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Chen J. Predicting memory from the network structure of naturalistic events. Nat Commun. 2022:13(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Aly M, Baldassano C. Anticipation of temporally structured events in the brain. elife. 2021:10:e64972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth R, Singmann H, Love J, Buerkner P, Herve M. Emmeans: estimated marginal means, aka least-squares means. 2020. [accessed 2023 February 20]. https://CRAN.R-project.org/package=emmeans.
- Lerner Y, Honey CJ, Silbert LJ, Hasson U. Topographic mapping of a hierarchy of temporal receptive windows using a narrated story. J Neurosci. 2011:31(8):2906–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loschky LC, Larson AM, Smith TJ, Magliano JP. The scene perception & event comprehension theory (SPECT) applied to visual narratives. Top Cogn Sci. 2020:12(1):311–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magliano JP, Zacks JM. The impact of continuity editing in narrative film on event segmentation. Cogn Sci. 2011:35(8):1489–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masís-Obando R, Norman KA, Baldassano C. Schema representations in distinct brain networks support narrative memory during encoding and retrieval. eLife. 2022:11:e70445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGatlin KC, Newberry KM, Bailey HR. Temporal chunking makes life’s events more memorable. Open Psychol. 2018:1(1):94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S, Wang D, Fox MD, Yeo BTT, Sepulcre J, Sabuncu MR, Shafee R, Lu J, Liu H. Individual variability in functional connectivity architecture of the human brain. Neuron. 2013:77(3):586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newtson D. Attribution and the unit of perception of ongoing behavior. J Pers Soc Psychol. 1973:28(1):28–38. [Google Scholar]
- Newtson D, Harvey JH, Ickes WJ, Kidd RF. New directions in attribution research. New Jersey: Lawrence Erlbaum Associates Hillsdale; 1976. [Google Scholar]
- Newtson D, Engquist GA, Bois J. The objective basis of behavior units. J Pers Soc Psychol. 1977:35(12):847–862. [Google Scholar]
- Peirce J, Gray JR, Simpson S, MacAskill M, Höchenberger R, Sogo H, Kastman E, Lindeløv JK. PsychoPy2: Experiments in behavior made easy. Behav Res. 2019:51:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raccah O, Doelling KB, Davachi L, Poeppel D. Acoustic features drive event segmentation in speech. J Exp Psychol Learn Mem Cogn. 2022. [DOI] [PubMed] [Google Scholar]
- Radvansky GA, Zacks JM. Event cognition, event cognition. New York, NY: Oxford University Press; 2014. [Google Scholar]
- Ristic J, Capozzi F. The role of visual and auditory information in social event segmentation. (preprint). Open Science Framework; 2022. [DOI] [PMC free article] [PubMed]
- Sargent JQ, Zacks JM, Hambrick DZ, Zacks RT, Kurby CA, Bailey HR, Eisenberg ML, Beck TM. Event segmentation ability uniquely predicts event memory. Cognition. 2013:129(2):241–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo X-N, Holmes AJ, Eickhoff SB, Yeo BTT. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb Cortex. 2018:28(9):3095–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan S, Garsoffky B, Hesse FW. Do film cuts facilitate the perceptual and cognitive organization of activity sequences? Mem Cogn. 2000:28(2):214–223. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Levin D, Cutting JE. A window on reality: perceiving edited moving images. Curr Dir Psychol Sci. 2012:21(2):107–113. [Google Scholar]
- Song K, Tan X, Qin T, Lu J, Liu T-Y. MPNet: masked and permuted pre-training for language understanding. 2020:abs/2004.09297.
- Song H, Finn ES, Rosenberg MD. Neural signatures of attentional engagement during narratives and its consequences for event memory. PNAS. 2021:118(33):e2021905118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer NK, Swallow KM, Zacks JM. Activation of human motion processing areas during event perception. Cogn Affect Behav Neurosci. 2003:3(4):335–345. [DOI] [PubMed] [Google Scholar]
- Speer NK, Zacks JM, Reynolds JR. Human brain activity time-locked to narrative event boundaries. Psychol Sci. 2007:18(5):449–455. [DOI] [PubMed] [Google Scholar]
- Speer NK, Reynolds JR, Swallow KM, Zacks JM. Reading stories activates neural representations of visual and motor experiences. Psychol Sci. 2009:20(8):989–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita TM, Barense MD, Honey CJ. The similarity structure of real-world memories (preprint). Anim Behav Cogn. 2021. [Google Scholar]
- Vanderwal T, Eilbott J, Finn ES, Craddock RC, Turnbull A, Castellanos FX. Individual differences in functional connectivity during naturalistic viewing conditions. NeuroImage. 2017:157:521–530. [DOI] [PubMed] [Google Scholar]
- Wang W, Wei F, Dong L, Bao H, Yang N, Zhou M. MiniLM: deep self-attention distillation for task-agnostic compression of pre-trained transformers. 2020:abs/2002.10957.
- Williams JA, Margulis EH, Nastase SA, Chen J, Hasson U, Norman KA, Baldassano C. High-order areas and auditory cortex both represent the high-level event structure of music. J Cogn Neurosci. 2022:34(4):699–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JN, Romero SG, Makale M, Grafman J. Category-specific representations of social and nonsocial knowledge in the human prefrontal cortex. J Cogn Neurosci. 2003:15(2):236–248. [DOI] [PubMed] [Google Scholar]
- Yates TS, Skalaban LJ, Ellis CT, Bracher AJ, Baldassano C, Turk-Browne NB. Neural event segmentation of continuous experience in human infants. Proc Natl Acad Sci USA. 2022:119(43): e2200257119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshurun Y, Nguyen M, Hasson U. Amplification of local changes along the timescale processing hierarchy. Proc Natl Acad Sci. 2017a:114(35):9475–9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshurun Y, Swanson S, Simony E, Chen J, Lazaridi C, Honey CJ, Hasson U. Same story, different story: the neural representation of interpretive frameworks. Psychol Sci. 2017b:28(3):307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshurun Y, Nguyen M, Hasson U. The default mode network: where the idiosyncratic self meets the shared social world. Nat Rev Neurosci. 2021:22(3):181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks JM, Sargent JQ. Chapter 7 - event perception: A theory and its application to clinical neuroscience. In: Ross BH, editors. The psychology of learning and motivation. Advances in research and theory. Burlington: Academic Press; 2010:53:253–299. [Google Scholar]
- Zacks JM, Swallow KM. Event segmentation. Curr Dir Psychol Sci. 2007:16(2):80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks JM, Braver TS, Sheridan MA, Donaldson DI, Snyder AZ, Ollinger JM, Buckner RL, Raichle ME. Human brain activity time-locked to perceptual event boundaries. Nat Neurosci. 2001:4(6):651–655. [DOI] [PubMed] [Google Scholar]
- Zacks JM, Speer NK, Vettel JM, Jacoby LL. Event understanding and memory in healthy aging and dementia of the Alzheimer type. Psychol Aging. 2006:21(3):466–482. [DOI] [PubMed] [Google Scholar]
- Zacks JM, Speer NK, Swallow KM, Braver TS, Reynolds JR. Event perception: a mind/brain perspective. Psychol Bull. 2007:133(2):273–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks JM, Kumar S, Abrams RA, Mehta R. Using movement and intentions to understand human activity. Cognition. 2009:112(2):201–216. [DOI] [PubMed] [Google Scholar]
- Zacks JM, Speer N, Swallow K, Maley C. The brain’s cutting-room floor: segmentation of narrative cinema. Front Hum Neurosci. 2010:4:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalla T, Verlut I, Franck N, Puzenat D, Sirigu A. Perception of dynamic action in patients with schizophrenia. Psychiatry Res. 2004:128(1):39–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from this study, including raw MRI data, is available on OpenNeuro at https://openneuro.org/datasets/ds004516/. Other data including the behaviorally reported boundaries and the full transcribed recall and appraisal (text) can be found at: https://github.com/thefinnlab/individual_event_seg.